Abstract
Objectives: The objective of our study was to assess the prognostic significance of the Pan-Immune-Inflammation Value (PIV) before concurrent chemoradiation (C-CRT) and prophylactic cranial irradiation (PCI) in patients with limited-stage small-cell lung cancer (SCLC). Methods: The medical records of LS-SCLC patients who underwent C-CRT and PCI between January 2010 and December 2021 were retrospectively analyzed. PIV values were calculated using the peripheral blood samples obtained within the past 7 days before the initiation of treatment: PIV = [neutrophils × platelets × monocytes] ÷ lymphocytes. Using receiver operating characteristic (ROC) curve analysis, the optimal pretreatment PIV cutoff values that can partition the study population into two groups with substantially distinct progression-free survival (PFS) and overall survival (OS) outcomes were determined. The relationship between PIV values and OS outcomes was the primary outcome measure. Results: Eighty-nine eligible patients were divided into two PIV groups at an optimal cutoff of 417 [Area under curve (AUC): 73.2%; sensitivity: 70.4%; specificity: 66.7%]: Group 1: PIV < 417 (N = 36) and Group 2: PIV ≥ 417 (N = 53). Comparative analyses revealed that patients with PIV < 417 had significantly longer OS (25.0 vs 14.0 months, p < .001) and PFS (18.0 vs 8.9 months, p = .004) compared to patients with PIV ≥ 417. The outcomes of the multivariate analysis have verified the independent significance of pretreatment PIV concerning PFS (p < .001) and OS (p < .001) outcomes. Conclusion: The findings of this retrospective study indicate that the pretreatment PIV is a reliable and independent prognostic biomarker for patients with LS-SCLC who were treated with C-CRT and PCI.
Keywords: small-cell lung cancer, biomarker, pan-immune-inflammation value, prognosis, survival
Introduction
Small-cell lung cancer (SCLC) is a significant cause of cancer-related morbidity and mortality on a global scale, constituting approximately 14% of all lung cancers. 1 The disease is classified as a neuroendocrine tumor with a highly aggressive biological phenotype, exhibiting indications of rapid growth and early dissemination. Despite the high efficacy of radiation therapy (RT) and chemotherapy in treating SCLC, most patients suffer from relapses shortly after undergoing these aggressive treatments, leading to a 5-years survival rate that is often below 10%. 2 Although the American Joint Committee on Cancer (AJCC) staging system, which is dependent on the tumor-node-metastasis (TNM) status of the disease, is recommended for the staging of SCLC patients, the non-surgical Veterans Administration Lung Study Group (VALSG) classification is preferred because SCLC rarely presents with a disease that is sufficiently localized to permit surgical resection. The VALSG classification system categorizes SCLC into two distinct stages: limited stage (LS-SCLC) and extensive stage (ES-SCLC). 3 Most ES-SCLC patients can benefit from systemic therapy as a standalone treatment, leading to symptom relief and improved survival rates. Recent studies have demonstrated that immunotherapy in combination with chemotherapy can marginally enhance survival outcomes.4–7 The standard treatment for patients with LS-SCLC is a combination of cisplatin and etoposide chemotherapy administered concurrently with thoracic RT (TRT), which may be followed by prophylactic cranial irradiation (PCI) in patients with an objective response.8,9 Despite the efficacy of concurrent chemoradiotherapy (C-CRT) in patients with LS-SCLC, the overall prognosis remains unfavorable, as evidenced by a median survival duration of only 16–24 months 3 The unfavorable results can be attributed mainly to the disease’s inability to provide a lasting response to existing treatment protocols and its tendency to cause early and widespread distant metastases.3,10
Persistent or chronic systemic inflammation suppresses host immune responses, complicates genetic imbalances, interferes with immune cell interactions, promotes tumorigenesis, and facilitates tumor growth, survival, and metastasis, contributing to a poor prognosis in various cancers.11,12 Different indices have been developed to assess the inflammatory response in cancer patients, utilizing peripheral blood counts of neutrophils, platelets, monocytes, and lymphocytes. These indices include the neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), monocyte-lymphocyte ratio (MLR), systemic immune-inflammation index (SII), and systemic inflammation response index (SIRI), which have demonstrated prognostic value in various types of cancer.13–20 Nonetheless, both two- and three-cell-based indices are subject to greater susceptibility to the influence of diverse factors, such as the artificial elevation of lymphocytes in viral diseases. Additionally, they do not account for the impact of remaining cells on the patient’s prognosis. However, each immune and inflammatory cell is known to exert substantial effects on cancer progression and metastasis, either independently or through its interactions with other cells or the various chemokines and cytokines that they secrete, which can either promote or suppress tumor growth. In this respect, the Pan-Immune-Inflammation Value (PIV), which has been recently introduced as an all-in-one cellular prognostic index, has demonstrated prognostic significance in various types of cancers, including colorectal-, breast-, esophageal-, small-cell lung-, and Merkel cell carcinomas, as well as malignant melanomas.21–32 A recent study by Zeng et al. found that a higher pretreatment PIV value was associated with poorer clinical outcomes in patients with ES-SCLC who received a combination of anti-PD-1/PD-L1 inhibitors and chemotherapy. 32 Interestingly, the prognostic value of PIV in LS-SCLC patients who receive standard C-CRT with PCI has not been investigated to date. Therefore, this knowledge gap motivated the conduct of this retrospective study, which aimed to determine the potential prognostic significance of pretreatment PIV in LS-SCLC patients who undergo standard C-CRT with PCI.
Patients and methods
Patients population
We searched for patients with LS-SCLC who underwent C-CRT followed by PCI between January 2010 and December 2021 in the databases of two distinct radiation oncology departments, namely Baskent University Faculty of Medicine and Mersin City Training and Research Hospital. Patients aged 18–80 years old with an Eastern Collaborative Oncology Group (ECOG) performance score of 0–2; available histopathological proof of SCLC, diagnostic computed tomography (CT), 18F-fluorodeoxyglucose positron emission CT (PET-CT), and brain magnetic resonance imaging (MRI) scans; staged as LS-SCLC according to VALSG criteria or T1-4N1-3MO per AJCC eighth edition; available records of TRT and computerized therapy datasets; and available complete blood count and biochemistry test results obtained within the past 7 days before the onset of C-CRT. This study excluded patients who had a prior history of chronic immunosuppressive medication or steroid usage, blood transfusions within the 90 days before the initiation of concurrent chemoradiotherapy (C-CRT), malignant pleural or pericardial effusion, insufficient pulmonary, cardiac, renal, or hepatic functions, or a previous history of RT or chemotherapy.
Ethics, consent, and permissions
The Baskent University Faculty of Medicine Institutional Review Board and the Mersin Provincial Health Directorate approved the retrospective research design, analyses of imaging scans, pathology findings, blood test results, and treatment outcomes (Project No: 2021/0134). Written Informed consent was obtained from all subjects before the study to collect and analyze blood samples and pathological specimens, as well as publish the resulting findings.
Treatment details
The standard procedure for LS-SCLC patients in both radiation oncology departments involved the utilization of co-registered PET-CT-based RT planning. TRT consisted of a total dosage of 54 Gy (1.8 Gy per fraction, 30 days), which was delivered using intensity-modulated RT (IMRT) or 3-dimensional conformal RT (3D-CRT) with megavoltage linear accelerators. The chemotherapy treatment plan included four cycles of the cisplatin and etoposide combination (cisplatin 60 mg/m2 IV on day 1 and etoposide 120 mg/m2 IV on days 1–3, delivered every 28 days), with the first two cycles being administered concurrently with TRT. Patients who did not exhibit any clinical or radiological signs of brain metastases, had no confirmed neurological abnormalities, and had at least a stable LS-SCLC response to the treatment protocol according to previously validated PET Response Criteria in Solid Tumors (PERCIST), version 1.0, were subjected to PCI with a total dose of 25 Gy (2.5 Gy per fraction, administered over a period of 10 days). 33
PIV measurements
The PIV values were computed following repeatedly validated Fucà and colleagues’ original formula, 21 utilizing the counts of neutrophils, platelets, monocytes, and lymphocytes obtained within the past 7 days before the initiation of treatment: PIV = [neutrophils × platelets × monocytes] ÷ lymphocytes.21–32
Evaluation of treatment response
Following the completion of the prescribed C-CRT and PCI, all patients underwent re-evaluations using PET-CT and brain MRI to determine treatment response and the presence of brain metastasis. The patients underwent assessments at regular intervals, with a frequency of every 3 months during the first 2 years, every 6 months from three to 5 years, and annually thereafter. Following the confirmation of a comprehensive metabolic response, the utilization of PET-CT evaluations was substituted with diagnostic thorax CT and abdominal ultrasonography examinations. Metabolic response was evaluated by the use of validated PET Response Criteria in Solid Tumors (PERCIST), version 1.0. 33
Statistical methods
The primary endpoint for this study was to determine the potential link between PIV and overall survival (OS), defined as the duration from the initial day of C-CRT until death or the final visit. The secondary endpoint was progression-free survival (DFS), which refers to the interval between the initial day of C-CRT and the occurrence of local or regional recurrence, distant metastasis, or death or last visit. The continuous numerical variables were presented using medians and ranges, while the categorical variables were expressed using frequency distributions. The study employed receiver operating characteristic (ROC) curve analysis to identify the optimal cutoff value of pretreatment PIV to stratify the research cohort into two groups with statistically significant differences in survival outcomes, if feasible. The statistical methods employed in this study to investigate the association between PIV groups and other variables included the chi-square test, Mann–Whitney U test, Student’s t-tests, and Spearman correlations as necessitated. The multivariate regression analysis was restricted to the covariates that had demonstrated statistical significance in the univariate analyses. Any p < .05 represented the statistical significance of the results in this study.34,35
Results
The present study involved the participation of 89 LS-SCLC patients from two radiation oncology centers. The vast majority of patients were male (84.3%) and ex-smokers (96%) (Table 1). Most patients had T3–4 (57.3%) or N2–3 (75.3%) disease, according to the AJCC eighth edition. Anemia was present in 56.2% of patients per the criteria established by the World Health Organization, which defines anemia as a hemoglobin level of <13 g/dL for males and <12 g/dL for females. All patients were able to receive the entire C-CRT regimen, and 13 (14.6%) and 76 (85.4%) of them could receive the three and four cycles of the prescribed chemotherapy, respectively.
Table 1.
Pretreatment and treatment patient and disease features for the whole study cohort and per Pan-Immune-Inflammation-Value groups.
| Variables | All patients (N = 89) | PIV < 417 (N = 36) | PIV ≥4 17 (N = 53) | p-value |
|---|---|---|---|---|
| Median age, years | 61 (37–79) | 63 (41–78) | 60 (37–79) | 0.62 |
| Age, n (%) | ||||
| <70 | 74 (83,1) | 33 (91.7) | 41 (77.4) | 0.091 |
| ≥70 | 15 (16.9) | 3 (8.3) | 12 (22.6) | |
| Gender, n (%) | ||||
| Male | 75 (84.3) | 29 (80.6) | 46 (86.8) | 0.56 |
| Female | 14 (15.7) | 7 (19.4) | 7 (13.2) | |
| ECOG, n (%) | ||||
| 0–1 | 70 (78.6) | 28 (77.8) | 42 (79.2) | 0.83 |
| 2 | 19 (21.4) | 8 (22.2) | 11 (20.8) | |
| Smoking status, n (%) | ||||
| Never smoked | 14 (15.7) | 6 (16.7) | 8 (15.1) | 0.78 |
| Ex-smoker | 75 (96.0) | 30 (83.3) | 45 (84.9) | |
| T-stage, n (%) | ||||
| 1–2 | 38 (42.7) | 17 (47.2) | 21 (39.6) | 0.32 |
| 3–4 | 51 (57.3) | 19 (52.8) | 32 (60.4) | |
| N-stage, n (%) | ||||
| 1 | 22 (24.7) | 10 (27.8) | 12 (22.6) | 0.39 |
| 2–3 | 67 (75.3) | 26 (72.2) | 41 (77.4) | |
| TNM stage, n (%) | ||||
| II | 20 (22.5) | 9 (25.0) | 11 (20.8) | 0.46 |
| III | 69 (77.5) | 27 (75.0) | 42 (79.2) | |
| Mean pretreatment blood parameters, 103 per μL | ||||
| Monocytes | 0.44 (0.21–1.17) | 0.27 (0.22–0.64) | 0.56 (0.21–1.17) | <0.001 |
| Neutrophils | 4.13.0 (2.17–9.37) | 3.11 (2.17–6.48) | 5.89 (3.68–9.37) | 0.001 |
| Platelets | 276 (155–549) | 198 (155–336) | 297 (197–549) | 0.003 |
| Lymphocytes | 3.26 (1.71–5.13) | 4.28 (2.67–5.13) | 2.07 (1.71–5.13) | <0.001 |
| Anemia, n (%) | ||||
| Absent | 39 (43.8) | 19 (52,8) | 20 (37.7) | 0.19 |
| Present | 50 (56.2) | 17 (47,2) | 33 (62.3) | |
| Total chemotherapy cycles, n (%) | ||||
| 3 | 13 (14.6) | 5 (13.9) | 8 (15.1) | 0.73 |
| 4 | 76 (85.4) | 31 (86.1) | 45 (84.9) | |
PIV: pan-immune-inflammation value; ECOG: eastern cooperative oncology group; T-stage: tumor stage, N-stage: nodal stage; TNM: tumor-node-metastasis.
The median follow-up duration was 19.7 months (range: 4.0–88.1) for the whole cohort. During the final analysis, 22 (24.7%) patients were still alive, and 17 (19.1%) were free from disease progression. The median PFS and OS times were 11.0 months [95% confidence interval (CI): 9.5–12.5) and 18.0 months (95% CI: 14.5–21.5)] for the entire research group, respectively. The corresponding 5-year PFS and OS rates were 11.3% and 18.5%, respectively.
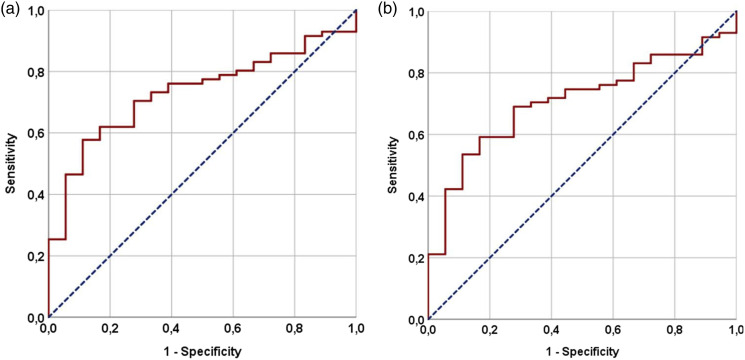
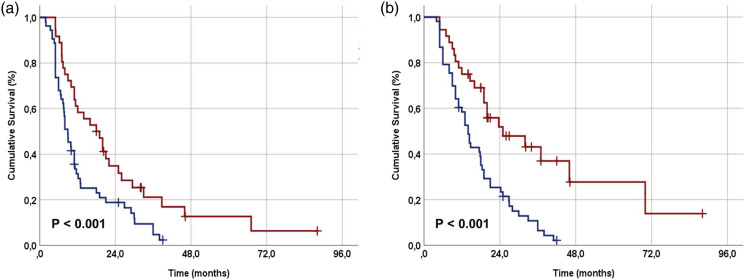
The ROC curve analysis determined 414 and 417 values as the ideal PIV cutoffs for PFS [Area under the curve (AUC): 70.8%; sensitivity: 69.4%; specificity: 67.1%] and OS (AUC: 73.2%; sensitivity: 70.4%; specificity: 67.7%) (Figure 1). We chose 417 as the common cutoff to divide patients into two groups for comparative analysis because the two cutoff values were so close: Group 1: L-PIV < 417 (N = 36) and Group 2: H-PIV ≥417 (N = 53). Comparing the two PIV groups based on their demographic and treatment characteristics revealed no significant differences, except for the cellular components of the PIV (Table 1). As expected, the results showed that the H-PIV group had significantly higher counts of monocytes (p = .017), neutrophils (p = .007), and platelets (p = .012), while the counts of lymphocytes (p = .004) were significantly lower in the same group (Table 1). The comparison of response rates based on the PERCIST showed that the L-PIV group had a significantly better overall objective response rate compared to the H-PIV group (55.6% vs. 18.9%; p = .002). According to the Kaplan–Meier analysis, L-PIV patients had significantly longer median PFS [18.0 (95% CI: 14.9–21.1) vs. 8.9 months (95% CI: (7.1–10.7); p = .004] and OS [25.0 (95% CI: 21.1–28.9) vs. 14.0 months (95% CI: 11.7–16.3); p < .001] durations than the H-PIV patients (Figure 2). Table 2 shows that the L-PIV group also had numerically better 3-year PFS and OS rates, suggesting a higher likelihood of achieving superior PFS and OS rates with L-PIV values in the long term.
Figure 1.
(a) Represents OS. (b) Represents PFS. Results of ROC analysis demonstrating the interaction between pretreatment Pan-Immune-Inflammation-Value measures and OS and PSS.
Figure 2.
Comparative survival outcomes per Pan-Immune-Inflammation Value (PIV) groups (a) progression-free survival, and (b) overall survival (red line: PIV < 417, and dark blue line: PIV ≥417).
Table 2.
Survival results per Pan-Immune-Inflammation value group.
| Outcome | Whole cohort (N = 89) | PIV < 417 (N = 36) | PIV ≥417 (N = 53) | p-value |
|---|---|---|---|---|
| Metabolic response rates, n (%) | ||||
| CMR | 18 (20.2) | 12 (33.3) | 6 (11.3) | 0.002 |
| PMR | 9 (10.1) | 6 (16.7) | 3 (5.7) | |
| SMD | 3 (3.4) | 2 (5.6) | 1 (1.9) | |
| PMD | 59 (66.3%) | 16 (44.4) | 43 (81.1) | |
| Progression-free survival | ||||
| Median (mo.) | 11.0 | 18.0 | 8.9 | <0.001 |
| 1-year (%) | 43.5 | 61.1 | 31.4 | |
| 2-years (%) | 25.4 | 34.8 | 18.8 | |
| 5-years (%) | 14.5 | 21.1 | 4.7 | |
| Overall survival | ||||
| Median (mo.) | 18.0 | 25.0 | 14.0 | <0.001 |
| 1-year (%) | 65.1 | 75.0 | 60.4 | |
| 2-years (%) | 35.9 | 51.9 | 25.3 | |
| 5-years (%) | 22.3 | 43.1 | 10.7 | |
PIV: pan-immune-inflammation value; CMR: complete metabolic response; PMR: partial metabolic response; SMD: stable metabolic disease; PMD: progressive metabolic disease; mo.: months.
ECOG performance status 0–1 (versus 2), T1–2 stage, N1 stage, absence of anemia (versus presence of anemia), and L-PIV value (versus H-PIV) were the factors identified that showed significant univariate connections with better PFS (p < .05 for each) and OS (p < .05 for each) outcomes (Table 3). According to the multivariate analysis shown in Table 3, all five factors retained their independent significance regarding their favorable effects on PFS (p < .05 for each) and OS (p < .05 for each).
Table 3.
The univariate and multivariate analyses results.
| Overall survival | Progression-free survival | |||||
|---|---|---|---|---|---|---|
| Factor | Univariate p-value |
Multivariate p-value |
HR | Univariate p-value |
Multivariate p-value |
HR |
| Gender (male vs female) | 0.24 | — | — | 0.34 | — | — |
| Age group (<70 vs. ≥70 years) | 0.20 | — | — | 0.49 | — | — |
| ECOG (0–1 vs. 2) | <0.001 | 0.008 | 1.96 | 0.002 | 0.011 | 1.78 |
| Anemia (absent vs present) | 0.016 | 0.024 | 1.64 | 0.032 | 0.036 | 1.52 |
| T-stage (1–2 vs. 3–4) | 0.009 | 0.017 | 1.42 | 0.004 | 0.007 | 1.51 |
| N-stage (1 vs. 2–3) | 0.006 | 0.009 | 1.63 | 0.002 | 0.005 | 1.74 |
| PIV (<417 vs. ≥ 417) | <0.001 | <0.001 | 3.28 | <0.001 | <0.001 | 2.39 |
HR: hazard ratio; ECOG: eastern cooperative oncology group; T-stage: tumor stage, N-stage: nodal stage; PIV: pan-immune-inflammation value.
Discussion
As a first attempt in LS-SCLC patients, this retrospective cohort analysis sought to determine the prognostic significance of pretreatment PIV values in terms of survival outcomes, namely PFS and OS, in a cohort of 89 LS-SCLC patients from two radiation oncology centers who underwent C-CRT followed by PCI. The study results validate that good performance status, lower T-stage, lower N-stage, and the lack of anemia are indicative of improved survival outcomes among this group of patients. More importantly, we discovered that pretreatment PIV ≥417 is associated with significantly lower median and 5-years PFS and OS rates.
The association between cancer initiation and progression and an exacerbated inflammatory response has been widely recognized. 36 The cellular constituents of the blood-borne inflammatory response include monocytes, neutrophils, platelets, lymphocytes, and their derivatives. Neutrophils facilitate the processes of tumor angiogenesis, adhesion of circulating tumor cells, and the occurrence of distant metastasis. 37 An elevation in neutrophil count may incite the discharge of substantial amounts of reactive oxygen and nitric oxide species, potentially leading to impaired T-cell function.38–40 The process of tumorigenesis comprises several stages, namely initiation, growth, migration, vascularization, invasion, and metastasis, all of which are susceptible to modulation by increased counts of platelets, monocytes, and macrophages.41,42 Platelets, in particular, play a crucial role in the development of distant metastases. 43 Lymphocytes are distinguished from other cell types due to their significant contribution to the immune response of the body. In advanced stages of cancer, a reduction in lymphocyte infiltration is a frequent observation, which leads to a favorable environment for metastasis.44,45 In the absence of analogous research, the indisputable fundamental data regarding the cellular constituents of the PIV, along with prior PIV investigations in different cancer categories, motivated us to explore its prognostic efficacy in LS-SCLC patients who underwent C-CRT and PCI.
The present study’s results, analogous to previous research, indicate that the initial ECOG performance, T-stage, N-stage, and anemia status are significant prognostic factors that impact the survival outcomes of these patients.46,47 The novel contribution of our research to the literature on SCLC is the initial evidence of a robust and independent connection between pretreatment L-PIV measurements and improved median progression-free survival (PFS) (18.0 vs 8.9 months; p < .001) and overall survival (OS) (25 vs 14 months; p < .001) durations in LS-SCLC patients who underwent C-CRT and PCI. Additionally, the L-PIV cohort’s comparatively higher 3-years survival rates indicate the sustained longevity of this survival benefit for patients exhibiting L-PIV measures prior to the commencement of C-CRT. These findings appear to be associated with higher objective response rates in the L-PIV group than in the H-PIV group (55.6% versus 18.9%; p = .002). The absence of PIV studies with a similar design in LS-SCLC patients presents a challenge for drawing definitive conclusions. However, the findings of our study are consistent with prior PIV investigations conducted at various tumor locations,21–32 and a recent PIV study reported by Zeng et al. for ES-SCLC patients. 32 Zeng and colleagues conducted a study wherein they combined chemotherapy with anti-PD-1/PD-L1 inhibitors to treat 53 patients with ES-SCLC. 32 The authors found that ES-SCLC patients who belonged to H-PIV had significantly shorter median PFS (3.37 months versus 7.70 months; p < .0001) and OS (7.27 months versus 16.07 months; p < .0001) compared to those who belonged to L-PIV. All these studies have consistently shown that PIV is a strong predictor of immune and inflammatory responses, regardless of the type or stage of the disease being studied. These findings hold even when considering the differences in tumor types, disease stages, treatment options, and research methods used across the studies. However, to substantiate this concluding statement, further research findings are required.
The precise relationship between lower PIV scores and better clinical results in LS-SCLC patients who undergo C-CRT and PCI remains uncertain. However, comparing our results with those of previously reported immune-inflammation biomarker studies in SCLC patients may yield reasonable conclusions. Previous studies have shown that PIV holds prognostic value in different solid tumors, including the ES-SCLC, regardless of the stage and treatment modalities.21–32 These findings suggest that the newly introduced PIV, encompassing the four principal cell categories involved in immune and inflammatory responses, constitutes a robust and dependable predictive indicator across almost all solid tumors, irrespective of disease stage or medical interventions, though the cutoffs used differ. Furthermore, given the definition of a prognostic factor, its independence from treatment choice validates its prognostic appropriateness. 48 And second, PIV is a four-cell-based biological marker that can be reformulated as PIV = [Platelets × SIRI (systemic inflammatory response index)] or PIV = [Monocytes × SII (systemic immune-inflammation index)]. Thus, the correlation between pretreatment PIV measures and patient prognosis can be elucidated by examining the well-established functions of platelets and monocytes in various stages of carcinogenesis and disease progression, along with the SIRI and SII outcomes found in SCLC patients. Wang et al. investigated the predictive value of SII in 228 SCLC patients and discovered that patients with SII 479 had worse OS (p < .001) and PFS (p < .001) independent of other variables. 49 To determine the clinical significance of pretreatment inflammation-based scores as prognostic indicators for SCLC patients’ survival, Hong et al. examined the medical records of 919 patients. 50 The authors found that SII ≥ 1600 was one of the inflammation-based scores that had a significant relationship with survival outcomes. The prognostic significance of pretreatment SII values was investigated by Wang et al. in a cohort of 653 patients with SCLC who received etoposide and platinum chemotherapy. The study findings revealed that patients with a pretreatment SII value of ≥748 had a higher incidence of metastasis and lower median OS durations. 19 The studies conducted on SIRI have also yielded similar results.18,20 Yilmaz et al. conducted a study on a cohort of 162 patients with ES-SCLC and found that a SIRI >1.5 was a significant independent predictor of poorer PFS and OS outcomes. 20 Kucuk and colleagues conducted a study to evaluate the prognostic value of SIRI in patients with LS-SCLC who underwent C-CRT. The study included 110 patients, and the results showed that the group with SIRI<1.93 had a significantly longer median OS (40.5 vs 14.2 months; p < .001) compared to the SIRI ≥1.93 group. 18 Undoubtedly, more investigation is required to elucidate the exact mechanism underlying the link between poor survival outcomes and high PIV values. Anyhow, as demonstrated in this study and other SCLC studies, the deteriorated PFS and OS outcomes in the H-PIV patient group may be associated with the effects of reduced immunogenic lymphocyte counts and higher inflammatory and immunosuppressive monocyte, platelet, and neutrophil counts.
There are several limitations to our research. In addition to its retrospective design, the study includes a small number of institutions and cohort size, making it susceptible to selection bias. Second, we did not perform power analysis for sample size calculation which may have under- or overestimate some results. Third, our study only focused on blood count data before treatment, despite the dynamic nature of PIV as a biomarker. And fourth, the unavoidable diversity in salvage therapies may have impacted the outcomes of each group, either positively or negatively. Therefore, conducting further research that addresses these limitations has the potential to produce significant outcomes. Although future multicenter prospective studies are necessary, our findings indicate that PIV based on simple blood-borne markers can reliably predict prognosis in LS-SCLC patients who undergo C-CRT and PCI.
Conclusions
The findings of this retrospective study suggest that a high pretreatment PIV can serve as a reliable prognostic biomarker for LS-SCLC patients, as it was found to be significantly and independently associated with unfavorable OS and DFS outcomes. If subsequent studies confirm it, pretreatment PIV could be an effective tool for determining LS-SCLC patients with a poor prognosis, allowing for tailored therapies specific to these groups in routine clinical practice.
Footnotes
Author contributions: All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval for this study was obtained from Baskent University Faculty of Medicine Institutional Review Board and the Mersin Provincial Health Directorate (2021/0134).
Informed consent: Written Informed Consent was obtained from all subjects before the study to collect and analyze blood samples and pathological specimens, as well as publish the resulting findings.
ORCID iDs
Erkan Topkan https://orcid.org/0000-0001-8120-7123
Duriye Ozturk https://orcid.org/0000-0002-3265-2797
Data availability statement
The data that support the findings of this study are available upon reasonable request. They are available from the Baskent University Department of Radiation Oncology Institutional Board.
References
- 1.Govindan R, Page N, Morgensztern D, et al. (2006) Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology 24(28): 4539–4544. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. (2019) Cancer statistics, 2019. CA: A Cancer Journal for Clinicians 69(1): 7–34. [DOI] [PubMed] [Google Scholar]
- 3.Van Meerbeeck JP, Fennell DA, De Ruysscher DK. (2011) Small-cell lung cancer. Lancet 378(9804): 1741–1755. [DOI] [PubMed] [Google Scholar]
- 4.Demedts IK, Vermaelen KY, van Meerbeeck JP. (2010) Treatment of extensive-stage small cell lung carcinoma: current status and future prospects. The European Respiratory Journal 35(1): 202–215. [DOI] [PubMed] [Google Scholar]
- 5.Liu SV, Reck M, Mansfield AS, et al. (2021) Updated overall survival and PD-L1 subgroup analysis of patients with extensive-stage small-cell lung cancer treated with atezolizumab, carboplatin, and etoposide (IMpower133). Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology 39: 619–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paz-Ares L, Dvorkin M, Chen Y, et al. CASPIAN Investigators (2019) Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet 394: 1929–1939. [DOI] [PubMed] [Google Scholar]
- 7.Horn L, Mansfield AS, Szczęsna A, et al. IMpower133 Study Group (2018) First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. The New England Journal of Medicine 379: 2220–2229. [DOI] [PubMed] [Google Scholar]
- 8.Turrisi AT, Kim K, Blum R, et al. (1999) Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. The New England Journal of Medicine 340: 265–271. [DOI] [PubMed] [Google Scholar]
- 9.Auperin A, Arriagada R, Pignon JP, et al. (1999) Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic cranial irradiation overview collaborative group. The New England Journal of Medicine 341: 476–484. [DOI] [PubMed] [Google Scholar]
- 10.Johnson BE, Bridges JD, Sobczeck M, et al. (1996) Patients with limited-stage small-cell lung cancer treated with concurrent twice-daily chest radiotherapy and etoposide/cisplatin followed by cyclophosphamide, doxorubicin, and vincristine. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology 14: 806–813. [DOI] [PubMed] [Google Scholar]
- 11.De Visser KE, Eichten A, Coussens LM. (2006) Paradoxical roles of the immune system during cancer development. Nature Reviews. Cancer 6(1): 24–37. [DOI] [PubMed] [Google Scholar]
- 12.Mantovani A, Allavena P, Sica A, et al. (2008) Cancer-related inflammation. Nature 454(7203): 436–444. [DOI] [PubMed] [Google Scholar]
- 13.Placke T, Salih HR, Kopp HG. (2012) GITR ligand provided by thrombopoietic cells inhibits NK cell antitumor activity. Journal of Immunology (Baltimore, MD, 1950) 189(1): 154–160. [DOI] [PubMed] [Google Scholar]
- 14.Labelle M, Begum S, Hynes RO. (2011) Direct signaling between platelets and cancer cells induces an epithelial–mesenchymal-like transition and promotes metastasis. Cancer Cell 20(5): 576–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schumacher D, Strilic B, Sivaraj KK, et al. (2013) Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis via P2Y2 receptor. Cancer Cell 24(1): 130–137. [DOI] [PubMed] [Google Scholar]
- 16.Gil-Bernabe AM, Ferjancic S, Tlalka M, et al. (2012) Recruitment of monocytes/macrophages by tissue factor-mediated coagulation is essential for metastatic cell survival and premetastatic niche establishment in mice. Blood 119(13): 3164–3175. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki R, Wei X, Allen PK, et al. (2019) Prognostic significance of total lymphocyte count, neutrophil-to-lymphocyte ratio, and platelet-to-lymphocyte ratio in limited-stage small-cell lung cancer. Clinical Lung Cancer 20(2): 117–123. [DOI] [PubMed] [Google Scholar]
- 18.Kucuk A, Ozkan EE, Eskici Oztep S, et al. (2020) The influence of systemic inflammation response index on survival outcomes of limited-stage small-cell lung cancer patients treated with concurrent chemoradiotherapy. Journal of Oncology 2020: 8832145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang C, Jin S, Xu S, et al. (2020) High systemic immune-inflammation index (SII) represents an unfavorable prognostic factor for small cell lung cancer treated with etoposide and platinum-based chemotherapy. Lung 198: 405–414. [DOI] [PubMed] [Google Scholar]
- 20.Yilmaz H, Yersal O. (2022) Prognostic significance of novel inflammatory markers in extensive-stage small-cell lung cancer. Journal of Cancer Research and Therapeutics 18(3): 691–696. [DOI] [PubMed] [Google Scholar]
- 21.Fucà G, Guarini V, Antoniotti C, et al. (2020) The pan-immune-inflammation value is a new prognostic biomarker in metastatic colorectal cancer: results from a pooled-analysis of the Valentino and TRIBE first-line trials. British Journal of Cancer 123(3): 403–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corti F, Lonardi S, Intini R, et al. (2021) The pan-immune-inflammation value in microsatellite instability-high metastatic colorectal cancer patients treated with immune checkpoint inhibitors. European Journal of Cancer (Oxford, England: 1990) 150: 155–167. [DOI] [PubMed] [Google Scholar]
- 23.Sato S, Shimizu T, Ishizuka M, et al. (2022) The preoperative pan-immune-inflammation value is a novel prognostic predictor for with stage I–III colorectal cancer patients undergoing surgery. Surgery Today 52(8): 1160–1169. [DOI] [PubMed] [Google Scholar]
- 24.Ligorio F, Fucà G, Zattarin E, et al. (2021) The pan-immune-inflammation-value predicts the survival of patients with human epidermal growth factor receptor 2 (HER2)-positive advanced breast cancer treated with first-line taxane-trastuzumab-pertuzumab. Cancers 13(8): 1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Şahin AB, Cubukcu E, Ocak B, et al. (2021) Low pan-immune-inflammation-value predicts better chemotherapy response and survival in breast cancer patients treated with neoadjuvant chemotherapy. Scientific Reports 11(1): 14662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Demir H, Demirci A, Eren SK, et al. (2022) A new prognostic index in young breast cancer patients. Journal of the College of Physicians and Surgeons-Pakistan: JCPSP 32(1): 86–91. [DOI] [PubMed] [Google Scholar]
- 27.Baba Y, Nakagawa S, Toihata T, et al. (2022) Pan-immune-inflammation value and prognosis in patients with esophageal cancer. Annals of Surgery Open 3(1): e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen X, Hong X, Chen G, et al. (2022) The pan-immune-inflammation value predicts the survival of patients with anaplastic lymphoma kinase-positive non-small cell lung cancer treated with first-line ALK inhibitor. Translational Oncology 17: 101338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gambichler T, Said S, Abu Rached N, et al. (2022) Pan-immune-inflammation value independently predicts disease recurrence in patients with Merkel cell carcinoma. Journal of Cancer Research and Clinical Oncology 148(11): 3183–3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fucà G, Beninato T, Bini M, et al. (2021) The pan-immune-inflammation value in patients with metastatic melanoma receiving first-line therapy. Targeted Oncology 16(4): 529–536. [DOI] [PubMed] [Google Scholar]
- 31.Guven DC, Sahin TK, Erul E, et al. (2022) The association between the pan-immune-inflammation value and cancer prognosis: a systematic review and meta-analysis. Cancers (Basel) 14(11): 2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeng R, Liu F, Fang C, et al. (2021) PIV and PILE score at baseline predict clinical outcome of anti-PD-1/PD-L1 inhibitor combined with chemotherapy in extensive-stage small cell lung cancer patients. Frontiers in Immunology 12: 724443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wahl RL, Jacene H, Kasamon Y, et al. (2009) From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. Journal of Nuclear Medicine: Official Publication, Society of Nuclear Medicine. 50(Suppl 1):122S–150S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caponio VCA, Troiano G, Togni L, et al. (2023) Pattern and localization of perineural invasion predict poor survival in oral tongue carcinoma. Oral Diseases 29(2): 411–422. [DOI] [PubMed] [Google Scholar]
- 35.Heinze G, Dunkler D. (2017) Five myths about variable selection. Transplant International: Official Journal of the European Society for Organ Transplantation 30: 6–10. [DOI] [PubMed] [Google Scholar]
- 36.Dolan RD, McSorley ST, Horgan PG, et al. (2017) The role of the systemic inflammatory response in predicting outcomes in patients with advanced inoperable cancer: systematic review and meta-analysis. Critical Reviews in Oncology/hematology 116: 134–146. [DOI] [PubMed] [Google Scholar]
- 37.Dvorak HF. (2015) Tumor stroma, tumor blood vessels, and antiangiogenesis therapy. Cancer Journal (Sudbury, Mass.) 21: 237–243. [DOI] [PubMed] [Google Scholar]
- 38.Tecchio C, Scapini P, Pizzolo G, et al. (2013) On the cytokines produced by human neutrophils in tumors. Seminars in Cancer Biology 23(3): 159–170. [DOI] [PubMed] [Google Scholar]
- 39.Kuper H, Adami HO, Trichopoulos D. (2000) Infections as a major preventable cause of human cancer. Journal of Internal Medicine 248(3): 171–183. [DOI] [PubMed] [Google Scholar]
- 40.Muller I, Munder M, Kropf P, et al. (2009) Polymorphonuclear neutrophils and T lymphocytes: strange bedfellows or brothers in arms? Trends in Immunology 30(11): 522–530. [DOI] [PubMed] [Google Scholar]
- 41.Sica A, Allavena P, Mantovani A. (2008) Cancer related inflammation: the macrophage connection. Cancer Letters 267(2): 204–215. [DOI] [PubMed] [Google Scholar]
- 42.Gouveia-Fernandes S. (2020) Monocytes and macrophages in cancer: unsuspected roles. Tumor Microenvironment Advances in Experimental Medicine and Biology. 1219: 161–185. [DOI] [PubMed] [Google Scholar]
- 43.Li Y, Li H, He J, et al. (2016) Platelets in cancer metastasis: To help the “villain” to do evil. Chemistry, an Asian Journal 11: 2078–2084.27305650 [Google Scholar]
- 44.Ferrone C, Dranoff G. (2010) Dual roles for immunity in gastrointestinal cancers. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology 28(26): 4045–4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hanahan D, Weinberg RA. (2011) Hallmarks of cancer: the next generation. Cell 144(5): 646–674. [DOI] [PubMed] [Google Scholar]
- 46.Ma X, Zhang Z, Chen X, et al. (2021) Prognostic factor analysis of patients with small cell lung cancer: real-world data from 988 patients. Thoracic Cancer 12(12): 1841–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laurie SA, Logan D, Markman BR, et al. Lung Cancer Disease Site Group of Cancer Care Ontario’s Program in Evidence-Based Care (2004) Practice guideline for the role of combination chemotherapy in the initial management of limited-stage small-cell lung cancer. Lung Cancer 43: 223–240. [DOI] [PubMed] [Google Scholar]
- 48.Clark GM. (2008) Prognostic factors versus predictive factors: examples from a clinical trial of erlotinib. Molecular Oncology 1(4): 406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang D, Guo D, Shi F, et al. (2019) The predictive effect of the systemic immune-inflammation index for patients with small-cell lung cancer. Future Oncology (London, England) 15(29): 3367–3379. [DOI] [PubMed] [Google Scholar]
- 50.Hong X, Cui B, Wang M, et al. (2015) Systemic immune-inflammation index, based on platelet counts and neutrophil–lymphocyte ratio, is useful for predicting prognosis in small cell lung cancer. The Tohoku Journal of Experimental Medicine 236(4): 297–304. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available upon reasonable request. They are available from the Baskent University Department of Radiation Oncology Institutional Board.