Abstract
A triester method for the synthesis of deoxynucleoside phosphorodithioate dimers is described. The phosphorodithioate linkage is introduced using a new dithiophosphorylating reagent DPSE-SP(S)Cl2 where DPSE = 2-diphenylmethylsilylethyl. This group is removed quickly using tetra-butylammonium fluoride leading to the quantitative formation of phosphorodithioate diesters uncontaminated with the corresponding phosphorothioates. The utility of this group is demonstrated by the synthesis of a pentadecathymidylic acid, [T(PS2)T(PO2)]7T, which contains alternating phosphorodithioate/phosphate diester internucleotide linkages.
Modified oligodeoxynucleotides have recently received much attention due to their therapeutic applications (1–3). Among the more interesting nucleic acid analogs are the phosphorodithioates in which both non-bridging oxygen atoms of the internucleotide linkage are replaced by sulfur. Deoxynucleoside phosphorodithioate dimers have been prepared in several ways using H-phosphonate (4–11), phosphordiamidite (12), phosphoramidite (13–15) and thiophosphoramidite (16–18) chemistries. Reports have also appeared on the synthesis of oligonucleotides with alternating phosphate and phosphorodithioate linkages (19), as well as one on ribonucleoside dimers (20). Of the above methods, the thiophosphoramidite method has been applied successfully to the preparation of mixed base oligonucleotides containing contiguous phosphorodithioate linkages. However, this method gives products which contain varying amounts of phosphorothioate linkages (2–10%) due to factors associated with the involvement of trivalent thiophosphorus compounds (21). In addition, the thiophosphoramidite synthons are difficult to purify on silica gel columns and have a tendency to dismutate in the presence of acidic catalysts such as tetrazole (15). The thiophosphite intermediate which is formed is also unstable to tetrazole. Similarly in thio- and dithio-H-phosphonate methodologies, the primary coupling products are unstable to reagents such as pivaloyl chloride (4) and toward the iodine mixtures routinely used for oxidation (7). Recently, Dahl et. al. reported (22–26) synthesis of dimers and oligomers up to the octamer level. In addition to being contaminated with low levels of phosphorothioate diester linkages, ∼1.2% per phosphorodithioate linkage of the oligomer was cleaved during deprotection. Octanucleotide phosphorodithioates T8(S2) (0.02 mmol) and C7T(S2) (0.12 mmol) have been synthesized on a large scale using dimers and tetramers (27). Phosphorodithioate linkages have also been synthesized utilizing the dithiaphospholane method in solution and on a solid support (28,29). We now report the preparation of deoxynucleoside phosphorodithioate dimers by a triester method using a new dithiophosphorylating reagent, DPSE-SP(S)Cl2, where DPSE = 2-diphenylmethylsilylethyl. This protecting group has recently been utilized (30–34) in the synthesis of oligonucleotides via the phosphoramidite approach.
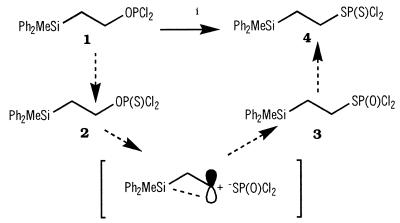
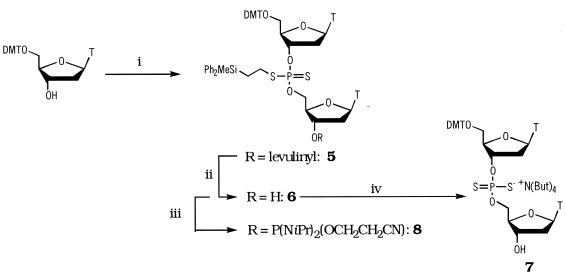
2-Diphenylmethylsilylethyldithiophosphorodichloridate (4) was prepared by reaction of commercially available 2-diphenylmethylsilyl ethanol with phosphorus trichloride to give phosphorodichloridite (1) [31P-NMR (δ): 175.5 p.p.m.] (31) which on refluxing with thiophosphoryl chloride, sulfur and charcoal afforded (4) (75% overall yield based on starting alcohol) [31P-NMR (δ): 71.45 p.p.m.] (35). The course of this interesting oxidative rearrangement was monitored by 31P-NMR. From spectra of the crude reaction mixture collected at various time points it appears as though the presumed initially formed O-alkylphosphorodichloridothioate (2) rearranges under the reaction conditions to the corresponding S-alkylphosphorodichloridothioate (3) [31P-NMR (δ): 32 p.p.m.] which is then converted to the product dichloridodithioate (4) [31P-NMR (δ): 71.5 p.p.m., Scheme 1]. Such migrations, commonly referred to as thiono-thiolo rearrangements (36–39), are known to involve (alkyl+) (thiophosphate–) ion pairs. The presence of a silicon atom in the 2 position in the above example would be expected to facilitate such a reaction (the β-silicon effect) (40). Treatment of dichloridothioate (4) with two equivalents of 1-hydroxy-6-trifluoromethylbenzotriazole gave putative O,O-bis(6-trifluoromethylbenzotriazol-1-yl)-S-2-diphenylmethylsilylethylphosphorodithioate [31P-NMR (δ): 114.1 p.p.m.] which upon sequential reaction with 5′-O-dimethoxytritylthymidine and 3′-O-levulinylthymidine gave the fully protected phosphorodithioate dimer (5) [31P-NMR (δ): 99.28, 98.5 p.p.m.] in 70% yield (Scheme 2).
Scheme 1. Reagents and conditions: (i) thiophosphoryl chloride, S8, charcoal, reflux, 12 h.
Scheme 2. Reagents and conditions: (i) Putative O,O-bis(6-trifluoromethylbenzotriazol-1-yl)-S-2-diphenylmethylsilylethylphosphorodiothiate, pyridine-dioxan, 2 h, 25°C then 3′-O-levulinylthymidine, N-methylimidazole, 18 h, 25°C; (ii) NH2NH2.H2O, pyridine–AcOH (3:2 v/v), 10 min, 0°C; (iii) 2-cyanoethyl-N,N,N′,N′-tetraisopropylphosphorodiamidite, I-H-tetrazole, CH2Cl2, 60 min, 25°C; (iv) TBAF (1 M in THF), 5 min, 25°C.
In a typical experiment compound (4) (5.9 g, 15 mmol) was coevaporated with dry dioxane (10 ml), then redissolved in dioxane (30 ml) and dry pyridine (2.4 ml, 30 mmol). 1-Hydroxy-6-trifluoromethylbenzotriazole (6.1 g, 30 mmol) was added and the products stirred at ambient temperature for 1 h. The above bis-phosphorylating reagent (25 ml, ∼12.5 mmol) was added to a stirred suspension of 5′-O-4,4′-dimethoxytritylthymidine (4.35 g, 8 mmol) in dry dioxane (5 ml). The mixture was allowed to stir for 2 h at room temperature, then 3′-O-levulinylthymidine (2.04 g, 6 mmol) and 1-methylimidazole (3.3 g, 40 mmol) were added. The mixture was allowed to stir overnight, then the solvent removed under reduced pressure. A solution of the residue in ethyl acetate (100 ml) was washed with saturated aqueous sodium hydrogen carbonate (2 × 50 ml) then dried (Na2SO4), filtered and concentrated in vacuo. The crude product was purified by silica gel chromatography, combination and evaporation of the fractions eluted with EtOAc–hexanes (4:1 v/v) gave compound (5) (5 g, 4.2 mmol, 70%) as a colourless glass. (5): Rf 0.4 (CHCl3-CH3OH, 9:1 v/v) 1H-NMR (CDCl3) δ 0.57 (s, 1.5 H), 0.61 (s, 1.5 H), 1.45 (m, 2H), 1.49–1.60 (m, 2H), 1.85 (s, 3H), 1.88 (s, 1.5H), 1.91 (s, 1.5H) 2.17 (s, 3H), 2.23–2.49 (m, 2H), 2.50–2.68 (m, 2H), 2.71–2.81 (m, 2H), 2.82–3.17 (m, 2H), 3.36–3.44 (m, 2H), 3.77 (s, 6H), 4.16–4.41 (m, 4H), 5.21 (m, 1H), 5.41 (m, 1H), 6.36 (m, 2H), 6.80–6.86 (m, 4H), 7.20–7.58 (m, 21H), 9.16 (m, br, 2H), 31P-NMR (CDCl3) δ 98.50, 99.30.
Removal of the 3′-O-levulinyl group under standard conditions (buffered hydrazine hydrate, 10 min, 0°C) gave the partially protected dimer (6) in 95% yield.
(6): 31P-NMR (CDCl3) δ 99.46, 99.66; [Found: m/z = 1127.3133 M + Na, C56H61N4O12PS2SiNa requires = 1127.3132].
As anticipated from our earlier studies on DPSE protected dinucleoside phosphorothioate triesters (34), treatment of dimer (6) with tetra-butylammonium fluoride (1 M, THF) at room temperature resulted in the quantitative formation in <5 min of the partially protected phosphorodithioate diester (7) via a β-fragmentation reaction.
(7): 31P-NMR (CDCl3) δ 113.8 [Found: m/z = 903.2143 M + Na, C41H45N4O12PS2Na requires = 903.2143].
The 31P-NMR spectrum of diester (7) (Fig. 1) clearly shows the material obtained to be essentially free from the corresponding dinucleoside phosphorothioate diester. It also proved possible to convert compound (6) into a phosphoramidite useful for the introduction of phophorodithioate linkages at predetermined sites within an oligonucleotide (vide infra). To this end, treatment of compound (6) with 2-cyanoethyl-N,N,N′,N′-tetra-isopropylphosphorodiamidite in the presence of 1H-tetrazole gave the dinucleoside phosphoramidite (8) in 90% isolated yield.
Figure 1.
31P-NMR spectrum of phosphorodithioate diester (7).
(8): 31P-NMR (CDCl3) δ 98.00, 98.40, 98.43, 99.56, 149.45, 149.65, 150.03 [Found: m/z = 1327.4165 M + Na, C65H78N6O13P2S2SiNa requires 1327.4211].
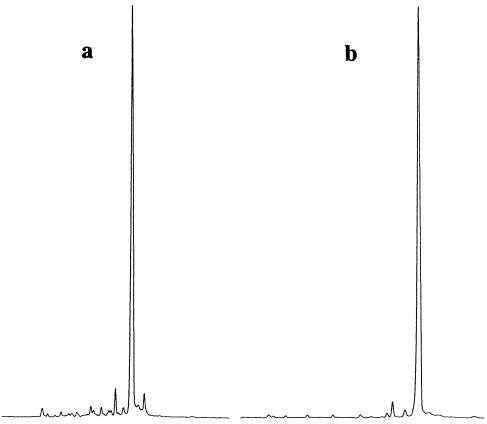
Synthesis of [T(PS2)T(PO2)]7T. In order to demonstrate the utility of the above dimer amidite (8), the synthesis of a pentadecathymidilylic acid, [T(PS2)T(PO2)]7T, containing alternating phosphorodithioate/phosphate diester internucleotide linkages was undertaken. Oligomerization was performed on an ABI 390Z DNA/RNA synthesizer (1 µmol scale on CPG) using a 0.1 M solution of amidite (8) (∼7 eq./coupling, 180 s wait time) in CH3CN utilizing a 0.45 M solution of 1H-tetrazole in CH3CN as activator. Cycle-wise oxidation was achieved by treatment with 90% aqueous tert-butyl hydroperoxide in CH3CN (15:85 v/v, 600 s wait time). Average coupling efficiencies were judged to be >98% by trityl assay. Treatment of the support-bound oligonucleotide with concentrated ammonium hydroxide (1 h, 25°C) to affect succinate cleavage and cyanoethyl removal was followed by incubation with 1 M tetra-butylammonium fluoride in THF (24 h, 25°C) to remove the DPSE groups. Desalting (SepPak™ C18 cartridge) of the lyophilized reaction mixture gave ∼100 A260 units of the crude oligomer, which was purified by reversed phase HPLC to afford ∼80 A260 units of the purified pentadecamer. Crude and purified oligonucleotides were detritylated with AcOH–water (1:4 v/v) and precipitated in the presence ammonium acetate with 10 vol of cold (–20°C) ethanol. Capillary gel electrophoresis (CGE) (Fig. 2) and electrospray mass spectrometry confirmed the identity of the product.
Figure 2.
Capillary gel electropherogram of (a) crude and (b) purified [T(PS2)T(PO2)]7T.
Found 4725.4, C150H196N30O89P14S14 requires 4725.2.
31P-NMR analysis (Fig. 3) of the crude material showed the expected 1:1 ratio of phosphorothioate:phosphate diester linkages (δ = 114.8 and –0.1 p.p.m., respectively). Figure 3 also shows the product to be virtually free of contaminating phosphorothioate diester linkages [31P-NMR (δ): ∼58 p.p.m.].
Figure 3.
31P-NMR spectrum of crude [T(PS2)T(PO2)]7T.
In summary, we have shown that protected dinucleoside phosphorodithioates can be obtained in good yields by a phosphotriester method using the new phosphorylating agent (2). The DPSE group can be removed cleanly under mild conditions which do not lead to formation of phosphorothioate impurities or lead to internucleotide cleavage. In addition we have demonstrated that implementation of this novel protecting group strategy leads to high quality oligonucleotides containing alternating phosphorodithioate/phosphate diester linkages.
REFERENCES
- 1.Crooke S.T. (1995) In Cuello,A.C. and Collier,B. (eds), Pharmacological Sciences, Perspectives for Research and Therapy in the late 1990s. Birkhäuser Verlag, Basel, Switzerland, pp. 393–399.
- 2.Crooke S.T. and Bennett,C.F. (1996) Annu. Rev. Pharmacol. Toxicol., 36, 107. [DOI] [PubMed] [Google Scholar]
- 3.Crooke S.T. (1995) In Wolff,M.E. (ed.), Burger’s Medicinal Chemistry and Drug Discovery. John Wiley & Sons, New York, Vol. 1, pp. 863.
- 4.Stawinsky J., Thelin,M. and Zain,R. (1989) Tetrahedron Lett., 30, 2157–2159. [Google Scholar]
- 5.Zain R., Stromberg,R. and Stawinski,J. (1995) J. Org. Chem., 60, 8241–8244. [Google Scholar]
- 6.Porrit G.M. and Reese,C.B. (1989) Tetrahedron Lett., 30, 4713–4715. [Google Scholar]
- 7.Brill W.K.-D., Yau,E.K. and Caruthers,M.H. (1989) Tetrahedron Lett., 30, 6621–6624. [Google Scholar]
- 8.Weisler W.T., Marshall,W.S. and Caruthers,M.H. (1993) In Agrawal,S. (ed.), Methods in Molecular Biology, Vol 20: Protocols for Oligonucleotides and Analogs, Synthesis and Properties. Humana Press Inc., Totowa, p. 191.
- 9.Yau E.K., Ma,Y.-X. and Caruthers,M.H. (1990) Tetrahedron Lett., 31, 1953–1956. [Google Scholar]
- 10.Beaton G., Brill,W.K.-D., Grandas,A., Ma,Y.-X., Nielsen,J., Yan,E. and Caruthers,M.H. (1991) Tetrahedron, 47, 2377–2388. [Google Scholar]
- 11.Greef C.H., Seeberger,P.H., Caruthers,M.H., Beaton,G. and Bankaitis-Davis,D. (1996) Tetrahedron Lett., 37, 4451–4454. [Google Scholar]
- 12.Nielsen J., Brill,W.K.-D. and Caruthers,M.H. (1998) Tetrahedron Lett., 29, 2911–2914. [Google Scholar]
- 13.Brill W.K.-D., Nielsen,J. and Caruthers,M.H. (1998) Tetrahedron Lett., 29, 5517–5520. [Google Scholar]
- 14.Farschschi N. and Gorenstein,D.G. (1998) Tetrahedron Lett., 29, 6843–6846. [Google Scholar]
- 15.Dahl B.H., Bjergarde,K., Sommer,V.B. and Dahl,O. (1989) Acta Chem. Scand., 43, 896–901. [Google Scholar]
- 16.Brill W.K.-D., Tang,J.-Y., Ma,Y.-X. and Caruthers,M.H. (1989) J. Am. Chem. Soc., 111, 2321–2322. [Google Scholar]
- 17.Dahl B.H., Bjergarde,K., Sommer,V.B. and Dahl,O. (1989) Nucl. Nucl., 8, 1023–1027. [Google Scholar]
- 18.Wiesler W.T. and Caruther,M.H. (1996) J. Org. Chem., 61, 4272–4281. [DOI] [PubMed] [Google Scholar]
- 19.Grandas A., Marshall,W.S., Nielsen,J. and Caruthers,M.H. (1989) Tetrahedron Lett., 30, 543–546. [Google Scholar]
- 20.Petersen K.H. and Nielsen,J. (1990) Tetrahedron Lett., 31, 911–914. [Google Scholar]
- 21.Bjergarde K. and Dahl,O. (1991) Nucleic Acids Res., 19, 5843–5850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dahl B.H., Bjergarde,K., Nielsen,J. and Dahl,O. (1990) Tetrahedron Lett., 31, 3489–3492. [Google Scholar]
- 23.Eldrup A.B., Bjergarde,K., Felding,J., Kehler,J. and Dahl,O. (1994) Nucleic Acids Res., 22, 1797–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eldrup A.B., Felding,J., Kehler,J. and Dahl,O. (1995) Tetrahedron Lett., 36, 6127–6130. [Google Scholar]
- 25.Kehler J., Püschl,A. and Dahl,O. (1996) Tetrahedron Lett., 37, 8041–8044. [Google Scholar]
- 26.Kehler J., Püschl,A. and Dahl,O. (1997) Nucl. Nucl., 16, 23–32. [Google Scholar]
- 27.Taktakishvilli M.O. and Caruthers,M.H. (1993) Bioorg. Khim., 19, 211–222. [Google Scholar]
- 28.Okruszek A., Sierzchala,A., Fearon,K.L. and Stec,W.J. (1995) J. Org. Chem., 60, 6998–7005. [Google Scholar]
- 29.Okruszek A., Sierzchala,A., Sochacki,M. and Stec,W.J. (1992) Tetrahedron Lett., 33, 7585–7588. [Google Scholar]
- 30.Krotz A.H., Cheruvallath,Z.S., Cole,D.L. and Ravikumar,V.T. (1998) Nucl. Nucl., 17, 2335–2338. [Google Scholar]
- 31.Ravikumar V.T., Wyrzykiewicz,T.K. and Cole,D.L. (1994) Tetrahedron, 50, 9255–9266. [Google Scholar]
- 32.Ravikumar V.T., Sasmor,H. and Cole,D.L. (1993) Bioorg. Med. Chem. Lett., 3, 2637–2640. [Google Scholar]
- 33.Ravikumar V.T. and Cole,D.L. (1994) Gene, 149, 157–161. [DOI] [PubMed] [Google Scholar]
- 34.Krotz A.H., Cole,D.L. and Ravikumar,V.T. (1996) Tetrahedron Lett., 37, 1999–2002. [Google Scholar]
- 35.Tolkmith H. (1958) J. Org. Chem., 23, 1685–1690. [Google Scholar]
- 36.Teichmann H. and Hilgetag,G. (1967) Angew. Chem Int. Ed. Engl., 6, 1013–1023. [Google Scholar]
- 37.Cooks R.G. and Gerrard,A.F. (1968) J. Chem. Soc B, 11,1327–1333. [Google Scholar]
- 38.Bruzik K. and Stec,W.J. (1981) J. Org. Chem., 46, 1625–1630. [Google Scholar]
- 39.Poulter C.D. and Mautz,D.S. (1991) J. Am. Chem. Soc., 113, 4895–4903. [Google Scholar]
- 40.Lambert J.B. (1990) Tetrahedron, 46, 2677–2689. [Google Scholar]