Abstract
Background
Chronic low back pain (CLBP) is an extremely common public health concern responsible for pain-related disability. CLBP is challenging to manage despite having a plethora of treatment options. Physiotherapy is a guideline-recommended treatment for CLBP. Furthermore, some forms of complementary medicines, such as dry needling, spinal manipulation, Tai Chi, and yoga are also recommended for CLBP treatment. We hypothesized that the combined treatment would be more effective when managing CLBP. Therefore, this randomized clinical trial aims to examine the impact of combined therapy of dry needling and physiotherapy compared to the treatment effect of only physiotherapy among patients with CLBP.
Methods
The study is a two-armed single-center, randomized controlled clinical superiority trial where participants are randomized to combined therapy of usual care physiotherapy and dry needling or only usual care physiotherapy (1:1). Individuals who are 18 years or older and experiencing LBP with or without leg pain for a minimum of three months will be considered eligible for the study. Pain severity, pain affective and physical interference, activity limitation, and insomnia symptoms of patients with CLBP will be measured at the baseline after four, 12 and 24-week treatment started.
Conclusion
Finding a better management strategy for managing CLBP is an ongoing challenge. Most of the novel techniques that try to manage CLBP are limitedly tested. This study will allow testing of the combined effect of usual care physiotherapy and dry needling when managing CLBP in terms of clinical efficacy. If the combined therapy is proven significantly effective, compared to usual care physiotherapy alone will provide plausible evidence of an effective treatment option to manage CLBP.
Trial registration
Clinical Trial Registry-India; trial registration number- CTRI/2022/09/045625.
Keywords: Chronic low back pain, Clinical trial, Dry needling, Evidence-based treatment, Physiotherapy
1. Background
Chronic low back pain (CLBP) is a major global public health problem affecting human life in many ways [1]. Globally, years lived with disability caused by low back pain increased by 54% between 1990 and 2019, and the point prevalence of low back pain was 7.3%, indicating that 540 million people were affected at any one time [2]. The economic burden of CLBP is significantly high, and this problem induces extra costs to the healthcare system. It also reduces the productivity of sufferers, thus impacting the economy in multiple ways [3].
CLBP is challenging to manage despite having a plethora of treatment options. Clinicians heavily relied on pharmacological treatment and surgery in the past. However, in the past three decades, significant changes were made to the main recommendations in many countries' national clinical practice guidelines to manage low back pain [4]. Some forms of complementary medicines, self-management, and physical and psychological therapies now receive greater emphasis than pharmacological and surgical treatments. For example, US national guidelines endorse the use of acupuncture, spinal manipulation, Tai Chi, and yoga for managing low back pain [5]. In addition, the US guidelines recommend non-pharmacological care as the first treatment option and reserve pharmacological care for patients for whom non-pharmacological care has not worked.
Prevalence of LBP is considerably high in Bangladesh [[6], [7], [8], [9], [10]]. However, low and middle-income countries (LMICs) such as Bangladesh has limited resources of healthcare facilities and does not have any guidelines to manage patients with low back pain [11]. Increased use of ineffective, potentially unsafe treatments, for example, the non-steroid anti-inflammatory drug, has wasted limited healthcare resources and harmed patients with CLBP [1]. A previous study conducted in Bangladesh suggested that clinicians engaged with managing back pain mainly rely on non-recommended and partially recommended treatments [12]. However, another clinical trial in Bangladesh revealed that evidence-based physiotherapy and combination therapy significantly reduce pain intensity and physical and affective interference among patients with musculoskeletal disorders, including low back pain [13].
Dry needling is an evidence-based treatment procedure and can reduce pain and related symptoms among patients with CLBP [14,15]. Similarly, usual care physiotherapy proved clinically effective when managing CLBP [16]. Nonetheless, researchers and clinicians are searching for more effective and widely accepted treatment options to manage this problem [17]. A clinical trial revealed that the application of dry needling in conjunction with physiotherapy resulted in notable enhancements in pain reduction, range of motion, functional capacity, and alleviation of myofascial trigger points among individuals experiencing persistent pain in other musculoskeletal conditions [18]. However, there is a data scarcity that evaluates the impact of combined treatment of physiotherapy and dry needling to manage patients with CLBP. We hypothesized that the combined treatment would be more effective when managing CLBP. Therefore, this randomized clinical trial aims to examine the impact of combined therapy of dry needling and physiotherapy compared to the treatment effect of only physiotherapy among patients with CLBP.
2. Methods and design
2.1. Study design
The study is a two-armed randomized controlled clinical superiority trial where participants are randomized to combined therapy of usual care physiotherapy and dry needling (PTDN) or only usual care physiotherapy (TPT) (1:1).
2.2. Settings
The trial will be conducted as a single-center trial at Dhaka Pain Physiotherapy & Rehabilitation Center Limited (DPRC), Dhaka, Bangladesh. This center has been operating since 2005 and offers multidisciplinary treatments, including physiotherapy and rehabilitation. The clinics will assess and recruit patients, and qualified and licensed physiotherapists and dry-needling practitioners will conduct the treatment.
2.3. Study population and recruitment
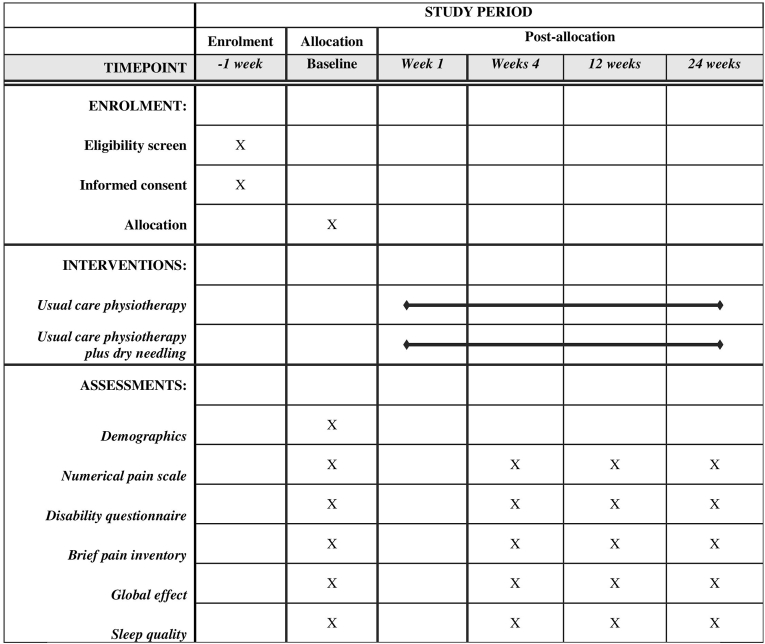
A total of 112 patients with CLBP will be included in the study over an estimated 6-months period between June 2023 and December 2023. Chronic or persistent low back pain is defined as pain that persists for ≥3 months (≥12 weeks) [4,19]. Patients meeting inclusion criteria will be recruited consecutively and identified at the first contact at the clinics during the assessment. Fig. 1 presents the flow of participants throughout the study. The sample size is calculated using the general formula of sample size calculation for randomized clinical trial [20]. Schedule of enrollment, intervention and assesments of study period can be found in Fig. 2.
Fig. 1.
Study flow chart of enrolment, allocation, intervention, and assessment.
Fig. 2.
Schedule of enrollment, interventions, and assessments for study period based on SPIRIT guidelines.
2.4. Inclusion criteria
Individuals who are 18 years or older and experiencing LBP with or without leg pain for a minimum of three months will be considered eligible for the study. They should also be fluent in Bangla so that they can complete the questionnaire and understand the interventions.
2.5. Exclusion criteria
The criteria for exclusion from the study were as follows: absence of pain and limited mobility in the lumbosacral region, presence of other spinal conditions such as spondylolisthesis, fractures, tumors, infections, rheumatic disease or cauda equina syndrome, pregnancy, implanted cardiac pacemaker, blood clotting disorders, use of anticoagulant or steroid therapy, metal implants in the treatment area, sensory disturbances, mental health disorders, cancer, changes in the skin at treatment site, viral or bacterial infections, fever, exhaustion, uncontrolled high blood pressure, fear of needles or refusal to give consent for the procedure.
2.6. Diagnostic assessment and informed consent
All the patients seeking treatment at the recruiting center will undergo a diagnostic interview before being considered for treatment. The interview will be conducted by qualified physiotherapists. Pathological and radiological tests will be conducted if necessary to evaluate the patient's condition. At the clinical assessment, patients will be provided with both verbal and written information about the study. Once a diagnosis has been made, patients will be asked for a written informed consent form to sign. Only after receiving written informed consent will participants be assigned to the study.
2.7. Randomization
Randomization to one of the two arms in the study: PTDN or TPT. Randomization occurs immediately after the patient has completed the baseline questionnaire; thus, the assessor is blinded to the allocation. Participants are randomly allocated to comparative groups (PTDN or TPT) by simple randomization with a 1:1 ratio through a computerized random sequence on the random.com website. The assignment to the group is independent of the time and the research staff performing the procedure. The randomization happens continuously, and treatment onset will occur no later than 1week after the diagnostic interview. A coordinator will monitor the number of patients assigned for the study in each center and stop recruitment when maximum numbers are secured. To control the study quality, the patients will be blinded by the two groups. Patients will be informed that they are receiving the best treatment available for their problem, and patients of two groups will be treated on two different floors of DPRC.
2.8. Ethical approval and trial registration
The ethical review committee of Institution of Physiotherapy, Rehabilitation & Research (IPRR) approved the trial proposal. The approval number is BPA-IPRR/IRB/June 08, 2036. This trial is registered in World Health Organization endorsed organization Clinical Trial Registry-India (https://ctri.nic.in/Clinicaltrials/login.php). The clinical trial registration number is CTRI/2022/09/045625; Registered on: September 19, 2022. This trial will follow the CONSORT guidelines [21] throughout the study.
3. Treatment
3.1. Dry needling
The dry needling program will be performed for the PTDN group according to the Five Regulatory Systems (FRS) concept [14]. The FRS encompasses five interrelated concepts in the context of therapeutic interventions such as dry needling. According to proponents of this concept, the approach primarily addresses the fascial tissues, which possess the ability to structurally adapt in response to external forces. When tissue is punctured, a relaxation response occurs, triggered by various nerve endings. This response leads to the relaxation of the punctured point and the surrounding area, forming the basis of the first regulatory system [22].
The second regulatory system relates to stasis, which involves the impaired flow of blood, lymph, and extracellular fluid within individual compartments. This stasis resembles the etiopathogenesis observed in compartment syndrome. Techniques focused on enhancing drainage and fluid return from the affected area may contribute to reducing pain [23].
The third regulatory system concerns the influence of the autonomic nervous system on the musculoskeletal system, particularly the locomotor organ, and the opposing actions of its two main branches, the sympathetic and parasympathetic [24]. According to the FRS principles, an increased sympathetic impulse can lead to a significant or complete reversal of pain, often accompanied by an improved range of motion and restoration of previously impaired function.
The fourth regulatory system involves proprioception and the extrapyramidal control of voluntary movements. The therapeutic emphasis in the FRS concept lies in reestablishing appropriate proprioceptive stimulation and restoring muscular balance through targeted interventions. Proper proprioception, mediated by deep sensory receptors, is vital for spatial orientation and movement patterns. Local or systemic proprioception impairments within the musculoskeletal system can affect movement patterns [25].
The fifth regulatory system posits the existence of reactive skin zones over the affected areas. These zones may exhibit altered reactivity compared to unaffected skin regions, potentially serving as indicators of underlying pathological processes or contributing to generating pain signals.
In summary, the FRS concept integrates the aforementioned five regulatory systems, highlighting the role of fascial tissues, stasis, autonomic nervous system influence, proprioception, and reactive skin zones in therapeutic interventions such as dry needling.
Maximum 8 sessions of dry needling will be conducted (maximum 2 sessions each week) for the patients attending in PTDN group for 4 weeks.
3.2. Physiotherapy
Physiotherapists will provide treatment for participants following their current standard physiotherapy practice. All the patients who participated in this study will be provided with similar usual care physiotherapy by the same physiotherapists. Usual care physiotherapy in Bangladesh for CLBP refers to the combination of exercise therapy (for example, McKenzie approach) and electrotherapy (for example, transcutaneous electric nerve stimulation) [12]. Participants will attend 21 x 30-min physiotherapy sessions for 4 weeks.
3.3. Unfavorable consequences or early completion of treatment
If any adverse events are observed during the study or if the patient experiences significant improvement in pain before completing 8 sessions of dry needling or 21 sessions of physiotherapy, their treatment will be stopped. Patients will have access to physicians for consultation in case of any complications during the trial.
3.4. Quality control of the study
All physiotherapists and dry needling practitioners involved in the study are qualified, licensed, and experienced professionals who will provide treatment in accordance with standard protocols in Bangladesh. The treatment providers are well-informed about potential adverse effects and will take necessary actions if such evets occur. Throughout the study, the corresponding supervisor will supervise the treatment procedure. A separate treatment log, exercise log, or adverse effect logbook will be maintained for individual patients. Furthermore, patients who enrolled in the study will receive free treatment sessions. Standard safety protocols for physiotherapy and dry needling will be maintained throughout the study.
3.5. Assessment of clinical outcomes
Patients will be assessed at the treatment centers at baseline, at the 4-weeks, 12-weeks and 24-weeks of treatment sessions started. Patients will complete a self-administered paper-based questionnaire translated in Bangla with the help of research assistants. Validated questionnaires that have been previously translated into Bangla will be used, and rest of the questionnaire will be translated into Bangla and checked by two professional language experts and a layman.
3.5.1. Primary outcomes
-
a)
Numerical rating pain scale
This study will utilize distinct 0–10 Numerical Rating Pain Scales to assess the severity of both back pain and leg pain. Participants will be asked to rate the intensity of their pain on these scales, considering the average level experienced over the preceding week. The scales will employ endpoints marked by descriptors of “no pain” and “most extreme pain imaginable” to capture the full range of pain intensity.
-
b)
Oswestry Disability Questionnaire
To measure the activity limitation, the second version of Oswestry Disability Questionnaire will be used. This scale received sufficient reliability and validity and used in multiple languages to measure the activity limitation in people with LBP with or without leg pain [[26], [27], [28], [29]].
3.5.2. Secondary outcomes
-
a)
Brief pain inventory
Pain severity: To measure self-reported overall pain severity, the pain severity subscale of the Bangla version of Brief Pain Inventory (BPI) will be used [13]. This subscale comprises four items that ask about the worst, least, and average pain intensity ratings experienced within the last 24 h, in addition to present pain ratings. Pain is rated using an 11-point numerical rating scale, with 0 indicating “no pain” and 10 indicating “pain as bad as you can imagine.” An overall pain severity rating is calculated as the mean of the four items on pain intensity. The BPI is a reliable and valid measure of pain severity and pain interference for individuals with musculoskeletal pain, with an internal consistency of 0.82 (Cronbach alpha) [30,31].
Pain Affective Interference: The pain interference subscale of BPI includes seven items that evaluate the degree to which pain affects mood, relationships with others, and enjoyment of life [32]. Responses are rated on an 11-point numerical rating scale, with 0 indicating “does not interfere” and 10 indicating “interferes completely.” Scores for pain effective interference will be determined by calculating the mean of the respective items.
Pain physical interference: The physical interference subscale of the BPI was derived from items on how pain affects a person's engagement in general activity, walking, and normal work-related activities, similar to previous studies [33]. The three items in this subscale are specifically designed to quantify the degree to which pain interferences with activity engagement, and scores range from 0 to 10, with higher scores indicating greater disruption to activity engagement due to pain.
Both the physical and affective factors of the BPI have been supported by confirmatory factor and Rasch analysis, and the two types of pain interference (affective and physical) are useful for guiding clinical assessment in pain conditions [32,33]. The BPI is widely used pain-relation outcome measure and is recommended for people living with pain [34,35].
-
b)
Global effect
The evaluation of overall change will be conducted by means of a 7-point Likert scale, wherein participants will indicate their level of improvement or worsen since the initial assessment using responses such as “completely recovered,” “much improved,” “slightly improved,” “no change”, “slightly worsened,” “much worsened,” or “vastly worsened.” Several validation for this scale have been deemed dependable, sensitive to change and legitimate [36,37].
-
c)
Sleep quality
Valid, reliable and previously used Bangla version [38,39] of the Insomnia Severity Index (ISI) will be administered to assess sleep quality. Each item is wreathed on a 0–4 scale, and the total score ranges from 0 to 28. A cumulative score of 8 is considered to have insomnia symptoms [40].
3.6. Analysis
The study will utilize arithmetic mean and standard deviation (SD) to describe continuous variables, and frequency and percentage to express categorical variables. The difference between groups in categorical variables will be determined using the chi-square and Fisher's exact tests. Within-group comparison at baseline, 4-week, 12-weeks and 24-weeks will be assessed using paired sample t-test with 95% confidence interval. A multivariable linear regression model will be used to investigate the combined effect of PTDN on the change in pain scores and mental health symptoms adjusted for confounders. Data will be analysed using IBM SPSS-V29.0 (SPSS Inc., Chicago, Illinois, USA) and Microsoft Excel® (2021; Microsoft Corporation, Redmond, WA, USA).
4. Discussion
Finding a better management strategy for managing CLBP is an ongoing challenge. Most of the novel techniques that try to manage CLBP are limitedly tested. This study will allow testing of the combined effect of usual care physiotherapy and dry needling when managing CLBP in terms of clinical efficacy in one of the LMICs that is Bangladesh.
The previous clinical trial suggested that physiotherapy combined with dry needling significantly reduced pain, improved range of motion and functional capacity, and alleviated myofascial trigger points in patients with total knee arthroplasty [18]. Nonetheless, a systematic review and meta-analysis concluded that there is limited evidence of varying quality suggesting that the use of dry needling may offer some degree of effectiveness in reducing pain and improving pressure pain threshold among individuals experiencing musculoskeletal pain, within a timeframe ranging from immediate after the treatment to a 12-week follow-up period. When compared to no treatment or sham dry needling, this technique appears to provide superior results, although the evidence supporting this is of low quality. Additionally, compared to other physical therapy treatments, there seems to be no significant difference in functional outcomes. Notably, there is currently a lack of evidence regarding the long-term benefits of dry needling [41]. Considering the limitations of past studies, the current clinical trial was designed to evaluate the combined effects of physiotherapy and dry needling for six months in terms of pain, disability, physical interference, and insomnia of the patients with CLBP.
4.1. Implication of the study
If the combined therapy is demonstrated to be significantly effective compared to physiotherapy alone, it will provide compelling evidence for an effective treatment option in managing CLBP in Bangladesh. Consequently, clinicians and treatment providers will have the confidence and impetus to adopt this procedure.
Funding
This study receives no specific funding.
Author contributions
Conceptualization: Mohammad Ali.
Data curation: N/A.
Formal analysis: N/A.
Investigation: Md Shafiullah Prodhania, Mohammad Ali.
Methodology: Mohammad Ali.
Project administration: Mohammad Ali, Gias Uddin Ahsan.
Resources: Shafiullah Prodhania, Mohammad Ali.
Software: Mohammad Ali.
Supervision: Mohammad Ali, G U Ahsan.
Validation: Shafiullah Prodhania, Mohammad Ali, G U Ahsan.
Visualization: Mohammad Ali.
Writing– original draft: Mohammad Ali.
Writing-review, and editing: Mohammad Ali.
Disclosure of potential conflicts of interest
All authors declare no conflicts of interest.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Acknowledgments
The authors are thankful to the participants for providing the information used to conduct the study.
Contributor Information
Md Shafiullah Prodhania, Email: shafiullahprodhan@gmail.com.
Gias Uddin Ahsan, Email: guahsan@gmail.com.
Mohammad Ali, Email: alibup2018@gmail.com, mohammad.ali@latrobe.edu.au.
References
- 1.Hartvigsen J., Hancock M.J., Kongsted A., Louw Q., Ferreira M.L., Genevay S., Hoy D., Karppinen J., Pransky G., Sieper J., Smeets R.J., Underwood M., Buchbinder R., Hartvigsen J., Cherkin D., Foster N.E., Maher C.G., Underwood M., van Tulder M., Anema J.R., Chou R., Cohen S.P., Menezes Costa L., Croft P., Ferreira M., Ferreira P.H., Fritz J.M., Genevay S., Gross D.P., Hancock M.J., Hoy D., Karppinen J., Koes B.W., Kongsted A., Louw Q., Öberg B., Peul W.C., Pransky G., Schoene M., Sieper J., Smeets R.J., Turner J.A., Woolf A. What low back pain is and why we need to pay attention. Lancet. 2018;391:2356–2367. doi: 10.1016/S0140-6736(18)30480-X. [DOI] [PubMed] [Google Scholar]
- 2.Abbafati C., Abbas K.M., Abbasi-Kangevari M., Abd-Allah F., Abdelalim A., Abdollahi M., Abdollahpour I., Abegaz K.H., Abolhassani H., Aboyans V., Abreu L.G., Abrigo M.R.M., Abualhasan A., Abu-Raddad L.J., Abushouk A.I., Adabi M., Adekanmbi V., Adeoye A.M., Adetokunboh O.O., Adham D., Advani S.M., Afshin A., Agarwal G., Aghamir S.M.K., Agrawal A., Ahmad T., Ahmadi K., Ahmadi M., Ahmadieh H., Ahmed M.B., Akalu T.Y., Akinyemi R.O., Akinyemiju T., Akombi B., Akunna C.J., Alahdab F., Al-Aly Z., Alam K., Alam S., Alam T., Alanezi F.M., Alanzi T.M., Alemu B.W., Alhabib K.F., Ali M., Ali S., Alicandro G., Alinia C., Alipour V., Alizade H., Aljunid S.M., Alla F., Allebeck P., Almasi-Hashiani A., Al-Mekhlafi H.M., Alonso J., Altirkawi K.A., Amini-Rarani M., Amiri F., Amugsi D.A., Ancuceanu R., Anderlini D., Anderson J.A., Andrei C.L., Andrei T., Angus C., Anjomshoa M., Ansari F., Ansari-Moghaddam A., Antonazzo I.C., Antonio C.A.T., Antony C.M., Antriyandarti E., Anvari D., Anwer R., Appiah S.C.Y., Arabloo J., Arab-Zozani M., Aravkin A.Y., Ariani F., Armoon B., Ärnlöv J., Arzani A., Asadi-Aliabadi M., Asadi-Pooya A.A., Ashbaugh C., Assmus M., Atafar Z., Atnafu D.D., Atout M.M.d.W., Ausloos F., Ausloos M., Ayala Quintanilla B.P., Ayano G., Ayanore M.A., Azari S., Azarian G., Azene Z.N., Badawi A., Badiye A.D., Bahrami M.A., Bakhshaei M.H., Bakhtiari A., Bakkannavar S.M., Baldasseroni A., Ball K., Ballew S.H., Balzi D., Banach M., Banerjee S.K., Bante A.B., Baraki A.G., Barker-Collo S.L., Bärnighausen T.W., Barrero L.H., Barthelemy C.M., Barua L., Basu S., Baune B.T., Bayati M., Becker J.S., Bedi N., Beghi E., Béjot Y., Bell M.L., Bennitt F.B., Bensenor I.M., Berhe K., Berman A.E., Bhagavathula A.S., Bhageerathy R., Bhala N., Bhandari D., Bhattacharyya K., Bhutta Z.A., Bijani A., Bikbov B., Bin Sayeed M.S., Biondi A., Birihane B.M., Bisignano C., Biswas R.K., Bitew H., Bohlouli S., Bohluli M., Boon-Dooley A.S., Borges G., Borzì A.M., Borzouei S., Bosetti C., Boufous S., Braithwaite D., Brauer M., Breitborde N.J.K., Breitner S., Brenner H., Briant P.S., Briko A.N., Briko N.I., Britton G.B., Bryazka D., Bumgarner B.R., Burkart K., Burnett R.T., Burugina Nagaraja S., Butt Z.A., Caetano Dos Santos F.L., Cahill L.E., Cámera L.A., Campos-Nonato I.R., Cárdenas R., Carreras G., Carrero J.J., Carvalho F., Castaldelli-Maia J.M., Castañeda-Orjuela C.A., Castelpietra G., Castro F., Causey K., Cederroth C.R., Cercy K.M., Cerin E., Chandan J.S., Chang K.L., Charlson F.J., Chattu V.K., Chaturvedi S., Cherbuin N., Chimed-Ochir O., Cho D.Y., Choi J.Y.J., Christensen H., Chu D.T., Chung M.T., Chung S.C., Cicuttini F.M., Ciobanu L.G., Cirillo M., Classen T.K.D., Cohen A.J., Compton K., Cooper O.R., Costa V.M., Cousin E., Cowden R.G., Cross D.H., Cruz J.A., Dahlawi S.M.A., Damasceno A.A.M., Damiani G., Dandona L., Dandona R., Dangel W.J., Danielsson A.K., Dargan P.I., Darwesh A.M., Daryani A., Das J.K., Das Gupta R., das Neves J., Dávila-Cervantes C.A., Davitoiu D.V., De Leo D., Degenhardt L., DeLang M., Dellavalle R.P., Demeke F.M., Demoz G.T., Demsie D.G., Denova-Gutiérrez E., Dervenis N., Dhungana G.P., Dianatinasab M., Dias da Silva D., Diaz D., Dibaji Forooshani Z.S., Djalalinia S., Do H.T., Dokova K., Dorostkar F., Doshmangir L., Driscoll T.R., Duncan B.B., Duraes A.R., Eagan A.W., Edvardsson D., El Nahas N., El Sayed I., El Tantawi M., Elbarazi I., Elgendy I.Y., El-Jaafary S.I., Elyazar I.R.F., Emmons-Bell S., Erskine H.E., Eskandarieh S., Esmaeilnejad S., Esteghamati A., Estep K., Etemadi A., Etisso A.E., Fanzo J., Farahmand M., Fareed M., Faridnia R., Farioli A., Faro A., Faruque M., Farzadfar F., Fattahi N., Fazlzadeh M., Feigin V.L., Feldman R., Fereshtehnejad S.M., Fernandes E., Ferrara G., Ferrari A.J., Ferreira M.L., Filip I., Fischer F., Fisher J.L., Flor L.S., Foigt N.A., Folayan M.O., Fomenkov A.A., Force L.M., Foroutan M., Franklin R.C., Freitas M., Fu W., Fukumoto T., Furtado J.M., Gad M.M., Gakidou E., Gallus S., Garcia-Basteiro A.L., Gardner W.M., Geberemariyam B.S., Ayalew Gebreslassie A.A.A., Geremew A., Gershberg Hayoon A., Gething P.W., Ghadimi M., Ghadiri K., Ghaffarifar F., Ghafourifard M., Ghamari F., Ghashghaee A., Ghiasvand H., Ghith N., Gholamian A., Ghosh R., Gill P.S., Ginindza T.G., Giussani G., Gnedovskaya E.V., Goharinezhad S., Gopalani S.V., Gorini G., Goudarzi H., Goulart A.C., Greaves F., Grivna M., Grosso G., Gubari M.I.M., Gugnani H.C., Guimarães R.A., Guled R.A., Guo G., Guo Y., Gupta R., Gupta T., Haddock B., Hafezi-Nejad N., Hafiz A., Haj-Mirzaian A., Haj-Mirzaian A., Hall B.J., Halvaei I., Hamadeh R.R., Hamidi S., Hammer M.S., Hankey G.J., Haririan H., Haro J.M., Hasaballah A.I., Hasan M.M., Hasanpoor E., Hashi A., Hassanipour S., Hassankhani H., Havmoeller R.J., Hay S.I., Hayat K., Heidari G., Heidari-Soureshjani R., Henrikson H.J., Herbert M.E., Herteliu C., Heydarpour F., Hird T.R., Hoek H.W., Holla R., Hoogar P., Hosgood H.D., Hossain N., Hosseini M., Hosseinzadeh M., Hostiuc M., Hostiuc S., Househ M., Hsairi M., Hsieh V.C.R., Hu G., Hu K., Huda T.M., Humayun A., Huynh C.K., Hwang B.F., Iannucci V.C., Ibitoye S.E., Ikeda N., Ikuta K.S., Ilesanmi O.S., Ilic I.M., Ilic M.D., Inbaraj L.R., Ippolito H., Iqbal U., Irvani S.S.N., Irvine C.M.S., Islam M.M., Islam S.M.S., Iso H., Ivers R.Q., Iwu C.C.D., Iwu C.J., Iyamu I.O., Jaafari J., Jacobsen K.H., Jafari H., Jafarinia M., Jahani M.A., Jakovljevic M., Jalilian F., James S.L., Janjani H., Javaheri T., Javidnia J., Jeemon P., Jenabi E., Jha R.P., Jha V., Ji J.S., Johansson L., John O., John-Akinola Y.O., Johnson C.O., Jonas J.B., Joukar F., Jozwiak J.J., Jürisson M., Kabir A., Kabir Z., Kalani H., Kalani R., Kalankesh L.R., Kalhor R., Kanchan T., Kapoor N., Matin B.K., Karch A., Karim M.A., Kassa G.M., Katikireddi S.V., Kayode G.A., Kazemi Karyani A., Keiyoro P.N., Keller C., Kemmer L., Kendrick P.J., Khalid N., Khammarnia M., Khan E.A., Khan M., Khatab K., Khater M.M., Khatib M.N., Khayamzadeh M., Khazaei S., Kieling C., Kim Y.J., Kimokoti R.W., Kisa A., Kisa S., Kivimäki M., Knibbs L.D., Knudsen A.K.S., Kocarnik J.M., Kochhar S., Kopec J.A., Korshunov V.A., Koul P.A., Koyanagi A., Kraemer M.U.G., Krishan K., Krohn K.J., Kromhout H., Kuate Defo B., Kumar G.A., Kumar V., Kurmi O.P., Kusuma D., La Vecchia C., Lacey B., Lal D.K., Lalloo R., Lallukka T., Lami F.H., Landires I., Lang J.J., Langan S.M., Larsson A.O., Lasrado S., Lauriola P., Lazarus J.V., Lee P.H., Lee S.W.H., Legrand K.E., Leigh J., Leonardi M., Lescinsky H., Leung J., Levi M., Li S., Lim L.L., Linn S., Liu S., Liu S., Liu Y., Lo J., Lopez A.D., Lopez J.C.F., Lopukhov P.D., Lorkowski S., Lotufo P.A., Lu A., Lugo A., Maddison E.R., Mahasha P.W., Mahdavi M.M., Mahmoudi M., Majeed A., Maleki A., Maleki S., Malekzadeh R., Malta D.C., Mamun A.A., Manda A.L., Manguerra H., Mansour-Ghanaei F., Mansouri B., Mansournia M.A., Mantilla Herrera A.M., Maravilla J.C., Marks A., Martin R.V., Martini S., Martins-Melo F.R., Masaka A., Masoumi S.Z., Mathur M.R., Matsushita K., Maulik P.K., McAlinden C., McGrath J.J., McKee M., Mehndiratta M.M., Mehri F., Mehta K.M., Memish Z.A., Mendoza W., Menezes R.G., Mengesha E.W., Mereke A., Mereta S.T., Meretoja A., Meretoja T.J., Mestrovic T., Miazgowski B., Miazgowski T., Michalek I.M., Miller T.R., Mills E.J., Mini G.K., Miri M., Mirica A., Mirrakhimov E.M., Mirzaei H., Mirzaei M., Mirzaei R., Mirzaei-Alavijeh M., Misganaw A.T., Mithra P., Moazen B., Mohammad D.K., Mohammad Y., Mohammad Gholi Mezerji N., Mohammadian-Hafshejani A., Mohammadifard N., Mohammadpourhodki R., Mohammed A.S., Mohammed H., Mohammed J.A., Mohammed S., Mokdad A.H., Molokhia M., Monasta L., Mooney M.D., Moradi G., Moradi M., Moradi-Lakeh M., Moradzadeh R., Moraga P., Morawska L., Morgado-Da-Costa J., Morrison S.D., Mosapour A., Mosser J.F., Mouodi S., Mousavi S.M., Khaneghah A.M., Mueller U.O., Mukhopadhyay S., Mullany E.C., Musa K.I., Muthupandian S., Nabhan A.F., Naderi M., Nagarajan A.J., Nagel G., Naghavi M., Naghshtabrizi B., Naimzada M.D., Najafi F., Nangia V., Nansseu J.R., Naserbakht M., Nayak V.C., Negoi I., Ngunjiri J.W., Nguyen C.T., Nguyen H.L.T., Nguyen M., Nigatu Y.T., Nikbakhsh R., Nixon M.R., Nnaji C.A., Nomura S., Norrving B., Noubiap J.J., Nowak C., Nunez-Samudio V., Oancea B., Odell C.M., Ogbo F.A., Oh I.H., Okunga E.W., Oladnabi M., Olagunju A.T., Olusanya B.O., Olusanya J.O., Omer M.O., Ong K.L., Onwujekwe O.E., Orpana H.M., Ortiz A., Osarenotor O., Osei F.B., Ostroff S.M., Otoiu A., Otstavnov N., Otstavnov S.S., Øverland S., Owolabi M.O., Mahesh P.A., Padubidri J.R., Palladino R., Panda-Jonas S., Pandey A., Parry C.D.H., Pasovic M., Pasupula D.K., Patel S.K., Pathak M., Patten S.B., Patton G.C., Toroudi H.P., Peden A.E., Pennini A., Pepito V.C.F., Peprah E.K., Pereira D.M., Pesudovs K., Pham H.Q., Phillips M.R., Piccinelli C., Pilz T.M., Piradov M.A., Pirsaheb M., Plass D., Polinder S., Polkinghorne K.R., Pond C.D., Postma M.J., Pourjafar H., Pourmalek F., Poznañska A., Prada S.I., Prakash V., Pribadi D.R.A., Pupillo E., Syed Z.Q., Rabiee M., Rabiee N., Radfar A., Rafiee A., Raggi A., Rahman M.A., Rajabpour-Sanati A., Rajati F., Rakovac I., Ram P., Ramezanzadeh K., Ranabhat C.L., Rao P.C., Rao S.J., Rashedi V., Rathi P., Rawaf D.L., Rawaf S., Rawal L., Rawassizadeh R., Rawat R., Razo C., Redford S.B., Reiner R.C., Reitsma M.B., Remuzzi G., Renjith V., Renzaho A.M.N., Resnikoff S., Rezaei N., Rezaei N., Rezapour A., Rhinehart P.A., Riahi S.M., Ribeiro D.C., Ribeiro D., Rickard J., Rivera J.A., Roberts N.L.S., Rodríguez-Ramírez S., Roever L., Ronfani L., Room R., Roshandel G., Roth G.A., Rothenbacher D., Rubagotti E., Rwegerera G.M., Sabour S., Sachdev P.S., Saddik B., Sadeghi E., Sadeghi M., Saeedi R., Saeedi Moghaddam S., Safari Y., Safi S., Safiri S., Sagar R., Sahebkar A., Sajadi S.M., Salam N., Salamati P., Salem H., Salem M.R., Salimzadeh H., Salman O.M., Salomon J.A., Samad Z., Samadi Kafil H., Sambala E.Z., Samy A.M., Sanabria J., Sánchez-Pimienta T.G., Santomauro D.F., Santos I.S., Santos J.V., Santric-Milicevic M.M., Saraswathy S.Y.I., Sarmiento-Suárez R., Sarrafzadegan N., Sartorius B., Sarveazad A., Sathian B., Sathish T., Sattin D., Saxena S., Schaeffer L.E., Schiavolin S., Schlaich M.P., Schmidt M.I., Schutte A.E., Schwebel D.C., Schwendicke F., Senbeta A.M., Senthilkumaran S., Sepanlou S.G., Serdar B., Serre M.L., Shadid J., Shafaat O., Shahabi S., Shaheen A.A., Shaikh M.A., Shalash A.S., Shams-Beyranvand M., Shamsizadeh M., Sharafi K., Sheikh A., Sheikhtaheri A., Shibuya K., Shield K.D., Shigematsu M., Il Shin J., Shin M.J., Shiri R., Shirkoohi R., Shuval K., Siabani S., Sierpinski R., Sigfusdottir I.D., Sigurvinsdottir R., Silva J.P., Simpson K.E., Singh J.A., Singh P., Skiadaresi E., Skou S.T., Skryabin V.Y., Smith E.U.R., Soheili A., Soltani S., Soofi M., Sorensen R.J.D., Soriano J.B., Sorrie M.B., Soshnikov S., Soyiri I.N., Spencer C.N., Spotin A., Sreeramareddy C.T., Srinivasan V., Stanaway J.D., Stein C., Stein D.J., Steiner C., Stockfelt L., Stokes M.A., Straif K., Stubbs J.L., Sufiyan M.B., Suleria H.A.R., Suliankatchi Abdulkader R., Sulo G., Sultan I., Tabarés-Seisdedos R., Tabb K.M., Tabuchi T., Taherkhani A., Tajdini M., Takahashi K., Takala J.S., Tamiru A.T., Taveira N., Tehrani-Banihashemi A., Temsah M.H., Tesema G.A., Tessema Z.T., Thurston G.D., Titova M.V., Tohidinik H.R., Tonelli M., Topor-Madry R., Topouzis F., Torre A.E., Touvier M., Tovani-Palone M.R., Tran B.X., Travillian R., Tsatsakis A., Tudor Car L.T., Tyrovolas S., Uddin R., Umeokonkwo C.D., Unnikrishnan B., Upadhyay E., Vacante M., Valdez P.R., van Donkelaar A., Vasankari T.J., Vasseghian Y., Veisani Y., Venketasubramanian N., Violante F.S., Vlassov V., Vollset S.E., Vos T., Vukovic R., Waheed Y., Wallin M.T., Wang Y., Wang Y.P., Watson A., Wei J., Wei M.Y.W., Weintraub R.G., Weiss J., Werdecker A., West J.J., Westerman R., Whisnant J.L., Whiteford H.A., Wiens K.E., Wolfe C.D.A., Wozniak S.S., Wu A.M., Wu J., Wulf Hanson S., Xu G., Xu R., Yadgir S., Yahyazadeh Jabbari S.H., Yamagishi K., Yaminfirooz M., Yano Y., Yaya S., Yazdi-Feyzabadi V., Yeheyis T.Y., Yilgwan C.S., Yilma M.T., Yip P., Yonemoto N., Younis M.Z., Younker T.P., Yousefi B., Yousefi Z., Yousefinezhadi T., Yousuf A.Y., Yu C., Yusefzadeh H., Moghadam T.Z., Zamani M., Zamanian M., Zandian H., Zastrozhin M.S., Zhang Y., Zhang Z.J., Zhao J.T., Zhao X.J.G., Zhao Y., Zheng P., Zhou M., Davletov K., Ziapour A., Mondello S., Lim S.S., Murray C.J.L., Wiangkham T., Amini S. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9/ATTACHMENT/96EE692B-E8A2-4D2B-AB04-5A9B1490A107/MMC2E.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maetzel A., Li L. The economic burden of low back pain: a review of studies published between 1996 and 2001. Best Pract. Res. Clin. Rheumatol. 2002;16:23–30. doi: 10.1053/berh.2001.0204. [DOI] [PubMed] [Google Scholar]
- 4.Foster N.E., Anema J.R., Cherkin D., Chou R., Cohen S.P., Gross D.P., Ferreira P.H., Fritz J.M., Koes B.W., Peul W., Turner J.A., Maher C.G., Buchbinder R., Hartvigsen J., Cherkin D., Foster N.E., Maher C.G., Underwood M., van Tulder M., Anema J.R., Chou R., Cohen S.P., Menezes Costa L., Croft P., Ferreira M., Ferreira P.H., Fritz J.M., Genevay S., Gross D.P., Hancock M.J., Hoy D., Karppinen J., Koes B.W., Kongsted A., Louw Q., Öberg B., Peul W.C., Pransky G., Schoene M., Sieper J., Smeets R.J., Turner J.A., Woolf A. Prevention and treatment of low back pain: evidence, challenges, and promising directions. Lancet. 2018;391:2368–2383. doi: 10.1016/S0140-6736(18)30489-6. [DOI] [PubMed] [Google Scholar]
- 5.Stochkendahl M.J., Kjaer P., Hartvigsen J., Kongsted A., Aaboe J., Andersen M., Andersen M.Ø., Fournier G., Højgaard B., Jensen M.B., Jensen L.D., Karbo T., Kirkeskov L., Melbye M., Morsel-Carlsen L., Nordsteen J., Palsson T.S., Rasti Z., Silbye P.F., Steiness M.Z., Tarp S., Vaagholt M. National Clinical Guidelines for non-surgical treatment of patients with recent onset low back pain or lumbar radiculopathy. Eur. Spine J. 2018;27:60–75. doi: 10.1007/s00586-017-5099-2. [DOI] [PubMed] [Google Scholar]
- 6.Ali M., Bonna A.S., Sarkar A., Islam A. Is coronavirus infection associated with musculoskeletal health complaints? Results from a comprehensive case-control study. J. Prim. Care Community Health. 2022;13 doi: 10.1177/21501319221114259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ali M., Ahsan G.U., Uddin Z., Hossain A. Road traffic delays in commuting workplace and musculoskeletal health among sedentary workers: a cross-sectional study in Dhaka city. J. Occup. Health. 2021;63 doi: 10.1002/1348-9585.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ali M., Ahsan G.U., Hossain A. Prevalence and associated occupational factors of low back pain among the bank employees in Dhaka City. J. Occup. Health. 2020 doi: 10.1002/1348-9585.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ali M., Bonna A.S., Sarkar A., Islam M.A., Rahman N.-A.-S. SARS-CoV-2 infection is associated with low back pain: findings from a community-based case-control study. Int. J. Infect. Dis. 2022;122:144–151. doi: 10.1016/j.ijid.2022.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ali M., Siddiq M.A.B., Pranto N.K., Amran N.H., Akter M., Munny M.A., Hossain M.I., Khan S.S., Mehedi M.M.H. Prevalence and predictors of musculoskeletal health complaints among sedentary, monotonous urban workers: a survey in Bangladesh. PLoS One. 2023;18 doi: 10.1371/journal.pone.0282922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ali M., Uddin Z., Hossain A. Economic stressors and mental health symptoms among Bangladeshi rehabilitation professionals: a cross-sectional study amid COVID-19 pandemic. Heliyon. 2021;7 doi: 10.1016/j.heliyon.2021.e06715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ali M., Uddin Z., Hossain A. Clinical practice pattern of managing low back pain among physiotherapists in Bangladesh: a cross-sectional study. Physiother. Pract. Res. 2022;43:275–282. doi: 10.3233/PPR-210549. [DOI] [Google Scholar]
- 13.Ali M., Uddin Z., Hossain A. Combined effect of vitamin D supplementation and physiotherapy on reducing pain among adult patients with musculoskeletal disorders: a quasi-experimental clinical trial. Front. Nutr. 2021;8:739. doi: 10.3389/fnut.2021.717473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajfur J., Rajfur K., Kosowski Ł., Walewicz K., Dymarek R., Ptaszkowski K., Taradaj J. The effectiveness of dry needling in patients with chronic low back pain: a prospective, randomized, single-blinded study. Sci. Rep. 2022;12 doi: 10.1038/s41598-022-19980-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu H.T., Gao H., Ma R.J., Zhao X.F., Tian H.F., Li L. 2018. Is Dry Needling Effective for Low Back Pain?: A Systematic Review and PRISMA-Compliant Meta-Analysis; p. 97. Medicine (Baltimore) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Middelkoop M., Rubinstein S.M., Kuijpers T., Verhagen A.P., Ostelo R., Koes B.W., Van Tulder M.W. A systematic review on the effectiveness of physical and rehabilitation interventions for chronic non-specific low back pain. Eur. Spine J. 2011;20:19. doi: 10.1007/S00586-010-1518-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foster N.E., Anema J.R., Cherkin D., Chou R., Cohen S.P., Gross D.P., Ferreira P.H., Fritz J.M., Koes B.W. 2018. Prevention and Treatment of Low Back Pain : Evidence , Challenges , and Promising Directions; p. 391. [DOI] [PubMed] [Google Scholar]
- 18.Núñez-Cortés R., Cruz-Montecinos C., Vásquez-Rosel Á., Paredes-Molina O., Cuesta-Vargas A. Dry needling combined with physical therapy in patients with chronic postsurgical pain following total knee arthroplasty: a case series. J. Orthop. Sports Phys. Ther. 2017;47:209–216. doi: 10.2519/jospt.2017.7089. [DOI] [PubMed] [Google Scholar]
- 19.Woods C.S., Kishino N.D., Haider T.T., Kay P.K. Effects of subacute versus chronic status of low back pain patients' response to a functional restoration program. J. Occup. Rehabil. 2000;10:229–233. doi: 10.1023/A:1026618519877. [DOI] [Google Scholar]
- 20.Zhong B. How to calculate sample size in randomized controlled trial? J. Thorac. Dis. 2009;1:51. /pmc/articles/PMC3256489/ [PMC free article] [PubMed] [Google Scholar]
- 21.Eysenbach G. Improving the quality of web surveys: the checklist for reporting results of internet E-surveys (CHERRIES) J. Med. Internet Res. 2004;6:e34. doi: 10.2196/jmir.6.3.e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zügel M., Maganaris C.N., Wilke J., Jurkat-Rott K., Klingler W., Wearing S.C., Findley T., Barbe M.F., Steinacker J.M., Vleeming A., Bloch W., Schleip R., Hodges P.W. Fascial tissue research in sports medicine: from molecules to tissue adaptation, injury and diagnostics: consensus statement. Br. J. Sports Med. 2018;52 doi: 10.1136/BJSPORTS-2018-099308. 1497–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vajapey S., Miller T.L. Evaluation, diagnosis, and treatment of chronic exertional compartment syndrome: a review of current literature. Phys. Sportsmed. 2017;45:391–398. doi: 10.1080/00913847.2017.1384289. Https://Doi.Org/10.1080/00913847.2017.1384289. [DOI] [PubMed] [Google Scholar]
- 24.Cruz-Díaz D., Lomas Vega R., Osuna-Pérez M.C., Hita-Contreras F., Martínez-Amat A. Effects of joint mobilization on chronic ankle instability: a randomized controlled trial. Disabil. Rehabil. 2015;37:601–610. doi: 10.3109/09638288.2014.935877. [DOI] [PubMed] [Google Scholar]
- 25.Hubbuch J.E., Bennett B.W., Dean J.C. Proprioceptive feedback contributes to the adaptation toward an economical gait pattern. J. Biomech. 2015;48:2925–2931. doi: 10.1016/j.jbiomech.2015.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davidson M., Keating J.L. A comparison of five low back disability questionnaires: reliability and responsiveness. Phys. Ther. 2002;82:8–24. doi: 10.1093/ptj/82.1.8. [DOI] [PubMed] [Google Scholar]
- 27.Fritz J.M., Irrgang J.J. A comparison of a modified Oswestry low back pain disability questionnaire and the quebec back pain disability scale. Phys. Ther. 2001;81:776–788. doi: 10.1093/ptj/81.2.776. [DOI] [PubMed] [Google Scholar]
- 28.Vincent J.I., MacDermid J.C., Grewal R., Sekar V.P., Balachandran D. Translation of Oswestry disability Index into Tamil with cross cultural adaptation and evaluation of reliability and validity. Open Orthop. J. 2014;8:11–19. doi: 10.2174/1874325001408010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu E.M., Nosova E.V., Falkenstein Y., Prasad P., Leasure J.M., Kondrashov D.G. Validation of a Russian language Oswestry disability Index questionnaire. Global Spine J. 2016;6:636–639. doi: 10.1055/s-0035-1570085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mendoza T., Mayne T., Rublee D., Cleeland C. Reliability and validity of a modified Brief Pain Inventory short form in patients with osteoarthritis. Eur. J. Pain. 2006;10:353. doi: 10.1016/j.ejpain.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 31.Keller S., Bann C.M., Dodd S.L., Schein J., Mendoza T.R., Cleeland C.S. Validity of the Brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin. J. Pain. 2004;20:309–318. doi: 10.1097/00002508-200409000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Walton D.M., Beattie T., Putos J., MacDermid J.C. A Rasch analysis of the Brief Pain Inventory Interference subscale reveals three dimensions and an age bias. J. Clin. Epidemiol. 2016;74:218–226. doi: 10.1016/j.jclinepi.2015.10.022. [DOI] [PubMed] [Google Scholar]
- 33.Walton D.M., Putos J., Beattie T., MacDermid J.C. Confirmatory factor analysis of 2 versions of the Brief Pain Inventory in an ambulatory population indicates that sleep interference should be interpreted separately. Scand. J. Pain. 2016;12:110–116. doi: 10.1016/j.sjpain.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 34.Dworkin R.H., Turk D.C., Farrar J.T., Haythornthwaite J.A., Jensen M.P., et al. Topical Review and Recommendations Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 35.Woznowski-Vu A., Uddin Z., Flegg D., Aternali A., Wickens R., Sullivan M.J.L., Sweet S.N., Skou S.T., Wideman T.H. Comparing novel and existing measures of sensitivity to physical activity among people with chronic musculoskeletal pain. Clin. J. Pain. 2019;35:656–667. doi: 10.1097/AJP.0000000000000732. [DOI] [PubMed] [Google Scholar]
- 36.Kamper S. Global rating of change scales. Aust. J. Physiother. 2009;55:289. doi: 10.1016/S0004-9514(09)70015-7. [DOI] [PubMed] [Google Scholar]
- 37.Kamper S.J., Maher C.G., Mackay G. Global rating of change scales: a review of strengths and weaknesses and considerations for design. J. Man. Manip. Ther. 2009;17:163–170. doi: 10.1179/JMT.2009.17.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mamun M.A., Alimoradi Z., Gozal D., Manzar M.D., Broström A., Lin C.Y., Huang R.Y., Pakpour A.H. Validating insomnia severity Index (ISI) in a Bangladeshi population: using classical test theory and Rasch analysis. Int. J. Environ. Res. Publ. Health. 2022;19:225. doi: 10.3390/IJERPH19010225. 19 (2021) 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ali M. Factors associated with COVID-19 fear among healthcare professionals in Bangladesh. Dialogues Heal. 2022;1 doi: 10.1016/j.dialog.2022.100037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morin C.M., Belleville G., Bélanger L., Ivers H. The insomnia severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34:601–608. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gattie E., Cleland J.A., Snodgrass S. The effectiveness of trigger point dry needling for musculoskeletal conditions by physical therapists: a systematic review and meta-analysis. J. Orthop. Sports Phys. Ther. 2017;47:133–149. doi: 10.2519/jospt.2017.7096. [DOI] [PubMed] [Google Scholar]