Abstract
Retroperitoneal tuberculosis is a rare disease that can mimic many conditions and lacks specific clinical manifestations, which makes it difficult to diagnose. As a consequence, it can be misdiagnosed as a malignant tumour. Endoscopic ultrasonography-guided fine-needle aspiration (EUS-FNA) can obtain specimens of the lesion site from areas that might be inaccessible using more traditional biopsy methods. A 60-year-old female patient was admitted with a 3-month history of intermittent upper abdominal pain accompanied by nausea. Imaging found pancreatic uncinate process and retroperitoneal lymph nodes at the horizontal part of the duodenum. EUS-FNA found necrotic matter, multinucleated giant cells and epithelioid cells consistent with the signs of tuberculosis bacilli infection, although typical noncaseous granuloma and Mycobacterium tuberculosis were not observed. Retroperitoneal tuberculosis was considered as the diagnosis. After anti-tubercular therapy, the signs and symptoms quickly improved and a repeat computed tomography scan found that the space-occupying lesion had reduced in size. By using EUS-FNA, the cytological and histopathological findings can be obtained in a timely manner to facilitate an earlier diagnosis and avoid unnecessary procedures such as laparotomy or surgery.
Keywords: Retroperitoneal tuberculosis, EUS-FNA, diagnosis, case report
Introduction
Tuberculosis is one of the most common public health problems worldwide.1 Abdominal tuberculosis, accounting for 5% of all cases of tuberculosis, is a severe extrapulmonary tuberculosis.2 Abdominal tuberculosis is intra-abdominal and involves the abdominal lymph nodes, spleen, liver and gastrointestinal tract.3 Abdominal tuberculous can be confused with other diseases, such as tumours, cysts, inflammation and lymphadenectasis.4 Thus, it is challenging to diagnose and manage abdominal tuberculosis because of the lack of specific clinical signs and the availability of imaging and pathological examinations. It is important to diagnose and treat abdominal tuberculosis earlier.
This current case report describes a rare case of a retroperitoneal space-occupying lesion in which the tuberculosis aetiology was examined by ultrasonography-guided fine-needle aspiration (EUS-FNA) biopsy and there was a favourable outcome after the use of antitubercular medications.
Case report
In May 2015, a 60-year-old female farmer was admitted to the Department of Gastroenterology, Lishui Municipal Central Hospital, Lishui, Zhejiang Province, China due to upper abdominal pain. Three months before admission, she suffered intermittent upper abdominal pain accompanied by nausea. Upon admission, she presented with paroxysmal middle to upper abdominal pain, which was characterized by paroxysmal colic not radiated to other sites. Her medical history revealed chronic viral hepatitis B for 3 years, which required medical treatment with lamivudine and adefovir dipivoxil. She denied a history of hepatitis and tuberculosis.
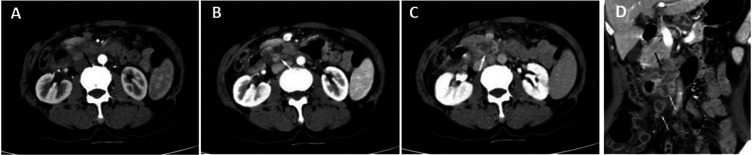
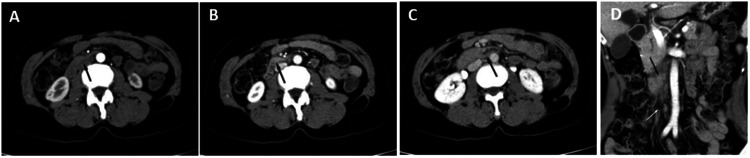
A physical examination revealed slight tenderness over the epigastric region. A routine laboratory examination revealed inflammatory leukocytosis (white blood counts of 4.0 × 109/l, neutrophil percentage of 80.0%), mild anaemia (haemoglobin of 105 g/l, mean corpuscular volume of 89.3 fl) and hepatitis B (surface antigen, hepatitis B e antigen and hepatitis B core antibody positive), which was associated with a weak positive T-SPOT test and a high erythrocyte sedimentation rate (ESR: 110 mm/h). Liver function tests, tumour biomarkers, renal function, electrolytes and purified protein derivative (PPD) tests were within normal limits. Chest computed tomography (CT) and magnetic resonance cholangiopancreatography (MRCP) were normal. After the completion of enhanced CT, the tube wall in the horizontal segment of the duodenum was irregularly thickened in the arterial phase axial position (Figures 1a and 1b), portal vein phase axial position (Figure 1c) and coronal position (Figure 1d). Multiple nonuniform enhancement and circular enhancement lymph nodes with different sizes were observed around it.
Figure 1.
Enhanced computed tomography imaging of the abdomen of a 60-year-old female farmer that presented with upper abdominal pain and a 3-month history of intermittent upper abdominal pain accompanied by nausea: (a & b) the tube wall in the horizontal segment of the duodenum was irregularly thickened in the arterial phase axial position (black arrow); (c) portal vein phase axial position and (d) coronal position. Multiple non-uniform enhancement and circular enhancement lymph nodes with different sizes can be seen around it (white arrows).
Based on the above results, clinicians initially suspected that this patient may have a duodenal tumour with multiple lymph node metastases in the abdominal cavity and partial involvement of the pancreatic uncinate process. The possibility of tuberculosis could not be excluded. Then a gastroscopy was performed and an ulcer of approximately 0.8 × 1.2 cm in size was observed in the duodenum with clear borders and slightly hyperplastic surrounding mucosa. The histopathological examinations were as follows (at the horizontal part of the duodenum): chronic mucositis (active) accompanied by erosion, with visible granulation tissue (Figure 2). Specific staining showed the following: acid-fast staining found no acid-fast bacilli; and periodic acid–Schiff found no fungus. So a duodenal tumour was excluded.
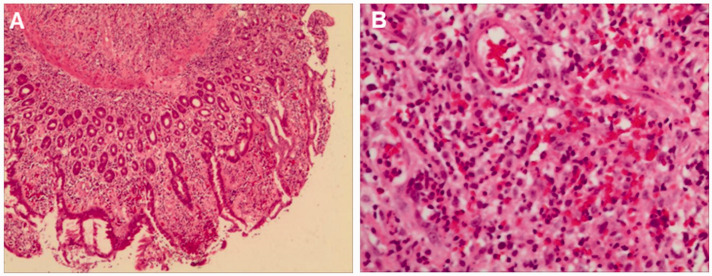
Figure 2.
Fine needle aspiration biopsy provided the following pathological findings in a 60-year-old female farmer that presented with upper abdominal pain and a 3-month history of intermittent upper abdominal pain accompanied by nausea: (a) chronic mucositis (active) accompanied by erosion (magnification × 100; haematoxylin & eosin) and (b) visible granulation tissue (magnification × 200; haematoxylin & eosin). The colour version of this figure is available at: http://imr.sagepub.com.
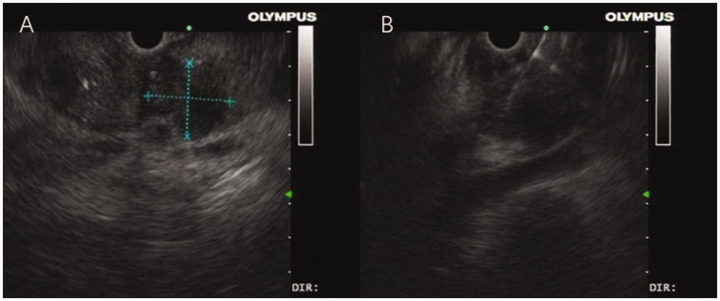
An endoscopic ultrasound revealed that the pancreatic body and tail were normal in structure, with the pancreatic duct at the pancreatic body and tail slightly dilated, with a diameter of approximately 0.3 cm. The pancreatic uncinate process was found to have a hypoechoic space-occupying lesion of unclear boundary (Figures 3a and 3b).
Figure 3.
Endoscopic ultrasound of the abdomen of a 60-year-old female farmer that presented with upper abdominal pain and a 3-month history of intermittent upper abdominal pain accompanied by nausea demonstrated the following: (a) the pancreatic body and tail were normal in structure, with the pancreatic duct at the pancreatic body and tail being slightly dilated with a diameter of approximately 0.3 cm. The pancreatic uncinate process was found to have a hypoechoic space-occupying lesion of unclear boundary and (b) the puncture needle accurately entered the process of the pancreas to obtain the fine needle aspiration sample.
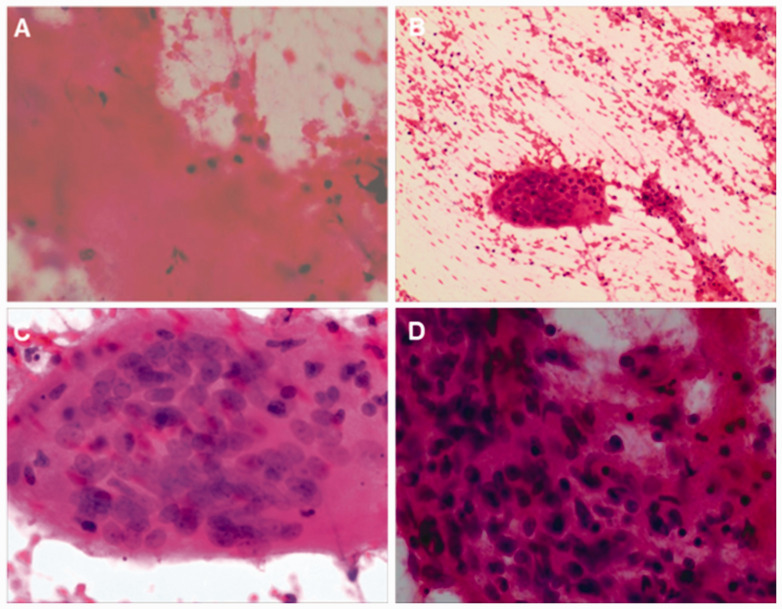
A further EUS-FNA was performed and an Olympus 22G ultrasound puncture needle was used for the procedure. A total of two passes were performed and the process was smooth. FNA cytological examination (FNA for space-occupying lesion at the uncinate process) found an eosinophilic necrotic substance in the smear, suspicious epithelioid cells and multinucleated giant cells, suggesting tuberculous inflammation (Figures 4a–4d). These results revealed that the lesions described in the uncinate process were retroperitoneal tuberculosis instead of lymph nodes.
Figure 4.
Fine needle aspiration biopsy provided the following cytological findings in a 60-year-old female farmer that presented with upper abdominal pain and a 3-month history of intermittent upper abdominal pain accompanied by nausea: (a) eosinophilic necrotic substance (magnification × 200; haematoxylin & eosin); (b & c) multinucleated giant cells (magnification × 100/400, respectively; haematoxylin & eosin) and (d) suspicious epithelioid cells (magnification × 400; haematoxylin & eosin). The colour version of this figure is available at: http://imr.sagepub.com.
Combining the histopathological examinations, high ESR and weak positive T-SPOT, retroperitoneal tuberculosis was first considered. A short course of anti-tubercular therapy was started with 300 mg isoniazid oral administration once a day, 450 mg rifampicin oral administration once a day, 1500 mg pyrazinamide oral administration once a day and 750 mg ethambutol oral administration once a day for 10 days. The abdominal pain gradually disappeared and the ESR was significantly decreased (ESR 50 mm/h). Approximately 5 months later, an abdominal CT showed no obvious thickening of the tube wall in the horizontal part of duodenum, clear fat space around the duodenum, small lymph nodes around the horizontal part of duodenum and retroperitoneum, showing uniform enhancement (Figures 5a–5d).
Figure 5.
Approximately 5 months after hospital admission, enhanced computed tomography imaging of the abdomen of a 60-year-old female farmer that presented with upper abdominal pain and a 3-month history of intermittent upper abdominal pain accompanied by nausea showed no obvious thickening of the tube wall in the horizontal part of duodenum (black arrows), a clear fat space around the duodenum, small lymph nodes around the horizontal part of duodenum and retroperitoneum, showing uniform enhancement (white arrows); (a & b) the arterial phase axial position; (c) portal vein phase axial position and (d) coronal position.
This case report was reviewed and approved by the Ethics Committee of Lishui Municipal Central Hospital, Lishui, Zhejiang Province, China (no. 2023-210). Written informed consent to publish this information was obtained from the patient. The reporting of this case report conforms to CARE guidelines. 5
Discussion
Abdominal tuberculosis is uncommon; it can be confused with many diseases, such as carcinoma, lymphoma, cystic neoplasia, and retroperitoneal tumours, which always causes a delay in its diagnosis.4 The intra-abdominal involvement of abdominal tuberculosis is generally observed in the abdominal lymph nodes, spleen, liver and gastrointestinal tract.3
In this current case, the tuberculosis was located in the retroperitoneal area, which is extremely rare. The patient denied a history of tuberculosis exposure and there was no associated lung involvement observed in the patient. For example, the chest CT was normal. Of all tuberculosis cases, gastrointestinal tuberculosis accounts for 5%; of which ileocecal tuberculosis cases are the most common, while duodenal tuberculosis cases are very rare, accounting for only 2.5% of gastrointestinal tract tuberculosis cases, and these mostly affect young women below 40 years of age.1–3 With regard to the types of extrapulmonary tuberculosis, the pancreas is the least involved organ and most relevant reports are case reports worldwide.6 Therefore, there are no exact statistics on the incidence rate. Even for systemic miliary tuberculosis involving the lung and liver, the pancreas is rarely involved, which is thought to be due to trypsin interfering with the implantation of Mycobacterium tuberculosis in the pancreas. 7 Due to the non-specific clinical manifestations, which lacked special symptoms such as abdominal pain, fever, night sweats, loss of appetite, weight loss, jaundice, diarrhoea or peripheral lymph node enlargement, pancreatic tuberculosis is often misdiagnosed as a malignant tumour. 8 The current patient was considered for tumour admission at the time. Several reported cases underwent laparotomy due to the preoperative suspicion of malignancy and the final postoperative pathological examination was pancreatic tuberculosis.9,10
Many cases of pancreatic tuberculosis are confirmed by postoperative pathological examination.11,12 Recent findings suggest that EUS-FNA is very important in the treatment process of pancreatic solid space-occupying lesions without pulmonary tuberculosis. 13 If the results of EUS-FNA indicate a granuloma, but there is no caseous necrosis and negative acid fast staining, then PPD, ESR and T-SPOT tests should be undertaken. 13 If these tests are positive, tuberculosis should be considered clinically and further tuberculosis tests should be conducted. 13 This current case also confirms this approach. Various non-typical symptoms are observed in abdominal tubercular infections, but this current case only presented with upper abdominal pain and nausea. The current treatment of tubercular infections has been well developed over many years; but the core challenge remains the precise diagnosis. When given the correct diagnosis and treatments, this current patient soon recovered.
Despite the increasing development of imaging technology and the availability of other diagnostic tests, the diagnosis of abdominal tuberculosis remains difficult. The diagnosis of duodenal tuberculosis still relies on deep tissue biopsy for finding granulomatous inflammation. 14 At present, the most common and effective means for this is just endoscopy and biopsy. An endoscopic examination of the current case observed no specific manifestations, but there were general ulcers at the lesion sites, multiple small ulcers, irregular boundaries that might have been accompanied by nodular thickening or peripheral nodules. At an intermediate or advanced stage of the infection, an obvious rigid canal wall and intestinal stenosis may be observed. 15 However, it is difficult to undertake a biopsy for tuberculosis when the infection is in the submucosa. During sampling, the specimen is shallow for the endoscopic forceps, so it is difficult to obtain the submucosal tuberculous granuloma. Thus, pathological results for relevant lesions often show inflammatory tissue changes and a correct diagnosis cannot be made by the results. 16
Endoscopic ultrasonography-guided fine-needle aspiration is an examination method that uses fine needle aspiration at the lesion site under the real-time guidance of endoscopic ultrasonography to obtain specimens of cells, tissues or body fluids for cytological and/or pathological diagnosis. In this current case, her symptoms and signs were non-specific and an imaging examination did not provide valid signs. The cytological results obtained by EUS-FNA should provide the most powerful evidence. The target organs for EUS-FNA include the mediastinum, perigastric area, pancreas and most of the peripancreatic area, perirectal and pelvic areas. EUS-FNA is a less invasive, safe and efficient technique for obtaining pathological tissue than traditional biopsy methods, which can be used to obtain histopathological evidence of the pancreatic biliary tract and surrounding lesions; and currently it might become the best method for the diagnosis of pancreatic space-occupying lesions of unknown origin. 17 A meta-analysis showed that the combined sensitivity and specificity of EUS-FNA in the diagnosis of pancreatic solid lesions were 86.8% and 95.8%, respectively. 18 EUS-FNA is considered to be a safe procedure with a complication rate of approximately 1% and a mortality rate of approximately 0.02%. 19 The main complications are haemorrhage, infection, pancreatitis and pneumothorax. 19 With a highly effective diagnostic rate, minimal trauma and a low complication rate, multiple or repeated passes may be performed by EUS-FNA for biopsy.
In conclusion, this current case report demonstrates that sometimes retroperitoneal space-occupying lesions involving multiple organs are not neoplastic lesions but tuberculosis of the lymph nodes. Extrapulmonary lymphatic tuberculosis can involve multiple organs and by using EUS-FNA, the cytological and histopathological findings can be obtained in a timely manner to facilitate an earlier diagnosis. This approach should also help to avoid laparotomy or surgery, making a definite diagnosis as early as possible, which should reduce the misdiagnosis rate and reduce the trauma to patients caused by various medical procedures.
Footnotes
Author contributions: Z.X., G.L., X.Y. and J.W.: conceptualization, methodology, investigation, writing and reviewing. All authors confirmed the submitting this article and data collected. All authors reviewed and approved the submitted version and further publication.
The authors declare that there are no conflicts of interest.
Funding: This research received no specific grant from funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Jianbo Wang https://orcid.org/0000-0002-0575-3023
References
- 1.Churchyard G, Kim P, Shah NS, et al. What We Know About Tuberculosis Transmission: An Overview. J Infect Dis 2017; 216(suppl_6): S629–S635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reader MM, Philip ESP.Infections and infestations. In: Margulis RA, Burbene JH. (eds) Alimentary tract radiology. St Louis: CV Mosby, 1989, pp.1478–1479. [Google Scholar]
- 3.Eraksoy H.Gastrointestinal and Abdominal Tuberculosis. Gastroenterol Clin North Am 2021; 50: 341–360. [DOI] [PubMed] [Google Scholar]
- 4.Kapoor VK.Abdominal tuberculosis. Postgrad Med J 1998; 74: 459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. Headache 2013; 53: 1541–1547. [DOI] [PubMed] [Google Scholar]
- 6.Sharma V, Rana SS, Kumar A, et al. Pancreatic tuberculosis. J Gastroenterol Hepatol 2016; 31: 310–318. [DOI] [PubMed] [Google Scholar]
- 7.De A, Lamoria S, Dhawan S, et al. Duodenal tuberculosis: dig deep to diagnose. Trop Doct 2016; 46: 172–174. [DOI] [PubMed] [Google Scholar]
- 8.Wolde TG, Huang S, Zhang K, et al. Evaluation of Twenty-One Cases of Abdominal Tuberculosis: A Single-Center Experience. Surg Infect (Larchmt) 2021;22: 299–304. [DOI] [PubMed] [Google Scholar]
- 9.Zengin K, Taskin M, Cicek Y, et al. Primary gastric tuberculosis mimicking gastric tumor that results in pyloric stenosis. Eur Surg 2003; 35: 220–221. [Google Scholar]
- 10.Franco-Paredes C, Leonard M, Jurado R, et al. Tuberculosis of the pancreas: report of two cases and review of the literature. Am J Med Sci 2002; 323: 54–58. [DOI] [PubMed] [Google Scholar]
- 11.Diaconu CC, Gheorghe G, Hortopan A, et al. Pancreatic Tuberculosis – A Condition That Mimics Pancreatic Cancer. Medicina (Kaunas) 2022; 58: 1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ben Hammouda S, Chaka A, Njima M, et al. Primary pancreatic tuberculosis mimicking pancreatic body cancer. A case report and review of the literature. Ann Med Surg (Lond) 2020; 58: 80–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu CX, Xiao LB, Luo ZF, et al. Diagnostic approaches for pancreatic tuberculosis. Hepatobiliary Pancreat Dis Int 2023; 22: 107–110. [DOI] [PubMed] [Google Scholar]
- 14.Udgirkar S, Surude R, Zanwar V, et al. Gastroduodenal tuberculosis: a case series and review of literature. Clin Med Insights Gastroenterol 2018; 11: 1179552218790566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rao YG, Pande GK, Sahni P, et al. Gastroduodenal tuberculosis management guidelines, based on a large experience and a review of the literature. Can J Surg 2004; 47: 364–368. [PMC free article] [PubMed] [Google Scholar]
- 16.Panic N, Maetzel H, Bulajic M, et al. Pancreatic tuberculosis: a systematic review of symptoms, diagnosis and treatment. United European Gastroenterol J 2020; 8: 396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsubayashi H, Matsui T, Yabuuchi Y, et al. Endoscopic ultrasonography guided-fine needle aspiration for the diagnosis of solid pancreaticobiliary lesions: Clinical aspects to improve the diagnosis. World J Gastroenterol 2016; 22: 628–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puli SR, Bechtold ML, Buxbaum JL, et al. How good is endoscopic ultrasound-guided fine-needle aspiration in diagnosing the correct etiology for a solid pancreatic mass? A meta-analysis and systematic review. Pancreas 2013; 42: 20–26. [DOI] [PubMed] [Google Scholar]
- 19.Wang KX, Ben QW, Jin ZD, et al. Assessment of morbidity and mortality associated with EUS-guided FNA: a systematic review. Gastrointest Endosc 2011; 73: 283–290. [DOI] [PubMed] [Google Scholar]