Abstract
Fluorine-18-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) is an essential tool in the diagnosis, staging, and assessment of treatment response in the management of lymphoma. Diffuse large B-cell lymphoma (DLBCL) represents the most common type of non-Hodgkin lymphoma (NHL). Although the curability rate is high, there are around 40% of patients exhibit relapse and present a therapeutic challenge. As important as 18F-FDG PET/CT is in the management of DLBCL, there are several limitations and potential pitfalls in assessing treatment response or relapse in patients who are also affected by active infectious disease concurrently. Hence, the knowledge of variable physiologic and altered physiologic uptake is of incredible essence when it comes to interpreting a complex scan. In this case report, we present a patient with relapsed DLBCL complicated by disseminated infection.
Keywords: 18-FDG PET/CT, Fluorine-18-fluorodeoxyglucose positron emission tomography, Non-Hodgkin lymphoma, NHL, Diffuse large B-cell lymphoma, DLBCL
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common lymphoid malignancy in adults, accounting for approximately 25% of non-Hodgkin lymphoma (NHL) cases [1]. It is defined as a heterogeneous group of malignancies arising from large cells with nuclei at least twice the size of a small lymphocyte [2]. DLBCL can occur as de novo; however, it may also arise from the progression or transformation of other types of lymphomas such as chronic lymphocytic leukemia, lymphoplasmacytic lymphoma, marginal zone lymphoma, follicular lymphoma and even lymphocyte-predominant Hodgkin lymphoma [2].
Patients commonly present with an enlarged mass or nodal enlargement in the abdomen, neck, or anywhere in the body. Some patients present with constitutional or B symptoms such as fever, weight loss, or drenching night sweats [2]. To diagnose DLBCL, an excisional biopsy or at least a core biopsy is obtained from an enlarged lymph node [2].
Although aggressive, DLBCL can be cured in 60%-70% of patients following first line immunochemotherapy with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) [1]. However, despite treatments, 30%-40% of patients still have insufficient responses or experience relapses after successful therapy [1]. The risk of relapse increases in DLBCL patients with bulky and residual tumors [1]. Therefore, it is important to recognize patients with diseases resistant to immunochemotherapy in the early phases of treatment.
Fluorine-18-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) scanning is now a standard of care for the assessment of pretreatment, interim, and post-treatment of response in most lymphomas [3]. However, its specificity is limited due to various pitfalls such as FDG uptake in organs with infiltrative changes postimmunochemotherapy or those affected by infections or inflammatory changes [3,4]. In addition, physiological FDG uptake is seen in metabolically active normal structures, as most human cells metabolize glucose for energy [5]. For example, physiological FDG biodistribution is present in the brain, urinary system, left ventricle, liver, skeletal muscle, brown fat, gastrointestinal mucosa, hematopoietic/lymphoid tissues, including the spleen and bone marrow [5]. Hence, distinguishing between physiological and pathological FDG uptake can be challenging when several overlapping processes are happening in the body.
In this paper, we present a case of relapsed DLBCL with disseminated infection including cerebral and splenic abscesses and discuss the challenge of evaluating concurrent active infection/abscesses in the background of physiological FDG uptake.
Case presentation
SL, a 43-year-old female patient, was referred by local general practitioner (GP) to the emergency department (ED) after having found to have diffuse abdominal lymphadenopathy from ultrasound (US) and computed tomography (CT) scan done for the investigation of deranged liver function tests on the background history of untreated hepatitis C. She presented with abdominal discomfort and chronic fatigue for more than 6 months without history of constitutional symptoms.
On physical examination, she had a palpable spleen with epigastric fullness, but no tenderness or hepatomegaly. Initial blood test showed borderline low cell counts with slight anemia, mild deranged liver function tests with mildly increased aspartate transaminase to 47 (normal reference range < 31), and an increased lactate dehydrogenase to 850 (normal reference range 120-250).
Initial imaging with US and CT revealed extensive abdominal lymphadenopathy with the largest conglomerate node measuring 110 mm x 43 mm and splenomegaly measuring 160-170 mm. She was urgently referred to hematology for further investigations and underwent her first staging PET/CT scan.
There was mild diffuse increased FDG activity in the enlarged spleen indicated splenic involvement (Figs. 1 and 2).
Fig. 1.
First staging PET/CT scan showed an enlarged spleen measuring 165 mm (A). Intensely FDG-avid lymphadenopathy below the diaphragm involving a bulky conglomerate in the upper abdomen (B). An enlarged gastrohepatic ligament node measuring 51 mm x 114 mm (C).
Fig. 2.
(A–C): First staging PET/CT revealed an enlarged para-aortic node measuring 49 mm x 41 mm (A). Lymphadenopathy above the diaphragm involving the right paratracheal node measuring 23 mm x 13 mm (B) and a left level IV cervical node measured 24 × 14 mm (C).
The core biopsy of the retroperitoneal lymph node confirmed the diagnosis of DLBCL. The bone marrow biopsy and flow cytometry revealed low levels of marrow involvement with large cell lymphoma. Consequently, the patient was diagnosed with stage IVB of DLBCL with involvement of the liver, spleen, and bone marrow.
The patient received chemotherapy with R-CHOP and radiotherapy to the abdomen. After 6 cycles of chemotherapy, an end of treatment PET/CT scan was performed, revealing a complete metabolic response to treatment.
There was a significant interval reduction in size of the upper abdominal lymphadenopathy, particularly within the gastrohepatic ligament and para-aortic nodal conglomerate masses. The spleen was markedly reduced in size (111 mm) from the staging PET/CT, which measured 165 mm (Fig. 3).
Fig. 3.
(A–C): End of treatment PET/CT demonstrated a notable decrease in the size of the spleen measuring 111 mm, and reduction in FDG avidity of the above and below the diaphragm lymphadenopathy. The left level IV cervical node measured 10 mm x 7 mm.
However, two months after the last PET/CT, the patient was brought to ED by her partner due to concerns of drowsiness and unexplained extreme lethargy. Biochemical tests showed hyponatremia and thrombocytopenia, and she was subsequently admitted under the Hematology team. While the patient was in the PET department for an urgent restaging PET/CT, she became increasingly drowsy and had unilateral right-sided anicosoria. As a result, the restaging PET/CT was deferred and an urgent CT head was performed instead. The CT head revealed new solid enhancing lesions in the interventricular, ependymal, and suprasellar regions, which confirmed the relapse of DLBCL with central nervous system (CNS) involvement (Fig. 4).
Fig. 4.
(A–C): CT head showed new interventricular, ependymal, suprasellar solid enhancing lesions consistent with CNS lymphoma.
Despite the new evidence of CNS lymphoma with neurological symptoms, she remained stable and was able to undergo a restaging PET/CT scan the following day. The results showed a relapse of lymphoma with involvement of the CNS and liver (Figs. 5 and 6).
Fig. 5.

Restaging PET/CT showed a new FDG-avid interventricular brain lesion.
Fig. 6.
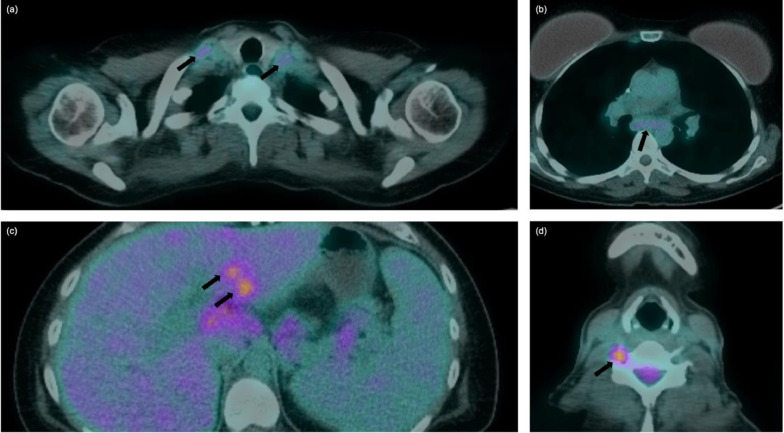
The restaging PET/CT demonstrated FDG uptake at the cervical spinal canal extending laterally to bilateral supraclavicular (A), and subcarinal lymphadenopathy (B), new FDG-avid foci within the liver (C) and intervertebral foramina at C5 and C6 (D).
After a multidisciplinary team (MDT) discussion, a treatment plan consisting of a combination of chemotherapy and targeted therapy (MATRIX) followed by autologous stem cell transplantation (ASCT) was decided upon as the next step in managing the patient's relapsed DLBCL.
During her hospital admission, another complication arose when nurses caring for her found a syringe and powder by her bedside suspecting IV drug use in the hospital. This presented an additional challenge for management, particularly when the patient experienced daily fevers in the setting of neutropenic sepsis having multiple medical emergency team (MET) calls throughout the admission and requiring antibiotic escalations. Despite this, she remained hemodynamically stable and was fluid responsive during the septic shock episodes. In addition, she was also tested positive for coronavirus disease 2019, which required antiviral treatment on top of the pre-existing antibiotics and antifungal therapy for an unknown source of infection.
The patient showed gradual signs of cell recovery and received MATRIX and intrathecal methotrexate with the plan for stem cell collections as agreed upon in the MDT discussion. However, due to her long hospital admission, the patient requested to be discharged home with plans to return for stem cell collection in a few days. Unfortunately, there was delay in her re-admission plan due to social circumstances and while at home, medications were not taken as directed. Following her readmission to the hospital, she developed persistent fevers but maintained stable hemodynamics. Intravenous Piperacillin with Tazobactam (Piptaz) was prescribed for the ongoing fevers.
Before stem cell collection, the patient underwent another restaging PET/CT scan, which showed multiple tiny scattered hypodensities throughout the spleen with some of the lesions demonstrated subtle increased FDG uptake to the diffusely increased background splenic FDG avidity concerning for multiple splenic abscesses in the current clinical context (Figs. 9 and 10). Additionally, there were new cerebral abscesses for which a same-day MRI brain was completed and confirmed the findings (Figs. 7 and 8).
Fig. 9.
(A–B): Tiny scattered hypodensities throughout the spleen demonstrating subtle increased FDG-uptake to the diffusely increased background splenic FDG avidity concerning for splenic abscesses.
Fig. 10.
Coronal CT view of the spleen measuring 130 mm craniocaudally with multiple tiny scattered hypodensities.
Fig. 7.
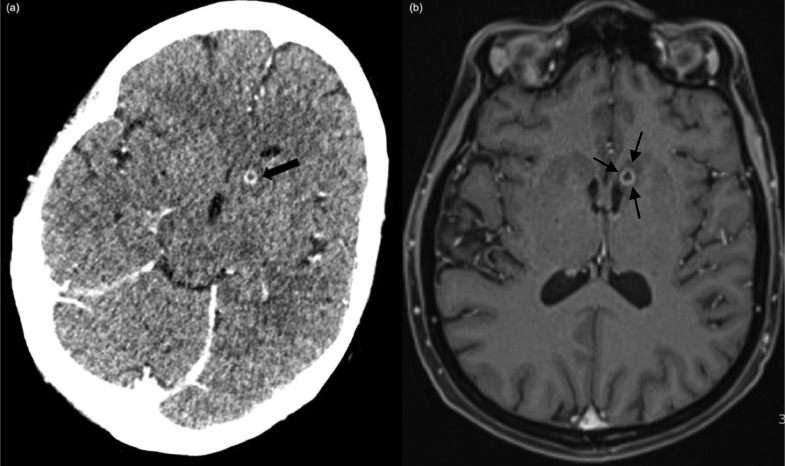
A new 5 mm ring-enhancing lesion inferior to the left head of caudate nucleus with surrounding mild vasogenic edema was visible on axial CT (A) and MR brain (B) concerning for a small cerebral abscess.
Fig. 8.
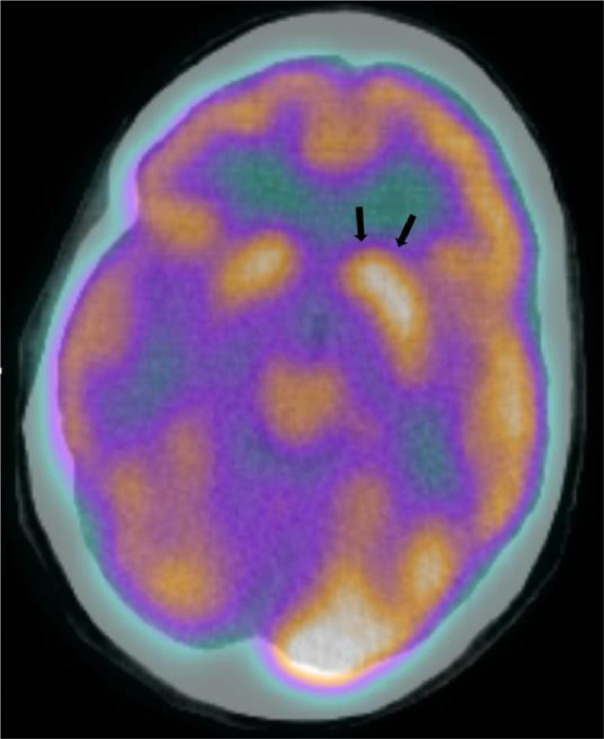
Subtly increased FDG uptake in the region of left head of caudate nucleus on restaging PET/CT that corresponded to the CT and MR brain findings of new cerebral abscess in the same area.
An urgent US-guided biopsy of the spleen was attempted, but unfortunately, the lesions were too small for useful sample collection. The surgeon was then consulted, and within a few days, the patient underwent laparoscopic total splenectomy. Intraoperatively, there were multiple lesions seen in the enlarged spleen which were densely adherent to surrounding structures making mobilization difficult.
Histopathological examination of the spleen revealed multiple small and round cream-colored lesions showing foci of necrosis and suppuration consistent with suppurative granulomatous inflammation scattered throughout the spleen. Microbiological culture results showed Candida tropicalis indicating a fungal infection.
The patient continued to make slow and gradual recovery and was subsequently discharged with at least three months of voriconazole as recommended by the infectious disease (ID) physicians and the plan for interval scans to assess progress. Follow-up appointments were scheduled, and postsplenectomy vaccine was planned after 6-12 months of Rituximab. The patient was strongly advised to comply with all follow-up reviews and treatments.
Discussion
FDG uptake is facilitated by cell surface carrier molecules called glucose transporters (GLUT1) and is quantified by the standardized uptake value (SUV) [6]. To differentiate between normal and pathological uptake, the relative FDG uptake of the tumor or infection is often compared to normal background tissue such as the liver and mediastinal blood pool [6]. Various patient-specific factors including age, gender, blood glucose level, and weight, must be considered while measuring SUV values [6]. As a result, every individual's SUV value is different and dependent on several factors. For example, the Deauville score is a 5-point scale system used in clinical reporting to stage and assess treatment response in lymphoma patients [3]. It is based on visual interpretation of FDG uptake and relies on 2 reference backgrounds of the individual patient, the mediastinum and the liver (Table 1) [3].
Table 1.
Deauville score [3]
| Deauville score (DS) | 18F-FDG uptake |
|---|---|
| 1 | No uptake |
| 2 | ≤ Mediastinal blood pool |
| 3 | > Mediastinum and ≤ liver |
| 4 | Moderately > liver at any site |
| 5 | Markedly > liver at any site and/or new sites of disease |
| X | New areas of uptake unlikely to be related to lymphoma |
The revised Ann Arbor staging system is used for staging patients with NHL by identifying the number of tumor sites including nodal or extranodal involvement and the location of the disease (Table 2) [7].
Table 2.
Ann Arbor staging system for lymphoma [7]
| Stage | Description |
|---|---|
| I | Involvement of a single lymph node region or single extranodal site |
| II | Involvement of 2 or more lymph node regions or lymphatic structures on the same side of the diaphragm alone or with involvement of limited, contiguous, extralymphatic organ or tissue |
| III | Involvement of lymph node regions on both sides of the diaphragm, which may include the spleen, limited, or contiguous, extralymphatic organ or tissue, or both |
| IV | Diffuse or disseminated foci of involvement of one or more extralymphatic organs or tissues, with or without associated lymphatic involvement |
All stages are further subdivided according to the absence (A) or presence (B) of disease-related symptoms [7].
Although DLBCL has high cure rates, a portion of patients experience relapse within twelve months after initial treatment, underscoring the importance of identifying patients with immunochemotherapy-resistant disease early on [1]. PET/CT is particularly valuable for assessing lymphoma response, but there are limitations in interpreting 18F-FDG PET/CT findings. One such pitfall is diffuse high uptake in bone marrow and/or spleen postimmunochemotherapy due to lymphocyte infiltration and reactive changes, which do not necessarily represent disease progression [4]. False positive FDG uptake findings at interim or response treatment scans can be a result from post-therapy inflammation [4].
Infection and inflammation can also cause elevated FDG uptake, as white blood cells are activated during systemic inflammatory disease and recruited to infected and inflamed tissue with high glucose metabolism [5]. While this can lead to nonspecific FDG uptake, there are instances where the uptake can be disease-specific enough to yield meaningful diagnostic outcomes, particularly when correlated with the current clinical context.
The case of SL presents a complex and challenging situation, involving relapsed DLBCL with CNS involvement, disseminated infection, slow cell recovery with ongoing fever despite escalation of therapy, complex social background with nonoptimal treatment compliance, and ongoing IV drug use. She remains acutely ill postsecond cycle of chemotherapy complicated by an unknown source of pyrexia despite on dexamethasone, antibiotics, antiviral and antifungal agents. In this clinical context, it may be argued that the PET/CT scan performed at that stage may have limited specificity to assess treatment response, detect active disease, or identify the source of infection.
However, the PET/CT scan did reveal diffuse increased FDG avidity within the spleen, which could reasonably be attributed to post-therapy infiltrative changes. Nevertheless, SL's lack of clinical improvement despite ongoing treatment raised suspicion of abscesses which were subsequently proven to be a fungal infection from the histopathology of the spleen.
While 18F-FDG PET/CT scans are essential in lymphoma evaluation, their application should be correlated to clinical context, considering the several factors that can mimic or obscure disease progression. High nonspecificity of elevated FDG uptake is often seen in inflammatory processes and physiological uptake throughout the body which can also confound interpretation [5]. Therefore, it is crucial to have strong knowledge of the physiological and abnormal FDG uptake seen in different processes to provide accurate clinical interpretation of the scan. Overlooking or dismissing the tiny hypodensities in the spleen as post-therapy reactive changes such as in this case, could have resulted in a very different clinical outcome for the patient.
Conclusion
In summary, SL's case highlights the importance of having a comprehensive understanding of both physiologic and altered physiologic FDG uptake throughout the body as well as the limitations of 18F-FDG PET/CT scans including the false positives and false negatives. In cases where there are overlap between active infection and post-therapy inflammation changes in an organ that is already known to have background physiological FDG uptake, this can easily lead to misinterpretation and ultimately, wrong treatment decisions. As such, careful and thorough interpretation of any potential confounding findings must be considered to prevent adverse patient outcomes.
Patient consent
Written and informed consent has been obtained from the patient for the case to be published for education, teaching, or research purposes.
Footnotes
Acknowledgments: No sources of funding are declared for this case report.
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Sarkozy C, Sehn LH. Management of relapsed/refractory DLBCL. Best Pract Res Clin Haematol. 2018;31(3):209–216. doi: 10.1016/j.beha.2018.07.014. https://pubmed.ncbi.nlm.nih.gov/30213390/ [accessed 20.10.22]Available from. [DOI] [PubMed] [Google Scholar]
- 2.Martelli M, Ferreri AJM, Agostinelli C, Di Rocco A, Pfereundschuh M, Pileri SA. Diffuse large B-cell lymphoma. Crit Rev in Onc/Hematol. 2013;87(2):146–171. doi: 10.1016/j.critrevonc.2012.12.009. https://www.sciencedirect.com/science/article/abs/pii/S1040842813000024?via/3Dihub [accessed 20.10.22]Available from. [DOI] [PubMed] [Google Scholar]
- 3.Dubreuil J, Salles G, Bozzetto J, Tordo J, Djaileb L, Berriolo-Riedinger A, et al. Usual and unusual pitfalls of 18F-FDG-PET/CT in lymphoma after treatment: a pictorial review. 2017 Nucl Med Commun. [accessed 8.10.22]; 38(7): 563-76. Available from: https://pubmed.ncbi.nlm.nih.gov/28570287/ doi: 10.1097/MNM.0000000000000697. [DOI] [PubMed]
- 4.Baba S, Abe K, Isoda T, Maruoka Y, Sasaki M, Honda H. Impact of FDG-PET/CT in the management of lymphoma. Ann Nucl Med [Internet] 2011;25(10):701–716. doi: 10.1007/s12149-011-0549-0. https://link.springer.com/article/10.1007/s12149-011-0549-0 [accessed 10.10.22]Available from: [DOI] [PubMed] [Google Scholar]
- 5.Pijil JP, Nienhuis PH, Kwee TC, Glaudemans AWJM, Slart RHJA, Gormsen LC. Limitations and pitfalls of FDG-PET/CT in infection and inflammation. Sem Nucl Med [Internet] 2021;51(6):633–645. doi: 10.1053/j.semnuclmed.2021.06.008. [DOI] [PubMed] [Google Scholar]; Available from: https://www.sciencedirect.com/science/article/pii/S0001299821000404. [Accessed 20.10.22].
- 6.Sharma P, Chatterjee P, Alvarado LA, Dwivedi AK. Standardized uptake value of normal organs on routine clinical [18F]FDG PET/CT: impact of tumor metabolism and patient-related factors. Nuc Med Rev. 2022;26:1–10. doi: 10.5603/NMR.a2022.0036. [DOI] [PubMed] [Google Scholar]; Available from https://journals.viamedica.pl/nuclear_medicine_review/article/view/NMR.a2022.0036/68817. [Accessed 02.04.23].
- 7.Ansell AM. Non-Hodgkin Lymphoma: diagnosis and treatment. Mayo Clin Proc. 2015;90(8):1152–1163. doi: 10.1016/j.mayocp.2015.04.025. [DOI] [PubMed] [Google Scholar]; Available from https://www.clinicalkey.com.au/#!/content/journal/1-s2.0-S0025619615004292. [Accessed 02.04.23].