Abstract
Stimulation of innate immunity can protect against infectious insult and could be used in combination with other therapies. Since antibiotic resistance is an increasing concern, strategies to reduce the dose or eliminate the need for these drugs are warranted. Lipo-CRX is a formulation in which the TLR4 agonist CRX-527 is incorporated into lipid membranes in liposomes. Lipo-CRX is less inflammatory than either CRX-527 or LPS, but retains unique capacity to enhance host defense responses. We compared lipo-CRX to other agonists in vitro using mammalian cells and in vivo in mice, and assessed indicators of innate immune responses and protection from bacterial infection. Lipo-CRX is similar to E. coli LPS in its capacity to activate bovine γδ T cells and to recruit neutrophils into mouse lungs, but with less reactivity in the LAL assay. However, lipo-CRX uniquely induced the production of systemic innate immune cytokines. In the mouse model of brucellosis, delivery of lipo-CRX to the lungs reduced the dissemination of B. abortus. While lipo-CRX or the antibiotic ampicillin alone did not alter B. abortus burdens in the lung, the combination had a synergistic beneficial effect. Our data suggest that stimulating the innate immune system with lipo-CRX, either alone or when combined with antibiotics, can enhance bacterial clearance in the mouse model of brucellosis.
Keywords: Adjuvant, antibiotics, Brucella, brucellosis, innate immunity
Introduction
Human brucellosis is one of the most common zoonotic diseases worldwide with more than 500,000 new cases annually, is associated with substantial residual disability, and is an important cause of travel-associated morbidity. 1 Brucellosis is a zoonotic infection that, in humans, is caused by Brucella abortus, B. melitensis, B. suis or B. canis. In the US, Brucella abortus still infects wild bison and elk and is a risk to cattle in the Greater Yellowstone Area (GYA). A National Academy of Sciences report from 2017 states that there is potential for both major economic impacts and public health threats if the issue in the GYA is not addressed. 2 Brucella spp. are considered potential bioweapons and there are no approved vaccines for use in humans. Approved vaccines exist for livestock; however, they are only 70–80% effective, they are infectious to humans, can be abortogenic, animals must be re-vaccinated, and one of the two approved vaccines can convolute diagnostic serology.3,4 Like many chronic bacterial infections, brucellosis can be treated with high dose, long-term antibiotics. This usually involves a combination of doxycycline and rifampin for a minimum of 6–8 weeks. However, in addition to development of antibiotic resistance, long-term antibiotic use has negative consequences for the microbiome, now appreciated as a critical component of health. Thus, there is a clear need for novel approaches to treat brucellosis and bacterial disease in general.
Cellular innate immune mechanisms are critical to defense against Brucella infection.5,6 The innate immune system is effective against a wide range of pathogens and is important for initiation of a long lasting adaptive response. Amplifying the innate immune response through use of various agonists, such as TLR agonists, has been shown to reduce bacterial burden in a number of infection models. 7 Similarly, this has been shown in brucellosis models when testing adjuvants for new vaccines where adjuvant was effective alone and augmented vaccine activity. 8 Thus, enhancing innate immune responses may represent an effective therapeutic approach to protect against Brucella infection, as we have demonstrated with Salmonella 9 and other bacterial infections. 10
Monophosphoryl lipid A (MPL) is an effective immune stimulant and has been used as an approved adjuvant for human and animal vaccines for many years.11–15 MPL is a less-toxic, TLR4 agonist developed as an alternative to lipopolysaccharide (LPS), a highly inflammatory compound produced by gram-negative bacteria. Novel lipid A mimetic compounds have also been synthesized as alternatives to MPL.16,17 These compounds are known as aminoalkyl glucosaminide phosphates (AGPs), the most active of which is available commercially (CRX-527, Invivogen). CRX-527 is similar to LPS in its capacity to stimulate immune responses and induce inflammation and fever. 18 Overt inflammation resulting in tissue damage is a concern when these agonists are used as treatments or adjuvants, but it has been shown that encapsulating LPS components, lipid A or MPL, greatly decreases these effects in vivo. 19 For example, human subjects tolerate much greater doses when MPL is in a liposome formulation. 20 Here we report on the application of a liposome formulation of CRX-527, called lipo-CRX, in models of bacterial infection. We found that lipo-CRX activated bovine immune cells in a fashion similar to other TLR agonists we have tested in the past.9,21–24 Lipo-CRX treatment of human THP-1 differentiated macrophages increased killing of Salmonella enterica serovar Typhimurium in vitro. Lipo-CRX was also effective in vivo, without inducing observable negative health implications. Intratracheal treatment with lipo-CRX recruited immune cells to the lungs of treated mice and induced cytokine responses detectable in both the lungs and blood. Lipo-CRX also provided partial protection against Brucella abortus infection in mice when used alone and increased the efficacy of suboptimal antibiotic treatment. Our results demonstrate that lipo-CRX has a unique immunostimulatory profile that may have significant benefit during bacterial infections in vivo. This may be due to a unique intracellular TLR4 signaling pathway in macrophages. 25 Herein, we have also demonstrated that stimulation of innate immunity combined with an antibiotic treatment synergizes to be more effective than either treatment by itself. Such advances are critical considering the looming issues surrounding the use and overuse of antibiotics.
Materials and methods
Liposome formulation
Stock solutions of CRX-527 (InvivoGen No.: tlrl-crx527), DOPC (Avanti Polar Lipids - 850375P-1 g) and cholesterol (Avanti Polar Lipids - 700100P-500 mg) were made by dissolving each substance in chloroform at the desired concentration. CRX-527 comes in 1 mg vials from InvivoGen and was carefully resuspended in chloroform. To prepare small 1.5 ml batches of lipo-CRX, 150μl of chloroform-suspended solutions of CRX-527 (20 mg/ml), DOPC (400 mg/ml), and cholesterol (100 mg/ml) were added to a depyrogenated round bottom flask. For liposomes without CRX-527 (vehicle control), only DOPC and cholesterol were added. The chloroform was removed by evaporation on a rotary evaporator with a 45°C water bath for at least 8 h, until a thin film was obtained and then left open in a biosafety cabinet for an additional 12 h for complete evaporation of residual solvent. The resulting film was resuspended in 1.5 ml hydration buffer (50 mM HEPES, 140 mM NaCl, 750 nM Na2HPO4 in injection grade water, pH 7.0) using sonication in a water bath (45 °C) with intermittent vortexing until all the film was completely dispersed into solution (1–2 h). The liposome preparation was then aseptically filtered using a 0.22 μM filter into a sterile depyrogenated container and stored at 4°C. Liposome preparations containing CRX-527 (lipo-CRX) had an opalescent sheen whereas liposomes without CRX-527 (vehicle) appeared cloudier and took longer to sonicate, suggesting a difference in the liposome composition. The final composition of the lipo-CRX is 2 mg/ml CRX-527, 40 mg/ml DOPC and 10 mg/ml cholesterol. Lipo-CRX was compared to LPS, CRX-527, and liposome only control in the Limulus Amebocyte Lysate (LAL) assay using the Chromo-LAL (Associates of Cape Cod) according to the manufacturer's instructions.
Bovine cell culture
Whole blood was drawn from Holstein bull calves (<2 years old) into sodium heparin tubes (Becton Dickinson, Franklin Lakes, NJ, USA). Histopaque 1077 (Sigma-Aldrich, St Louis, MO, USA) was used to separate the PBMCs from whole blood, as per the manufacturer's instructions, as previously described. 26 Cell preparations were maintained in X-VIVO 15 medium (Cambrex) at 1 × 106 cells/ml at 37°C, 5% CO2 in the presence of known or test agonists, or medium and/or vehicle only as controls for 24–48 h. Samples from a minimum of three calves were compared. Bovine PBMCs were cultured with lipo-CRX, E. coli LPS (Sigma) or MPL or CRX-527 (Invivogen) at the indicated concentrations along with media and vehicle only controls. Vehicle is the same liposome preparation, but without added CRX-527 incorporated within the liposome membrane. Following incubation, the supernatant fluids were collected and cells were stained for γδ TCR (clone GD3.8) and IL-2R (clone LCBT2A, VMRD) as previously described.21–24 and assessed on a BD FACS-Caliber cytometer.
THP-1 culture and in vitro infection
THP-1 cells were acquired from ATCC and maintained in complete RPMI with non-heat-inactivated 10% fetal bovine serum and a total of 4.5 g/L glucose (THP-1 media). THP-1 cells were passaged for less than 4–5 weeks in order to maintain cell integrity. Cells were cultured at 1 × 106 cells/well in 24-well plates with 40 ng/ml PMA (Fisher) for 4–5 days to allow for development of adherent macrophages then rested before treatment. Cells were stimulated 24 h prior to infection with varying concentrations of LPS, MPL, CRX-527, lipo-CRX or vehicle only. Following stimulation, cells were infected with S. Typhimurium (MOI of 5) from log phase cultures in 200μl THP-1 medium, or B. abortus (vaccine strain S19, MOI 25) from an overnight culture, as described elsewhere. 27 Cells were infected for 30 min, medium removed and replaced with medium containing gentamicin at 100μg/ml for another hour. Medium was removed again and cells were washed with PBS then lysed in 200ul PBS containing 0.1% Triton-X. Lysates were serially diluted and plated on LB agar for S. Typhimurium or PIA for B. abortus for CFU enumeration.
Mice and infection
All animal studies were carried out in compliance with the Montana State University Institutional Animal Care and Use Committee, following the NIH Guide for Care and Use of Laboratory Animals, Eighth Edition. The mice (at least 5 mice per group) were 6- to 10-week-old C57BL/6. The experiments were performed with both male and female mice, in approximately equal numbers.
For assessment of innate immune responses in the lung and serum, mice were treated with compounds by intratracheal delivery and euthanized 24 h later. At sacrifice, blood and bronchoalveolar lavage (BAL) fluids were collected. The blood cells were separated from serum, and the BAL cells were separated from the BAL supernatant fluids. Cells from BAL and blood were assessed for neutrophil content by staining with antibodies specific for Ly6G (FITC), CD11c (PE), MHCII (PerCP) and CD11b (APC) from commercial sources and assessed using the FACS Calibur. Serum and BAL fluids were assessed by sandwich ELISAs for expression of the cytokines IFN-γ (BD OptiEIA) and IL-12 (MABTECH).
To assess B. abortus infection in vivo, mice were treated with specific innate immune system agonists by the intraperitoneal or intratracheal routes at the stated intervals before or after infection. Mice were infected with 1 × 105 CFU of B. abortus strain 2308 by the intraperitoneal or intratracheal routes under BSL-3 containment. For intratracheal delivery, mice were deeply anesthetized with isoflurane, and bacteria or treatments were delivered into the trachea at a volume of 100 µl/mouse using a 24-gauge gavage needle. Specifically, 50 µl was instilled and allowed to be aspirated by a deep breath, followed by two instillations of 25 µl each followed by deep breaths. Intratracheal delivery results in consistent and even distribution in the lung. 28 In some experiments, mice were treated with ampicillin sulfate delivered in drinking water to achieve approximately 50–200 mg/kg per day. Antibiotic treatment started on day 6 post-infection and antibiotic water was replaced every 3 days. This antibiotic is effective against Brucella in vitro, but has not been used extensively in vivo. 29 Of note, our CDC-regulated Select Agent facility restricts experimental use of the commonly utilized antibiotic doxycycline, to prevent the generation of resistant strains. Thus, this antibiotic, one of the few that can access intracellular space to counter B. abortus infection, was not used in our studies. Mice infected with B. abortus develop few symptoms and generally did not lose substantial mass (data not shown). Mice were monitored daily and euthanized on day 14 post-infection. Lungs and spleens were collected, weighed, homogenized, serially diluted and plated on PIA or brucella agar plates . The plates were incubated for 5–6 days and colonies were quantified.
Statistics
Statistical analyses were performed using Prism (GraphPad Software, San Diego, CA, USA). The Kolmogorov-Smirnov test (with Dallal-Wilkinson-Liliefor P value) was used to confirm that the data formed a Gaussian distribution. Normally distributed data were analyzed with Student's t-test when comparing only 2 groups, and one-way ANOVA with Bonferroni post-test when comparing multiple groups within an experiment. Otherwise, groups were compared using the Mann-Whitney test.
Results
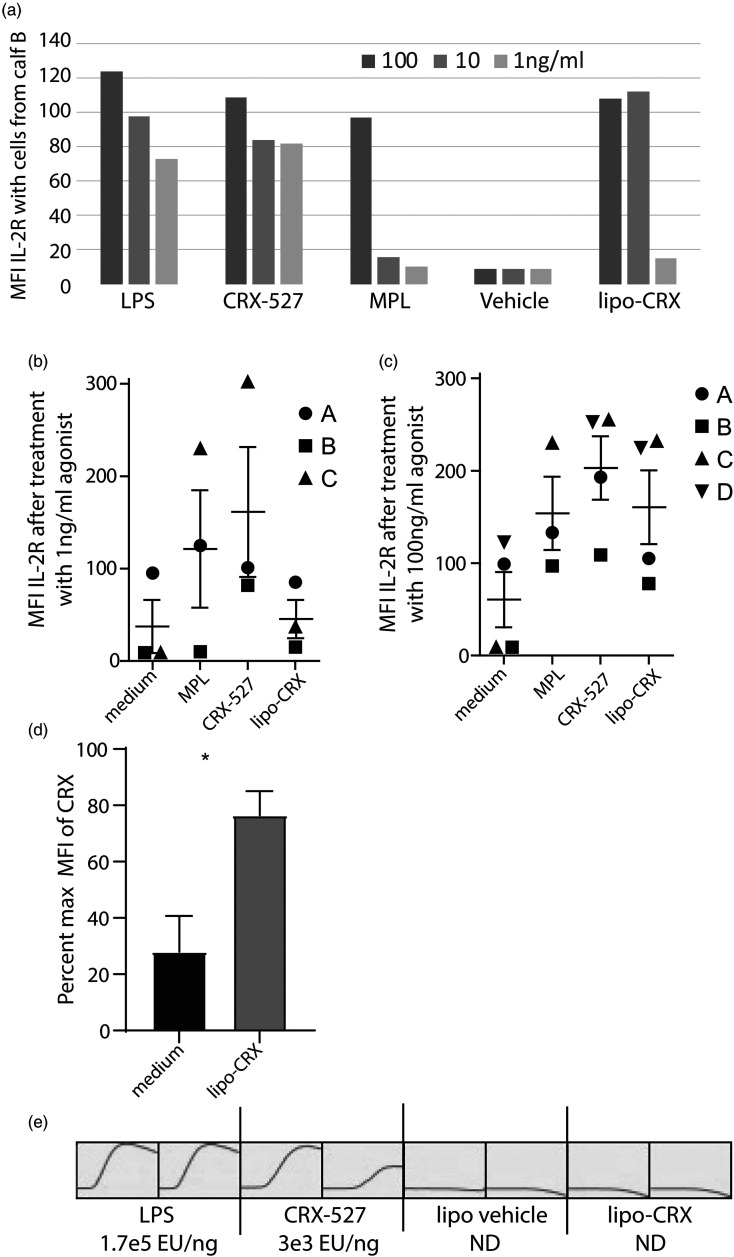
Prior to testing lipo-CRX in vivo, we first screened for activation in two different in vitro functional assays. The first assay was based on our previous studies where we showed that agonists such as LPS, various plant polysaccharides, and the TLR4/TLR2 agonist amphotericin B upregulated IL-2R on resting bovine peripheral blood γδ T cells in dose-dependent manners.9,21–24 PBMCs were separated from bovine blood and cultured with lipo-CRX, E. coli LPS, CRX-527 or MPL at 100, 10, or 1 ng/ml and medium- or vehicle-only controls for 24 h. Following incubation, cells were stained for γδ TCR and IL-2R as previously described and assessed on the FACS Caliber cytometer. 9 Expression of IL-2R was elevated on γδ T cells stimulated with all agonists (Figure 1). Although activation dropped off with 1 ng/ml lipo-CRX, lipo-CRX activated bovine γδ T cells to a similar extent as the same concentrations of LPS and increased activation over vehicle control and the same concentrations of MPL (Figure 1A). Similar responses were noted in cells from four different calves, although variability in the magnitude of responses as reflected in mean fluorescence precluded significance (Figure 1B and C). To normalize the data between experiments, we calculated percent of maximum with the CRX value being the maximum value, for control and lipo-CRX treatment for each calf experiment. The difference between groups achieved significance by unpaired Student's t-test (Figure 1D). These results indicated that lipo-CRX can activate innate immunity in a sterile environment. We also compared 100ul of LPS, CRX-527, lipo-CRX and the liposome vehicle, each at 10 ng/ml, for reactive capacity in an LAL assay done in duplicate. Figure 1E shows that LPS was strongly reactive, CRX-527 less so, and lipo-CRX had little to no reactivity and appeared similar to vehicle only. These data suggest that in the lipo-CRX formulation, the added CRX-527 is efficiently incorporated into the liposomes, and that lipo-CRX retains immune reactive capacity, despite its less reactive LAL status.
Figure 1.
Lipo-CRX activates bovine γδ T cells in mixed cultures in vitro, despite lack of LAL reactivity. a. Cells were treated with agonists as shown at varying concentrations. After 24 h, IL-2R expression was measured by flow cytometry on bovine γδ T cells. This assay was performed in single wells precluding statistical analyses. This procedure was repeated on cells from 3 (1 ng/ml) or 4 (100 ng/ml) different bovine calves at two concentrations of agonist. b. 1 ng/ml and c. 100 ng/ml. Standard error bars are shown. d. To normalize the data between experiments shown in Figure 1C, we calculated % of maximum, with the CRX value being the maximum value, for control and lipo-CRX treatment for each calf experiment. Data were pooled, means and SEM calculated and significance tested by unpaired Student's t-test. e. LPS, CRX-527, lipo-CRX and vehicle only control (liposomes without CRX-527) were compared in an LAL assay in duplicate showing development of Endotoxin Units (EU) over time and EU values as calculated by comparison to a standard curve are shown for each. LPS was strongly reactive, CRX-527 less so, and lipo-CRX had no reactivity and appeared similar to vehicle only (ND-not detected).
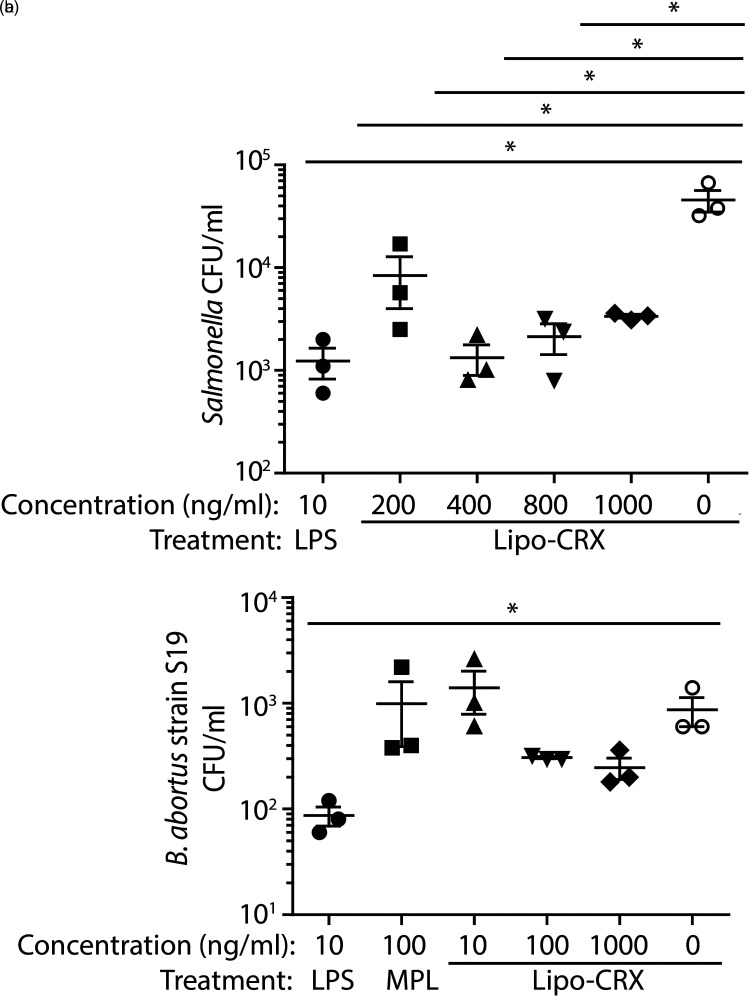
Next, we tested if lipo-CRX could activate human THP-1 macrophages, leading to increased anti-bacterial responses against the intracellular bacterium S. Typhimurium and the S19 vaccine strain of B. abortus. THP-1 monocytes were first stimulated with 40 ng/ml of PMA for 4–5 days at which point they developed into adherent macrophages. Cells were rested, and then pretreated with varying doses of agonists for 24 h before the treatments were removed and cells were infected with either S. Typhimurium or B. abortus (strain S19). Cells were incubated with gentamycin-containing medium, then rinsed, lysed, serially diluted and plated on the appropriate agar plates to determine CFUs. Pre-treatment with lipo-CRX reduced Salmonella CFUs compared to vehicle only at all concentrations tested, but the most dramatic effects were at 400, 800 and 1000 ng/ml of lipo-CRX (Figure 2A). At these higher doses of lipo-CRX, CFUs were reduced almost as effectively as that achieved by 10 ng/ml of LPS. Apparent reductions of B. abortus were also achieved, although these differences were not large enough to achieve statistical significance (Figure 2B). These results suggest that lipo-CRX can activate cellular innate immunity in a manner that enhances protection in response to intracellular infection with bacteria.
Figure 2.
Lipo-CRX treatment contributes to protection of THP-1 macrophages from in vitro infection with two different intracellular bacterial pathogens. a. THP-1 macrophages were pretreated with agonists then infected with S. Typhimurium. Intracellular bacteria were measured in triplicate wells for each agonist. b. A similar titration experiment was performed in THP-1 macrophages using the S19 vaccine strain of B. abortus. These experiments were each repeated 3 times and representative data are shown. For A,*p < 0.05 as measured by one-way ANOVA. For B, *p < 0.05 between LPS positive and medium negative controls as measured by Student's t-test.
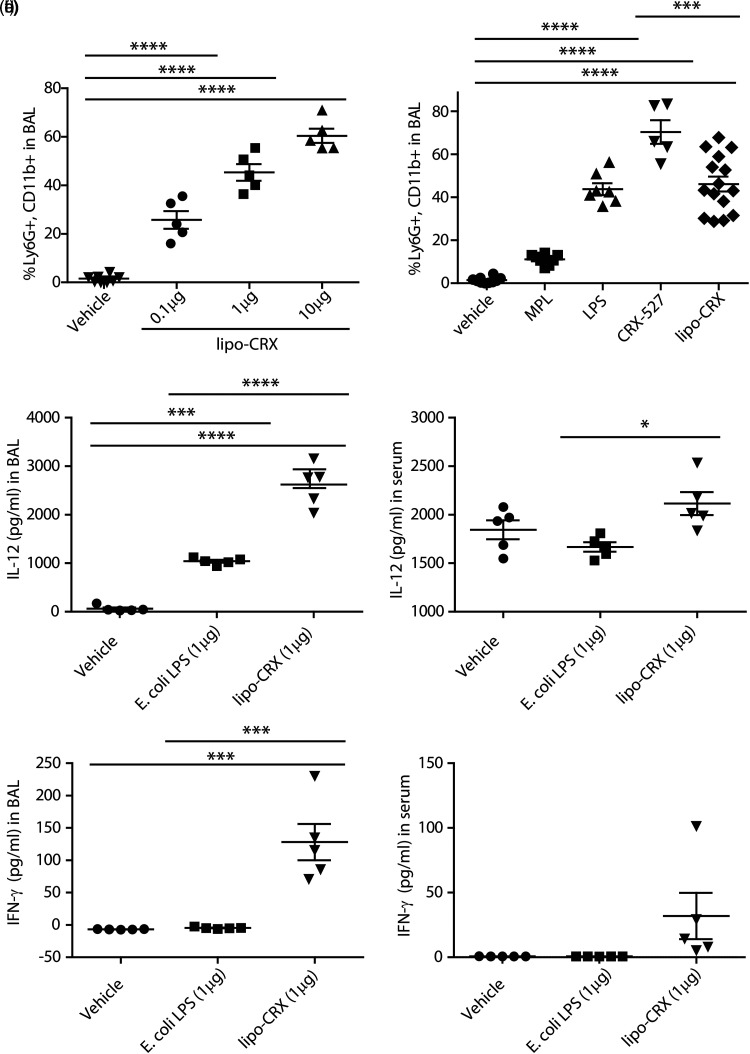
We next wanted to determine the in vivo effects of lipo-CRX delivery in the absence of infection to better understand its immune activation potential. The infection models we planned to use involve direct lung infection and pathogenesis; thus, we chose to examine the effect of delivering lipo-CRX directly to the lung. C57BL/6 mice were treated intratracheally (i.t.) with different concentrations of lipo-CRX, vehicle only, or other agonists for comparison. After 24 h, the mice were sacrificed and bronchoalveolar lavage (BAL) fluids and blood were collected. The cells collected from BAL supernatant fluid and blood were assessed by flow cytometry, and BAL cells were also visualized by cytospin and differential staining. IL-12 and IFN-γ in BAL supernatant fluids and serum were measured by ELISA. Numbers of macrophages in the lung were comparable between all concentrations of lipo-CRX and vehicle control (data not shown). There was an increase in neutrophils recruited to the lungs of mice treated with varying concentrations of lipo-CRX compared to the vehicle only control (Figure 3A). Using 1μg as the optimal concentration, lipo-CRX was compared to MPL, LPS, and CRX-527 at the same concentration for neutrophil recruitment into the lung (Figure 3B). CRX-527 induced the most robust neutrophil response, consistent with its potent capacity to stimulate via TLR4. Lipo-CRX induced significantly less neutrophil influx into the lungs than did CRX-527 and was more comparable to LPS than to MPL (Figure 3B). None of the agonists induced changes in the percentages of blood neutrophils or macrophages at this interval after i.t. treatment (data not shown).
Figure 3.
Lipo-CRX delivered i.t. induces rapid immune stimulation in the lung. a. A titration of Lipo-CRX was applied to mouse lungs and compared to vehicle (delivered at the equivalent volume as the 10μg lipo-CRX dose). After 24 h, neutrophil recruitment to the lungs was measured by flow cytometry. b. A 1μg dose of each agonist was delivered into lungs of mice and neutrophils in lungs were measured by flow cytometry 24 h later. This figure displays combined data from three similar experiments. c-f. Cytokine levels were measured in BAL supernatant fluids and serum collected 24 h after treatment, using ELISA assays in two repeat experiments. *p < 0.1, **p < 0.01, ****p < 0.0001, as calculated by one-way ANOVA with Bonferroni's Multiple Comparison Test.
In addition to neutrophil recruitment to the lung 24 h post-treatment, lipo-CRX also uniquely induced the innate immune system cytokines IL-12 and IFNγ, which are important in host defense against intracellular bacterial pathogens. 30 In these experiments, we compared lipo-CRX to equivalent doses of vehicle and LPS. Although LPS induced a low-level expression of IL-12 in BAL fluids, lipo-CRX induced significantly more IL-12 both in BAL fluids and in circulation (Figure 3C and D). Lipo-CRX also induced greater expression of IFN-γ in BAL fluid and the periphery compared to LPS (Figure 3E and F). These results demonstrate that lipo-CRX is an effective immunostimulatory agent in the mouse lung. Furthermore, lipo-CRX is more effective than LPS at initiating global innate responses following lung delivery.
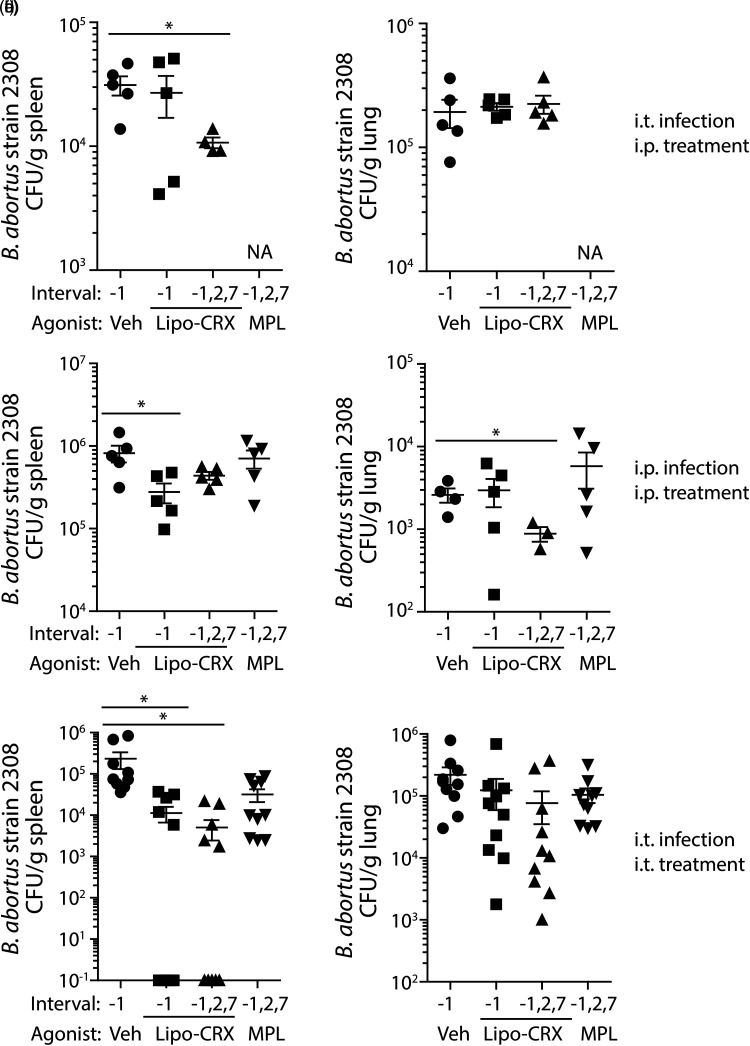
To assess the potential for lipo-CRX as a treatment in the face of bacterial infection in vivo, we utilized B. abortus strain 2308 to challenge mice. Multiple experiments were conducted to determine the most effective route of delivery for lipo-CRX. Initially, mice were either treated one day before infection, or one day before and 2 and 7 days after infection with 1μg lipo-CRX, MPL or vehicle only. Mice were infected i.t. with 1 × 105 CFU B. abortus and weighed intermittently during infection. There were no consistent differences in body weight between the groups during infection (data not shown). At day 14 post-infection, spleens and lungs were collected, weighed, homogenized and diluted to quantify CFU/gram of tissue. Following intratracheal infection, multiple lipo-CRX treatments by the intraperitoneal route had a significant effect on bacterial burden in the spleen, but no apparent effect in the lung (Figure 4A and B). When mice were infected and treated by the intraperitoneal route, the reduction of bacteria in the spleen was apparent with one prophylactic lipo-CRX treatment, and only with repeated doses of lipo-CRX was there a reduction in bacterial burdens in the lungs (Figure 4C and D). When mice were both infected and treated intratracheally, lipo-CRX had its most dramatic effects (Figure 4E and F). A reduction of bacterial burden in the lungs was noted, but was not significant. However, approximately half of the mice treated either just before or before and during infection had no detectable B. abortus in spleens. When bacteria were detectible, they were reduced by approximately 2 logs. The findings were consistent, considering that in each of 2 experiments, 2 or 3 of the 5 mice in lipo-CRX-treated groups had undetectable levels of bacteria in spleens. Thus, a primary effect of lipo-CRX treatment is reduction of dissemination from the lungs to the spleens in infected mice. This may be a function of the route and interval of treatment.
Figure 4.
Lipo-CRX reduces bacterial burden in virulent B. abortus-infected mice. Mice were treated either i.t. or i.p. with 1μg lipo-CRX, MPL (C-F only), or the same volume of vehicle only on days −1, 2 and 7 post- i.t. or i.p. infection with 105 CFU B. abortus, or with lipo-CRX just on day −1. Fourteen days after infection mice were euthanized and bacteria in lungs and spleens were enumerated. a, b. Agonists were delivered intraperitoneally and mice were infected with B. abortus in the lung. NA-not assessed. c, d. Mice were both treated and infected by the intraperitoneal route. e, f. Mice were both treated and infected by the intratracheal route. Figures 4E and F are combined data from two different experiments, while A-D were single experiments with n = 5 mice per group. *p < 0.05 as calculated by Student's t test.
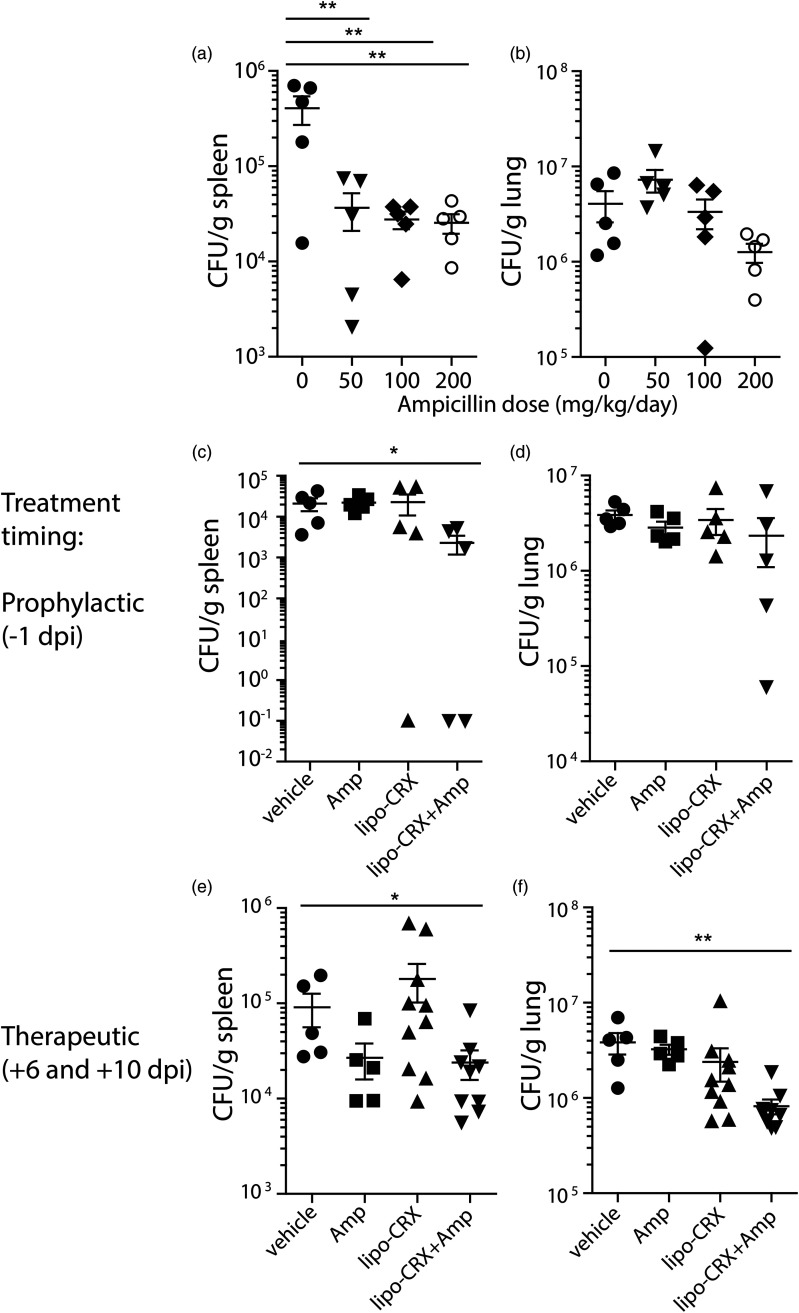
Finally, we assessed the combination of lipo-CRX with a suboptimal dose of the antibiotic ampicillin. To do this, we titrated an oral dose of ampicillin (provided in drinking water) in the mouse model of brucellosis after i.t. delivery of the bacteria. All doses partially reduced bacterial burdens in the spleens while only the highest dose of approximately 200 mg/kg/day partially affected burdens in the lungs (Figure 5A and B). Of note, all antibiotic doses induced an abnormal effect in the gastrointestinal tract resulting in enlarged ceca (data not shown). Since doses of 50 or 100 mg/kg/day ampicillin delivered orally did not significantly reduce bacterial counts in the lungs, we used these doses for two different combination treatment studies. First, we combined lipo-CRX and ampicillin treatment in Brucella-infected mice. Mice were treated with 1μg of lipo-CRX by i.t. delivery on the day before infection, and then infected with Brucella by i.t. delivery. On day 5 post-infection, 100 mg/kg/day ampicillin was delivered in drinking water. Mice were monitored and then euthanized on day 14 post-infection, as described above. The combination of lipo-CRX with ampicillin resulted in a minor, though statistically significant, reduction in bacterial burdens in the spleen, and a trend toward reduction in the lung as well (Figure 5C and D).
Figure 5.
Innate agonists synergize with a suboptimal dose of antibiotics to reduce B. abortus burdens in mouse spleens and lungs. a, b. Ampicillin was delivered to mice in the drinking water at the indicated doses starting at day 6 post infection with 105 CFU B. abortus. c, d. A dose of 100 mg/kg of ampicillin was delivered starting at day 6 post infection combined with lipo-CRX, 1μg i.t. on the day before infection (−1 dpi) and compared to vehicle, lipo-CRX or ampicillin alone. e, f. A combination treatment was also delivered i.t. at therapeutic intervals, 6 and 10 days after infection combined with a lower dose of ampicillin (50 mg/kg) and compared to vehicle, lipo-CRX or ampicillin alone. In each experiment, mice were euthanized 14 days post infection and bacterial burdens in spleens and lungs were assessed. Figures 5E and F are combined data from two different experiments, while A-D were single experiments with n = 5 mice per group. *p < 0.0 5, **p < 0.01 as calculated by the Mann-Whitney test.
Since a therapeutic dosing strategy for Brucella infection would be the most practical real-world application of a new treatment, we assessed treatment of infected mice at various intervals post infection. Delivery of lipo-CRX on days 4, 5, or 6 post-infection did not reduce bacterial burdens (data not shown). Thus, we combined a multiple dose strategy together with suboptimal antibiotic delivery (50 mg/kg/day was used this time to possibly show a greater benefit when combined with lipo-CRX). The combination of multiple treatments with lipo-CRX and suboptimal antibiotics resulted in a beneficial effect measured in both the spleens and lungs (Figure 5E and F). The results suggest that lipo-CRX and ampicillin delivered therapeutically synergize to provide protection against Brucella infection. Of note, ampicillin is not used to treat Brucella infection, because it cannot access intracellular bacteria, as can the antibiotic doxycycline. These data suggest that the combination of a liposome with innate agonist in the membrane, such as lipo-CRX, together with an antibiotic, even one not usually considered for infection with intracellular bacteria, may be an effective combination treatment.
Discussion
Here we showed a novel TLR4 agonist formulation, lipo-CRX, effectively stimulates innate lymphocytes and monocytes/macrophages leading to either upregulation of IL-2 receptor or increased bacterial killing, respectively. Activity was comparable to or greater than two other TLR4 agonist preparations, E. coli LPS or MPL. Lipo-CRX is a liposome preparation of CRX-527. While CRX-527 is a highly inflammatory agonist, incorporation into lipid membranes is thought to increase in vivo adjuvant activity through enhanced interaction and uptake into cells, while minimizing toxic inflammation. This was demonstrated by the reduced capacity of lipo-CRX to recruit neutrophils compared to CRX-527. However, we do not suggest that lipo-CRX is signaling via the TLR4-TRIF pathway intracellularly. This effect increases the expression of type I IFN, which may in turn enhance rather than suppress B. abortus infection in vivo. 31 Our results are consistent with the reported benefit of encapsulating lipid A or MPL in reducing pyrogenicity in both rabbits and monkeys. 19 Furthermore, in a malaria vaccine study in which human subjects were given liposomal vaccines, the subjects tolerated much greater levels of liposome encapsulated MPL with minimal to no toxic effects systemically. 20 We found that lipo-CRX administration to the mouse lung led to neutrophil recruitment and cytokine responses within the lung and, impressively, systemically as well. No overt negative effects such as ruffling or hunching were noted in the mice, suggesting minimal toxicity was associated with the lipo-CRX treatments. Also consistent with our findings, a similar liposome formulation of CRX-527 showed reduced toxicity in dogs (Baldridge, J. unpublished).
Upon Brucella infection in the lung, dendritic cells and migratory alveolar macrophages uptake Brucella and disseminate to the mediastinal draining lymph nodes. From there, the Brucella-infected cells can enter the bloodstream via the thoracic duct and migrate to extra-pulmonary locations, such as the spleen. 32 Likely, lung-directed treatment with lipo-CRX affects macrophage infection in the lung resulting in reduced dissemination from that site to the spleen, whereas later during infection, i.p. treatment may be more effective at targeting systemic infection. Intratracheal treatment of mice with lipo-CRX induced the recruitment of neutrophils. While neutrophils are called to sites of initial Brucella infection and ingest Brucella, the bacteria actually resist their bactericidal activity and cause the neutrophils to present “eat me” signals. These signals attract macrophages to phagocytose the Brucella-infected neutrophils, allowing the bacteria to now replicate and thrive inside of macrophages. 33 Thus, we cannot definitively say that the recruitment of neutrophils to the site of Brucella infection would be beneficial. Lipo-CRX also induced IL-12 and IFN-γ in BAL fluids and blood measured 24 h after delivery to the mouse lung. These cytokines are relevant for protection from intracellular bacterial infections, including brucellosis. 30 Previous research has shown that the presence of IL-12 and IFN- γ helps reduce Brucella burdens in mouse spleens. 34 Specifically, administering recombinant IL-12 to infected mice led to a significant reduction in splenic bacterial counts three weeks post-infection. When anti-IFN- γ antibody was simultaneously administered with recombinant IL-12, bacterial counts increased, suggesting that IL-12 and IFN- γ work together to reduce bacterial counts in the spleen. In the lungs of Brucella-infected mice, increased IL-12 and IFN- γ was detected at day seven post-infection 35 and these cytokines help control Brucella infection in human patients as well. 36 Thus, the induction of these cytokines by lipo-CRX suggest a potential mechanism for the protection against brucellosis.
Our long-term research goals have been to identify approaches to increase immune cell clearance of intracellular pathogens, such as B. abortus. The LPS from Brucella does not activate the innate immune system, and thus facilitates intracellular infection without raising alarms, contributing to its pathogenesis.37,38 Other Brucella bacterial components and proteins also conspire to block effective immune responses to infection.35,39,40 Therefore, we reasoned that activating a normal antibacterial response with an external adjuvant might be beneficial. This has rarely been shown in brucellosis models. 8 Hielpos et al. determined that LPS treatment before Brucella infection could increase antibacterial inflammatory responses and enhance the clearance of a mutant B. abortus strain lacking Btp proteins, which are responsible for suppressing TLR4 responses. However, this treatment was only assessed at 24 h pre-infection, and did not affect wild type Brucella replication at this interval. 35 Lipid A liposome formulations have also been shown to overcome immunosuppression in other models of disease. 41 Such TLR4-directed liposome formulations have been studied as vaccine adjuvants in several models, 42 but less so as innate agonists for preventative or therapeutic application. MPL was incorporated into liposomes and the capacity for enhanced CD8 T cell responses was assessed, but direct innate responses were not compared. 43 Our data suggests both that external augmentation of the innate immune system can decrease bacterial burdens and that such augmentation can synergize with antibiotics towards a treatment for brucellosis. This approach may well also be effective in other bacterial infections, some of which have diminishing antibiotic options.
It is unlikely that use of a therapeutic adjuvant in a bacterial infection would be used alone clinically, because complete clearance would not be expected by the innate immune system alone. The combination of innate agonists with antibiotics has been discussed. 18 This approach has rarely been applied in models of bacterial disease. In one such example, TLR5 agonist (flagella) enhanced the activity of antibiotics, providing an additive effect. 44 We predicted a synergistic effect, where lower concentrations of both the adjuvant and antibiotic could be used in clearing infection. This is a critically important issue, particularly for the treatment of brucellosis in developing countries. Currently, effective control of infection can be achieved by use of a cocktail of high dose antibiotics for extended periods of time in infected individuals. 45 There are very few antibiotics known to access bacteria in the intracellular space. One of them is doxycycline, currently used clinically for brucellosis. Combination therapies as we have described may expand the repertoire of antibiotics to treat intracellular bacteria to those that are not necessarily known to access intracellular space, such as ampicillin. Many developing countries where brucellosis is endemic lack the resources or access to sufficient antibiotics to effectively treat the disease. 1 Further, use of antibiotics for extended periods of time leads to dysbiosis and selection of antibiotic resistance. As such, reducing the amount of antibiotic and the length of time for treatment would provide a huge benefit to combat brucellosis worldwide. Our data suggests that lipo-CRX enhances clearance by inducing the generation of innate immune cytokines and increasing the anti-bacterial effects of monocytes/macrophages. Similar effects were seen with plant-derived polysaccharides in vivo that we showed activate innate immune cells partially through a MyD88-dependent pathway. 22 These plant polysaccharides induced IL-12 and IFN-γ, leading to increased clearance of Francisella tularensis and Burkholderia pseudomallei. 10
In this study, we assessed the activity of lipo-CRX for innate immune stimulation and for its capacity to affect the mouse model of brucellosis. Stimulation of innate immunity is a rational approach to a bioweapons situation as it is non-specific, thus, the cause of the infection or outbreak need not be specifically identified. In this era of increasing resistance to antibiotics and deeper understanding of the impact of the microbiome, it is critical to discover and apply novel preventative and therapeutic measures to bacterial infection. Augmenting the innate immune system using specific agonists can decrease bacterial burdens. This may be particularly useful when applied together with antibiotics that are not expected to be effective. For brucellosis, such a combined treatment could reduce the length of time that infected patients have to take antibiotics and accelerate complete recovery.
Footnotes
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by funding from the Montana Research and Economic Development Initiative (MREDI), NIH (R21 AI144496-01), USDA/NIFA Animal Health, and the Montana Agricultural Experiment Station.
ORCID iD: Jodi F. Hedges https://orcid.org/0000-0002-7461-3972
References
- 1.Pappas G, Papadimitriou P, Akritidis N, et al. The new global map of human brucellosis. Lancet 2006; 6: 91–99. [DOI] [PubMed] [Google Scholar]
- 2.National Academies of Sciences EaM. Revisiting Brucellosis in the Greater Yellowstone Area. Washington, DC: The National Academies Press, 2017. [PubMed] [Google Scholar]
- 3.Dorneles EMS, Sriranganathan N, Lage AP. Recent advances in Brucella abortus vaccines. Vet Res 2015; 46: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodwin ZI, Pascual DW. Brucellosis vaccines for livestock. Vet Immunol Immunopathol 2016; 181: 51–58. [DOI] [PubMed] [Google Scholar]
- 5.Skyberg JA, Thornburg T, Rollins M, et al. Murine and bovine ((T cells enhance innate immunity against Brucella abortus infections. PLoS ONE 2011; 6: e21978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pei J, Ding X, Fan Y, et al. Toll-like receptors are critical for clearance of Brucella and play different roles in development of adaptive immunity following aerosol challenge in mice. Front Cell Infect Microbiol 2012; 2: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mifsud E, Tan A, Jackson D. TLR Agonists as modulators of the innate immune response and their potential as agents against infectious disease. Front Immunol 2014; 5: 79. DOI: 10.3389/fimmu.2014.00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montaraz JA, Winter AJ. Comparison of living and nonliving vaccines for Brucella abortus in BALB/c mice. Infect Immun 1986; 53: 245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hedges JF, Mitchell AM, Jones K, et al. Amphotericin B stimulates (( T and NK cells, and enhances protection from Salmonella infection. Innate Immun 2015; 21: 598–608. [DOI] [PubMed] [Google Scholar]
- 10.Skyberg JA, Rollins MF, Holderness JS, et al. Nasal Acai polysaccharides potentiate innate immunity to protect against pulmonary Francisella tularensis and Burkholderia pseudomallei infections. PLoS Pathog 2012; 8: e1002587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao Z, Foster PA, Gross GJ. Monophosphoryl lipid A protects against endotoxic shock via inhibiting neutrophil infiltration and preventing disseminated intravascular coagulation. CircShock 1994; 43: 107–114. [PubMed] [Google Scholar]
- 12.Carpati CM, Astiz ME, Rackow EC, et al. Monophosphoryl lipid A attenuates the effects of endotoxic shock in pigs. J Lab Clin Med 1992; 119: 346–353. [PubMed] [Google Scholar]
- 13.Astiz ME, Rackow EC, Kim YB, et al. Monophosphoryl lipid A induces tolerance to the lethal hemodynamic effects of endotoxemia. CircShock 1991; 33: 92–97. [PubMed] [Google Scholar]
- 14.Kiener PA, Marek F, Rodgers G, et al. Induction of tumor necrosis factor, IFN-gamma, and acute lethality in mice by toxic and non-toxic forms of lipid A. Journal of Immunology (Baltimore, Md: 1950) 1988; 141: 870–874. [PubMed] [Google Scholar]
- 15.Madonna GS, Peterson JE, Ribi EE, et al. Early-phase endotoxin tolerance: induction by a detoxified lipid A derivative, monophosphoryl lipid A. Infect Immun 1986; 52: 6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stöver AG, Da Silva Correia J, Evans JT, et al. Structure-activity relationship of synthetic toll-like receptor 4 agonists. J Biol Chem 2004; 279: 4440–4449. [DOI] [PubMed] [Google Scholar]
- 17.Cluff CW, Baldridge JR, Stover AG, et al. Synthetic toll-like receptor 4 agonists stimulate innate resistance to infectious challenge. Infect Immun 2005; 73: 3044–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernandez A, Patil NK, Stothers CL, et al. Immunobiology and application of toll-like receptor 4 agonists to augment host resistance to infection. Pharmacol Res 2019; 150: 104502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richards RL, Swartz GM, Schultz C, et al. Immunogenicity of liposomal malaria sporozoite antigen in monkeys: adjuvant effects of aluminium hydroxide and non-pyrogenic liposomal lipid A. Vaccine 1989; 7: 506–512. [DOI] [PubMed] [Google Scholar]
- 20.Fries LF, Gordon DM, Richards RL, et al. Liposomal malaria vaccine in humans: a safe and potent adjuvant strategy. Proc Natl Acad Sci U S A 1992; 89: 358–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holderness J, Jackiw L, Kimmel E, et al. Select plant tannins induce IL-2R( up-regulation and augment cell division in (( T cells. The Journal of Immunology 2007; 179: 6468–6478. [DOI] [PubMed] [Google Scholar]
- 22.Holderness J, Schepetkin IA, Freedman B, et al. Polysaccharides isolated from Acai fruit induce innate immune responses. PLoS ONE 2011; 6: e17301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graff JC, Kimmel EM, Schepetkin IA, et al. Polysaccharides derived from Yamoa (Funtumia elastica) affect innate immunity in part by priming (( T cells. Int Immunopharmacol 2009; 9: 1313–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kerns HMM, Jutila MA, Hedges JF. The distinct response of (( T cells to the Nod2 agonist, muramyl dipeptide. Cell Immunol 2009; 257: 38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shibata T, Motoi Y, Tanimura N, et al. Intracellular TLR4/MD-2 in macrophages senses Gram-negative bacteria and induces a unique set of LPS-dependent genes. Int Immunol 2011; 23: 503–510. 2011/06/30. [DOI] [PubMed] [Google Scholar]
- 26.Hedges JF, Cockrell D, Jackiw L, et al. Differential mRNA expression in circulating (( T lymphocyte subsets defines unique tissue-specific functions. J Leukocyte Biol 2003; 73: 306–314. [DOI] [PubMed] [Google Scholar]
- 27.Czyz DM, Willett JW, Crosson S. Brucella abortus induces a Warburg shift in host metabolism that is linked to enhanced intracellular survival of the pathogen. J Bacteriol 2017; 199: e00227-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Driscoll KE, Costa DL, Hatch G, et al. Intratracheal instillation as an exposure technique for the evaluation of respiratory tract toxicity: uses and limitations. Toxicol Sci 2000; 55: 24–35. [DOI] [PubMed] [Google Scholar]
- 29.Mortensen JE, Moore DG, Clarridge JE, et al. Antimicrobial susceptibility of clinical isolates of Brucella. Diagn Microbiol Infect Dis 1986; 5: 163–169. [DOI] [PubMed] [Google Scholar]
- 30.Xing Z, Zganiacz A, Santosuosso M. Role of IL-12 in macrophage activation during intracellular infection: iL-12 and mycobacteria synergistically release TNF-α and nitric oxide from macrophages via IFN-γ induction. J Leukocyte Biol 2000; 68: 897–902. [PubMed] [Google Scholar]
- 31.de Almeida LA, Carvalho NB, Oliveira FS, et al. Myd88 and STING signaling pathways are required for IRF3-mediated IFN-( induction in response to Brucella abortus infection. PLoS ONE 2011; 6: e23135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Archambaud C, Salcedo SP, Lelouard H, et al. Contrasting roles of macrophages and dendritic cells in controlling initial pulmonary Brucella infection. Eur J Immunol 2010; 40: 3458–3471. 2010/11/26. [DOI] [PubMed] [Google Scholar]
- 33.Gutiérrez-Jiménez C, Mora-Cartín R, Altamirano-Silva P, et al. Neutrophils as Trojan Horse vehicles for Brucella abortus macrophage infection. Front Immunol 2019; 10: 1012. doi: 10.3389/fimmu.2019.01012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sathiyaseelan J, Goenka R, Parent M, et al. Treatment of Brucella-susceptible mice with IL-12 increases primary and secondary immunity. Cell Immunol 2006; 243: 1–9. 2006/12/23. [DOI] [PubMed] [Google Scholar]
- 35.Hielpos MS, Ferrero MC, Fernández AG, et al. Btp proteins from Brucella abortus modulate the lung innate immune response to infection by the respiratory route. Front Immunol 2017; 8: 1011. DOI: 10.3389/fimmu.2017.01011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eskandari-Nasab E, Moghadampour M, Asadi-Saghandi A, et al. Levels of interleukin-(IL)-12p40 are markedly increased in brucellosis among patients with specific IL-12B genotypes. Scand J Immunol 2013; 78: 85–91. [DOI] [PubMed] [Google Scholar]
- 37.Goldstein J, Hoffman T, Frasch C, et al. Lipopolysaccharide (LPS) from Brucella abortus is less toxic than that from Escherichia coli, suggesting the possible use of B. abortus or LPS from B. abortus as a carrier in vaccines. Infect Immun 1992; 60: 1385–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lapaque N, Moriyon I, Moreno E, et al. Brucella lipopolysaccharide acts as a virulence factor. Curr Opin Microbiol 2005; 8: 60–66. [DOI] [PubMed] [Google Scholar]
- 39.Dornand J, Gross A, Lafont V, et al. The innate immune response against Brucella in humans. Vet Microbiol 2002; 90: 383–394. 2002/11/05. [DOI] [PubMed] [Google Scholar]
- 40.Jakka P, Namani S, Murugan S, et al. The Brucella effector protein TcpB induces degradation of inflammatory caspases and thereby subverts non-canonical inflammasome activation in macrophages. J Biol Chem 2017; 292: 20613–20627. 2017/10/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alving CR, Richards RL. Liposomes containing lipid A: a potent nontoxic adjuvant for a human malaria sporozoite vaccine. Immunol Lett 1990; 25: 275–279. [DOI] [PubMed] [Google Scholar]
- 42.Tandrup Schmidt S, Foged C, Korsholm KS, et al. Liposome-based adjuvants for subunit vaccines: formulation strategies for subunit antigens and immunostimulators. Pharmaceutics 2016; 8: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nordly P, Agger EM, Andersen P, et al. Incorporation of the TLR4 agonist monophosphoryl lipid A into the bilayer of DDA/TDB liposomes: physico-chemical characterization and induction of CD8+ T-cell responses in vivo. Pharm Res 2011; 28: 553–562. journal article. DOI: 10.1007/s11095-010-0301-9. [DOI] [PubMed] [Google Scholar]
- 44.Porte R, Fougeron D, Muñoz-Wolf N, et al. A toll-like receptor 5 agonist improves the efficacy of antibiotics in treatment of primary and influenza virus-associated pneumococcal mouse infections. Antimicrob Agents Chemother 2015; 59: 6064–6072. 2015/07/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Corbel MJ. Brucellosis in humans and animals. World Health Organization, 2006. https://apps.who.int/iris/handle/10665/43597. [Google Scholar]