Abstract
ZJ-101 is a structurally simplified analog of marine natural product superstolide A that was previously designed and synthesized in our laboratory. Biological investigation shows that ZJ-101 maintains the potent anticancer activity of the original natural product with an undefined mechanism of action. To facilitate chemical biology study, a biotinylated ZJ-101 was synthesized and biologically evaluated.
Keywords: Superstolide A, ZJ-101, Anticancer agent, Biotinylated probe
Graphical Abstract
Natural products and their analogs play a very important role in drug discovery and development, especially for cancer and infectious diseases.1 Natural products isolated from marine invertebrates are promising lead compounds to develop 1st-in-class anticancer drugs and a new source of unique chemical probes to discover novel drug targets.2 Over the past two decades a number of marine natural products-inspired anticancer drugs have been approved by regulatory agencies and some novel drug targets have been identified.3 Moreover, a number of marine natural products-inspired drug candidates are in clinical trials.4
Superstolide A (1) (Figure 1) isolated in minute amounts from the deep-water marine sponge Neosiphonia superstes exhibits potent antiproliferative activity against several tumor cell lines with IC50 values ranging from 4.8 to 64 nM.5 Its unprecedented chemical structure suggests that it might have a unique cellular target(s) and a novel mechanism of action. However, like many biologically active marine natural products, the scarcity of superstolide A has hampered the preclinical evaluation and chemical biology investigation of this structurally distinct and mechanistically unique marine natural product.
Figure 1.
Superstolide A and ZJ-101
To solve its supply problem, we recently designed and synthesized a truncated analog of superstolide A, which was named as ZJ-101 (Figure 1).6 Most importantly, ZJ-101 maintains the potent anticancer activity of the original natural product. Thus, our synthetic approach has, for the first time, successfully solved the bottleneck of supply of superstolide A, albeit indirectly. In the NCI-60 cell screen ZJ-101 exhibited broadly potent anticancer activity. Some of the most aggressive CNS (SF-295 cell line) and triple-negative breast cancers (MDA-MB-231/ATCC cell line) that are refractory to most existing therapies are particularly sensitive to ZJ-101 with GI50 values less than 10 nM.7 Intriguingly, cell lines with p53 mutations, which often cause drug resistance, do not affect ZJ-101’s sensitivity. In fact, five out of six most sensitive cell lines among NCI-60 cell lines (with GI50 < 10 nM) are those with p53 mutations.7 Among the colon cancer cell lines tested, HT29 that harbors p53 mutation (IC50 = 7.5 nM) is significantly more sensitive to ZJ-101 than HCT116 with wild type p53 (IC50 = 52.7 nM).
To gain mechanistic insight, we employed the COMPARE pattern-recognition analysis of the NCI 60-cell mean graph screening profile of ZJ-101. However, it did not reveal strong correlation to the profiles of any other known anticancer compounds in the NCI’s database, suggesting ZJ-101 represents a new class of anticancer agent with a novel and, as yet, undefined mechanism of action.7
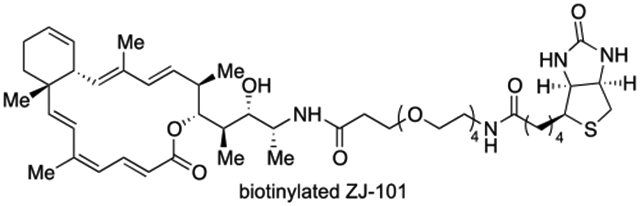
To fully exploit the therapeutic potential of ZJ-101, it is imperative to identify its drug target and elucidate its mechanism of action. To facilitate our chemical biology study, a biotinylated ZJ-101 was designed (Figure 2). The biotinylated ZJ-101 offers the feature of a reversible solid-support reagent when combined with monomeric-avidin sepharose or avidin-coated solid surface.8 Because our previous structure-activity relationship studies have shown that the 16-membered macrolactone, especially the trienyl conjugated lactone moiety of the molecule, is the key pharmacophore, biotin should be anchored at a site away from this region.6b, 6c In addition, amide is an ideal functional group to connect a small molecule to biotin via a PEG linker. Therefore, we decided to attach the PEG and biotin at the amide position of ZJ-101. Herein, we report our synthesis and biological evaluation of biotinylated ZJ-101.
Figure 2.
Design of biotinylated ZJ-101 (3)
Our original synthetic strategy for ZJ-101 was modified for the synthesis of biotinylated ZJ-101 (3).6 The retrosynthetic analysis of biotinylated ZJ-101 (3) is outlined in Scheme 1. This synthetic route is advantageous as it is flexible and convergent. It can also be used for future structural optimization in our medicinal chemistry study.
Scheme 1.
Retrosynthetic analysis of biotinylated ZJ-101 (3)
The synthesis of the key fragment 6 is shown in Scheme 2. Compound 8, an advanced intermediate in the synthesis of the linear polypropionate (C20-C60) portion of superstolide A,9 reacted with di-tert-butyl dicarbonate to give compound 9 in 85% yield. Compound 9 underwent cross metathesis with vinylboronic acid pinacol ester 10 catalyzed by Hoveyda-Grubbs catalyst 2nd generation to provide compound 6 in 71% yield.10
Scheme 2.
Synthesis of fragment 6
The synthesis of biotinylated ZJ-101 (3) is shown in Scheme 3. Compound 5, an advanced intermediate in our original synthesis of ZJ-101,6 was the point of departure. Stereoselective Suzuki coupling between compound 5 and vinyl boronate 6 gave compound 11 in 71% yield. Negishi coupling between vinyl bromide 11 and Me2Zn provided the requisite trisubstituted olefin 12 in 85% yield with complete stereoselectivity. TBAF-mediated deprotection of three silyl protecting groups afforded alkyne 13 in 90% yield. Compound 13 underwent a regio- and stereoselective hydrostannylation to furnish vinyl stannane 14 in 85% yield. It should be noted that the mixed solvent system of THF and hexanes is far superior, in terms of reaction yield, to dichloromethane which was used in our original synthesis of ZJ-101. Regioselective esterification between alcohol 14 and anhydride 7 provided compound 15 in 90% yield.6c Ti(O-iPr)4-promoted isomerization of compound 15 furnished the desired ester 16 in 82% yield. Compound 16 underwent a facile intramolecular Stille coupling to give compound 4, the key precursor of biotinylated ZJ-101, in 88% yield. Upon the treatment of trifluoroacetic acid (TFA) the Boc protecting group of compound 4 was removed and the resulting primary amine reacted with NHS-PEG4-Biotin under the standard conditions to finally afford biotinylated ZJ-101 (3) in 36% yield.
Scheme 3.
Synthesis of biotinylated ZJ-101 (3)
To determine if biotinylated ZJ-101 has any biological activity, we used Alamar Blue assay to evaluate its anti-proliferative effect in three human cancer cell lines. As shown in Table 1, the IC50 values of biotinylated ZJ-101 against human glioblastoma cell line (SF296), human breast cancer cell line (MCF-7), and human triple-negative breast cancer cell line (MDA-MB-231) are all greater than 5000 nM, indicating that this compound has lost anticancer activity. The biological tests suggest that anchoring biotin to the amide position of ZJ-101 via a PEG linker disrupts the interaction of molecule with its putative target(s) in the cells. While the testing result is disappointing, we have for the first time discovered that the acetamide group is one of the key pharmacophores, which is critically important information for future medicinal chemistry study of ZJ-101 and will guide the design of future chemical probes of ZJ-101.
Table 1.
Antiproliferative effect of biotinylated ZJ-101 on various malignant tumor cells (Alamar Blue assay)
| Entry | Cell Line | Biotinylated ZJ-101 IC50 (nM) |
ZJ-101 IC50 (nM) |
|---|---|---|---|
| 1 | SF295 | > 5000 | 63.5 |
| 2 | MCF-7 | > 5000 | 40.8 |
| 3 | MDA-MB-231 | > 5000 | 61.1 |
In conclusion, the biotinylated ZJ-101 has been synthesized from compound 5 in 9 steps in good yield. The loss of anti-proliferative effect of this biotinylated ZJ-101 suggests that in order to synthesize a bioactive ZJ-101-based probe, we will have to avoid both the trienyl conjugated lactone region and the acetamide moiety for the attachment of biotin or photoaffinity groups. A comprehensive structure-activity relationship study is likely needed to identify suitable positions for affinity or proximity-based probes. In addition, the discovery that the acetamide group is one of the key pharmacophores provides us important structure-activity relationship information for future medicinal chemistry study of ZJ-101. Furthermore, the current synthetic approach can be used to carry out structural optimization on the amide moiety, which may lead to the discovery of new analogs with improved potency and ADMET profiles. Research in these directions is currently underway and will be reported in due course.
Supplementary Material
Acknowledgments
This work was made possible by the generous support of the GAP funding from the Office of the Vice President for Research and Economic Development (OVPRED) at the University of Iowa, funding from InnoBioPharma, LLC (D2015070002), and a grant (1R21CA204836-01) from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
Dr. Zhendong Jin is the founder and a shareholder of InnoBioPharma, LLC that sponsored the project, “Development of novel anticancer agents based on natural products” at the University of Iowa.
References
- 1.Beutler JA. Curr Protoc Pharmacol. 2009. Sep;Chapter 9:Unit 9.11. [DOI] [PubMed] [Google Scholar]
- 2.(a) Montaser R; Luesch H Future Med Chem 2011, 3, 1475–1489; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Qi Y; Ma S ChemMedChem 2011, 6, 399; [DOI] [PubMed] [Google Scholar]; (c) Bhatnagar I; Kim SK Mar. Drugs 2010, 8, 2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Lu WY; Li HJ; Li QY; Wu YC Bioorg Med Chem. 2021, 35,116058; [DOI] [PubMed] [Google Scholar]; (b) Haque N; Parveen S; Tang T; Wei J; Huang Z Mar Drugs 2022, 20, 528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghareeb MA; Tammam MA; El-Demerdash A; Atanasov AG Curr. Res. in Biotechnol 2020, 2, 88. [Google Scholar]
- 5.(a) D'Auria MV, Debitus C, Paloma LG, Minale L, Zampella A, J. Am. Chem. Soc 1994, 116, 6658; [Google Scholar]; (b) D'Auria MV, Debitus C, Paloma LG, Minale L, Zampella A, J. Nat. Prod 1994, 57, 1595. [DOI] [PubMed] [Google Scholar]
- 6.(a) Chen L; Riaz Ahmed KB; Huang P; Jin Z Angewandte Chemie Int. Ed 2013, 52, 3446; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Qian S; Shah AK; Head SA; Liu JO; Jin Z Bioorg. Med. Chem. Lett 2016, 26, 3411; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Qiu H; Qian S; Head SA; Liu JO; Jin Z Bioorg. Med. Chem. Lett 2016, 26, 4702. [DOI] [PubMed] [Google Scholar]
- 7.Unpublished results.
- 8.Sche PP; McKenzie KM; White JD; Austin DJ Chemistry & Biology 1999, 6, 707 and references cited therein. [DOI] [PubMed] [Google Scholar]
- 9.Yu W; Zhang Y; Jin Z Org. Lett 2001, 3, 1447. [DOI] [PubMed] [Google Scholar]
- 10.(a) Morrill C, Grubbs RH, J. Org. Chem 2003, 68, 6031; [DOI] [PubMed] [Google Scholar]; (b) Morrill C, Funk TW, Grubbs RH, Tetrahedron Lett. 2004, 45, 7733. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.







