Abstract
Objective:
The author’s objective was to evaluate sex and race representation in temporal bone histopathology studies.
Design:
PubMed, Embase, Cochrane, Web of Science, and Scopus were searched for studies written in English examining temporal bone histopathology specimens from US-based institutions from January 1st, 1947, to September 1st, 2021. Two authors then performed “snowballing” by reviewing references from the initial search and included the studies that fulfilled the inclusion criteria. For each study, the following information was collected: publication details, study design, funding, institution from where temporal bone specimens were procured, number of study specimens, and donor demographical information.
Results:
The authors found that out of 300 studies, 166 (55%) report sex while only 15 (5%) reported race information. Over the past 70 years, the ratio of studies reporting sex to those that do not has increased from 1.00 to 2.19 and the number of female temporal bone histopathology subjects relative to male has increased from 0.67 to 0.75. Over 90% of studies that do report this information feature participant racial compositions that do not reflect the diversity of the US population.
Conclusion:
Studies of temporal bone histopathology often do not report participant sex or race. The reporting of participant sex and the inclusion of specimens from female donors have both increased over time. However, temporal bone histopathology study cohorts are not representative of the racial diversity of the US population. The otolaryngology community must strive to build temporal bone histopathology libraries that are representative of the diverse US population.
Keywords: Sex, Race, Temporal bone, Histopathology, Otopathology, Health Disparities
INTRODUCTION
The study of temporal bone histopathology (TBH) is a critical tool for increasing our understanding of the pathologic basis for many otologic and neurotologic diseases (Schuknecht 1996; da Costa Monsanto et al 2018). Due to the location of the inner ear within the temporal bone and the inability to use traditional techniques such as biopsy or surgical excision (Merchant et al 2008), many otologic diseases were poorly understood until the 19th century. During the late 19th and early 20th centuries methods were developed in Europe, and later the United States, to study post-mortem temporal bone sections (Schuknecht 1996; da Costa Monsanto et al 2018). TBH studies provided key insights into several important otologic diseases, including cholesteatoma, otosclerosis, and age-related hearing loss (Pappas 1996; Schuknecht 1996; Nogueira et al 2007; Merchant et al 2008; da Costa Monsanto et al 2018).
The knowledge gained from studies of TBH continues to shape modern diagnostic and therapeutic approaches; however, it is unknown whether TBH specimens represent the US population. Preclinical and clinical studies investigating various otologic topics such as hearing loss and hearing loss management underreport sex, race, and ethnicity and have study populations that are skewed and, thus, misrepresent the actual US population (Lauer & Schrode 2017; Villavisanis et al 2018; Villavisanis et al 2020; Pittman et al 2021). Further, many otologic diseases occur more commonly in different sex or racial groups (e.g., vestibular disorders in females or hearing loss in White males). To address this problem, the National Institutes of Health (NIH) have made concerted efforts since 1993 to have researchers report demographic data and include female participants as well as racial and ethnic minority participants (National Institutes of Health 1993). The NIH mandate should apply to NIH-funded TBH studies in which a diverse cohort of subjects inclusive of all demographical features, such as sex and race, are necessary to develop a complete understanding of otologic disease.
To our knowledge, there have been no systematic reviews investigating the representation of sex and race in TBH studies. Characterizing patient demographics from existing TBH studies will highlight which populations are not currently represented and may guide the future recruitment of temporal bone donors. The inclusion of a diversity of subjects in TBH studies will strengthen the external validity of findings, improve our understanding of the influence of sex and race in disease processes, and may guide future efforts to address health disparities. The objective of this study was to determine the sex and race of individuals included in US-based TBH studies to understand how accurately human temporal studies reflect the diversity of the US population.
MATERIALS AND METHODS
Study Search Strategy
We queried five electronic databases (PubMed, Embase, Cochrane, Web of Science, and Scopus) for studies examining human TBH, including the spectrum of otologic and vestibular pathology. Inclusion criteria were studies written in English, temporal bone specimens from US-based institutions, and original research (excluding systematic reviews and meta-analyses). We excluded studies using specimens from non-US-based institutions or if the origin was unclear. Other exclusion criteria included non-human subjects, case reports or case series (fewer than five cases). The Population, Intervention, Comparator, Outcome, and Study Design (PICOS) framework was employed, defining the scope of this study to include all study populations from US-based TBH studies and specimen population demographics as a relevant comparison. No particular intervention, outcome or study design was selected for. This study was designed and executed as a systematic review according to Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (Figure S1). This study was Institutional Review Board exempt.
Our intention was to include all known TBH studies after 1947, the year when the National Library of Medicine began indexing articles. Thus, we included articles published between January 1st, 1947, to September 1st, 2021. The search was performed on October 14, 2021, by a university informationist searching terms and Boolean operators such as (“Temporal bone” OR “Stylomastoid foramen”) AND “Pathology” AND “specimen”. Complete search strategies can be found in the Supplementary information (Table S1).
Data Extraction
After duplicates from different databases were removed, studies were uploaded to Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia). Subsequently, abstracts, then full-text studies were screened by one author (PSK). Studies were excluded if full texts were unavailable or did not agree with inclusion/exclusion criteria. Studies obtained from the initial search strategy were compiled. Two authors (PSK, NSA) then performed “snowballing” by systematically reviewing references from the initial search, as some studies may have been missed by our initial search strategy (Greenhalgh and Peacock 2005). The additional references that fulfilled the inclusion criteria were then included in the review. Risk of bias assessments were not performed because the purpose of this review was to determine the representation of donor populations, rather than to assess the efficacy of a diagnostic or therapeutic intervention. For the domains risk of bias due to confounding and selection of participants: studies were described as “unknown” if donor demographic data were not provided, “low risk” if data were provided and accurately reflected the US population, and “high risk” if data were provided and did not reflect the US population.
Coding Study Characteristics
For each study the following information was recorded: study title, year of publication, publication type (otolaryngology, audiology, geriatric, neurology/neuroscience), study design (case series or case control), source of funding (industry, nonprofit, government, private, no funding/not listed), institution from which specimens were procured, study aim(s), number of study specimens, average age of temporal bone donors, whether sex and racial demographics were reported and the numbers of specimens from each category reported. All study designs were either case series or case control due to the nature of histopathology studies.
Sex, Race, and Ethnicity Definitions
The sex classification used for this review was derived from the US Census Bureau: male or female. According to the US Census Bureau 2019 and 2021 Population Estimates program, 50.8% and 76.3% of the US population identify as female and White, respectively (Bureau, U. C. n.d.; U.S. Census Bureau QuickFacts: United States. n.d.). Thus, studies that included a cohort of less than 50% female subjects were considered to underrepresent females. Similarly, studies that included a cohort of less than 24% non-White subjects were considered to underrepresent racial and ethnic minorities. We used these definitions to categorize studies as adequately representing the US population.
In 1997, the federal Office of Management and Budget (OMB) issued a federal register revising the standards for race and ethnicity classifications. These standards are currently used by the US Department of Human Health Services and US Census Bureau (Standards for the Classification of Federal Data on Race and Ethnicity n.d.). These categories include White, Black or African American, American Indian or Alaska Native, Asian, Native Hawaiian or Other Pacific Islander, Other, or Two or more race (Grieco and Cassidy 2001). Ethnicity is a social-political construct describing cultural identification. In 2000, the federal government mandated that agencies are required to use a minimum of two ethnicities: Hispanic or Latino or not Hispanic or Latino. For the purposes of this review, we assessed studies’ populations based on the OMB’s race classification and the mandate’s ethnicity classifications (Oh et al 2015). However, our review found that no TBH studies reported on ethnicity of study cohorts therefore analysis of this variable was not possible.
Data Analysis
Chi-squared tests were performed to assess the effects of publication type, study design, and funding on sex and race representation, with a p<0.05 significance criterion. All counts, calculations, and statistical tests were performed on Microsoft Excel 2017 (Version 2201).
RESULTS
One thousand four-hundred thirty-nine studies were initially imported from which 99 duplicates were removed. The abstracts of 1340 studies (93% of initial import) were screened, and 961 studies were deemed irrelevant. Subsequent full-text screening was performed on 379 studies (26%) and 229 were excluded: 92 studies used specimens not from a US-based institution, 65 were not temporal bone histopathology studies, 34 were not available in full-text format, 23 were case reports or case series (less than 5 cases), 12 were unscreened duplicates, and 3 were not original research. One-hundred fifty (10%) studies were included as the “start set.” After “snowballing” was performed, 303 studies were compiled for analysis. Of these, 3 studies were excluded since they did not provide the number of specimens examined nor the demographic information of the specimens.
Due to nature of histopathology studies, many domains in the risk of bias analysis do not apply, therefore are “low risk”. However, several earlier studies did not report all data collected on each specimen. For example, some of these studies only reported observations of specimens with artifacts of interest. These were considered “high risk” in the domain of bias in selection of the reported results.
Reporting of Sex
Out of 300 studies, 166 (55%) reported the sex of study subjects, while 134 (45%) did not (Figure 1). Out of the 166 studies, a total of 8119 study subjects were included, of which 4254 (52.4%) were male and 3865 (47.6%) were female. Of note, some temporal bone specimens may have been used in more than one study published by a single research group. Fifty-five (18.3%) of these studies featured greater than or equal to 50% female representation. Five studies (1.7%) included a solely male group, all others included both males and females.
Figure 1.
Number and proportion of TBH studies that that report age, sex, and race
Journal type, evidence quality, and funding distributions were not significantly different (p>0.05) among the total set of studies, studies reporting sex, and studies with ≥ 50% female representation (Table 1). Out of the 166 studies that reported on sex, 7 (4.2%) used the term “gender” while reporting sex information (Male vs. Female vs. Other). We inferred that the authors of these studies intended to report sex information, a biological attribute, rather than gender, a complex social construction.
Table 1.
Publication characteristics of temporal bone histopathology studies reporting race.
| Study | Evidence qualitya |
Journal type |
Funding | Number of participants |
Number of male participants |
Number of female participants |
Number of participants reported to be: |
||
|---|---|---|---|---|---|---|---|---|---|
| Black or African American |
White | Other | |||||||
| Nager & Nager (1953) | 4 | Otolaryngology | No funding/Not listed | 5 | 3 (60%) | 2 (40%) | 1 (20%) | 4 (80%) | 0 |
| Paparella & Lim (1967) | 4 | Otolaryngology | Nonprofit, Government | 5 | 2 (40%) | 3 (60%) | 1 (20%) | 4 (80%) | 0 |
| Johnsson (1971) | 4 | Otolaryngology | Nonprofit, Government | 9 | 7 (78%) | 2 (22%) | 0 | 9 (100%) | 0 |
| LeFerriere et al (1974)b | 4 | Otolaryngology | Nonprofit, Government | 216 | NA | NA | 16 (7.4%) | 200 (92.6%) | 0 |
| Sando et al (1975) | 4 | Otolaryngology | No funding/Not listed | 7 | 3 (43%) | 4 (57%) | 0 | 7 (100%) | 0 |
| Hawkins et al (1978) | 4 | Otolaryngology | No funding/Not listed | 7 | 7 (100%) | 0 | 0 | 7 (100%) | 0 |
| Johnsson et al (1981) | 4 | Otolaryngology | Government | 5 | 2 (40%) | 3 (60%) | 1 (20%) | 4 (80%) | 0 |
| Simms & Neely (1989)c | 4 | Otolaryngology | No funding/Not listed | 103 | 54 (52%) | 49 (48%) | 0 | 45 (43.7%) | 58 (56.3%) |
| Nelson & Hinojosa (2006) | 3 | Otolaryngology | No funding/Not listed | 31 | 21 (68%) | 10 (32%) | 9(29%) | 22 (71%) | 0 |
| Yew et al (2011) | 4 | Otolaryngology | No funding/Not listed | 102 | 68 (67%) | 34 (33%) | 49 (48.0%) | 53 (52.0%) | 0 |
| Gluth & Nelson (2017) | 3 | Otolaryngology | No funding/Not listed | 38 | 21 (55%) | 17 (45%) | 9 (23.7%) | 29 (76.3%) | 0 |
| Pauno et al (2017)d | 3 | Otolaryngology | Nonprofit, Government | 24 | 5 (42%) | 7 (58%) | 0 | 12 (100%) | 0 |
Per the modified criteria from the Oxford Centre for Evidence-based Medicine for ratings of individual studies.
LeFerriere et al (1974) did not report sex.
Simms & Neely (1989) divided the study cohort as “White” and “NonWhite.”
Pauno et al (2017) only reported control group data demographics.
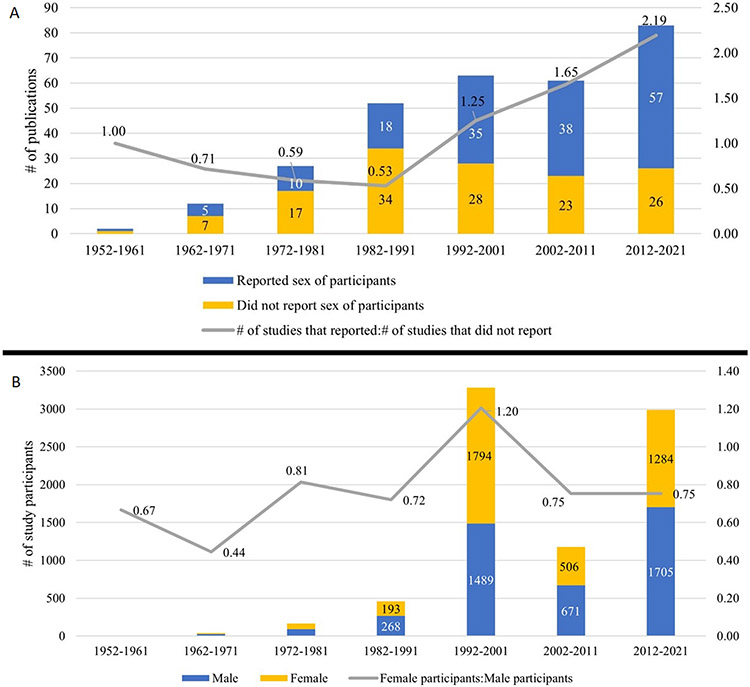
From the 1952-61 period to 1982-91 period, the ratio of the number of studies that reported sex to the number of studies which did not report sex first decreased from 1.00 to 0.53. After the 1982-91 period, the proportion increased steeply to 2.19 in 2012-21. In 2012-21, 57 (74%) studies reported sex while 20 (26%) did not (Figure 2A).
Figure 2.
A. Number and proportion of TBH studies that report sex versus those that do not report sex over time. B. Number and proportion of female versus male participants in TBH study cohorts over time.
From the 1952-61 period to 2012-21, the ratio of female study subjects to male study subjects rose from 0.67 to 0.75 (ratio 1.0 = even sex representation). Across this time span, the lowest ratio occurred during 1962-71 at 0.44 and the peak occurred during 1992-2001 at 1.20. During the nearly 70-year time frame, the number of study subjects in TBH studies increased from 5 (60% male, 40% female) to 2989 (45.3% male, 54.7% female) (Figure 2B).
Reporting of Race
Out of 300 studies, 15 (5%) reported the race of subjects while 285 (95%) did not (Figure 1). No studies reported the ethnicity of subjects. Three of these 15 studies only described the sex and race of specific subjects whose temporal bones displayed a pathology of interest. Out of the other 12 studies, 11 described the sex and race of all subjects while 1 described these demographic data only for the non-control group, which constituted half of the total number of study subjects.
The date of publication of these 12 studies ranged from 1953 to 2017 and all studies were published in Otolaryngology-focused journals. Seven out of the 12 were either not funded or the authors did not list funding sources, 4 were funded by non-profit and government agencies, while 1 was solely funded by a government agency. Eleven studies had less than 34% non-White subjects, and in 4 of these 100% of the subjects were White. One study divided their population into “White” and “Non-White” subjects. This study did not discuss the racial composition of the “Non-White” group. Apart from the study that divided their population into “White” and “Non-White” subjects, all study subjects in the 12 studies were reported to be solely White or Black or African American (Table 2).
Table 2.
Publication characteristics of temporal bone histopathology studies for all reviewed studies, studies reporting sex, studies with cohorts comprising of greater than or equal to 50% female participant representation, and studies reporting race.
| Total set | Studies reporting sex |
p-value | Studies with >=50% female participant representation |
p-value | Studies reporting race |
p-value | ||
|---|---|---|---|---|---|---|---|---|
| # of publications | 303 | 164 | 55 | 12 | ||||
| Journal type | Otolaryngology | 265 (87.5%) | 138 (84.1%) | p=0.99 | 47 (85.5%) | p=0.91 | 12 (100%) | p=0.63 |
| Audiology | 18 (5.9%) | 11 (6.7%) | 3 (5.5%) | 0 | ||||
| Neurology/Neuroscience | 15 (4.9%) | 11 (6.7%) | 4 (7.3%) | 0 | ||||
| Other | 5 (1.7%) | 4 (2.4%) | 1 (1.8%) | 0 | ||||
| Evidence quality † | Case series (4) | 199 (65.7%) | 98 (59.8%) | p=0.20 | 35 (63.6%) | p=0.77 | 9 (75%) | p=0.50 |
| Case control (3) | 104 (34.3%) | 66 (40.2%) | 20 (36.4%) | 3 (25%) | ||||
| Funding | Government | 211 (54.2%) | 115 (54.2%) | p=0.99 | 44 (60.3%) | p=0.99 | 5 (33.3%) | p=0.07 |
| Nonprofit | 72 (18.5%) | 37 (17.5%) | 12 (16.4%) | 4 (26.7%) | ||||
| Private | 37 (9.5%) | 21 (9.9%) | 9 (12.3%) | 0 | ||||
| No funding/not listed | 69 (17.7%) | 39 (18.4%) | 8 (11.0%) | 6 (40%) | ||||
Per the modified criteria from the Oxford Centre for Evidence-based Medicine for ratings of individual studies
DISCUSSION
Through a systematic review of all published TBH studies in the US since 1947, we found 300 studies that reported information about temporal bone specimen donors. We found that 166 (55%) studies reported sex while 15 (5%) reported race information. There has been an increase in sex reporting and the number of female TBH subjects relative to males over the past 70 years. Given the infrequent reporting of race information it is unclear whether there is increasing minority representation in these studies. However, 11 out of the 12 studies that reported race information underrepresented racial minority populations. To the authors’ knowledge, this is the first systematic review of all TBH studies in the US since 1947, and the first to characterize the representation of sex and race populations in such studies.
Conducting research including diverse patient cohorts that represent the US population’s varied backgrounds, exposures, and experiences increases the generalizability of findings. This is especially applicable for otologic disease, in which TBH studies heavily inform understanding of pathophysiology and clinical approaches to disease (Merchant et al 2008). Making scientific conclusions based on results from non-representative cohorts can mislead the scientific community and prevent minority populations from receiving the benefits of the research thereby exacerbating health inequities (Neuhauser et al 2005). To fully understand the spectrum of otologic disease and incidence of histopathologic disease in different sub-populations it is necessary that we study TBH from a diverse donor cohort. Additionally, many otologic diseases occur with varying frequency in certain demographic groups. For example, otosclerosis and vestibular disorders are more common in females (Guild 1950; Lin et al 2012) and susceptibility to hearing loss is associated with sex and skin pigmentation (Arnegard et al, 2020). In such cases, it would be reasonable to include study cohorts that are representative of the sub-population(s) in which specific pathologies are common, according to non-TBH epidemiological studies. Conclusions based on such study designs would necessitate robust reporting of sub-population characteristics, something which is lacking based on the present review. It is also important to note the additional benefits of adequate representation such as understanding the heterogeneity of treatment effects, increasing trust of the research enterprise and medical establishment, and increasing quality of health care decision making for all people.
The NIH Revitalization Act of 1993 mandated, for the first time, the inclusion of women and minority populations in NIH-funded research in order to increase representation of women and members of racial and ethnic minority groups in clinical research (National Institutes of Health 1993; Tahhan et al 2018). This was extended by the 2016 Sex As a Biological Variable (SABV) policy which required all applicants to factor sex as a biological variable into vertebrate animal and human research designs and analyses (Tahhan et al 2018; Arnegard et al 2020). Our results indicate that for TBH studies, there was a decrease in reporting sex until 1982-91 followed by a steep increase in reporting until the present period when 69% of studies reported sex. This increase may be attributed to the NIH Revitalization Act enacted in 1993.The inclusion of females has also increased, albeit at a slower rate. However, it is impossible to assess the extent to which TBH studies evaluate specimens that represent the racial demographics of the general population if studies do not report such information. Despite the mandate, the proportion of racial and ethnic minorities in research study populations across varied fields has remained lower than that of the US population. Our TBH review data concurs with data in other fields, including cardiology (Charrow et al 2017), dermatology (Robbins and Bernat 2017), neurology (Loree et al 2019), oncology (Burkhard et al 2021), psychiatry (Burchard et al 2015), pulmonology (Falasinnu et al 2018), and rheumatology (Corazzi et al 2020). In fact, only 3 (2.5%) of the 120 TBH studies published after 1993 report racial demographic information. Hence, we can conclude that there is continued inadequacy in racial minority reporting and likely underrepresentation.
Numerous studies have examined the role of sex as a biological variable in hearing and vestibular disorders (Bowman et al 2000; Lauer and Schrode 2017; Milon et al 2018; Villavisanis et al 2018; Schuster et al 2019; Kobrina et al 2020; Nolan 2020; Gandhi et al 2021; García-Liñeira et al 2021; Wang et al 2021; Mucci et al 2022). In addition to the anatomical differences in the length of the cochlea, it has been hypothesized that sex hormones may play a role in affecting outer hair cells, causing sex-based differences in click-evoked auditory brainstem response and otoacoustic emissions that are reversed in menopausal females (Ciorba et al 2016). Males are more commonly affected than females by presbycusis (Villavisanis et al 2020; Ciorba et al 2016), sudden sensorineural hearing loss (Pinto et al 2010), noise-induced hearing loss (Ciorba et al 2016), and tinnitus (Crowe et al 1934). TBH may provide insights into some of these differences. For example, Schuknecht expanded the work of Crowe et al (1934) (Shuknecht 1964) to classify presbycusis by cochlear histological damage patterns (1964) (Nelson and Hinojosa 2006). Only more recently have there been efforts to correlate histological damage patterns with specific presbycusis audiogram subtypes (Wu et al 2020; Landegger et al 2016; Sun et al 2014). There have been no attempts to determine the influence of sex on these histologic elements in the context of age-related hearing loss in human temporal bones.
In 2011, a National Health and Nutritional Examination Survey study found that individuals who self-identify as Black had decreased odds of hearing loss (Arnegard et al 2020). Further, studies have described an association between skin color and inner ear pigmentation, where race was often used as a surrogate for skin color (Erbele et al 2016; Andresen et al 2021; Barrenäs and Axelsson 1992). TBH studies in animals, then humans, postulated that melanin in the stria vascularis may be responsible for this effect by acting like a free-radical scavenger and metal ion chelator (Erbele et al 2016; Strain 2004; Ohlemiller et al 2006; Ohlemiller et al 2009; Oakes and Kaufman 2017). However, limitations of these studies include a lack of power based on the small sample size of temporal bones available. Further, the role of melanin in the cochlea remains unknown. Pigmentary changes in the vestibular system are even less understood (Barrenäs and Axelsson 1992). These differences by skin pigmentation, where race is employed as a proxy, support the importance of including a range of subjects in these studies, particularly when potentially protective mechanisms have been largely unexplored. Such scientific inquiries have the potential to reveal basic mechanisms of sensorineural hearing loss such as recovery from sensorineural hearing loss in patients with sickle cell crisis (Urban et al 1973; Alkindi et al 2011; Kim et al 2018). The pathophysiology of functional hearing loss in sickle cell disease may be due to expanded bone marrow in the petrous temporal bone, ischemia of hair cells secondary to micro-occlusive crisis, or hypoxic injury secondary to decreased total hemoglobin (Kapoor et al 2021). Investigations into the reversibility of hearing loss in this context has the potential to reveal basic mechanisms of cochlear physiology.
Race is considered a social construct, not a biological construct (Baye et al 2011). Furthermore, studies indicate that there are greater genetic differences between individuals of the same race than between individuals of different races (Borrell et al 2021; Flanagin et al 2021). Here, we wish to comment on the disparities that may exist in TBH literature, not on the influence of demographic variables, such as sex and race, on histopathology. However, it would be remiss to state that the disparities in the TBH literature do not impact our understanding of otologic physiology and pathophysiology. Biased sample populations result in biased conclusions. Future investigations must not only report these vital demographic data but also include underrepresented groups in their analyses. It may also be prudent to use genetic profiles and longitudinal health information as a more robust and precise variable as opposed to sex, race, and ethnicity in examining TBH findings. However, these data are not typically available for archival human temporal bone specimens. Looking forward, recruitment of human temporal bone donors enrolled in longitudinal health studies will allow for critical opportunities to associate the complexities of human health conditions with histopathological correlates of otologic disease. These foundational efforts are necessary to produce meaningful findings that are generalizable to the US population, fuel potentially novel insights, and, ultimately, work to improve health equity in otolaryngology research and patient outcomes.
Beyond race and ethnicity, more detailed information from donors should be collected, such as individual- and environmental-level factors, for example skin pigmentation, occupation, or self-reported exposures. In our review, data regarding where donors lived, their occupations, and the temporal bone donation process were often not discussed. This may be due to the lack of standardization in the temporal bone procurement process. In 1960, the Hearing Health Foundation and the American Academy of Ophthalmology and Otolaryngology created the National Temporal Banks Program and formalized the temporal bone donation process on a national level. The work was continued in 1992 with the formation of the National Temporal Bone, Hearing and Balance Pathology Resource Registry (the Registry), a non-profit organization created by the National Institute on Deafness and Other Communication Disorders. Currently, the process of temporal bone donation requires the patient to call a hotline and fill out a 28-page enrollment packet. A donor card is issued after the packet is received by the National Temporal Bone Register in Boston, Massachusetts. Fortunately, the Register does allow for prospective collection of valuable data such as donor addresses, medical history, and biologic materials which allow for molecular analyses (da Costa Monsanto et al 2018). However, our community should take steps to mitigate bias in donor selection. It is possible that solely patients with otologic and neurotologic disorders and their close friends or family make up the vast majority of donors. Race and socioeconomic status, however, deeply impact medical care-seeking behavior (Arnett et al 2016). Outreach efforts may help increase the diversity of temporal bone donors. Such efforts could include targeted advertisements such as distribution of enrollment packets at the local departments of motor vehicles, and education of physicians beyond those caring for patients with otologic and neurotologic disorders. These initiatives would also allow for the recruitment of those without otologic disease as well as potentially younger individuals.
There are several limitations in this study. TBH studies that did report race information did not comment on how this information was obtained. Authors assuming the race of a subject could oversimplify demographic distributions (e.g., Hispanic Black or African American assumed to be solely Black or African American). Additionally, we do not know whether sex and race information for the specimens were included in records associated with the harvested temporal bones. This demographic information may simply not be recorded for specimens. Finally, the small number of studies that reported racial demographic information (5%) severely limited our ability to perform further analyses. We also recognize the limitation related to the use of the term “non-white” and accompanying simplification and support published recommendations to avoid such terminology when possible (Armstrong et al 2007).
Several questions remain. It is unclear to what extent our data represents the available TBH specimens from the National Temporal Bone, Hearing, and Balance Pathology Resource Registry. Increased understanding of the demographics of available TBH specimens in the US may better guide future donor recruitment, however, this assessment of the published literature is likely a reasonable approximation. In our review, the temporal bone donation and collection methodologies used in the included studies are largely unknown. Deep mistrust of healthcare and medical research exists among many Black and Hispanic communities (Armstrong et al 2007) and likely affects the diversity of available specimens. Future reviews should consider classification of temporal bone studies by otologic and neurotologic condition such as otosclerosis and presbycusis, to further investigate the extent to which the scientific community’s understanding of this disease may be impacted by sample populations. We have additionally found that there are simply not enough studies on certain conditions to perform a systematic assessment. To our knowledge, there have been no significant outreach initiatives made to address the lack of diversity among temporal bone donors. Efforts should be made to expand and enhance initiatives to address the underlying mechanisms which cause distrust of the research community.
From 2016 to 2060, it is anticipated that the non-White population in the US will increase from 23.1% to 32%. Though non-Hispanic Whites are projected to be the single largest demographic through the next 50 years, this population is projected to shrink by about 19 million individuals and will cease to constitute the majority by 2045 (Vespa et al 2020). The demographics of available TBH specimens lags behind the rapidly changing demographics of the US. We can expect this disparity to magnify if adequate measures to report and ensure representation are not taken. Efforts must be made to provide the best possible care to all people--informed by TBH studies--regardless of sex or race.
CONCLUSIONS
Though participant sex reporting and inclusion in TBH studies may be increasing, only 5% of studies reported racial information of their participants. Further, most of the studies' participant racial breakdown does not reflect the diversity of the US population. Thus, we highlight the lack of knowledge regarding otologic findings among a diverse cohort of individuals and the limited generalizability of existing TBH findings. Collective efforts among clinicians, scientists, and funders must be made to address possible underlying inequities in otologic disorders in both research and clinical practice.
Supplementary Material
Financial disclosures/Conflicts of interest:
This was supported in-part by funding from the American Otologic Society (Fellowship Grant, P.S.K.), David M. Rubinstein Fund for Research (A.M.L.); K23DC018302, B.K.W.; U24DC020850, A.M.L. & B.K.W.; T32DC000027, N.S.A.), and National Institute on Aging (K23AG059900, C.L.N.). No funding organizations had a role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the decision to submit the article for publication; or in the preparation, review, or approval of the article. A.M.L. receives an honorarium from the Creighton Translational Hearing Center External Advisory Committee. C.L.N. is a volunteer board member of the nonprofit organizations, Access HEARS and the Hearing Loss Association of America.
REFERENCES
- Alkindi S, Arafa N, Al Okbi M, & Pathare A (2011). Complete recovery following sudden sensorineural hearing loss in a patient with sickle cell disease. Hematology/Oncology and Stem Cell Therapy, 4(2), 97–99. 10.5144/1658-3876.2011.97 [DOI] [PubMed] [Google Scholar]
- Andresen NS, Coreas S, Villavisanis DF, & Lauer AM (2021). Comparison of Age-Related Pigmentary Changes in the Auditory and Vestibular Systems Within Mouse and Human Temporal Bones. Frontiers in Neuroscience, 15. https://www.frontiersin.org/articles/10.3389/fnins.2021.680994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong K, Ravenell KL, McMurphy S, & Putt M (2007). Racial/Ethnic Differences in Physician Distrust in the United States. American Journal of Public Health, 97(7), 1283–1289. 10.2105/AJPH.2005.080762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnegard ME, Whitten LA, Hunter C, & Clayton JA (2020). Sex as a Biological Variable: A 5-Year Progress Report and Call to Action. Journal of Women’s Health (2002), 29(6), 858–864. 10.1089/jwh.2019.8247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett MJ, Thorpe RJ, Gaskin DJ, Bowie JV, & LaVeist TA (2016). Race, Medical Mistrust, and Segregation in Primary Care as Usual Source of Care: Findings from the Exploring Health Disparities in Integrated Communities Study. Journal of Urban Health : Bulletin of the New York Academy of Medicine, 93(3), 456–467. 10.1007/s11524-016-0054-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrenäs M-L, & Axelsson A (1992). The Development of Melanin in the Stria Vascularis of the Gerbil. Acta Oto-Laryngologica, 112(1), 50–58. 10.3109/00016489209100782 [DOI] [PubMed] [Google Scholar]
- Baye TM, He H, Ding L, Kurowski BG, Zhang X, & Martin LJ (2011). Population structure analysis using rare and common functional variants. BMC Proceedings, 5 Suppl 9, S8. 10.1186/1753-6561-5-S9-S8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrell LN, Elhawary JR, Fuentes-Afflick E, Witonsky J, Bhakta N, Wu AHB, Bibbins-Domingo K, Rodríguez-Santana JR, Lenoir MA, Gavin JR, Kittles RA, Zaitlen NA, Wilkes DS, Powe NR, Ziv E, & Burchard EG (2021). Race and Genetic Ancestry in Medicine—A Time for Reckoning with Racism. The New England Journal of Medicine, 384(5), 474–480. 10.1056/NEJMms2029562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman DM, Brown DK, & Kimberley BP (2000). An examination of gender differences in DPOAE phase delay measurements in normal-hearing human adults. Hearing Research, 142(1–2), 1–11. 10.1016/s0378-5955(99)00212-9 [DOI] [PubMed] [Google Scholar]
- Burchard EG, Oh SS, Foreman MG, & Celedón JC (2015). Moving toward true inclusion of racial/ethnic minorities in federally funded studies. A key step for achieving respiratory health equality in the United States. American Journal of Respiratory and Critical Care Medicine, 191(5), 514–521. 10.1164/rccm.201410-1944PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau, U. C. (n.d.). Age and Sex Tables. Census.Gov. Retrieved August 4, 2022, from https://www.census.gov/topics/population/age-and-sex/data/tables.html
- Burkhard C, Cicek S, Barzilay R, Radhakrishnan R, & Guloksuz S (2021). Need for Ethnic and Population Diversity in Psychosis Research. Schizophrenia Bulletin, 47(4), 889–895. 10.1093/schbul/sbab048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrow A, Xia FD, Joyce C, & Mostaghimi A (2017). Diversity in Dermatology Clinical Trials: A Systematic Review. JAMA Dermatology, 153(2), 193–198. 10.1001/jamadermatol.2016.4129 [DOI] [PubMed] [Google Scholar]
- Ciorba A, Benatti A, Bianchini C, Aimoni C, Volpato S, Bovo R, & Martini A (2011). High frequency hearing loss in the elderly: Effect of age and noise exposure in an Italian group. The Journal of Laryngology and Otology, 125(8), 776–780. 10.1017/S0022215111001101 [DOI] [PubMed] [Google Scholar]
- Ciorba A, Corazzi V, Bianchini C, Aimoni C, Skarzynski H, Skarzynski PH, & Hatzopoulos S (2016). Sudden sensorineural hearing loss: Is there a connection with inner ear electrolytic disorders? A literature review. International Journal of Immunopathology and Pharmacology, 29(4), 595–602. 10.1177/0394632016673845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corazzi V, Ciorba A, Skarżyński PH, Skarżyńska MB, Bianchini C, Stomeo F, Bellini T, Pelucchi S, & Hatzopoulos S (2020). Gender differences in audio-vestibular disorders. International Journal of Immunopathology and Pharmacology, 34, 2058738420929174. 10.1177/2058738420929174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Costa Monsanto R, Pauna HF, Paparella MM, & Cureoglu S (2018). Otopathology in the United States: History, Current Situation and Future Perspectives. Otology & Neurotology : Official Publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology, 39(9), 1210–1214. 10.1097/MAO.0000000000001942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbele ID, Lin FR, Agrawal Y, Francis HW, Carey JP, & Chien WW (2016). Racial differences of pigmentation in the human vestibular organs. Otolaryngology - Head and Neck Surgery (United States), 155(3), 479–484. 10.1177/0194599816645764 [DOI] [PubMed] [Google Scholar]
- Falasinnu T, Chaichian Y, Bass MB, & Simard JF (2018). The Representation of Gender and Race/Ethnic Groups in Randomized Clinical Trials of Individuals with Systemic Lupus Erythematosus. Current Rheumatology Reports, 20(4), 20. 10.1007/s11926-018-0728-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagin A, Frey T, Christiansen SL, & AMA Manual of Style Committee. (2021). Updated Guidance on the Reporting of Race and Ethnicity in Medical and Science Journals. JAMA, 326(7), 621–627. 10.1001/jama.2021.13304 [DOI] [PubMed] [Google Scholar]
- Gandhi P, Biju K, Klatt BN, Simonsick E, & Agrawal Y (2021). Self-Reported Sense of Direction and Vestibular Function in the Baltimore Longitudinal Study of Aging (BLSA). Journal of the Association for Research in Otolaryngology: JARO, 22(2), 207–214. 10.1007/s10162-020-00780-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Liñeira J, Leirós-Rodríguez R, Romo-Pérez V, & García-Soidán JL (2021). Sex differences in postural control under unstable conditions in schoolchildren with accelerometric assessment. Gait & Posture, 87, 81–86. 10.1016/j.gaitpost.2021.04.027 [DOI] [PubMed] [Google Scholar]
- Greenhalgh T, & Peacock R (2005). Effectiveness and efficiency of search methods in systematic reviews of complex evidence: Audit of primary sources. BMJ (Clinical Research Ed.), 331(7524), 1064–1065. 10.1136/bmj.38636.593461.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieco EM, Cassidy RC (2001). Overview of Race and Hispanic Origin. Census 2000 Brief. https://www2.census.gov/library/publications/decennial/2000/briefs/c2kbr01-01.pdf [Google Scholar]
- Guild SR (1950). Incidence, location and extent of otosclerotic lesions. A.M.A. Archives of Otolaryngology, 52(6), 848–852. 10.1001/archotol.1950.00700030875002 [DOI] [PubMed] [Google Scholar]
- C. S. J, G. S. R, & P. L. M (1934). Observations on the Pathology of High-Tone Deafness. The Journal of Nervous and Mental Disease, 80(4), 480. [Google Scholar]
- Kapoor E, Strum D, Shim T, Kim S, Sabetrasekh P, & Monfared A (2021). Characterization of Sensorineural Hearing Loss in Adult Patients With Sickle Cell Disease: A Systematic Review and Meta-analysis. Otology & Neurotology, 42(1), 30–37. 10.1097/MAO.0000000000002825 [DOI] [PubMed] [Google Scholar]
- Kim SJ, Taheri MR, Merkison M, & Monfared A (2018). Cochlear Implantation in a Patient With Sickle Cell Disease With Early Cochlear Sclerosis. Otology & Neurotology, 39(2), e87. 10.1097/MAO.0000000000001660 [DOI] [PubMed] [Google Scholar]
- Kobrina A, Schrode KM, Screven LA, Javaid H, Weinberg MM, Brown G, Board R, Villavisanis DF, Dent ML, & Lauer AM (2020). Linking anatomical and physiological markers of auditory system degeneration with behavioral hearing assessments in a mouse (Mus musculus) model of age-related hearing loss. Neurobiology of Aging, 96, 87–103. 10.1016/j.neurobiolaging.2020.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landegger LD, Psaltis D, & Stankovic KM (2016). Human Audiometric Thresholds do not Predict Specific Cellular Damage in the Inner Ear. Hearing Research, 335, 83–93. 10.1016/j.heares.2016.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer AM, & Schrode KM (2017). Sex Bias in Basic and Preclinical Noise-Induced Hearing Loss Research. Noise & Health, 19(90), 207–212. 10.4103/nah.NAH_12_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FR, Maas P, Chien W, Carey JP, Ferrucci L, & Thorpe R (2012). Association of Skin Color, Race/Ethnicity, and Hearing Loss Among Adults in the USA. JARO: Journal of the Association for Research in Otolaryngology, 13(1), 109–117. 10.1007/s10162-011-0298-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loree JM, Anand S, Dasari A, Unger JM, Gothwal A, Ellis LM, Varadhachary G, Kopetz S, Overman MJ, & Raghav K (2019). Disparity of Race Reporting and Representation in Clinical Trials Leading to Cancer Drug Approvals From 2008 to 2018. JAMA Oncology, 5(10), e191870. 10.1001/jamaoncol.2019.1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant SN, McKenna MJ, Adams JC, Nadol JB Jr., Fayad J, Gellibolian R, Linthicum FH Jr., Ishiyama A, Lopez I, Ishiyama G, Baloh R, & Platt C (2008). Human Temporal Bone Consortium for Research Resource Enhancement. JARO: Journal of the Association for Research in Otolaryngology, 9(1), 1–4. 10.1007/s10162-008-0111-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Methods in Social Epidemiology, 2nd Edition ∣ Wiley. (n.d.). Wiley.Com. Retrieved August 6, 2022, from https://www.wiley.com/en-us/Methods+in+Social+Epidemiology%2C+2nd+Edition-p-9781118505595 [Google Scholar]
- Milon B, Mitra S, Song Y, Margulies Z, Casserly R, Drake V, Mong JA, Depireux DA, & Hertzano R (2018). The impact of biological sex on the response to noise and otoprotective therapies against acoustic injury in mice. Biology of Sex Differences, 9(1), 12. 10.1186/s13293-018-0171-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucci V, Hamid M, Jacquemyn Y, & Browne CJ (2022). Influence of sex hormones on vestibular disorders. Current Opinion in Neurology, 35(1), 135–141. 10.1097/WCO.0000000000001019 [DOI] [PubMed] [Google Scholar]
- National Institute of Health (NIH) (1993). S.1 - National Institutes of Health Revitalization Act of 1993 Subtitle B--Clinical Research Equity Regarding Women and Minorities. National Institute of Health. https://orwh.od.nih.gov/sites/orwh/files/docs/NIH-Revitalization-Act-1993.pdf [Google Scholar]
- Nelson EG, & Hinojosa R (2006). Presbycusis: A human temporal bone study of individuals with downward sloping audiometric patterns of hearing loss and review of the literature. The Laryngoscope, 116(9 Pt 3 Suppl 112), 1–12. 10.1097/01.mlg.0000236089.44566.62 [DOI] [PubMed] [Google Scholar]
- Neuhauser HK, von Brevern M, Radtke A, Lezius F, Feldmann M, Ziese T, & Lempert T (2005). Epidemiology of vestibular vertigo: A neurotologic survey of the general population. Neurology, 65(6), 898–904. 10.1212/01.wnl.0000175987.59991.3d [DOI] [PubMed] [Google Scholar]
- Nogueira JF, Hermann DR, Américo R. dos R., Barauna Filho IS, Stamm AEC, & Pignatari SSN (2007). A brief history of otorhinolaryngolgy: Otology, laryngology and rhinology. Brazilian Journal of Otorhinolaryngology, 73(5), 693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan LS (2020). Age-related hearing loss: Why we need to think about sex as a biological variable. Journal of Neuroscience Research, 98(9), 1705–1720. 10.1002/jnr.24647 [DOI] [PubMed] [Google Scholar]
- Oh SS, Galanter J, Thakur N, Pino-Yanes M, Barcelo NE, White MJ, Bruin D. M. de, Greenblatt RM, Bibbins-Domingo K, Wu AHB, Borrell LN, Gunter C, Powe NR, & Burchard EG (2015). Diversity in Clinical and Biomedical Research: A Promise Yet to Be Fulfilled. PLOS Medicine, 12(12), e1001918. 10.1371/journal.pmed.1001918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller KK, Dahl AR, & Gagnon PM (2010). Divergent Aging Characteristics in CBA/J and CBA/CaJ Mouse Cochleae. JARO: Journal of the Association for Research in Otolaryngology, 11(4), 605–623. 10.1007/s10162-010-0228-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller KK, Rice MER, Lett JM, & Gagnon PM (2009). Absence of strial melanin coincides with age-associated marginal cell loss and endocochlear potential decline. Hearing Research, 249(1–2), 1–14. 10.1016/j.heares.2008.12.005 [DOI] [PubMed] [Google Scholar]
- Humes KR, Jones NA, Ramirez RA (2011, March). Overview of Race and Hispanic Origin: 2010. 2010 Census Briefs. https://www.census.gov/content/dam/Census/library/publications/2011/dec/c2010br-02.pdf [Google Scholar]
- Pappas DG (1996). Otology through the ages. Otolaryngology--Head and Neck Surgery: Official Journal of American Academy of Otolaryngology-Head and Neck Surgery, 114(2), 173–196. 10.1016/s0194-5998(96)70162-6 [DOI] [PubMed] [Google Scholar]
- Pinto PCL, Sanchez TG, & Tomita S (2010). The impact of gender, age and hearing loss on tinnitus severity. Brazilian Journal of Otorhinolaryngology, 76(1), 18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman CA, Roura R, Price C, Lin FR, Marrone N, & Nieman CL (2021). Racial/Ethnic and Sex Representation in US-Based Clinical Trials of Hearing Loss Management in Adults: A Systematic Review. JAMA Otolaryngology-- Head & Neck Surgery, 147(7), 656–662. 10.1001/jamaoto.2021.0550 [DOI] [PubMed] [Google Scholar]
- Revisions to the Standards for the Classification of Federal Data on Race and Ethnicity. (n.d.). The White House. Retrieved August 4, 2022, from https://obamawhitehouse.archives.gov/node/15626 [Google Scholar]
- Robbins NM, & Bernat JL (2017). Minority Representation in Migraine Treatment Trials. Headache, 57(3), 525–533. 10.1111/head.13018 [DOI] [PubMed] [Google Scholar]
- Schuknecht HF (1964). FURTHER OBSERVATIONS ON THE PATHOLOGY OF PRESBYCUSIS. Archives of Otolaryngology (Chicago, Ill.: 1960), 80, 369–382. 10.1001/archotol.1964.00750040381003 [DOI] [PubMed] [Google Scholar]
- Schuknecht HF (1996). Otopathology: The Past, Present, and Future. Auris Nasus Larynx, 23, S43–S45. 10.1016/S0385-8146(96)80032-0 [DOI] [Google Scholar]
- Shuster BZ, Depireux DA, Mong JA, & Hertzano R (2019). Sex differences in hearing: Probing the role of estrogen signaling. The Journal of the Acoustical Society of America, 145(6), 3656. 10.1121/1.5111870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standards for the Classification of Federal Data on Race and Ethnicity. (n.d.). The White House. Retrieved August 6, 2022, from https://obamawhitehouse.archives.gov/node/15639 [Google Scholar]
- Strain GM (2004). Deafness prevalence and pigmentation and gender associations in dog breeds at risk. Veterinary Journal (London, England: 1997), 167(1), 23–32. 10.1016/s1090-0233(03)00104-7 [DOI] [PubMed] [Google Scholar]
- Sun DQ, Zhou X, Lin FR, Francis HW, Carey JP, & Chien WW (2014). Racial difference in cochlear pigmentation is associated with hearing loss risk. Otology & Neurotology: Official Publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology, 35(9), 1509–1514. 10.1097/MAO.0000000000000564 [DOI] [PubMed] [Google Scholar]
- Tahhan AS, Vaduganathan M, Greene SJ, Fonarow GC, Fiuzat M, Jessup M, Lindenfeld J, O’Connor CM, & Butler J (2018). Enrollment of Older Patients, Women, and Racial and Ethnic Minorities in Contemporary Heart Failure Clinical Trials: A Systematic Review. JAMA Cardiology, 3(10), 1011–1019. 10.1001/jamacardio.2018.2559 [DOI] [PubMed] [Google Scholar]
- Urban GE Jr. (1973). Reversible sensori-neural hearing loss associated with sickle cell crisis. The Laryngoscope, 83(5), 633–638. 10.1288/00005537-197305000-00001 [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau QuickFacts: United States. (n.d.). Retrieved August 6, 2022, from https://www.census.gov/quickfacts/fact/table/US/PST045221 [Google Scholar]
- Vespa J, Medina L, & Armstrong DM (2020). Demographic Turning Points for the United States: Population Projections for 2020 to 2060. Population Estimates and Projections. https://www.census.gov/content/dam/Census/library/publications/2020/demo/p25-1144.pdf [Google Scholar]
- Villavisanis DF, Berson ER, Lauer AM, Cosetti MK, & Schrode KM (2020a). Sex-based Differences in Hearing Loss: Perspectives From Non-clinical Research to Clinical Outcomess. Otology & Neurotology: Official Publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology, 41(3), 290–298. 10.1097/MAO.0000000000002507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villavisanis DF, Berson ER, Lauer AM, Cosetti MK, & Schrode KM (2020b). Sex-based Differences in Hearing Loss: Perspectives From Non-clinical Research to Clinical Outcomess. Otology & Neurotology: Official Publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology, 41(3), 290–298. 10.1097/MAO.0000000000002507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villavisanis DF, Schrode KM, & Lauer AM (2018). Sex bias in basic and preclinical age-related hearing loss research. Biology of Sex Differences, 9(1), 23. 10.1186/s13293-018-0185-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Wang X, Yang L, Han K, Huang Z, & Wu H (2021). Sex differences in noise-induced hearing loss: A cross-sectional study in China. Biology of Sex Differences, 12(1), 24. 10.1186/s13293-021-00369-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, O’Malley JT, Gruttola V. de, & Liberman MC (2020). Age-Related Hearing Loss Is Dominated by Damage to Inner Ear Sensory Cells, Not the Cellular Battery That Powers Them. Journal of Neuroscience, 40(33), 6357–6366. 10.1523/JNEUROSCI.0937-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.