Abstract
Background and aims
Familial hypercholesterolemia (FH) is among the most common genetic disorders in primary care. However, only 15% or less of patients are diagnosed, and few achieve the goals for low-density lipoprotein cholesterol (LDL-C). In this analysis of the German Cascade Screening and Registry for High Cholesterol (CaRe High), we examined the status of lipid management, treatment strategies, and LDL-C goal attainment according to the ESC/EAS dyslipidemia guidelines.
Methods
We evaluated consolidated datasets from 1501 FH patients diagnosed clinically and seen either by lipid specialists or general practitioners and internists. We conducted a questionnaire survey of both the recruiting physicians and patients.
Results
Among the 1501 patients, 86% regularly received lipid-lowering drugs. LDL-C goals were achieved by 26% and 10% of patients with atherosclerotic cardiovascular disease (ASCVD) according to the 2016 and 2019 ESC/EAS dyslipidemia guidelines, respectively. High intensity lipid-lowering was administered more often in men than in women, in patients with ASCVD, at higher LDL-C and in patients with a genetic diagnosis of FH.
Conclusions
FH is under-treated in Germany compared to guideline recommendations. Male gender, genetic proof of FH, treatment by a specialist, and presence of ASCVD appear to be associated with increased treatment intensity. Achieving the LDL-C goals of the 2019 ESC/EAS dyslipidemia guidelines remains challenging if pre-treatment LDL-C is very high.
Keywords: Familial hypercholesterolemia, Cascade screening, Patient registry, Low-density lipoproteins, Cardiovascular risk, Treatment, Adherence
Highlights
-
•
FH is undertreated in Germany compared to guideline recommendations.
-
•
Male gender, genetic proof of FH, treatment by a specialist, and ASCVD are associated with increased treatment intensity.
-
•
Achieving the LDL-C goals of the latest dyslipidemia guidelines remains challenging if pre-treatment LDL-C is very high.
1. Introduction
Familial hypercholesterolemia (FH) is a genetic, mostly co-dominant disorder of lipoprotein metabolism causing elevated low-density lipoprotein cholesterol (LDL-C) from childhood and a high risk of premature atherosclerotic cardiovascular disease (ASCVD) if left untreated. FH is estimated to cause up to 20% of myocardial infarctions before the age of 45 and up to 5% of myocardial infarctions before the age of 60 [1,2].
The most common genetic mutations affect the genes for the low-density lipoprotein receptor (LDLR), accounting for approximately 90%, as well as apolipoprotein B (APOB), or proprotein convertase subtilisin/kexin type 9 (PCSK9) [[3], [4], [5]].
Due to underdiagnosis and undertreatment of FH, a premature cardiovascular event often is the first clinical manifestation of FH with therapeutic implications, [6,7]. Early and effective treatment with lipid-lowering drugs may allow a normal life expectancy [8,9].
The estimated prevalence of FH in Germany is 1:300; thus, heterozygous FH may affect 270,000 Germans [10]. However, only a small proportion of FH patients in Germany seem to be diagnosed and adequately treated [11]. The latest guidelines for the management of dyslipidemia was issued on 2019 and classifies FH per se as high cardiovascular risk. For FH patients with pre-existing ASCVD or another risk factor, the LDL-C goal is < 55 mg/dl (<1.4 mmol/l), whereas for FH patients without pre-existing cardiovascular disease (CVD) or any other risk factor the LDL-C goal is < 70 mg/dl (<1.8 mmol/l) [12]. The 2016 EAS/ESC dyslipidemia guidelines with higher LDL-C target values were still valid when this study started [13].
To overcome underdiagnosis and undertreatment of FH in Germany, the Cascade Screening and Registry for High Cholesterol (CaRe High) project was established in 2015 [14]. This interim analysis of the registry examines the status of treatment and achievement of LDL-C goals according to the 2016 and 2019 ESC/EAS dyslipidemia guidelines in the first 1501 patients included in the study.
2. Patients and Methods
2.1. Study design and population
The design of CaRe High has been described previously [11,14]. Written informed consent was obtained from each patient. The study protocol complies with the 1975 Declaration of Helsinki and was approved by the ethics committee of the physicians’ chamber of the Federal State of Baden-Württemberg and the ethics committees responsible for each of the participating centers. Inclusion criteria were LDL-C ≥190 mg/dl (4.9 mmol/L) without lipid-lowering therapy (LLT) or total cholesterol >290 mg/dl (7.5 mmol/l) and at least one of the following criteria: tendon xanthomas, family history of hypercholesterolemia, family history of myocardial infarction before the age of 50 in grandparents, uncles, or aunts or before the age of 60 in parents, siblings, or children. These inclusion criteria are in line with previous recommendations made by Klose et al. [15]. For descriptive purposes, we also calculated the Dutch Lipid Clinic Network (DLCN) Score for FH [6].
FH patients without and with CVD at the time of study inclusion were assigned to the category “primary prevention” and “secondary prevention”, respectively. CVD was defined as a history of at least one myocardial infarction, coronary revascularization, angina pectoris peripheral arterial disease (PAD), or transient ischemic attack, or stroke.
The study data considered here were collected from September 2015 to the end of October 2020.
For this evaluation, diabetes mellitus (including type 1 and 2) was defined by either the physicians’ diagnosis, HbA1c ≥ 6.5%, fasting glucose ≥125 mg/dl, or the use of antidiabetic medications (insulin, sulfonylurea, alpha-glucosidase inhibitors, GLP-1 receptor agonists, SGTL2-2 inhibitors, metformin, glinides, glitazones, DPP4- inhibitors). Obesity was defined by a body mass index (BMI) of 30 kg/m2 or higher [16]. Hypertension was defined by a written diagnosis in reports, intake of antihypertensive drugs, or systolic (resp. diastolic) blood pressure >140 mmHg (resp. 90 mmHg) in a single measurement during an actual visit.
2.2. Treating physicians
We distinguished between two groups of treating physicians: general practitioners (GPs) and internists without special training in lipids, or lipid specialists (cardiologists, angiologists, nephrologists, endocrinologists, and physicians in lipid outpatient clinics).
For patients referred to lipid clinics or lipid specialists, we used the laboratory values and medication at the time of study inclusion; this may coincide with the initial consultation with the lipid expert and reflect the treatment strategy of the referring physician. The treatment status without lipid-lowering therapy, designated as “no LLT”, applies to the treatment status at the time of registry entry and does not rule out that a patient had been treated earlier and discontinued therapy or received treatment after study inclusion.
2.3. Laboratory parameters
Current laboratory data rely on questionnaires filled out by physicians. The following parameters were assessed: LDL-C, total cholesterol, high-density lipoprotein-cholesterol (HDL-C), triglycerides, thyroid-stimulating hormone (TSH), hemoglobin A1c (HbA1c), fasting plasma glucose (FPG). High sensitivity C-reactive protein (CRP), alanine-amino transferase (ALAT), aspartate-amino transferase (ASAT), bilirubin, alkaline phosphatase, uric acid, urea, and creatinine.
2.4. Genetic testing
Genetic testing was performed by different laboratories throughout Germany without common strategy.
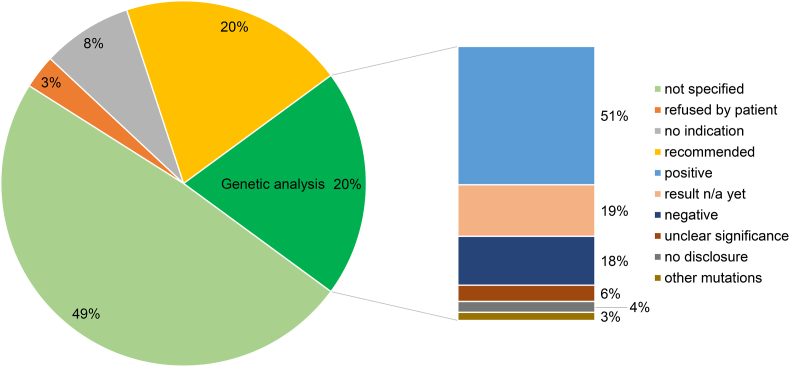
Functional mutations found in LDLR, APOB, or PCSK9 genes and homozygous recessive mutations in the low-density lipoprotein receptor adapter protein 1 (LDLRAP1) gene were considered as positive genetic test result for FH (Fig. 1). Patients with two apoliprotein E (APOE) genotypes ε3/ε4 and ε4/ε4 (n = 9), but no major FH mutation were assigned to a separate category designated “other mutations”.
Fig. 1.
Status, availability and results of genetic analyses of FH patients participating in the CaRe High registry.
According to the inclusion criteria mentioned above, a positive genetic test was not required for participation in CaRe High. Results of any genetic analysis were obtained when available and only if written informed consent was obtained from the patient.
2.5. Treatment status
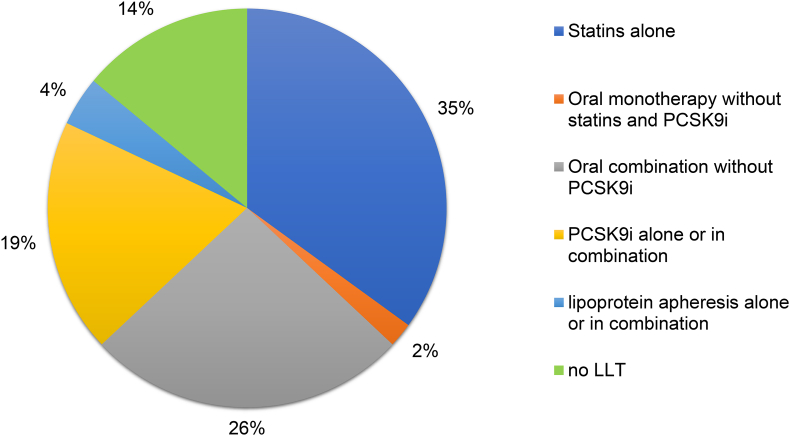
The treatment status refers to the status at the time of study inclusion (often the first visit at a lipid outpatient clinic) and is categorized as follows (Fig. 2, Fig. 3, Supplementary Fig. 1): a) no LLT, b) statins alone, c) oral combinations without PCKS9i, i.e. two or more of the following oral agents: statins, ezetimibes, fibrates, bile acid sequestrants, d) oral monotherapy without statins and without PCSK9 inhibitors (PCSK9i), e) PCSK9i alone or in combination with other lipid-lowering agents, f) apheresis alone or in combination with other lipid-lowering therapy.
Fig. 2.
Lipid-lowering therapy (LLT) of patients with FH at the time of study inclusion: a) no LLT, b) statins alone; c) combinations without PCKS9i, i.e. two or more of the following agents: statins, ezetimibes, fibrates, bile acid sequestrants; d) oral monotherapy without statins and without PCSK9 inhibitors (PCSK9i); e) PCSK9i alone or in combination with other lipid-lowering agents; f) apheresis alone or in combination with other lipid-lowering therapy.
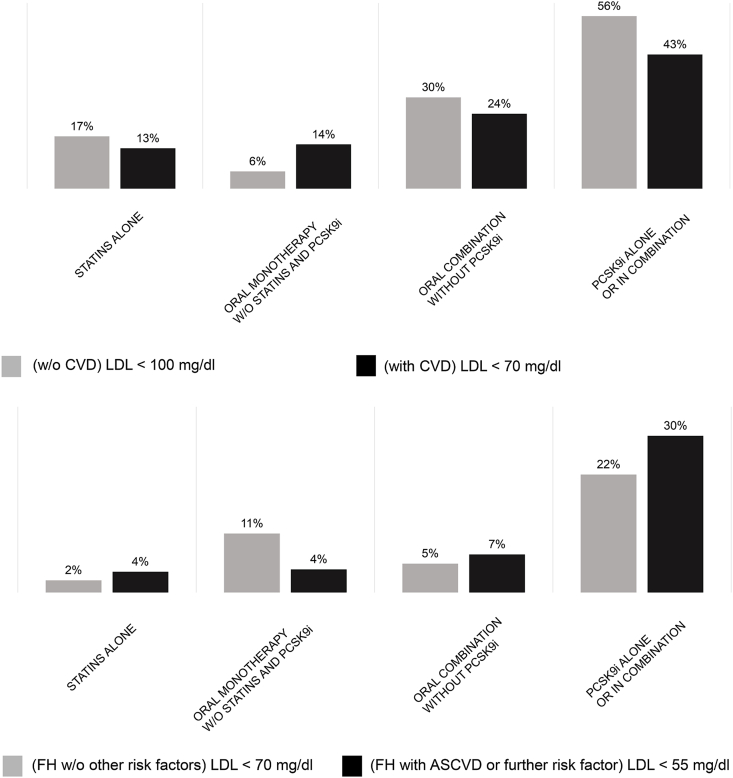
Fig. 3.
LDL-C goal attainment according to EAS/ESC dyslipidemia guidelines 2016 (A), 2019 (B). Treatment goals were: (A) less than 100 mg/dl (2.6 mmol/l) without pre-existing CVD and less than 70 mg/dl (1.8 mmol/l) for FH patients with pre-existing CVD; (B) less than 70 mg/dl (1.8 mmol/l) for FH without pre-existing CVD or any other cardiovascular risk factor and less than 55 mg/dl (1.4 mmol/l) for FH with pre-existing CVD or another cardiovascular risk factor.
2.6. LDL-C goals
The 2016 EAS/ESC dyslipidemia guidelines [13] applied when 88% of patients were recruited. We also applied the 2019 EAS/ESC guidelines [12] to the analysis of LDL-C goal attainment. Based on original therapy-naïve or therapy-corrected baseline LDL-C levels, we estimated how many FH patients achieved a 50% LDL-C reduction with therapy, which is recommended as an additional goal by the 2019 version of the EAS/ESC dyslipidemia guidelines.
2.7. Multivariate regression analysis for treatment
To categorize drug treatment into different intensities, we made use of published data for the efficacy of statins [17]. In the case of additional PCSK9i treatment, we assumed an LDL-C reduction of 50% by PCSK9 inhibition [[18], [19], [20], [21]], corresponding to an index value of 2. For ezetimibe, fibrates, and bile acid sequestrants, the corresponding intensities were set to 1.2, 1.15, and 1.1, respectively [[22], [23], [24], [25]]. We assigned the intensity value 1 to the treatment category “no LLT”. The intensities of drug combinations were represented by the product of their individual. For instance, a patient receiving atorvastatin 80 mg per day (intensity 2.2) plus 10 mg ezetimibe (intensity 1.2) plus a PCSK9i (intensity 2) was assigned a treatment intensity of 5.28. No intensity score was assigned to apheresis (4% of the population). High intensity therapy was defined as a regimen offering an LDL-C reduction on average by ≥ 50% (index ≥2).
To examine which factors are significantly associated with treatment intensity, we estimated odds ratios (ORs) and their 95% confidence intervals (CIs) as part of a multivariate logistic regression making the following comparisons: A) low-medium intensity treatment versus no treatment, B) high intensity therapy versus low-medium intensity treatment [26].
The independent variables were age, sex (reference: female), presence of ASCVD (reference: no ASCVD), diabetes mellitus (reference: no diabetes mellitus), obesity (reference: no obesity), hypertension (reference: no hypertension), therapy-corrected LDL-C, genetic test (with the categories 'positive', 'negative' and 'not available or not performed' with ‘negative’ as reference category).
We also conducted two supplementary analyses. In the first of these analyses, we compared patients receiving monotherapy with those receiving no treatment (Supplementary Fig. 2A), and those receiving combination therapy with those receiving monotherapy (Supplementary Fig. 2B). In the second supplementary analysis, we compared patients receiving generic agents (statins, ezetimibe, fibrates, bile acid sequestrants) with those receiving no treatment (Supplementary Fig. 3A), and those receiving PCSK9i alone or in combination (Supplementary Fig. 3B) with those treated with monotherapy.
2.8. Other statistical analysis
Pearson's chi-squared test and the Fisher exact test for small samples were used to analyze the associations between two categorical variables. Metric data were compared between two groups using the Wilcoxon rank sum test. To compare metric data between more than two groups, we performed the Kruskal-Wallis rank sum test, followed by pairwise comparisons with the Wilcoxon rank sum tests.
All computations were performed in R version 4.0.2 (R functions fisher.test, kruskal.test, wilcox.test, and glm from the R library stats and the R function vglm from the R library VGAM) [[27], [28], [29]].
3. Results
3.1. Study population
A total of 1501 patients with cleared datasets by November 2020 were included in this analysis. Median age (25th and 75th percentiles) at the time of registry entry and at first diagnosis of FH was 56 years (46–64 years) and 40 years (29–50 years), respectively. The median (25th and 75th percentiles) corrected LDL-C concentration was 238 mg/dl (184–339 mg/dl) or 6.15 mmol/l (4.76–8.77 mmol/l), median actual LDL-C 140 mg/dl (92–201 mg/dl) or 3.62 mmol/l (2.38–5.2 mmol/l), median HDL-C 54 mg/dl (44–66 mg/dl) or 1.46 mmol/l (1.14–1.71 mmol/l), and median triglycerides 127 mg/dl (89–186 mg/dl) or 1.44 mmol/l (1.01–2.1 mmol/l). Most laboratory values were within the clinical reference range. A total of 13.3% patients had diabetes mellitus, 34.6% had hypertension, and 22.1% were obese (Table 1).
Table 1.
Characteristics of patients in the CaRe High registry.
| BINARY/CATEGORICAL VARIABLES | |
|---|---|
| Variable | N (%) |
| Male | 682 (45.4%) |
| Female | 819 (54.6%) |
| Treatment by specialists | 1311 (87.3%) |
| DLCN, FH unlikely | 72 (5.8%) |
| DLCN, possible FH | 522 (42%) |
| DLCN, probable FH | 358 (28.8%) |
| DLCN, definite FH | 291 (23.4%) |
| Diabetes mellitus (type 1 or 2) | 200 (13.3%) |
| Hypertension | 520 (34.6%) |
| Obesity | 332 (22.1%) |
| No additional cardiovascular risk factor | 728 (48.5%) |
| One additional cardiovascular risk factor | 531 (35.4%) |
| Two cardiovascular risk factors | 205 (13.7%) |
| Three or more cardiovascular risk factors | 37 (2.5%) |
|
METRIC VARIABLES | |||
|---|---|---|---|
| Parameter, unit | #(defined values) | mean ±SDa | median (25th-75th percentile)a |
| Age, years | 1501 | 54 ± 14 | 56 (46–64) |
| Age at first diagnosis, years | 978 | 40 ± 14 | 40 (29–50) |
| BMI, kg/m2 | 1427 | 26.6 ± 5 | 26 (23–29) |
| LDL-C, mg/dl | 1434 | 151 ± 73 | 140 (92–201) |
| LDL-C, mmol/l | 1434 | 3.91 ± 1.89 | 3.62 (2.38–5.20) |
| LDL-C (corrected for treatment), mg/dl | 1434 | 278 ± 147 | 238 (184–339) |
| LDL-C (corrected for treatment), mmol/l | 1434 | 7.19 ± 3.79 | 6.15 (4.76–8.77) |
| HDL-C, mg/dl | 1405 | 57 ± 18 | 54 (44–66) |
| HDL-C, mmol/l | 1405 | 1.46 ± 0.47 | 1.40 (1.14–1.71) |
| Total cholesterol (with treatment), mg/dl | 1429 | 226 ± 81 | 215 (165–282) |
| Total cholesterol (on treatment), mmol/l | 1429 | 5.84 ± 2.09 | 5.56 (4.27–7.29) |
| Triglycerides, mg/dl | 1372 | 149 ± 86 | 127 (89–186) |
| Triglycerides, mmol/l | 1372 | 1.68 ± 0.97 | 1.44 (1.01–2.10) |
| Thyroid stimulating hormone (TSH), μIU/ml | 705 | 1.83 ± 1.02 | 1.59 (1.10–2.30) |
| HbA1c, % | 776 | 5.74 ± 0.77 | 5.6 (5.3–5.9) |
| Fasting glucose, mg/dl | 790 | 98 ± 21 | 94 (87–103) |
Only metric values ≥ 0.2 x median and ≤5 x median value (related to the totality of persons with defined values) are taken into account.
3.2. Genetic testing
Genetic testing for FH had been conducted in 297 (20%) of 1501 individuals (Fig. 1). Among the 1204 patients without genetic testing, testing had been recommended in 297 but had not (yet) been performed. In 128 patients (8% of the total cohort), the treating physician indicated “no indication for genetic testing” on our questionnaires and 41 patients (3% of entire cohort) refused to undergo genetic testing.
In 150 (51%) tested individuals, FH-causing LDLR, APOB, PCSK9 or LDLRAP1 mutations were found. Nine (3%) patients carried at least one ε4 allele at the APOE locus in the absence of a major FH mutation (“other mutations”). In 18 (6%) patients, the genetic findings were of unclear significance.
No FH-causing mutation was found in 54 (18%) tested individuals, and 11 (4%) patients refused to provide their results. For 55 (19%) patients, genetic testing had been ordered but the result was still pending at the time of our analysis.
3.3. Treatment status at the time of study inclusion
At the time of study inclusion (often the first visit at a lipid outpatient clinic), 35% of all patients received statins, 26% received oral combination therapy, 19% were treated with PCSK9i alone or in combination with other lipid-lowering drugs (no apheresis), 4% of patients received apheresis treatment with or without oral therapy, and 14% of patients were not treated with any lipid-lowering medication (Fig. 2).
3.4. Target value attainment
According to the 2016 EAS/ESC guidelines, 26% of patients with CVD and 23% of patients without CVD achieved LDL-C goals. In contrast, only 10% of patients with ASCVD or another cardiovascular risk factor (CVRF) and only 4% of FH patients without ASCVD or one other CVRF achieved their LDL-C goals according to the 2019 EAS/ESC guidelines (data not shown). However, 37% of FH patients without previous cardiovascular events achieved a 50% reduction of LDL-C levels versus 60% of FH patients with ASCVD (data not shown).
FH patients treated with PCSK9i or combination therapies achieved their LDL-C goals according to the 2016 EAS/ESC guidelines more often than those treated with oral lipid-lowering drug monotherapy (Fig. 3A). Approximately one-fifth of patients without CVRFs and one-third of patients with ASCVD and another risk factor achieved LDL-C goals according to the 2019 EAS/ESC guidelines if they were treated with PCSK9i, whereas LDL-C goals were only achieved by a small proportion of patients using other therapies (Fig. 3B).
Overall, treatment with PCSK9i alone or in combination with other therapies led to achieving LDL-C goals for the largest proportion of patients in this cohort, regardless of whether we applied the EAS/ESC guidelines of 2016 (Fig. 3A) or 2019 (Fig. 3B), or the 50% reduction in LDL-C criterion (data not shown).
3.5. Factors associated with treatment
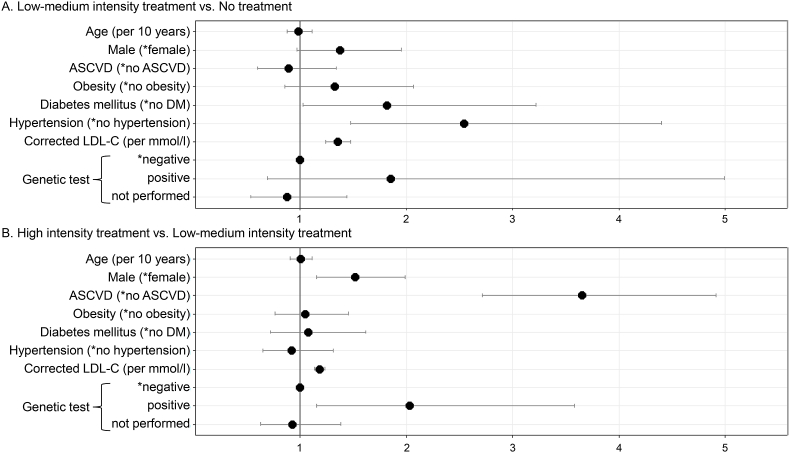
To examine which factors were associated with treatment intensity (cf. section “Patients and Methods”), we estimated odds ratios (ORs) and their 95% confidence intervals (CIs) as part of a multivariate logistic regression making the following comparisons: “low-medium intensity treatment” versus “no treatment”, “high intensity therapy” versus “low-medium intensity treatment”. The independent variables were age, sex, presence of ASCVD, diabetes mellitus, obesity, hypertension, therapy-naïve LDL-C, and genetic test status.
Significantly increased ORs for low-medium intensity versus no treatment were found for diabetes mellitus (OR = 1.82), hypertension (OR = 2.55), corrected LDL-C (OR = 1.35 per mmol/l). For gender (OR = 1.37, reference: female), the p-value is in the borderline range between 0.05 and 0.1 (Fig. 4 A).
Fig. 4.
Multivariate logistic-regression analysis of therapy intensity adjusted for age, gender, ASVD, obesity, DM, hypertension, corrected LDL-C and genetic test result. The odds ratios (with 95% CIs) to the binary outcomes are shown. ∗ indicates the reference category.
Significantly increased ORs for high intensity versus low-medium intensity treatment were found for male gender (OR = 1.52, reference: female), ASCVD (OR = 3.65), corrected LDL-C (OR = 1.18 per mmol/l) and a positive genetic test result (OR = 2.03, reference: genetic test negative) (Fig. 4 B) associated with high intensity treatment.
In the supplementary analyses, significantly increased ORs for monotherapy versus no treatment were found for male gender, diabetes mellitus, hypertension, corrected LDL-C. Significantly increased ORs for combination therapy versus monotherapy were found for male gender, ASCVD, corrected LDL-C and a positive genetic test result. For age, the p-value is in the borderline range between 0.05 and 0.1.
Significantly increased ORs for generic agents versus no treatment were found for male gender, diabetes mellitus, hypertension, corrected LDL-C. For positive genetic test results, the p-value to is in the borderline range between 0.05 and 0.1. Significantly increased ORs for PCSK9i versus generic agents were found for age, ASCVD, corrected LDL-C, and positive genetic test results.
In Germany, the prescription of PCSK9 inhibitors is restricted to board-licensed cardiologists, angiologists, nephrologists and endocrinologists, or lipid outpatient clinics. Therefore, patients treated by these specialists might be at a higher likelihood to receive more intensive treatment. We therefore conducted a sensitivity analysis in which we included only those patients treated by specialists. This did not materially change our results (treatment intensities associated with age, gender, ASCVD, obesity, diabetes mellitus, hypertension, treatment naïve LDL C, genetic test result, respectively).
GPs and internists treated more patients with statins alone (44% vs 33%), whereas patients in lipid clinics were more often treated with PCSK9i alone or in combination (21% vs 8%) and more often received apheresis treatment alone or in combination (2% vs 5%) (Supplementary Fig. 1).
This suggests that treatment by specialists might also have been associated with a higher treatment intensity. However, because the majority of patients (n = 1311, 87%) was treated by specialists rather than non-specialists (GPs and internists), we did not include this distinction into the multivariate regression analysis.
4. Discussion
4.1. Study population
The CaRe High registry studied a population with FH in Germany and a high prevalence of CVD (42% in women and 45% in men). Some of the patients had other risk factors, but the proportion of patients with diabetes mellitus, hypertension, and obesity was lower than in the German general population [10].
4.2. Genetic testing
A study of more than 20,000 patients showed that at approximately equal LDL-C (200 mg/dl, 5.2 mmol/l) the atherosclerosis risk is higher in genetically proven FH than in those without and that 55% of those patients with genetically confirmed FH are below the threshold value of 190 mg/dl [30]. Thus, the genetic detection of FH has far-reaching therapeutic consequences.
Though genetic testing is consequentially recommended by current international (EAS 2019) [12,13] and national guidelines [31], only one-fifth of patients in the study population underwent genetic testing. In another fifth, genetic analysis had been recommended to the patients but had not been completed at the time of analysis. One reason for this may be that GPs are afraid to exceed their assigned budget for laboratory services, disregarding that genetic testing is reimbursed by the German statutory health insurance funds outside the regular laboratory budgets. Notably, some German university outpatient departments are not entitled to order genetic tests at the expense of the statutory health insurance and, therefore, are dependent on cooperation with GPs for prescribing genetic tests.
Some patients may have also rejected genetic testing either because they are in fear of disadvantages (e.g., health insurance) or because they reinforce their right not to know. Another reason for the low uptake of genetic testing may be that physicians lack the time to cope with the formal requirements of carrying out genetic diagnosis.
Several systematic studies have shown that adherence to statins is improved by genetic evidence of FH [32], as the motivation to take the primarily prescribed statins regularly and over the long-term increases significantly. In turn, adherence correlates with the effectiveness of statins in reducing the number of cardiovascular events [33]. Taken together, further efforts are therefore warranted to enhance the uptake of genetic testing. Genetic testing should be considered more regularly by patients and treating physicians, as genetic testing for FH also provides a starting point for familial cascade screening, as described in the large, nationwide, publicly funded, cascade screening program in the Netherlands [34].
4.3. Treatment status at time of study inclusion
At the time of study inclusion, 86% of study participants regularly received lipid-lowering medication. The reasons for not providing regular treatment in 14% of study participants were not specifically investigated but may be related to an underestimation of the vascular risk conferred by FH and intolerance to previous medication. Notably, the data regarding treatment status were only recorded once at the time of inclusion, often at the first visit to the lipid outpatient clinic so that changes may have been implemented afterwards. In line with this, preliminary data from a follow-up of our patients have shown that most patients will be treated over time.
4.4. Target value attainment
Overall, only one quarter of FH patients in the CaRe High registry achieve the LDL-C goals according to the 2016 EAS/ESC guidelines and only a tenth of FH patients in the CaRe High registry achieve the LDL-C target values according to the 2019 guidelines.
Data from the Greek FH registry and an Italian observational study indicate a very low rate of target value attainment among FH patients as well [35,36]. In Italy, the electronic health records of more than 200,000 individuals referred to a laboratory for routine assessment and of more than 500 ASCVD patients (age ⩽65 years) who underwent percutaneous transluminal coronary angioplasty (PTCA) were studied [37]. The 50% LDL-C reduction goal was achieved by 70.6% of patients in the laboratory survey, but only 18.5% of PTCA patients achieved the LDL-C < 55 mg/dl goal. This is in good agreement with the 26% and 10% of our patients with ASCVD at goal according to the 2016 and 2019 ESC/EAS dyslipidemia guidelines, respectively, and with the 60% of FH patients with ASCVD achieving a 50% reduction in LDL-C.
Most patients in CaRe High registry achieving the LDL-C goals received PCSK9i in combination with other oral LLTs These results are in line with data from the global FHSC registry. Therein, higher numbers of therapies used are significantly associated with higher goal attainment rates, particularly when PCSK9i were included [38].
This illustrates that a tremendous gap still exists between guideline recommendations and their implementation in clinical practice, and that the implementation of the 2019 guidelines will be challenging.
4.5. Factors associated with treatment
Basically, two comparisons were made to identify factors associated with treatment intensity: low-medium to no treatment and high intensity to low-medium intensity. First, male sex, baseline LDL-C, diabetes mellitus and hypertension increased the likelihood of being treated at all. Second, male sex, baseline LDL-C, ASCVD, and a positive genetic test result were associated with escalated treatment. This is in line with a study reporting an increased treatment adherence in patients with previous cardiovascular events [39]. In Germany, the occurrence of ASCVD related may open the regulatory window for PCSK9i prescription, which is otherwise limited, and genetic testing may be used to support their prescription.
A theoretical possibility to explain the association of a positive genetic test with treatment intensity may be that very high LDL concentrations themselves increase the probability of ordering a genetic test and are treated more intensely. Both, however, treatment naïve LDL cholesterol and outcomes of genetic testing have been included simultaneously as “predictors” of treatment intensity into our logistic regression models. This means that both factors are mutually adjusted for each other and that the odds ratios reported can be considered independent from each other without confounding.
Studies suggest that genetic findings are associated with improved treatment [11,40]. In consistence, our results cross-sectionally indicate associations of high-intensity treatment, combination therapy and PCSK9i prescription with positive results of genetic tests.
Potentially, LDL-C goal attainment and intensity of LLT may be associated with treatment by lipid specialist, as suggested by the results in Supplementary Fig. 1. While non-specialists focused on monotherapy with statins and oral lipid-lowering drugs, specialists more frequently used PSCK9i. This discrepancy may be related to the largely conflicting guidelines of scientific societies (2016 and 2019 ESC/EAS dyslipidemia guidelines, DEGAM S3 guideline “General practitioner risk consultation for cardiovascular prevention”) [12,13,30] which may address different audiences and/or reflect the G-BA (Federal Joint Committee)-issued restrictions on prescribing PCSK9is in Germany, stating that only board-licensed specialists (lipidologists, nephrologists, cardiologists, angiologists, endocrinologists) are entitled to initiate treatment with a PCSK9i, whereas GPs are permitted to issue follow-up prescriptions only [41].
Because only a minor percentage of patients in our study was included by non-specialists (GPs and internists) who would not be entitled for an initial prescription of PCSKi, we did not include the distinction between non-specialists and specialists into our regression analysis for treatment intensity.
4.6. Limitations
Our study has several limitations [14]. Genetic testing was not mandatory for inclusion in our registry. Nevertheless, compared to our previous interim report, we were able to substantially increase the available number of results from genetic testing (from 11% to 20%). An FH-causing mutation was found in 51% of the 297 tested individuals. In patients who underwent genetic testing, diagnostic strategies were heterogenous (Sanger sequencing, next generation sequencing, panel diagnostics) because of a lack of standardization amongst the different human genetic laboratories in Germany. The genes that were analyzed were mostly selected by the treating physicians or may have been limited by the available methods; therefore, they may not have included all candidate genes, and the rate of genetically detectable FH may be higher than reported here. As 87% of patients in the CaRe High registry are treated by lipid specialists, data from the current study are not representative of the ambulatory sector in general. Many patients were included in the study either at their first appointment with the treating physicians or a lipid clinic. Therefore, the information on their treatment status provides point estimate that may be subject to adjustment and improvement. Furthermore, the patients in this registry are probably not representative of the FH population in Germany, as both the patients and physicians who took part in this study may have had a higher-than-average awareness of FH.
We applied both the 2016 and 2019 ESC/EAS dyslipidemia guidelines [12,13]. However, most patients in this interim analysis were included before the 2019 guidelines were published. Therefore, the proportion of patients not achieving the LDL-C goals according to the 2019 guidelines may be overestimated.
The current data rely on information given by patients and their physicians. Not all of the information or laboratory values were available in all cases. Thus, the total number of datasets varies for each analysis. The LDL-C values before treatment were not always known and we had to use correction factors depending on specific medication use to calculate treatment-naive LDL-C values.
5. Conclusions
The interim analysis confirms that lipid-lowering treatment of FH patients in Germany is still insufficient with potential for improvement. Treatment intensity is significantly associated with sex, ASCVD, therapy-naïve LDL-C, and a positive genetic test result. This should prompt physicians to consider genetic testing more often in patients with clinical suspicion of FH in order to improve awareness and treatment intensity for ultimately achieving treatment goals and reducing cardiovascular risk.
Financial support
CaRe High is a project of D•A•CH Society for the Prevention of Cardiovascular Diseases (registered society). It is supported by funding from AMGEN GmbH, Munich, and Sanofi-Aventis GmbH, Berlin, to the D•A•CH Society for the Prevention of Cardiovascular Diseases (registered society).
Author contributions
NS and WM drafted the manuscript and revised the statistical analysis. IaH revised the manuscript. AD performed the statistical analysis. TBG and WM designed the study and wrote the study protocol. NS, WM, and US predefined the research questions. AB, UJ, GK, WK, ACK, UL, JLK BO, WR, EST, AV, IGB, CBO, HH, and SK provided patient data and/or revised the manuscript. FUB, UK, CK, MM, KGP, HS, and KSJ provided patient data. WM and US revised the manuscript.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: NS was project lead of the CaRe High registry, which is financed in cooperation with Amgen GmbH and Sanofi-Aventis GmbH. IaH reports lecture fees from Synlab. NS reports non-financial support from Amgen GmbH during the conduct of the study. AD is employed by the CaRe High registry. TBG, AB, ACK, WR, CK, MM, KSJ, JLK, and CBO have nothing to disclose. IGB has received honoraria for consulting and/or lecture fees from Amgen, Sanofi-Aventis, Aegereon, Regeneron, Novartis, Pfizer, Synlab, Akcea Therapeutics, and Daiichi-Sankyo and institutional support from Amgen, Sanofi-Aventis, Akcea Therapeutics, DACH, and Novartis. HH reports lecture fees from Amgen, Berlin-Chemie, Daiichi-Sankio, Pfizer, and Sanofi-Aventis. SK received honoraria as a speaker and member of advisory boards from Amgen, AstraZeneca, Berlin Chemie, Boehringer Ingelheim, Daiichi Sankyo, Medical Association North Rhine Germany, Novartis, Roche, Sanofi-Aventis, and Servier. FUB reports lecture fees from Amgen, Sanofi-Aventis, and Synlab outside the submitted work. UK reports grants from D.A.C.H. Society for Prevention of cardiovascular disease during the conduct of the study; others from Amgen, Sanofi-Aventis, Alexion, Berlin Chemie, and Fresenius Medical Care outside the submitted work. GK reports lecture/advisory board fees from Akcea, Amgen, BMS, MSD, Sanofi-Aventis, and Synlab outside the submitted work. UJ reports honoraria from Aegerion, Akcea, Amgen, Chiesi, Sanofi-Aventis, Kaneka, Diamed, and Fresenius Medical Care outside the submitted work. WK reports personal fees from AstraZeneca, Novartis, Pfizer, The Medicines Company, DalCor, Kowa, Amgen, Corvidia, Berlin-Chemie, and Sanofi-Aventis, and grants and non-financial support from Beckmann, Singulex, Abbott, and Roche Diagnostics outside the submitted work; BO reports lecture/advisory board fees from Amgen, Amryt, Berlin Chemie, Hexal, MSD, and Sanofi-Aventis outside the submitted work. UL reports personal honoraria for lecture fees and other from Amgen, BerlinChemie, MSD, and Sanofi-Aventis outside the submitted work. EST received speakers' honoraria for presentations, advisory board activities, and funding of research projects by Fresenius Medical Care Germany, Amgen, Sanofi-Aventis, and Berlin Chemie. HS reports personal fees from AMGEN, Sanofi-Aventis, and MSD SHARP&DOHME outside the submitted work. KP reports personal fees from Akcea, Amarin, Amgen, Berlin-Chemie, Boehringer-Ingelheim, MSD, Daiichi-Sankyo, and Sanofi-Aventis outside the submitted work. AV reports lecture/advisory board fees from Aegerion, Amgen, Berlin Chemie, Fresenius, MSD, Regeneron/Sanofi-Aventis, and Synlab outside the submitted work. DMW received honoraria as speaker and member of advisory boards from Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, MSD, Novo Nordisk, Novartis, Sanofi-Aventis, and Sanofi-Pasteur. US reports honoraria for consultancy and lecturing from Amgen, Amarin, AstraZeneca, Berlin Chemie, Boehringer Ingelheim, Daiichi Sankyo, MSD, Sanofi-Aventis, Synlab, Novartis, and NovoNordisk.WM reports grants and personal fees from Sanofi-Aventis during the conduct of the study; grants from Siemens Healthineers, Astra-Zeneca, Bayer Vital GmbH, bestbion dx GmbH, Boehringer Ingelheim Pharma GmbH Co KG, Immundiagnostik GmbH, Merck Chemicals GmbH, MSD Sharp and Dohme GmbH, Novartis Pharma GmbH, and Olink Proteomics, grants and personal fees from Aegerion Pharmaceuticals, AMGEN, Alexion Pharmaceuticals, BASF, Abbott Diagnostics, Numares AG, Berlin-Chemie, and Akzea Therapeutics, and other from Synlab Holding Deutschland GmbH outside the submitted work.
Acknowledgements
We wish to thank Dr. Gereon Böll and Dr. Anja Kirschbaum, Clinic Königsfeld der DRV Westfalen, Ennepetal; Dr. Merke, Bensheim; Dr. Segiet, Speyer; Dr. Mayer, Mannheim; Dr. Leistikow, Mannheim; Dr. Füllgraf-Horst, Mannheim; Dr. Schüler, Hirschberg; Dr. Wiener, Weinheim; Dr. Hein, Böblingen; Dr. Baumgartner, Schwetzingen; Dr. Schömig, Heilbronn; Dr. Schweizer, Adelsried; Dr. Scholl, Prevention First, Frankfurt a.M.; Dr. Gebauer and Dr. Predica, Median-Park-Klinik, Bad Dürkheim; Dr. Müller, Mannheim; Dr. Kopf, Heidelberg; Dr. Busch, Heidelberg; Dr. Allendorf-Ostwald, Bruchsal; Dr. Fink, Elzach; Dr. Böhm, Marbach, Dr. Jäkel, Wiehre; Dr. Stadelmann, Nürnberg; Dr. Schrader, Nürnberg; Dr. Paulsen, Berlin; Dr. Homberger, Berlin; Dr. Zemmrich, Berlin; Dres. Seeger, Berlin; Dr. Scholl, Mühlhausen; Dres. Morguet and Majeed, Berlin; Dres. Biolik and Leimbach, Berlin; Dr. Deiss, Berlin; Mrs. Richter, Berlin; Dr. Pantchechnikova, Berlin; Mrs. Dorn, Berlin; Dr. Vallée, Berlin; Dr. Spens, Leipzig; Dr. Wiesner, Leipzig; Mrs. Gräsl and Dr. Herbst, University Clinic Würzburg; Dr. Strack and Dr. Tafelmeier, University Clinic Regensburg for recruiting patients into the CaRe High registry.We thank the CaRe High-Team: Isabel Klein, Dominika Pienkowska, Lydia Frenzke, Antonia Sonntag, Alica Gopon, Anika Paprotny, Jutta Christmann, Christine Stumpp, Bianka Erbe, Aniko Bosch, Felix Fath, and Tobias Sietmann, D•A•CH Society for the Prevention of Cardiovascular Diseases, Hamburg, Germany. This article is dedicated to the Memory of Kurt Franz Maria Oette (∗ March 29, 1927, † November 9, 2022) who was the Director of the Institute of Clinical Chemistry at the University of Cologne from 1971 to 1994. Dr. Oette made major contributions to lipid biochemistry, the development of immunoabsorption for lipoprotein apheresis and the genetics of familial hypercholesterolemia. He was awarded the Heinrich Wieland prize in 1972.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.athplu.2023.06.001.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
Comparison of lipid-lowering therapy (LLT) of patients treated by GPs and internists (light grey bars) and by lipid specialists (dark bars)
References
- 1.Brænne I., Kleinecke M., Reiz B., Graf E., Strom T., Wieland T., Fischer M., Kessler T., Hengstenberg C., Meitinger T., Erdmann J., Schunkert H. Systematic analysis of variants related to familial hypercholesterolemia in families with premature myocardial infarction. Eur J Hum Genet. 2016;24:191–197. doi: 10.1038/ejhg.2015.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldberg A.C., Hopkins P.N., Toth P.P., Ballantyne C.M., Rader D.J., Robinson J.G., Daniels S.R., Gidding S.S., De Ferranti S.D., Ito M.K., McGowan M.P., Moriarty P.M., Cromwell W.C., Ross J.L., Ziajka P.E. Familial hypercholesterolemia: screening, diagnosis and management of pediatric and adult patients: clinical guidance from the national lipid association expert panel on familial hypercholesterolemia. J. Clin. Lipidol. 2011:133–140. doi: 10.1016/j.jacl.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Whitfield A.J., Barrett P.H.R., Van Bockxmeer F.M., Burnett J.R. Lipid disorders and mutations in the APOB gene. Clin Chem. 2004 doi: 10.1373/clinchem.2004.038026. [DOI] [PubMed] [Google Scholar]
- 4.Defesche J.C., Pricker K.L., Hayden M.R., Van Der Ende B.E., Kastelein J.J.P. Familial defective apolipoprotein B-100 is clinically indistinguishable from familial hypercholesterolemia. Arch Intern Med. 1993 doi: 10.1001/archinte.1993.00410200071008. [DOI] [PubMed] [Google Scholar]
- 5.Abifadel M., Varret M., Rabès J.P., Allard D., Ouguerram K., Devillers M., Cruaud C., Benjannet S., Wickham L., Erlich D., Derré A., Villéger L., Farnier M., Beucler I., Bruckert E., Chambaz J., Chanu B., Lecerf J.M., Luc G., Moulin P., Weissenbach J., Prat A., Krempf M., Junien C., Seidah N.G., Boileau C. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003 doi: 10.1038/ng1161. [DOI] [PubMed] [Google Scholar]
- 6.Nordestgaard B.G., Chapman M.J., Humphries S.E., Ginsberg H.N., Masana L., Descamps O.S., Wiklund O., Hegele R.A., Raal F.J., Defesche J.C., Wiegman A., Santos R.D., Watts G.F., Parhofer K.G., Hovingh G.K., Kovanen P.T., Boileau C., Averna M., Borén J., Bruckert E., Catapano A.L., Kuivenhoven J.A., Pajukanta P., Ray K., Stalenhoef A.F.H., Stroes E., Taskinen M.R., Tybjærg-Hansen A. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease. Eur Heart J. 2013;34:3478–3490. doi: 10.1093/eurheartj/eht273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bates T.R., Burnett J.R., Bockxmeer F.M. va, Hamilton S., Arnolda L., Watts G.F. Detection of familial hypercholesterolaemia: a major treatment gap in preventative cardiology. Heart Lung Circ. 2008;17:411–413. doi: 10.1016/j.hlc.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Versmissen J., Oosterveer D.M., Yazdanpanah M., Defesche J.C., Basart D.C.G., Liem A.H., Heeringa J., Witteman J.C., Lansberg P.J., Kastelein J.J.P., Sijbrands E.J.G. Efficacy of statins in familial hypercholesterolaemia: a long term cohort study. BMJ. 2009;338:223–226. doi: 10.1136/bmj.a2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luirink I.K., Wiegman A., Kusters D.M., Hof M.H., Groothoff J.W., de Groot E., Kastelein J.J.P., Hutten B.A. 20-Year follow-up of statins in children with familial hypercholesterolemia. N Engl J Med. 2019 doi: 10.1056/nejmoa1816454. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt N., Schmidt B., Dressel A., Gergei I., Klotsche J., Pieper L., Scharnagl H., Kleber M.E., März W., Lehnert H., Pittrow D., Stalla G., Wittchen H.-U.U., Grammer T.B. Familial hypercholesterolemia in primary care in Germany. Diabetes and cardiovascular risk evaluation: targets and Essential Data for Commitment of Treatment (DETECT) study. Atherosclerosis. 2017;266:24–30. doi: 10.1016/j.atherosclerosis.2017.08.019. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt N., Grammer T., Gouni-Berthold I., Julius U., Kassner U., Klose G., König C., Laufs U., Otte B., Steinhagen-Thiessen E., Wanner C., März W. CaRe high – cascade screening and registry for high cholesterol in Germany. Atherosclerosis Suppl. 2017;30:72–76. doi: 10.1016/j.atherosclerosissup.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 12.Mach F., et al. ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2019;41:111–188. doi: 10.1093/eurheartj/ehz455. 2020. [DOI] [PubMed] [Google Scholar]
- 13.Catapano L., et al. ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016;37:2999–3058l. doi: 10.1093/eurheartj/ehw272. 2016. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt N., Dressel A., Grammer T.B., Gouni-Berthold I., Julius U., Kassner U., Klose G., König C., Koenig W., Otte B., Parhofer K.G., Reinhard W., Schatz U., Schunkert H., Steinhagen-Thiessen E., Vogt A., Laufs U., März W. Lipid-modifying therapy and low-density lipoprotein cholesterol goal attainment in patients with familial hypercholesterolemia in Germany: the CaReHigh Registry. Atherosclerosis. 2018;277:314–322. doi: 10.1016/j.atherosclerosis.2018.08.050. [DOI] [PubMed] [Google Scholar]
- 15.Klose G., Laufs U., März W., Windler E. Familial hypercholesterolemia: developments in diagnosis and treatment. Dtsch. Arztebl. Int. 2014;111 doi: 10.3238/arztebl.2014.0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Obesity: preventing and managing the global epidemic Report of a WHO consultation. World Health Organization technical report series. 2000;894 i–253. [PubMed] [Google Scholar]
- 17.Ep W., Tj W., Wet H. NHG-Standpunt diagnostiek en behandeling van familiaire hypercholesterolemie. Huisarts Wet. 2006;49:202–204. doi: 10.1007/bf03084705. [DOI] [Google Scholar]
- 18.Gouni-Berthold I., Descamps O.S., Fraass U., Hartfield E., Allcott K., Dent R., März W. Systematic review of published Phase 3 data on anti-PCSK9 monoclonal antibodies in patients with hypercholesterolaemia. Br J Clin Pharmacol. 2016;82:1412–1443. doi: 10.1111/bcp.13066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toth P.P., Worthy G., Gandra S.R., Sattar N., Bray S., Cheng L.I., Bridges I., Worth G.M., Dent R., Forbes C.A., Deshpande S., Ross J., Kleijnen J., Stroes E.S.G. Systematic review and network meta-analysis on the efficacy of evolocumab and other therapies for the management of lipid levels in hyperlipidemia. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.116.005367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt A.F., Pearce L.S., Wilkins J.T., Overington J.P., Hingorani A.D., Casas J.P. PCSK9 monoclonal antibodies for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2017;2017 doi: 10.1002/14651858.CD011748.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alhajri L., Alhadhrami A., Almheiri S., Almutawa Y., Alhashimi Z. The efficacy of evolocumab in the management of hyperlipidemia: a systematic review. Ther. Adv. Cardiovasc. Dis. 2017;11:155–169. doi: 10.1177/1753944717698925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pirillo A., Norata G.D., Catapano A.L. LDL-Cholesterol-Lowering therapy. Handb Exp Pharmacol. 2022;270:73–101. doi: 10.1007/164_2020_361. [DOI] [PubMed] [Google Scholar]
- 23.Staels B., Handelsman Y., Fonseca V. Bile acid sequestrants for lipid and glucose control. Curr Diabetes Rep. 2010;10(1):70–77. doi: 10.1007/s11892-009-0087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farnier M., Roth E., Gil-Extremera B., Mendez G.F., Macdonell G., Hamlin C., Perevozskaya I., Davies M.J., Kush D., Mitchel Y.B., Ezetimibe/Simvastatin + Fenofibrate Study Group Efficacy and safety of the coadministration of ezetimibe/simvastatin with fenofibrate in patients with mixed hyperlipidemia. Am Heart J. 2007;153(2):335. doi: 10.1016/j.ahj.2006.10.031. e1–335.e3358. [DOI] [PubMed] [Google Scholar]
- 25.Farnier M., Dejager S. Effect of combined fluvastatin-fenofibrate therapy compared with fenofibrate monotherapy in severe primary hypercholesterolemia. French Fluvastatin Study Group. Am J Cardiol. 2000;85(1):53–57. doi: 10.1016/s0002-9149(99)00606-2. [DOI] [PubMed] [Google Scholar]
- 26.Mazhar F., Hjemdahl P., Clase C.M., Johnell K., Jernberg T., Carrero J.J. Lipid-lowering treatment intensity, persistence, adherence and goal attainment in patients with coronary heart disease. Am Heart J. 2022;251:78–90. doi: 10.1016/j.ahj.2022.05.021. [DOI] [PubMed] [Google Scholar]
- 27.McCrum-Gardner E. Which is the correct statistical test to use? Br J Oral Maxillofac Surg. 2008;46(1):38–41. doi: 10.1016/j.bjoms.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Yee T.W., Wild C.J. Vector generalized additive models. J. R. Stat. Soc. Ser. B. 1996 doi: 10.1111/j.2517-6161.1996.tb02095.x. [DOI] [Google Scholar]
- 29.Yee T.W. 2015. Vector generalized linear and additive models: with an implementation in R. [DOI] [Google Scholar]
- 30.Khera A.V., Won H.H., Peloso G.M., Lawson K.S., Bartz T.M., Deng X., van Leeuwen E.M., Natarajan P., Emdin C.A., Bick A.G., Morrison A.C., Brody J.A., Gupta N., Nomura A., Kessler T., Duga S., Bis J.C., van Duijn C.M., Cupples L.A., Psaty B., Rader D.J., Danesh J., Schunkert H., McPherson R., Farrall M., Watkins H., Lander E., Wilson J.G., Correa A., Boerwinkle E., Merlini P.A., Ardissino D., Saleheen D., Gabriel S., Kathiresan S. Diagnostic yield and clinical utility of sequencing familial hypercholesterolemia genes in patients with severe hypercholesterolemia. J Am Coll Cardiol. 2016;67:2578–2589. doi: 10.1016/J.JACC.2016.03.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ludt S., Angelow A., Baum E., Chenot J.-F., Donner-Banzhoff N., Egidi G., Fessler J., Haasenritter J., Popert U. vol. 19. 2017. (Hausärztliche Risikoberatung zur kardiovaskulären Prävention S3 Leitlinie AWMF-Register-Nr. 053-024 DEGAM-Leitlinie Nr). [Google Scholar]
- 32.Lee S., Akioyamen L.E., Aljenedil S., Rivière J.B., Ruel I., Genest J. Genetic testing for familial hypercholesterolemia: impact on diagnosis, treatment and cardiovascular risk. Eur. J. Prev. Cardiol. 2019;26:1262–1270. doi: 10.1177/2047487319829746. [DOI] [PubMed] [Google Scholar]
- 33.Shalev V., Chodick G., Silber H., Kokia E., Jan J., Heymann A.D. Continuation of statin treatment and all-cause mortality: a population-based cohort study. Arch Intern Med. 2009;169:260–268. doi: 10.1001/ARCHINTERNMED.2008.552. [DOI] [PubMed] [Google Scholar]
- 34.Huijgen R., Kindt I., Fouchier S.W., Defesche J.C., Hutten B.A., Kastelein J.J.P., Vissers M.N. Functionality of sequence variants in the genes coding for the low-density lipoprotein receptor and apolipoprotein B in individuals with inherited hypercholesterolemia. Hum Mutat. 2010;31:752–760. doi: 10.1002/HUMU.21258. [DOI] [PubMed] [Google Scholar]
- 35.Rizos C.V., Skoumas I., Rallidis L., Skalidis E., Tziomalos K., Garoufi A., Anagnostis P., Sfikas G., Kotsis V., Doumas M., Kolovou G., Lambadiari V., Dima I., Kiouri E., Zacharis E., Agapakis D., Attilakos A., Antza C., Vlachopoulos C., Liberopoulos E.N. LDL cholesterol target achievement in heterozygous familial hypercholesterolemia patients according to 2019 ESC/EAS lipid guidelines: implications for newer lipid-lowering treatments. Int J Cardiol. 2021;345:119–124. doi: 10.1016/J.IJCARD.2021.10.024. [DOI] [PubMed] [Google Scholar]
- 36.D'Erasmo L., Commodari D., Di Costanzo A., Minicocci I., Polito L., Ceci F., Montali A., Maranghi M., Arca M. Evolving trend in the management of heterozygous familial hypercholesterolemia in Italy: a retrospective, single center, observational study. Nutr Metabol Cardiovasc Dis. 2020;30:2027–2035. doi: 10.1016/J.NUMECD.2020.06.028. [DOI] [PubMed] [Google Scholar]
- 37.Fasano T., Trenti C., Negri E.A., Guiducci V., Foracchia M., Bonelli E., Canovi S., Besutti G., Bertolini S., Calandra S. Search for familial hypercholesterolemia patients in an Italian community: a real-life retrospective study. Nutr Metabol Cardiovasc Dis: Nutr Metabol Cardiovasc Dis. 2022;32(3):577–585. doi: 10.1016/j.numecd.2021.12.024. [DOI] [PubMed] [Google Scholar]
- 38.EAS Familial Hypercholesterolaemia Studies Collaboration (FHSC) Global perspective of familial hypercholesterolaemia: a cross-sectional study from the EAS Familial Hypercholesterolaemia Studies Collaboration (FHSC) Lancet (London, England) 2021;398(10312):1713–1725. doi: 10.1016/S0140-6736(21)01122-3. [DOI] [PubMed] [Google Scholar]
- 39.Pérez De Isla L., Alonso R., Mata N., Fernández-Pérez C., Muñiz O., Díaz-Díaz J.L., Saltijeral A., Fuentes-Jiménez F., De Andrés R., Zambón D., Piedecausa M., Cepeda J.M., Mauri M., Galiana J., Brea Á., Sanchez Muñoz-Torrero J.F., Padró T., Argueso R., Miramontes-González J.P., Badimón L., Santos R.D., Watts G.F., Mata P. Predicting cardiovascular events in familial hypercholesterolemia: the SAFEHEART registry (Spanish Familial Hypercholesterolemia Cohort Study) Circulation. 2017;135:2133–2144. doi: 10.1161/CIRCULATIONAHA.116.024541. [DOI] [PubMed] [Google Scholar]
- 40.Kassner U., Wühle-Demuth M., Missala I., Humphries S.E., SteinhagenThiessen E., Demuth I. Clinical utility gene card for: hyperlipoproteinemia, TYPE II. Eur J Hum Genet. 2014;22:953. doi: 10.1038/ejhg.2013.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.G. Bundesausschuss, Anlage III Übersicht über Verordnungseinschränkungen und -ausschlüsse, [n.d].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of lipid-lowering therapy (LLT) of patients treated by GPs and internists (light grey bars) and by lipid specialists (dark bars)