Abstract
Background
There are few clinical symptoms in early colorectal cancer, so it is necessary to find a simple and economical tumor detection index for auxiliary diagnosis. This study aims to explore the diagnostic value of preoperative inflammation-related indicators, such as neutrophil, lymphocyte, platelet count, platelet to lymphocyte ratio (PLA), neutrophil to lymphocyte ratio (NLR), and systemic immune-inflammation index (SII), for early colorectal cancer, and determine whether inflammation-related indicators can provide more accurate diagnostic judgment for patients.
Methods
This study was a retrospective study. Patients who were first diagnosed with colorectal cancer or colorectal adenomatous polyp at Beijing Friendship Hospital from October 2016 to October 2017 were retrospectively collected. According to inclusion and exclusion criteria, a total of 342 patients were included, including 216 patients with colorectal cancer and 126 patients with colorectal adenomatous polyp. Fasting venous blood and other clinical features were collected to compare the differences between colorectal cancer and colorectal adenoma.
Results
There were statistically significant differences in age, carcinoembryonic antigen, albumin, hemoglobin, mean platelet volume, lymphocyte, monocyte, NLR, PLA, SII, and mean platelet volume to platelet count ratio between colorectal cancer group and colorectal adenoma group (P < .05), and a Nomogram model was established. Using inflammatory markers to differentiate colorectal and colorectal polyps produced greater AUC than using tumor markers alone (.846 vs .695).
Conclusion
Inflammation-related indicators, such as lymphocyte, monocyte, and mean platelet volume, may serve as potential indicators to assist in the diagnosis of early colorectal cancer.
Keywords: colorectal cancer, adenomatous polyps, inflammation-related indicators, mean platelet volume, lymphocyte, monocyte, nomogram
Introduction
Colorectal cancer is one of the most common malignancies in the world, with the third highest incidence and cancer-related mortality of all cancers. 1 Many people don’t detect early colorectal cancer in time until complications such as abdominal pain, bleeding, and intestinal obstruction occur, because it has few clinical symptoms.2,3 Although colonoscopy greatly improves the detection rate of colorectal cancer, its high invasiveness and cost limit its widespread use in screening. Tumor markers, such as carcinoembryonic antigen (CEA) and carbohydrate antigen199 (CA199), are often used for screening, but their sensitivity is not high enough.4,5 In addition, colonoscopy can sometimes be difficult to distinguish between early colorectal cancer and adenoma. Therefore, it is necessary to find a simple and easy-to-obtain and economic tumor detection index for auxiliary diagnosis.
More and more evidence supports the important role of inflammatory and thrombotic processes in all stages of colorectal cancer development.6-8 A large number of studies have shown that inflammation-related indicators based on routine blood tests, such as platelet to lymphocyte ratio (PLR), neutrophil to lymphocyte ratio (NLR), systemic immune-inflammation index (SII), and mean platelet volume to platelet count ratio (MPV/PC), can be used for the diagnosis and prognosis of liver cancer, cervical cancer, endometrial cancer, lung cancer, nasopharyngeal cancer, esophageal cancer, colorectal cancer, and other malignant tumors.9-15 These indicators are widely used as routine tests for outpatient and inpatient patients because they are cheap and easy to obtain. However, most studies have focused on patients with operable primary or advanced tumors.16,17 Few studies have reported whether inflammatory-related indicators can assist the diagnosis of early colorectal cancer.
The aim of this study was to explore the diagnostic value of preoperative inflammatory-related indicators in early colorectal cancer, and to compare with traditional tumor markers to determine whether inflammatory-related indicators can provide more accurate diagnostic judgment for patients.
Materials and Methods
Study Population
This study was a retrospective study. This study consecutively recruited patients who were first diagnosed with colorectal cancer or colorectal adenomatous polyp at Beijing Friendship Hospital from October 2016 to October 2017. Inclusion criteria were as follows: Patients undergoing endoscopic or surgical resection; the pathological diagnosis was colorectal cancer or colorectal adenomatous polyp; peripheral blood venous specimens were collected within 1 week before treatment; and the pathological stage of colorectal cancer was stage I or II. The exclusion criteria are as follows: pregnancy or lactation, other malignancies, diabetes, thyroid disease, cardiovascular disease, and autoimmune diseases (such as idiopathic thrombocytopenic purpura, systemic lupus erythematosus), kidney disease, liver disease, blood disease, blood transfusion within 6 months before surgery, no other organ involvement or distant metastasis, no lymph node metastasis, and preoperative neoadjuvant chemoradiotherapy. The gold standard for diagnosis of colorectal cancer and adenomatous polyps in this study was pathology results after surgical or endoscopic resection. All colorectal cancer patients were staged according to the eighth edition of the American Joint Committee on Cancer (AJCC) TNM staging criteria. 18 We defined early colorectal cancer as pathological stage I or II disease.19-21 A total of 342 patients were enrolled, including 216 colorectal cancer patients and 126 colorectal adenomatous polyp patients. The following clinical data were collected: sex, age, height, weight, tumor location, T stage, and inflammation-related indicators. This study was approved by the Ethics Committee of Beijing Friendship Hospital, Capital Medical University (2018-P2-045-01). All the patients have signed written informed consent forms for their data to be used in the study and the data were kept confidential. We have deidentified all patient details. This study was conducted in accordance with the Declaration of Helsinki.
Clinical Measurements and Calculation
Fasting venous blood was collected in the morning within 1 week before surgery. The following data will be collected: neutrophil count, lymphocyte count, monocyte count, hemoglobin (HGB), albumin (ALB), platelet count (PC), mean platelet volume (MPV), CEA, and CA199. MPV/PC is the ratio of mean platelet volume to platelet count, NLR is the ratio of neutrophils to lymphocytes, PLR is the ratio of platelets to lymphocytes, and SII is calculated by (neutrophils * platelets)/lymphocytes.
Statistical Analyses
All data were analyzed using SPSS 22.0 software and R 3.6.2 for statistical analysis. Patients with missing important data or ambiguous diagnoses were simply deleted. Continuous variables were represented by median (interquartile range), and categorical variables were represented by frequency. Kruskal–Wallis H test was used to compare the 2 groups of data. Spearman’s correlation test was used for correlation analysis. Cox proportional risk regression model was used for multivariate analysis to find out the independent risk factors. Based on multivariate Cox and other proportional risk models, a Nomogram prediction model was established to screen for independent prognostic factors and predict the possibility of colorectal cancer. Sensitivity, specificity, and area under the curve (AUC) were calculated by receiver operating characteristic (ROC) curve to evaluate the diagnostic value of inflammatory indicators in colorectal cancer. P < .05 was considered statistically significant.
Results
Clinical Characteristics of the Study Population
Differences in clinical features between the colorectal cancer group and the adenomatous polyp group were shown in Table 1. There were no significant differences in gender, BMI, neutrophil, PC, and CA199 between the 2 groups (P > .05). The age and CEA of colorectal cancer group were significantly higher than that of adenomatous polyp group, but ALB and HGB were significantly lower than that of adenomatous polyp group (P < .05). In terms of inflammation-related indicators, the differences between the colorectal cancer group and the adenomatous polyp group are shown in Figure 1. There were statistically significant differences in MPV, lymphocyte, monocyte, NLR, PLR, SII, and MPV/PC between the 2 groups (P < .05), but there were no statistically significant differences in neutrophil and PC (P > .05).
Table 1.
Differences in Clinical Characteristics Between Colorectal Cancer and Adenomatous Polyp Groups. Continuous Variables Were Represented by Q2 (Q1, Q3) and Categorical Variables Were Represented by Cases (Percentage).
| Parameters | Colorectal Cancer Group (n = 216) | Adenomatous Polyp Group (n = 126) | /Z | P-Value |
|---|---|---|---|---|
| Gender | 2.879 | .090 | ||
| Male | 140 (64.8%) | 70 (55.6%) | ||
| Female | 76 (35.2%) | 56 (44.4%) | ||
| Age | 64 (59, 72) | 61 (52, 66) | −4.756 | <.001 |
| BMI | 23.62 (21.76, 25.97) | 24.05 (22.01, 26.52) | −1.325 | .185 |
| ALB | 38.65 (36.00, 40.50) | 40.05 (38.57, 41.52) | −4.578 | <.001 |
| Neutrophil | 3.43 (2.68, 4.24) | 3.30 (2.66, 3.99) | −.882 | .378 |
| Lymphocyte | 1.67 (1.23, 2.06) | 1.86 (1.54, 2.23) | −3.955 | <.001 |
| Monocyte | .27 (.14, .38) | .34 (.26, .41) | −4.893 | <.001 |
| MPV | 8.7 (7.7, 9.6) | 9.2 (8.6, 10.2) | −4.558 | <.001 |
| PC | 217.5 (180.5, 269) | 212 (186, 253.25) | −.461 | .645 |
| SII | 433.34 (306.00, 715.62) | 366.30 (286.91, 490.33) | −3.410 | .001 |
| MPV/PC | .0391 (.0295, .0498) | .0431 (.0361, .0516) | −2.937 | .003 |
| HGB | 129 (114.25, 143) | 142 (132.75, 152.25) | −6.523 | <.001 |
| NLR | 2.04 (1.56, 3.10) | 1.74 (1.42, 2.15) | −3.717 | <.001 |
| PLR | 134.34 (101.98, 186.01) | 116.12 (94.52, 137.76) | −3.861 | <.001 |
| CEA | 2.27 (1.51, 4.47) | 1.44 (.94, 2.15) | −5.881 | <.001 |
| CA199 | 6.8 (3.56, 17.3) | 7.4 (4.5, 11.7) | −.080 | .936 |
Figure 1.
Comparison of inflammation-related indexes between colorectal cancer group and adenomatous polyp group. (A) Comparison of neutrophil count, lymphocyte count, monocyte count, MPV, MPV/PC, and NLR between colorectal cancer group and adenomatous polyp group; (B) comparison of PC, PLR, and SII between colorectal cancer group and adenomatous polyp group.
Differences in Inflammation-Related Indicators Between Subgroups of Colorectal Cancer
The differences of inflammation-related indicators among different subgroups of colorectal cancer were shown in Table 2. ALB, HGB, MPV, MPV/PC, NLR, PLR, and SII were significantly different in patients with T stage <3 and ≥3. Subgroup analysis based on tumor location showed significant differences in HGB, MPV/PC, PLR, and SII, but no significant differences in ALB, MPV, and NLR.
Table 2.
Differences of Inflammation-Related Indexes in Different Subgroups of Colorectal Cancer. All Variables Were Represented by Q2 (Q1, Q3). The Right and Left Side of the Tumor Location Were Bounded by the Splenic Flexure.
| Parameters | n | ALB | P-Value | HGB | P-Value | MPV | P-Value | MPV/PC | P-Value |
|---|---|---|---|---|---|---|---|---|---|
| T stage | |||||||||
| <3 | 113 | 39.20 (37.25, 41.20) | <.001 | 133 (121, 149) | <.001 | 9.00 (8.10, 9.65) | .023 | .0422 (.0349, .0517) | <.001 |
| ≥3 | 103 | 37.20 (33.70, 39.90) | 122 (104, 135) | 8.20 (7.40, 9.60) | .0327 (.0268, .0467) | ||||
| Tumor location | |||||||||
| right | 51 | 37.30 (33.90, 40.20) | .169 | 116 (91, 135) | .003 | 8.60 (7.40, 10.00) | .62 | .0301 (.0265, .0404) | <.001 |
| left | 64 | 38.80 (36.80, 40.22) | 131 (116, 148) | 9.00 (7.95, 9.57) | .0424 (.0301, .0500) | ||||
| rectum | 101 | 38.80 (36.05, 41.05) | 130 (118, 143) | 8.50 (7.60, 9.60) | .0411 (.0335, .0520) | ||||
The Diagnostic Value of Inflammation-Related Indicators in Early Colorectal Cancer
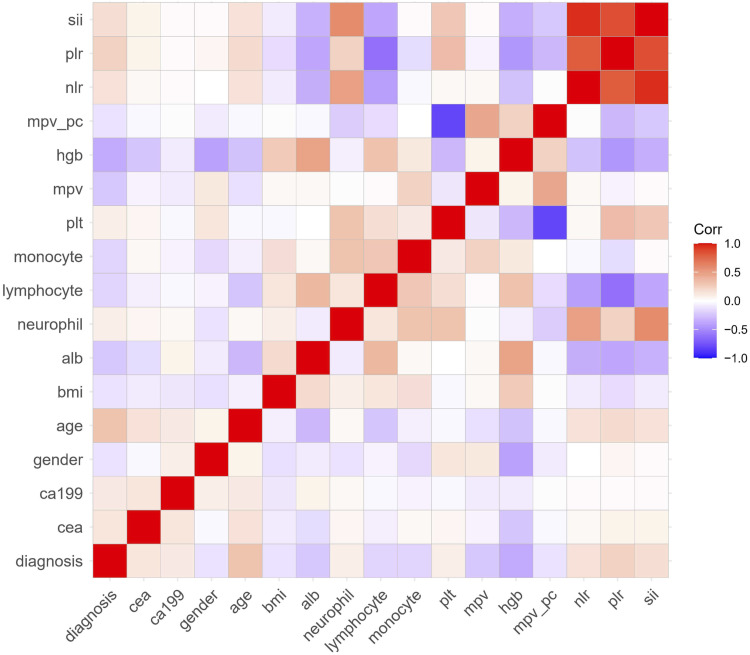
The correlation analysis among various factors is shown in Figure 2. MPV/PC was negatively correlated with PC and positively correlated with MPV. NLR, PLR, and SII were positively correlated with neutrophil and negatively correlated with lymphocyte, respectively. Significantly correlated variables were excluded from the logistic regression model. Finally, age, sex, lymphocyte, monocyte, HGB, MPV, and CEA were selected for multivariate analysis to establish a Nomogram model (Figure 3A). The traditional tumor marker CEA and inflammation-related indicators were separately extracted to establish a Nomogram model (Figure 3B). Figure 3C combined all factors.
Figure 2.
The correlation analysis among inflammation-related indexes and significant factors.
Figure 3.
Nomogram model. (A) A nomogram composed of inflammation-related indicators; (B) A nomogram composed of CEA; (C) A nomogram composed of inflammation-related indicators and CEA.
Inflammation-related indicators and CEA were used to draw ROC correction curves (Figure 4). The red curve shows CEA, the blue curve shows inflammation-related indicators, and the green curve combines all factors. The AUC of CEA was .695, the AUC of inflammation-related indicators was .846, and the AUC of all factors was .854. .707 is the cut-off value, and the Jorden index is the highest at this time. At this time, the specificity is .864 and the sensitivity is .725. Using inflammation-related indicators to differentiate colorectal cancer from colorectal polyps produced greater AUC than using tumor markers alone. The AUC produced by all factors was not significantly different from that produced by inflammation-related indicators alone.
Figure 4.
ROC curves of inflammatory markers and CEA.
The Diagnostic Value of Inflammation-Related Indicators in Stage I Colorectal Cancer
Differences in clinical features between the stage I colorectal cancer group and the adenomatous polyp group were shown in Table 3. There were significant differences in age, ALB, lymphocyte, MPV, HGB, and CEA between the 2 groups (P < .05). These factors were included for multivariate analysis (Table 4). Multivariate analysis showed that age, CEA, less monocyte count, and HGB were independent risk factors for the diagnosis of stage I colorectal cancer. The ROC correction curve was drawn and the AUC was .739 (Figure 5).
Table 3.
Differences in Clinical Characteristics Between Stage I Colorectal Cancer and Adenomatous Polyp Groups.
| Stage I Group | Adenomatous Polyp Group | t/c/Z | P-Value | |
|---|---|---|---|---|
| Gender | 1.646 | .2 | ||
| Male | 72 (63.7%) | 70 (55.6%) | ||
| Female | 41 (36.3%) | 56 (44.4%) | ||
| Age | 64 (59, 70) | 61 (52, 66) | −3.26 | .001 |
| BMI | 24.26 ± 3.37 | 24.46 ± 3.38 | −.443 | .658 |
| ALB | 39.20 (37.25, 41.20) | 40.05 (38.57, 41.52) | −2.089 | .037 |
| Neutrophil | 3.16 (2.55, 3.87) | 3.30 (2.66, 3.99) | −.932 | .351 |
| Lymphocyte | 1.73 (1.35, 2.14) | 1.86 (1.54, 2.23) | −2.226 | .026 |
| Monocyte | .28 (.16, .38) | .34 (.26, .41) | −3.678 | <.001 |
| MPV | 9.0 (8.1, 9.6) | 9.2 (8.6, 10.2) | −2.85 | .004 |
| PC | 211.61 ± 58.71 | 218.78 ± 51.94 | −1.001 | .318 |
| SII | 386.83 (273.19, 539.41) | 366.30 (286.91, 490.33) | −.377 | .706 |
| MPV/PC | .0422 (.0349, .0518) | .0431 (.0361, .0516) | −.53 | .596 |
| HGB | 133.64 ± 18.81 | 141.90 ± 13.91 | −3.889 | <.001 |
| NLR | 1.73 (1.46, 2.58) | 1.74 (1.42, 2.15) | −.979 | .328 |
| PLR | 118.44 (91.77, 161.98) | 116.12 (94.52, 137.76) | −.951 | .342 |
| CEA | 2.04 (1.23, 3.02) | 1.44 (.93, 2.16) | −3.569 | <.001 |
| CA199 | 7.09 (4.07, 13.84) | 7.40 (4.50, 11.70) | −.026 | .979 |
Table 4.
Multivariate Analysis of Clinical Characteristics of Stage I Colorectal Cancer and Adenomatous Polyp Group.
| B | SE | Wald | P | OR | 95%CI | |
|---|---|---|---|---|---|---|
| Age | .039 | .016 | 6.242 | .012 | 1.04 | 1.008, 1.072 |
| ALB | −.008 | .052 | .025 | .875 | .992 | .895, 1.099 |
| Lymphocyte | .151 | .264 | .325 | .568 | 1.162 | .693, 1.950 |
| Monocyte | −2.517 | 1.247 | 4.077 | .043 | .081 | .007, .929 |
| MPV | −.164 | .127 | 1.659 | .198 | .849 | .661, 1.089 |
| HGB | −.027 | .01 | 7.599 | .006 | .973 | .955, .992 |
| CEA | .272 | .111 | 5.971 | .015 | 1.313 | 1.055, 1.633 |
Figure 5.
ROC curve of multivariate analysis of stage I colorectal cancer group and adenomatous polyp group.
Discussion
Currently, the main screening methods for colorectal cancer include colonoscopy, guaiac fecal occult blood test (gFOBT), fecal immunochemical test (FIT), fecal DNA test, blood test, and computed tomography colonography (CTC). 22 Colonoscopy is currently recognized as the gold standard for colorectal cancer prevention. However, it requires bowel preparation, resulting in poor patient compliance. In addition, colonoscopy carries a high risk of perforation and bleeding. 23 The gFOBT test has an acceptable sensitivity and specificity but is highly influenced by drugs and diet. 24 FIT is superior to gFOBT because it detects human globulin and is therefore not affected by diet. 25 Because CRC cells are shed in the stool, abnormal DNA changes during CRC can be detected by stool samples. The stool multitarget DNA (mt-sDNA) test is a combination of FIT and testing for aberrant DNA methylation. 26 In recent years, a large number of studies have shown that inflammation is closely related to the occurrence and development of tumors, which can be reflected by blood test.27-29 The mechanism may be related to the following factors. Inflammatory cells secrete a variety of cytokines, chemokines, and adhesion molecules that promote cell proliferation and angiogenesis. In addition, inflammatory cells induce the production of reactive oxygen species (ROS), which causes DNA damage and inhibits DNA repair after injury.30,31 These events promote the formation of tumor microenvironment and promote tumor development and metastasis. 32
Systemic inflammatory response (SIR) can be measured by a simple index of peripheral blood morphological parameters. 33 Inflammation-related indicators are associated with adverse tumor behavior and survival outcomes in a variety of malignant solid tumors, including colorectal cancer.34-36 A meta-analysis showed that elevated PLR was associated with poor CRC differentiation, high tumor stage, lymphatic vascular invasion, and CRC recurrence. 37 Copija et al found that patients with metastatic colorectal cancer were more likely to present with high PC and PLR than those with stage II-III colorectal cancer. 11 Chen et al reported that SII was an independent predictor of OS and DFS in colorectal cancer patients. 17 Patients with low NLR, PLR, and SII had better overall survival (OS) and disease-free survival (DFS). Shi et al found that preoperative MPV/PC is a new independent prognostic index for gastric cancer, and the Nomogram survival prediction model based on tumor size TNM stage and MPV/PC is more accurate than TNM stage in predicting the 3–5 year survival rate of patients with resectable gastric cancer. 15
Few studies have reported the role of inflammation-related indicators in differentiating early colorectal cancer from colorectal polyps. Wu et al conducted hematological examinations in 186 patients with colorectal cancer, 132 patients with adenomatous polyps and healthy controls, and found that inflammation-related indicators such as MPV/PC and PLR can be effective diagnostic indicators for distinguishing between benign and malignant colorectal tumors and between early and advanced colorectal cancer. 10 This conclusion is consistent with that of our study. However, Wu et al enrolled patients with stage III-IV colorectal cancer. The elevated inflammation-related indicators are not entirely due to colorectal cancer itself, as complications of advanced colorectal cancer such as bleeding, perforation, and intestinal obstruction can activate systemic inflammatory responses. Our study included only patients with stage I-II colorectal cancer and compared inflammation-related indicators with traditional tumor markers. This study found that a Nomogram model constructed by MPV, lymphocytes, and monocytes can effectively predict early colorectal cancer, and its diagnostic efficiency is significantly higher than that of tumor markers. In the Nomogram model, monocytes are negatively associated with the risk of malignancy. Peripheral blood monocyte can be activated by some cytokines (such as IFN-γ or IFN-α) to produce TRAIL protein, which directly induces cell death of TRAIL-sensitive cancer cells.38,39 Monocyte can also direct lymphocyte recruitment in the tumor microenvironment through paracrine signaling and interact with adaptive immunity as antigen-presenting cells.39,40 MPV was also negatively correlated with the risk of malignant tumor. A decrease in MPV has also been associated with locally advanced esophageal squamous cell carcinoma, gastric cancer, and bone marrow metastasis with solid tumors.41-43 Increased degradation of large platelets under the influence of chronic inflammation and tumors may lead to reduced MPV, possibly because larger platelets are more responsive to stimulation and more susceptible to selective degradation. 43 However, lymphocytes were positively correlated with the risk of malignant tumor. This may be because patients with tumors are more likely to activate their lymph nodes to produce neutralizing antibodies. In the subgroup analysis between the stage I colorectal cancer group and the adenomatous polyp group, decreased monocyte count and increased CEA were independent risk factors for the diagnosis of early colorectal cancer, which suggested that monocyte count and CEA could still differentiate stage I colorectal cancer from adenomatous polyps. These results suggest that, to a certain extent, inflammation-related markers and CEA can reflect the initiation and progression of tumors.
This study has the following limitations. Firstly, this was a single-center retrospective study, which could not exclude some confounding factors. Secondly, relatively few cases were included in this study, so more and larger studies are necessary to verify our results.
Conclusions
Inflammation-related indicators, such as lymphocyte, monocyte, and MPV, may serve as potential indicators to assist in the diagnosis of early colorectal cancer. The colorectal cancer prediction model based on inflammation-related indicators may be more objective and reliable than traditional tumor markers to predict early colorectal cancer.
Acknowledgments
We are grateful to all the patients who participated in this study.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval: This study was approved by the Ethics Committee of Beijing Friendship Hospital, Capital Medical University, Beijing, China (the approval number: 2018-P2-045-01, date: 2018/04/04). All the patients have signed written informed consent forms for their data to be used in the study and the data were kept confidential. This study was conducted in accordance with the Declaration of Helsinki.
ORCID iD
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA A Cancer J Clin. 2021;71:7-33. doi: 10.3322/caac.21654 [DOI] [PubMed] [Google Scholar]
- 2.Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490-1502. doi: 10.1016/S0140-6736(13)61649-9 [DOI] [PubMed] [Google Scholar]
- 3.Mahasneh A, Al-Shaheri F, Jamal E. Molecular biomarkers for an early diagnosis, effective treatment and prognosis of colorectal cancer: Current updates. Exp Mol Pathol. 2017;102:475-483. doi: 10.1016/j.yexmp.2017.05.005 [DOI] [PubMed] [Google Scholar]
- 4.Bagaria B, Sood S, Sharma R, Lalwani S. Comparative study of CEA and CA19-9 in esophageal, gastric and colon cancers individually and in combination (ROC curve analysis). Cancer Biol Med. 2013;10:148-157. doi: 10.7497/j.issn.2095-3941.2013.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang SY, Lin M, Zhang HB. Diagnostic value of carcinoembryonic antigen and carcinoma antigen 19-9 for colorectal carcinoma. Int J Clin Exp Pathol. 2015;8:9404-9409. [PMC free article] [PubMed] [Google Scholar]
- 6.Balkwill F, Mantovani A. Inflammation and cancer: Back to Virchow. Lancet. 2001;357:539-545. doi: 10.1016/S0140-6736(00)04046-0 [DOI] [PubMed] [Google Scholar]
- 7.Borsig L. The role of platelet activation in tumor metastasis. Expert Rev Anticancer Ther. 2008;8:1247-1255. doi: 10.1586/14737140.8.8.1247 [DOI] [PubMed] [Google Scholar]
- 8.Neurath MF, Finotto S. IL-6 signaling in autoimmunity, chronic inflammation and inflammation-associated cancer. Cytokine Growth Factor Rev. 2011;22:83-89. doi: 10.1016/j.cytogfr.2011.02.003 [DOI] [PubMed] [Google Scholar]
- 9.Diem S, Schmid S, Krapf M, et al. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer. 2017;111:176-181. doi: 10.1016/j.lungcan.2017.07.024 [DOI] [PubMed] [Google Scholar]
- 10.Wu YY, Zhang X, Qin YY, Qin JQ, Lin FQ. Mean platelet volume/platelet count ratio in colorectal cancer: A retrospective clinical study. BMC Cancer. 2019;19:314. doi: 10.1186/s12885-019-5504-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Copija A, Nowakowska-Zajdel E, Janion K, Walkiewicz K. Clinical characteristics of colorectal cancer patients in terms of selected platelet indices. Dis Markers. 2020;2020:6145604. doi: 10.1155/2020/6145604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao X, Xue J, Yang H, Han X, Zu G. Association of clinical parameters and prognosis with the pretreatment systemic immune-inflammation index (SII) in patients with gastric cancer. J Coll Physicians Surg Pak. 2021;31:83-88. doi: 10.29271/jcpsp.2021.01.83 [DOI] [PubMed] [Google Scholar]
- 13.Deng Q, Long Q, Liu Y, Yang Z, Du Y, Chen X. Prognostic value of preoperative peripheral blood mean platelet volume/platelet count ratio (MPV/PC) in patients with resectable cervical cancer. BMC Cancer. 2021;21:1282. doi: 10.1186/s12885-021-09016-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Y, Chen Y, Zhu Y, et al. Postoperative systemic immune-inflammation index (SII): A superior prognostic factor of endometrial cancer. Front Surg. 2021;8:704235. doi: 10.3389/fsurg.2021.704235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi H, Wang H, Pan J, Liu Z, Li Z. Comparing prognostic value of preoperative platelet indexes in patients with resectable gastric cancer. Sci Rep. 2022;12:6480. doi: 10.1038/s41598-022-10511-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stegeman I, de Wijkerslooth TR, Stoop EM, et al. Colorectal cancer risk factors in the detection of advanced adenoma and colorectal cancer. Cancer Epidemiol. 2013;37:278-283. doi: 10.1016/j.canep.2013.02.004 [DOI] [PubMed] [Google Scholar]
- 17.Chen JH, Zhai ET, Yuan YJ, et al. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol. 2017;23:6261-6272. doi: 10.3748/wjg.v23.i34.6261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiser MR. AJCC 8th edition: Colorectal cancer. Ann Surg Oncol. 2018;25:1454-1455. doi: 10.1245/s10434-018-6462-1 [DOI] [PubMed] [Google Scholar]
- 19.Knapen DG, de Haan JJ, Fehrmann R, de Vries E, de Groot D. Opportunities on the horizon for the management of early colon cancer. Crit Rev Oncol Hematol. 2023;183:103918. doi: 10.1016/j.critrevonc.2023.103918 [DOI] [PubMed] [Google Scholar]
- 20.Parikh AR, Van Seventer EE, Siravegna G, et al. Minimal residual disease detection using a plasma-only circulating tumor DNA assay in patients with colorectal cancer. Clin Cancer Res. 2021;27(20):5586-5594. doi: 10.1158/1078-0432.CCR-21-0410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tie J, Wang Y, Tomasetti C, et al. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci Transl Med. 2016;8(346):346ra92. doi: 10.1126/scitranslmed.aaf6219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ladabaum U, Dominitz JA, Kahi C, Schoen RE. Strategies for colorectal cancer screening. Gastroenterology. 2020;158(2):418-432. doi: 10.1053/j.gastro.2019.06.043 [DOI] [PubMed] [Google Scholar]
- 23.Lin JS, Perdue LA, Henrikson NB, Bean SI, Blasi PR. Screening for colorectal cancer: Updated evidence report and systematic review for the US preventive services task force. JAMA. 2021;325(19):1978-1998. doi: 10.1001/jama.2021.4417 [DOI] [PubMed] [Google Scholar]
- 24.Levi Z, Birkenfeld S, Vilkin A, et al. A higher detection rate for colorectal cancer and advanced adenomatous polyp for screening with immunochemical fecal occult blood test than guaiac fecal occult blood test, despite lower compliance rate. A prospective, controlled, feasibility study. Int J Cancer. 2011;128(10):2415-2424. doi: 10.1002/ijc.25574 [DOI] [PubMed] [Google Scholar]
- 25.Vart G, Banzi R, Minozzi S. Comparing participation rates between immunochemical and guaiac faecal occult blood tests: A systematic review and meta-analysis. Prev Med. 2012;55(2):87-92. doi: 10.1016/j.ypmed.2012.05.006 [DOI] [PubMed] [Google Scholar]
- 26.Lee JK, Liles EG, Bent S, Levin TR, Corley DA. Accuracy of fecal immunochemical tests for colorectal cancer: Systematic review and meta-analysis. Ann Intern Med. 2014;160(3):171. doi: 10.7326/M13-1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herszényi L, Lakatos G, Hritz I, Varga MZ, Cierny G, Tulassay Z. The role of inflammation and proteinases in tumor progression. Dig Dis. 2012;30:249-254. doi: 10.1159/000336914 [DOI] [PubMed] [Google Scholar]
- 28.Rasic I, Radovic S, Aksamija G. Relationship between chronic inflammation and the stage and histopathological size of colorectal carcinoma. Med Arch. 2016;70:104-107. doi: 10.5455/medarh.2016.70.104-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Şahin F, Aslan AF. Relationship between inflammatory and biological markers and lung cancer. J Clin Med. 2018;7:160. doi: 10.3390/jcm7070160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hold GL, El-Omar EM. Genetic aspects of inflammation and cancer. Biochem J. 2008;410:225-235. doi: 10.1042/BJ20071341 [DOI] [PubMed] [Google Scholar]
- 31.Maletzki C, Emmrich J. Inflammation and immunity in the tumor environment. Dig Dis. 2010;28:574-578. doi: 10.1159/000321062 [DOI] [PubMed] [Google Scholar]
- 32.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436-444. doi: 10.1038/nature07205 [DOI] [PubMed] [Google Scholar]
- 33.Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20:6212-6222. doi: 10.1158/1078-0432.CCR-14-0442 [DOI] [PubMed] [Google Scholar]
- 34.Sato R, Oikawa M, Kakita T, et al. The prognostic value of the prognostic nutritional index and inflammation-based markers in obstructive colorectal cancer. Surg Today. 2020;50:1272-1281. doi: 10.1007/s00595-020-02007-5 [DOI] [PubMed] [Google Scholar]
- 35.Toyokawa T, Muguruma K, Yoshii M, et al. Clinical significance of prognostic inflammation-based and/or nutritional markers in patients with stage III gastric cancer. BMC Cancer. 2020;20:517. doi: 10.1186/s12885-020-07010-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin YW, Kang WP, Huang BL, et al. Nomogram based on clinical characteristics and serological inflammation markers to predict overall survival of oral tongue squamous cell carcinoma patient after surgery. BMC Oral Health. 2021;21:667. doi: 10.1186/s12903-021-02028-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang XZ, Chen WJ, Zhang X, et al. An elevated platelet-to-lymphocyte ratio predicts poor prognosis and clinicopathological characteristics in patients with colorectal cancer: A meta-analysis. Dis Markers. 2017;2017:1053125. doi: 10.1155/2017/1053125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hartwig T, Montinaro A, von Karstedt S, et al. The TRAIL-induced cancer secretome promotes a tumor-supportive immune microenvironment via CCR2. Mol Cell. 2017;65:730-742. doi: 10.1016/j.molcel.2017.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olingy CE, Dinh HQ, Hedrick CC. Monocyte heterogeneity and functions in cancer. J Leukoc Biol. 2019;106:309-322. doi: 10.1002/JLB.4RI0818-311R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jakubzick CV, Randolph GJ, Henson PM. Monocyte differentiation and antigen-presenting functions. Nat Rev Immunol. 2017;17:349-362. doi: 10.1038/nri.2017.28 [DOI] [PubMed] [Google Scholar]
- 41.Aksoy S, Kilickap S, Hayran M, et al. Platelet size has diagnostic predictive value for bone marrow metastasis in patients with solid tumors. Int J Lab Hematol. 2008;30:214-219. doi: 10.1111/j.1751-553X.2007.00947.x [DOI] [PubMed] [Google Scholar]
- 42.Yun ZY, Li N, Zhang X, et al. Mean platelet volume, platelet distribution width and carcinoembryonic antigen to discriminate gastric cancer from gastric ulcer. Oncotarget. 2017;8:62600-62605. doi: 10.18632/oncotarget.15898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun SY, Zhao BQ, Wang J, et al. The clinical implications of mean platelet volume and mean platelet volume/platelet count ratio in locally advanced esophageal squamous cell carcinoma. Dis Esophagus. 2018;31:31. doi: 10.1093/dote/dox125 [DOI] [PubMed] [Google Scholar]