Abstract
The inferior vena cava agenesis (IVCA) is a rare and often asymptomatic malformation due to the abundant development of the collateral circulation. However, it is frequently found in young people and carries a significant risk of deep venous thrombosis (DVT). It is estimated that about 5% of patients under 30 years of age presenting with DVT have this condition. We report a case of a previously healthy 23-year-old patient presenting with signs of acute abdomen and hydronephrosis due to the thrombophlebitis of an unusual iliocaval venous collateral, which developed secondary to IVCA. After treatment, the iliocaval collateral and hydronephrosis completely regressed on a 1-year follow-up. To our knowledge, this is the first such case reported in the literature.
Keywords: Acute abdomen, Deep vein thrombosis, Hydronephrosis, Inferior vena cava agenesis, Thrombophlebitis, Collateral vein
Introduction
The inferior vena cava (IVC) is the largest vein in the human body, located in the retroperitoneal space. It transports deoxygenated blood from the abdomen, pelvis, and lower extremities to the right atrium [1]. Its embryonic development is complex and consists of several interconnected phases between the sixth and eighth gestational weeks: formation, regression, and anastomosis of 4 pairs of veins (supracardinal and subcardinal veins, posterior cardinal vein, and vitelline [omphalomesenteric] veins). Understandably, many different malformations can occur, such as duplication, malposition, or agenesis, most of which have no clinical significance [1], [2], [3], [4]. Their incidence in the general population ranges from 0.05% to 8.7% [5]. The inferior vena cava agenesis (IVCA), however, carries a significantly higher risk of deep venous thrombosis (DVT) [6].
Herein, we report a case of a young male patient presenting with signs of acute abdomen due to thrombophlebitis of the iliocaval collateral, which developed secondary to IVCA and caused compression of the right ureter with subsequent hydronephrosis. We extensively reviewed the literature, and to our knowledge, this is the first such case reported.
Case report
A 23-year-old male presented to the emergency department with complaints of fever and right lumbar pain radiating to the right groin area. His medical history was unremarkable. Physical examination revealed an axillary body temperature of 38°C, a diffusely tender abdomen, and a positive Blumberg sign. Laboratory tests showed signs of inflammation, with raised leukocyte levels of 25 × 109/L (reference levels <9.7) and C-reactive protein levels of 327.1 mg/L (<5.0).
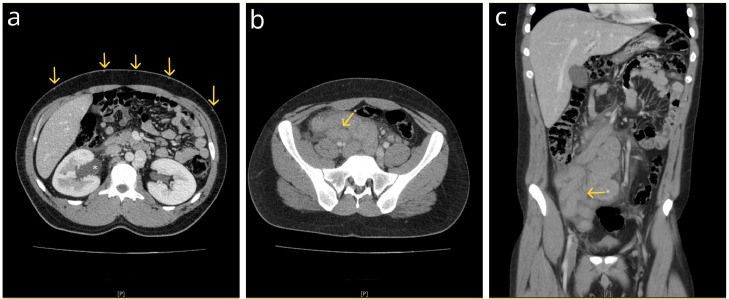
Acute appendicitis was suspected, and an ultrasound (US) examination showed a large, hypoechoic, and serpiginous mass in the retroperitoneal space, located mainly in the right abdomen. A computed tomography (CT) scan revealed infrarenal agenesis of the inferior vena cava segment, with multiple, tortuous, and partially thrombosed vascular channels draining into paravertebral and lumbar veins. Both common iliac veins (CIV) formed an IVC stump and drained through extensive collateral circulation, mainly lumbar, paravertebral, and abdominal wall collateral veins, into compensatory dilated azygos and hemiazygos veins. The right renal vein drained into a renal segment of the IVC, while the left drained into the hemiazygos vein, which further drained into the azygos vein (Fig. 1). A strikingly enlarged, thrombosed, and serpiginous iliocaval venous collateral developed, originating from the left CIV and draining into the renal segment of the IVC. Surrounding visceral fat showed signs of stranding, and moderate amounts of free intraperitoneal fluid were found, indicating inflammation, that is, thrombophlebitis (Fig 2). External, internal, and common iliac veins on both sides and the left testicular vein were thrombosed (Fig. 3).
Fig. 1.
Postcontrast coronal (A, C, D) and axial (B) reformatted CT images of the thorax, abdomen, and pelvis in the portal-venous (PV) phase. (A) A blind-ending IVC (*) and partially thrombosed retroperitoneal collaterals are seen (arrow). (B) The enlarged azygous vein (*) is comparable in size to the aorta (Ao). (C) The hemiazygos vein drains into the azygos vein. Both vessels are significantly enlarged. (D) Markedly enlarged paravertebral veins are seen (arrows).
Fig. 2.
Postcontrast coronal reformatted CT images of the abdomen and pelvis in the PV phase. (A) A strikingly enlarged, thrombosed, and serpiginous iliocaval venous collateral fills the large part of the abdomen, with the small intestine pushed into the left upper quadrant. Note the surrounding fat stranding and free fluid, indicating thrombophlebitis. (B) The arrow points to the origin point of the iliocaval collateral at the left CIV. (C) The arrow points to the termination point of the iliocaval collateral at the IVC, just above the blind end.
Fig. 3.
Postcontrast axial (A, B) and coronal (C) CT images of the abdomen and the pelvis in the PV phase. (A, B) Extensive thrombosis is seen in the external (EIV) and internal iliac veins (IIV), as well as the common iliac veins (CIV). (C) A dilated and thrombosed left testicular vein (*) drains into the left renal vein (RV) at the level of the renal-hemiazygos vein junction. HaV – hemiazygos vein.
The iliocaval collateral compressed the right ureter. Consequently, grade 1 hydronephrosis developed, and the right kidney showed slightly delayed contrast uptake (Fig. 4).
Fig. 4.
Postcontrast axial (A,B) and coronal (C) CT images of the abdomen and pelvis. (A) Grade I hydronephrosis (*) with a delayed contrast uptake of the right kidney is seen. Note the prominent superficial abdominal wall veinous collaterals (arrows) and an enlarged hemiazygos vein (HaV). (B, C) The right ureter (*) is compressed by the thrombosed iliocaval collateral. Note the reactive inflammation of the ureteral wall.
He was hospitalized and first referred to a vascular surgeon, who deemed the patient unsuitable for surgical intervention. Echocardiography revealed mitral valve prolapse with moderate mitral regurgitation. Thrombophilia workup showed homozygotic mutations in A1298C methylenetetrahydrofolate reductase (MTHFR) and plasminogen activator inhibitor type 1 (PAI-1).
The treatment consisted of low-molecular-weighted heparin, broad-spectrum antibiotics, and a urologist placement of an endoscopy-guided JJ stent which decompressed the right ureter. Since the patient did not complain of lower extremity swelling and pain on presentation, a color Doppler ultrasound of his lower extremities was performed 20 days after admission and showed no signs of DVT. Due to the results of the thrombophilia workup, a hematologist recommended lifelong anticoagulant prophylaxis with dabigatran (150 mg twice daily). One month after admission, he was discharged symptom-free and in good general condition.
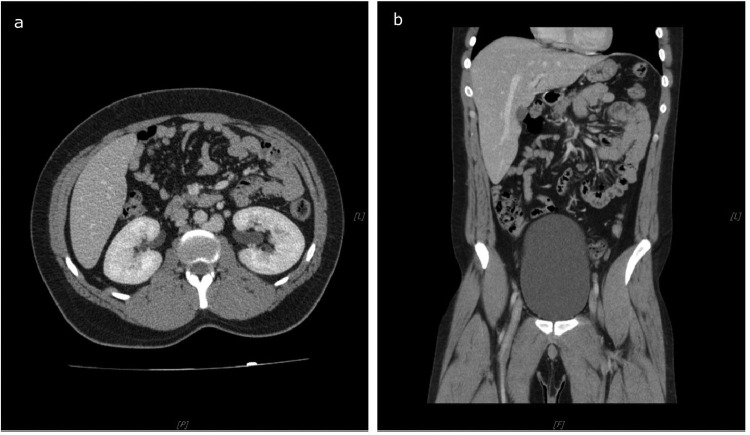
On a 1-year follow-up, the patient was symptom-free, showed no signs of DVT, and was without adverse events related to anticoagulant therapy. Surprisingly, a follow-up CT scan showed complete regression of thrombosed iliocaval venous collateral with fully patent and enlarged other developed collateral pathways. Hydronephrosis also regressed, and both kidneys showed concurrent contrast uptake (Fig. 5). Unfortunately, the left testicular vein remained thrombosed, and the patient developed a left-sided varicocele on a 2-year follow-up, which required surgical ligation.
Fig. 5.
Postcontrast axial (A) and coronal (B) CT images of the abdomen in a PV phase on a 1-year follow-up. Surprisingly, iliocaval venous collateral completely regressed. Therefore, the right kidney shows no signs of hydronephrosis with prompt contrast uptake.
Discussion
The inferior vena cava agenesis is a rare malformation with a prevalence between 0.0005% and 1% in the general population. It can be complete or segmental. The latter is divided based on location into intrahepatic, suprarenal, renal, and infrarenal segment agenesis. Only 6% of all cases are renal or infrarenal variants [1], [2], [3],5,7]. Some authors suggest that agenesis of the infrarenal IVC segment develops because of intrauterine thrombosis rather than a congenital malformation, but there is still no clear evidence for either theory [2], [3], [4],8,9].
Extensive collateral circulation develops to bypass the interruption, of which 4 pathways are the most significant: the paravertebral plexus draining into the vena cava superior (VCS) through the azygos-hemiazygos system, the hemorrhoidal plexus draining into the portal vein, the gonadal system draining into suprarenal VCI, and the superficial system draining into subclavian veins or VCS through anterior abdominal wall veins [10], [11], [12]. Besides the unusual iliocaval collateral, the paravertebral and abdominal wall collaterals were the primary pathways in our case.
Tufano et al. [13] report in their study from 2020 that patients with IVCA and DVT were more likely to be young, male, have unprovoked proximal or bilateral DVT, and were less likely to have a pulmonary embolism (PE), probably due to the difficulty of passing the embolus through the network of collaterals, as first reported in the review of the literature by Lambert et al. [14]. In the same paper, Lambert stated that only about 10% of cases had PE.
Although IVCA is frequently asymptomatic and incidentally found on imaging or during surgeries [9], it is often associated with other gastrointestinal, vascular, pulmonary, and cardiac malformations [15]. In our case, echocardiography revealed MVP. As far as we know, no other case report described a similar finding, so it is still unclear if there is a connection between the 2 anomalies.
Yugueros et al. [10] described a connection between IVCA and obstructive pyelonephritis in a case report from 2013, where an enlarged and thrombosed right gonadal vein led to the right ureter obstruction. Usta Atmaca et al. [16] reported a case in 2014 where retroperitoneal collaterals led to compression of both ureters and hydronephrosis. Similarly, in our case, an enlarged and inflamed iliocaval collateral compressed the right ureter, which led to mild hydronephrosis and delayed contrast uptake of the right kidney.
The true prevalence of thrombophilia in patients with IVC anomalies is unknown [17]. As mentioned above, the pelvic and lower extremity DVT risk is elevated (10-fold increase) due to often insufficiently developed collateral pathways, leading to inadequate venous return, venous hypertension, and venous stasis [6,15,18]. About 5% of people under 30 with DVT have this condition [5,8,9,14,19]. Symptoms may develop after rigorous physical activity, typically as lower back or abdominal pain due to lumbar vein thrombosis or lower extremity pain, swelling, and erythema due to DVT [4,8,14,20]. It exceptionally presents as an acute abdomen of uncertain etiology [10]. In our case, acute abdomen developed due to extensive thrombosis and thrombophlebitis of the iliocaval collateral. Lumbar veins were patent and dilated. Since the patient did not complain of lower extremity swelling and pain, a color Doppler ultrasound was performed late after admission, when the patient was under anticoagulation therapy and displayed no signs of DVT. It remains unclear if he did not develop DVT at all or if the thrombi had cleared by then.
Signs of upstream congestion have also been reported, such as pelvic congestion syndrome in females and varicocele in males [21], which also occurred in our case.
There should be a high degree of suspicion, and one should examine the abdominal and pelvic venous pool anatomy in young patients presenting with bilateral DVT (50% of all cases), pelvic DVT, recurrent episodes, or idiopathic thrombosis without known risk factors, especially in males (82% of all cases) [7], [8], [9],13,14].
Color Doppler US is usually the first diagnostic modality used because it is relatively cheap, widely available, and can demonstrate pelvic and lower extremity DVT. Unfortunately, it is often challenging to visualize VCI due to overlaying structures and bowel gas. Therefore, CT or MRI angiography is done because these modalities have much higher sensitivity and can easily show the exact anatomy of the collateral network [3,8,9,13,19,21]. In addition, genetic testing for thrombophilia and searching for other causes of hypercoagulability, such as prolonged immobilization, recent trauma, surgery, or vascular catheterization, are highly recommended [8].
Administration of anticoagulant medications, compression stockings, lower extremity elevation, and thrombolysis in selected cases is the most common treatment strategy, but since the condition is rare, there is still debate about the therapeutic strategy [8,9,14,15,20,21]. While many studies suggest lifelong anticoagulation [3,21], others suggest against it. Riera-Mestre et al. [22] state in their 2014 research that in such patients, the risk of DVT recurrence after anticoagulant therapy cessation is equal to the general population with unprovoked DVT, so the positives and the negatives of the long-term prophylaxis should be carefully assessed.
Conclusion
It is necessary to think about and search for IVCA and other venous malformations in young people, especially males, presenting with bilateral lower extremity or pelvic DVT and without any known risk factors. Unrecognized preoperatively, such anomalies can lead to profuse and potentially fatal bleeding during surgeries [7]. Adequate medications could reduce the risk of potential recurrent thromboembolic incidents [8,9,14], but further research is necessary to determine the best therapeutic strategy, the optimal duration of treatment, and the long-term outcome of such patients.
Patient consent
Written informed consent was obtained from the patient for the publication of this case report and accompanying images. The anonymity of all clinical and graphical data used is ensured.
Footnotes
Acknowledgments: The authors declare that they have not received funding of any sort.
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Tucker WD, Shrestha R, Anatomy Burns B. StatPearls. StatPearls Publishing; Treasure Island (FL): 2023. Abdomen and pelvis: inferior vena cava.https://www.ncbi.nlm.nih.gov/books/NBK482353/ [accessed July 25, 2022] [PubMed] [Google Scholar]
- 2.Gensas CS, Pires LM, Kruse ML, Leiria TL, Gomes DG, Lima GG. Agenesis of the inferior vena cava. Rev Bras de Cardiol Invasiva (English Edition) 2012;20(no. 4):427–430. doi: 10.1016/S2214-1235(15)30090-9. [DOI] [Google Scholar]
- 3.Gerges P, Mian A, Singh G, Aziz M, Guirguis S, Koteish A. Agenesis of the intrahepatic inferior vena cava: a case report and literature review. Cureus. 2023;15(2):e35589. doi: 10.7759/cureus.35589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knipp B, Knechtges P, Gest T, Wakefield T. Inferior vena cava: embryology and anomalies. Aortic Aneurysms. 2009;1:289–307. doi: 10.1007/978-1-60327-204-9_20. [DOI] [Google Scholar]
- 5.Ramos Aranda J, Ramírez Cerda C, Cohen Mussali S, Valdés Flores J. Inferior vena cava agenesis: an unusual cause of deep vein thrombosis and pulmonary embolism in young adult patients. EJVES Short Rep. 2018;39:12–15. doi: 10.1016/j.ejvssr.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menning M, Yousef M. Congenital inferior vena cava agenesis with ulceration and deep vein thrombosis. Eur J Case Rep Intern Med. 2021;8(3) doi: 10.12890/2021_002312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grillo VTRDS, Mellucci PL, Jaldin RG, Bertanha M, Pimenta REF, Sobreira ML. Agenesis of the infra-hepatic segment of the inferior vena cava associated with recurrent deep venous thrombosis: case report. J Vasc Bras. 2021;20 doi: 10.1590/1677-5449.210006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cruz IE, Ferreira P, Silva R, Silva F, Madruga I. Inferior vena cava agenesis and deep vein thrombosis: a pharmacological alternative to vitamin k antagonists. Eur J Case Rep Intern Med. 2019;6(12) doi: 10.12890/2019_001310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paddock M, Robson N. The curious case of the disappearing IVC: a case report and review of the etiology of inferior vena cava agenesis. J Radiol Case Rep. 2014;8(4):38–47. doi: 10.3941/jrcr.v8i4.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yugueros X, Alvarez B, Fernández E, Boqué M, Matas M. Compressive symptoms due to thrombosed or hypertrophic collateral circulation in infrarenal inferior vena cava agenesis. Ann Vasc Surg. 2013;27(2):238.e9–238.e13. doi: 10.1016/j.avsg.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 11.Menezes T, Haider EA, Al-Douri F, El-Khodary M, Al-Salmi I. Pelvic congestion syndrome due to agenesis of the infrarenal inferior vena cava. Radiol Case Rep. 2018;14(1):36–40. doi: 10.1016/j.radcr.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morosetti D, Picchi E, Calcagni A, Lamacchia F, Cavallo AU, Bozzi A, et al. Anomalous development of the inferior vena cava: case reports of agenesis and hypoplasia. Radiol Case Rep. 2018;13(4):895–903. doi: 10.1016/j.radcr.2018.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tufano A, López-Jiménez L, Bikdeli B, García-Bragado F, Mazzolai L, Amitrano M, et al. Inferior vena cava agenesis in patients with lower limb deep vein thrombosis in the RIETE registry. When and why to suspect. Int J Cardiol. 2020;305:115–119. doi: 10.1016/j.ijcard.2020.01.013. [DOI] [PubMed] [Google Scholar]
- 14.Lambert M, Marboeuf P, Midulla M, Trillot N, Beregi JP, Mounier-Vehier C, et al. Inferior vena cava agenesis and deep vein thrombosis: 10 patients and review of the literature. Vasc Med. 2010;15(6):451–459. doi: 10.1177/1358863X10391355. [DOI] [PubMed] [Google Scholar]
- 15.Arıkan AA, Emre S, Avni BF. The use of rivaroxaban in deep venous thrombosis associated with vena cava inferior agenesis. Turk Gogus Kalp Damar Cerrahisi Derg. 2019;27(4):583–585. doi: 10.5606/tgkdc.dergisi.2019.17672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atmaca Usta H, Akbaş F, Erdenen F, Karagöz Y, Pekgüç E. Congenital agenesis of the inferior vena cava presenting with acute renal failure. Turk Gogus Kalp Dama. 2014;22:182–186. doi: 10.5606/tgkdc.dergisi.2014.8046. [DOI] [Google Scholar]
- 17.Nseir W, Mahamid M, Abu-Rahmeh Z, Markel A. Recurrent deep venous thrombosis in a patient with agenesis of inferior vena cava. Int J Gen Med. 2011;4:457–459. doi: 10.2147/IJGM.S21423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tufano A, Cannavacciuolo F, Gianno A, Cerbone AM, Mangiacapra S, Coppola A, et al. Inferior vena cava agenesis and deep vein thrombosis in the young: a review of the literature and local experience. Semin Thromb Hemost. 2017;43(8):827–835. doi: 10.1055/s-0037-1603363. [DOI] [PubMed] [Google Scholar]
- 19.Pasqui E, de Donato G, Camarri S, Molinari R, Cascinelli I, Pelini V, et al. Case report: inferior vena cava agenesia in a young male patient presenting with bilateral iliac veins thrombosis. Front Surg. 2022;9 doi: 10.3389/fsurg.2022.832336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langer F, Dos Santos D, Suertegaray G, Haygert C. Bilateral deep vein thrombosis associated with inferior vena cava agenesis in a young patient manifesting as low back pain. Acta Med Port. 2017;30(4):333–337. doi: 10.20344/amp.7744. [DOI] [PubMed] [Google Scholar]
- 21.Protti G, Elia F, Bosco F, Aprà F. An eminent absence: agenesis of inferior vena cava underlying bilateral iliac vein thrombosis. Eur J Case Rep Intern Med. 2020;7(12) doi: 10.12890/2020_001999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riera-Mestre A, Romera A, Fernández A, Corbella X. Long-term follow-up after anticoagulant treatment withdrawal in patients with deep venous thrombosis and inferior vena cava agenesis. Eur J Intern Med. 2014;25(9):e113–e114. doi: 10.1016/j.ejim.2014.10.008. [DOI] [PubMed] [Google Scholar]