Abstract
Background:
Fascioliasis is a parasitic zoonosis that can infect humans and be a source of significant morbidity. The World Health Organization lists human fascioliasis as a neglected tropical disease, but the worldwide prevalence of fascioliasis data is unknown.
Objective:
We aimed to estimate the global prevalence of human fascioliasis.
Data sources and methods:
We performed a systematic review and prevalence meta-analysis. We used the following inclusion criteria: articles published in the English, Portuguese, or Spanish languages from December 1985 to October 2022 and studies assessing the prevalence of Fasciola in the general population with an appropriate diagnostic methodology, including longitudinal studies, prospective and retrospective cohorts, case series, and randomized clinical trials (RCTs). We excluded animal studies. Two reviewers independently reviewed the selected studies for methodological quality, performing critical standard measures from JBI SUMARI. A random-effects model was conducted of the summary extracted data on the prevalence proportions. We reported the estimates according to the GATHER statement.
Results:
In all, 5617 studies were screened for eligibility. Fifty-five studies from 15 countries were selected, including 154,697 patients and 3987 cases. The meta-analysis revealed a pooled prevalence of 4.5% [95% confidence interval (CI): 3.1–6.1; I2 = 99.4%; T2 = 0.07]. The prevalence in South America, Africa, and Asia was 9.0%, 4.8%, and 2.0%, respectively. The highest prevalence was found in Bolivia (21%), Peru (11%), and Egypt (6%). Subgroup analysis showed higher prevalence estimates in children, in studies from South America, and when Fas2-enzyme-linked immunosorbent assay (ELISA) was used as a diagnostic method. A larger study sample size (p = 0.027) and an increase in female percentage (p = 0.043) correlated with a decrease in prevalence. Multiple meta-regression showed a higher prevalence for hyperendemic than hypoendemic (p = 0.002) or mesoendemic (p = 0.013) regions.
Conclusion:
The estimated prevalence and projected disease burden of human fascioliasis are high. Study findings support that fascioliasis continues to be a globally neglected tropical disease. Strengthening epidemiological surveillance and implementing measures to control and treat fascioliasis is imperative in the most affected areas.
Keywords: epidemiology, F. gigantica, F. hepatica, human fascioliasis, meta-analysis, neglected tropical disease, prevalence, systematic review
Introduction
Fascioliasis is an emerging global parasitic disease caused by Fasciola hepatica and F. gigantica. Fasciola spp. have a complex life cycle that involves intermediate aquatic gastropod hosts and definitive mammalian hosts such as humans. 1 Eating habits are the most significant risk factor of infection by Fasciola spp., with the consumption of wild watercress contaminated with infective metacercariae being the most reported source of infection. Likewise, studies in the highlands of Peru indicate that drinking untreated water is associated with a higher risk of Fasciola spp. infection.2,3
Fascioliasis significantly impacts the livestock industry, with wild ruminant reservoirs as a source of disease introduction. Between 10% and 80% of cattle are infected globally. 2 Livestock production losses and increased associated treatment costs contribute to lowered meat, milk, and wool production and a predisposition for peracute mortality caused by Clostridium noyvi. Worldwide studies have reported losses of up to millions of US dollars annually.4,5 These losses perpetuate poverty and deny smallholder farmers much-needed income and subsistence. 6
Human fascioliasis has been a public health concern for the last three decades, prompting the World Health Organization’s (WHO) declaration as a neglected tropical disease.7,8 Fascioliasis is asymptomatic in most patients, but right upper quadrant discomfort and anorexia can occur. It is associated with anemia and weight loss in children, who are especially vulnerable to devastating long-term complications, such as delayed growth and poor neurocognitive development.2,9 In addition, the disease is estimated to incur 90,000 disability-adjusted life years (DALYs) due to associated abdominal symptoms such as nausea, vomiting, diarrhea, and pain. 10 Infestation has also been associated with liver fibrosis in humans and animals.11,12 The complications following Fasciola infections may include acute cholecystitis, biliary obstruction, and liver abscesses, often requiring abdominal surgeries. 13
The number of humans infected by Fasciola spp. in 1998 increased in 51 countries on 5 continents, with 7071 reported human cases. 14 In 2012, the estimated number was 2.6 million cases reported in 81 countries worldwide. The prevalence varies by continent, but the highest has been reported in the Andes region of Latin America. 9
Human fascioliasis may be emerging due to more favorable wet weather for fluke egg survival due to climate change. There is a gap in knowledge about the global status of this neglected parasitic disease. Current studies are mainly limited to the regional level, but cost-effective serological tests are lacking in the most affected areas.15–17 There is an urgent need for assessments of disease burden to monitor the prevalence dynamics of human fascioliasis to promote stakeholders’ engagement in implementing effective public health programs aimed at disease prevention. Given changes in climate and food habits that could increase the presence of intermediate hosts and suitable conditions for their growth, it is essential to assess changes to fascioliasis human cases worldwide. This study aims to perform a systematic review and meta-analysis to estimate the global prevalence of human fascioliasis and examine prevalence variation by demographic and clinical characteristics.
Methods
Search strategy
The Joanna Briggs Institute (JBI) methodology for systematic reviews and meta-analysis with a three-phase search strategy was utilized. 18 Initial keywords were identified, database-specific search filters were constructed, and the included studies’ reference list was searched. We considered articles published in English, Portuguese, or Spanish from December 1985 to October 2022. Results of a web of science core collection search of the topic field “Fascioliasis” listed by language revealed that 95% of entries were in English, Portuguese, or Spanish (supplementary material). An initial comprehensive literature search was conducted in May 2022 by a Medical Librarian, with an update on October 2022. The following databases were searched: MEDLINE, Web of Science Core, Scopus, Cochrane Library, SciELO, Crossref, LILACS, and Google Scholar. Relevant publications were identified by a search strategy using a combination of keywords related to fascioliasis in humans were used, such as “Fascioliasis,”, “F. hepatica,”, “F. gigantica,” “helminthiasis,”, “liver fluke,” “Fasciola,” “prevalence,”, “seroprevalence.” See detailed MEDLINE search strategy (supplementary material). Search terms included Fasciola AND Prevalence, excluding animal studies. This review considered longitudinal studies, prospective and retrospective cohorts, case series, and randomized clinical trials (RCTs). Filters were used to limit results to human studies. A search for additional research and the manual addition of other significant papers in the field was done on the reference list of every study chosen.
Study selection
After the systematic search, all registered articles were uploaded to “ProQuest RefWorks” (Ann Arbor, Michigan, USA), where duplicate reports were removed. Then, a screening of the title and abstract was carried out, which were reviewed by two authors, while a third one resolved the differences. Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia) was used for screening and full-text review. Through Covidence, a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram was generated with the number of results found, the number excluded during title/abstract screening, and the number excluded during full-text assessments and methodological appraisals, along with reasons for exclusion. We reported the estimates according to the GATHER statement. 19
Eligibility criteria
We included studies with evidence of endemicity data for F. hepatica or F. gigantica reported worldwide in the general population (Table 1). We assessed each study for an adequate estimated population size, 20 appropriate serological and coprological diagnostic methodology, and availability of prevalence data. We excluded studies of participants with comorbidities or significant risk factors that may alter the course of the disease, such as relatives diagnosed with the disease, significant eosinophilia, occupational exposure, or who have been previously diagnosed with fascioliasis, and also those that did not provide enough pertinent outcome data or were determined not to have an acceptable quality methodologic assessment. We also excluded any gray literature or expert opinion data due to the absence of a peer-reviewed quality evaluation.
Table 1.
Eligibility criteria per the POS criterion.
| Criterion | Definition |
|---|---|
| Population | We included studies with evidence of endemicity data for F. hepatica or F. gigantica reported worldwide in the general population. |
| Outcome | Prevalence, number of positive samples divided by the total number of patients assessed on each study expressed as a percentage |
| Study | Full text primary studies published in English, Spanish or Portuguese in eligible Databases from December 1985 to October 2022 |
Data analysis
Study data were collected and managed using Microsoft Excel 2020 electronic data capture tools. We performed data visualization and quality control in GraphPad (version 9.4.1 for Windows, GraphPad Software, San Diego, California, USA). Extracted data included the year of the study, type of study, country/continent of origin, duration of the study, number of infected patients, number of participants, length of follow-up, population demographics, diagnostic technique (Fas2-enzyme-linked immunosorbent assay [ELISA], microscopy on stool samples and antibody ELISA test), type of infection (symptomatic, asymptomatic or both), and kind of endemicity of the area studied. Endemicity was defined by the percentage of the arithmetic mean intensity of eggs per gram of feces (EPG). Hypoendemic if the prevalence is less than 1%, mesoendemic region if the prevalence is between 1% and 10% (50–300 EPG), and hyperendemic area if prevalence >10% (>300 EPG). 21 If a study reported stool microscopy and serology, we extracted numbers for serology only, the most sensitive methodology. The primary outcome was the prevalence.
Quality assessment
Two reviewers independently reviewed the selected studies for methodological quality, performing quality critical standard measures from the JBI System for the Unified Management, Assessment and Review of Information (JBI SUMARI; Joanna Briggs Institute, Adelaide, Australia). A third independent reviewer resolved assessment differences between the two reviewers. Critical appraisals were performed utilizing the JBI Reviewer’s Manual checklists for longitudinal studies. All studies with greater than 60% of “yes” answers to the essential appraisal questions were subject to data extraction and synthesis per JBI guidelines. The risk of bias was assessed using the QUIPS tool. 22
Statistical analysis
The prevalence proportion was calculated by dividing the cases of Fasciola in each study by the total number of participants. We computed each study’s Freeman–Tukey double-arcsine-transformed proportion to obtain the effect sizes using the meta-analysis of prevalence package.23,24 Confidence intervals for individual studies were calculated with the exact or the score (Wilson) method. A random-effects model was performed in the meta-analysis as prevalence and estimated effect sizes are expected to change between different studied populations.
To calculate the heterogeneity and variability of the meta-analysis, we estimated the I² statistic and the τ² coefficient. We established a heterogeneity of ⩾75% as considerable heterogeneity based on the Cochrane Handbook for Systematic Reviews of Interventions. The studies were analyzed by subgroups: type of study, age group, symptomatology, the decade in which the study was carried out, country, continent, female percentage, study population size, diagnostic method, endemicity, and study duration. The pooled effect was recalculated after excluding one study from the analysis and repeating this single-study exclusion for each study.
The variables associated with the pooled prevalence (p < 0.05) and continuous explanatory variables (study population size, study year, and percentage of women) were included in a random-effects multi meta-regression analysis. In addition, Egger’s regression test and a Galbraith plot were performed to generate a funnel plot that assesses publication bias and the existence of minor study effects. A p-value < 0.05 for the Egger test was considered significant for possible publication bias. Statistical analysis was performed using the STATA software program, version 18.0 (StataCorp, College Station, Texas, USA).
Results
Study population and characteristics
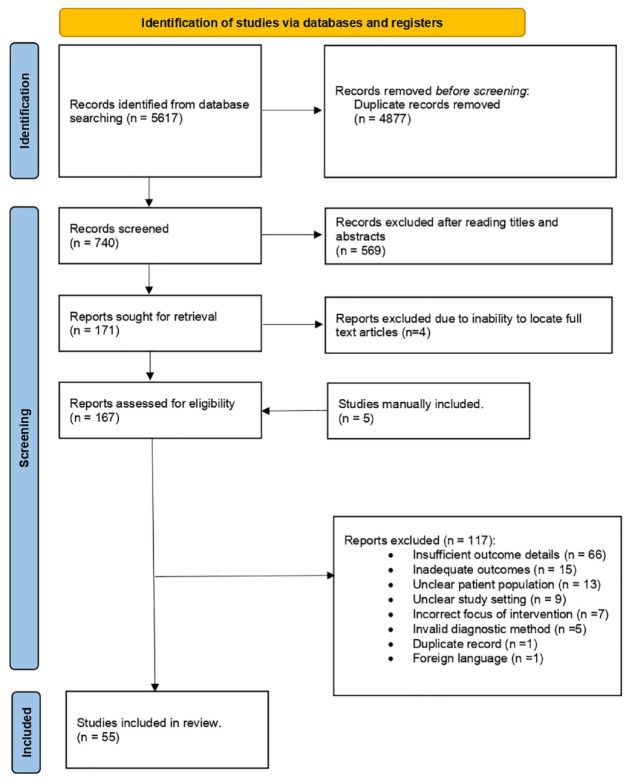
We initially identified 5617 studies. After deduplication, 740 studies were screened for eligibility based on titles and abstracts. Of these, 171 full-text articles were assessed, of which four were excluded due to lack of accessibility to the full text. The remaining 167 studies were thoroughly evaluated, considering the inclusion and exclusion criteria. They were also subjected to quality assessment using the JBI Critical Appraisal tool. We manually included 5 studies and excluded 117 for different reasons (Figure 1). A total of 55 articles were eligible. Among them, 52 cross-sectional studies, 2 retrospectives, and 1 clinical trial, composed of 154,697 patients and 3987 cases of fascioliasis, were utilized for the meta-analysis (Figure 1).
Figure 1.
PRISMA flow diagram.
Of the 55 studies, most were cross-sectional seroprevalence from South America and Asia and enrolled patients between 1990 and 2019. Studies sample sizes varied from 42 to 69,633 patients, with a mean of 2,812 participants per study. Fascioliasis cases per study ranged from 1 to 932, with a mean of 73 cases per study. Gender distribution had a slight female predominance at 57.7% (44 studies). Twenty-six (47%) studies included information from patients between 0 and 17 years old. The prevalence of human fascioliasis ranged from 0.03% to 32.5% (Table 2). A few studies had missing data for demographic data. The mean follow-up study duration was 1.8 years, ranging from 1 to 11 years.
Table 2.
Studies characteristics and Fasciola prevalence.
| Study | Study type | Study size (n) | Cases (n) | Study duration (years) | Prevalence %, (CI 95%) | Age | Symptoms | Diagnostic method | Country |
|---|---|---|---|---|---|---|---|---|---|
| Abdi et al. 25 | Cross-sectional | 600 | 4 | 1 | 0.7 (0.3–1.7) | 18–60 years old | Asymptomatic | ELISA | Iran |
| Abo-Madyan et al. 26 | Clinical trial | 1019 | 17 | 2 | 1.7 (1–2.7) | Mixed ages | Mixed | Stool microscopy | Egypt |
| Afshan et al. 27 | Cross-sectional | 546 | 130 | 1 | 23.8 (20.4–27.6) | Mixed ages | Mixed | ELISA | Pakistan |
| Aguiar et al. 28 | Cross-sectional | 558 | 11 | 1 | 2 (1.1–3.5) | 0–17 years old | Mixed | Stool microscopy | Brazil |
| Apt et al. 29 | Cross-sectional | 5861 | 41 | 4 | 0.7 (0.5–0.9) | Mixed ages | Asymptomatic | ELISA | Chile |
| Asadian et al. 30 | Cross-sectional | 458 | 9 | 1 | 2 (1–3.7) | Mixed ages | Mixed | ELISA | Iran |
| Ashrafi et al. 31 | Cross-sectional | 1984 | 30 | 3 | 1.5 (1.1–2.2) | Mixed ages | Mixed | ELISA | Iran |
| Bahram et al. 32 | Cross-sectional | 612 | 11 | 1 | 1.8 (1–3.2) | Mixed ages | — | ELISA | Iran |
| Bekana et al. 33 | Cross-sectional | 798 | 44 | 1 | 5.5 (4.1–7.3) | 0–17 years old | Mixed | Stool microscopy | Ethiopia |
| Beyhan et al. 34 | Cross-sectional | 817 | 45 | 8 | 5.5 (4.1–7.3) | Mixed ages | Mixed | ELISA | Turkey |
| Bless et al. 35 | Cross-sectional | 221 | 1 | 1 | 0.5 (0.1–2.5) | 0–17 years old | Symptomatic | ELISA | Cambodia |
| Bozorgomid et al. 36 | Cross-sectional | 975 | 5 | 3 | 0.5 (0.2–1.2) | 18–60 years old | Mixed | ELISA | Iran |
| Cabada et al. 37 | Cross-sectional | 2515 | 253 | 5 | 10.1 (8.9–11.3) | 0–17 years old | Mixed | Fas2-ELISAlisa | Peru |
| Cabada et al. 38 | Cross-sectional | 227 | 22 | 1 | 9.7 (6.5–14.2) | 0–17 years old | Mixed | Stool microscopy | Peru |
| Carnevale et al. 39 | Cross-sectional | 42 | 5 | 1 | 11.9 (5.2–25) | 18–60 years old | Symptomatic | ELISA | Argentina |
| Cengiz et al. 40 | Cross-sectional | 1600 | 89 | 2 | 5.6 (4.5–6.8) | Mixed ages | Mixed | ELISA | Turkey |
| Curtale et al. 41 | Cross-sectional | 1783 | 54 | 1 | 3 (2.3–3.9) | 0–17 years old | Mixed | Stool microscopy | Egypt |
| Curtale et al. 42 | Cross-sectional | 21,477 | 932 | 4 | 4.3 (4.1–4.6) | 0–17 years old | Mixed | Stool microscopy | Egypt |
| Curtale et al. 43 | Cross-sectional | 1331 | 72 | 1 | 5.4 (4.3–6.8) | Mixed ages | Asymptomatic | Stool microscopy | Egypt |
| Davoodi et al. 44 | Cross-sectional | 2418 | 60 | 2 | 2.5 (1.9–3.2) | Mixed ages | Mixed | ELISA | Iran |
| Eshrati et al. 45 | Cross-sectional | 1053 | 28 | 1 | 2.7 (1.8–3.8) | Mixed ages | Mixed | ELISA | Iran |
| Esteban et al. 21 | Cross-sectional | 2723 | 419 | 6 | 15.4 (14.1–16.8) | 0–17 years old | Mixed | Stool microscopy | Bolivia |
| Esteban et al. 46 | Cross-sectional | 558 | 154 | 1 | 27.6 (24.1–31.5) | 0–17 years old | Symptomatic | Stool microscopy | Bolivia |
| Esteban et al. 47 | Cross-sectional | 338 | 82 | 1 | 24.3 (20–29.1) | 0–17 years old | Mixed | Stool microscopy | Peru |
| Esteban et al. 48 | Cross-sectional | 678 | 87 | 1 | 12.8 (10.5–15.6) | Mixed ages | Mixed | Stool microscopy | Egypt |
| Fentie et al. 49 | Cross-sectional | 520 | 17 | 1 | 3.3 (2.1–5.2) | 0–17 years old | Mixed | Stool microscopy | Ethiopia |
| Gonzalez et al. 50 | Cross-sectional | 476 | 116 | 1 | 24.4 (20.7–28.4) | 0–17 years old | Mixed | Stool microscopy | Peru |
| Hassan et al. 51 | Cross-sectional | 1350 | 147 | 1 | 10.9 (9.3–12.7) | 0–17 years old | Mixed | ELISA | Egypt |
| Heydarian et al. 52 | Cross-sectional | 1256 | 16 | 1 | 1.3 (0.8–2.1) | Mixed ages | – | ELISA | Iran |
| Hosseini et al. 53 | Cross-sectional | 1025 | 2 | 1 | 0.2 (0.1–0.7) | Mixed ages | Symptomatic | ELISA | Iran |
| Kaya et al. 54 | Cross-sectional | 586 | 26 | 1 | 4.4 (3–6.4) | 18–60 years old | Mixed | ELISA | Turkey |
| Kheirandish et al. 55 | Cross-sectional | 801 | 6 | 1 | 0.7 (0.3–1.6) | Mixed ages | Asymptomatic | ELISA | Iran |
| Lopez et al. 56 | Cross-sectional | 223 | 23 | 1 | 10.3 (7–15) | 0–17 years old | Asymptomatic | Stool microscopy | Peru |
| Maciel et al. 57 | Cross-sectional | 434 | 36 | 1 | 8.3 (6.1–11.3) | Mixed ages | Mixed | ELISA | Brazil |
| Manouchehri et al. 58 | Cross-sectional | 1475 | 2 | 3 | 0.1 (0–0.5) | >60 years old | Mixed | ELISA | Iran |
| Manrique et al. 59 | Cross-sectional | 507 | 2 | 1 | 0.4 (0.1–1.4) | 0–17 years old | Mixed | Stool microscopy | Colombia |
| Mantari et al. 60 | Cross-sectional | 312 | 16 | 1 | 5.1 (3.2–8.2) | 0–17 years old | Mixed | Stool microscopy | Peru |
| Marcos et al. 61 | Cross-sectional | 157 | 51 | 1 | 32.5 (25.7–40.2) | 0–17 years old | Mixed | Fas2-ELISAlisa | Peru |
| Marcos et al. 62 | Cross-sectional | 291 | 25 | 1 | 8.6 (5.9–12.4) | 0–17 years old | Mixed | Stool microscopy | Peru |
| Natividad et al. 63 | Cross-sectional | 132 | 4 | 1 | 3 (1.2–7.5) | Mixed ages | Mixed | Stool microscopy | Peru |
| Nguyen et al. 64 | Retrospective | 10,084 | 590 | 1 | 5.9 (5.4–6.3) | Mixed ages | Mixed | ELISA | Vietnam |
| Nxsana et al. 65 | Cross-sectional | 162 | 1 | 1 | 0.6 (0.1–3.4) | 0–17 years old | Asymptomatic | Stool microscopy | South Africa |
| Özturhan et al. 66 | Cross-sectional | 884 | 7 | 1 | 0.8 (0.4–1.6) | Mixed ages | Mixed | ELISA | Turkey |
| Qureshi et al. 67 | Cross-sectional | 540 | 4 | 1 | 0.7 (0.3–1.9) | 0–17 years old | Mixed | Stool microscopy | Pakistan |
| Qureshi et al. 68 | Cross-sectional | 7200 | 85 | 2 | 1.2 (1–1.5) | Mixed ages | Asymptomatic | Stool microscopy | Pakistan |
| Rodríguez et al. 69 | Cross-sectional | 253 | 13 | 1 | 5.1 (3–8.6) | 0–17 years old | Mixed | Stool microscopy | Peru |
| Rodríguez et al. 70 | Cross-sectional | 270 | 17 | 1 | 6.3 (4–9.9) | 0–17 years old | Mixed | Stool microscopy | Peru |
| Saberinasab et al. 71 | Cross-sectional | 471 | 8 | 1 | 1.7 (0.9–3.3) | Mixed ages | Mixed | ELISA | Iran |
| Steinmann et al. 72 | Cross-sectional | 1262 | 24 | 1 | 1.9 (1.3–2.8) | 0–17 years old | Mixed | Stool microscopy | Kyrgyzstan |
| Taş Cengiz et al. 73 | Retrospective | 69,633 | 25 | 11 | 0 (0–0.1) | Mixed ages | Mixed | ELISA | Turkey |
| Valencia et al. 74 | Cross-sectional | 842 | 33 | 1 | 3.9 (2.8–5.5) | 0–17 years old | Mixed | Fas2-ELISAlisa | Peru |
| Wilches et al. 75 | Cross-sectional | 61 | 3 | 1 | 4.9 (1.7–13.5) | Mixed ages | Mixed | ELISA | Colombia |
| Yilmaz and Godekmerdan 76 | Cross-sectional | 500 | 9 | 1 | 1.8 (0.9–3.4) | 0–17 years old | Asymptomatic | ELISA | Turkey |
| Zoghi et al. 77 | Cross-sectional | 933 | 24 | 1 | 2.6 (1.7–3.8) | Mixed ages | Mixed | ELISA | Iran |
| Zumaquero et al. 78 | Cross-sectional | 865 | 50 | 1 | 5.8 (4.4–7.5) | 0–17 years old | Symptomatic | ELISA | Mexico |
CI, confidence interval; ELISA, enzyme-linked immunosorbent assay.
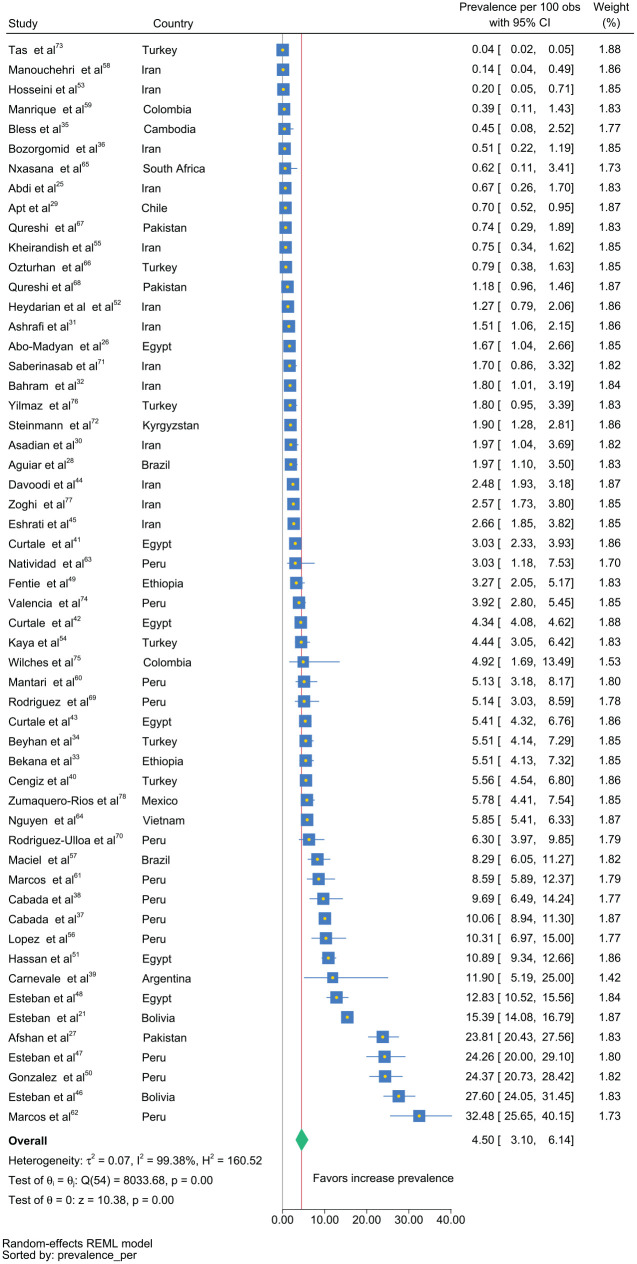
We found a pooled estimated prevalence of 4.5% [95% confidence interval (CI): 3.1–6.1; I2 = 99.4%; T2 = 0.07] (Figure 2). The I2 variable suggested significant heterogeneity among these studies, as well as the τ 2 , which represents the variability of the prevalence of each study.
Figure 2.
Forest plot of global estimated prevalence of fascioliasis by study.
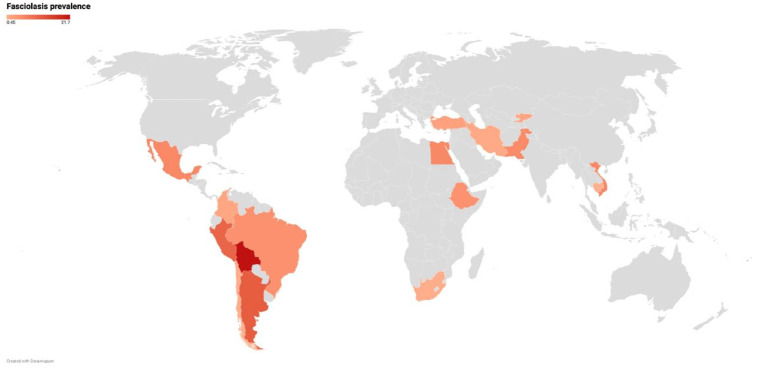
The final dataset included data from 16 countries, with generally only a few studies from each represented country (Figure 3). More studies were included from Peru (12) and Iran (13) than other countries. The prevalence in South America, Africa, and Asia was 9.0%, 4.7%, and 2.0%, respectively. (Table 3, Supplementary material Figures 1 S and 2 S). The highest prevalence was found in Bolivia (21%), Peru (11%), and Egypt (6%) (Figure 3, Heat map).
Figure 3.
Heat map of unique datasets from each country depicting the prevalence of fascioliasis.
Table 3.
Subgroup analysis of Fasciola prevalence.
| Characteristic | Studies (n) | Prevalence (%) | 95% CI | I 2 | p-value |
|---|---|---|---|---|---|
| Type of study | 99.4% | 0.431 | |||
| Retrospective | 2 | 1.7 | 0.0–0.12 | ||
| Cross-sectional | 52 | 4.7 | 3.2–6.4 | ||
| Age groups | 99.4% | 0.049 | |||
| 0–17 years old | 26 | 6.8 | 4.2–10.0 | ||
| 18–60 years old | 4 | 2.6 | 0.0–7.8 | ||
| Mixed ages | 24 | 3.0 | 1.7–4.7 | ||
| Symptoms | 99.4% | 0.058 | |||
| Symptomatic | 5 | 6.2 | 0.2–18.1 | ||
| Asymptomatic | 8 | 2.0 | 0.6–4.1 | ||
| Mixed | 40 | 5.1 | 3.4–7.2 | ||
| Decade | 99.4% | 0.148 | |||
| 1985–1994 | 2 | 9.8 | 0.0–49.3 | ||
| 1995–2004 | 12 | 8.3 | 4.2–13.5 | ||
| 2005–2014 | 28 | 3.2 | 1.9–4.9 | ||
| 2015–2021 | 13 | 3.7 | 1.6–6.8 | ||
| Country a | 99.4% | <0.0001 | |||
| Bolivia | 2 | 21.1 | 10.5–34.2 | ||
| Peru | 12 | 10.7 | 6.3–15.9 | ||
| Egypt | 6 | 5.7 | 2.8–9.6 | ||
| Pakistan | 3 | 5.4 | 0.0–23.3 | ||
| Brazil | 2 | 4.6 | 0.4–12.7 | ||
| Ethiopia | 2 | 4.4 | 2.5–6.8 | ||
| Turkey | 6 | 2.4 | 0.6–5.1 | ||
| Colombia | 2 | 1.6 | 0.0–8.9 | ||
| Iran | 13 | 1.2 | 0.8–1.8 | ||
| Continent | 99.4% | <0.0001 | |||
| South America | 20 | 9.0 | 5.5–13.2 | ||
| Asia | 25 | 2.0 | 1.1–3.2 | ||
| Africa | 9 | 4.7 | 2.6–7.5 | ||
| Diagnostic method | 99.4% | 0.022 | |||
| ELISA | 28 | 2.7 | 1.5–4.2 | ||
| Fas2-ELISA | 3 | 13.3 | 1.8–32.6 | ||
| Stool Microscopy | 24 | 6.1 | 3.7–9.0 | ||
| Endemicity | 99.4% | 0.0002 | |||
| Hypoendemic | 6 | 1.0 | 0.4–1.8 | ||
| Mesoendemic | 37 | 3.6 | 2.4–5.0 | ||
| Hyperendemic | 11 | 12.0 | 6.3–19.2 | ||
| Study duration | 99.4% | 0.524 | |||
| ⩽1 year | 42 | 4.8 | 3.2–6.7 | ||
| >1 year | 13 | 3.7 | 1.3–7.3 |
CI, confidence interval; ELISA, enzyme-linked immunosorbent assay.
Including countries with at least 2 studies.
Subgroup analysis
We performed a subgroup analysis to explore the high heterogeneity. The subgroup was separated by decades from 1985 to 1994, 1995 to 2004, 2005 to 2014, and 2015 to 2021. Older decades had higher prevalences but were not statistically significant (Table 3, Figure 3 S). Across age groups, the prevalence was higher and statistically different in patients younger than 18 years compared with those 18 years or older and of mixed ages (Table 3, Figure 4 S). Prevalence was also higher when Fas2-ELISA was used as a diagnostic tool compared with microscopy stool study or antibody ELISA test (Table 3, Figure 5 S). When comparing the results by endemicity of the place where the study was conducted, a significantly higher prevalence was found in hyperendemic areas compared to hypoendemic and mesoendemic (Table 3, Figure 6 S). We did not find differences in prevalence when comparing according to the type of study, symptomatic status, or study duration (Supplemental Figures 7S–9S). A larger study sample size (p = 0.027) and an increase in female percentage (p = 0.043) correlated with a decrease in prevalence.
Multiple meta-regression
Prevalence was higher for hyperendemic than hypoendemic (p = 0.002) or mesoendemic (p = 0.013). Estimated differences on the scale of the Freeman-Tukey double arcsin transform were 0.536 (95% CI: 0.208, 0.865) and 0.344 (0.077, 0.610), respectively.
Sensitivity analysis
Sensitivity analysis of the 55 studies (after excluding each study) did not significantly change the overall prevalence. There was also no significant change in heterogeneity. Furthermore, prevalence effect sizes did not vary after decreasing the variance to 0.25. The rates declined to 2.5%, assuming an I2 of 10%. The Egger test showed a p-value of 0.03. A funnel plot for publication bias indicated asymmetry on the right side of the graph, and more studies are found in the upper part, suggesting possible publication bias (Figure 10 S). Galbraith plot showed a cluster of studies close to the Y axis, suggesting low precision for them (supplementary material Figure 11 S). Our assessment of the certainty of the estimated prevalence is low, based on the limited geographic studies included, the potential publication bias, and the high heterogeneity. The actual effect may be substantially different from the estimated effect.
Discussion
Our systematic review found a prevalence of global fascioliasis of 4.5%. However, most studies came from Iran and Peru; ELISA testing could have detected prior resolved infections, and included studies could have sampled populations already at risk. The variable most strongly associated with increased prevalence was a known area of hyperendemicity, suggesting some studies targeted at-risk populations. The current burden estimate of infected patients is unclear, but nearly 50 million people represent 4.5% of the population of countries reporting prevalence for this study. 79
Human fascioliasis is an emerging zoonosis due to the increased reported cases in non-endemic countries. The global prevalence of this disease was unknown.1,80 Global estimates performed in 2012 found a much higher estimated prevalence of fascioliasis of 14%, mainly using expert opinion studies. 81 In the early 1990s, 2594 cases were reported in approximately 42 countries—the WHO estimated about 2.4 million infected people worldwide after surveying experts. Currently, about 2.6 to 17 million people with fascioliasis are estimated worldwide. However, these estimates used outdated reports.9,80,82 Conversely, some experts argued that an increase in diagnosis artificially drives the emergence observed.
We found a decreasing trend in the fascioliasis prevalence from 1985 to 1994 through 2015 to 2021. In 2006, a plan for epidemiological surveillance, control, and treatment was launched by the WHO. 83 The WHO promoted a mass drug administration of triclabendazole to decrease the human prevalence of fascioliasis in high-burden countries such as Bolivia, Egypt, Peru, and Vietnam. In two districts of Bolivia, a significant reduction in prevalence was observed, being 26.9% and 12.6% in 1999 to only 0.7% and 1% in 2017, respectively. 84 Also, in Egypt, a decrease in prevalence was observed from 1998 to 2002, from 5.6% to 1.2%, respectively. 85 A recent systematic review from Pakistan, including two studies, found a prevalence of 0.3% among humans. 86 The availability of livestock and human antiparasitic treatment can potentially affect or decrease the disease prevalence. However, a surge of new cases is expected since the intermediate host (Lymmnae spp.) and untreated animals, particularly wildlife species, continue to contaminate the environment with fluke eggs and cercariae, not to mention the emergent problem of triclabendazole-resistant fascioliasis in both humans and animals (the only drug effective against Fasciola). 12
Our results suggest the prevalence of fascioliasis is high. However, the number of infected people could still be higher since only a few prevalence studies are available, especially in the most affected areas. 87 Furthermore, the population studied in hyperendemic regions is relatively small and commonly has school-age population groups. Since fascioliasis is not a notifiable disease, its prevalence in many countries is unavailable. Also, the prevalence in endemic areas is heterogeneous, with local prevalence as high as 62%. In contrast, close-proximity regions may have a prevalence as low as 0%. 21 Therefore, further well-powered epidemiological surveillance studies are needed to estimate the number of infected individuals per region and globally. 80
In all, 81 countries have reported the presence of fascioliasis. The most affected regions are South America and Africa; however, no country is free of Fasciola spp. infection. 88 In our analysis, the prevalence of only 16 nations was available, far lower than the actual number of affected countries, reflecting the pronounced lack of epidemiological and clinical data. We found a high prevalence in Bolivia (21%), Peru (10.7%), and Egypt (5.7%). In South America, a global prevalence reached 15.4% in 24 communities. Peru, one of the countries with the highest prevalence, reported numbers up to 24.3% in 3 communities, classified as hyperendemic areas.89,90
We found the highest prevalence in South America and Africa. These results are within the range reported by other systematic reviews, such as in Africa, with prevalence studies ranging from 0.29% to 19.3%. In South America, previous reports indicated a high prevalence ranging from 15% to 66% annually.91,92 Fascioliasis predominantly affects impoverished human populations lacking essential resources and infrastructure, such as deficient health systems. 93
We found a higher prevalence when using the Fas2 ELISA than ELISA and coprological methods. Coprological methods (microscopic visualization of eggs in the stool) are the most commonly used techniques for diagnosing fascioliasis since they are more accessible in hyperendemic areas with lower technological input required. Among these methods, the WHO recommends using the Kato-Katz technique in regions of high prevalence; however, the Lumbreras rapid sedimentation test has a higher sensitivity than other methods. 83 Nonetheless, these techniques are limited by the stage of the disease, being more sensitive during chronic infection, given a long pre-patent period of many months before egg production in feces. Coprological studies are often misused during the early stages of disease—often asymptomatic and with very low to no egg production. As a result, coprological tests have lower sensitivity during this stage, raising concerns about an increase in false negative rates in asymptomatic patients. 94 Due to its higher positive predictive value, the Fas2 ELISA could be considered the method of choice for large-scale prevalence screening tests.2,16 However, the MM3 coproantigen and serological CL-1 ELISA test are commercially available with an increased performance.95,96
We found an increased prevalence of fascioliasis with a decreased percentage of women. A study from Egypt observed a higher prevalence in women than men, with 5.1% and 3.6%, respectively, while a study in Peru found no gender differences.42,50
In most prevalence studies, children are the most predominant group infected, which peaks between 9 and 11 years. 1 Similarly, we found that children aged 0 and 17 years had a higher prevalence compared to the groups of 18 and 60 years. The higher prevalence of fascioliasis in children could be due to their habit of placing aquatic plants in their mouths, lack of hygiene, and proximity to rivers and drains.93,97 Although not statistically significant, we also found a higher prevalence among symptomatic subjects, who may not seek medical attention until biliary complications occur.
Finally, our systematic review suggests more people have been infected by fascioliasis than previously reported. These results concern public health since fascioliasis is not considered a fatal disease but rather a disabling one, like most neglected tropical diseases. WHO calculated 90,041 DALYs and a global loss of 3.2 billion dollars annually in animal production.98,99 We expect an increase in global disease burden given associations with climate change, ecotourism, exports, agriculture, sociocultural factors, and eating habits. Additional funding and epidemiological studies are needed to specify regional disease burden for implementing surveillance, health promotion, disease control, and adequate treatment programs according to each country’s health policies.
Limitations
The potential limitations of this study may be attributed to the low number of published studies included, which could have introduced selection bias. If studies were biased toward at-risk populations, that could overestimate the overall prevalence, which can explain the large estimated number of people with a history of infection worldwide. These studies varied in sample size, study design, epidemiologic settings, population characteristics, disease stages, and follow-up durations, translating to high heterogeneity. Many studies were performed in South America and Asia, where there is a systemic lack of diagnostic tests for fascioliasis, which may have been selected for populations with greater access to diagnostics. Also, we only included reports published in English, Portuguese, or Spanish, limiting the inclusion of additional regional studies as revealed in the funnel plot; however, we covered >95% of the published literature. Finally, the obtained global estimates are intended to inform a projected global disease burden and by no means a particular local geographic zone. It is well known that the prevalence of fascioliasis is patchy and can even vary drastically from adjacent areas.
Conclusion
The estimated global prevalence of human fascioliasis was 4.5% in the included studies, translating into a high disease burden. Based on our findings, fascioliasis continues to expand as a globally neglected tropical disease. A clear data gap persists for human fascioliasis prevalence worldwide. High-quality studies in those settings are crucial to improving the burden of disease estimates. As this neglected tropical disease affects the most underprivileged populations, strengthening epidemiological surveillance, and implementing measures to control and treat fascioliasis is imperative in the most affected areas to prevent long-term complications.
Supplemental Material
Supplemental material, sj-docx-1-tai-10.1177_20499361231185413 for The global prevalence of human fascioliasis: a systematic review and meta-analysis by Luis Raúl Rosas-Hostos Infantes, Guillermo Andres Paredes Yataco, Yeimer Ortiz-Martínez, Treana Mayer, Angelica Terashima, Carlos Franco-Paredes, Esteban Gonzalez-Diaz, Alfonso J. Rodriguez-Morales, D. Katterine Bonilla-Aldana, Lilian Vargas Barahona, Alyssa A. Grimshaw, Daniel B. Chastain, Stefan Sillau, Luis A. Marcos and Andrés F. Henao-Martínez in Therapeutic Advances in Infectious Disease
Acknowledgments
None.
Footnotes
ORCID iDs: Alfonso J. Rodriguez-Morales  https://orcid.org/0000-0001-9773-2192
https://orcid.org/0000-0001-9773-2192
Lilian Vargas Barahona  https://orcid.org/0000-0003-3330-808X
https://orcid.org/0000-0003-3330-808X
Daniel B. Chastain  https://orcid.org/0000-0002-4018-0195
https://orcid.org/0000-0002-4018-0195
Andrés F. Henao-Martínez  https://orcid.org/0000-0001-7363-8652
https://orcid.org/0000-0001-7363-8652
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Luis Raúl Rosas-Hostos Infantes, Facultad de Medicina Alberto Hurtado, Universidad Peruana Cayetano Heredia, Lima, Perú.
Guillermo Andres Paredes Yataco, Facultad de Medicina Alberto Hurtado, Universidad Peruana Cayetano Heredia, Lima, Perú.
Yeimer Ortiz-Martínez, Department of Internal Medicine, Universidad Industrial de Santander and Hospital Universitario de Santander, Bucaramanga, Colombia.
Treana Mayer, Department of Microbiology, Immunology & Pathology, Colorado State University, Fort Collins, CO, USA.
Angelica Terashima, Laboratorio de Parasitología, Instituto de Medicina Tropical Alexander von Humboldt, Universidad Peruana Cayetano Heredia, Lima, Perú; Departamento de Enfermedades Infecciosas, Tropicales y Dermatológicas, Hospital Nacional Cayetano Heredia, Lima, Perú.
Carlos Franco-Paredes, Hospital Infantil de México Federico Gómez, México City, México; Instituto Conmemorativo Gorgas de Estudios de la Salud, Panamá.
Esteban Gonzalez-Diaz, Epidemiological Surveillance and Preventive Medicine Unit, Hospital Civil de Guadalajara Fray Antonio Alcalde, Guadalajara, Mexico.
Alfonso J. Rodriguez-Morales, Grupo de Investigación Biomedicina, Faculty of Medicine, Fundacion Universitaria Autónoma de las Américas-Institucion Universitaria Vision de las Americas, Pereira, Risaralda, Colombia Gilbert and Rose-Marie Chagoury School of Medicine, Lebanese American University, Beirut, Lebanon; Master of Clinical Epidemiology and Biostatistics, Universidad Cientifica del Sur, Lima, Peru.
D. Katterine Bonilla-Aldana, Research Unit, Universidad Continental, Huancayo, Peru.
Lilian Vargas Barahona, Division of Infectious Diseases, University of Colorado Anschutz Medical Campus, Aurora, CO, USA.
Alyssa A. Grimshaw, Yale University, New Haven, CT, USA
Daniel B. Chastain, Department of Clinical & Administrative Pharmacy, University of Georgia College of Pharmacy, Albany, GA, USA
Stefan Sillau, Department of Neurology and Biostatistics, University of Colorado School of Medicine, Aurora, CO, USA.
Luis A. Marcos, Division of Infectious Diseases, Departments of Medicine, Microbiology and Immunology, Stony Brook University, Stony Brook, NY, USA
Andrés F. Henao-Martínez, University of Colorado Anschutz Medical Campus, 12700 E. 19th Avenue, Mail Stop B168, Aurora, CO 80045, USA. Universidad Cientifica del Sur, Lima, Peru andres.
Declarations
Ethics approval and consent to participate: As the research project is a systematic review, it does not involve participation or action on humans or animals. The Institutional Ethics Committee exonerated the project, so it only required the approval of the University Directorate of Research, Science, and Technology (DUICT) of the Universidad Peruana Cayetano Heredia, Lima, Peru.
Consent for publication: Not applicable.
Author contributions: Luis Raúl Rosas-Hostos Infantes: Conceptualization; Data curation; Formal analysis; Investigation; Writing—original draft.
Guillermo Andres Paredes Yataco: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing—original draft.
Yeimer Ortiz-Martínez: Resources; Visualization; Writing—review & editing.
Treana Mayer: Writing—review & editing.
Angelica Terashima: Conceptualization; Project administration; Resources; Supervision; Writing—review & editing.
Carlos Franco-Paredes: Conceptualization; Project administration; Supervision; Writing—review & editing.
Esteban Gonzalez-Diaz: Writing—review & editing.
Alfonso J. Rodriguez-Morales: Writing—review & editing.
D. Katterine Bonilla-Aldana: Writing—review & editing.
Lilian Vargas Barahona: Writing—review & editing.
Alyssa A. Grimshaw: Data curation; Methodology; Resources; Validation; Writing—review & editing.
Daniel B. Chastain: Data curation; Supervision; Writing—review & editing.
Stefan Sillau: Formal analysis; Investigation; Methodology; Writing—review & editing.
Luis A. Marcos: Conceptualization; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Writing—review & editing.
Andrés F. Henao-Martínez: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Software; Supervision; Validation; Visualization; Writing—original draft; Writing—review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by grant UL1 RR025780 from the National Institutes of Health/National Center for Research Resources, Colorado Clinical Translational Science Institute.
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: We declare no competing interests related to this work. Dr. Sillau reported receiving grants from the Alzheimer’s Association, the Benign Essential Blepharospasm Research Foundation, the Colorado Department of Public Health, the Davis Phinney Foundation, the Hewitt Family Foundation, the Michael J. Fox Foundation, the National Institutes of Health, the National Institute of Nursing Research, the Patient-Centered Outcomes Research Institute, and the Rocky Mountain Alzheimer’s Disease Center outside the submitted work. Dr. Henao-Martínez reported being the recipient of a K12-clinical trial award as a co-principal investigator for the Expanded Access IND Program (EAP) to provide the Yellow Fever vaccine (Stamaril) to Persons in the United States outside the submitted work. The Editor in Chief and Associate Editor of Therapeutic Advances in Infectious Disease are authors of this paper. Therefore, the peer review process was managed by alternative members of the Editorial Board and the submitting Editors had no involvement in the decision-making process. No other disclosures were reported.
Availability of data and materials: The corresponding author had full access to data in the study and had final responsibility for the decision to submit the manuscript for publication. The datasets generated and analyzed in the current study are available from the corresponding author at reasonable request. The protocol was sought to be registered in PROSPERO. However, it was declined to give COVID-19-related research priority
References
- 1.Mas-Coma S, Valero MA, Bargues MD. Fascioliasis. Adv Exp Med Biol 2019; 1154: 71–103. [DOI] [PubMed] [Google Scholar]
- 2.Caravedo MA, Cabada MM. Human Fascioliasis: current epidemiological status and strategies for diagnosis, treatment, and control. Res Rep Trop Med 2020; 11: 149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marcos L, Maco V, Terashima A, et al. Fascioliasis in relatives of patients with Fasciola hepatica infection in Peru. Rev Inst Med Trop Sao Paulo 2005; 47: 219–222. [DOI] [PubMed] [Google Scholar]
- 4.Charlier J, Rinaldi L, Musella V, et al. Initial assessment of the economic burden of major parasitic helminth infections to the ruminant livestock industry in Europe. Prev Vet Med 2020; 182: 105103. [DOI] [PubMed] [Google Scholar]
- 5.Arias-Pacheco C, Lucas JR, Rodriguez A, et al. Economic impact of the liver condemnation of cattle infected with Fasciola hepatica in the Peruvian Andes. Trop Anim Health Prod 2020; 52: 1927–1932. [DOI] [PubMed] [Google Scholar]
- 6.Terashima A, Canales M, Maco V, et al. Observational study on the effectiveness and safety of multiple regimens of triclabendazole in human fascioliasis after failure to standard-of-care regimens. J Glob Antimicrob Resist 2021; 25: 264–267. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Research priorities for zoonoses and marginalized infections: WHO TRS N°971. Geneva: World Health Organization, 2012. [PubMed] [Google Scholar]
- 8.Mas-Coma S, Funatsu IR, Angles R, et al. Domestic pig prioritized in one health action against fascioliasis in human endemic areas: experimental assessment of transmission capacity and epidemiological evaluation of reservoir role. One Health 2021; 13: 100249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Webb CM, Cabada MM. Recent developments in the epidemiology, diagnosis, and treatment of Fasciola infection. Curr Opin Infect Dis 2018; 31: 409–414. [DOI] [PubMed] [Google Scholar]
- 10.Havelaar AH, Kirk MD, Torgerson PR, et al. World Health Organization Global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med 2015; 12: e1001923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Machicado C, Machicado JD, Maco V, et al. Association of Fasciola hepatica infection with liver fibrosis, cirrhosis, and cancer: a systematic review. PLoS Negl Trop Dis 2016; 10: e0004962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marcos LA, Terashima A, Yi P, et al. Mechanisms of liver fibrosis associated with experimental Fasciola hepatica infection: roles of Fas2 proteinase and hepatic stellate cell activation. J Parasitol 2011; 97: 82–87. [DOI] [PubMed] [Google Scholar]
- 13.Chang Wong MR, Pinto Elera JO, Guzman Rojas P, et al. Demographic and clinical aspects of hepatic fascioliasis between 2013–2010 in National Hospital Cayetano Heredia, Lima, Peru. Rev Gastroenterol Peru 2016; 36: 23–28. [PubMed] [Google Scholar]
- 14.Mas-Coma S. Epidemiology of fascioliasis in human endemic areas. J Helminthol 2005; 79: 207–216. [DOI] [PubMed] [Google Scholar]
- 15.Marcos L, Maco V, Terashima A. Triclabendazole for the treatment of human fascioliasis and the threat of treatment failures. Expert Rev Anti Infect Ther 2021; 19: 817–823. [DOI] [PubMed] [Google Scholar]
- 16.Flores VM, Raymundo LM, Iwashita AT, et al. Fas2-ELISA y la técnica de sedimentación rápida modificada por lumbreras en el diagnóstico de la infección por Fasciola hepática. Rev Med Hered 2002; 13: 49–57. [Google Scholar]
- 17.Terashima A, Marcos LA. Fracaso de dosis única de triclabendazole para el tratamiento de fasciolosis humana. Acta Médica Peruana 2016; 33: 228–231. [Google Scholar]
- 18.Aromataris EMZ. Joanna Briggs institute reviewer’s manual, 2017, https://jbi.global/sites/default/files/2020-08/Checklist_for_Prevalence_Studies.pdf
- 19.Stevens GA, Alkema L, Black RE, et al. Guidelines for accurate and transparent health estimates reporting: the GATHER statement. Lancet 2016; 388: e19–e23. [DOI] [PubMed] [Google Scholar]
- 20.Pourhoseingholi MA, Vahedi M, Rahimzadeh M. Sample size calculation in medical studies. Gastroenterol Hepatol Bed Bench 2013; 6: 14–17. [PMC free article] [PubMed] [Google Scholar]
- 21.Esteban JG, Flores A, Angles R, et al. High endemicity of human fascioliasis between Lake Titicaca and La Paz valley, Bolivia. Trans R Soc Trop Med Hyg 1999; 93: 151–156. [DOI] [PubMed] [Google Scholar]
- 22.Hayden JA, Van der Windt DA, Cartwright JL, et al. Assessing bias in studies of prognostic factors. Ann Intern Med 2013; 158: 280–286. [DOI] [PubMed] [Google Scholar]
- 23.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health 2014; 72: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barendregt JJ, Doi SA, Lee YY, et al. Meta-analysis of prevalence. J Epidemiol Community Health 2013; 67: 974–978. [DOI] [PubMed] [Google Scholar]
- 25.Abdi J, Naserifar R, Rostami Nejad M, et al. New features of fascioliasis in human and animal infections in Ilam Province, Western Iran. Gastroenterol Hepatol Bed Bench 2013; 6: 152–155. [PMC free article] [PubMed] [Google Scholar]
- 26.Abo-Madyan A, Morsy TA, Motawea SM, et al. Clinical trial of Mirazid in treatment of human fascioliasis, Ezbet El-Bakly (Tamyia Center) Al-Fayoum Governorate. J Egypt Soc Parasitol 2004; 34: 807–818. [PubMed] [Google Scholar]
- 27.Afshan K, Kabeer S, Firasat S, et al. Seroepidemiology of human fascioliasis and its relationship with anti-Fasciola IgG and liver enzymes as biomarkers of pathogenicity. Afr Health Sci 2020; 20: 208–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aguiar Oliveira A, Da SilvaNascimento A, Dos Santos TAM, et al. Estudo de prevalência e fatores associados à fasciolose no Município de Canutama, Estado do Amazonas, Brasil. Epidemiolservsaúde 2007; 16: 251–259. [Google Scholar]
- 29.Apt W, Aguilera X, Vega F, et al. Prevalence of fascioliasis in humans, horses, pigs, and wild rabbits in 3 Chilean provinces. Bol Oficina Sanit Panam 1993; 115: 405–414. [PubMed] [Google Scholar]
- 30.Asadian S, Mohebali M, Moudi M, et al. Seroprevalence of human fascioliasis in Meshkin-Shahr district, Ardabil province, northwestern Iran in 2012. Iran J Parasitol 2013; 8: 516–521. [PMC free article] [PubMed] [Google Scholar]
- 31.Ashrafi K, Saadat F, O’Neill S, et al. The endemicity of human fascioliasis in Guilan Province, Northern Iran: the baseline for implementation of control strategies. Iran J Public Health 2015; 44: 501–511. [PMC free article] [PubMed] [Google Scholar]
- 32.Bahram N, Sharbatkhori M, Tohidi F, et al. Serological study of Fascioliasis using indirect ELISA in Gorgan City, Golestan Province, Northern Iran. Iran J Parasitol 2020; 15: 418–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bekana T, Berhe N, Eguale T, et al. Prevalence and factors associated with intestinal schistosomiasis and human fascioliasis among school children in Amhara Regional State, Ethiopia. Trop Med Health 2021; 49: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beyhan YE, Yilmaz H. Seroprevalence of fascioliasis in the eastern region of Turkey: an eight-year investigation. Turk J Gastroenterol 2020; 31: 746–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bless PJ, Schar F, Khieu V, et al. High prevalence of large trematode eggs in schoolchildren in Cambodia. Acta Trop 2015; 141: 295–302. [DOI] [PubMed] [Google Scholar]
- 36.Bozorgomid A, Nazari N, Kia EB, et al. Epidemiology of fascioliasis in Kermanshah Province, Western Iran. Iran J Public Health 2018; 47: 967–972. [PMC free article] [PubMed] [Google Scholar]
- 37.Cabada MM, Morales ML, Webb CM, et al. Socioeconomic factors associated with fasciola hepatica infection among children from 26 communities of the Cusco Region of Peru. Am J Trop Med Hyg 2018; 99: 1180–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cabada MM, Goodrich MR, Graham B, et al. Fascioliasis and eosinophilia in the highlands of Cuzco, Peru and their association with water and socioeconomic factors. Am J Trop Med Hyg 2014; 91: 989–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carnevale S, Cabrera MG, Cucher MA, et al. Direct, immunological and molecular techniques for a fasciolosis survey in a rural area of San Luis, Argentina. J Parasit Dis 2013; 37: 251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cengiz ZT, Yilmaz H, Dulger AC, et al. Seroprevalence of human fascioliasis in Van Province, Turkey. Turk J Gastroenterol 2015; 26: 259–262. [DOI] [PubMed] [Google Scholar]
- 41.Curtale F, Nabil M, El Wakeel A, et al. Anaemia and intestinal parasitic infections among school age children in Behera Governorate, Egypt. Behera survey team. J Trop Pediatr 1998; 44: 323–328. [DOI] [PubMed] [Google Scholar]
- 42.Curtale F, Hassanein YA, Barduagni P, et al. Human fascioliasis infection: gender differences within school-age children from endemic areas of the Nile Delta, Egypt. Trans R Soc Trop Med Hyg 2007; 101: 155–160. [DOI] [PubMed] [Google Scholar]
- 43.Curtale F, Abd El-Wahab Hassanein Y, El Wakeel A, et al. Distribution of human fascioliasis by age and gender among rural population in the Nile Delta, Egypt. J Trop Pediatr 2003; 49: 264–268. [DOI] [PubMed] [Google Scholar]
- 44.Davoodi L, Mizani A, Najafi-Vosough R, et al. A descriptive study of human fascioliasis in Qaemshahr, Mazandaran Province, Iran: its prevalence and risk factors. Arch Clin Infect Dis 2022; 17: e123682. [Google Scholar]
- 45.Eshrati B, Mokhayeri H, Rokni MB, et al. Seroepidemiology of human fascioliasis in rural and nomad areas of Lorestan province, western Iran, in 2016 and 2017. J Parasit Dis 2020; 44: 806–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Esteban JG, Flores A, Aguirre C, et al. Presence of very high prevalence and intensity of infection with Fasciola hepatica among Aymara children from the Northern Bolivian Altiplano. Acta Trop 1997; 66: 1–14. [DOI] [PubMed] [Google Scholar]
- 47.Esteban JG, Gonzalez C, Bargues MD, et al. High fascioliasis infection in children linked to a man-made irrigation zone in Peru. Trop Med Int Health 2002; 7: 339–348. [DOI] [PubMed] [Google Scholar]
- 48.Esteban JG, Gonzalez C, Curtale F, et al. Hyperendemic fascioliasis associated with schistosomiasis in villages in the Nile Delta of Egypt. Am J Trop Med Hyg 2003; 69: 429–437. [PubMed] [Google Scholar]
- 49.Fentie T, Erqou S, Gedefaw M, et al. Epidemiology of human fascioliasis and intestinal parasitosis among schoolchildren in Lake Tana Basin, northwest Ethiopia. Trans R Soc Trop Med Hyg 2013; 107: 480–486. [DOI] [PubMed] [Google Scholar]
- 50.Gonzalez LC, Esteban JG, Bargues MD, et al. Hyperendemic human fascioliasis in Andean valleys: an altitudinal transect analysis in children of Cajamarca Province, Peru. Acta Trop 2011; 120: 119–129. [DOI] [PubMed] [Google Scholar]
- 51.Hassan MM, Moustafa NE, Mahmoud LA, et al. Prevalence of fasciola infection among school children in Sharkia Governorate, Egypt. J Egypt Soc Parasitol 1995; 25: 543–549. [PubMed] [Google Scholar]
- 52.Heydarian P, Ashrafi K, Mohebali M, et al. Seroprevalence of human fasciolosis in Lorestan Province, Western Iran, in 2015–16. Iran J Parasitol 2017; 12: 389–397. [PMC free article] [PubMed] [Google Scholar]
- 53.Hosseini G, Sarkari B, Moshfe A, et al. Epidemiology of human fascioliasis and intestinal helminthes in rural areas of Boyer-Ahmad Township, Southwest Iran; a population based study. Iran J Public Health 2015; 44: 1520–1525. [PMC free article] [PubMed] [Google Scholar]
- 54.Kaya S, Demirci M, Demirel R, et al. Seroprevalence of fasciolosis and the difference of fasciolosis between rural area and city center in Isparta, Turkey. Saudi Med J 2006; 27: 1152–1156. [PubMed] [Google Scholar]
- 55.Kheirandish F, Kayedi MH, Ezatpour B, et al. Seroprevalence of human fasciolosis in Pirabad, Lorestan Province, Western Iran. Iran J Parasitol 2016; 11: 24–29. [PMC free article] [PubMed] [Google Scholar]
- 56.Lopez M, White AC, Jr, Cabada MM. Burden of fasciola hepatica infection among children from Paucartambo in Cusco, Peru. Am J Trop Med Hyg 2012; 86: 481–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maciel MG, Lima WDS, De Almeida FLM, et al. Cross-sectional serological survey of human fascioliasis in Canutama municipality in Western Amazon, Brazil. J Parasitol Res 2018; 2018: 6823638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Naeini KM, Nasiri FM, Rokni MB, et al. Seroprevalence of human fascioliasis in Chaharmahal and Bakhtiyari Province, Southwestern Iran. Iran J Public Health 2016; 45: 774–780. [PMC free article] [PubMed] [Google Scholar]
- 59.Manrique-Abril F, Suescun-Carrero S. Prevalencia de parasitismo intestinal y situacion nutricional en escolares y adolescentes de Tunja.(Perspectiva general de la enfermedad/trastorno). Cesmedicina 2011; 25: 20–30. [Google Scholar]
- 60.Mantari TC, Chávez V A, Suárez A F, et al. Fasciolasis en ninos de tres distritos del departamento de Junin, Peru. Rev Investig Vet Perú 2012; 23: 454–461. [Google Scholar]
- 61.Marcos Raymundo LA, Flores VM, Terashima A, et al. Hyperendemicity of human fasciolosis in the Mantaro Valley, Peru: factors for infection with Fasciole hepatica. Rev Gastroenterol Peru 2004; 24: 158–164. [PubMed] [Google Scholar]
- 62.Marcos L, Romani L, Florencio L, et al. Hyperendemic and mesoendemic zones of Fasciola infection surrounding urban Lima: an emerging disease?. Rev Gastroenterol Peru 2007; 27: 31–36. [PubMed] [Google Scholar]
- 63.Natividad Carpio I, Iwashita A. Prevalencia de infección humana por Fasciola hepática en pobladores del distrito de Caujul provincia de Oyon, region de Lima, Perú. Acta Méd Peru 2008; 25: 77–80. [Google Scholar]
- 64.Nguyen T, Cheong FW, Liew JW, et al. Seroprevalence of fascioliasis, toxocariasis, strongyloidiasis and cysticercosis in blood samples diagnosed in Medic Medical Center Laboratory, Ho Chi Minh City, Vietnam in 2012. Parasit Vectors 2016; 9: 486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nxasana N, Baba K, Bhat V, et al. Prevalence of intestinal parasites in primary school children of Mthatha, eastern Cape Province, South Africa. Ann Med Health Sci Res 2013; 3: 511–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ozturhan H, Emekdas G, Sezgin O, et al. Seroepidemiology of Fasciola Hepatica in Mersin province and surrounding towns and the role of family history of the Fascioliasis in the transmission of the parasite. Turk J Gastroenterol 2009; 20: 198–203. [DOI] [PubMed] [Google Scholar]
- 67.Qureshi AW, Zeb A, Mansoor A, et al. Fasciola hepatica infection in children actively detected in a survey in rural areas of Mardan district, Khyber Pakhtunkhawa province, northern Pakistan. Parasitol Int 2019; 69: 39–46. [DOI] [PubMed] [Google Scholar]
- 68.Qureshi AW, Tanveer A, Mas-Coma S. Epidemiological analysis of human fascioliasis in northeastern Punjab, Pakistan. Acta Trop 2016; 156: 157–164. [DOI] [PubMed] [Google Scholar]
- 69.Rodríguez-Ulloa C, Rivera-Jacinto M, Chilón YS, et al. Infección por Fasciola hepatica en escolares del distrito de Condebamba, Cajamarca. Rev Invest Vet Perú 2018; 29: 1411–1420. [Google Scholar]
- 70.Rodríguez-Ulloa C, Rivera-Jacinto M, Del Valle-Mendoza J, et al. Risk factors for human fascioliasis in schoolchildren in Banos del Inca, Cajamarca, Peru. Trans R Soc Trop Med Hyg 2018; 112: 216–222. [DOI] [PubMed] [Google Scholar]
- 71.Saberinasab M, Mohebali M, Molawi G, et al. Seroprevalence of human fascioliasis using indirect ELISA in isfahan district, central iran in 2013. Iran J Parasitol 2014; 9: 461–465. [PMC free article] [PubMed] [Google Scholar]
- 72.Steinmann P, Usubalieva J, Imanalieva C, et al. Rapid appraisal of human intestinal helminth infections among schoolchildren in Osh oblast, Kyrgyzstan. Acta Trop 2010; 116: 178–184. [DOI] [PubMed] [Google Scholar]
- 73.Taş Cengiz Z, Yilmaz H, Beyhan YE, et al. A Comprehensive retrospective study: intestinal parasites in human in Van Province. Turkiye Parazitol Derg 2019; 43: 70–73. [DOI] [PubMed] [Google Scholar]
- 74.Valencia M Nicasio, Pariona D Andrea, Huaman A Margarita, et al. Seroprevalencia de fasciolosis en escolares y en ganado vacuno en la provincia de Huancavelica, Perú. Rev Peru Med Exp Salud Públ 2005; 22: 96–102. [Google Scholar]
- 75.Wilches C, Jaramillo JG, Muñoz DL, et al. Presencia de infestación por Fasciola hepatica en habitantes del valle de San Nicolás, oriente antioqueño. Infectio 2009; 13: 92–99. [Google Scholar]
- 76.Yilmaz H, Godekmerdan A. Human fasciolosis in Van Province, Turkey. Acta Trop 2004; 92: 161–162. [DOI] [PubMed] [Google Scholar]
- 77.Zoghi S, Emami M, Shahriarirad S, et al. Human fascioliasis in nomads: a population-based serosurvey in southwest Iran. Infez Med 2019; 27: 68–72. [PubMed] [Google Scholar]
- 78.Zumaquero-Rios JL, Sarracent-Perez J, Rojas-Garcia R, et al. Fascioliasis and intestinal parasitoses affecting schoolchildren in Atlixco, Puebla State, Mexico: epidemiology and treatment with nitazoxanide. PLoS Negl Trop Dis 2013; 7: e2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Worldometer. Population by country, 2022, https://www.worldometers.info/
- 80.Alba A, Vazquez AA, Hurtrez-Bousses S. Towards the comprehension of fasciolosis (re-)emergence: an integrative overview. Parasitology 2021; 148: 385–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Furst T, Keiser J, Utzinger J. Global burden of human food-borne trematodiasis: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12: 210–221. [DOI] [PubMed] [Google Scholar]
- 82.Nyindo M, Lukambagire AH. Fascioliasis: an ongoing zoonotic trematode infection. Biomed Res Int 2015; 2015: 786195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.World Health Organization. Report of the WHO informal meeting on use of triclabendazole in fascioliasis control, WHO headquarters, Geneva, Switzerland, 17–18October2006, 2007, https://www.who.int/publications/i/item/WHO-CDS-NTD-PCT-2007.1 [Google Scholar]
- 84.Mollinedo S, Gutierrez P, Azurduy R, et al. Mass drug administration of triclabendazole for Fasciola Hepatica in Bolivia. Am J Trop Med Hyg 2019; 100: 1494–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Curtale F, Hassanein YA, Savioli L. Control of human fascioliasis by selective chemotherapy: design, cost and effect of the first public health, school-based intervention implemented in endemic areas of the Nile delta, Egypt. Trans R Soc Trop Med Hyg 2005; 99: 599–609. [DOI] [PubMed] [Google Scholar]
- 86.Rizwan M, Khan MR, Afzal MS, et al. Prevalence of fascioliasis in livestock and humans in Pakistan: a systematic review and meta-analysis. Trop Med Infect Dis 2022; 7: 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhou XN. Prioritizing research for ‘one health—one world’. Infect Dis Pover 2012; 1: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Savioli L DD, Crompton DWT. Sustaining the drive to overcome the global impact of neglected tropical diseases. Second WHO report on neglected tropical diseases. Geneva: World Health Organization, 2013. [Google Scholar]
- 89.Parkinson M, O’Neill SM, Dalton JP. Endemic human fasciolosis in the Bolivian Altiplano. Epidemiol Infect 2007; 135: 669–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Marcos L, Romani L, Florencio L, et al. Zonas hiperendémicas y mesoendémicas de la infección por fasciola hepática aledañas a la ciudad de lima: una enfermedad emergente. Rev de Gastroenterol del Perú 2007; 27: 31–36. [PubMed] [Google Scholar]
- 91.Carmona C, Tort JF. Fasciolosis in South America: epidemiology and control challenges. J Helminthol 2017; 91: 99–109. [DOI] [PubMed] [Google Scholar]
- 92.Dermauw V, Muchai J, Al Kappany Y, et al. Human fascioliasis in Africa: a systematic review. PLoS ONE 2021; 16: e0261166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sabourin E, Alda P, Vazquez A, et al. Impact of human activities on fasciolosis transmission. Trends Parasitol 2018; 34: 891–903. [DOI] [PubMed] [Google Scholar]
- 94.Mas-Coma S, Bargues MD, Valero MA. Diagnosis of human fascioliasis by stool and blood techniques: update for the present global scenario. Parasitology 2014; 141: 1918–1946. [DOI] [PubMed] [Google Scholar]
- 95.Martinez-Sernandez V, Orbegozo-Medina RA, Gonzalez-Warleta M, et al. Rapid Enhanced MM3-COPRO ELISA for detection of fasciola coproantigens. PLoS Negl Trop Dis 2016; 10: e0004872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Munita MP, Rea R, Martinez-Ibeas AM, et al. Comparison of four commercially available ELISA kits for diagnosis of Fasciola hepatica in Irish cattle. BMC Vet Res 2019; 15: 414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Marcos L, Terashima A, Leguia G, et al. La infección por Fasciola Hepática en el Perú: una enfermedad emergente. Rev Gastroenterol Perú 2007; 27: 389–396. [PubMed] [Google Scholar]
- 98.Torgerson PR, Devleesschauwer B, Praet N, et al. World Health Organization estimates of the global and regional disease burden of 11 foodborne parasitic diseases, 2010: a data synthesis. PLoS Med 2015; 12: e1001920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mehmood K, Zhang H, Sabir AJ, et al. A review on epidemiology, global prevalence and economical losses of fasciolosis in ruminants. Microb Pathog 2017; 109: 253–262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tai-10.1177_20499361231185413 for The global prevalence of human fascioliasis: a systematic review and meta-analysis by Luis Raúl Rosas-Hostos Infantes, Guillermo Andres Paredes Yataco, Yeimer Ortiz-Martínez, Treana Mayer, Angelica Terashima, Carlos Franco-Paredes, Esteban Gonzalez-Diaz, Alfonso J. Rodriguez-Morales, D. Katterine Bonilla-Aldana, Lilian Vargas Barahona, Alyssa A. Grimshaw, Daniel B. Chastain, Stefan Sillau, Luis A. Marcos and Andrés F. Henao-Martínez in Therapeutic Advances in Infectious Disease