Abstract
Multiple sclerosis (MS) is a chronic, progressive neurological disease involving neuroinflammation, neurodegeneration, and demyelination. Cladribine tablets are approved for immune reconstitution therapy in patients with highly active relapsing–remitting MS based on favorable efficacy and tolerability results from the CLARITY study that have been confirmed in long-term extension studies. The approved 4-year dosing regimen foresees a cumulative dose of 3.5 mg/kg administered in two cycles administered 1 year apart, followed by 2 years of observation. Evidence on managing patients beyond year 4 is scarce; therefore, a group of 10 neurologists has assessed the available evidence and formulated an expert opinion on management of the growing population of patients now completing the approved 4-year regimen. We propose five patient categories based on response to treatment during the first 4-year regimen, and corresponding management pathways that envision close monitoring with clinical visits, magnetic resonance imaging (MRI) and/or biomarkers. At the first sign of clinical or radiological disease activity, patients should receive a highly effective disease-modifying therapy, comprising either a full cladribine regimen as described in regulatory documents (cumulative dose 7.0 mg/kg) or a comparably effective treatment. Re-treatment decisions should be based on the intensity and timing of onset of disease activity, clinical and radiological assessments, as well as patient eligibility for treatment and treatment preference.
Keywords: cladribine tablets, expert opinion, immune reconstitution therapy, relapsing multiple sclerosis, re-treatment
Introduction
Multiple sclerosis and new treatment options: focus on immune reconstitution therapies
Multiple sclerosis (MS) is a chronic immune-mediated disease characterized by inflammation and neurodegeneration, affecting over 2.8 million people worldwide, with a mean age at diagnosis of 20–30 years. 1 Although the disease remains incurable, in the past three decades several disease-modifying therapies (DMTs) have been developed, 2 predominantly for the relapsing forms, with the aim of reducing disease activity and slowing disease progression by modulating, sequestering, or depleting immune cells. Many DMTs are immunomodulators and immunosuppressors that are administered continuously as maintenance therapy, while pulsed immune reconstitution therapies (IRTs) are administered intermittently, allowing long periods of drug-free remission and providing efficacy beyond the active treatment period. 3 Among IRTs licensed for the treatment of MS, alemtuzumab 4 and cladribine 5 are administered for a short period each year (i.e. pulsed) and have durable but different effects on the immune system.3,6,7 Unlike maintenance therapies, in which the risk of adverse events (AEs) increases with continued exposure, the risk of AEs with IRTs is higher in the post-treatment period.6,8 Moreover, IRT therapies, especially oral IRT (e.g. cladribine tablets), require less intense monitoring, which positively impacts treatment adherence. Data from the randomized controlled CLARITY trial showed a positive effect of cladribine tablets treatment on quality of life, 9 in accordance with recent evidence showing good tolerability and treatment satisfaction with cladribine tablets both in treatment naïve and DMT-experienced patients. 10 Thus, the pulsed administration and favorable risk-benefit profile of cladribine tablets have a positive impact on treatment adherence.
Cladribine: mechanism of action
Cladribine (2-chlorodeoxyadenosine) is a nucleoside analog pro-drug that, when activated intracellularly through phosphorylation by deoxycytidine kinase (DCK), interferes with DNA synthesis and repair in dividing and quiescent cells, leading to cell death. 11 Cladribine is inactivated through de-phosphorylation by the enzyme 5’-nucleotidase (5’-NTase), preventing its accumulation in most cell types. 11 Activated cladribine preferentially accumulates in T and B lymphocytes due to the high ratio of DCK to 5’-NTase in these cells, 12 and induces a marked reduction of CD19+ B cells within 4-8 weeks after initiation of treatment. 13 Although recovery of the overall CD19+ B-cell pool is driven by naïve and regulatory B cells, levels of memory B cells remain reduced for at least 12 months,14,15 providing a long-term effect of cladribine on disease control. Furthermore, the rapid reduction of CD19+ B cells after cladribine administration may be responsible for the early reduction in combined unique activity (CUA) lesions at MRI starting 2 months after treatment initiation. 16 In contrast to the rapid effect on B cells, cladribine has a slower and more moderate effect on the reduction of CD4+ and CD8+ T cells, reaching nadir 3-6 months after treatment initiation. 15
Unlike the pronounced effect on mature and memory B cells, cladribine does not affect plasma cells, 14 thereby ensuring preservation of acquired humoral response. This explains why cladribine-treated patients have positive humoral responses to COVID-19 vaccines,17–21 and Hepatitis B vaccines. 22 Finally, the minimal effect of cladribine on the innate immune system ensures general cellular immune competency, providing a response to infection. Patients treated with cladribine are not at a greater risk of developing severe COVID-19. 23
Cladribine efficacy and safety: focus on long-term data
The approved cumulative cladribine dose for the treatment of relapsing MS is 3.5 mg/kg over 2 years, administered orally for two treatment courses, with each course comprising two treatment weeks in years 1 and 2, followed by 2 years without treatment. 5 The efficacy of cladribine tablets was initially demonstrated in the randomized, placebo-controlled CLARITY study that showed a significant reduction in both clinical and MRI activity with a higher proportion of patients achieving no evidence of disease activity (NEDA-3) compared with the placebo group.24,25 The efficacy observed in CLARITY was maintained in the CLARITY Extension. The majority of patients remained relapse-free without need of additional treatment beyond years 1 and 2, 26 further demonstrating the durable efficacy of cladribine tablets in reducing frequency and severity of relapses. 27 Post hoc analysis showed that the Expanded Disability Status Scale (EDSS) score remained stable for up 5 years both in patients receiving no further treatment, or in those receiving additional courses of cladribine in years 3 and 4 of the CLARITY Extension study. 28 Consistent with these findings, cladribine tablets also resulted in a benefit for achieving and maintaining NEDA-3. 29 In addition to the exploratory analyses on efficacy, the CLARITY Extension study mainly investigated the safety profile of cladribine tablets. 26 Lymphopenia, expected due to the mechanism of action, constitutes the most common AE with cladribine; 30 however, with proper management, including pre-treatment screening for active or latent chronic infections (e.g. hepatitis, herpes zoster, tuberculosis, varicella zoster) and assessment of vaccinations, the risk of severe opportunistic infection is low. 5 Lymphopenia occurred more frequently in patients treated with additional courses of cladribine tablets in years 3 and 4, compared with patients receiving placebo in the Cladribine Tablets Treating Multiple Sclerosis Orally (CLARITY) study extension. 26 It is important to note that, because of the randomized study design, the additional treatment with cladribine tablets was administered without observing the requirement to achieve a lymphocyte count of at least 800 cells/mm3 before initiating further treatment. 5 Analysis of only patients who had lymphocyte counts ⩾ 800 cells/mm3 before receiving additional treatment with cladribine tablets revealed a lymphopenia incidence of 11% to 12% in years 3 and 4. 31
The lower incidence of autoimmune AEs after treatment with cladribine tablets, 30 compared with alemtuzumab, is likely due to a rapid recovery of regulatory B cells and to the repopulation occurring in the context of sufficient regulatory T cells. 32
Clinical trial data revealed a higher frequency of malignancies in patients treated with cladribine tablets compared with placebo; however, further analyses showed that the incidence does not increase over time, is independent of the cladribine dose, and does not involve clusters of malignancies.30,33 Patients should adhere to local cancer screening programs and cladribine tablets should not be administered to patients with active malignancy. 5
Finally, data on cladribine tablets from the real-world MS setting confirm efficacy and safety comparable to findings in phase 3 clinical trials and extensions.34–37 Real-world data from an Israeli cohort of patients with highly active relapsing/remitting MS (⩾ 1 relapse in the previous year, and ⩾ 1 T1 gadolinium-enhancing lesion or ⩾ 9 T2 lesions while on treatment with another DMT; or ⩾ 2 relapses in the previous year regardless of treatment status) provided evidence that 2 years of cladribine (cumulative dose 3.5 mg/kg) is effective for preventing relapse activity and neurological worsening in year 3 (59.0%; 36/61) and year 4 (74.3%; 26/35). 38 All these findings raise the question of whether additional treatment should be administered after year 4, based on the patient-profile, to provide better disease control.
Aim
Currently, many of the first patients who initiated treatment with cladribine tablets have transitioned through the approved 4-year treatment regimen described in regulatory documents. 5 Management of these patients beyond year 4 needs to be addressed, 39 also considering that the Summary of Product Characteristic (SmPC) does not specify a maximum number of cladribine courses or list previous cladribine treatment as a contraindication for cladribine treatment. 5 In this respect, little evidence is available about re-treatment after year 4. Additional courses of cladribine tablets were administered between the end of the second and the third year in 5/270 patients in a German cohort who experienced disease reactivation, defined as clinical relapse, 6-month confirmed worsening of disability, or new/enlarging T2-hyperintense MRI lesions. 35 Similarly, nine patients in a UK real-world cohort were re-treated with a third course of cladribine (subcutaneous or oral) after year 2 because of disease activity based on MRI-evidence (n = 6), clinical relapse (n = 1), or both (n = 2). 40 A retrospective study conducted in 41 patients with MS assessed subcutaneous cladribine administered (off-label) for longer than 4 years, and/or at cumulative doses exceeding the approved dose of cladribine tablets, reporting long-term effectiveness and a favorable safety profile. 41 A systematic review of real-world evidence on the use of cladribine tablets for MS revealed that more real-world studies with longer follow-up periods are needed. 42
Meanwhile, the ongoing ‘Cladribine, a Multicenter, Long-term Efficacy and Biomarker Australian Study’ (CLOBAS) is collecting prospective real-world data on the safety and efficacy (NEDA-3) of cladribine tablets, including the option of early re-treatment in year 3. 43 Monitoring will include MRI, as well as cognitive performance and biomarkers, including lymphocyte subsets, serum neurofilament light chain (sNfL), DNA methylation, and RNA analysis.
Regulatory agencies do not provide guidance on re-treatment with cladribine tablets after the initial regimen is completed, and it is unclear how to manage these patients. To address this issue, a group of 10 experts, including neurologists and radiologists with extensive experience in managing patients with MS in Italy, reviewed the available clinical trial data and real-world evidence during a virtual meeting held on 30 June 2022. The group proposed a classification for patients treated with cladribine tablets and suggested criteria to identify cladribine-treated patients who might benefit from treatment beyond year 4. The meeting was recorded and subsequently consolidated into a draft manuscript that was extensively revised and approved by the participants.
Proposed patient classification and management after year 4
Patient monitoring and classification
Considering the chronic and incurable nature of MS and that patients who receive cladribine have been diagnosed with highly active MS, the panel agreed that close monitoring and/or re-treatment with cladribine tablets or another highly effective DMT is necessary.
NEDA is a concept used in monitoring disease activity in patients with relapsing-remitting multiple sclerosis (RRMS). 44 Components of this composite measure may depend on the intended use and local resource availability. NEDA-3 criteria include (i) absence of clinical relapses, (ii) absence of Gd + T1 lesions and ⩽ 1 new T2 lesion within one year of the last MRI, and (iii) absence of confirmed disability (EDSS) over 3 months of ⩾ 1 point in patients with baseline score ⩽ 4.0, or ⩾ 0.5 points in patients with baseline score > 4.5. We suggest NEDA-3 as the minimum criteria for monitoring, with additional assessments used when available (Table 1). Disability progression can occur in the absence of overt inflammatory activity, 45 and it has been suggested that monitoring of outcomes in addition to inflammation is needed to guide treatment to delay or prevent progression. 46 Addition of brain atrophy as a fourth NEDA component has been proposed, 47 and shown to improve prediction of disability progression; 48 however, standardized methods for serial measurement of brain volume are not widely available.
Table 1.
Patient monitoring for treatment efficacy.
| Minimum set of assessments | Frequency |
|---|---|
| • MRI assessment with a standardized acquisition protocol ○ New or enlarging T2-weighted lesions ○ T1-weighted gadolinium-enhancing a lesions |
6–12 months |
| • Clinical assessment ○ Symptoms, signs of relapse ○ Changes in disability (e.g., EDSS) |
3 months |
| Additional assessments to consider, when available | |
| • MRI assessment of brain volume loss | 6–12 months |
| • Biomarker: serum neurofilament light chain | 3–6 months |
| • Functional assessments ○ Physical function (e.g., timed 25-foot walk, activity tracking) ○ Cognitive function (e.g., symbol digit modalities test) |
3–6 months |
| • Patient-reported outcomes (e.g., quality of life, fatigue) | 3 months |
EDSS, Expanded Disability Status Scale; MRI, magnetic resonance imaging.
Follow guidelines and use gadolinium judiciously.
Low sensitivity of clinical and imaging parameters may result in delayed diagnosis of reactivation or progression. 49 Neurofilament light chain represents an established biomarker for acute MS disease activity and a good predictor of disease progression. Importantly, recent findings from Benkert et al. 50 demonstrated that sNfL can also be used as a biomarker to monitor drug response, making it potentially useful as an indicator of the need for additional cladribine treatment courses after year 4. We agree that, when available, sNfL should be measured at the end of year 4 to establish a baseline, and every 3–6 months thereafter.
Data from the CLASSIC-MS study suggest that the effect of cladribine tablets on mobility and disability outcomes is sustained in the long term: 90.0% of patients with relapsing MS were not bedridden or wheelchair users, and 55.8% of the exposed cohort had not received further treatment after a median follow-up of 10.9 years from CLARITY/CLARITY Extension; 51 however, approximately 25% of patients who had received cladribine tablets (3.5 mg/kg) in CLARITY and received placebo in the CLARITY Extension had relapsed by the end of CLARITY Extension (≈ 4 years after starting cladribine therapy). 26
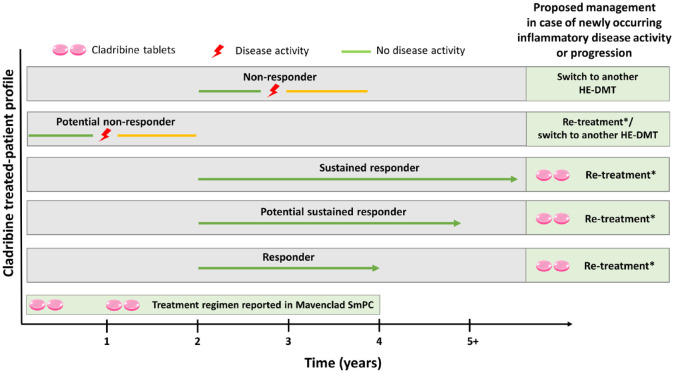
The group of experts proposed a classification for patients based on their response to cladribine over time following the initiation of treatment (Table 2), and a treatment strategy (Figure 1).
Table 2.
Proposed treatment management according to response to cladribine treatment.
| Classification | Description | Management |
|---|---|---|
| Responder | A patient who is stable a after completing two cycles of cladribine tablets (years 1 and 2) and remains so throughout years 3 and 4 | Continue close disease monitoring
b
Re-treatment c with cladribine tablets in case of any newly occurring inflammatory disease activity or disease progression. d |
| Potential sustained responder | A patient remaining stable a after year 4 and up to year 5 from the beginning of cladribine tablets treatment | Close disease monitoring
b
Re-treatment c with cladribine tablets in case of any early sign of newly occurring inflammatory disease activity or disease progression. d |
| Sustained responder | A patient remaining stable a for at least 1 year beyond year 5 | Close disease monitoring
b
Re-treatment c with cladribine tablets in case of any early sign newly occurring inflammatory disease activity or disease progression. d |
| Potential non-responder | A patient showing signs of disease reactivation within 24 months after starting cladribine tablets treatment (i.e., before completing two therapy cycles) | Cladribine re-treatment c /switch to other highly effective DMT according to clinical judgment, based on relapse severity and treatment history |
| Non-responder | A patient with clinical and/or MRI activity vs basal disease activity after year 2 but before year 4 | Switch to another highly effective DMT |
DMT, disease-modifying therapies; MRI, magnetic resonance imaging.
No signs of disease activity or progression during close monitoring.
Minimum criteria for close monitoring: No evidence of disease activity (NEDA-3) defined as absence of new or enlarging T2 and/or T1 Gd+ lesions (every 6 months), clinical relapses and disability progression (every 3 months). Consider monitoring serum neurofilament light chain levels every 3–6 months, where available.
Re-treatment is defined as a complete 4-year regimen of cladribine tablets, corresponding to 3.5 mg/kg over the course of 2 years in patients meeting criteria for treatment, 5 followed by a treatment-free period of 2 years for responders, 3 years for potential sustained responders, and 4 years for sustained responders.
Persistent inflammatory disease activity could be an indication to switch to another highly effective DMT.
Figure 1.
Proposed patient management based on response to cladribine tablets.
*Re-treatment, defined as a full course of cladribine tablets comprising 2 treatment years followed by 2 years of observation, 5 is envisioned for responders, potential sustained responders, and sustained responders without contraindications who show any signs of new disease activity or progression. For potential non-responders, consider cladribine re-treatment or switching to another highly effective disease-modifying therapy DMT (HE-DMT); for non-responders [e.g. those with clinical and/or magnetic resonance imaging (MRI) disease activity or disease progression after year 2], consider switching to another HE-DMT.
The panel stressed the importance of continuous monitoring for disease activity during the treatment-free period, agreeing that NEDA-3 is the minimum acceptable criterion for response when administering a highly effective DMT like cladribine tablets. Patients who do not achieve or maintain NEDA-3 should be candidates for re-treatment with cladribine tablets if they meet criteria, 5 or for another highly effective DMT based on patient and disease characteristics.52,53
Patient management
The choice of treatment should be guided by clinical and MRI parameters; other parameters (e.g. biomarkers) should be considered only when clinical and radiological parameters are stable (i.e. no evidence of relapses, disability progression, or new or enlarging MRI T2 and MRI T1Gd + lesions).
MRI should be performed 6 months after the start of treatment, to establish a new baseline after residual disease has subsided, and from this point, there should be minimal tolerance for signs of MRI activity;54,55 (re-)treatment should be considered at the first sign of activity.
Conclusions
Results from the CLARITY Extension study suggest that there may be a subpopulation of patients (e.g. responders, potential sustained responders, sustained responders) who could benefit from further treatment with cladribine tablets beyond year 4. 29 The absence of guidance on this issue prompted us to elaborate a practical approach for identifying and managing such patients.
We suggest that long-term management is guided by responder-type classification as defined by clinical and MRI activity, and biomarker evaluation. This approach would require regular disease monitoring for the onset of any sign of inflammatory disease activity or progression, at which the reintroduction of cladribine tablets in the therapeutic sequence could ensure better treatment response and a durable effect on disease control.
The long-term safety of cladribine tablets and any cumulative effects of additional courses is of interest. Integrated safety analyses from the cladribine tablets clinical program showed no increased rates of malignancies or infections in patients receiving higher doses and additional courses of cladribine tablets during clinical studies, however, more real-world cohort and registry data are needed.
The prolonged disease-free periods with cladribine tablets, without the necessity for continuous treatment, provide flexibility in redosing or switching to another highly effective DMT, which may be an advantage, for example when planning a pregnancy and breastfeeding. Cladribine tablets represent a therapeutic option to take time without wasting time.
Acknowledgments
None.
Footnotes
ORCID iDs: Diego Centonze  https://orcid.org/0000-0002-8390-8545
https://orcid.org/0000-0002-8390-8545
Carlo Pozzilli  https://orcid.org/0000-0002-6360-4798
https://orcid.org/0000-0002-6360-4798
Contributor Information
Diego Centonze, Department of Systems Medicine, Tor Vergata University, Via Montpellier, 1, 00133 Rome, Italy; IRCCS Neuromed, Pozzilli, Italy.
Maria Pia Amato, Department NEUROFARBA, University of Florence, Florence, Italy; IRCCS Fondazione Don Carlo Gnocchi, Florence, Italy.
Vincenzo Brescia Morra, Multiple Sclerosis Clinical Care and Research Center and Department of Neuroscience (NSRO), Federico II University, Naples, Italy.
Eleonora Cocco, Department of Medical Science and Public Health and Centro Sclerosi Multipla, University of Cagliari, Cagliari, Italy.
Nicola De Stefano, Department of Medicine, Surgery and Neuroscience, University of Siena, Siena, Italy.
Claudio Gasperini, Department of Neurosciences, S Camillo Forlanini Hospital Rome, Rome, Italy.
Paolo Gallo, Department of Neuroscience, University of Padova, Padua, Italy.
Carlo Pozzilli, Department of Human Neuroscience, Sapienza University, Rome, Italy.
Maria Trojano, University of Bari ‘Aldo Moro’, Bari, Italy.
Massimo Filippi, Neuroimaging Research Unit, Division of Neuroscience, IRCCS San Raffaele Scientific Institute, Milano, Italy.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Diego Centonze: Investigation; Methodology; Writing – review & editing.
Maria Pia Amato: Conceptualization; Investigation; Methodology; Writing – review & editing.
Vincenzo Brescia Morra: Conceptualization; Investigation; Methodology; Writing – review & editing.
Eleonora Cocco: Conceptualization; Investigation; Methodology; Writing – review & editing.
Nicola De Stefano: Conceptualization; Investigation; Methodology; Writing – review & editing.
Claudio Gasperini: Conceptualization; Investigation; Methodology; Writing – review & editing.
Paolo Gallo: Conceptualization; Investigation; Methodology; Writing – review & editing.
Carlo Pozzilli: Conceptualization; Investigation; Methodology; Writing – review & editing.
Maria Trojano: Conceptualization; Investigation; Methodology; Writing – review & editing.
Massimo Filippi: Conceptualization; Investigation; Methodology; Writing – review & editing.
Funding: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: This work was supported by Merck Serono S.p.A., Rome, Italy, an affiliate of Merck (CrossRef Funder ID: 10.13039/100009945). Merck had no influence in the design, interpretation of the data, or content of the manuscript. No payments were made to the authors for the writing of this manuscript. Editorial assistance was provided by medical writer Richard Vernell of Ethos S.r.l. and supported by an independent medical writing grant from Merck Serono S.p.A., Rome, Italy, an affiliate of Merck (CrossRef Funder ID: 10.13039/100009945)
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MPA has served on Scientific Advisory Boards for Biogen, Novartis, Roche, Merck, Sanofi-Genzyme, and Teva; has received speaker honoraria from Biogen, Merck, Sanofi-Genzyme, Roche, Novartis, and Teva; has received research grants for her Institution from Biogen, Merck, Sanofi-Genzyme, Novartis, and Roche. She is a co-editor of the Multiple Sclerosis Journal and associate editor of Frontiers in Neurology. VBM has received honoraria from Almirall, Bayer, Biogen, Merck, Mylan, Novartis, Roche, Sanofi-Genzyme, and Teva. DC is an advisory board member or has given advice to Almiral, Bayer Schering, Biogen, GW Pharmaceuticals, Merck Serono, Novartis, Roche, Sanofi-Genzyme, and Teva; has received honoraria for speaking or consultation fees from Almiral, Bayer Schering, Biogen, GW Pharmaceuticals, Merck Serono, Novartis, Roche, Sanofi-Genzyme, and Teva; is the principal investigator in clinical trials for Bayer Schering, Biogen, Merck Serono, Mitsubishi, Novartis, Roche, Sanofi-Genzyme, and Teva. His preclinical and clinical research was supported by grants from Bayer Schering, Biogen Idec, Celgene, Merck Serono, Novartis, Roche, Sanofi-Genzyme, and Teva. EC has served on scientific advisory boards from Bayer, Biogen, Merck, Novartis, Roche, Sanofi-Genzyme, and Teva. NDS declares no conflict of interest. MF is the Editor-in-Chief of the Journal of Neurology and associate editor of Radiology, Human Brain Mapping and Neurological Sciences; received compensation for consulting services and/or speaking activities from Almiral, Alexion, Bayer, Biogen, Celgene, Eli Lilly, Genzyme, Merck Serono, Novartis, Roche, Sanofi, Takeda, and Teva Pharmaceutical Industries; and receives research support from Biogen Idec, Merck Serono, Novartis, Roche, Sanofi, Almiral, Eli Lilly, Teva Pharmaceutical Industries, Italian Ministry of Health, Fondazione Italiana Sclerosi Multipla, and ARiSLA (Fondazione Italiana di Ricerca per la SLA). CG has served on scientific advisory boards for Biogen, Novartis, Roche, Merck, and Sanofi-Genzyme and has received speaker honoraria from Biogen, Merck, Bayer, Sanofi-Genzyme, Roche, Novartis, Almiral, and Mylan. PG has been a consultant and member of advisory board for Biogen Italy, Sanofi, Merck, Almiral, Roche, and Novartis; has received funding for travel and speaker honoraria from Merck Serono, Biogen Idec, Sanofi, Novartis-Pharma, and Roche; has received research support from Bayer, Biogen Italy, Merk, Sanofi, Roche, and Novartis. CP is involved in scientific advisory boards for Alexion, Biogen, Roche, Merck, Novartis, Janssen, and Almiral; receives consulting and/or speaking fees from Alexion, Almiral, Biogen, Bristol Myers, Janssen, Roche, Merck, Novartis, and Biogen; and research support from Merck, Roche, Novartis, Biogen, Bristol Myers, and Almiral. MT has served on the scientific AB for Biogen, Novartis, Roche, Merck, BMS, and Genzyme; has received speaker honoraria from Biogen, Roche, Sanofi, Merck, Genzyme, Janssen, and Novartis; and has received research grants for her institution from Biogen, Merck, Novartis, and Roche.
Availability of data and materials: Not applicable.
References
- 1.Multiple Sclerosis International Federation. Atlas of MS. 3rd ed.London: Multiple Sclerosis International Federation, 2020. [Google Scholar]
- 2.McGinley MP, Goldschmidt CH, Rae-Grant AD. Diagnosis and treatment of multiple sclerosis: a review. JAMA 2021; 325: 765–779. [DOI] [PubMed] [Google Scholar]
- 3.Giovannoni G. Disease-modifying treatments for early and advanced multiple sclerosis: a new treatment paradigm. Curr Opin Neurol 2018; 31: 233–243. [DOI] [PubMed] [Google Scholar]
- 4.European Medicines Agency. LEMTRADA epar-product-information, https://www.ema.europa.eu/en/documents/product-information/lemtrada-epar-product-information_en.pdf (2022, accessed 21 October 2022).
- 5.European Medicines Agency. Mavenclad product information, https://www.ema.europa.eu/en/documents/product-information/mavenclad-epar-product-information_en.pdf (2022, accessed 21 October 2022).
- 6.Boyko AN, Boyko OV. Cladribine tablets’ potential role as a key example of selective immune reconstitution therapy in multiple sclerosis. Degener Neurol Neuromuscul Dis 2018; 8: 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.AlSharoqi IA, Aljumah M, Bohlega S, et al. Immune reconstitution therapy or continuous immunosuppression for the management of active relapsing-remitting multiple sclerosis patients? A narrative review. Neurol Ther 2020; 9: 55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giovannoni G, Mathews J. Cladribine tablets for relapsing-remitting multiple sclerosis: a clinician’s review. Neurol Ther 2022; 11: 571–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Afolabi D, Albor C, Zalewski L, et al. Positive impact of cladribine on quality of life in people with relapsing multiple sclerosis. Mult Scler 2018; 24: 1461–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brochet B, Hupperts R, Langdon D, et al. Treatment satisfaction, safety, and tolerability of cladribine tablets in patients with highly active relapsing multiple sclerosis: CLARIFY-MS study 6-month interim analysis. Mult Scler Relat Disord 2022; 57: 103385. [DOI] [PubMed] [Google Scholar]
- 11.Leist TP, Weissert R. Cladribine: mode of action and implications for treatment of multiple sclerosis. Clin Neuropharmacol 2011; 34: 28–35. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs BM, Ammoscato F, Giovannoni G, et al. Cladribine: mechanisms and mysteries in multiple sclerosis. J Neurol Neurosurg Psychiatry 2018; 89: 1266–1271. [DOI] [PubMed] [Google Scholar]
- 13.Baker D, Marta M, Pryce G, et al. Memory B cells are major targets for effective immunotherapy in relapsing multiple sclerosis. EBioMedicine 2017; 16: 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ceronie B, Jacobs BM, Baker D, et al. Cladribine treatment of multiple sclerosis is associated with depletion of memory B cells. J Neurol 2018; 265: 1199–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiendl H, Schmierer K, Hodgkinson S, et al. Specific patterns of immune cell dynamics may explain the early onset and prolonged efficacy of cladribine tablets: a MAGNIFY-MS substudy. Neurol Neuroimmunol Neuroinflamm 2023; 10: e200048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Stefano N, Barkhof F, Montalban X, et al. Early reduction of MRI Activity during 6 months of treatment with cladribine tablets for highly active relapsing multiple sclerosis: MAGNIFY-MS. Neurol Neuroimmunol Neuroinflamm 2022; 9: e1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rieckmann P, Centonze D, Giovannoni G, et al. Expert opinion on COVID-19 vaccination and the use of cladribine tablets in clinical practice. Ther Adv Neurol Disord 2021; 14: 17562864211058298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Achiron A, Mandel M, Dreyer-Alster S, et al. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther Adv Neurol Disord 2021; 14: 17562864211012836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dreyer-Alster S, Menascu S, Mandel M, et al. COVID-19 vaccination in patients with multiple sclerosis: safety and humoral efficacy of the third booster dose. J Neurol Sci 2022; 434: 120155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grothe C, Steffen F, Bittner S. Humoral immune response and lymphocyte levels after complete vaccination against COVID-19 in a cohort of multiple sclerosis patients treated with cladribine tablets. J Cent Nerv Syst Dis 2021; 13: 11795735211060118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brill L, Rechtman A, Zveik O, et al. Effect of cladribine on COVID-19 serology responses following two doses of the BNT162b2 mRNA vaccine in patients with multiple sclerosis. Mult Scler Relat Disord 2022; 57: 103343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landi D, Nicoletti CG, Di Mauro G, et al. Anti-HBs titers are not decreased after treatment with oral Cladribine in patients with multiple sclerosis vaccinated against hepatitis B virus. Mult Scler Relat Disord 2022; 57: 103334. [DOI] [PubMed] [Google Scholar]
- 23.Albanese A, Sormani MP, Gattorno G, et al. COVID-19 severity among patients with multiple sclerosis treated with cladribine: a systematic review and meta-analysis. Mult Scler Relat Disord 2022; 68: 104156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giovannoni G. Cladribine to treat relapsing forms of multiple sclerosis. Neurotherapeutics 2017; 14: 874–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giovannoni G, Comi G, Cook S, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med 2010; 362: 416–426. [DOI] [PubMed] [Google Scholar]
- 26.Giovannoni G, Soelberg Sorensen P, Cook S, et al. Safety and efficacy of cladribine tablets in patients with relapsing-remitting multiple sclerosis: results from the randomized extension trial of the CLARITY study. Mult Scler 2018; 24: 1594–1604. [DOI] [PubMed] [Google Scholar]
- 27.De Stefano N, Sormani MP, Giovannoni G, et al. Analysis of frequency and severity of relapses in multiple sclerosis patients treated with cladribine tablets or placebo: the CLARITY and CLARITY extension studies. Mult Scler 2022; 28: 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giovannoni G, Comi G, Rammohan K, et al. Long-term disease stability assessed by the expanded disability status scale in patients treated with cladribine tablets 3.5 mg/kg for relapsing multiple sclerosis: an exploratory post Hoc analysis of the CLARITY and CLARITY extension studies. Adv Ther 2021; 38: 4975–4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giovannoni G, Singer BA, Issard D, et al. Durability of no evidence of disease activity-3 (NEDA-3) in patients receiving cladribine tablets: the CLARITY extension study. Mult Scler 2022; 28: 1219–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cook S, Leist T, Comi G, et al. Safety of cladribine tablets in the treatment of patients with multiple sclerosis: an integrated analysis. Mult Scler Relat Disord 2019; 29: 157–167. [DOI] [PubMed] [Google Scholar]
- 31.Cook S, Comi G, Giovannoni G, et al. 039 Rates of lymphopenia in years 1–4 in patients with relapsing multiple sclerosis treated annually with cladribine tablets. J Neurol Neurosurg Psychiatry 2018; 89: A16. [Google Scholar]
- 32.Costelloe L, Jones J, Coles A. Secondary autoimmune diseases following alemtuzumab therapy for multiple sclerosis. Expert Rev Neurother 2012; 12: 335–341. [DOI] [PubMed] [Google Scholar]
- 33.Leist T, Cook S, Comi G, et al. Long-term safety data from the cladribine tablets clinical development program in multiple sclerosis. Mult Scler Relat Disord 2020; 46: 102572. [DOI] [PubMed] [Google Scholar]
- 34.Moser T, Ziemssen T, Sellner J. Real-world evidence for cladribine tablets in multiple sclerosis: further insights into efficacy and safety. Wien Med Wochenschr 2022; 172: 365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pfeuffer S, Rolfes L, Hackert J, et al. Effectiveness and safety of cladribine in MS: real-world experience from two tertiary centres. Mult Scler 2022; 28: 257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petracca M, Ruggieri S, Barbuti E, et al. Predictors of cladribine effectiveness and safety in multiple sclerosis: a real-world, multicenter, 2-year follow-up study. Neurol Ther 2022; 11: 1193–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rauma I, Viitala M, Kuusisto H, et al. Finnish multiple sclerosis patients treated with cladribine tablets: a nationwide registry study. Mult Scler Relat Disord 2022; 61: 103755. [DOI] [PubMed] [Google Scholar]
- 38.Magalashvili D, Mandel M, Dreyer- Alster S, et al. Cladribine treatment for highly active multiple sclerosis: real-world clinical outcomes for years 3 and 4. J Neuroimmunol 2022; 372: 577966. [DOI] [PubMed] [Google Scholar]
- 39.Meuth SG, Bayas A, Kallmann B, et al. Long-term management of multiple sclerosis patients treated with cladribine tablets beyond year 4. Expert Opin Pharmacother 2022; 23: 1503–1510. [DOI] [PubMed] [Google Scholar]
- 40.Allen- Philbey K, Marta M, Gnanapavan S, et al. Disease activity after cladribine immune reconstitution therapy: to repeat or to retreat? (P9-4.002). Neurology 2022; 98: 3135. [Google Scholar]
- 41.Rejdak K, Zasybska A, Pietruczuk A, et al. Long-term safety and efficacy of subcutaneous cladribine used in increased dosage in patients with relapsing multiple sclerosis: 20-year observational study. J Clin Med 2021; 10: 5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oreja-Guevara C, Brownlee W, Celius EG, et al. Expert opinion on the long-term use of cladribine tablets for multiple sclerosis: systematic literature review of real-world evidence. Mult Scler Relat Disord 2023; 69: 104459. [DOI] [PubMed] [Google Scholar]
- 43.Maltby VE, Lea RA, Monif M, et al. Efficacy of cladribine tablets as a treatment for people with multiple sclerosis: protocol for the CLOBAS study (cladribine, a multicenter, long-term efficacy and biomarker Australian study). JMIR Res Protoc 2021; 10: e24969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giovannoni G, Turner B, Gnanapavan S, et al. Is it time to target no evident disease activity (NEDA) in multiple sclerosis? Mult Scler Relat Disord 2015; 4: 329–333. [DOI] [PubMed] [Google Scholar]
- 45.Kappos L, Wolinsky JS, Giovannoni G, et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol 2020; 77: 1132–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giovannoni G, Popescu V, Wuerfel J, et al. Smouldering multiple sclerosis: the ‘real MS’. Ther Adv Neurol Disord 2022; 15: 17562864211066751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kappos L, De Stefano N, Freedman MS, et al. Inclusion of brain volume loss in a revised measure of ‘no evidence of disease activity’ (NEDA-4) in relapsing-remitting multiple sclerosis. Mult Scler 2016; 22: 1297–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rotstein D, Solomon JM, Sormani MP, et al. Association of NEDA-4 with no long-term disability progression in multiple sclerosis and comparison with NEDA-3: a systematic review and meta-analysis. Neurol Neuroimmunol Neuroinflamm 2022; 9: e200032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Biernacki T, Kokas Z, Sandi D, et al. Emerging biomarkers of multiple sclerosis in the blood and the CSF: a focus on neurofilaments and therapeutic considerations. Int J Mol Sci 2022; 23: 3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benkert P, Meier S, Schaedelin S, et al. Serum neurofilament light chain for individual prognostication of disease activity in people with multiple sclerosis: a retrospective modelling and validation study. Lancet Neurol 2022; 21: 246–257. [DOI] [PubMed] [Google Scholar]
- 51.Giovannoni G, Leist T, Aydemir A, et al. Long-term efficacy for patients receiving cladribine tablets in CLARITY/CLARITY extension: primary results from 9–15 years of follow-up in the CLASSIC-MS study. Mult Scler Relat Disord 2022; 59: 103633. [Google Scholar]
- 52.Rae-Grant A, Day GS, Marrie RA, et al. Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2018; 90: 777–788. [DOI] [PubMed] [Google Scholar]
- 53.Montalban X, Gold R, Thompson AJ, et al. ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler 2018; 24: 96–120. [DOI] [PubMed] [Google Scholar]
- 54.Filippi M, Agosta F. Imaging biomarkers in multiple sclerosis. J Magn Reson Imaging 2010; 31: 770–788. [DOI] [PubMed] [Google Scholar]
- 55.Wattjes MP, Ciccarelli O, Reich DS, et al. 2021 MAGNIMS-CMSC-NAIMS consensus recommendations on the use of MRI in patients with multiple sclerosis. Lancet Neurol 2021; 20: 653–670. [DOI] [PubMed] [Google Scholar]