Abstract
Medicinal plants have been extensively exploited for their immense pharmacological and immune-supporting potential. Fruit of Citrullus colocynthis has several active secondary metabolites such as phenolics, flavonoids, and essential oils that are used in traditional medicines as antidiabetic, anti-inflammatory, antioxidant, and antimicrobial agents. In this study, phytoconstituents in organic fractions (n-hexane, chloroform, and ethyl acetate) of the methanolic extract of C. colocynthis were analyzed and identified by FT-IR, HPLC, and GC-MS analysis. Ethyl acetate fraction showed the highest antioxidant scavenging (76 ± .769%) and anti-inflammatory (40 ± .473%) activities at the concentration of 3 mg/mL. Similarly, antidiabetic effect was measured by inhibition of α-amylase where, ethyl acetate fraction (77 ± .844%) exhibited the highest antidiabetic activity. Among all organic fractions, ethyl acetate exhibited strong antimicrobial potential followed by n-hexane and chloroform fractions against selected pathogenic bacteria. Various concentrations of the ethyl acetate extract were tested in-vivo for cytotoxicity and results indicated minor morphological changes in liver cells including ballooning, fatty droplets, and slight accumulation of extracellular matrix even at concentrations of 400 mg/kg. In-silico study showed that stigmasta-7,16-dien-3-ol had a strong interaction with COX-1 and COX-2 to reduce inflammation. The abovementioned results indicate the pharmacological strengths of C. colocynthis to fight several diseases.
Keywords: Citrullus colocynthis, GC-MS, Antioxidant, Anti-inflammatory, Antimicrobial, Cytotoxicity
Introduction
Medicinal plants have been used to improve overall human health and are known to cure multiple ailments. The extracts of medicinal plants are used for the formulation of novel traditional medicines or food supplements. 1 Plant-based chemicals are further classified as primary and secondary metabolites. Primary metabolites consist of proteins, amino acids, purines, pyrimidines, and chlorophyll, whereas, secondary metabolites consist of several bioactive compounds with immense biological potential. C. colocynthis has been used for various ethnopharmacological purposes for centuries. A major compound from C. colocynthis named as cucurbitin or cucurbit, has shown effectiveness in a wide range of diseases, including skin, gynecological, pulmonary infections, inflammatory conditions, cardiovascular problems, and immune-related illnesses. 2
Extracts of C. colocynthis contain phytochemicals such as phenolics, flavonoids, alkaloids, terpenes, and tannins that show good potential as antimicrobial, antidiabetic,3,4 and anticancer agents. 5 The antioxidant properties of polyphenols enable them to scavenge free radicals and prevent their future formation. Free radical scavenging activities of C. colocynthis extract are broad-spectrum because of the large proportion of polyphenols as its phytoconstituent. 6 All these secondary metabolites from medicinal plants can include phenols, alkaloids, terpenoids, acetogenins, and saponins. 7 Bioactive compounds derived from Citrullus plants, categorized as alkaloids, flavonoids, and phenolic acids also show antimalarial, analgesic, and diuretic activities.5,8 Terpenoids in extracts of medicinal plants are known for their anthelmintic, antiviral, antibacterial, antimalarial, anti-inflammatory, and anticancer potential.9,10 Phenols and flavonoids exhibit antiallergic, antibacterial, and antioxidant activities. Saponins are reported to have antiviral, anti-inflammatory, and immune responses. 8 Globally, people use these plants as primary source of medicine based upon their folkloric uses and to have protection against infectious diseases. 11 In addition to have remedial effects, these medicinal plants are able to scavenge harmful radicals such as peroxyl radical from the body. 12
Citrullus is commonly consumed by diabetic people. Diabetes is a common metabolic disorder leading to death of large number of people every year. Due to stress and insufficient amount of enzyme, glycation of protein leads to formation of reactive oxygen species (ROS). 13 Although several synthetic drugs are used to cure diabetes, nonetheless, they are associated with certain limitations and side effects. Natural products are rich source of novel and effective antidiabetic compounds, which significantly cure diabetes and can serve as inhibitors of α-glucosidase and considerably lower blood glucose levels. 14 C. colocynthis has been used extensively to cure diabetes in folk medicine and contains various biologically active compounds that cause hypoglycaemic effect by inhibiting activity of α-glucosidase. 15 Phenolic compounds (galloylepicatechin) derived from C. colocynthis exhibit antidiabetic potential with no side effects and are attributed as a very low-cost treatment of diabetes and related disorders. 16
The aim of this research was to perform the detailed and in-depth phytochemical analysis and to explore the biological potential of bioactive compounds isolated from active fractions of methanolic extract of C. colocynthis. The study also aimed to evaluate the potentially active compounds with antimicrobial, antioxidant, antidiabetic, and cytotoxic effects in-vitro.
Materials and Methods
Collection of Plant Material
C. colocynthis plants were purchased from the local market of Lahore, Pakistan. Plants were cross-verified and approved from a taxonomist of the Department of Botany, University of Central Punjab, Lahore, Pakistan.
Preparation of Methanolic Extract
The C. colocynthis fruit (100 g) was washed with sterile water and shade dried. The dried fruit was ground to make a fine powder. Ten-grams of the powdered sample were soaked into 100 mL of methanol for 14 days at room temperature. The mixture was filtered and the residue was resuspended in 100 mL of methanol. The process was repeated three times and the filtrate was combined. The filtrate was subjected to concentration in a rotary evaporator (Rota Vapor R-200 Buchi, Switzerland) at pressure of 100 mbar and 40 °C temperature. 17
Organic Extraction and Fractionation
The methanolic extract was fractionated by using organic solvents including n-hexane, chloroform, and ethyl acetate on polarity pattern. After fractionation, samples were concentrated to dryness using a rotary evaporator at a temperature 40–45 °C and stored in a refrigerator at 4 °C for further analysis. 18
Qualitative and Quantitative Analysis
Phytoconstituents Analysis
Qualitative phytochemical profiling of n-hexane, chloroform, and ethyl acetate fractions of the methanolic extract of C. colocynthis was performed to detect the presence of alkaloids, saponins, tannins, flavonoids, terpenoids, glycosides, and quinones by following the methods as described before [19].
Fourier Transform Infrared (FT-IR) Spectroscopic Analysis
FT-IR analysis of n-hexane, chloroform, and ethyl acetate fractions of C. colocynthis was performed using Perkin Elmer spectrophotometer system. The characteristic peaks and functional groups at resolution of 4 cm−1 within a peak a range of 500 to 4000 cm−1 were found out. 20
Determination of Total Phenolic and Flavonoid Content
Folin-ciocalteu assay was used to determine the total phenolic content by using a UV-visible spectrophotometer at 760 nm, and the total flavonoid content of fractions was estimated by AlCl3 at 415 nm.19,21
Characterization of Phytochemical Components
High-Performance Liquid Chromatography Analysis of Phenolics and Flavonoids
The phenolics and flavonoids were analyzed using high-performance liquid chromatography (HPLC). The HPLC instrument equipped with a quaternary pump (model 1260 Agilent, USA) was used in this analysis. A 20-μL volume of the filtered sample was injected into a Zorbax Eclips Plus reverse phase (C18) column [4.6 × 250 mm; 5 μm particle size]. The mobile phase used contained methanol (solvent A) and 1% acetic acid (Solvent B). 22 The run time of each sample was 60 minutes with a flow rate of 1 mL/min. Each sample was run in triplicate to ensure validity.
GC-MS Analysis
Volatile bioactive compounds were isolated and identified through GC-MS analysis on the basis of their molecular weight, and profiling of C. colocynthis fruit organic fractions was determined by GC-MS System 23 (GC-MS model: 7890B, 5977A, Agilent USA). Inlet temperature was kept at 280 °C and sample volume of 1 μL was injected in the column (DB 5MS 30 cm, .25 mm, .25 μm). Helium was used as a carrier gas and flow rate was maintained at 1 mL/min. The temperature of the oven was programmed as 50 °C for 1min, 25 °C to 120 °C for 5min, 10 °C to 160 °C for 0min, 6 °C to 240 °C for 0min, and finally 2 °C to 290 °C for 0min. Run time was maintained at 51.133min. Chromatograms were processed for identification of volatile compounds.
Biological Activities of Active Fractions
Total polyphenol and flavonoid contents in organic fractions of C. colocynthis were determined as mg of gallic acid equivalent/g or as mg/100g dry matter. The highest values of polyphenols were recorded in ethyl acetate fraction (718.02 ± 1.89), while, minute concentrations of flavonoids were recorded in this fraction (28.654 ± .303) mg/100gm. Previous studies have suggested the presence of polyphenols in the methanolic extract of C. colocynthis other than the fruit part. A study has shown polyphenols as 740 mg of gallic acid/100g and flavonoids as 130 mg/100g of catechin in C. colocynthis extract. 24
Antioxidant Free Radical Scavenging Activity by DPPH Assay
The DPPH assay of organic fractions of C. colocynthis was measured by following the previously reported methods.21,25 For the reaction, .001 g of DPPH was dissolved into 50 mL of methanol. Next, 1 mL of DPPH solution was added in each fraction of prepared concentrations (.5, 1.0, 1.5, 2.0, 2.5, and 3.0 mg/mL) of the plant extract. Samples were incubated at 28 °C for 30 minutes and observed for color change from purple into yellowish, showing the scavenging of free radicals. Absorbance was measured at 517 nm by using a spectrophotometer. Scavenging activity was expressed as the percentage inhibition using the following formula
Scavenging Activity of Hydroxyl Radical
Scavenging activity of hydroxyl radical was performed by following the previously published method. 26 From each fraction (n-hexane, ethyl acetate, and chloroform) of C. colocynthis, concentrations ranging from .5, 1.0, 1.5, 2.0, 2.5, and 3.0 mg/mL, were separately added in a test tube. To it, .5 mL of DMSO (80%), .5 mL of iron-EDTA, .25 mL of EDTA solution, and .05 molL−1 phosphate buffer of pH 7.4, were added in each test tube. The test tubes were kept in a water bath for 10 min at 85 °C. The reaction was stopped by adding .5 mL of cold tri-chloroacetic acid (TCA). Finally, .5 mL of Nash’s reagent was added in each test tube and incubated for 10 minutes at room temperature. Absorbance was taken at 517 nm using a spectrophotometer. By using the following formula, % age inhibition was determined as:
Anti-inflammatory Activity
In-vitro anti-inflammatory activity was examined by following the previously described method. 27 Concentrations comprising 0.5, 1.0, 1.5, 2.0, 2.5, and 3.0 mg/mL were tested against diclofenac sodium as a standard drug. The absorbances were measured at 660 nm by using a UV-visible spectrophotometer. The experiment was repeated 3 times and results were compared with the standard drug. The % age of inhibition was found by using the following formula:
Antimicrobial Activity
Antimicrobial activity was performed by disc-diffusion method. 28 Each fraction was tested individually against five bacterial pathogens including Bacillus subtilis, Enterococcus faecalis, Staphylococcus aureus, E. coli, and Klebsiella pneumoniae, obtained from the Microbial Culture Bank of University of Central Punjab. Overnight grown fresh bacterial cultures were spread on nutrient agar plates. The discs (6 mm) coated with specific concentration were placed on an agar plate containing test pathogens. Discs coated with DMSO were used as a negative control. Ampicillin and Ciprofloxacin were taken as standard drug controls. Plates were incubated at 37 °C for 24 h. Antibacterial activity was assessed by measuring the zone of inhibition by comparing with standard.
Minimum Inhibitory Concentration
C. colocynthis fractions (ethyl acetate and chloroform) were assessed for minimum inhibitory effect against selected pathogens by microdilution method using resazurin-based assay. 29 In a microtitre plate, 100 μL of nutrient broth was added in each well. Following this, 100 μL of 250 mg/mL ethyl acetate fraction was added in each first well of row A-D and two-fold diluted till column 10. In each well, 20 μL of individual bacterial cultures was used as; rows A and E= E. coli, rows B and F = Klebsiella pneumoniae, rows C and G= Staphylococcus aureus, rows D and H = Bacillus subtilis. Ciprofloxacin (10 μL/mL) was taken as positive control in column 12, whereas DMSO was taken as negative control in column 11. The plate was well wrapped with aluminum foil and incubated at 37 °C for 24 hrs. Finally, 20 μL of resazurin was added in each well and re-incubated for 4 hrs for colorimetric indication. Change in color from purple to light blue was visualized, and the MIC of a compound was recorded.
Antidiabetic Activity
Antidiabetic activity was measured by α-amylase inhibition. Organic fractions of C. colocynthis (n-hexane, ethyl acetate, and chloroform) were dissolved in DMSO in concentrations ranging from 0.5, 1.0, 1.5, 2.0, 2.5, and 3.0 mg/mL. Ten-µL of α-amylase solution was supplemented with 390 μL of DMSO containing each individual concentration and kept at 37 °C in an incubator for 10 minutes. The mixture was re-incubated for one hour following mixing of 100 μL of starch solution (1%). Following incubation, .1 mL of iodine solution (1%) was added. Absorbance was observed at 565 nm using a spectrophotometer and results were compared with standard of metformin. 30 The % inhibition was determined by the given formula:
Hemolytic Activity
Hemolytic activity was examined by the method described by Shahzadi et al. (2017).31,32 Three milliliter of blood sample was centrifuged at 4000 rpm for 5 minutes following the addition of chilled phosphate buffer saline (pH 7.4) for washing of blood cells. From each organic fraction [concentration = 0.5, 1.0, 1.5, 2.0, 2.5, and 3.0 mg/mL], 100 μL was separately mixed with blood cells. Samples were incubated at 37°C for 30 minutes and chilled for 4–7 minutes and re-centrifuged for 5 minutes at 4000 rpm. Next, 100 μL of supernatant from each test tube was used and diluted ten times using cold PBS. Triton X-100 (.1%) and PBS were taken as positive control and negative control, respectively. The absorbance was recorded at 576 nm using a spectrophotometer (HALO DB-20 Dynamica). The % RBCs lysis for each sample was measured as below:
Thrombolytic Activity
As reported previously, for thrombolytic activity, pre-weighed micro-centrifuge tubes (we) were added with 0.5 mL of blood sample and incubated for 40 min at 37 °C to observe blood clotting33-35 Tubes containing clots (wm) were weighed again after removal of the serum to calculate weight of clot before lysis (wm-we). Previously prepared concentration of each organic fraction was separately added in tubes containing clots and incubated for 85 min at 37 °C for lysis of clot. Tubes were left overnight and weighted to measure the clot weight (wl) after lysis. Following lysis, difference of weight between after lysis (wl) and weight of empty tube (we) was recorded as the weight of the clot. Weight before lysis and weight after lysis were indicated (wb) and (wl), respectively. Streptokinase was taken as positive control, and distilled water was taken as negative control.
% Clot lysis was calculated by the given formula:
Cytotoxicity Assay
Hepatotoxicity assay was performed to check the toxic effects of bioactive compounds derived from C. colocynthis. 36 Swiss-albino male rats of approximately of the same age group, having weight of 110–130 g, were taken from the animal house of the University of Central Punjab, Lahore. Sixteen rats were divided in four groups as one control and three test groups. Different doses of each organic fraction were suspended in normal saline to prepare four doses of concentrations; 100 mg/kg, 200 mg/kg, 300 mg/kg, and 400 mg/kg according to animal body weight. One group was treated with n-hexane, second with ethyl acetate, and third group was treated with chloroform fraction. Group I: Control group was incorporated with 1 mL/kg normal saline as oral dose for 15 days; Group II, Group III, and Group IV were subjected to dose concentrations of 100 mg/kg, 200 mg/kg, 300 mg/kg, and 400 mg/kg of n-hexane extract, ethyl acetate, and chloroform, respectively. The dose was given orally to rats by gavage for 15 days (once a day). Blood samples of all rats were drawn, rats were slaughtered, and livers were used to examine toxicity.
Statistical Analysis
Statistical data was presented as means ± SD, and one way ANOVA was applied to observe difference between biological activities of different experimental fractions of C. colocynthis. Throughout the obtained results, *P < .05 and ***P < .001 were considered statistically significant and highly significant, respectively.
Molecular Docking
Phyto-compounds shown by GC-MS analysis were used as ligands for molecular docking analysis against COX-1 and COX-2 proteins. PubChem database was used to fetch 2D molecular structures of compounds which were converted to PDB file format using PyMOL software. The crystal structure of COX-1 (6Y3C) and COX-2 (SC-558) was retrieved from the RCSB, PDB database, whereas the (3D) structure of selected target proteins was presented by the AutoDock Vina tool. 37 Retrieved files were saved as Target.pdb., whereas Kollman partial charges and polar hydrogen atoms were inserted to the 3D structures. By using AutoDock Vina software 1.2.0, docking was performed. 38
Molecular Dynamics
The MD complexes were imposed to check any residues which may still be deformed or unstable after MD. Characterization of MD complexes such as deformability, β-factor, and co-variance analysis was performed using iMODS software. 39
ADMET Analysis
Assumption of the toxicity and pharmacokinetics of the compounds identified from C. colocynthis extract was carried out via SwissADME.40,41
Results and Discussion
Phytochemical Screening
Phytochemicals such as alkaloids, steroids, terpenoids, saponins, and tannins were confirmed in all three organic fractions (n-hexane, ethyl acetate, and chloroform). Flavonoids were present in chloroform fraction, however, found missing in ethyl acetate and n-hexane fractions. Quinones were detected in ethyl acetate and n-hexane fractions, and were absent in chloroform fraction. The results are in line with the previously published literature, where extracts of C. colocynthis fruit have shown the presence of secondary metabolites including alkaloids, steroids, terpenoids, saponins, and tannins. 21 Significant flavonoids in C. colocynthis include C-glycosides, quercitrin, iso-vitexin, iso-orientin, iso-orientin-3′methylether, C-p-hydroxybenzyl derivatives, 8-Cp-hydroxybenzoylisovitexin, 6-C-phydroxybenzoylvitexin, 8-C-p-hydroxybenzoyl, and isovitexin-4′-O-glucoside have been reported from stem, fruit, and leaf extracts of the plant. These flavonoids have shown considerable antioxidant potential and can scavenge the toxic radicals.42-44 Screening of the extracts on gas chromatography revealed the presence of albumin, ascorbic acid, β-carotenes, vicilins, tocotrienols, lutins, 2-tetradcyclobutane, and 2-dodecyclobutane. In addition, presence of various amino acids such as arginine, leucine, isoleucine, and fatty acids including palmitic acid, linoleic acid, and linolenic acid was also observed in the extract. Previously reported literature on C. colocynthis has shown the presence of these compounds through chromatographic techniques and their biological potential as antidiabetic, hepatoprotective, and anti-tumor agents. 45 The results of GC-MS of C. colocynthis fractions are summarized in Table S1.
FT-IR Analysis
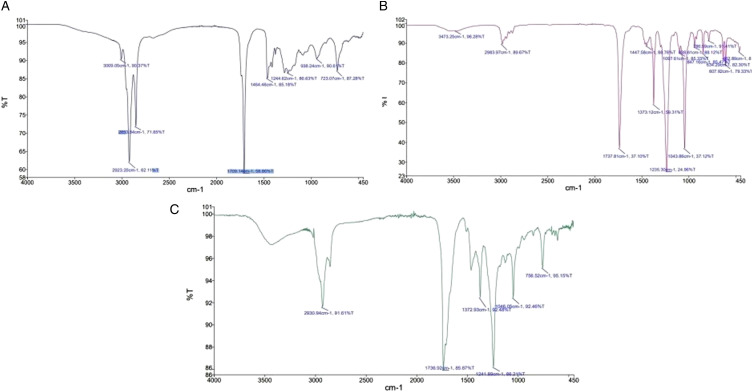
Characterization of phenolic constituents by spectroscopic analysis provides vital detail about the formulation of plant species and their motif identification. The UV-VIS spectroscopy is a simple technique used for identification and segregation of phytochemicals on the basis of polarity (lipophilic and hydrophilic molecules). Spectroscopic (FT-IR) analysis can thus be used for the analysis of phytoconstituents of the plant extracts. 20 For the structural elucidation and identification of biologically active compounds, FT-IR, which is a non-destructive analytical technique, was used offering a prompt analysis. 46 The results of FT-IR analysis revealed the presence of alkaloids (N-H stretching), polyphenols, flavonoids (O-H stretching), and terpenes (CH group). 47 The FT-IR spectrum verified the existence of alkanes, alkynes, phenols, alcohols, alkyl halides, aldehydes, carboxylic acids, and amines in C. colocynthis. When previously studied through FT-IR for functional groups analysis, C. colocynthis has shown the abovementioned compounds with broad-spectrum bioactive potential. The FT-IR analysis in this study served as the confirmational tool to re-evaluate the presence of these functional groups in organic fractions.43,48 The FT-IR spectrum profile of n-hexane, ethyl acetate, and chloroform fraction has been illustrated in (Figure 1, Table S2).
Figure 1.
FT-IR Spectrum of methanolic fractions of C. colocynthis.
Total Phenolic and Flavonoid Content
Total phenolic and flavonoid content in C. colocynthis fractions is shown in Table 1. The highest concentration of phenolics was observed in ethyl acetate fraction (718.02 ± 1.89 mg/100 gm) followed by chloroform and n-hexane fractions. The highest quantity of flavonoids was estimated in ethyl acetate fraction (28.654 ± 0.303 mg/100 gm), whereas, least quantity was present in n-hexane fraction. Previous studies on C. colocynthis extracts have shown the presence of polyphenols and flavonoids which was also confirmed in this research. 43 A similar study was conducted on the extract of C. colocynthis, which showed 10.7 + .1 mg/g of total flavonoid content. 49 The total amount of phenolic compounds present in the methanolic extract of C. colocynthis was found to be 58.76 mg/g. Also, the flavonoids content of C. colocynthis extract was determined and found to be 2.01 mg/g and .13 mg/g of rutin equivalent, respectively. 45
Table 1.
Total polyphenol and flavonoid content.
| Fractions | Mean ± S.D TFC as CE mg/100 gm) | Mean ± S.D TPC as CE mg/100 gm) |
|---|---|---|
| n-Hexane | 15.142 ± 0.148 | 146 ± 1.89 |
| Ethyl acetate | 28.654 ± 0.303 | 718.02 ± 1.89 |
| Chloroform | 17.637 ± 0.084 | 186.24 ± 0.145 |
HPLC Analysis
A detailed phytochemical characterization of the organic fractions (n-hexane, chloroform, and ethyl acetate) of the methanolic extracts of C. colocynthis fruit confirmed the existence of phenolic and flavonoid metabolites such as quercetin, gallic acid, vanillic acid, chlorogenic acid, syringic acid, m-coumaric acid, p-coumaric acid, ferulic acid, benzoic acid, cinnamic acid, and sinapic acid. These compounds have been previously explored in C. colocynthis fruit extract and were demonstrated for biological potential in previous investigations.50,51 Dominant compounds observed through HPLC analysis were caffeic acid, vanillic acid, and benzoic acid. The highest concentration of vanillic acid (173.3708 ppm), caffeic acid (32.3764 ppm), and benzoic acid (129.5395 ppm) was observed in n-hexane fraction followed by ethyl acetate and chloroform fractions, respectively (Figure 2, Table S3). One of the recent studies on C. colocynthis in Alexandria showed the presence of benzoic acid, rhamnetin, isoquercetin, hydroxycaffeic acids, and caffeic acid in several organic fractions, however, the highest quantities of caffeic acid (32.3764 ppm) in this study are exceptional as compared to previous (10.3764 ppm), where its presence was shown to be minimal.52,53
Figure 2.
HPLC chromatogram methanolic fractions of C. colocynthis.
GC-MS Analysis
Outcome of GC-MS analysis showed the presence of valuable volatile biologically active compounds in all active fractions of C. colocynthis. Bioactive constituents identified in n-hexane fraction included 9,12-octadecadienoic acid (Z, Z)-methyl ester (20.78%) and linoleic acid ethyl ester (20.58%) which were in significantly higher concentration. Eleven different metabolites were quantified in ethyl acetate fraction, and among all constituents, linoleic acid ethyl ester (26.71%) and 9,12-octadecadienoic acid (Z, Z)-, methyl ester (26.63%) were significant. In chloroform fraction, linoleic acid ethyl ester (45.51%) and 9,12-octadecadienoic acid (Z, Z)-, methyl ester (21.73%) were noted in significant amounts (Figure 3 and Table S4).
Figure 3.
GC-MS chromatogram methanolic fractions of C. colocynthis.
Biological Activities of C. colocynthis Methanolic Extract
Antioxidant Activity
The free radical scavenging activity was performed by DPPH assay to quantify the percentage inhibitory effect of bioactive compounds. The quotients of antioxidant activity are shown in Table 2. Results suggested that ethyl acetate fraction gave the highest DPPH scavenging activity among all the tested fractions with the value of 76 ± 0.769% at concentration of 3 mg/mL. It was observed that by increasing the concentration from 0.5 mg/mL to 3 mg/mL, DPPH scavenging activity was significantly increased. IC 50 values of n-hexane, ethyl acetate, and chloroform fractions were 2.29 mg/mL, 1.72 mg/mL, and 2.06 mg/mL, respectively. Previous studies have shown that C. colocynthis possesses antioxidant potential and related biological activities. DPPH scavenging activity of the organic leaf extract of C. colocynthis has been reported as 88.0 ± 2.7% at concentration of 250 mg/mL. 26 DPPH analysis of some other medicinal plants has demonstrated the highest IC 50 values of R. stricta and M. crassifolia as 0.335 and 0.448 mg/mL, respectively. 54 A recent analysis of C. colocynthis showed that the ethanolic extract of roots and leaves had high free radical inhibition activity which was 36.52 and 33.83%, respectively. 21 Nonetheless, in this research study, the values of antioxidant activity of all three organic fractions were considerably high as compared to previously reported ones.
Table 2.
Antioxidant activity of methanolic fractions of C. colocynthis by DPPH and H2O2 assay.
| Fractions | Conc. mg/mL | DPPH Scavenging % | H2O2 Scavenging % | IC 50 (mg/mL) | |
|---|---|---|---|---|---|
| DPPH | H2O2 | ||||
| n-Hexane | 0.5 | 11 ± 0.050 | 9 ± 0.103 | 2.22 | 2.40 |
| 1 | 24 ± 0.234 | 21 ± 0.275 | |||
| 1.5 | 32 ± 0.367 | 27 ± 0.219 | |||
| 2 | 45 ± 0.388 | 42 ± 0.465 | |||
| 2.5 | 54 ± 0.514 | 53 ± 0.514 | |||
| 3 | 64 ± 0.693 | 59 ± 0.486 | |||
| Ethyl acetate | 0.5 | 22 ± 0.241 | 18 ± 0.168 | 1.86 | 2.03 |
| 1 | 29 ± 0.218 | 27 ± 0.242 | |||
| 1.5 | 41 ± 0.366 | 40 ± 0.345 | |||
| 2 | 54 ± 0.485 | 49 ± 0.485 | |||
| 2.5 | 63 ± 0.571 | 57 ± 0.529 | |||
| 3 | 76 ± 0.769 | 72 ± 0.612 | |||
| Chloroform | 0.5 | 12 ± 0.109 | 11 ± 0.050 | 2.22 | 2.47 |
| 1 | 27 ± 0.251 | 19 ± 0.101 | |||
| 1.5 | 39 ± 0.305 | 33 ± 0.301 | |||
| 2 | 46 ± 0.415 | 43 ± 0.540 | |||
| 2.5 | 55 ± 0.503 | 50 ± 0.514 | |||
| 3 | 65 ± 0.594 | 62 ± 0.634 | |||
| Ascorbic acid | 1 | 91 ± 0.878 | 82 ± 0.792 | ||
Hydrogen Peroxide Activity
Free radicals (H2O2) scavenging assay was performed by using various concentrations of methanolic fractions of C. colocynthis as described in Table 2. The ethyl acetate fraction showed strong scavenging activity (72 ± 0.612%) at the concentration of 3.0 mg/mL, whereas chloroform and n-hexane fraction showed 62 ± 0.634% and 59 ± .486% scavenging activities, respectively. The significant difference in percentage inhibition of H2O2 of all fractions was noted. H2O2 is produced in the human body in various metabolic processes and may also exist in air and water vapors in response to redox reactions. Free radicals of H2O2 decompose into reactive oxygen species (ROS) which initiate the lipid peroxidation and consequently desecration of DNA. The ethyl acetate fraction of C. colocynthis was capable to scavenge hydrogen peroxide in the presence of phenolic compounds which donates the electrons to the peroxides and neutralize it into water. 55
Anti-inflammatory Activity
Results of denaturation of BSA have been summarized in Table 3. In-vitro anti-inflammatory activity was determined by comparing the results with standard diclofenac sodium to check biopotential of secondary metabolites found in fractions of C. colocynthis. n-hexane, ethyl acetate, and chloroform fractions of C. colocynthis with various concentrations in the range of 0.5 mg/mL–3 mg/mL were used. The maximum inhibitory activity was recorded at a concentration of 3 mg/mL of ethyl acetate fraction (40 ± 0.473%), followed by chloroform (39 ± 0.336%), and n-hexane fraction (37 ± 0.413%). As reported by a previous study, the anti-inflammatory potential of phenolic compounds such as vanillic acid and quercetin in C. colocynthis was explored. The data showed that compounds were involved in cure, and demonstrated anti-inflammatory, antimicrobial, antioxidant, and antidiabetic activities. 56 Major cause of auto-antigens in several arthritic disorders might be due to protein denaturation. 57 Denaturation of proteins are known to be inhibited by anti-inflammatory antidots which have been significantly found in organic extracts of C. colocynthis. Moreover, besides showing anti-inflammatory activities, some of these compounds additionally possess antimicrobial potential. Increasing antibiotic resistance and because of evolving pathogens, existing antimicrobials are losing their efficacy. Hence, several therapeutic compounds from medicinal plants have been tested as an alternative source of antimicrobials for pathogenic microbes because of low side-effects and cost-effectiveness. 58 C. colocynthis, a medicinal plant, simultaneously possesses therapeutic, antimicrobial, and anti-inflammatory potential and can be a preferred choice to fight microbial and inflammatory diseases. 11
Table 3.
Anti-inflammatory, antidiabetic, hemolytic, and thrombolytic effect of methanolic fractions C. colocynthis fruit.
| Fractions | Conc. mg/mL | Anti-inflammatory% Inhibition | Antidiabetic % Inhibition | % Hemolysis | % Thrombolysis |
|---|---|---|---|---|---|
| n-Hexane | 0.5 | 11 ± 0.112 | 8 ± 0.007 | .41 ± 0.343 | 0.35 |
| 1 | 12 ± 0.106 | 14 ± 0.137 | 0.82 ±0 .055 | 0.7 | |
| 1.5 | 22 ± 0.212 | 33 ± 0.326 | 1.16 ± 0.195 | 0.7 | |
| 2 | 26 ± 0.286 | 39 ± 0.321 | 1.68 ± 0.577 | 1.4 | |
| 2.5 | 31 ± 0.369 | 45 ± 0.419 | 2.24 ± 0.967 | 2.45 | |
| 3 | 37 ± 0.413 | 55 ± 0.528 | 2.26 ±0 .945 | 3.85 | |
| Ethyl acetate | 0.5 | 13 ± 0.102 | 13 ± 0.137 | 0.34 ± .092 | 1.4 |
| 1 | 13 ± 0.160 | 23 ± 0.220 | 1.05 ± 0.140 | 2.1 | |
| 1.5 | 21 ± 0.247 | 37 ± 0.329 | 1.80 ± 0.092 | 4.91 | |
| 2 | 27 ± 0.286 | 51 ± 0.547 | 2.40 ± 0.140 | 8.07 | |
| 2.5 | 32 ± 0.321 | 65 ± 0.526 | 2.92 ± 0.092 | 11.22 | |
| 3 | 40 ± .0473 | 77 ± 0.844 | 3.37 ± 0.159 | 13.68 | |
| Chloroform | 0.5 | 9 ± 0.052 | 8 ± 0.134 | 0.37 ± 0.367 | 0.7 |
| 1 | 13 ± 0.189 | 16 ± 0.086 | 0.52 ± 0.103 | 1.4 | |
| 1.5 | 21 ± 0.237 | 30 ± 0.313 | 0.99 ± 0.266 | 1.4 | |
| 2 | 26 ± 0.236 | 39 ± 0.359 | 1.51 ±0 .594 | 1.75 | |
| 2.5 | 31 ± 0.310 | 44 ± 0.423 | 1.97 ± 0.885 | 2.45 | |
| 3 | 39 ± 0.336 | 57 ± 0.573 | 2.23 ± 0.992 | 4.91 | |
| Diclofenac Na | 1 | 84.8 ± 0.059 | — | — | — |
| Metformin | 1 | — | 91 ± 1 | — | — |
| Triton X-100 | 1 | — | — | 89 ± 1 | — |
| Streptokinase | 1 | — | — | — | 86 |
Antidiabetic Activity
Results showed that methanolic fraction of C. colocynthis contained active phytochemicals, which were characterized by various spectroscopic analyses, and validated for antidiabetic potential through α-amylase assay. The highest inhibition of α-amylase was noted by ethyl acetate fraction (77 ± 0.844%), followed by chloroform fraction (57 ± 0.573%), and n-hexane fraction (55 ± 0.528%). A previous experimental work done to evaluate antidiabetic potential by reduction of enzyme α-amylase and α-glucosidase showed significant antidiabetic potential of C. colocynthis. 59 Studies showed that an in-vitro α-glucosidase inhibition assay indicated marked inhibition of α-glucosidase by C. colocynthis extract at 355.5 μg/mL concentration. 15 Both of these enzymes play a significant role to increase the blood glucose levels, and lead to diabetes. Results of this study also confirm the antidiabetic activities of all organic fractions and demonstrated that the antidiabetic effect subsequently increased with the increase in the concentration. This study along with previously reported literature provides the scientific basis to use the whole fruit of C. colocynthis as a drug against hyperglysemia and dyslipidemia (Table 3).
Hemolytic Activity
At the highest concentration of 3 mg/mL, n-hexane, ethyl acetate, and chloroform fractions showed 2.26 ± 0.945%, 3.37± 0.159%, and 2.23 ± 0.992% hemolysis of blood cells, respectively. The reference Triton X-100 showed 89 ± 1% blood cell lysis. This data indicates that ethyl acetate fraction showed the significant blood cell lysis followed by n-hexane and chloroform fractions. A similar study was performed to test hemolytic potential of various medicinal plants and showed that the methanolic extract of E. officinalis exhibited least hemolytic activity (5.89 ± 0.78%), whereas T. chebula exhibited highest percent hemolysis (13.12 ± 1.2%). 60 However, the hemolytic activity of C. colocynthis extract is in safe range and does not affect its therapeutic potential (Table 3). The methanolic extract of E. officinalis exhibited the highest radical scavenging potential with percent inhibition of 90.11 ± 7.2 than the other tested methanolic extracts of selected medicinal plants in that study. 60 While the lowest percent inhibition of free radical production was shown by methanolic extract with an average ± SD value of 63.23 ± 5.8%. 60
Thrombolytic Activity
Highest thrombolytic activity was shown by ethyl acetate fraction with the values of 13.68% followed by chloroform (4.91%) and n-hexane (3.85%). The reference streptokinase showed 86% clot lysis at 30,000 IU concentration. This data indicates that ethyl acetate fraction showed the significant clot lysis demonstrating the potential of C. colocynthis fruit as a good choice as thrombolytic agent. A previous study on in-vitro thrombolytic activity of various medicinal plants using methanolic extracts of Buchholzia coriaceae (27.062%) at a dose concentration of 100 μg/mL highlighted their thrombolytic potential; however, there are less reports on the thrombolytic potential of C. colocynthis. 61 Thrombolysis potential of medicinal plants suggest that these plants can be employed for remedial purposes and have advantages over traditional therapeutic drugs. Besides thrombolysis potential, anticoagulant activity of plant extracts has also been evaluated by the PT, INR, and PTT assays, using normal human plasma and showed promising results complementing their therapeutic roles and can be a future research prospect for C. colocynthis.
Antimicrobial Activity
Antimicrobial effect of (n-hexane, ethyl acetate, and chloroform) fractions of C. colocynthis fruit against five microbial strains (Enterococcus faecalis, Bacillus subtilus, Staphylococcus aureus, Escherichia coli, and Klebsiella pneumoniae) was observed. Ethyl acetate fraction showed the highest zone of inhibition against K. pneumoniae (19.63 ± .32 mm). The other fractions (n-hexane and chloroform) exhibited comparably less antimicrobial potential, whereas Enterococcus faecalis showed resistance against n-hexane fraction. K. pneumoniae showed resistance against chloroform fraction. The results have been illustrated in Figure S1 and Table S7. Organic fractions of C. colocynthis fruits have shown significant antimicrobial effects in previous research studies, and hence, the said plant can be used against several disease-causing microbes. Previously, the presence of phenolics such as quercetin, myricetin, glycosides, and terpenoids has been shown in several medicinal plants by biochemical profiling of leaves. 62 Hence, these phenolic compounds can be the potential reason for showing antimicrobial activities. Similar experimental work was performed using C. colocynthis organic fractions to assess the antimicrobial activity against different strains. 63 In that, n-hexane and ethyl acetate fraction showed its effect only against B. cereus, L. monocytogenes, and S. aureus, whereas butanol and methanol showed no activity against the bacterial strains. Maximum antimicrobial activity was observed using chloroform fraction which was selected to isolate the active compounds of C. colocynthis. Highest zone of inhibition against A. fumigatus of 11 mm (a 25 mg/1mL DMSO) and 21 mm (at 100 mg/1 mL DMSO) was observed. The highest zone of inhibition of seed extract of C. colocynthis against B. subtilis was noted as 11 mm (at 25 mg/1 mL DMSO) and 22 mm (at 100 mg/1 mL DMSO). Highest zone of inhibition against F. oxysporum was 15 mm (at 25 mg/1mL DMSO) and 21 mm (at 100 mg/1 mL DMSO). Both of strains showed greater zones of inhibition by increasing the concentrations of the extracts. Gram-negative bacteria Pseudomonas aeruginosa showed sensitivity against the ethanolic extract of C. colocynthis contrary. 64
Minimum Inhibitory Concentration
MIC was assessed against two Gram positive (S. aureus and B. subtilis) and two Gram-negative bacteria (E. coli and K. pneumonia) using ethyl acetate and chloroform fraction of the methanolic extract. MIC of ethyl acetate fraction was observed as 125 μg/mL, 62.5 μg/mL, 31.25 μg/mL, and 31.25 μg/mL against E. coli, K. pneumoniae, S. aureus, and B. subtilis, respectively. Similarly, MIC values of chloroform fraction of the methanolic extract were assessed as 250 μg/mL, 125 μg/mL, and 125 μg/mL for E. coli, S. aureus and B. subtilis, respectively. K. pneumoniae indicated resistance against chloroform fraction. Ciprofloxacin was taken as positive control that exhibits MIC against all tested pathogens except K. pneumoniae. According to previous studies, the extract of C. colocynthis fruits exhibited .10 mg/mL MIC against Candida albicans, .20 mg/mL MIC against E. coli, and .23 mg/mL MIC against P. aeruginosa. 63 Similarly, another research conducted using C. colocynthis extract showed MIC of less than 2 mg/mL against B. cereus and B. subtilis which the lowest MIC values (.5 mg/mL and 2.0 mg/mL, followed by Micrococcus spp. (1.5 mg/mL) and S. aureus (2.0 mg/mL), respectively. 65
Cytotoxicity Assay
Hepatotoxicity was performed to confirm the biopotential of natural compounds derived from C. colocynthis. It was observed that ethyl acetate fraction showed the promising profile of biologically active compounds among the selected fractions of the methanolic extract. Cytotoxic effect of secondary metabolites was observed by performing histopathological examination of liver tissues of albino rats, which showed minor changes in the liver tissues such as ballooning, fatty droplets, and accumulation of extracellular matrix at various concentration of ethyl acetate, proving that bioactive compounds in ethyl acetate fraction have no toxic effect. Furthermore, studies indicate that ethyl acetate fraction has good concentration of antioxidants which may impart hepatoprotective effect in reducing free radicals. So, it is inferred that all biologically active compounds found in ethyl acetate fractions of C. colocynthis are safe to be used (Figure 4). The ethanolic extract possesses a promising antioxidant power with an IC50 value of 4.56 mg/mL. These results are highly justified by the presence of bioactive compounds in the plant extract characterized with a GC-MS, which showed the presence of polyphenols and flavonoids as responsible agents for the antioxidant activity. Iso-orientin 3-O-methylether, isosaponarin, and isovitexin isolated from C. colocynthis possess a strong antioxidant power with an IC50 value ranging from 5.62 × 10−4 to 7.13 × 10−2 mg/mL. 66
Figure 4.
Hepatoprotective effect of ethyl acetate fractions of C. colocynthis.
Molecular Docking
Deformability, β-Factor, and Co-variance Computations
The major deformability was observed in the stigmasta-7,16-dien-3-ol, (3. beta. ,5. alpha) of COX-1 and COX-2 complex and had many peaks of approximate value 1.0 deformability index. The results of MD are showed in Figure 5 that demonstrates the normal mode analysis (NMA) of stigmasta-7,16-dien-3-ol, (3. beta. ,5. alpha) of COX-1 and COX-2 complex. The graph of deformability (5A) of complexes of COX-1 and COX-2 showed the peaks in the graph correspond to the deformability region of protein. Comparison of NMA and PDB field has been illustrated in the β-factor graph in Figure 5(B). Variance, co-variance, residue index, and atom index have been shown in Figures 5C-5F. Co-variance provided sufficient results at elastic network map.
Figure 5.
Molecular dynamics of COX-1 and COX-2. (A) Molecular dynamics of stigmasta-7,16-dien-3-ol, (3. beta. ,5. alpha.) showed the docking compound. (B) presents a low level of deformation of all residues. (C) shows the beta factor. (D) Eigon values are shown. (E) shows the variance explained in both red and green. (F) shows the variance and elastic of complex.
ADMET Analysis
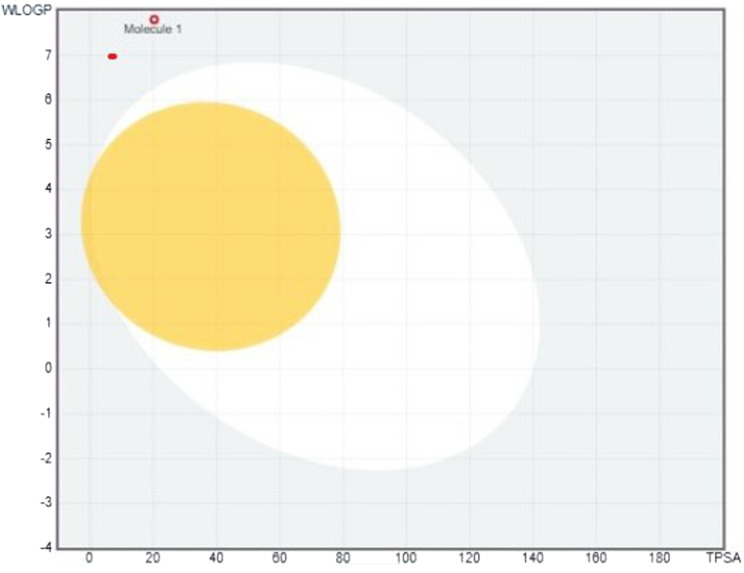
SwissADME analysis showed that stigmasta-7,16-dien-3-ol, (3. beta. ,5. alpha.) is the good anti-inflammatory compound. It had fair lipophilicity, Lop P greater than 4.94, and was slightly water soluble. Physiochemical properties showed that it had a molecular formula of C29H48O and molecular weight of 412.69 g/mol. The pharmacokinetic analysis gave moderate GI absorption and did not violate Lipinski’s rule. Compounds interaction and pharmacokinetics evaluated low glycoprotein, permeability, and no inhibitory effect on CYP2C19 and CYP2C9. The boiled-egg image of stigmasta-7,16-dien-3-ol, (3. beta. ,5. alpha.) complex is presented in Figure 6.
Figure 6.
SWISS ADMET analysis of Stigma sterol. The yolk indicates the blood-brain barrier and the white area indicates gastrointestinal absorption.
Molecular Docking
For the preparation of binding site, PDB format of the drug was used and AutoDock Vina was used for virtual screening. Molecular docking was performed to visualize interaction between ligands and target proteins following the assessment of their binding affinities. The binding energies are demonstrated in Table S8 and Figure S2. The top two ligands with the least binding energy were hexadecanoic acid methyl ester and 9,12-octadecadienoic acid (Z, Z)-, methyl ester, showing scores of −4.9 and −5.1. The highest binding energy was shown by stigmasta-7,16-dien-3-ol, (3. beta. ,5. alpha), which was −7.5 and −8.4, 10 E,12(Z)-conjugated linoleic acid −7 and −6.5 was the most effective inhibitor. Further molecular simulation (MD) of top compounds with COX-1 and COX-2 was performed by iMOD simulation which showed the stability of the docked complexes between the phytochemicals and protein through strong hydrogen bonding. 67 According to the ADMET analysis and drug-like characteristics of the molecules as describe above, GIT bioavailability of the molecules is moderate, however, they are impermeable through the blood-brain barrier (BBB). An efficient drug possesses six physicochemical characteristics such as polarity, flexibility, lipophilicity, saturation, size, and solubility. 68 The In-silico data also indicated that stigmasta-7,16-dien-3-ol, (3. beta. ,5. alpha) might be good in anti-inflammation.
Conclusions
This study summarizes the biological activities of C. colocynthis fruits, and a comprehensive analysis of phytochemicals such as phenolics, flavonoids, and primary and secondary metabolites. Organic fractions of methanolic extract showed significant antioxidant, antimicrobial, antidiabetic, and anti-inflammatory activities. Hepatoprotective effect was evaluated by using animal modeling test which showed its safety to be used as medicine. The research comprehends the previously reported literature with new evidences and can be a potential cure for the local patients and pharmaceutical industries. The aforesaid study appraised that the fruit of C. colocynthis has potential for diverse biological activities and can be used to cure various ailments and may be used in future to design different drugs by in-silico approach.
Supplemental Material
Supplemental Material, sj-pdf-1-dos-10.1177_15593258231187357 for Pharmacological Activities and In-Silico Studies of Bioactive Compounds Identified in Organic Fractions of the Methanolic Extract of Citrullus Colocynthis by Haseeb Akram Sindhu, Muhammad Afzal, and Izzah Shahid in Dose-Response
Acknowledgments
The authors are highly thankful to the Textile Testing Industry (TTI) of Lahore, Pakistan, for providing the facility to carry out FT-IR and GC-MS analysis.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Haseeb Akram Sindhu https://orcid.org/0000-0001-5472-1186
Muhammad Afzal https://orcid.org/0000-0002-2559-3450
References
- 1.Van Wyk B-E, Wink M. Medicinal Plants of the World. 1st ed. Wallingford, UK: CABI; 2018:362. [Google Scholar]
- 2.Marzouk B, Refifà M, Montalbano S, et al. Vitro Sprouted Plantlets of Citrullus colocynthis (L.) Schrad Shown to Possess Interesting Levels of Cucurbitacins and Other Bioactives against Pathogenic Fungi. Plants 2022;11:2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roy A, Ahuja S, Bharadvaja N. A review on medicinal plants against cancer. Journal of Plant Sciences and Agricultural Research. 2017;2:1-8. [Google Scholar]
- 4.Abdulridha MK, Al-Marzoqi AH, Al-Awsi GRL, Mubarak SM, Heidarifard M, Ghasemian A. Anticancer effects of herbal medicine compounds and novel formulations: A literature review. J Gastrointest Cancer. 2020;51:765-773. [DOI] [PubMed] [Google Scholar]
- 5.Ghasemian A, Eslami M, Hasanvand F, Bozorgi H, Al-Abodi HR. Eucalyptus camaldulensis properties for use in the eradication of infections. Comp Immunol Microbiol Infect Dis 2019;65:234-237. [DOI] [PubMed] [Google Scholar]
- 6.Al-Nablsi S, El-Keblawy A, Ali MA, et al. Phenolic contents and antioxidant activity of Citrullus Colocynthis fruits, growing in the hot arid desert of the UAE, influenced by the fruit parts, accessions, and seasons of fruit collection. Antioxidants 2022;11:656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehra N, Tamta G, Nand V. A review on nutritional value, phytochemical and pharmacological attributes of Foeniculum vulgare Mill. J Pharmacogn Phytochem 2021;10:1255-1263. [Google Scholar]
- 8.De Silva GO, Abeysundara AT, Aponso MMW. Extraction methods, qualitative and quantitative techniques for screening of phytochemicals from plants. American Journal of Essential Oils and Natural Products. 2017;5:29-32. [Google Scholar]
- 9.Shahin-Kaleybar B, Niazi A, Afsharifar A, et al. Isolation of cysteine-rich peptides from Citrullus colocynthis. Biomolecules 2020;10:1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdulridha MK, Al-Marzoqi A-H, Ghasemian A. The anticancer efficiency of Citrullus colocynthis toward the colorectal cancer therapy. J Gastrointest Cancer 2020;51:439-444. [DOI] [PubMed] [Google Scholar]
- 11.Hussain AI, Rathore HA, Sattar MZ, Chatha SA, Sarker SD, Gilani AH. Citrullus colocynthis (L.) Schrad (bitter apple fruit): A review of its phytochemistry, pharmacology, traditional uses and nutritional potential. J Ethnopharmacol 2014;155:54-66. [DOI] [PubMed] [Google Scholar]
- 12.Chekroun E, Benariba N, Adida H, Bechiri A, Azzi R, Djaziri R. Antioxidant activity and phytochemical screening of two Cucurbitaceae: Citrullus colocynthis fruits and Bryonia dioica roots. Asian Pacific Journal of Tropical Disease 2015;5:632-637. [Google Scholar]
- 13.Karimabad MN, Niknia S, Golnabadi MB, Poor SF, Hajizadeh MR, Mahmoodi M. Effect of Citrullus colocynthis extract on glycated hemoglobin formation (in vitro). The Eurasian Journal of Medicine 2020;52:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaudhury A, Duvoor C, Reddy Dendi VS, et al. Clinical review of antidiabetic drugs: implications for type 2 diabetes mellitus management. Front Endocrinol. 2017;8:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghauri AO, Ahmad S, Rehman T. In vitro and in vivo anti-diabetic activity of Citrullus colocynthis pulpy flesh with seeds hydro-ethanolic extract. J Compl Integr Med 2020;17:34. [DOI] [PubMed] [Google Scholar]
- 16.Shi C, Karim S, Wang C, Zhao M, Murtaza G. A review on antidiabetic activity of Citrullus colocynthis Schrad. Acta Pol Pharm 2014;71:363-367. [PubMed] [Google Scholar]
- 17.Soni A, Sosa S. Phytochemical analysis and free radical scavenging potential of herbal and medicinal plant extracts. J Pharmacogn Phytochem. 2013;2:22-29. [Google Scholar]
- 18.Riaz M, Rasool N, Bukhari IH, et al. In vitro antimicrobial, antioxidant, cytotoxicity and GC-MS analysis of Mazus goodenifolius. Molecules 2012;17:14275-14278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tlili H, Hanen N, Ben Arfa A, et al. Biochemical profile and in vitro biological activities of extracts from seven folk medicinal plants growing wild in southern Tunisia. PLoS One. 2019;14:e0213049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dhivya S, Kalaichelvi K. UV-Vis spectroscopic and FTIR analysis of Sarcostemma brevistigma, wight. and arn. International Journal of Herbal Medicine. 2017;9:46-49. [Google Scholar]
- 21.Ahmed M, Ji M, Qin P, et al. Phytochemical screening, total phenolic and flavonoids contents and antioxidant activities of Citrullus colocynthis L. and Cannabis sativa L. Appl Ecol Environ Res. 2019;17:6961-6979. [Google Scholar]
- 22.Mradu G, Saumyakanti S, Sohini M, Arup M. HPLC profiles of standard phenolic compounds present in medicinal plants. International Journal of Pharmacognosy and Phytochemical Research. 2012;4:162-167. [Google Scholar]
- 23.Cardenia V, Toschi TG, Scappini S, Rubino RC, Rodriguez-Estrada MT. Development and validation of a Fast gas chromatography/mass spectrometry method for the determination of cannabinoids in Cannabis sativa L. Journal of Food and Drug Analysis. 2018;26:1283-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar S, Kumar D, Saroha K, Singh N, Vashishta B. Antioxidant and free radical scavenging potential of Citrullus colocynthis (L.) Schrad. methanolic fruit extract. Acta Pharm. 2008;58:215-220. [DOI] [PubMed] [Google Scholar]
- 25.Dhanani T, Shah S, Gajbhiye N, Kumar S. Effect of extraction methods on yield, phytochemical constituents and antioxidant activity of Withania somnifera. Arab J Chem. 2017;10:S1193. [Google Scholar]
- 26.Gurudeeban S, Satyavai K, Ramanathan T. Antioxidant and radical scavenging effect of Citrullus colocynthis. Inven Impact Nutraceuticals. 2010;1:145. [Google Scholar]
- 27.Williams L, O'connar A, Latore L, et al. The in vitro anti-denaturation effects induced by natural products and non-steroidal compounds in heat treated (immunogenic) bovine serum albumin is proposed as a screening assay for the detection of anti-inflammatory compounds, without the use of animals, in the early stages of the drug discovery process, W Indian Med J 2008;57:35. [PubMed] [Google Scholar]
- 28.Riaz T, Abbasi MA, Shazadi T, Shahid M. Assessment of Fumaria indica, Dicliptera bupleuroides and Curcuma zedoaria for their antimicrobial and hemolytic effects. Pak J Pharm Sci. 2019;32:697-703. [PubMed] [Google Scholar]
- 29.Rafiq MA, Shahid M, Jilani K, Aslam MA. Antibacterial, antibiofilm, and anti-quorum sensing potential of novel synthetic compounds against pathogenic bacteria isolated from chronic sinusitis patients. Dose Response. 2022;20:15593258221135731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vyas M. Nutritional profile of spinach and its antioxidant & antidiabetic evaluation. Int J Green Pharm. 2017;1:11. [Google Scholar]
- 31.Shahzadi T, Zaib M, Riaz T, Shehzadi S, Abbasi MA, Shahid M. Synthesis of eco-friendly cobalt nanoparticles using Celosia argentea plant extract and their efficacy studies as antioxidant, antibacterial, hemolytic and catalytical agent. Arabian J Sci Eng. 2019;44:6435-6444. [Google Scholar]
- 32.Rubab K, Abbasi MA, Siddiqui SZ, et al. Synthesis, pharmacological screening and computational analysis of some 2-(1H-Indol-3-yl)-N'-[(un) substituted phenylmethylidene] acetohydrazides and 2-(1H-Indol-3-yl)-N'-[(un) substituted benzoyl/2-thienylcarbonyl] acetohydrazides. Pak J Pharm Sci. 2017;30:1263-1275. [PubMed] [Google Scholar]
- 33.Noreen A, Hussain F, Shahid M. Insights on the antioxidant, antidiabetic, antiamnesic, cytotoxic, thrombolytic and antibiofilm activities of Stevia rebaudiana leaves. Prog Nutr. 2020;22:e2020027. [Google Scholar]
- 34.Nighat F, Mushtaq Z, Maqsood M, Shahid M, Hanif MA, Jamil A. Cytotoxic, a-amylase inhibitory and thrombolytic activities of organic and aqueous extracts of Bacillus clausii KP10. Pak J Pharm Sci. 2020;33. [PubMed] [Google Scholar]
- 35.Khan SG, Bokhari TH, Anjum F, et al. Synthesis, Characterization, Antibacterial, Hemolytic and Thrombolytic Activity Evaluation of 5-(3-chlorophenyl)-2-((N-(substituted)-2-acetamoyl) Sulfanyl)-1, 3, 4-oxadiazole Derivatives. Pakistan J Pharmaceutical Sciences 2020. Online ahead of print. [PubMed] [Google Scholar]
- 36.Vakiloddin S, Fuloria N, Fuloria S, Dhanaraj SA, Balaji K, Karupiah S. Evidences of hepatoprotective and antioxidant effect of Citrullus colocynthis fruits in paracetamol induced hepatotoxicity. Pak J Pharm Sci. 2015;28:951-957. [PubMed] [Google Scholar]
- 37.Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31:455-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gul F, Khan I, Iqbal J, et al. Phytochemistry, biological activities and in silico molecular docking studies of Oxalis pes-caprae L. compounds against SARS-CoV-2. J King Saud Univ Sci. 2022;34:102136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qazi S, Das S, Khuntia BK, et al. silico molecular docking and molecular dynamic simulation analysis of phytochemicals from Indian foods as potential inhibitors of SARS-CoV-2 RdRp and 3CLpro. Nat Prod Commun. 2021;16:1934578X211031707.In. [Google Scholar]
- 40.http://www.swissadme.ch/. SwissADME. 2022.
- 41.Awadelkareem AM, Al-Shammari E, Elkhalifa AEO, et al. Phytochemical and In Silico ADME/Tox Analysis of Eruca sativa Extract with Antioxidant, Antibacterial and Anticancer Potential against Caco-2 and HCT-116 Colorectal Carcinoma Cell Lines. Molecules. 2022;27:1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Q-Y, Munawar M, Saeed M, et al. Citrullus colocynthis (L.) Schrad (Bitter Apple Fruit): Promising traditional uses, pharmacological effects, aspects, and potential applications. Front Pharmacol. 2022;12:3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Benariba N, Djaziri R, Bellakhdar W, et al. Phytochemical screening and free radical scavenging activity of Citrullus colocynthis seeds extracts. Asian Pac J Trop Biomed. 2013;3:35-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thangavel P, Ramasamy RK. Phytochemical screening and antibacterial and antifungal activity of the stem, leaf and fruit extracts using different solvent of Citrullus colocynthis (L.) Schrad. J Pharmacogn Phytochem. 2019;8:189-192. [Google Scholar]
- 45.Owoade AO, Adetutu A, Olorunnisola OS, Ayinde KS. The in-vitro antioxidant properties and phytochemical constituents of Citrullus colocynthis methanolic extract. Elixir Appl Botany. 2018;121:51556-51562. [Google Scholar]
- 46.Kumar JK, Prasad AD. Identification and comparison of biomolecules in medicinal plants of Tephrosia tinctoria and Atylosia albicans by using FTIR. Rom J Biophys. 2011;21:63-71. [Google Scholar]
- 47.Sahu N, Saxena J. Phytochemical analysis of Bougainvillea glabra Choisy by FTIR and UV-VIS spectroscopic analysis. Int J Pharm Sci Rev Res. 2013;21:196-198. [Google Scholar]
- 48.Nyakuma B, Oladokun O, Dodo Y, Wong S, Uthman H, Halim M. Fuel characterization and thermogravimetric analysis of melon (Citrullus Colocynthis L.) seed husk. Chem Chemical Technol. 2016;10:493-497. [Google Scholar]
- 49.Mehrzadi S, Shojaii A, Pur SA, Motevalian M. Anticonvulsant activity of hydroalcoholic extract of Citrullus colocynthis fruit: involvement of benzodiazepine and opioid receptors. Journal of evidence-based complementary & alternative medicine. 2016;21:NP31. [DOI] [PubMed] [Google Scholar]
- 50.Ogbuji K, McCutcheon GS, Simmons AM, Snook ME, Harrison HF, Levi A. Partial leaf chemical profiles of a desert watermelon species (Citrullus colocynthis) and heirloom watermelon cultivars (Citrullus lanatus var. lanatus). Hortscience. 2012;47:580-584. [Google Scholar]
- 51.Chawech R, Jarraya R, Girardi C, et al. Cucurbitacins from the leaves of Citrullus colocynthis (L.) Schrad. Molecules 2015;20:18001-18015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hussain AI, Rathore HA, Sattar MZ, et al. Phenolic profile and antioxidant activity of various extracts from Citrullus colocynthis (L.) from the Pakistani flora. Ind Crop Prod 2013;45:416-422. [Google Scholar]
- 53.Elansary HO, Szopa A, Kubica P, et al. Bioactivities of traditional medicinal plants in Alexandria. Evid base Compl Alternative Med, 2018;1:2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yonbawi AR, Abdallah HM, Alkhilaiwi FA, Koshak AE, Heard CM. Anti-proliferative, cytotoxic and antioxidant properties of the methanolic extracts of five Saudi Arabian flora with folkloric medicinal use: Aizoon canariense, Citrullus colocynthis, Maerua crassifolia, Rhazya stricta and Tribulus macropterus. Plants. 2021;10:2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ramanathan T, Gurudeeban S, Satyavai K. Antioxidant and Radical Scavenging Effect of Citrullus colocynthis. Journal of Current Research. 2010;2:067-069. [Google Scholar]
- 56.Choudhury S, Sharan L, Sinha MP. Phytochemical and antimicrobial screening of Psidium guajava L. leaf extracts against clinically important gastrointestinal pathogens. J Nat Prod Plant Resour. 2012;2:524-529. [Google Scholar]
- 57.Kariawasam K, Pathirana R, Ratnasooriya W, Handunnetti S, Abeysekera W. Phytochemical profile and in vitro anti-inflammatory activity of aqueous leaf extract of Sri Lankan variety of Psidium guajava L. J Pharmacogn Phytochem. 2017;6:22-26. [Google Scholar]
- 58.Uzunlu S, Niranjan K. Laboratory antimicrobial activity of cinnamaldehyde and pomegranate-based polycaprolactone films. J Appl Polym Sci. 2017;134:45347. [Google Scholar]
- 59.Attar U, Ghane S. In vitro antioxidant, antidiabetic, antiacetylcholine esterase, anticancer activities and RP-HPLC analysis of phenolics from the wild bottle gourd (Lagenaria siceraria (Molina) Standl.). South Afr J Bot. 2019;125:360-370. [Google Scholar]
- 60.Kauser A, Shah SMA, Iqbal N, et al. In vitro antioxidant and cytotoxic potential of methanolic extracts of selected indigenous medicinal plants. Prog Nutr. 2018;20:706-712. [Google Scholar]
- 61.Ponsankar A, Sahayaraj K, Senthil-Nathan S, et al. Toxicity and developmental effect of cucurbitacin E from Citrullus colocynthis L.(Cucurbitales: Cucurbitaceae) against Spodoptera litura Fab. and a non-target earthworm Eisenia fetida Savigny. Environ Sci Pollut Control Ser. 2020;27:23390-23401. [DOI] [PubMed] [Google Scholar]
- 62.Ramos IL, Bandiola TMB. Phytochemical screening of Syzygium cumini (myrtaceae) leaf extracts using different solvents of extraction. Der Pharm Lett. 2017;9:74-78. [Google Scholar]
- 63.Kim MG, Lee SE, Yang JY, Lee HS. Antimicrobial potentials of active component isolated from Citrullus colocynthis fruits and structure–activity relationships of its analogues against foodborne bacteria. J Sci Food Agric. 2014;94:2529-2533. [DOI] [PubMed] [Google Scholar]
- 64.Hameed B, Ali Q, Hafeez M, Malik A. Antibacterial and antifungal activity of fruit, seed and root extracts of Citrullus colocynthis plant. Biological and Clinical Sciences Research Journal. 2020;9:2020. [Google Scholar]
- 65.Al Qaisi YT, Khleifat KM, Oran SA, et al. Ruta graveolens, Peganum harmala, and Citrullus colocynthis methanolic extracts have in vitro protoscolocidal effects and act against bacteria isolated from echinococcal hydatid cyst fluid. Arch Microbiol. 2022;204:228. [DOI] [PubMed] [Google Scholar]
- 66.Bourhia M, Messaoudi M, Bakrim H, et al. Citrullus colocynthis (L.) Schrad: Chemical characterization, scavenging and cytotoxic activities. Open Chemistry 2020;18:986-994. [Google Scholar]
- 67.Das NC, Labala RK, Patra R, Chattoraj A, Mukherjee S. In silico identification of new anti-SARS-CoV-2 agents from bioactive phytocompounds targeting the viral spike glycoprotein and human TLR4. Lett Drug Des Discov. 2022;19:175-191. [Google Scholar]
- 68.Shaikh IA, Muddapur UM, Badiger S, et al. Silico Molecular Docking and Simulation Studies of Protein HBx Involved in the Pathogenesis of Hepatitis B Virus-HBV. Molecules. 2022;27:1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-dos-10.1177_15593258231187357 for Pharmacological Activities and In-Silico Studies of Bioactive Compounds Identified in Organic Fractions of the Methanolic Extract of Citrullus Colocynthis by Haseeb Akram Sindhu, Muhammad Afzal, and Izzah Shahid in Dose-Response