Abstract
Hepatic trematodosis by opisthorchiid flukes has been reported sporadically in North American fish-eating raptors. Bald eagles (Haliaeetus leucocephalus) infected by these flukes often have various degrees of granulomatous cholangitis, pericholangitis, necrosis of adjacent hepatocytes, and subsequent hepatic fibrosis. Species identification has been complicated by the inability to dissect intact specimens from liver tissue. Between 2007 and 2018, 5 juvenile bald eagles with massive hepatic trematodosis were identified at autopsy. Histologically, flukes were non-spinous. Parasitologic identification revealed ventral suckers (80–93 µm diameter), and uteri containing golden, operculated eggs (~25.0 × 12.0 µm). An unfixed frozen liver sample of one eagle was analyzed by PCR and DNA sequencing targeting the large subunit rRNA, ITS region, and cox1 genes of the parasite. The fluke DNA sequences shared 99.6%, 98.4%, and 87.0% similarity, respectively, with Erschoviorchis anuiensis, a newly described opisthorchiid species infecting the liver and pancreas of fish-eating birds in Europe and Asia. Infection by E. anuiensis is highly pathogenic in several piscivorous bird species. The clinical significance of trematodosis in our 5 cases is uncertain because all birds had comorbidities.
Keywords: bald eagles, liver flukes, Opisthorchis, phylogenetic, PCR, raptors
North American birds of prey are affected by a wide array of metazoan parasites, including trematodes. 16 Research on raptor parasitology is limited, leaving unanswered questions about morbidity, mortality, and speciation of trematodes that affect raptors. Trematodes of the family Opisthorchiidae, including Opisthorchis sp., Metorchis bilis, and Amphimerus elongatus, have been reported to cause liver lesions in raptors and other fish-eating birds. 16 The life cycle of opisthorchiid flukes utilizes 2 intermediate hosts (typically a snail and a fish as the first and second host, respectively). 9 Definitive hosts of opisthorchiid flukes include a wide variety of vertebrates including humans, mammals, and fish-eating birds. 9
Four hatch-year bald eagles (Haliaeetus leucocephalus; died of, or were euthanized because of, West Nile virus infection) and one emaciated hatch-year bald eagle were diagnosed with massive hepatic trematodosis at autopsy between 2007 and 2018 at the University of Minnesota Veterinary Diagnostic Laboratory (St Paul, MN, USA). All 5 eagles were presented for autopsy in late summer/early fall (August–October). The liver of all autopsied eagles was moderately enlarged and brown-to-green with myriad white-to-tan, subcapsular serpentine streaks. On cross-section, the parenchyma was widely occupied by numerous flukes that were tubular, thin-walled, fragile, and < 1.0 mm diameter and < 2.0 mm long (Fig. 1A). The fragility of the flukes precluded the removal of intact whole-worm specimens from the liver parenchyma. Fluke fragments recovered from one of the eagles were fixed in 10% neutral-buffered formalin solution, stained with Semichon acetocarmine, and mounted in Canada balsam for morphologic evaluation. Sections of liver were fixed in 10% neutral-buffered formalin solution, embedded in paraffin wax, serially sectioned, and stained with H&E. A sample of liver was saved frozen from one eagle. Microscopically, 25–75% of the liver parenchyma was occupied by flukes in all eagles. Flukes were non-spinous. The ventral suckers were 80–93 µm diameter, and the uteri contained many golden, operculated eggs (~25.0 × 12.0 µm). The flukes were often surrounded by large numbers of lymphocytes, plasma cells, multinucleate giant cells, and heterophilic granulocytes with various degrees of hepatic cord dissociation, adjacent necrosis, and fibroplasia (Fig. 1B). These histologic changes were limited to the hepatic parenchyma directly associated with the flukes.
Figure 1.
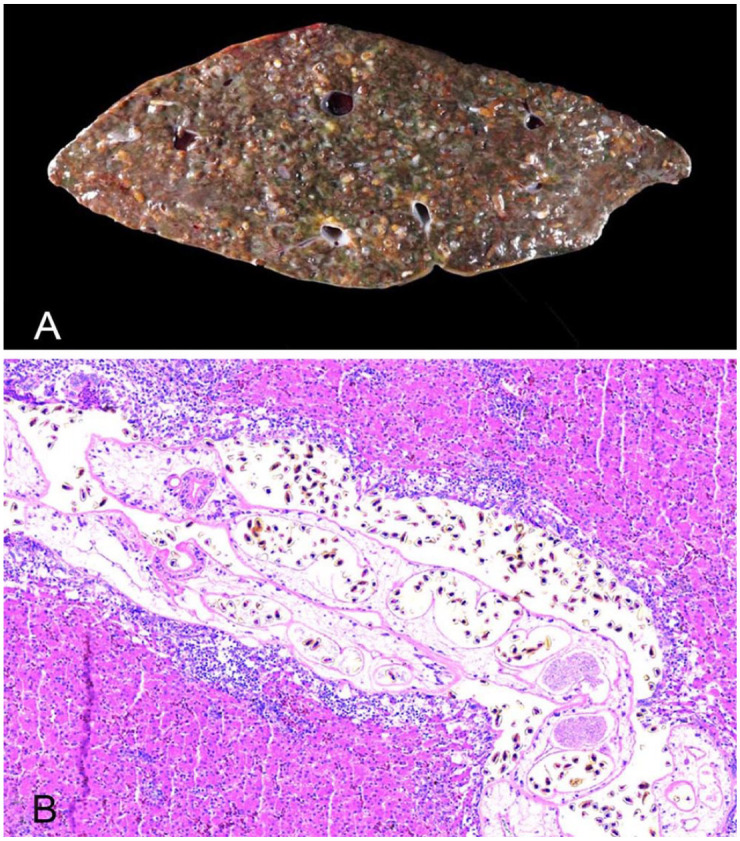
Liver of a bald eagle with trematodosis. A. The entire liver is invaded by massive numbers of trematodes. B. The hepatic parenchyma is invaded by trematodes that are surrounded by lymphocytes, plasma cells, multinucleate giant cells, and heterophils. H&E.
Examination of the Semichon acetocarmine–stained fluke fragments revealed that they were posterior-ends from 3 different flukes each containing a pair of testes arranged in tandem. In one of the fragments, an ovary was also present located anterior to the testes. In addition, there was a middle section of a fluke containing a ventral sucker. A vitellarium was present in all 3 of the posterior fluke fragments. The anterior testes were 525–1,180 μm long, and the posterior testes were 707–1,220 μm. The ovary was 343 μm long by 192 μm wide; the ventral sucker was 70 μm long by 94 μm wide. The vitellarium consisted of numerous follicles and was prominent in the posterior worm fragments lateral to the testes and extending anterior to the ovary. The full extent of the vitellarium could not be determined from the fragments.
Fluke genomic DNA was extracted from the unfixed frozen liver sample of one eagle (DNeasy blood and tissue kit; Qiagen) per the manufacturer’s instructions. The complete nucleotide sequences of the ITS1-5.8S-ITS2 rDNA region, the partial sequence of the large subunit (LSU) rRNA gene, and the partial sequence of the сох1 gene were amplified using the following primers: BD1F (5′-GTCGTAACAAGGTTTCCGTA-3′) 11 and 28S4R (5′-TATTTAGCCTTGGATGGAGTTTACC-3′) 3 for the ITS region; LSU5 (5′-TAGGTCGACCCGCTGAAYTTAAGCA-3′) and 1500R (5′-GCTATCCTGAGGGAAACTTCG-3′) 15 for the LSU rRNA gene; and JB3F (5′-TTTTTTGGGCATCCTGAGGTTTAT-3′) and Trema-cox1/R (5′-CAGCAAATCATGATGCAAAAGGTA-3′)2,4 for the cox1 gene.
Each PCR reaction was performed in triplicate using a total volume of 50 μL, consisting of 2 μL (20–50 ng) of DNA, 5 μL of 10× PCR buffer (Qiagen), 1 μL of dNTP, 10 μL of Q solution, 3 μL of MgCl2, 5 μL of each primer set (10 μM), 0.5 μL (2.5 units) of HotStarTaq DNA polymerase (Qiagen), and the remaining volume of nuclease-free water. Negative control samples with 2 μL of nuclease-free water were conducted in place of DNA.
PCR conditions comprised an initial denaturation and activation at 95°C for 1 min, followed by 40 cycles of 30 s at 94°C, 30 s at 55°C, 56°C, and 50°C (respectively for ITS, LSU, and cox1), 2 min at 72°C, and a final extension at 72°C for 7 min followed by cooling to 12°C. PCR amplicons were visualized after 1% agarose gel electrophoresis (SYBR Safe DNA gel stain; Thermo Fisher) under a UV light source.
Amplicons of the expected size were sequenced bidirectionally at Psomagen (Rockville, MD, USA) using the PCR primers. Assembly of the combined corresponding forward and reverse sequences was used to derive the consensus sequences that were aligned using the ClustalW accessory application with subsequent pairwise similarity calculations determined using BioEdit 7.0. 8 A BLAST search (https://blast.ncbi.nlm.nih.gov/Blast.cgi) determined similarity with other sequences available in GenBank; sequences with > 90% similarity and > 95% query coverage were included in the alignment.
Phylogenies were constructed by maximum likelihood (ML) using MEGA v.11.0. 13 The aligned DNA sequences of 1216, 272, and 347 bp were used for the LSU rRNA gene (21 nucleotide sequences), ITS2 region (16 nucleotide sequences), and cox1 region (14 nucleotide sequences), respectively. All positions containing gaps and missing data were eliminated. Statistical support for topologies was bootstrap-sampled 1,000 times, and support values (%) analysis was superimposed on the tree. The ML trees were assembled using the General Time Reversible + Gamma (G) + Invariable (I) model, Kimura 2-parameter model + G, Hasegawa-Kishino-Yano + G + I, respectively, for the LSU rRNA gene, ITS2 region, and cox1 gene as determined by the lowest Bayesian Information Criterion (BIC) score and highest Akaike Information Criterion corrected (AICc) value. 13
The partial LSU rRNA gene sequence (1,295 bp), ITS region (1,070 bp), and cox1 gene (392 bp) generated from the liver of the juvenile bald eagle (GenBank ON604859, OP325462, OP328808) share 99.6%, 98.4%, and 87.0% sequence identity with Erschoviorchis anuiensis (GenBank MK877245–MK877249, LSU & ITS; MK77240–MK877244, cox1) isolated from domestic Muscovy ducklings (Cairina moschata) from Russia. The phylogenetic analyses based on the LSU rRNA gene (shown), ITS2 region, and cox1 gene all place the eagle trematode sequences at the base of the branch containing the cluster of E. anuiensis sequences (Fig. 2).
Figure 2.
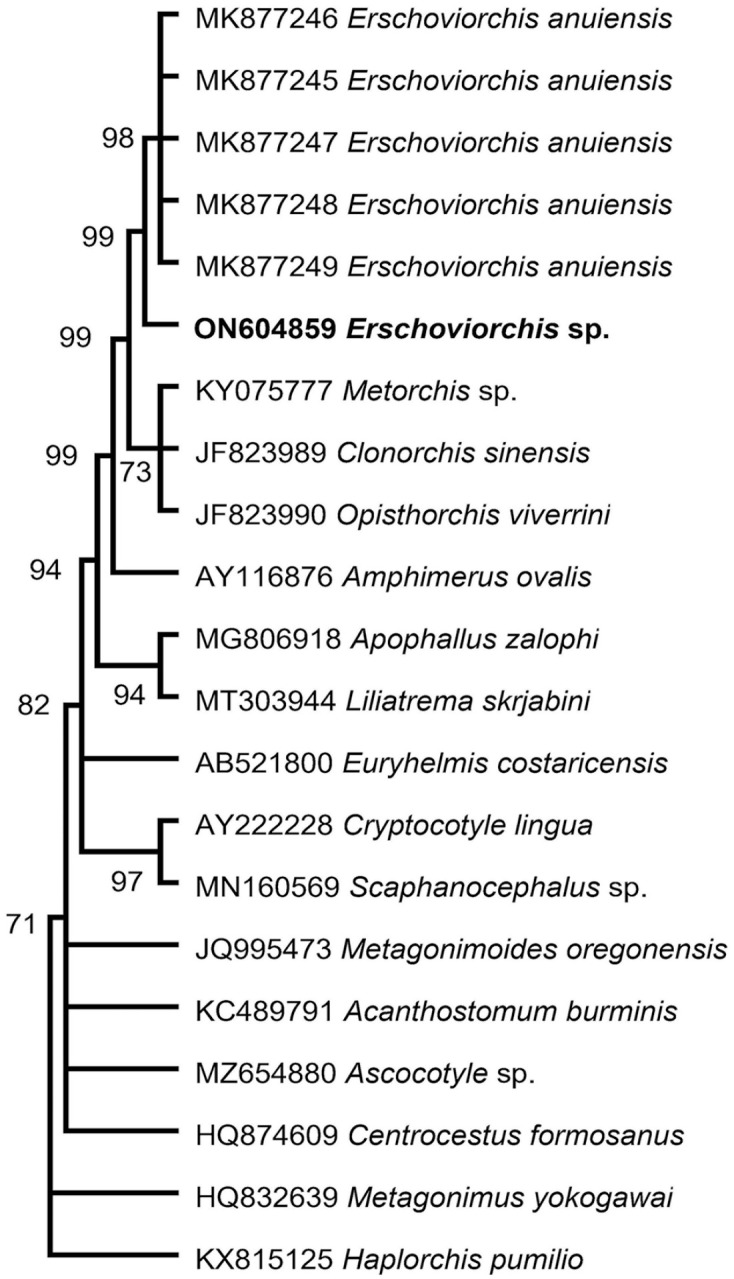
Phylogenetic position of the eagle trematode. Our newly described Erschoviorchis sp. LSU rRNA sequence is in bold.
The nucleotide sequence variation in the 3 genetic loci suggested that the trematode from the eagle could be a new species within the genus Erschoviorchis. 14 Until recently, the only species within this genus was E. lintoni infecting the pancreas of the common loon (Gavia immer) in North America. 7 E. lintoni has also been reported in the pancreas and bile ducts of various fish-eating birds in Western Eurasia, Eastern Siberia, and East Asia. 14 In our case, the molecular comparison between E. lintoni and this new species remains uncertain because molecular characterization of E. lintoni has not been performed. However, E. lintoni infection is unlikely in our cases based on the available limited morphologic data. Our measurements of the ventral suckers on histopathology and the testes, ovary, and ventral sucker in the worm fragments were all significantly larger than those reported for E. lintoni. 6 In addition, the structure of the vitellarium reported for E. lintoni (a few follicles restricted to the posterior half of the fluke) 6 was inconsistent with our observations of the fragments recovered in our case. The vitellarium consisted of numerous follicles spanning from the testes to some level anterior to the ovary.
E. anuiensis, the other species within this genus, was described from experimental infections of Muscovy ducks using metacercaria recovered from fish from the Amur River basin in the Russian Far East. 14 Amphimerus elongatus, a morphologically similar opisthorchiid fluke, has been reported to cause severe liver damage in various species of ducks, whooping crane (Cygnus cygnus), black swan (Chenopsis atrata), belted kingfisher (Megaceryle alcyon alcyon), double-crested cormorant (Phalacrocorax auritus), and bald eagle.1,5,6,10,12 Although A. elongatus shares similarities with the liver fluke identified in our cases, A. elongatus was reported to lack oral suckers. 6 Given the difficulty in dissecting out intact specimens, the identifications in most reports of A. elongatus have been based on examination of worm fragments and/or detection on histopathology and therefore were not definitive. Whether some of these reports were actually of infection with the same species of Erschoviorchis as described here remains unknown.
Ultimately, the recovery of fresh intact adult flukes from affected North American bald eagles is required for a complete morphologic characterization and description of a new Erschoviorchis sp. A complete morphologic characterization paired with molecular analysis will aid in determining the role that this Erschoviorchis sp. may have as a cause of liver (and pancreas) infection in North American fish-eating birds. Our case marks a starting point for further determining the significance of trematodosis in North American raptors, as well as accurately speciating those that affect bald eagles. Whether the Erschoviorchis sp. described here was newly introduced or has been long established in North America remains unknown. The specific intermediate host species are uncertain, but likely are snail and fish species endemic to the upper Midwest.
Although E. anuiensis appears to be highly pathogenic based on experimental infections, the clinical significance of massive hepatic trematodosis in our cases remains uncertain. Liver-specific clinical chemistry was not performed in any of these 5 eagles, and 4 of the 5 birds also had West Nile virus infection, which was considered to be the cause of their clinical deterioration. Based on the morphologic similarities of the flukes observed on histology, the shared seasonality of the infection, and the similar age of each of the 5 eagles, we postulate that all 5 eagles were infected with the same fluke species, but genetic analysis of trematodes from the other 4 affected bald eagles was not performed. Although various species of opisthorchiid flukes are known to infect humans, the zoonotic risk for Erschoviorchis spp. remains uncertain.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Kathleen A. McDermott  https://orcid.org/0000-0002-2386-5467
https://orcid.org/0000-0002-2386-5467
Arno Wünschmann  https://orcid.org/0000-0003-4292-4896
https://orcid.org/0000-0003-4292-4896
Contributor Information
Kathleen A. McDermott, Minnesota Veterinary Diagnostic Laboratory, University of Minnesota, Saint Paul, MN, USA
Spencer J. Greenwood, Departments of Biomedical Sciences, Atlantic Veterinary College, University of Prince Edward Island, Charlottetown, PEI, Canada
Gary A. Conboy, Pathology and Microbiology, Atlantic Veterinary College, University of Prince Edward Island, Charlottetown, PEI, Canada
Dana M. Franzen-Klein, The Raptor Center, University of Minnesota, Saint Paul, MN, USA
Arno Wünschmann, Minnesota Veterinary Diagnostic Laboratory, University of Minnesota, Saint Paul, MN, USA.
References
- 1.Atkinson CT, et al., eds. Parasitic Diseases of Wild Birds. Wiley, 2008:233. [Google Scholar]
- 2.Atopkin DM, et al. Morphological and molecular data for species of Lecithaster Lühe, 1901 and Hysterolecithoides Yamaguti, 1934 (Digenea: Lecithasteridae) from fish of East Asia and phylogenetic relationships within the Hemiuroidea Looss, 1899. J Helminthol 2018;94:e14. [DOI] [PubMed] [Google Scholar]
- 3.Besprozvannykh VV, et al. Life cycle, morphology of developmental stages of Metorchis ussuriensis sp. nov. (Trematoda: Opisthorchiidae), and phylogenetic relationships with other opisthorchiids. J Zool Syst Evol Res 2019;57:24–40. [Google Scholar]
- 4.Bowles J, McManus DP.Genetic characterization of the Asian Taenia, a newly described taeniid cestode of humans. Am J Trop Med Hyg 1994;50:33–44. [PubMed] [Google Scholar]
- 5.Boyd EM, Fry AE.Metazoan parasites of the Eastern belted kingfisher, Megaceryle alcyon alcyon. J Parasitol 1971;57:150–156. [Google Scholar]
- 6.Gower WC.Studies on the trematode parasites of ducks in Michigan with special reference to the mallard. PhD dissertation. Department of Zoology. Michigan State College of Agriculture and Applied Science, 1937. doi: 10.25335/M5K22S. [DOI] [Google Scholar]
- 7.Gower WC.A new trematode from the loon, Gavia immer, and its relationship to Haematotrephus fodiens Linton, 1928. Proc US National Museum 1939;87:139–143. [Google Scholar]
- 8.Hall TA.BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 1999;41:95–98. [Google Scholar]
- 9.King S, Scholz T.Trematodes of the family Opisthorchiidae: a minireview. Korean J Parasitol 2001;39:209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuiken T, et al. Causes of morbidity and mortality and their effects on reproductive success in double-crested cormorants from Saskatchewan. J Wildl Dis 1999;35:331–346. [DOI] [PubMed] [Google Scholar]
- 11.Morgan JA, Blair D.Nuclear rDNA ITS sequence variation in the trematode genus Echinostoma: an aid to establishing relationships within the 37-collar-spine group. Parasitology 1995;111:609–615. [DOI] [PubMed] [Google Scholar]
- 12.Pence DB, Childs GE.Pathology of Amphimerus elongatus (Digenea: Opisthorchiidae) in the liver of the double-crested cormorant. J Wildl Dis 1972;8:221–224. [DOI] [PubMed] [Google Scholar]
- 13.Tamura K, et al. MEGA 11: Molecular Evolutionary Genetics Analysis version 11. Mol Biol Evol 2021;38:3022–3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tatonova YV, et al. Morphological and molecular data for highly pathogenic avian parasite Erschoviorchis anuiensis sp. n. and phylogenetic relationships within the Opisthorchiidae (Trematoda). Parasitol Int 2020;75:102055. [DOI] [PubMed] [Google Scholar]
- 15.Tkach VV, et al. Molecular phylogenetic analysis of the Microphalloidea Ward, 1901 (Trematoda: Digenea). Syst Parasitol 2003;56:1–15. [DOI] [PubMed] [Google Scholar]
- 16.Wünschmann A, et al. Birds of prey. In: Terio KA, et al., eds. Pathology of Wildlife and Zoo Animals. Elsevier, 2018:738. [Google Scholar]


