Abstract
Acute myocardial infarction and pulmonary embolism can have life-threatening consequences such as congestive heart and respiratory failure, respectively. Cancer patients are at great risk of both acute myocardial infarction and pulmonary embolism complications because the malignancy sparks the patient’s blood hypercoagulable state. Nevertheless, the literature currently offers only a few reports on acute myocardial infarction associated with pulmonary embolism, and two of them occurred in the same cancer patient. Here, we present a case of a 60-year-old woman who had been diagnosed with lung cancer. She was admitted to the emergency department twice. She was diagnosed with acute myocardial infarction at her first admission, when she experienced sudden-onset chest pain. Electrocardiography showed ST-segment elevation in leads V1–V3 with inverted T wave and pathological Q wave, suggesting an acute myocardial infarction. Coronary angiography revealed a thrombus in the left anterior descending coronary artery, and thrombus aspiration was performed. After 1 month, she had an attack of pulmonary embolism with syncope upon the second admission. A computed tomographic pulmonary angiography showed branches of right and left pulmonary embolism. Anticoagulation and antiplatelet measures were taken. In this article, we discuss the relationship between cancer and thrombosis with a special focus on the conservative management strategy regarding anticoagulant and antiplatelet therapy in our case.
Keywords: Acute myocardial infarction, pulmonary embolism, cancer-associated thrombosis
Introduction
Cancer-associated thromboembolism (CAT) is one of the prevalent causes of mortality among cancer patients. 1 The association between cancer and thromboembolism has been confirmed and reported over the past few years, but the pathological mechanism is less explored. The hypercoagulable state is a well-known independent risk factor for venous thromboembolism (VTE) in patients with malignancy.2,3 However, the relationship between cancer and arterial thromboembolism (ATE) remains unclear, and there have only been a few systematic attempts to analyze therapies for these patients. We herein report an unusual lung cancer case with acute myocardial infarction (AMI) and pulmonary embolism (PE) effectively treated via pharmacotherapy. In addition, we highlight the importance of treating CAT with a conservative management strategy involving anticoagulant and antiplatelet therapy in patients with malignancy and AMI.
Case presentation
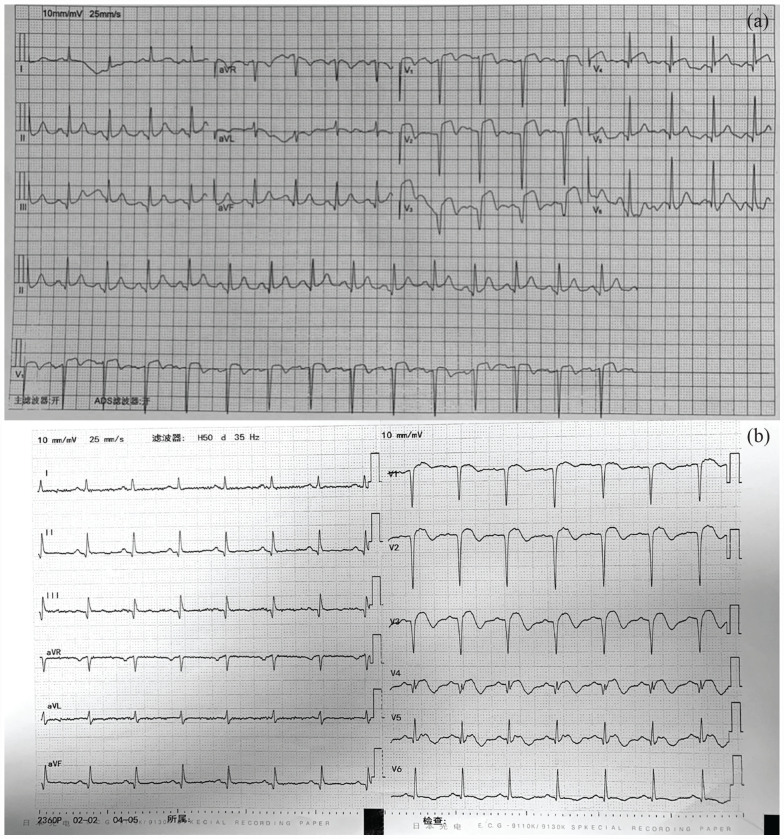
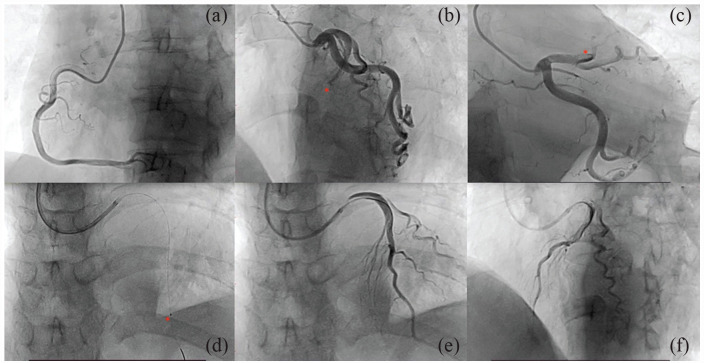
A 60-year-old woman with advanced lung cancer was admitted to the Affiliated Hospital of Jilin Medical University for 3 h with a sudden onset of chest pain behind her sternum that spread to her back and shoulders. She suffered from hypertension as a cardiovascular risk factor. Emergency physical examination revealed a blood pressure of 98/65 mm Hg, with a pulse of 92 beats/min. The oxygen saturation upon finger oximetry was 95% with the patient breathing ambient air in the emergency room. The cardiac examination revealed a weakening of the first heart sound in the apical area without murmurs. An electrocardiograph (ECG) showed sinus rhythm with ST-segment elevation in leads V1–V3 and pathological Q wave formation in V1–V4 leads (Figure 1). Emergency laboratory findings reported cardiac troponin I, >50.00 ng/mL (reference range < 0.5 ng/mL); myoglobin fraction, 136.62 ng/mL (reference range < 50 ng/mL); creatine kinase MB, 55.93 ng/mL (reference range < 5.0 ng/mL); NT-proBNP, 1938.92 (reference range < 125 pg/mL). Echocardiography revealed abnormal segmental motion of the left ventricular wall, decreased left ventricular systolic function (ejection fraction 31%), and pericardial effusion. Coronary angiography revealed 100% occlusion of the mid-left anterior descending artery (LAD) with TIMI 0 flow. Due to thrombus overload, thrombus aspiration was immediately performed, as well as TIMI III flow (Figure 2). This patient was given aspirin (100 mg/qd), clopidogrel (75 mg/qd), and low-molecular-weight heparin (LMWH) (5000 IU/bid). Simultaneously, valsartan and propranolol were routinely applied.
Figure 1.
(a) Electrocardiogram (ECG) displaying ST-segment elevation in leads V1–V4 with pathological Q waves. (b) ECG showing sinus rhythm with ST-segment elevation in leads V1–V4 with inverted T waves and pathological Q wave formation in chest leads.
Figure 2.
Still-frame images of coronary angiography: (a) Normal right coronary artery. (b) and (c) Normal left circumflex artery and total occlusion of the proximal left anterior descending coronary artery (asterisk). (d) Placement of aspiration catheter (asterisk). (e) and (f) Post-aspiration angiography demonstrating thrombolysis in myocardial infarction 3 flow.
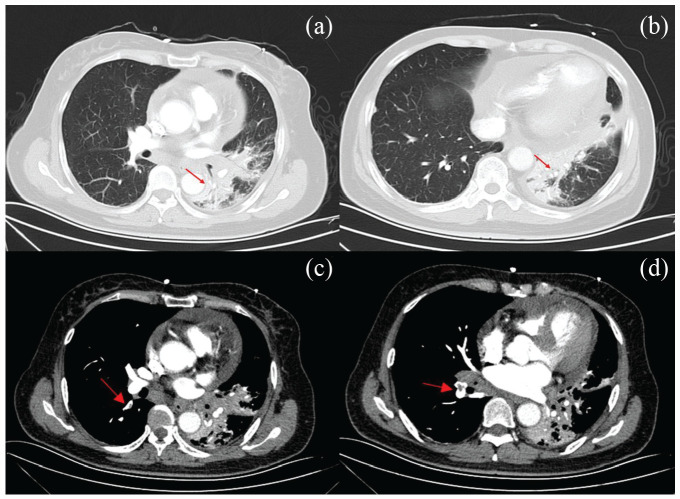
After 1 month, the patient was sent to the emergency department a second time because she experienced syncope for 2 min. Her blood pressure was 122/75 mm Hg, with a pulse of 92 beats/min and oxygen saturation of 92%. Accentuation of the second heart sound in the pulmonary valve area was performed. The ECG showed sinus rhythm with ST-segment elevation in leads V1–V4 with inverted T waves and pathological Q wave formation in chest leads (Figure 1(b)). Her laboratory test results revealed cardiac troponin I, 2.660 ng/mL (reference range, 0–0.034 ng/mL); D-dimmer, 90,450.00 (reference range, 0–500 mg/L). Ultrasonic cardiogram (UCG) revealed that the thickness of the left ventricle extensive anterior wall, anteroseptal wall, and apex area becomes thinner. The apex of the heart showed a significant tumor-like bulge during the systolic phase, which was suspected to be MI with a ventricular aneurysm. Furthermore, a small amount of mitral and tricuspid regurgitation and pericardial effusion were detected. The ejection fraction remained at 48%. Computed tomographic pulmonary angiography (CTPA) showed PE in the basal segment of the right upper and lower lobe and its branches and in the anterior segment of the left upper lobe (Figure 3). After further diagnostic evaluation, the patient was given rivaroxaban. Meanwhile, aspirin and clopidogrel were also applied. The patient’s condition dramatically improved. At her 30-day follow-up, she did not report any further episodes of chest pain or thrombotic events.
Figure 3.
(a) and (b) CT scans displaying signs of central bronchogenic carcinoma in the inferior lobe of the left lung with atelectasis and obstructive pneumonia (red arrows). (c) and (d) Computed tomographic pulmonary angiography showing pulmonary embolism in the basal segment of the right upper and lower lobe and its branches, as well as in the anterior segment of the left upper lobe (red arrows).
Discussion
The relationship between cancer and the increased incidence of thrombosis has been widely recognized as a major public health issue. 4 Great efforts have been made to reduce the incidence of CAT over the years, but its incidence rate is still increasing in patients with active malignancy, including both VTE and ATE. Here, we describe a 60-year-old woman with lung cancer who suffered from ATE and then VTE in succession. The hypercoagulable state of malignancy was considered a reasonable explanation for intracoronary thrombosis and PE in the present case.
VTE is the second most common cause of death in cancer patients, which always manifests as deep venous thrombosis (DVT) and PE. 5 Kim et al. 6 summarized the occurrence mechanisms of VTE in CAT. Notably, cancer cells can secrete extracellular vesicles (EVs), of which the negatively charged plasma membrane provides a catalytic surface for vitamin K-dependent clotting factors such as VII, IX, X, and prothrombin. Moreover, neutrophils and neutrophil extracellular traps (NETs) induced by IL-8, TNF-α, and G-CSF act as scaffolds for red blood cells, platelets, and procoagulants.7,8 However, most pathways converge to activate coagulation factors through extrinsic coagulation pathways to generate thrombin. In our case, the patient experienced syncope for 2 min at her second admission, which was suspected to be due to PE or arrhythmogenic reasons. To guide diagnosis and treatment, we introduced a commonly used decision approach, the Wells score. 9 The patient was assessed as having a score greater than 6 points, indicating that PE was likely. Next, we arranged CTPA for the patient considering their high levels of D-dimmer, as well as their clinical characteristics of chest pain and respiratory problems. Elevated procoagulants, fibrinolytic activity inhibition, and platelet activation all trigger the progress of ATE. 10 ATE is less common than VTE, with an accumulative incidence within 6 months of malignancy diagnosis of no more than 5%. 11 A prospective study reported a similar finding that patients with malignancies who died from ATE accounted for only 5.6% of total deaths. 12 Grilz et al. 11 showed that the cumulative incidence of ATE in 1 year was 1.7% among cancer patients. Wang et al. 2 showed that the cumulative incidence of ATE was 2.36% in 330 days. However, ATE, including myocardial infarction (MI) and cerebral stroke, is still the primary cause of death and disability worldwide. Thus, AMI resulting from cancer-induced hypercoagulability is extremely rare. According to a retrospective cohort study, the risk of ATE increased before cancer diagnosis, with the most remarkable increase occurring during the diagnosis of malignant tumors. 2 A large randomized controlled trial (RCT) showed that the incidence of ATE at 6 months of lung cancer remained high (8.3%), with MI accounting for 2.0%. 13 In our case, the patient was diagnosed with lung cancer at the first admission, which confirmed the relationship between advanced cancer and a high risk of ATE. Moreover, we hold the same view as Wang et al. 2 in that a patient newly diagnosed with lung cancer may have an increased risk of ATE.
CAT management is well worth researching, practicing, and developing constantly, especially in terms of medical therapy (including anticoagulants) and interventional operation. Numerous RCTs have shown that LMWH, a representative anticoagulant, significantly decreases the risk of VTE; meanwhile, the risk of major bleeding increases slightly. 14 In addition, international guidelines and most specialists recommend the administration of LMWH for at least 6 months in patients with CAT. 15 The newly updated guidelines recommended direct-acting oral anticoagulants (DOACs), such as rivaroxaban, as an alternative therapy to LMWH for VTE for patients without contraindications or drug interactions. 16 However, the driving force behind the DOAC treatment option primarily relies on the development of cancer, cancer sites, comorbidities, and drug interactions. 17 Therefore, patient conditions should be extensively considered. Costa et al. 18 reported that rivaroxaban significantly decreases the incidence of recurrent thrombosis compared with LMWH. An RCT found that rivaroxaban can reduce the risk of death from cardiovascular disease, MI, or stroke. 19 These data provide a practical basis for our therapeutic regimen. After ruling out other potential drug interactions and factors, our patient was administered rivaroxaban. In effect, there is limited clinical data regarding ATE that is secondary to malignant tumors, which makes its treatment more challenging. Pharmacotherapy (anticoagulants and antiplatelets) and catheter-based revascularization are widely used in acute coronary thrombosis treatment worldwide. 20 Although aspirin can reduce the incidence of arterial thrombosis in multiple myeloma, 21 the role of antiplatelet therapy and anticoagulant therapy in preventing ATE occurrence in patients with solid tumors needs to be further verified. Based on the above finding, we adopted the use of anticoagulants and antiplatelets in our patient. In addition, thrombus aspiration was performed instead of stent implantation and balloon dilatation. This approach was considered due to the heavy burden of thrombus in AMI, infarct-related vessel enlargement, and the persistent retention of a contrast agent distal to the occlusion. We did not administer thrombolytic therapy in this case because the patient showed no cardiac insufficiency or low blood pressure. The British Thoracic Society (BTS) guidelines for the management of acute PE do not recommend thrombolytic therapy as the first-line treatment for non-massive PE. 22
Early intervention is the most effective way to prevent the occurrence of CAT. Although the incidence of CAT is increasing each year, there exists a knowledge gap concerning the optimal surveillance strategies for thromboprophylaxis in hospitalized patients with malignancy. Risk assessment models can predict VTE occurrence and contribute to clinical decision-making, but there is no unified criterion standardizing these models to effectively improve their ability to identify high-risk patients.6,23 Biomarkers might be promising parameters for the monitoring and prevention of CAT. 6 A cohort study demonstrated that the rearrangement of anaplastic lymphoma kinase (ALK) in lung cancer represents a higher risk for thrombogenesis. 24 Low hemoglobin levels and high platelet and leukocyte counts preceding chemotherapy are closely related to VTE incidence. 25 With the revelation of the key mechanism of VTE, subsequent research concentrated on related factors, such as P-selectin and plasminogen activator inhibitor-1. 26 However, because there are relatively few studies on primary ATE and recurrent VTE and because there are limited specific cancer data and guidelines, usual care is required.
Conclusion
We provide an uncommon case of thrombosis located in two different areas: the coronary and pulmonary arteries. The thrombosis in these two sites then caused two fatal diseases within a month: AMI and PE, respectively. The treatment strategy for a clinical case varies depending on the prothrombotic state of malignancies. Therefore, we adopted a conservative treatment with anticoagulation for the patient during their hospital stay and achieved a prominent curative effect before discharge. CAT precaution measures and therapeutic management in cancer patients are the targets for future studies, which could effectively reduce the risk of primary ATE and recurrent VTE and prolong mean survival time.
Footnotes
Author contributions: Conceptualization: Jiacheng Jin, Xin Qi
Supervision: Guangyin Shen
Validation: Xin Qi, Hongyu Zhang, Min Li, Shuangbin Li
Writing – original draft: Jiacheng Jin
Writing – review & editing: Guangyin Shen, Shuangbin Li
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed consent: Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
ORCID iD: Guangyin Shen  https://orcid.org/0000-0001-5442-6881
https://orcid.org/0000-0001-5442-6881
References
- 1.Khorana AA. Cancer and coagulation. Am J Hematol 2012; 87: S82–S87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang J, Kim YD, Kim CH. Incidence and risk of various types of arterial thromboembolism in patients with cancer. Mayo Clin Proc 2021; 96(3): 592–600. [DOI] [PubMed] [Google Scholar]
- 3.Khorana AA, Mackman N, Falanga A, et al. Cancer-associated venous thromboembolism. Nat Rev Dis Primers 2022; 8: 11. [DOI] [PubMed] [Google Scholar]
- 4.Gervaso L, Dave H, Khorana AA. Venous and arterial thromboembolism in patients with cancer: JACC: CardioOncology state-of-the-art review. JACC Cardiooncol 2021; 3(2): 173–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weitz JI, Haas S, Ageno W, et al. Cancer associated thrombosis in everyday practice: perspectives from GARFIELD-VTE. J Thromb Thrombolysis 2020; 50(2): 267–277. [DOI] [PubMed] [Google Scholar]
- 6.Kim AS, Khorana AA, McCrae KR. Mechanisms and biomarkers of cancer-associated thrombosis. Transl Res 2020; 225: 33–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demers M, Krause DS, Schatzberg D, et al. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc Natl Acad Sci U S A 2012; 109: 13076–13081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuchs TA, Brill A, Duerschmied D, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A 2010; 107: 15880–15885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and d-dimer. Ann Intern Med 2001; 135: 98–107. [DOI] [PubMed] [Google Scholar]
- 10.Tuzovic M, Herrmann J, Iliescu C, et al. Arterial thrombosis in patients with cancer. Curr Treat Options Cardiovasc Med 2018; 20: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grilz E, Königsbrügge O, Posch F, et al. Frequency, risk factors, and impact on mortality of arterial thromboembolism in patients with cancer. Haematologica 2018; 103(9): 1549–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khorana AA, Francis CW, Culakova E, et al. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost 2007; 5(3): 632–634. [DOI] [PubMed] [Google Scholar]
- 13.Navi BB, Reiner AS, Kamel H, et al. Risk of arterial thromboembolism in patients with cancer. J Am Coll Cardiol 2017; 70: 926–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lyman GH, Kuderer NM. Clinical practice guidelines for the treatment and prevention of cancer-associated thrombosis. Thromb Res 2020; 191(Suppl. 1): S79–S84. [DOI] [PubMed] [Google Scholar]
- 15.Posch F, Königsbrügge O, Zielinski C, et al. Treatment of venous thromboembolism in patients with cancer: a network meta-analysis comparing efficacy and safety of anticoagulants. Thromb Res 2015; 136(3): 582–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Key NS, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol 2020; 38: 496–520. [DOI] [PubMed] [Google Scholar]
- 17.Mahe I, Chapelle C, Plaisance L, et al. Management of cancer-associated thrombosis in France: a national survey among vascular disease and supportive care specialists. Cancers (Basel) 2022; 14: 4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Costa OS, Kohn CG, Kuderer NM, et al. Effectiveness and safety of rivaroxaban compared with low-molecular-weight heparin in cancer-associated thromboembolism. Blood Adv 2020; 4: 4045–4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ntaios G, Papavasileiou V, Diener HC, et al. Nonvitamin-K-antagonist oral anticoagulants in patients with atrial fibrillation and previous stroke or transient ischemic attack: a systematic review and meta-analysis of randomized controlled trials. Stroke 2012; 43: 3298–3304. [DOI] [PubMed] [Google Scholar]
- 20.Oren O, Herrmann J. Arterial events in cancer patients—the case of acute coronary thrombosis. J Thorac Dis 2018; 10: S4367–S4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palumbo A, Cavo M, Bringhen S, et al. Aspirin, warfarin, or enoxaparin thromboprophylaxis in patients with multiple myeloma treated with thalidomide: a phase III, open-label, randomized trial. J Clin Oncol 2011; 29: 986–993. [DOI] [PubMed] [Google Scholar]
- 22.Howard LS. BTS Guidelines for the initial outpatient management of pulmonary embolism: there’s no place like home. Thorax 2018; 73: 607–608. [DOI] [PubMed] [Google Scholar]
- 23.Pabinger I, van Es N, Heinze G, et al. A clinical prediction model for cancer-associated venous thromboembolism: a development and validation study in two independent prospective cohorts. Lancet Haematol 2018; 5(7): e289–e298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roopkumar J, Poudel SK, Gervaso L, et al. Risk of thromboembolism in patients with ALK- and EGFR-mutant lung cancer: a cohort study. J Thromb Haemost 2021; 19(3): 822–829. [DOI] [PubMed] [Google Scholar]
- 25.Khorana AA, Francis CW, Culakova E, et al. Risk factors for chemotherapy-associated venous thromboembolism in a prospective observational study. Cancer 2005; 104: 2822–2829. [DOI] [PubMed] [Google Scholar]
- 26.Khorana AA, DeSancho MT, Liebman H, et al. Prediction and prevention of cancer-associated thromboembolism. Oncologist 2021; 26(1): e2–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]