Abstract
Background:
Sleep difficulties and rhythm disturbances are some of the problems associated with adolescent binge drinking. Recently, animal models of alcohol-induced insomnia have been developed, however, studies in human subjects have recently focused not only on nighttime EEG findings but also on daytime sleepiness and disrupted activity levels as typically measured by activity tracking devices such as a “Fitbit”. We sought to develop and test a Fitbit-like device in rats and use it to track rest-activity cycles following adolescent alcohol exposure.
Methods:
The effects of 5 weeks of adolescent ethanol vapor or control conditions were evaluated in 48 male and female Wistar rats, on FitBite activity while intoxicated, and over acute (24 hours post vapor exposure) and chronic withdrawal (four weeks post vapor exposure). Data were analyzed using activity count and cosinor analyses. Fourteen rats were subsequently implanted with cortical electrodes and data from the FitBite were compared to EEG data to determine how well the FitBite could identify sleep and activity cycles.
Results:
Female rats were generally found to be more active than males with higher circadian rhythm amplitudes and mesors (rhythm adjusted means) across a 24h period. Significant correlations between EEG estimated sleep and activity counts using the FitBite were found. When the rats were tested during intoxication after 4 weeks of ethanol vapor exposure, they had significantly less overall activity. Disruptions in circadian rhythm was also found with significant decreases in the circadian amplitude, mesor, and a later shift in the acrophase. At 24 hours of ethanol withdrawal, rats had more episodes of activity with shorter durations during the daytime, when rats are expected to spend more of their time sleeping. This effect remained at 4 weeks following withdrawal, but circadian rhythm disruptions were no longer present.
Conclusions:
A Fitbit-like device can be successfully used in rats to assess rest-activity cycles. Adolescent alcohol exposure was found to produce circadian rhythm disturbance that were not observed after withdrawal. Fragmentation of ultradian rest-activity cycles during the light period were found at 24hrs and 4 weeks after withdrawal and support data demonstrating sleep disturbance long after alcohol withdrawal.
Keywords: adolescence, alcohol, sleep, activity rhythms, Fitbit
INTRODUCTION
Disturbance in sleep regulation is one of the health risks associated with adolescent alcohol and drug use. While adolescence is a time when both drug- and alcohol-seeking behaviors emerge, this age group may be particularly vulnerable to sleep disturbance associated with substance use (Hasler and Clark, 2013, Ehlers et al., 2018b, Bartel et al., 2015, Colrain et al., 2009, Hasler et al., 2015, Hasler et al., 2014b, Hasler et al., 2014a). There is a growing body of evidence indicating that alcohol use may be associated with disturbances in circadian rest activity rhythms, however, little is known about the effects in adolescents (see Meyrel et al., 2020, Hasler et al., 2012, Tamura et al., 2021). It has long been demonstrated that the “evening chronotype”, as compared to the “morning chronotype” is associated with greater risk of developing an AUD (Gulick and Gamsby, 2018, Lemoine et al., 2013). If such individuals are staying up late drinking, they may shift their sleep and food consumption time and be exposed to light at night, all factors that may induce rhythm disruption (Escobar et al., 2011). Teenagers may also be especially at risk for rhythm disruption due to their evening preference and weekday schedules that promote late night hours, exposure to light at night, and sleep deprivation due to the need to attend school early in the morning (Adan et al., 2012). This weekday schedule may be followed by irregular and “catch up” sleep patterns on the weekends (Hagenauer and Lee, 2012, Touitou, 2013). These irregular sleep cycles seen in some teens may also be associated with drug and alcohol use as demonstrated in a Finnish study of adolescents where the presence of daytime sleepiness combined with irregular sleep schedules was found to account for 12% of the variance in substance use in 15 year old girls and 26% in boys (Tynjala et al., 1997). While many investigators posit the theory that a reciprocal relationship exists between disrupted sleep and circadian rhythms and alcohol usage (Meyrel et al., 2020, Hasler et al., 2012, Tamura et al., 2021), in human studies it is difficult to disentangle the importance of lifestyle factors in determining what is causal in the relationship between substance use and sleep and activity disruptions.
Recently, animal models of alcohol-induced insomnia have been developed that allow for study of the effects of ethanol on sleep independent of factors that may confound human studies, such as premorbid conditions, lifestyle choices, and other substance use. Investigations conducted in our laboratory as well as others, in rodents, have shown that chronic ethanol exposure can produce persistent and long-term changes in sleep, similar to what has been reported in humans with alcohol use disorders (AUD) (Sanchez-Alavez et al., 2018, Criado and Ehlers, 2010, Ehlers et al., 2013a, Ehlers and Slawecki, 2000, Sharma et al., 2022). More recently an animal model of sleep disturbance, resulting from alcohol administration during adolescence, has been developed (see Ehlers et al., 2018a, Ehlers et al., 2011, Criado et al., 2008b). We have shown that alcohol exposure during adolescence, in this rat model, can result in persistent electrophysiological, behavioral and neuroanatomical deficits in young adulthood (see Ehlers et al., 2011, Ehlers et al., 2014, Ehlers et al., 2013b, Ehlers et al., 2013c) that parallel findings seen in human adolescent binge drinkers (Ehlers et al., 2020, Hanson et al., 2011, Schweinsburg et al., 2011, Squeglia et al., 2009).
However, studies in human subjects have recently focused not only on nighttime EEG findings but also on daytime sleepiness and disrupted activity levels as typically measured by activity tracking devices such as a “Fitbit” (see de Zambotti et al., 2019, Godino et al., 2020). In fact, a recent review suggested that management of AUD should include the assessment of circadian rhythms using actigraphy, and any abnormalities identified should be corrected using rhythm-focused therapeutic measures (Hasler et al., 2012). So far, such studies have not been fully translated to animal models. Rodents are nocturnal and thus are more active during the dark phase, and in addition have so called “polyphasic” patterns of sleep-wake behavior and rest-activity rhythms that are ultradian, or less than 24 hours in length (see Stephenson et al., 2012). Such rhythms are not well characterized (Ehlers et al., 1983) and have not been studied following chronic ethanol administration.
In the present study, we describe the use of a “fitbit-like” device that was originally developed for use in dogs (FitBark®-2), that we have modified for use in rats to track activity patterns that we call a “FitBite”. First, we describe, in male and female rats, the successful use of this device to collect multiple days of data and describe how the data differ over the adolescent adult transition. Secondly, we compare the data collected from the FitBite to simultaneously recorded EEG data obtained from rats with implanted cortical electrodes in order to determine how well the FitBite can identify sleep episodes. Thirdly, we used the FitBite to study differences in activity in young adult male and female rats during prolonged exposure (5 weeks) to ethanol vapor as adolescents and following acute and protracted withdrawal (or control conditions) in adulthood.
MATERIALS AND METHODS
Animal subjects
Forty-eight adolescent Wistar rats (24 males, 24 females) (Charles River (USA) arrived with their dams on postnatal day (PD) 21. They were double housed under a 12h light/dark cycle (lights on 08:00), with water and food available ad libitum. The study was approved by The Scripps Research Institute’s Animal Care and Use Committee and adheres to the guidelines outlined in the NIH Guide for the Care and Use of Laboratory Animals (NIH publication No. 80–23, revised 1996).
The FitBite consists of a non-invasive activity monitor called a FitBark®-2 (FitBark Inc. Kansas City, MO). For our application, the fitbark was embedded in a Velcro pouch on a spandex jacket (Lomir Biomedical, Inc.). Jackets were fitted over the front two legs and torso of the rat allowing for the FitBark activity monitor to be attached to the dorsal side of the rat. Animals were habituated to the jackets one week prior to activity monitoring for approximately 4 hours during their light cycle and monitored to ensure jackets were tolerated. Clear plexiglass partial cage dividers were used to prevent cage mates from removing or damaging the activity devices or jackets. Activity monitors were worn for 24 to 48 hours to encompass an entire circadian (light/dark) cycle. Activity data (counts per minute) from each animal were available immediately via the FitBark® app and later downloaded for further analysis. The battery of the activity monitor can last for up to six months before needing to be recharged which allows for longitudinal studies to be accomplished.
Ethanol vapor exposure
The methodology for ethanol vapor inhalation during adolescence have been previously described (see Ehlers et al., 2011, Ehlers et al., 2013a, Ehlers et al., 2013c). Briefly, the vapor chambers used to expose rats to ethanol were titrated to produce constant levels of high to moderate BECs (Blood Ethanol Concentrations) between 175–350 mg/dL. Blood ethanol concentration were 337 mg/dL at 4 weeks of ethanol vapor exposure and did not significantly differ between the males and females. These levels are similar to the BEC levels of binge drinking seen in some studies in adolescent humans (Patrick et al., 2013). Male and female rats in the ethanol vapor group were exposed to vaporized 95% ethanol from 8 p.m. (onset of dark cycle) to 10 a.m. daily for a 5-week period (P22–57). Tail blood samples were collected during this time every 3–4 days to assess BECs. Male and female rats in the air control group were not placed in the ethanol vapor chambers in order to avoid potential residual exposure to ethanol but were handled identically to ethanol rats including tail blood collection. An Analox micro-statAM1 (Analox Instr. Ltd., Lunenberg, MA) was used to estimate BECs. After the 5-week ethanol or control exposure, rats were housed in standard cages for the rest of the experiment.
Surgical procedure
At the end of the 5-week ethanol vapor experiment, 16 animals (4 male and 2 female vapor exposed; 4 male and 4 female air controls) were surgically implanted with EEG electrodes. Two screw electrodes were implanted in the calvarium, one overlying the frontal cortex (FCTX, AP: 1.5 mm, ML: ± 3.0 mm, FR1), and another over the parietal cortex (PCTX, AP: −4.5mm, ML: ± 4.5 mm) with a reference implanted over lambda, guided by the (Paxinos and Watson, 1986) atlas. An electromyography (EMG) wire electrode was inserted into the rats’ neck muscle. A multi-pin Plastics One® connector was used to make electrical connections. The surgical procedures performed in this study have also been previously described in detail elsewhere (Ehlers et al., 2013c, Ehlers et al., 2018a, Ehlers and Slawecki, 2000).
Electrophysiological recordings
After a two-week recovery from surgery, rats were habituated to the electrophysiological recording chambers prior to EEG recordings. All EEG sessions began at the onset of the light cycle (08:00 a.m.) after at least 8 weeks of protracted withdrawal from ethanol vapor or air conditions The EEG was recorded from the 2 leads (frontal cortex and parietal cortex) that were referenced to the lambda ground using a Sensorium preamplifier/amplifier unit (Shelburne, VT). Signals were digitized at a rate of 256 Hz using software described previously (Ehlers et al., 2013b, Ehlers et al., 2018a, Ehlers and Slawecki, 2000).
Sleep EEG analyses
Slow-wave sleep (SWS) (1–4 Hz) was scored for the 5-h EEG recording sessions in 4 second epochs. SWS episodes were defined as an increase in EEG power that exceeded twice the amplitude of waking baseline EEG power lasting longer than 8 s. Rapid eye movement (REM) sleep was visually identified as synchronized theta activity (4–8 Hz) that was preceded by an episode of SWS in the absence of muscle activity. Sleep patterns were identified and analyzed for SWS and REM states. SWS and REM states were combined to define “sleep” while lack of SWS and REM was labelled “active”. Using the scored EEG states of each 4 second epoch, every 1-minute epoch of EEG was designated as a percentage identified as “active”.
Activity data collection
FitBite activity data was collected for a 36-hour time periods at three time points: 4 weeks into vapor or air control exposure, 24 hours following cessation of vapor/control during acute withdrawal, and 4 weeks following cessation of vapor/control during protracted withdrawal. The FitBite data were analyzed by two methods: 1) quantification of the FitBite activity data by identifying active episodes (two or more activity counts per minute epoch) and measuring their overall intensity, number of episodes (also called bouts) and mean durations, as well as counting the number of total active episodes in each light/dark period and 2) cosinor analyses of combined light/dark periods that included measures of: Mesor (Midline Statistic of Rhythm, or rhythm adjusted mean), period, amplitude, and acrophase using the program called Cosinor (https://www.circadian.org/softwar.html). Mesor is defined by the mean value based on the distribution of values across the circadian rhythm, whereas the amplitude is the difference between the maximum value and the mesor of the fitted curve. Acrophase is the time (hours) in which the peak value of activity occurred starting from 12:00am (0:00). The two data analysis methods used one-minute bins of FitBark® activity data that began 12 hours after the beginning of the 36-hour recording for the remaining 24 hour period which began at light-onset (08:00) for the three time points.
At the end of the vapor experiment, overall FitBite activity was compared to the simultaneously collected 5-h EEG recording period during the onset of the light cycle in the 14 animals. FitBite activity was analyzed using a minimum threshold, to account for minimum movement or noise. Three FitBite activity thresholds were investigated (any activity, 2 or more activity counts, 3 or more activity counts) for best correlation to EEG user designated activity. EEG was considered active if 50% or more of the minute was scored as active (as opposed to sleep) EEG. Using Pearson Chi-Square analyses, FitBite data at each threshold level was compared to EEG user scored activity and their statistics were compared for efficacy. Results showed similarly high correlations between 2 and 3 or more activity counts (Pearson statistic, range = 59–154.37, mean = 104.9). Looking at just these two thresholds, 2 or more activity counts correlated most closely with user scored EEG in 8 out of the 14 subjects (4 vapor, 4 control) while 3 or more activity counts correlated most closely with 6 out of 14 subjects (5 vapor, 1 control). Two or more activity counts was used as the threshold for “active” in the Fitbite activity analyses as it was the most frequent and more equally represented with vapor and control animals.
Statistical analyses
Data analyses were based on the three aims of the study. The first aim we analyzed the FitBite data using the activity and cosinor measures described above, in the control male and female animals over the three time points to determine if there were any sex or time effects. To accomplish this, we used a within subjects 2-way ANOVA with sex and time as the independent variables for each activity measure and used post hoc analyses to determine if the time points differed from each other. In the second aim, we compare the data collected from the FitBite to EEG data as described above from 14 rats with implanted cortical electrodes and used Spearman correlations to determine how the two measures compared in defining sleep cycles. In the third aim, we used the FitBite data from the activity and cosinor analyses to study differences in activity in young adult male and female rats during prolonged exposure to alcohol vapor as adolescents and following acute and protracted withdrawal (or control conditions) after their adolescence. To accomplish this, we used a 2-way ANOVA (sex, time) with posthoc analyses as in aim 1. In all analyses significance was set at p<0.01.
RESULTS
In these experiments, rats were exposed to 5 weeks of ethanol vapor or control conditions during adolescence and activity was collected (while intoxicated), then withdrawn from ethanol and subsequent recordings were collected over acute (24 hours post vapor exposure) and chronic withdrawal (four weeks post vapor exposure). FitBite activity data were collected at the three time points each for a period of 36 hours, 24 hours of which was subjected to activity and cosinor analyses. After those studies were completed 14 rats were implanted with cortical EEG electrodes and following recovery, 5 hours of EEG and FitBite data were analyzed and compared.
The first aim analyzed the FitBite data using the activity and cosinor measures described in the methods, in the control male and female animals over the three time points to determine if there were any sex or time effects. Two figures in the supplement show 24-hour FitBite recordings from a representative male and female rat. The rats, being nocturnal, were more active during the dark phase, and have what are called “polyphasic” patterns of rest-activity rhythms that are ultradian, or less than 24 hours in length (see Stephenson et al., 2013). Figures 1 and 2 depict the time and sex effects over the duration of the study in the controls for FitBite and cosinor data, respectively. Female rats were generally found to be overall more active than males in the light (F=14.6, p<0.001) but not in the dark. Females also had longer individual active episodes in the light (F=28.3, p<0.0001) and in the dark (F=8.9, p<0.01), but the two sexes did not differ in the number of activity episodes they had in either the light or the dark.
Figure 1:
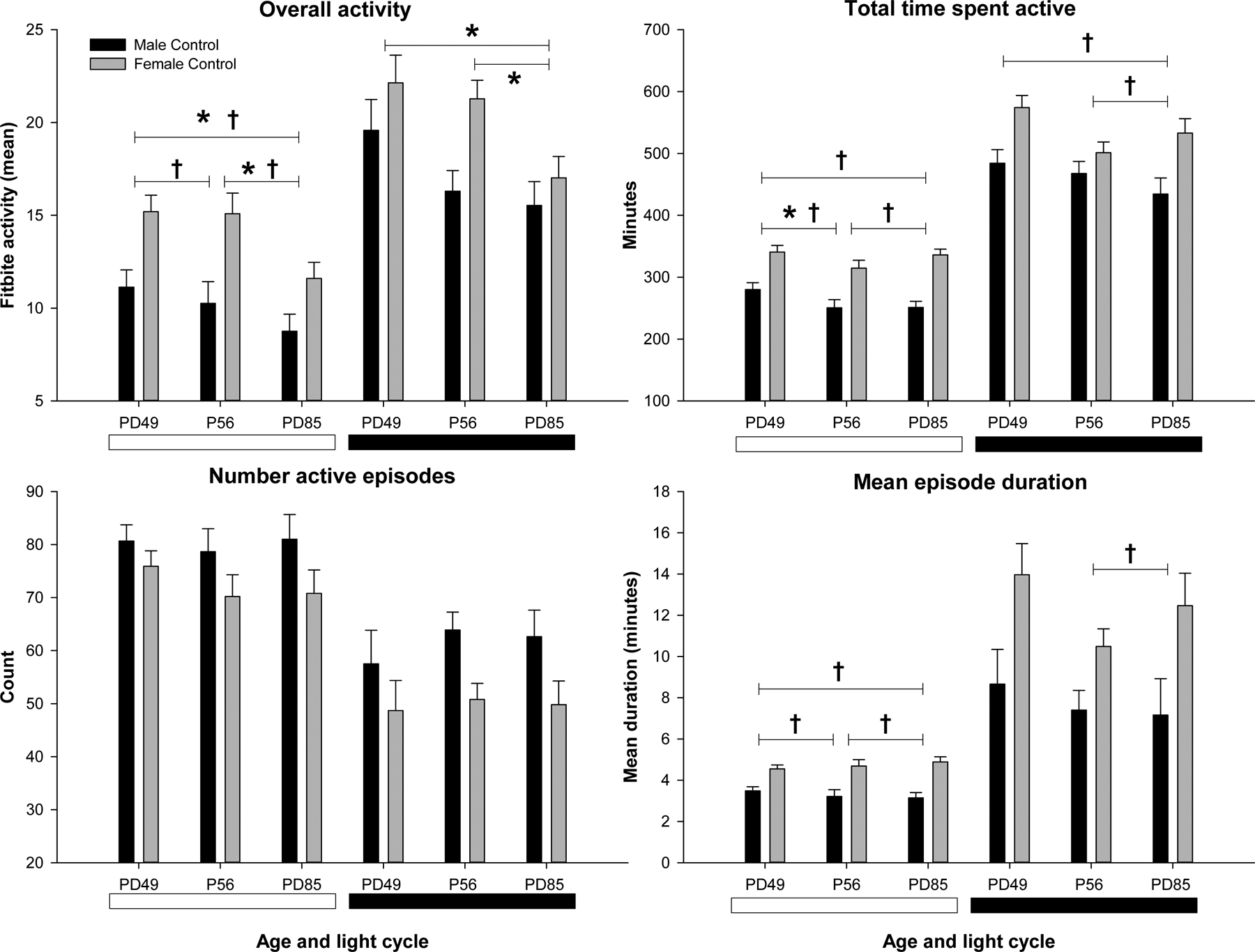
Fitbite activity in the control group in males (black bars) and females (shaded bars) at three time points (4 weeks vapor, 24hr withdrawn, 4 weeks withdrawn). Activity during the light (white bar) and dark (black bar) period is shown for each measure. * Indicates significant (p < 0.01) time effect. † Indicates significant (p < 0.01) sex effect.
Figure 2:
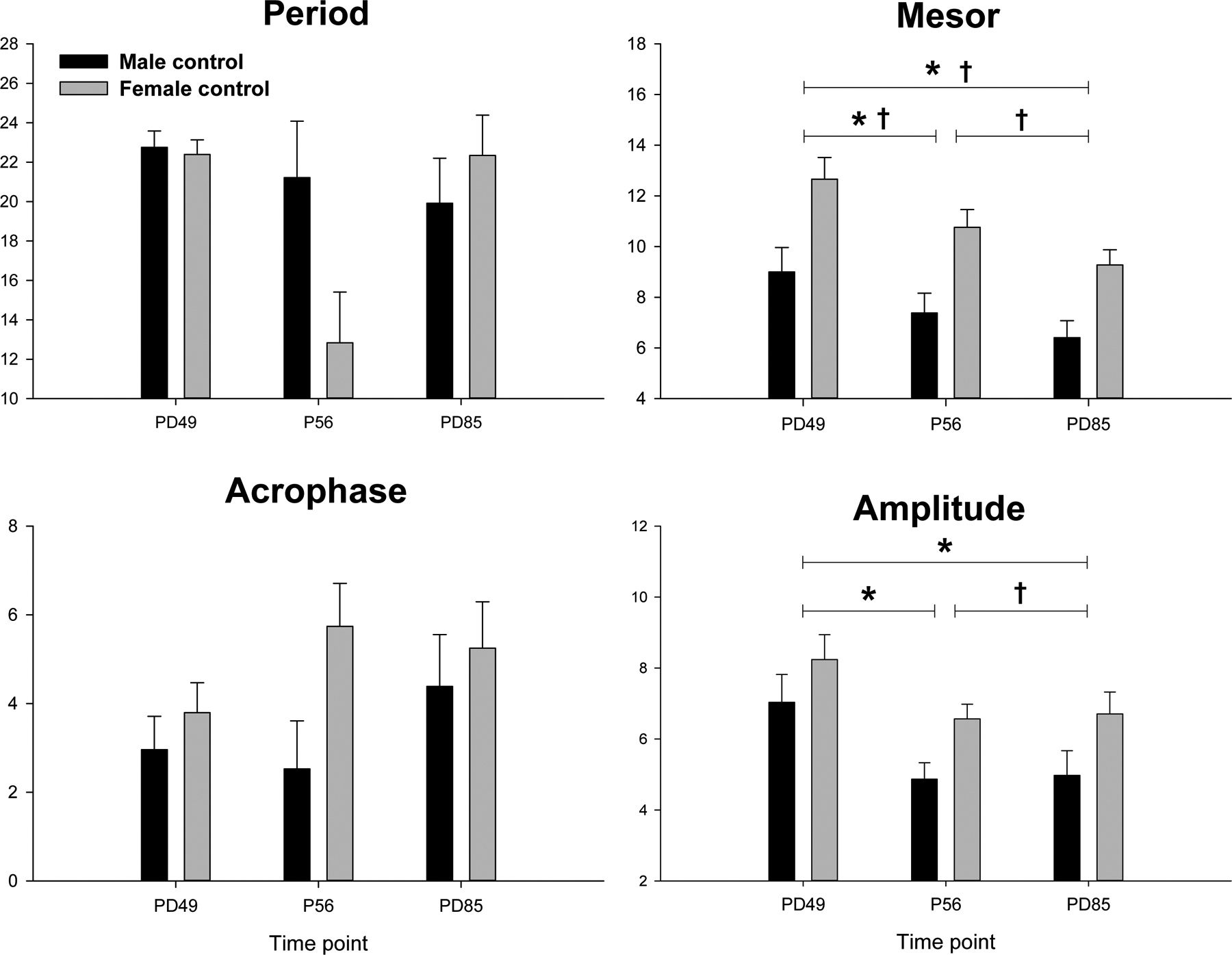
Cosinor data in the control group in males (black bars) and females (shaded bars) at three time points (4 weeks vapor, 24hr withdrawn, 4 weeks withdrawn). * Indicates significant (p < 0.01) time effect. † Indicates significant (p < 0.01) sex effect
Several activity measures also significantly changed over the time of the study in the controls. Overall, the rats became less active over the time points from P49-P85 in both the light (F=7.6, p<0.003) and in the dark (F=12.3, p<0.001), and spent less total time active in the light (F=6.4, p<0.005) but not significantly so during the dark period. However, there were no differences in the number of active episodes or in the duration of the episodes between the three time points. There were also some differences seen in the controls between the sexes and over time, in the cosinor analyses, as seen in figure 3. While there were no significant sex or time effects on period or acrophase, there were significant sex effects on amplitude (F=5.8, p<0.05) and mesor (F=13.3, p<0.01) with females having higher amplitudes and mesors. With respect to time, both amplitude (F=8.3, p<0.01) and mesor (F=16.1, p<0.001) significantly decreased over the time of the experiment. There were no sex by time interactions in any of the measures.
Figure 3:
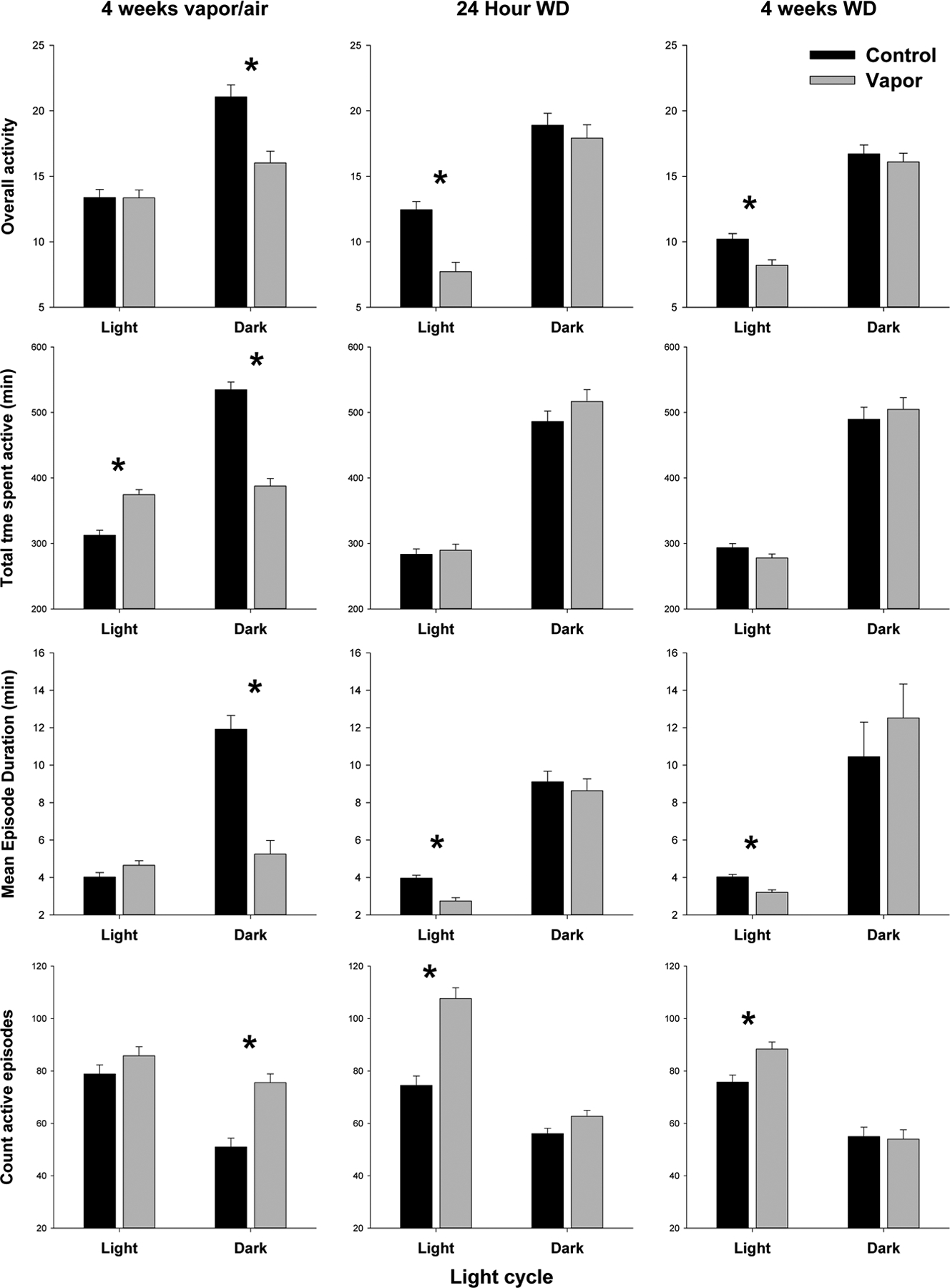
Alcohol treated (shaded bars) and control (black bars) animals’ activity measures during light and dark periods compared for each time point (4 weeks vapor, 24hr withdrawn, 4 weeks withdrawn). * Indicates significant (p < 0.01) treatment effect.
In the second aim, we compare the data collected directly from the FitBite to EEG user scored data (% active) from 14 rats with implanted cortical electrodes and used Spearman correlations to determine how the two measures compared in defining sleep cycles. As seen in table 1 we found highly significant correlations (p<0.00001) between estimation of sleep by use of the EEG and activity counts using the FitBite. The Spearman correlations between the two measures ranged between 0.513 and 0.797.
Table 1.
Spearman’s rho for FitBite activity correlations with active scored EEG
| Group | Spearman’s rho |
|---|---|
| Male control | 0.658, 0.661, 0.741, 0.797 |
| Male vapor | 0.513, 0.544, 0.571, 0.616 |
| Female control | 0.633,0.728, 0.730, 0.750 |
| Female vapor | 0.597, 0.625 |
In the third aim, we used the FitBite data from the activity and cosinor analyses to study differences in activity in young adult male and female rats during prolonged exposure to alcohol vapor as adolescents and following acute and protracted withdrawal (or control conditions) after their adolescence. Analyses of the FitBite activity data peaks revealed that the sex effects seen in the analyses of the controls in aim 1 remained significant in the 2×2 analyses with females being more active than males in the light at all three time points (F=7.2–22.6, p<0.01) and during protracted withdrawal (F=8.7, p<0.005) in the dark (see supplemental table 1). Females also had longer individual active episodes in the light during acute and protracted withdrawal (F=11.3,41.0, p<0.002) and in the dark during protracted withdrawal (F=8.7, p<0.005). The two sexes only differed in the number of active episodes they had with females having fewer episodes during protracted withdrawal (F=12.8, p<0.001). Females also spent more time active during the dark period, and during vapor exposure and protracted withdrawal, than males (F=10.7,12.9, p<0.002). As seen in figure 3, analyses of the effects of alcohol on activity measures revealed significant effects depending on the time point studied. When the rats were tested while they were intoxicated after 4 weeks of vapor exposure, they had significantly less overall activity in the dark (F=15.7, p<0.001) and spent less time active in the dark (F=79.4, p<0.0001) but spent more time active in the light (F=33.3, p<0.001). They also had significantly more active episodes in the dark (F=27.0, p<0.0001) that were of shorter duration (F=41.5, p<0.0001). The changes seen in the activity measures after 4 weeks of alcohol intoxication were also seen in the cosinor analyses, as depicted in figure 4. During vapor exposure, significant decreases were seen in period (F=53.3, p<0.0001), mesor (F=15.4, p<0.001), and amplitude (F=25.0, p<0.0001) and a later shift in acrophase (F=22.8, p<0.0001). Overall, females displayed increased mesor compared to males (9.1, p<0.01), with a significant interaction driven by higher mesor for vapor females compared to sex-matched controls (F=9.1, p<0.05).
Figure 4:
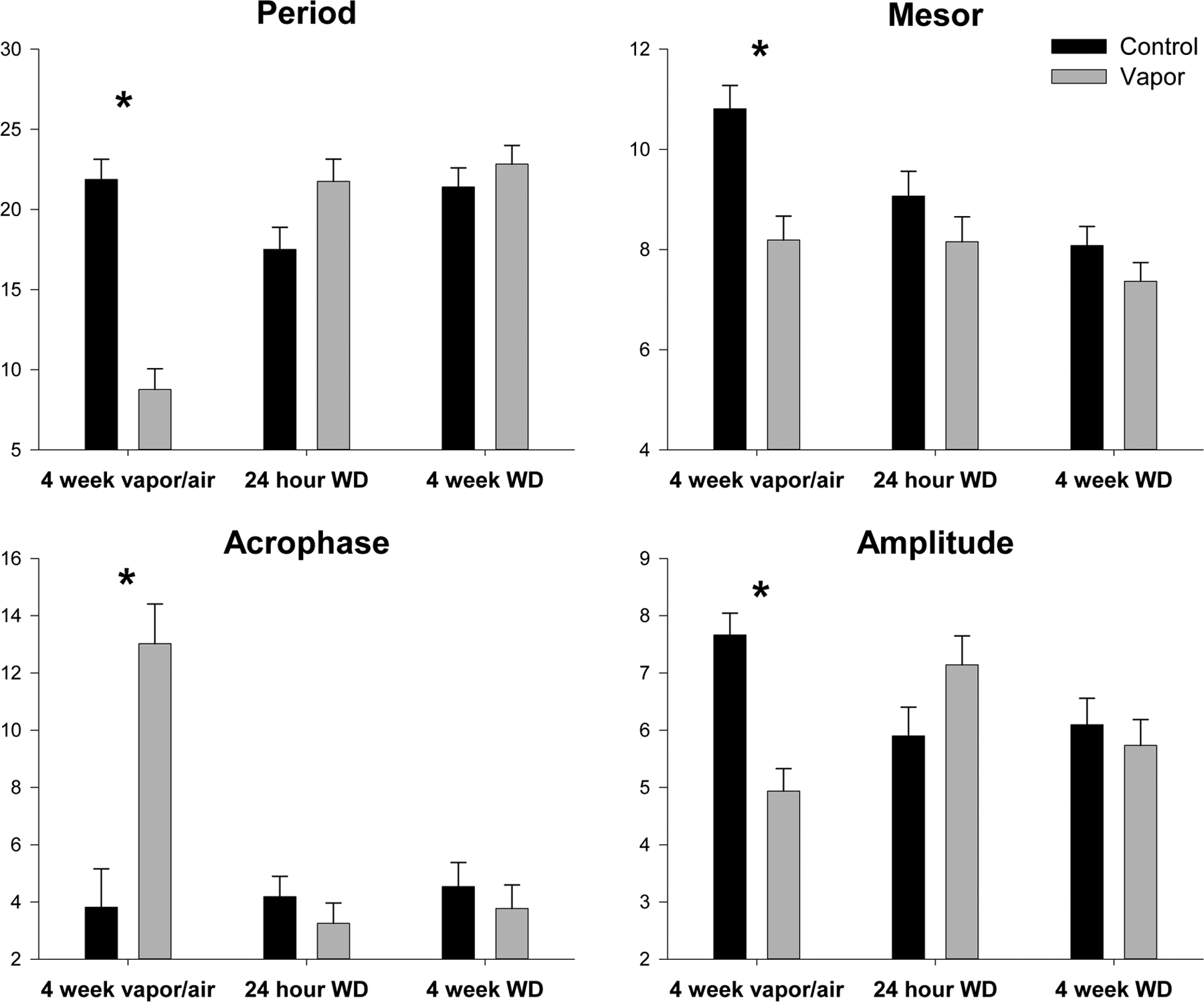
Alcohol treated (shaded bars) and control (black bars) animals’ cosinor results compared for each time point (4 weeks vapor, 24hr withdrawn, 4 weeks withdrawn). * Indicates significant (p < 0.01) treatment effect.
One week later after 24 hours of withdrawal, rats were found to be overall less active in the light (F=24.7, p<0.001) and had more activity episodes in the light (F=18.1, p<0.001) that were shorter in duration (F=10.8, p<002). This effect was also evident at 4 weeks following withdrawal where rats were found to be overall less active in the light (F=11.1, p<0.002) and had more activity episodes in the light (F=10.21, p<0.003) that were shorter in duration (F=18.1, p<0.01). Evaluation of the cosinor data revealed that at 24 hour of withdrawal the period was increased in the vapor animals (F=4.8, p<0.05) but no other cosinor variables were affected when comparing groups at the 24-hour withdrawal or at the 4-week withdrawal time point. There were overall differences between sexes with females displayed a shorter period (F=9.7, p<0.05) and increased acrophase (8.5, p<0.05) compared to males at the 24hr withdrawal time point and an increased mesor (32.9, p<0.001) and amplitude (13.2, p<0.01) compared to males at the 4 week withdrawal time point.
DISCUSSION
The use of alcohol during early adolescence has been associated with a number of long-term health consequences, in addition to elevated risk for alcohol use disorders in adulthood (Dawson et al., 2008, Ehlers et al., 2006). The relationship between adolescent maturation, the beginning of alcohol use and the development of binge drinking is often attributed to social and environmental factors. However, changing physiological mechanisms could also contribute to the risk for initiating excessive alcohol drinking during adolescence. For example, endogenous circadian rhythms naturally shift with puberty so that adolescents are staying up later at night but need to attend school early in the morning potentially causing reductions in sleep quantity and quality (Hagenauer et al., 2009). While there are several lines of evidence demonstrating that disruptions in circadian rhythms and sleep may occur following ethanol exposure in human adolescents (see (Hasler et al., 2012, Meyrel et al., 2020) there are a number of unstudied biological variables that could lead to alcohol-induced sleep disturbances. For instance, most studies investigating circadian rhythms have focused on measures of melatonin and cortisol and few studies have simultaneously measured sleep and circadian activity rhythms (see Hasler et al., 2012, Meyrel et al., 2020).
Preclinical models allow for study of ethanol exposure during adolescence on sleep independent of factors that may confound human studies such as differences in genetic risk, psychiatric comorbidity as well as lifestyle and cultural factors. However, studies in human subjects have recently focused not only on nighttime EEG findings but also on day time sleepiness and disrupted activity levels as typically measured by activity tracking devices such as a “Fitbit” (see de Zambotti et al., 2019, de Zambotti et al., 2020, Godino et al., 2020). In the present study, we describe the use of a “fitbit-like” like device that was developed for use in dogs called a FitBark, that we modified for use in rats to track activity in rats that we call a FitBite. We demonstrated that we could collect multiple days of data. The FitBite jackets are easy to fit or remove and adjust for the rat’s growth without any sedation; thus, rats avoid the stress of a surgical implant or a heavy monitor. This system has several advantages to implantable monitors in that it can be modified as the animal ages, and the output is wireless and does not require that animals be single housed on plates. Importantly, the measurements can be made simultaneously in a large number of animals housed in their home cages, in the alcohol vapor chambers, or any other behavioral chamber. Other forms of activity monitors have been used to quantify circadian rhythms after alcohol use in rodents. Specifically, non-invasive ambulatory systems have been able to capture differences in activity after ethanol exposure using specialized arenas with infrared motion sensors (Ruby et al., 2009, Zhou et al., 2015) and home-cage devices that count wheel rotations (Rosenwasser et al., 2014). The use of these specialized arenas requires expensive equipment and dedicated laboratory space leading to potentially shorter recording sessions, sampling only a fraction of the daily activity. Home-cage methods are ideal and allow for more continuous monitoring. However, wheel running itself can alter the activity patterns and circadian rhythm in rodents (O’Neal et al., 2017). While there are advantages and disadvantages to many of these methods, the fitbite system has been validated as an affordable alternative to the current non-invasive models because it is home-cage compatible, allowing continuous data collection that can be scaled to a large number of cages.
Using the FitBite we found that female rats were generally more active than males spending more time in individual activity episodes in the light and dark and had higher amplitudes and mesors. Several activity measures also significantly changed over the time of the study in the controls. The rats became overall more active over the time points from P49-P85 in both the light and in the dark. However, there were no differences in the number of active episodes or in the duration of the episodes between the three time points. In the cosinor analyses both amplitude and mesor significantly decreased over the time of the experiment, however, no differences were found in period or acrophase. Thus, while demonstrating amplitude differences, our data did not reveal period or timing changes in ultradian or 24-hour rhythms as a function of the age of the animal. However, the limited number of measurement points may have obscured any subtle rhythm changes associated with development over P49–85.
Secondly, we compare the data collected from the FitBite to EEG data obtained from rats with implanted cortical electrodes in order to determine how well the FitBite can identify sleep episodes. We found highly significant correlations between estimation of sleep by use of the EEG and activity counts using the FitBite. The Spearman correlations between the two measures ranged between 0.513 and 0.797. In fact, the correlations between the measures were significantly higher in our study than what has been reported previously from humans using Fitbits® and EEG (0.28–0.67) in a systematic review (Van de Water et al., 2011). One factor that decreased the accuracy of the FitBite was the fact that FitBark monitors software average activity over 1-minute intervals and EEG data in our studies are averaged over 4 second intervals. In the rat, slow wave sleep intervals can be very short. Over a 1-minute period there may be several episodes of slow wave sleep, REM, rest, and activity thus making it difficult to categorize activity over that time period. Thus, if the FitBark software were able to average over shorter intervals the data, or used additional physiological measures, such as breathing or heart rate, we might be even more precise in identifying sleep episodes.
Thirdly, we used the FitBite data from the activity and cosinor analyses to study differences in activity in young adult male and female rats during prolonged exposure to alcohol vapor during adolescence and following acute and protracted withdrawal (or control conditions) after adolescence. When the rats were tested while they were intoxicated after 4 weeks of vapor exposure, they had significantly less overall activity and spent less time active in the dark but spent more time active in the light. Reductions in 24-hour body temperature and locomotor activity during the dark cycle has been shown after vapor ethanol exposure in C57BL/6J mice (Damaggio and Gorman, 2014a) and after chronic ethanol pair-feeding of liquid diet in transgenic rats (Guo et al., 2016). The changes seen in the activity measures after 4 weeks of alcohol intoxication in the present study were also seen in the cosinor analyses where significant decreases were seen in circadian period, mesor, and amplitude and an increased shift was seen in acrophase. Shortening of the free-running period has been previously shown during adult ethanol access in rodents (Rosenwasser et al., 2014) Others have also shown that chronic alcohol exposure can shift the acrophase of the activity rhythm later leading to desynchrony in the normal phase relationship between body temperature and activity (Guo et al., 2016). Chronic ethanol has been shown to attenuate circadian photic phase resetting in hamsters (Ruby et al., 2009). It has also been demonstrated that “binge” levels of ethanol administration can reverse sleep-wake cycles in rats (Sharma et al., 2014a). Desynchronization of circadian rhythms and molecular clocks following chronic ethanol exposure has been reported previously in other studies in rodents, however, reviews of the topic have concluded that the effects are typically modest and that the results vary between species and strain (for reviews see (Rosenwasser et al., 2015, Sarkar, 2012, Spanagel et al., 2005, Damaggio and Gorman, 2014b).
Twenty-four hours after alcohol withdrawal, the main findings in our study were that rats had more activity episodes in the light that were of shorter duration. This effect was observed to remain at 4 weeks following withdrawal. However, the rhythm disturbance as indexed by cosinor analyses, seen during alcohol exposure did not remain at 24-hour or at the 4-week withdrawal time points. These data suggest that while alcohol can disrupt activity rhythms during exposure, following withdrawal, the effects that remain are disruptions in activity during the light phase of the cycle when the rats typically sleep. Adult ethanol exposure has also shown reductions in wheel-running activity but no prolonged effects on circadian waveforms after 1 week of withdrawal (Logan et al., 2010). Previous studies from our laboratory using rats with implanted EEG electrodes have shown that adolescent alcohol exposure can lead to a disruption in slow-wave sleep (SWS) and sleep fragmentation (Criado et al., 2008a, Ehlers et al., 2013a, Ehlers et al., 2018a). The present study is a replication and confirmation of those studies, in that we found that young adult rats with a history of adolescent alcohol vapor exposure demonstrated a fragmentation of waking activity that consisted of a decrease in the duration and an increase in the number of activity episodes during the light period.
While there is good evidence that alcohol disrupts sleep and may be a risk factor for relapse to alcohol use disorder in humans (Brower, 2015), and may also alter circadian rhythms (Hasler et al., 2012, Meyrel et al., 2020), there is less known about the neurophysiological mechanisms by which this occurs. The hypothalamic orexin system has been demonstrated to be important in the initiation and maintenance of wakefulness, since knocking down or depleting orexin neurons /receptors results in the sleep-wake dysfunction (McCarley, 2007). Previous research in our laboratory has demonstrated that exposure to ethanol during adolescence produces increases in the hypothalamic orexin system in the anterior hypothalamus (Amodeo et al., 2020). Increased hypothalamic orexin mRNA and/or orexin-A+IR expression has also been previously shown after chronic ethanol consumption in alcohol-preferring iP rats (Lawrence et al., 2006), and in Long-Evans rats (Barson et al., 2015).
Adolescent alcohol exposure most likely causes changes in multiple brain circuits and neurotransmitter systems that could ultimately lead to disrupted rhythms and sleep in young adults (see Damaggio and Gorman, 2014b, Rosenwasser et al., 2015). Several authors have provided data to suggest that ethanol may alter activity of the per1 and per2 clock genes in peripheral tissue, but not in the suprachiasmatic nucleus (SCN) which is considered the master pacemaker for maintaining phase synchrony and circadian organization (Filiano et al., 2013, Guo et al., 2016, Spanagel et al., 2005). However, other studies have suggested that chronic alcohol exposure could interfere with normal SCN functions potentially through altered neuropeptide systems (Madeira et al., 1997). Adenosinergic regulation of striatal clock gene expression has also been demonstrated to be associated with ethanol intake in constant light (Ruby et al., 2014), and following ethanol induced sleep promotion (Sharma et al., 2014b). We have additionally demonstrated that reduced numbers of ChAT- immunoreactive (ChAT-IR) neurons in the basal forebrain, an area important in sleep regulation, are observed in adulthood after adolescent ethanol exposure (Ehlers et al., 2011). Taken together these data suggest that adolescent alcohol exposure influences multiple rhythm and sleep systems.
These studies describe data showing that a Fitbit-like device can be successfully used in rats to assess rest-activity cycles. Adolescent alcohol exposure was found to produce rhythm disturbance using cosinor analyses that was not observed after withdrawal. Fragmentation of ultradian rest-activity cycles during the light period were found at 24hrs and 4 weeks after withdrawal and support data demonstrating sleep disturbance long after alcohol withdrawal in rats who had been exposed to alcohol as adolescents. However, these studies do not model self-administration of alcohol over a range of drinking levels (Ehlers et al., 2020), nor do they mimic the complex genetic and environmental risks for rhythm disturbance and/or insomnia, seen in adolescent and young adult humans (de Zambotti et al., 2018).
Supplementary Material
ACKNOWLEDGEMENTS
The authors wish to acknowledge the technical support of Mellany Santos and Philip Lau. National Institutes of Health (NIH) funding for this study was provided by the National Institute on Alcoholism and Alcohol Abuse (NIAAA) U01 AA19969, AA006059 to CLE.
Source of support:
This work was supported by National Institute of Health (NIH) grants, U01 AA019969; R01 AA006059 to Cindy L. Ehlers from the National Institute on Alcohol Abuse and Alcoholism (NIAAA).
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicts of interest. The experiments comply with the current laws of the country in which they were performed.
REFERENCES
- Adan A, Archer SN, Hidalgo MP, Di Milia L, Natale V, Randler C (2012) Circadian typology: a comprehensive review. Chronobiol. Int 29:1153–1175. [DOI] [PubMed] [Google Scholar]
- Amodeo LR, Liu W, Wills DN, Vetreno RP, Crews FT, Ehlers CL (2020) Adolescent alcohol exposure increases orexin-A/hypocretin-1 in the anterior hypothalamus. Alcohol 88:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barson JR, Ho HT, Leibowitz SF (2015) Anterior thalamic paraventricular nucleus is involved in intermittent access ethanol drinking: role of orexin receptor 2. Addict. Biol 20:469–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel KA, Gradisar M, Williamson P (2015) Protective and risk factors for adolescent sleep: a meta-analytic review. Sleep Med. Rev 21:72–85. [DOI] [PubMed] [Google Scholar]
- Brower KJ (2015) Assessment and treatment of insomnia in adult patients with alcohol use disorders. Alcohol 49:417–427. [DOI] [PubMed] [Google Scholar]
- Colrain IM, Turlington S, Baker FC (2009) Impact of alcoholism on sleep architecture and EEG power spectra in men and women. Sleep 32:1341–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criado JR, Ehlers CL (2010) Effects of adolescent ethanol exposure on event-related oscillations (EROs) in the hippocampus of adult rats. Behav. Brain Res 210:164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criado JR, Wills DN, Walker BM, Ehlers CL (2008a) Effects of adolescent ethanol exposure on sleep in adult rats. Alcohol 42:631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criado JR, Wills DN, Walker BM, Ehlers CL (2008b) Electrophysiological effects of dizocilpine (MK-801) in adult rats exposed to ethanol during adolescence. Alcohol. Clin. Exp. Res 32:1752–1762. [DOI] [PubMed] [Google Scholar]
- Damaggio AS, Gorman MR (2014a) Circadian phase determines effects of repeated ethanol vapor exposure and withdrawal on body temperature and activity rhythms of male mice. Alcohol. Clin. Exp. Res 38:879–888. [DOI] [PubMed] [Google Scholar]
- Damaggio AS, Gorman MR (2014b) The circadian timing system in ethanol consumption and dependence. Behav. Neurosci 128:371–386. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Goldstein RB, Chou SP, Ruan WJ, Grant BF (2008) Age at first drink and the first incidence of adult-onset DSM-IV alcohol use disorders. Alcohol. Clin. Exp. Res 32:2149–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zambotti M, Cellini N, Goldstone A, Colrain IM, Baker FC (2019) Wearable Sleep Technology in Clinical and Research Settings. Med. Sci. Sports Exerc 51:1538–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zambotti M, Cellini N, Menghini L, Sarlo M, Baker FC (2020) Sensors Capabilities, Performance, and Use of Consumer Sleep Technology. Sleep Med. Clin 15:1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zambotti M, Goldstone A, Colrain IM, Baker FC (2018) Insomnia disorder in adolescence: Diagnosis, impact, and treatment. Sleep Med. Rev 39:12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Criado JR, Wills DN, Liu W, Crews FT (2011) Periadolescent ethanol exposure reduces adult forebrain ChAT+IR neurons: correlation with behavioral pathology. Neuroscience 199:333–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Desikan A, Wills DN (2013a) Developmental differences in EEG and sleep responses to acute ethanol administration and its withdrawal (hangover) in adolescent and adult Wistar rats. Alcohol 47:601–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Desikan A, Wills DN (2014) Event-related potential responses to the acute and chronic effects of alcohol in adolescent and adult Wistar rats. Alcohol. Clin. Exp. Res 38:749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Liu W, Wills DN, Crews FT (2013b) Periadolescent ethanol vapor exposure persistently reduces measures of hippocampal neurogenesis that are associated with behavioral outcomes in adulthood. Neuroscience 244:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Oguz I, Budin F, Wills DN, Crews FT (2013c) Peri-adolescent ethanol vapor exposure produces reductions in hippocampal volume that are correlated with deficits in prepulse inhibition of the startle. Alcohol. Clin. Exp. Res 37:1466–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Phillips E, Wills D, Benedict J, Sanchez-Alavez M (2020) Phase locking of event-related oscillations is decreased in both young adult humans and rats with a history of adolescent alcohol exposure. Addict. Biol 25:e12732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Russo PV, Mandell AJ, Bloom FE (1983) Architecture of rat nocturnal locomotion: A predictive descriptor of the effects of antidepressant and antimanic treatments. Psychopharmacol 19:692–695. [Google Scholar]
- Ehlers CL, Sanchez-Alavez M, Wills D (2018a) Effect of gabapentin on sleep and delta and theta EEG power in adult rats exposed to chronic intermittent ethanol vapor and protracted withdrawal during adolescence. Psychopharmacology (Berl.) 235:1783–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Slawecki CJ (2000) Effects of chronic ethanol exposure on sleep in rats. Alcohol 20:173–179. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Slutske WS, Gilder DA, Lau P, Wilhelmsen KC (2006) Age at first intoxication and alcohol use disorders in Southwest California Indians. Alcohol. Clin. Exp. Res 30:1856–1865. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Wills D, Gilder DA (2018b) A history of binge drinking during adolescence is associated with poorer sleep quality in young adult Mexican Americans and American Indians. Psychopharmacology (Berl.) 235:1775–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar C, Salgado R, Rodriguez K, Blancas Vazquez AS, Angeles-Castellanos M, Buijs RM (2011) Scheduled meals and scheduled palatable snacks synchronize circadian rhythms: consequences for ingestive behavior. Physiol. Behav 104:555–561. [DOI] [PubMed] [Google Scholar]
- Filiano AN, Millender-Swain T, Johnson R Jr., Young ME, Gamble KL, Bailey SM (2013) Chronic ethanol consumption disrupts the core molecular clock and diurnal rhythms of metabolic genes in the liver without affecting the suprachiasmatic nucleus. PLoS One 8:e71684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godino JG, Wing D, de Zambotti M, Baker FC, Bagot K, Inkelis S, Pautz C, Higgins M, Nichols J, Brumback T, Chevance G, Colrain IM, Patrick K, Tapert SF (2020) Performance of a commercial multi-sensor wearable (Fitbit Charge HR) in measuring physical activity and sleep in healthy children. PLoS One 15:e0237719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulick D, Gamsby JJ (2018) Racing the clock: The role of circadian rhythmicity in addiction across the lifespan. Pharmacol. Ther 188:124–139. [DOI] [PubMed] [Google Scholar]
- Guo R, Simasko SM, Jansen HT (2016) Chronic Alcohol Consumption in Rats Leads to Desynchrony in Diurnal Rhythms and Molecular Clocks. Alcohol. Clin. Exp. Res 40:291–300. [DOI] [PubMed] [Google Scholar]
- Hagenauer MH, Lee TM (2012) The neuroendocrine control of the circadian system: adolescent chronotype. Front. Neuroendocrinol 33:211–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenauer MH, Perryman JI, Lee TM, Carskadon MA (2009) Adolescent changes in the homeostatic and circadian regulation of sleep. Dev. Neurosci 31:276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson KL, Medina KL, Padula CB, Tapert SF, Brown SA (2011) Impact of Adolescent Alcohol and Drug Use on Neuropsychological Functioning in Young Adulthood: 10-Year Outcomes. J. Child Adolesc. Subst. Abuse 20:135–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Clark DB (2013) Circadian misalignment, reward-related brain function, and adolescent alcohol involvement. Alcohol. Clin. Exp. Res 37:558–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Martin CS, Wood DS, Rosario B, Clark DB (2014a) A longitudinal study of insomnia and other sleep complaints in adolescents with and without alcohol use disorders. Alcohol. Clin. Exp. Res 38:2225–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Smith LJ, Cousins JC, Bootzin RR (2012) Circadian rhythms, sleep, and substance abuse. Sleep Med. Rev 16:67–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Soehner AM, Clark DB (2014b) Circadian rhythms and risk for substance use disorders in adolescence. Curr Opin Psychiatry 27:460–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Soehner AM, Clark DB (2015) Sleep and circadian contributions to adolescent alcohol use disorder. Alcohol 49:377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B (2006) The orexin system regulates alcohol-seeking in rats. Br. J. Pharmacol 148:752–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemoine P, Zawieja P, Ohayon MM (2013) Associations between morningness/eveningness and psychopathology: an epidemiological survey in three in-patient psychiatric clinics. J. Psychiatr. Res 47:1095–1098. [DOI] [PubMed] [Google Scholar]
- Logan RW, Seggio JA, Robinson SL, Richard GR, Rosenwasser AM (2010) Circadian wheel-running activity during withdrawal from chronic intermittent ethanol exposure in mice. Alcohol 44:239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeira MD, Andrade JP, Lieberman AR, Sousa N, Almeida OF, Paula-Barbosa MM (1997) Chronic alcohol consumption and withdrawal do not induce cell death in the suprachiasmatic nucleus, but lead to irreversible depression of peptide immunoreactivity and mRNA levels. J. Neurosci 17:1302–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarley RW (2007) Neurobiology of REM and NREM sleep. Sleep Med 8:302–330. [DOI] [PubMed] [Google Scholar]
- Meyrel M, Rolland B, Geoffroy PA (2020) Alterations in circadian rhythms following alcohol use: A systematic review. Prog. Neuropsychopharmacol. Biol. Psychiatry 99:109831. [DOI] [PubMed] [Google Scholar]
- O’Neal TJ, Friend DM, Guo J, Hall KD, Kravitz AV (2017) Increases in Physical Activity Result in Diminishing Increments in Daily Energy Expenditure in Mice. Curr. Biol 27:423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick ME, Schulenberg JE, Martz ME, Maggs JL, O’Malley PM, Johnston LD (2013) Extreme binge drinking among 12th-grade students in the United States: prevalence and predictors. JAMA Pediatr 167:1019–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates 2nd. ed., Academic Press, Sydney, Australia. [Google Scholar]
- Rosenwasser AM, Fixaris MC, McCulley WD 3rd (2015) Photoperiodic modulation of voluntary ethanol intake in C57BL/6 mice. Physiol. Behav 147:342–347. [DOI] [PubMed] [Google Scholar]
- Rosenwasser AM, McCulley WD 3rd, Fecteau M (2014) Circadian activity rhythms and voluntary ethanol intake in male and female ethanol-preferring rats: effects of long-term ethanol access. Alcohol 48:647–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby CL, Brager AJ, DePaul MA, Prosser RA, Glass JD (2009) Chronic ethanol attenuates circadian photic phase resetting and alters nocturnal activity patterns in the hamster. Am. J. Physiol. Regul. Integr. Comp. Physiol 297:R729–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby CL, Vadnie CA, Hinton DJ, Abulseoud OA, Walker DL, O’Connor KM, Noterman MF, Choi DS (2014) Adenosinergic regulation of striatal clock gene expression and ethanol intake during constant light. Neuropsychopharmacology 39:2432–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Alavez M, Wills DN, Amodeo L, Ehlers CL (2018) Effect of Gabapentin on Sleep and Event-Related Oscillations (EROs) in Rats Exposed to Chronic Intermittent Ethanol Vapor and Protracted Withdrawal. Alcohol. Clin. Exp. Res 42:624–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar DK (2012) Circadian genes, the stress axis, and alcoholism. Alcohol Res 34:362–366. [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Schweinsburg BC, Nagel BJ, Eyler LT, Tapert SF (2011) Neural correlates of verbal learning in adolescent alcohol and marijuana users. Addiction 106:564–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Bradshaw K, Sahota P, Thakkar MM (2014a) Acute binge alcohol administration reverses sleep-wake cycle in Sprague Dawley rats. Alcohol. Clin. Exp. Res 38:1941–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Parikh M, Mishra V, Zuniga A, Sahota P, Thakkar M (2022) Sleep, sleep homeostasis and arousal disturbances in alcoholism. Brain Res. Bull 182:30–43. [DOI] [PubMed] [Google Scholar]
- Sharma R, Sahota P, Thakkar MM (2014b) Role of adenosine and the orexinergic perifornical hypothalamus in sleep-promoting effects of ethanol. Sleep 37:525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Rosenwasser AM, Schumann G, Sarkar DK (2005) Alcohol consumption and the body’s biological clock. Alcohol. Clin. Exp. Res 29:1550–1557. [DOI] [PubMed] [Google Scholar]
- Squeglia LM, Spadoni AD, Infante MA, Myers MG, Tapert SF (2009) Initiating moderate to heavy alcohol use predicts changes in neuropsychological functioning for adolescent girls and boys. Psychol. Addict. Behav 23:715–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson R, Lim J, Famina S, Caron AM, Dowse HB (2012) Sleep-wake behavior in the rat: ultradian rhythms in a light-dark cycle and continuous bright light. J. Biol. Rhythms 27:490–501. [DOI] [PubMed] [Google Scholar]
- Tamura EK, Oliveira-Silva KS, Ferreira-Moraes FA, Marinho EAV, Guerrero-Vargas NN (2021) Circadian rhythms and substance use disorders: A bidirectional relationship. Pharmacol. Biochem. Behav 201:173105. [DOI] [PubMed] [Google Scholar]
- Touitou Y (2013) Adolescent sleep misalignment: a chronic jet lag and a matter of public health. J. Physiol. Paris 107:323–326. [DOI] [PubMed] [Google Scholar]
- Tynjala J, Kannas L, Levalahti E (1997) Perceived tiredness among adolescents and its association with sleep habits and use of psychoactive substances. J. Sleep Res 6:189–198. [DOI] [PubMed] [Google Scholar]
- Van de Water AT, Holmes A, Hurley DA (2011) Objective measurements of sleep for non-laboratory settings as alternatives to polysomnography--a systematic review. J. Sleep Res 20:183–200. [DOI] [PubMed] [Google Scholar]
- Zhou P, Werner JH, Lee D, Sheppard AD, Liangpunsakul S, Duffield GE (2015) Dissociation between diurnal cycles in locomotor activity, feeding behavior and hepatic PERIOD2 expression in chronic alcohol-fed mice. Alcohol 49:399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


