Abstract
Introduction
Red blood cell (RBC) transfusion may affect the recipient immune system. During RBC storage in an unphysiological environment, RBC quality and function are impaired, the cells bleb extracellular vesicles (EVs), and other bioactive substances accumulate in the storage medium. EVs can carry reactive biomolecules and mediate cell-cell interactions. Thus, EVs could explain RBC transfusion related immunomodulation, particularly after prolonged storage.
Methods
We exposed peripheral blood mononuclear cells (PBMCs) to allogeneic RBC supernatant (SN) and EVs from fresh and longer-stored RBC units, diluted plasma, and storage solution SAGM, and studied activation and proliferation of T-cells by flow cytometry, and cytokine secretion of LPS-stimulated PBMCs by enzyme-linked immunosorbent assay (ELISA).
Results
Both fresh and longer-stored RBC SN but not EVs induced immunomodulation in recipient cells. RBC SN and diluted plasma augmented the proliferation of particularly CD8<sup>+</sup> T-cells in a 4-day proliferation assay. T-cell activation by SN was evident already after 5 h as shown by upregulation of CD69. SN suppressed monocyte TNF-α and increased IL-10 secretion while diluted plasma increased secretion of both cytokines.
Conclusion
This in vitro study demonstrates that stored RBC SN will have mixed immunomodulatory effects depending on responder cells and conditions, independent of RBC storage age. Fresh RBCs containing relatively few EVs can induce immune responses. Residual plasma in the products may contribute to these effects.
Key Words: Red blood cells, Extracellular vesicles, T-cell proliferation, Transfusion-related immunomodulation
Introduction
Stored red blood cells (RBCs) may affect the immune system, referred as transfusion-related immunomodulation (TRIM) [1, 2]. Studies have demonstrated the immunomodulatory effects of stored RBCs in vitro. Depending on assay conditions and cells, these effects can be immunosuppressive [3, 4] or proinflammatory [5, 6, 7, 8, 9].
RBCs are stored up to 35–42 days at 5°C. RBC storage lesions are well documented in laboratory settings [10], and observational clinical studies suggest that transfusing longer-stored RBCs could be harmful, especially for the critically ill patients [11, 12]. Randomized clinical trials have demonstrated, however, that the freshest RBCs are not superior to standard-time stored RBCs [13, 14]. Nevertheless, transfused RBCs of any storage age can potentially induce immune responses. TRIM-mediating bioactive substances may be present already early on storage and perhaps more abundantly in aged products.
During storage, lipid bilayer extracellular vesicles (EVs) bleb from cell membranes and accumulate in RBC units [7, 15, 16]. EVs may transfer bioactive molecules and mediate cell-to-cell interactions [15, 17]; thus, EVs are suspected to act as mediators of TRIM. RBC EVs can enhance T-cell responses [7], whereas in another study, this effect was seen with stored RBC supernatant (SN), but not with EVs alone [5]. RBC EVs also have immunosuppressive properties [3]. Furthermore, RBC SN, but not EVs, has been shown to suppress monocyte function in vitro [4]. These disparate results may reflect the varying effects of RBC EVs and SN on distinct cell types as well as differences in manufacturing and storage conditions.
We initially hypothesized that EVs in RBC products would mediate TRIM and be proinflammatory, and due to RBC storage lesions and increasing EV concentrations, the magnitude of the effects would increase with prolonged storage. Accordingly, we studied the effect of fresh and longer-stored RBC EVs and SN on T-cell and monocyte activation. Furthermore, we tested the role of residual plasma as a potential contributing factor in SN on the observed T-cell responses.
Materials and Methods
Overview of Study Design
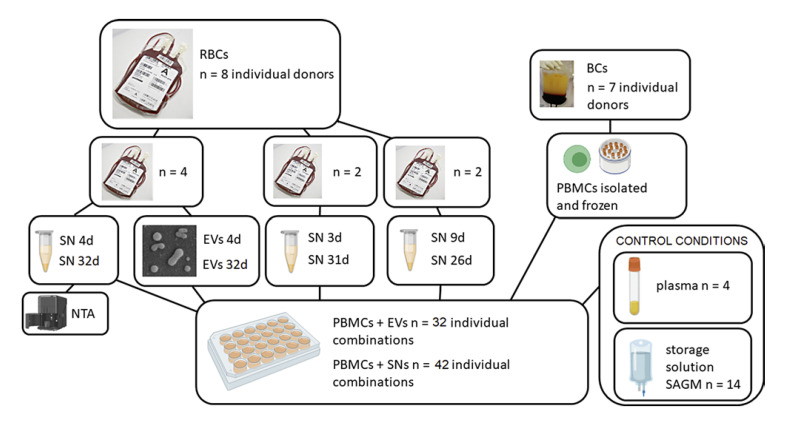
At first, we studied how exposure to EVs and SN obtained from RBC units (n = 4) at fresh (4 days) and 28 days later at end-stage storage (32 days) will affect proliferation and differentiation of anti-CD3-activated T-cells in peripheral blood mononuclear cell (PBMC) cultures. After seeing that storage age was irrelevant and that only SN was effective, we later repeated experiments utilizing SN from available RBC units of varying storage ages. The number of EVs in the centrifugate samples from the four RBC units utilized in the first experiment was measured with nanoparticle tracking analysis (Fig. 1). After detecting that SN augments T-cell proliferation, we studied whether CD69-expression can be exploited as a quicker way of showing T-cell activation.
Fig. 1.
T-cell proliferation study design. PBMCs were stimulated with anti-CD3 and cultured exposed to either PBS, SN, EVs, SAGM, or diluted plasma. The first experiment included four RBC units from which EVs, SN, and samples for NTA were obtained at early and late phases of storage. The experiments were continued utilizing SN (but no more EVs) sampled at various RBC unit storage ages. Altogether seven individual PBMCs were used in the experiments, utilizing always the same individuals when comparing fresh versus old RBCs. RBCs, stored red blood cells; BCs, buffy coats; SN, supernatant; EVs, extracellular vesicles; PBMCs, peripheral blood mononuclear cells; NTA, nanoparticle tracking analysis; SAGM, saline-adenine-glucose-mannitol – solution.
We also wanted to see whether LPS-stimulated leukocytes in PBMC cultures will alter their cytokine secretion after exposure to, again, EVs and SN obtained from RBC units at early (5 days) and late storage (33 days) (study design not included in Fig. 1). The same RBC units (n = 4) were used in the first T-cell proliferation and cytokine assay. Both experiments, T-cell proliferation and cytokine assay, were later repeated adding diluted plasma conditioning to test whether residual plasma in RBC units might be behind the observed immune responses.
Packed RBCs and PBMCs
Whole blood was collected and RBCs were produced according to standard operating procedures at FRC Blood Service. Briefly, whole blood units were collected from eligible volunteer donors and manufactured by using red cell filtration (RCF; top/bottom). Whole blood (460 mL) was collected with 63 mL of citrate-phosphate-dextrose (CPD) anticoagulant using CompoFlow sets (Fresenius), and units were rapidly cooled to 20–24°C and held overnight. Whole blood units were centrifuged for 12 min at 4,389 g (Hettich centrifuges, Germany) and processed into RBCs, plasma, and buffy coats using an automatic extractor (Compomat G5; Fresenius) and saline-adenine-glucose-mannitol (SAGM, 100 mL) was added to the extracted RBCs. The RBC units were leukoreduced by filtration at room temperature. All RBC units produced were stored at 1–6°C. Maximum storage time for RBCs is 35 days in Finland.
Figure 1 presents the study design regarding T-cell proliferation. In the first experiment (n of RBCs = 4), samples (6 + 35 mL) were aseptically drawn from the bags at day 4 (early) and day 32 (prolonged storage). The assay was later repeated with samples from RBC units (n = 4) of variable storage times (Fig. 1).
PBMCs (n = 7 individual donors for T-cell proliferation [frozen], and n = 16 for cytokine assays [used instantly]) were isolated from buffy coats. Ficoll-Paque PLUS (Amersham Biosciences) density gradient centrifugation was used according to the manufacturer's instructions and the cells were frozen in 10% dimethyl sulfoxide (Wak-Chemie Medical) – fetal bovine serum (Gibco, Life Technologies) media. When thawed the cells were washed three times with phosphate-buffered saline (PBS; Sigma-Aldrich), pH 7.2, and suspended to assay medium (RPMI 1640 Medium (1X) with L-glutamine and HEPES buffer, with 5% fetal bovine serum and 1% penicillin-streptomycin; Gibco, Life Technologies).
EV and SN Isolation
To obtain cell-free SN, a 6 mL aliquot of an RBC concentrate was centrifuged two times, first for 20 min at 805 g without brake and subsequently 3,000 g 20 min. SNs used in assays were not further manipulated and thus included EVs too. For EV isolation, 35 mL of an RBC concentrate was diluted with 35 mL of PBS (Sigma-Aldrich) and centrifuged two times as described. The resulting supernatant was ultracentrifuged for 1 h at 100,000 g (+4°C, fixed-angle MLA-50 rotor; Beckman Coulter) to obtain EVs. The EVs were washed once with PBS, ultracentrifuged for 1 h at 100,000 g (+4°C, swing out MLS-50 rotor; Beckman Coulter) and resuspended in 1.75 mL of PBS. SNs (n = 8) and EVs (n = 4) were stored at +5°C and used in assays within 24 h.
Nanoparticle Tracking Analysis
Concentration of EVs in RBC SNs (n = 4, each analyzed two times, at day 4 and at day 32) was examined by Nanoparticle Tracking Analysis (NTA). Of the RBC concentrates, 0.5 mL was resuspended in 2 mL of SAGM solution and centrifuged two times for 20 min at RT and 805 g without a brake (Eppendorf centrifuge 5810R). The resulting supernatant was analyzed for particle content by NTA (instrument LM14C with violet laser 405 nm, 70 mW; Malvern Instruments Ltd., Malvern, UK) and a sCMOS camera (Hamamatsu Photonics K.K., Hamamatsu City, Japan). The samples were injected manually, and data acquisition was conducted at ambient temperature in triplicate using the NanoSight software v. 2.3 (Malvern Instruments Ltd.). Settings for data acquisition were: basic, camera level 14, autosettings off, polydispersity and reproducibility high, with particles per image 40–100 (acquisition time 90 s). Data were analyzed in the NanoSight software v. 3.0 (Malvern Instruments Ltd.) with threshold 5 and gain 10.
T-Cell Proliferation Assay and CD69 Expression
One million PBMCs were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE; Life Technologies) at a final concentration of 5 μM for 5 min. Labeled cells were combined in 550 μL assay media with 50 μL of either EVs, SN, PBS, SAGM, or diluted plasma. T-cell proliferation was activated with anti-CD3 (LEAF Purified anti-human CD3; BioLegend) at a final concentration of 0.5 μg/mL. Proliferation was analyzed after 4 days as a dilution of CFSE intensity. Non-stimulated CFSE-labeled and stimulated/non-stimulated non-labeled MNC cells were used as controls. All conditions were in triplicate. EVs, SNs, and plasma were allogeneic to PBMCs. At the end, PBMCs were labeled with fluorescent-conjugated anti-CD4 (eBioscience, Thermo Fischer Scientific), and anti-CD8 (BD Biosciences) antibodies according to the manufacturer's recommendations. Samples were run on BD FACS Aria IIu (BD) and the acquired data were analyzed with FlowJo v10.0.7 (BD) software. T-cell proliferation was analyzed first by gating the lymphocyte population and then evaluating CFSE intensity as median fluorescence intensity in each sample. CD4 and CD8 positive cells were gated to get proportions of cells in each condition.
To test early T-cell activation, PBMCs were activated as described above and incubated with 50 μL of either SN, PBS, or SAGM. SNs were obtained from 3- to 31-day-stored RBCs. After for 5 h, cells were stained for CD69 surface marker (BD) expression, as well as with anti-CD3, anti-CD4, anti-CD8 and corresponding isotype control antibodies and run on BD FACS Aria IIu. CD69 expression was analyzed first by gating the lymphocyte population, and the percentage of CD69-positive cells was obtained based on gating to the corresponding isotype control-labeled cells. CD69 median fluorescence intensity was obtained from the whole lymphocyte population.
Cytokine Assay
Freshly isolated PBMCs (n = 16 individual responders) were suspended in assay medium (RPMI) to a final concentration of 500,000 cells per 0.5 mL. Fifty µl aliquot of EV, SN, PBS, SAGM, or diluted plasma was added to each well. EVs, SNs, and plasma were allogeneic. Altogether, 8 responders received EVs or SN from 5 days (fresh) RBC units (n = 4) and 8 responders received EVs or SN from the same 33-day-stored RBC units (n = 4). Experiments with diluted plasma were done separately and involved two individual PBMC responders and three plasma donors (n = 6 individual combinations). PBMCs were stimulated with 5 ng/mL LPS (Escherichia coli O55:B5; Sigma-Aldrich) at 37°C in 5% CO2. After 20 h, the supernatants were collected after centrifugation (300 g 15 min) and stored at −80°C. All conditions were performed in triplicate.
Concentrations of TNFα and IL-10 in supernatants were determined using a standard cytokine sandwich ELISA protocol, according to the manufacturer's instructions. All antibodies, standards, and other reagents were purchased from BD Pharmingen (San Jose, CA). Briefly, 96-well Maxi Sorp microtiter plates (Nunc) were coated with a monoclonal capture antibody (mouse anti-human TNFα or rat anti-human IL-10), and after blocking with 1% bovine serum albumin (Sigma-Aldrich) in PBS, the samples were incubated in the wells. The bound cytokines were detected by biotinylated mouse anti-human TNFα or rat anti-human IL-10 antibody, avidin-horseradish peroxidase conjugate and tetramethylbenzidine substrate. The color reactions were stopped by adding 1 M phosphoric acid. The intensities of the color reactions were measured at 450 nm with Clariostar (BMG Labtech, Ortenberg, Germany). Two-fold dilutions of purified recombinant human TNFα and IL-10 were used as standards.
Diluted Plasma
Whole blood in citrate tubes (n = 4 individuals for T-cell proliferation, and n = 3 for cytokine assay) was collected from standard blood donation. Blood was centrifuged 2,200 g for 11 min to obtain plasma. FRC Blood Service quality control statistics show that RBC unit cell-free supernatant contains on average 15.6% of residual plasma, thus we diluted plasma in SAGM in ratios 1:5 and 1:10.
Statistical Analysis
The Friedman test was used to study related samples, and to compare independent groups of samples, the Kruskal-Wallis test was used. Dunn's multiple comparisons were calculated for both tests. p value <0.05 was considered statistically significant. Data are presented as median (IQR). Analyses were performed with GraphPad Prism (version 8.1.2, GraphPad Software).
Results
Nanoparticle Tracking Analysis
The number of EVs increased in each tested RBC product during storage. The median number of EVs was 67,120 × 106 (IQR 22,680–101,710 × 106) at day 4, and 119,300 × 106 (IQR 89,740–139,610 × 106) at day 32.
Activation and Proliferation of T-Cells
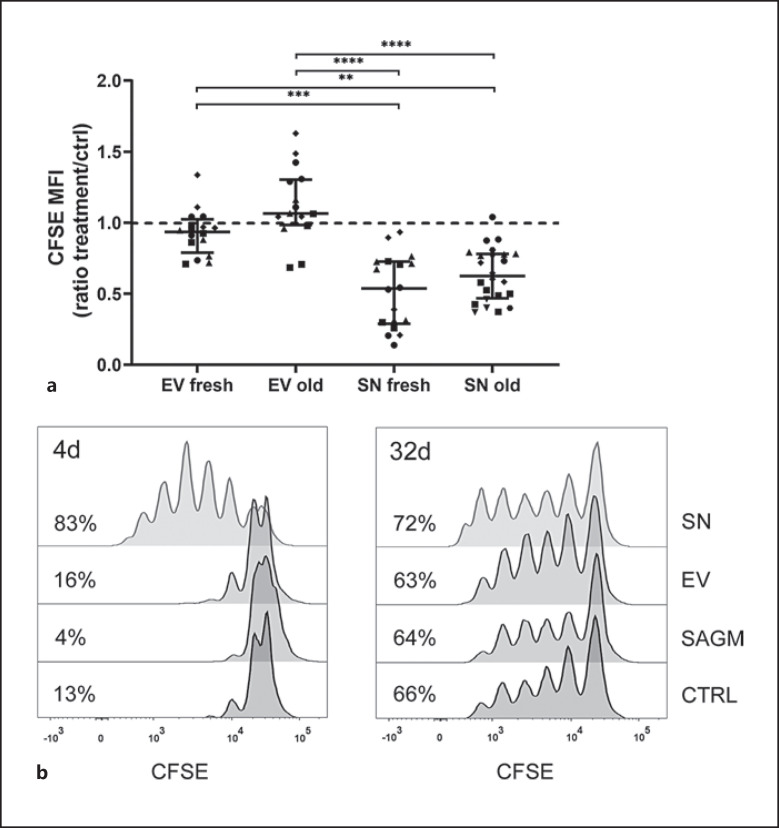
Independent of RBC storage age, SN but not EVs enhanced proliferation of anti-CD3-activated T-cells within PBMC cultures (Fig. 2). SNs from both fresh and longer-stored RBC units increased T-cell proliferation compared with EVs from fresh and longer-stored RBC units and with control (all SNs vs. ctrl p = 0.012, SN fresh vs. SN old non-significant, EV fresh vs. SN fresh p = 0.005, EV fresh vs. SN old p = 0.007, EV old vs. SN fresh p < 0.001, and EV old vs. SN old p < 0.001). EVs had a varying, statistically non-significant, effect in such a way that individual PBMCs responded uniquely, and proliferation was boosted in some, while suppressed in some responders. No RBC-donor dependent variation was seen, and RBC storage age was irrelevant (EVs fresh vs. EV old non-significant) (Fig. 2a). SN-induced proliferation augmentation was evident in intermediate RBC storage ages too. SAGM suppressed T-cell proliferation (p = 0.032). The response of T-cells from individual donors to activation had high variation. No distinguishable RBC-donor-dependent variation existed.
Fig. 2.
PBMCs were labeled with CFSE, stimulated with anti-CD3 and cultured for 4 days. CFSE is halved with each cell division; a lower CFSE MFI indicates augmented proliferation. a The data are presented as median (IQR). PBMCs exposed to fresh (n = 18) and longer stored RBC SN (n = 24) proliferated significantly more in relation to control (n = 20), while fresh (n = 16) and longer stored RBC EVs (n = 16) had varying effects on proliferation with no statistical significance in relation to control. Both fresh and old SNs increased cell proliferation significantly compared with fresh and old EVs and with control (Kruskal-Wallis test, p = 0.005 EV fresh vs. SN fresh, p = 0.007 EV fresh vs. SN old, p < 0.001 EV old vs. SN fresh and p < 0.001 EV old vs. SN old). RBC storage age was not significant. The number of samples differs among groups due to the data were extracted from several individual experiments. Symbols represent individual PBMCs. b Representative figure of T-cell proliferation of PBMCs exposed to SAGM, and SN and EVs from an RBC unit at days 4 and 32 of storage. Percentages of proliferating cells from lymphocyte population are shown. Individual PBMCs responded to anti-CD3 stimulation very differently and different level of proliferation was observed among experiments, which is seen. Thus, results are always compared to their own control. MFI, median fluorescence intensity.
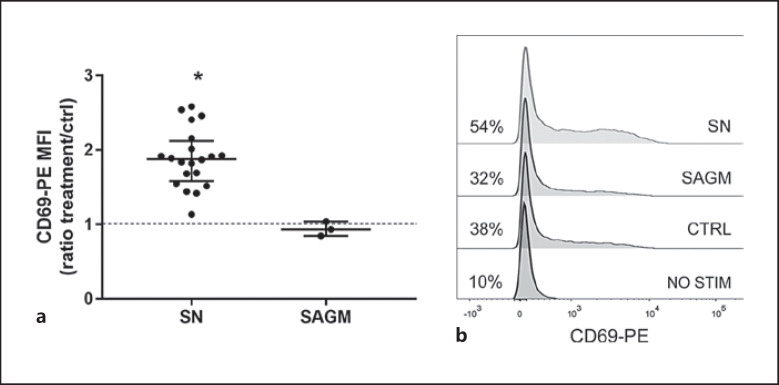
CD69-staining demonstrated that incubation with SN (storage age 3–31 days) for as short as 5 h resulted in increased activation of T-cells (Fig. 3). Increase in CD69 expression was seen equally in CD4+ and CD8+ T-cells (data not shown).
Fig. 3.
a Five-hour incubation with SN resulted in significantly increased expression of early activation marker CD69 in anti-CD3 activated T-cells (CD69-PE MFI values were compared with the Friedman test, p = 0.01 SN vs. ctrl). The data are shown as ratios to control and presented as median (IQR). b Representative examples of CD69 expression in non-activated, anti-CD3-activated, and SAGM- and SN-exposed anti-CD3-activated T cells. Percentages of positive cells from lymphocyte population are shown. CD69 gates were assigned based on isotype control.
T-Cell Differentiation
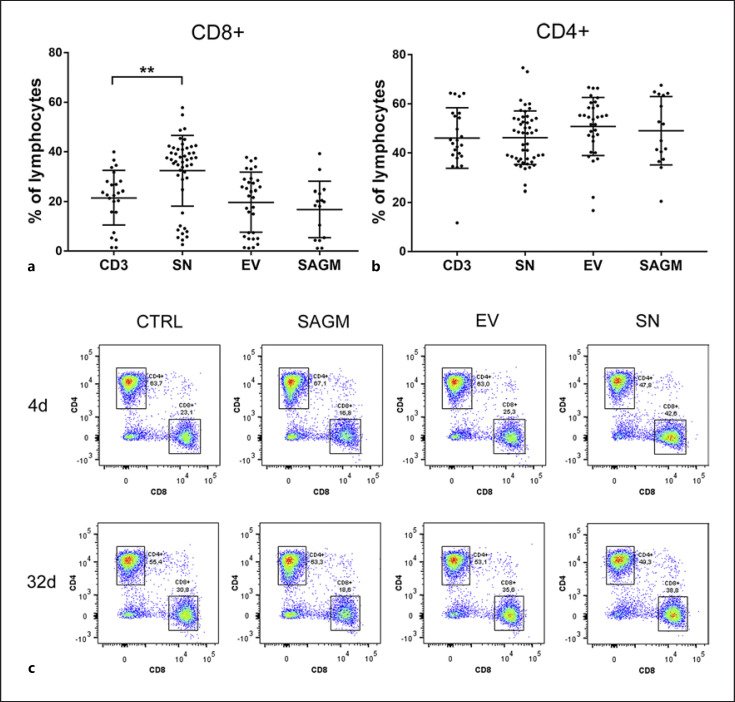
Exposing PBMCs to SN resulted in increased proportion of CD8+ T-cells among lymphocytes after four-day incubation (p = 0.01) while EVs alone and SAGM did not have a significant effect (Fig. 4a, c). The proportion of CD4+ T-cells was similar among exposures (Fig. 4b). RBC storage age had no effect and thus not displayed in Figure 4a, b.
Fig. 4.
Exposure to RBC SN resulted in (a) increased percentage of CD8+ T-cells among lymphocytes (p = 0.001). b Proportion of CD4+ cells did not differ among treatment groups. The data are presented as median (IQR). c Representative figure of CD4+ and CD8+ T-cell percentages among lymphocytes. 4- and 32-day-stored SN and EV, and SAGM and control are shown.
PBMC Cytokine Secretion
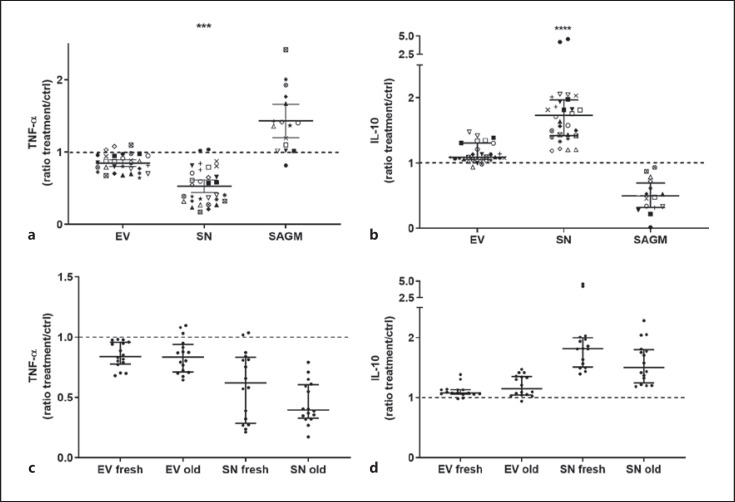
Both fresh and longer stored SN but not EVs decreased secretion of pro-inflammatory cytokine TNF-α (SN vs. ctrl p = 0.0003) and increased secretion of IL-10 (SN vs. ctrl p < 0.0001) in LPS-stimulated PBMC cultures (Fig. 5). No significant differences were found in relation to RBC storage time (Fig. 5c, d). There was no observable variation among SNs and among EVs from individual RBC-donors, but there was high individual variation in the tendency of individual donor PBMCs to produce cytokines. When the data are examined visually, EVs seem to have a small effect in the same direction as SNs, whereas SAGM seems to affect PBMCs conversely; however, these results remained statistically non-significant (Fig. 5a, b).
Fig. 5.
All PBMCs were stimulated with LPS. Cytokine levels were compared with the Friedman test. The data are presented as median (IQR). Exposure to SN decreased the secretion of TNF-α (a) significantly (SN vs. control p = 0.0003, other comparisons ns) and increased the secretion of IL-10 (b) significantly (SN vs. control p < 0.0001, other comparisons ns). The data are here shown as ratios in relation to control. Sixteen individual PBMCs (represented by individual symbols in a, b) and four individual RBCs (each at storage day 5 and 33) were used in the assays. High individual variation of responder PBMCs cytokine secretion is seen. RBC storage age (c, d) did not affect cytokine secretion.
Residual Plasma
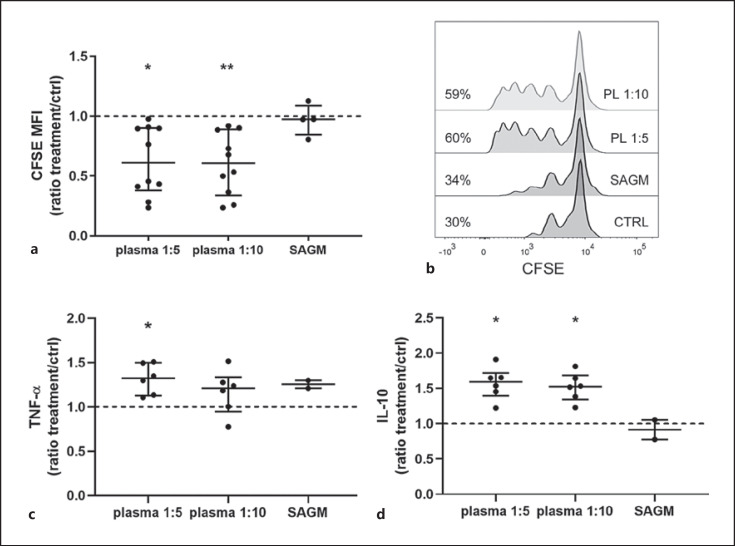
Finally, we went on to test whether residual plasma in stored RBCs would be behind the immune responses. Indeed, diluted plasma in concentrations mimicking residual plasma in RBC SNs resulted in a boost in proliferation of activated T cells. Exposure to plasma, like SN, made PBMCs to secrete more IL-10, but opposed to SN, plasma resulted in increased secretion of TNF-α too (Fig. 6).
Fig. 6.
Diluted plasma (PL; a n = 4 and c, d n = 3 individual donors) mimicking residual plasma in SN within RBC units augmented T-cell proliferation (a, b). CFSE-labeled PBMCs (n = 4) were stimulated with anti-CD3 for 4 days. CFSE is halved with each cell division; a lower CFSE MFI indicates augmented proliferation. a Both dilutions of plasma enhanced proliferation significantly in relation to control (PL 1:5 p = 0.016, PL 1:10 p = 0.003). Plasma was diluted in storage solution SAGM 1:5 and 1:10 to imitate residual plasma in RBC SN. b Representative example of T-cell proliferation of the same PBMCs exposed to the two different dilutions of plasma. Percentages of proliferating cells from lymphocyte population are shown. c, d In comparison to control, diluted plasma increased LPS-stimulated PBMCs TNF-α (c) (PL 1:5 p = 0.04) and IL-10 (d) secretion (PL 1:5 p = 0.04, PL 1:10 p = 0.04). The data are presented as median (IQR). MFI, median fluorescence intensity.
Discussion
This study demonstrates that regardless of age within clinical storage period, RBC SN but not RBC EVs alone, have both anti- and proinflammatory effects depending on the cell type that is exposed. These findings add to and are in line with current evidence from clinical studies that fresh RBCs are not superior to longer-stored ones [13, 14] and demonstrate some of the potential mechanisms of adverse effects of RBC transfusion. Contrary to our initial hypothesis, the role of EVs as a mediating factor could not be confirmed. Exposure to SN boosts T-cell proliferation. SN activates especially CD8+ T-cells, and activation is detected as early as at 5 h of incubation. SN alters monocyte cytokine secretion in an anti-inflammatory direction. Diluted plasma has partly similar properties compared with RBC SN, suggesting that residual plasma in RBC units could contribute to, but not fully explain, the immunomodulation. The TRIM-inducing substance or substances within SN are unknown.
Unexpectedly, fresh and longer-stored RBC SNs equally provoked immune responses, indicating that the mediating factors within SN are present already early on storage. Prolonged storage with more numerous EVs or bioactive substances did not affect the immune responses. Based on the initial results, we suspected that the mediating factor could be located in the residual plasma fraction of an RBC unit and, thus, be present immediately after RBC manufacture. Plasma diluted in SAGM indeed, like SN, augmented T-cell proliferation, but affected TNF-α secretion opposingly, proposing that residual plasma may induce some but does not explain all the immune responses seen here. Obviously, there may be several bioactivities in the RBC storage medium that can induce immunomodulation, perhaps some derived from residual plasma, while others from RBCs or residual cells. Karsten et al. [18] detected numerous cytokines and chemokines when erythrocytes were conditioned in PBS for 24 h, so apparently, although anucleate, erythrocytes themselves may quickly release cytokines in their storage medium.
Particularly, CD8+ T-cells proliferated when exposed to SN. Generally, CD8+ T-cells defend the body against viruses and cancer by killing infected or damaged cells and their activation requires antigen-presentation and co-stimulation by direct cell contact [19]. Soluble anti-CD3 represents the first signal to CD3-T-cell receptor. SN induces the second, co-stimulatory signal, which requires cell-to-cell contact. Hence, a bioactive substance within SN likely activates T-cells indirectly via antigen-presenting cells (APCs), supported by the findings of another study [7]. SN did not affect PBMCs without anti-CD3 stimulation (data not included), in agreement with that CD8+-T-cell-activation requires multiple signals. Interestingly, intact erythrocytes have been shown to enhance CD8+-T-cell expansion preferentially over CD4+ T-cells [20], and later the same group demonstrated erythrocyte-conditioned EV-free media to support proliferation of all T cells [5]. Although not carried out with packed RBCs, those results are in line with ours and propose that T-cell boosting bioactivities within SN or plasma may originate from erythrocytes. Antunes et al. [5] argued that potential bioactive proteins include peroxiredoxins, hemoglobin in oxy and deoxy states, and β-globin.
SN increased the expression of early activation marker CD69 in T-cells already after 5 h of incubation, suggesting that the immune cells, likely in vivo too, will react to transfusion quickly. Staining with CD69 might be utilized in laboratory experiments of TRIM as a faster sign of T-cell activation instead of full four-day cell proliferation assay. The expression of CD69 increased equally in both CD4+ and CD8+ T-cells, although augmented proliferation was only seen in CD8+ cells.
PBS and SAGM were used as control conditions for SN and EV samples in our experiments. Unexpectedly, storage solution SAGM independently suppressed T-cell proliferation compared with anti-CD3-stimulated control incubated in PBS. In addition to saline, SAGM contains adenine, glucose, and mannitol, some of which affects the recipient cells in this in vitro assay, and clearly the immune response to SN exposure is not caused by SAGM.
While CD8+ T-cells were activated, indicating a proinflammatory response, cytokine assays with monocytes revealed somewhat opposing results. Monocytes secreted more anti-inflammatory cytokine IL-10 and less proinflammatory TNF-α when exposed to SN. Similarly to the T-cell proliferation assay, the effect was seen with SN but not EVs, and product storage age did not have an impact. When the data are examined visually, EVs seem to have slight effect in similar direction with SN but much more subtle and statistically non-significant. Likewise, SAGM seemed visually to affect cytokine secretion conversely to SN.
Comparison with the literature is challenging due to various study design, and we consider that the seemingly disparate results in the field of ex vivo TRIM studies are mostly explained by different study settings. Some researchers have used fresh blood, some packed RBCs with various manufacturing methods and storage solutions, and recipient cells and assays also vary. Muszynski et al. [4], similarly to us, found only RBC SN but not EVs functional when they studied monocytes in vitro. Other groups have demonstrated, utilizing distinct types of assays, that RBC-derived EVs or other EVs within RBC units may be functional and be either immunosuppressive [3] or -stimulatory [6, 7, 8, 21]. Indeed, Danesh et al. [7] found specifically that exosomes are proinflammatory and T-cell boosting. Our results likely differ due to unalike EV-definition and isolation technique, and dissimilar handling of source RBCs.
An in vitro RBC transfusion model suppressed proliferation of purified T-cells [22], which is explained by the absence of APCs. When using soluble instead of plate-bound anti-CD3 in T-cell proliferation assay, like we did, accessory cells are required for T-cell activation [23]. Small changes in laboratory study arrangements can lead to seemingly contradicting results, and immune cells affect one another in complex ways; therefore, ex vivo studies must be compared and interpreted cautiously.
In this study, we observed T-cell and monocyte responses with the same RBC units at different ages and repeated with RBC units from several individual donors. These findings suggest that the effects are not based on the donor's individual qualities or RBC storage age. Instead, there was wide variation in the magnitude of immune cell responses of different PMBC-donors, and interestingly, the effect of EVs varied so that they enhanced T-cell proliferation in some while suppressing the proliferation in other PBMCs, resulting in statistically non-significant results (Fig. 2a). This could reflect that individual patients in their immune states at the time of transfusion could react differently to RBC transfusion, i.e., depending on whether their body is under inflammation, resolution, or immunosuppression. Therefore, it would be intriguing to repeat these studies using PBMCs from patients having, e.g., sepsis or other inflammatory or immunosuppressive states. EVs could provoke an effect in some but not all immune states.
Considering study strengths, we included diluted plasma and SAGM-controls which the latest related studies lack, providing novel findings that SAGM-stored RBC SN and diluted plasma boost T-cell proliferation while SAGM itself suppresses it. We provide further evidence that EVs seem not to be the main immune cell activating factor within stored RBCs but recipient cells may react to EVs in varying ways. Our numerous samples reliably exclude RBC donor-dependent and storage-time dependent variation in the effects. The results were further confirmed and early activation of T-cells was demonstrated by CD69-staining.
Our study has some limitations. First, SAGM and diluted plasma controls per se are not perfect controls because SAGM and residual plasma within RBC products may be altered due to cell metabolism. Second, EV characterization was limited to NTA. We have no reason, however, to expect the EVs qualities to differ from the RBC EVs from the several RBC products that we have previously thoroughly characterized [16]. EVs did not have consistent functionality in our experiments; hence, further characterization would not add value to the results. However, we cannot exclude the theoretical effect of the EVs of the lowest density that may not be included in the centrifuged EV aliquot. It would have been valuable to carry out experiments with EV-depleted supernatant which we were unable to do. A recent study demonstrated that washing reduced monocyte secretion of proinflammatory cytokines/chemokines after exposure to RBCs [21] and it would be interesting to study also T-cell proliferation with SNs from washed RBCs. To limit TRIM, in theory, washing would remove most of SN and bioactivities within, but a large randomized trial would be needed to prove clinical benefit.
The purpose of RBC transfusion is to increase oxygen delivery to tissues, not to cause undesired immune response such as TRIM. Our results indicate that RBCs of any storage age have immunomodulatory potential, mediated by bioactivities contained in SN. Residual plasma in RBC units may contribute to immune responses. The direction and magnitude of immune responses may vary depending on baseline immune state and disease course of transfusion recipient.
Statement of Ethics
RBC units and buffy coats were obtained from the Finnish Red Cross Blood Service and handled anonymously. The research use of RBCs and buffy coats is in accordance with the rules of the Finnish Supervisory Authority for Welfare and Health, and the use of buffy coats and RBCs for the study has been approved by the FRCBS Institutional Review Board (0906/7/2015).
Conflict of Interest Statement
The authors declare no conflict of interests.
Funding Sources
This study was supported by the Finnish Funding Agency for Innovation (TEKES) as part of SalWe research program Personalized Diagnostics and Care (GET IT DONE, Grant No. 3986/31/2013), and Clinical Research funding (EVO/VTR) from Helsinki University Hospital.
Author Contributions
Eva Laurén, Lotta Sankkila, Ville Pettilä, and Erja Kerkelä conceived and designed the study; Eva Laurén and Lotta Sankkila carried out the experiments and collected the data; Eva Laurén and Erja Kerkelä performed the analysis; Eva Laurén wrote the paper with input from Lotta Sankkila, Ville Pettilä, and Erja Kerkelä.
Data Availability Statement
The data can be requested from the corresponding author, Erja Kerkelä, with the permission of FRC Blood Service.
Acknowledgments
Figure 1 was in part created with BioRender.com under a paid subscription. We would like to thank Sami Valkonen for performing NTA and Juha Eronen for providing RBC manufacturing method.
Funding Statement
This study was supported by the Finnish Funding Agency for Innovation (TEKES) as part of SalWe research program Personalized Diagnostics and Care (GET IT DONE, Grant No. 3986/31/2013), and Clinical Research funding (EVO/VTR) from Helsinki University Hospital.
References
- 1.Muszynski JA, Spinella PC, Cholette JM, Acker JP, Hall MW, Juffermans NP, et al. Transfusion-related immunomodulation: review of the literature and implications for pediatric critical illness. Transfusion. 2017;57((1)):195–206. doi: 10.1111/trf.13855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Remy KE, Hall MW, Cholette J, Juffermans NP, Nicol K, Doctor A, et al. Mechanisms of red blood cell transfusion-related immunomodulation. Transfusion. 2018;58((3)):804–815. doi: 10.1111/trf.14488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sadallah S, Eken C, Schifferli JA. Erythrocyte-derived ectosomes have immunosuppressive properties. J Leukoc Biol. 2008 Nov;84((5)):1316–1325. doi: 10.1189/jlb.0108013. [DOI] [PubMed] [Google Scholar]
- 4.Muszynski JA, Bale J, Nateri J, Nicol K, Wang Y, Wright V, et al. Supernatants from stored red blood cell (RBC) units, but not RBC-derived microvesicles, suppress monocyte function in vitro. Transfusion. 2015 Aug;55((8)):1937–1945. doi: 10.1111/trf.13084. [DOI] [PubMed] [Google Scholar]
- 5.Antunes RF, Brandão C, Maia M, Arosa FA. Red blood cells release factors with growth and survival bioactivities for normal and leukemic T cells. Immunol Cell Biol. 2011 Jan;89((1)):111–121. doi: 10.1038/icb.2010.60. [DOI] [PubMed] [Google Scholar]
- 6.Zecher D, Cumpelik A, Schifferli JA. Erythrocyte-derived microvesicles amplify systemic inflammation by thrombin-dependent activation of complement. Arterioscler Thromb Vasc Biol. 2014 Feb 1;34((2)):313–320. doi: 10.1161/ATVBAHA.113.302378. [DOI] [PubMed] [Google Scholar]
- 7.Danesh A, Inglis HC, Jackman RP, Wu S, Deng X, Muench MO, et al. Exosomes from red blood cell units bind to monocytes and induce proinflammatory cytokines, boosting T-cell responses in vitro. Blood. 2014 Jan 30;123((5)):687–696. doi: 10.1182/blood-2013-10-530469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Straat M, Böing AN, Tuip-De Boer A, Nieuwland R, Juffermans NP. Extracellular vesicles from red blood cell products induce a strong pro-inflammatory host response, dependent on both numbers and storage duration. Transfus Med Hemother. 2016;43((4)):302–305. doi: 10.1159/000442681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischer D, Büssow J, Meybohm P, Weber CF, Zacharowski K, Urbschat A, et al. Microparticles from stored red blood cells enhance procoagulant and proinflammatory activity. Transfusion. 2017 Nov;57((11)):2701–2711. doi: 10.1111/trf.14268. [DOI] [PubMed] [Google Scholar]
- 10.D'Alessandro A, Kriebardis AG, Rinalducci S, Antonelou MH, Hansen KC, Papassideri IS, et al. An update on red blood cell storage lesions, as gleaned through biochemistry and omics technologies. Transfusion. 2015 Jan;55((1)):205–219. doi: 10.1111/trf.12804. [DOI] [PubMed] [Google Scholar]
- 11.Koch CG, Li L, Sessler DI, Figueroa P, Hoeltge GA, Mihaljevic T, et al. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008 Mar 20;358((12)):1229–1239. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 12.Goel R, Johnson DJ, Scott AV, Tobian AAR, Ness PM, Nagababu E, et al. Red blood cells stored 35 days or more are associated with adverse outcomes in high-risk patients. Transfusion. 2016;56((7)):1690–1698. doi: 10.1111/trf.13559. [DOI] [PubMed] [Google Scholar]
- 13.Heddle NM, Cook RJ, Arnold DM, Liu Y, Barty R, Crowther MA, et al. Effect of short-term vs. long-term blood storage on mortality after transfusion. N Engl J Med. 2016 Nov 17;375((20)):1937–1945. doi: 10.1056/NEJMoa1609014. [DOI] [PubMed] [Google Scholar]
- 14.Cooper DJ, McQuilten ZK, Nichol A, Ady B, Aubron C, Bailey M, et al. Age of red cells for transfusion and outcomes in critically ill adults. N Engl J Med. 2017 Sep 27;377((19)):1858–1867. doi: 10.1056/NEJMoa1707572. [DOI] [PubMed] [Google Scholar]
- 15.Antonelou MH, Seghatchian J. Update on extracellular vesicles inside red blood cell storage units: adjust the sails closer to the new wind. Transfus Apher Sci. 2016;55((1)):92–104. doi: 10.1016/j.transci.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 16.Lauren E, Tigistu-Sahle F, Valkonen S, Westberg M, Valkeajarvi A, Eronen J, et al. Phospholipid composition of packed red blood cells and that of extracellular vesicles show a high resemblance and stability during storage. Biochim Biophys Acta Mol Cell Biol Lipids. 2018;1863((1)):1–8. doi: 10.1016/j.bbalip.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Yáñez-Mó M, Siljander PRM, Andreu Z, Zavec AB, Borràs FE, Buzas EI, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karsten E, Breen E, Herbert BR. Red blood cells are dynamic reservoirs of cytokines. Sci Rep. 2018;8((1)):3101. doi: 10.1038/s41598-018-21387-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abbas A, Lichtman A, Pillai S. In: Basic immunology: functions and disorders of the immune system. 6th ed. Amsterdam, Netherlands: Elsevier Inc; 2020. T cell–mediated immunity: activation of T lymphocytes; pp. p. 96–118. [Google Scholar]
- 20.Fonseca AM, Pereira CF, Porto G, Arosa FA. Red blood cells promote survival and cell cycle progression of human peripheral blood T cells independently of CD58/LFA-3 and heme compounds. Cell Immunol. 2003;224((1)):17–28. doi: 10.1016/s0008-8749(03)00170-9. [DOI] [PubMed] [Google Scholar]
- 21.Almizraq RJ, Kipkeu BJ, Acker JP. Platelet vesicles are potent inflammatory mediators in red blood cell products and washing reduces the inflammatory phenotype. Transfusion. 2020;60((2)):378–390. doi: 10.1111/trf.15590. [DOI] [PubMed] [Google Scholar]
- 22.Long K, Meier C, Bernard A, Williams D, Davenport D, Woodward J. T-cell suppression by red blood cells is dependent on intact cells and is a consequence of blood bank processing. Transfusion. 2014;54((5)):1340–1347. doi: 10.1111/trf.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kruisbeek AM, Shevach E, Thornton AM. Proliferative assays for T cell function. Curr Protoc Immunol. 2004 May; doi: 10.1002/0471142735.im0312s60. Chapter 3: Unit 3.12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data can be requested from the corresponding author, Erja Kerkelä, with the permission of FRC Blood Service.