Abstract
Background
Our prior open trial showed the feasibility of a smartphone-based support system coupled with a Bluetooth breathalyzer (SoberDiary) in assisting recovery for patients with alcohol dependence (AD). In this 24-week follow-up study, we further explored the efficacy of supplementing SoberDiary to treatment as usual (TAU) over 12 weeks of intervention and whether the efficacy persisted in the post-intervention 12 weeks.
Methods
51 patients who met the DSM-IV criteria of AD were randomly assigned to the technological intervention group (TI group, receiving technology intervention of SoberDiary plus TAU, n = 25) or those receiving only TAU (TAU group, n = 26). After 12 weeks of intervention (Phase I), all participants were followed for another post-intervention 12 weeks (Phase II). We collected the drinking variables and psychological assessment data every 4 weeks (i.e., weeks 4, 8, 12, 16, 20, and 24). In addition, the cumulative abstinence days and retention rates were recorded. We used mixed-model analysis to compare the difference in outcomes between groups.
Results
In Phase I or Phase II, we did not find differences in drinking variables, alcohol craving, depression, or anxiety severity between the two groups. However, the TI group showed greater self-efficacy for drinking refusal in Phase II than the TAU group.
Conclusions
Although our system (SoberDiary) did not demonstrate benefits in drinking or emotional outcomes, we found the system holds promise to enhance self-efficacy on drinking refusal. Whether the benefit in promoting self-efficacy persists longer than 24 weeks requires further investigation.
Keywords: Alcohol dependence, Breathalyzer, Smartphone-based treatment intervention, Relapse prevention, Self-efficacy
Highlights
-
•
This 24-week trial examined the benefit of SoberDiary system to treat AD.
-
•
We randomized patients to receive SoberDiary (TI) or not (TAU group) for 12 weeks.
-
•
Both groups were followed for another 12 weeks without SoberDiary intervention.
-
•
The system did not yield better drinking outcomes over the 24 weeks of follow-up.
-
•
The system The TI group exhibited higher post-intervention self-efficacy in drinking refusal.
1. Introduction
Alcohol dependence (AD) is a chronic relapsing disorder characterized by persistent, compulsive, and uncontrolled alcohol consumption despite clinically significant impairment or distress (Roerecke and Rehm, 2014; Grant et al., 2015). AD is the most prevalent disorder of substance dependence, affecting 63.5 million people worldwide (Peacock et al., 2018). It has been associated with increased mortality rates and greater risks of various physical and psychiatric disorders (Archibald et al., 2019; Hasin et al., 2007; Rehm, 2011). AD poses a severe threat to public health, and significant unmet clinical needs exist in managing AD.
Treating AD is a challenging task for clinical practitioners. The efficacy of treatment for AD is inconsistent among studies or merely presents in a subpopulation of patients, like those with less severe of AD or co-occurring depression (Laaksonen et al., 2008; Morley et al., 2006; Richardson et al., 2008; Rosner et al., 2008). In addition, people often seek treatment at a relatively late stage when the disease severity has become extreme (Bruffaerts et al., 2007). Furthermore, the efficacy of the therapy is disappointedly limited. Only 16 % of individuals with AD receiving treatment achieve abstinence (Fan et al., 2019). Up to 50–80 % of the patients with AD have periods of relapse to alcohol use within two years after treatment (Bradizza et al., 2006; McKay et al., 2006). Some evidence also showed that 40 %–60 % of patients treated for alcohol problems relapse within three months, and the rate of relapse increases to 70 %–80 % within 12 months (Bradizza et al., 2006; McLellan et al., 2000; Witkiewitz and Marlatt, 2004). These observations suggest a strong need to look for alternative treatment strategies to improve the access and efficacy of relapse prevention (Mitchell et al., 2020).
Currently, care providers for AD treatment do not typically offer aftercare treatment for relapse prevention for patients who have achieved initial abstinence from detoxification (Hasin et al., 2007). Research suggests extended intervention or increased patients' access to prolonged participation in continuing care is associated with better outcomes, including personal health, quality of life, and a decrease in the frequency of acute-care treatment (McKay, 2005; McLellan et al., 2005). Continued aftercare management that offers support from outside clinics may foster the effectiveness of treatment (McKay, 2005). In line with this, digital health technology delivered via smartphone is a promising means of overcoming the barriers of conventional treatment options, which are often financially overburdened, labor-intensive, and unstable, to support people in recovery whenever and wherever needed (Muench, 2014).
AD involves the patient's impairment to exert cognitive control over drinking behavior (Coriale et al., 2018). Cognitive-behavioral therapy methodology has the advantage of using well-defined treatment practices that can be easily implemented and monitored over various specific emotional or behavioral problems. Fast-growing smartphone technologies offer excellent opportunities to engage patients using the cognitive behavioral model and offer the advantage of broad access and appealing interaction mechanisms, thereby facilitating long-term self-management in a cost-effective manner. To build a continuous care system based on smartphone technologies to support relapse prevention of AD after detoxification, we created an integrated system that includes a smartphone application, a portable Bluetooth breathalyzer, and a back-ended server (SoberDiary) (You et al., 2017). In a pilot study based on an open trial design, we demonstrated that the SoberDiary system might be a feasible supplement to conventional treatment in assisting patients with recovery (You et al., 2017). In addition, through intra-group comparison, we found that patients with a high adherence displayed better outcomes in drinking behavior than those with a low adherence to SoberDiary. However, without a comparison group receiving only treatment as usual (TAU), we did not know whether the benefit of SoberDiary holds.
As such, we conducted a randomized controlled trial to determine whether supplemental SoberDiary combined with TAU could be superior in relapse prevention and reinforcing alcohol abstinence than TAU alone. We also would like to explore whether the benefit persists after the SoberDiary system is withdrawn. We followed patients with AD for 24 weeks: Phase I of SoberDairy intervention for 12 weeks and Phase II of SoberDiary discontinuation for another post-intervention 12 weeks. We hypothesized that patients assisted by SoberDiary have better alcohol-related (abstinence as well as drinking frequency and quantity) and psychological (alcohol craving, depression, anxiety, and self-efficacy) outcomes than those who received TAU alone. In addition, the benefit of SoberDiary could sustain in Phase II following the discontinuation of the support system. We believe our study could leverage a better practice for AD treatment in the technology platform.
2. Methods
2.1. Study design and participants
This 24-week follow-up study was approved by the Institutional Review Board at Taipei City Hospital (TCH) (IRB No. TCHIRB-1020701) and registered at clinicaltrial.gov (NCT02385643) before the study began. The study has two phases. In Phase I (i.e., intervention phase) of the 12-week follow-up, we randomly divided the patients with AD into those receiving technology intervention plus TAU (TI group) and those receiving TAU only (TAU group). In Phase II (i.e., post-intervention), we followed all participants for another 12 weeks.
We recruited treatment-seeking patients with AD from the Department of Addiction Sciences, Taipei City Psychiatry Center (TCPC) of Taipei City Hospital. They were screened and given a structured interview according to the Structured Clinical Interview for DSM-IV by the principal investigator for inclusion in the study. The inclusion criteria were: (1) age between 20 and 65 years, (2) fulfilling at least five of the DSM-IV criteria of AD, (3) completed abstinence for at least 10 days and free of any withdrawal symptoms, (4) drug screening test results negative for opiates, amphetamines, and ketamine, and (5) being able to use an Android smartphone as their primary phone because the support system is established only on the Android system. The exclusion criteria were: (1) a current DSM-IV diagnosis of dependence or abuse on other substances except for tobacco, (2) a current mental or psychiatric impairment or disease that required chronic psychotropic medication or inpatient treatment on a psychiatric ward, (3) a prior history of opioid or psychostimulant abuse, (4) a history of schizophrenia spectrum disorder, bipolar I or bipolar II disorder, or major depressive disorder with psychotic features, (5) evidence of severe neurologic or medical illnesses, (6) homelessness, and (7) cognitive deficit and not thus being able to comprehend the informed consent and study procedure. A total of 51 participants were included in the study and randomized into the TI group (n = 25) and TAU group (n = 26).
2.2. Procedures
The eligible individuals provided informed consent after a full explanation of the study. We assigned unmasked randomization generated by the study statistician, who was the only member aware of each participant's treatment group allocation. Participants who had been allocated to the TI group received a 50-minute tutorial to ensure they understood how to operate the smartphone application and the Bluetooth sensor facility properly. A note was provided on the phone that included the operation procedure and how to access the technical support team if there were any problems. Also, we built a website for the participants to review all research procedures and the operation details of the SoberDiary system (http://mll.csie.ntu.edu.tw/soberdiary). The details of SoberDiary have been described in our prior study and on our website http://mll.csie.ntu.edu.tw/soberdiary (You et al., 2017). Through the Bluetooth interface, the breathalyzer wirelessly sends the test results to their phones, which are installed with a SoberDiary app. To ensure that all participants could take full advantage of the SoberDiary system, we provided smartphones to those who did not own one or could not afford it. This step ensured the inclusivity of our study and allowed us to evaluate the efficacy of our intervention across a broader range of socioeconomic backgrounds.
During Phase I, both groups followed up at the outpatient care unit and were assessed at weeks 4, 8, and 12. The main functions of the SoberDiary included: (1) sending reminder prompts to complete the Breath Alcohol Concentration (BrAC) tests (i.e., breathing into a Bluetooth alcohol gas sensor that detects alcohol consumption by measuring the alcohol concentration in the participant's breath) at least twice per day, (2) providing a feedback program that visualizes the participant's progress over the intervention period, and (3) introducing a social aspect of rehabilitation by enabling a group of participants to share their progress and send anonymously encouraging messages to each other in a mutual attempt to maintain sobriety. In Phase II (post-intervention), all the participants continued receiving TAU in the outpatient department. They were assessed every four weeks (i.e., weeks 16, 20, 24) to evaluate the maintenance of the intervention effect in Phase I.
2.3. Clinical assessment
2.3.1. Alcohol drinking outcomes
The frequency and quantity of alcohol consumption were determined using the Time-Line Follow-Back (TLFB) method (Maisto et al., 1982). Participants were asked to complete a structured drinking diary recording precisely the sort and the amount of alcohol intake recorded in grams per day of pure alcohol. One drink in this study is defined as equivalent to 10 g of pure alcohol. In addition, alcohol craving was measured by VAS, a self-rated craving scale with a ten-point Likert scale from 0 to 9, 0 being no craving and 9 being so severe that the participant could not resist a drink if it was available. We collected the data at baseline and every four weeks thereafter.
2.3.2. Severity of alcohol dependence
Severity of alcohol dependence was measured by the Chinese version of the Severity of Alcohol Dependence Questionnaire (SADQ), which has been validated before (Cheng et al., 2009).
2.3.3. The depression and anxiety in the past month
The depression and anxiety in the past month were assessed using the Beck Depression Inventory-II (BDI-II) (Lu et al., 2002) and the Beck Anxiety Inventory (BAI) (Che et al., 2006) at baseline. Then they followed up every four weeks until the end of the study.
2.3.4. The self-efficacy on alcohol abstinence
The self-efficacy on alcohol abstinence was assessed by Drinking Refusal Self-Efficacy Questionnaire (DRSEQ) (range: 31–186) (Oei and Burrow, 2000) and Alcohol Abstinence Self-Efficacy Scale (AASE) (range: 20–100) (DiClemente et al., 1994), both of which are self-rated questionnaires focusing on the capability of keeping alcohol abstinence despite triggering situational cues or emotional states. The DRSEQ examines whether an individual can subjectively refuse relapse alcohol drinking, while the AASE examines whether participants can tolerate alcohol cravings in different situations. The data were collected starting at week four and every four weeks after that.
2.4. Outcome measurements
The outcome measurement included drinking variables: number of drinks per drinking day, number of heavy drinking days (defined as alcohol consumption above 60 g per day for males and above 40 g per day for females), and number of drinks per drinking day since the last visit. We also collected data using psychological assessments, including the SADQ, VAS, BDI, BAI, DRSEQ, and AASE, and the cumulative abstinence days and retention rates. Drop-out was defined as participants choosing to leave the study, not taking BrAC tests for at least two visits (as evidenced by records of BrAC tests stored in the back-end server of the mobile support system), or needing to receive withdrawal treatment again.
2.5. Statistical analysis
We used Student's t and chi-squared tests for continuous and categorical data, respectively, to examine the differences in demographic characteristics between TAU and TI groups. To evaluate the intervention's effect on drinking behaviors and psychological ratings, we used repeated-measures regression models (PROC MIXED; SAS Institute) with the type of treatment (TI or TAU), time (week 4, week 8, week 12, week 16, week 20, week 24), and the respective interaction as independent variables. We also used mixed modeling to analyze repeated measures over time, which is advantageous due to its ability to retain cases with missing data points. All models were adjusted for the years of education and marital status, which were shown to be different between the TAU and TI groups at baseline. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, North Carolina). A two-sided P-value ≤0.05 was deemed statistically significant.
3. Results
3.1. Clinical characteristics of participants
Table 1 summarizes the baseline clinical characteristics of participants. We did not find significant differences in the demographic and clinical variables (p > 0.05 for all) between TI and TAU groups, except that the years of education and percentage of the married population were higher in the TI group (p = 0.01 and 0.03, respectively).
Table 1.
Baseline demographic and clinical variables in the technology intervention (TI) and treatment as usual (TAU) groups.
| TI group (n = 25) |
TAU group (n = 26) |
p-Valuea | |
|---|---|---|---|
| Gender (M/F), N (%) | 20 (80.0)/5 (20.0) | 18 (69.2)/8 (30.8) | 0.52 |
| Age (years), mean (SD) | 44.0 (9.8) | 42.2 (8.7) | 0.49 |
| Education (years), mean (SD) | 14.4 (2.9) | 12.5 (2.1) | 0.01 |
| Employment, | |||
| Full-time job, N (%) | 21 (84.0) | 21 (80.8) | 1.00 |
| Marital status, | |||
| Married, N (%) | 17 (68.0) | 8 (30.8) | 0.03 |
| Alcohol use variables, mean (SD) | |||
| Age at first alcohol use (y/o) | 17.7 (4.5) (n = 24) | 18.1 (3.8) (n = 24) | 0.78 |
| Age at first alcohol intoxication (y/o) | 31.4 (10.2) | 30.5 (8.0) | 0.75 |
| Number of drinking days in the past 90 days | 64.6 (28.5) | 73.3 (23.0) | 0.24 |
| Number of heavy drinking days in the past 90 days | 59.1 (32.6) | 71.8 (26.4) | 0.13 |
| Number of drinks per drinking day in the past 90 days | 10.6 (5.1) | 15.9 (13.2) | 0.07 |
| Psychological variables | |||
| SADQ (range: 0–60), mean (SD) | 38.8 (12.9) | 35.2 (6.3) | 0.21 |
| BDI (range:0–63), mean (SD) | 18.6 (13.2) | 16.8 (9.8) | 0.57 |
| BAI (range: 0–63), mean (SD) | 14.0 (14.1) | 11.7 (9.7) | 0.49 |
| VAS (range 0–9), mean (SD) | 2.1 (2.6) | 3.1 (3.6) | 0.28 |
| DRSEQ (range: 31–186), mean (SD) | 142.1 (34.7) (n = 25) | 149.6 (40.0) (n = 20) | 0.51 |
| AASE (range: 20–100), mean (SD) | 71.6 (19.9) (n = 25) | 77.9 (21.0) (n = 20) | 0.31 |
| Laboratory data | |||
| AST (U/L) | 65.5 (111.9) | 42.3 (31.3) | 0.33 |
| ALT (U/L) | 43.4 (48.9) | 33.3 (30.8) | 0.38 |
| GGT (U/L) | 228.3 (418.1) (n = 25) | 259.4 (281.3) (n = 25) | 0.76 |
| T-Bil (mg/dL) | 0.94 (0.51) (n = 22) | 0.86 (0.58) (n = 26) | 0.60 |
Abbreviations: AASE: Alcohol Abstinence Self-Efficacy Scale; ALT: alanine aminotransferase; AST: aspartate aminotransferase; BAI: Beck Anxiety Inventory; BDI: Beck Depression Inventory; DRSEQ: Drinking Refusal Self-Efficacy Questionnaire; GGT: gamma-glutamyl transpeptidase; Hb: hemoglobin; MCV: mean corpuscular volume; SADQ: Severity of Alcohol Dependence Questionnaire; SD: standard deviation; T-Bil: total bilirubin; VAS: Visual Analog Score for cravings.
Note: DRSEQ and AASE ratings started at week four.
P values were calculated using Student's t-test (for continuous data) or Fisher's exact test (for categorical data). A two-sided P-value ≤ 0.05 was deemed statistically significant.
3.2. Drinking outcomes
Mixed-model analyses examining a time effect in both the TI and TAU groups demonstrated an improvement in drinking outcomes for both groups over time. However, there was no significant difference in the group x time interaction effect between the two groups over these variables, including the number of drinking days per week, number of heavy drinking days per week, and number of drinks per drinking day (Table 2) (p-value of the interaction effect: 0.49, 0.14, and 0.39, respectively). This suggests that supplementing SoberDiary did not result in significantly different drinking outcomes when compared to TAU alone.
Table 2.
Effects of treatment on drinking outcomes over time. Data are presented as mean (SD).
| Week 4 |
Week 8 |
Week 12 |
Week 16 |
Week 20 |
Week 24 |
p valuea |
|||
|---|---|---|---|---|---|---|---|---|---|
| Number of patients |
Group effect | Time effect | Interaction effect | ||||||
| TI group |
25 |
23 |
20 |
19 |
18 |
17 |
|||
| TAU group | 20 | 16 | 15 | 12 | 12 | 11 | |||
| Number of drinking days/week, | |||||||||
| TI group | 1.6 (3.1) | 3.4 (5.7) | 4.5 (7.0) | 3.5 (6.7) | 2.6 (5.3) | 3.4 (6.1) | 0.10 | <0.01 | 0.49 |
| TAU group | 4.3 (5.9) | 7.5 (10.3) | 7.5 (8.8) | 6.5 (8.1) | 7.0 (7.6) | 5.2 (5.4) | |||
| Number of heavy drinking days/week | |||||||||
| TI group | 1.0 (2.9) | 1.8 (3.6) | 2.7 (5.5) | 0.6 (1.4) | 1.5 (2.1) | 1.5 (3.0) | 0.18 | <0.01 | 0.14 |
| TAU group | 1.2 (2.9) | 4.1 (7.0) | 4.0 (6.4) | 0.7 (0.9) | 4.3 (7.3) | 0.6 (0.7) | |||
| Number of drinks per drinking day | |||||||||
| TI group | 1.1 (1.6) | 1.3 (1.7) | 1.8 (2.0) | 0.9 (1.3) | 1.2 (1.6) | 1.2 (1.3) | 0.46 | 0.05 | 0.39 |
| TAU group | 1.9 (3.8) | 1.8 (2.4) | 1.8 (2.0) | 0.7 (0.9) | 2.0 (1.8) | 0.9 (0.8) | |||
All variables were adjusted for education and marital status.
Abbreviations: SD: standard deviation; TAU: treatment as usual; TI: technology intervention.
A two-sided P-value ≤ 0.05 was deemed statistically significant.
3.3. Psychological outcomes
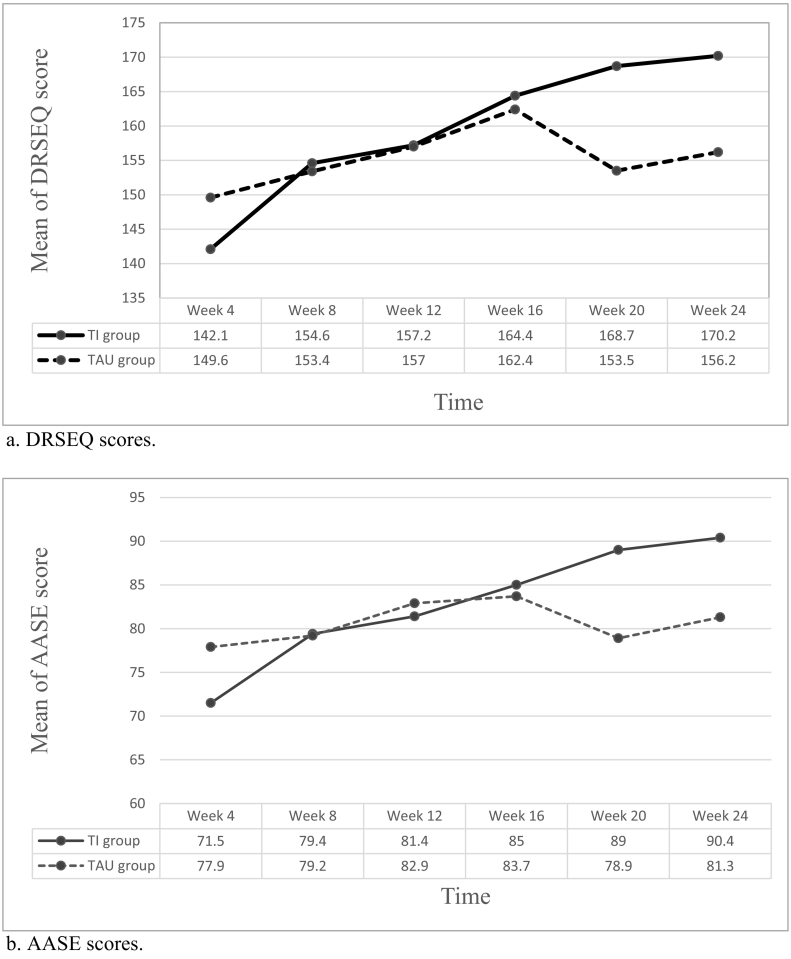
We found no significant differences between the TI and TAU groups in alcohol craving severity and depression and anxiety symptoms over time either in Phase I or Phase II of treatment (p > 0.05) (Table 3). In addition, the TI and TAU groups displayed a similar increase in DRSEQ and AASE scores during Phase I; however, the TI group showed greater DRSEQ and AASE scores in the TI group (Fig. 1). The score changes of DRSEQ and AASE from week 4 to week 24 in the TI group were greater than in the TAU group (28.0 vs. 6.7 and 18.8 vs. 3.4, respectively, p < 0.05). Using the scores at week 4 as the reference, the mixed-model analysis revealed a significant difference in the DRSEQ between the TI and TAU groups at week 20 and week 24 (Supplementary Table 1). These observations suggest a steady increase in DRSEQ and AASE scores in the TI group, even after the withdrawal of SoberDiary.
Table 3.
Effects of treatment on psychological outcomes over time. Data are presented as mean (SD).
| Week 4 |
Week 8 |
Week 12 |
Week 16 |
Week 20 |
Week 24 |
p valued |
|||
|---|---|---|---|---|---|---|---|---|---|
| Number of participants |
Group effect | Time effect | Interaction effect | ||||||
| TI group |
25 |
23 |
20 |
19 |
18 |
17 |
|||
| TAU group | 20 | 16 | 15 | 12 | 12 | 11 | |||
| VAS | |||||||||
| TI group | 1.4 (2.3) | 1.1 (2.3) | 1.7 (2.4) | 1.2 (2.3) | 0.8 (1.0) | 0.9 (1.5) | 0.23 | 0.05 | 0.51 |
| TAU group | 1.8 (2.5) | 2.0 (2.5) | 1.7 (1.8) | 1.3 (1.6) | 1.6 (1.5) | 0.9 (1.0) | |||
| BDI | |||||||||
| TI group | 10.4 (11.4) | 7.4 (6.9) | 9.4 (9.8) | 6.9 (6.5) | 5 (3.8) | 9.1 (12.5) | 0.29 | 0.15 | 0.13 |
| TAU group | 9.9 (8.3) | 9.8 (9.9) | 9.5 (8.1) | 8.1 (7.1) | 9.8 (9.5) | 7.9 (7.5) | |||
| BAI | |||||||||
| TI group | 7.7 (8.0) | 5.2 (5.6) | 6.7 (10.2) | 3.5 (4.4) | 3.0 (3.1) | 7.2 (12.8) | 0.53 | <0.01 | 0.15 |
| TAU group | 5.7 (5.4) | 7.3 (7.4) | 6.6 (5.2) | 4.5 (4.9) | 5.2 (6.0) | 5.7 (5.3) | |||
| DRSEQ | |||||||||
| TI group | 142.1 (34.7) | 154.6 (26.5) | 157.2 (30.1) | 164.4 (24.2) | 168.7 (22.4) | 170.2 (26.9) | 0.12 | <0.01 | <0.01 |
| TAU group | 149.5 (40.0) | 153.4 (40.3) | 157 (35.0) | 162.4 (27.7) | 153.5 (29.6) | 156.2 (29.3) | |||
| AASE | |||||||||
| TI group | 71.6 (19.9) | 79.4 (17.6) | 81.4 (17.0) | 85 (16.3) | 89.0 (12.4) | 90.4 (14.0) | 0.13 | <0.01 | <0.01 |
| TAU group | 77.9 (21.0) | 79.2 (23.0) | 82.9 (17.7) | 83.7 (14.9) | 78.9 (15.7) | 81.3 (16.6) | |||
All variables were adjusted for education and marital status.
Abbreviations: AASE: Alcohol Abstinence Self-Efficacy Scale; BAI: Beck Anxiety Inventory; BDI: Beck Depression Inventory; DRSEQ: Drinking Refusal Self-Efficacy Questionnaire; Severity of Alcohol Dependence Questionnaire; SD: standard deviation; VAS: Visual Analog Score for cravings.
A two-sided P-value ≤ 0.05 was deemed statistically significant.
Fig. 1.
Changes of self-efficacy (a: DRSEQ; b AASE) scores over the 24-week follow-up.
a. DRSEQ scores.
b. AASE scoores.
Abbreviations: AASE: Alcohol Abstinence Self-Efficacy Scale; TAU: treatment as usual; TI: technology intervention.
3.4. Abstinence and retention rate
Table 4 shows the abstinence and retention rate results in the TI and TAU groups. We did not observe a significant difference between the two groups in cumulative abstinence days in Phase I or II. Sixteen participants dropped out during Phase I, and twenty-two participants dropped out during Phase II. The retention rate in the TI group was higher than the TAU group in Phase I (92.0 % vs. 61.5 %, p = 0.08) and Phase II (76.0 % vs. 46.2 %, p = 0.06), though this difference did not reach statistical significance.
Table 4.
Cumulative abstinence days and retention rates in the technology intervention (TI) and treatment as usual (TAU) groups.
| Weeks | Cumulative abstinence days |
Retention rate |
||||
|---|---|---|---|---|---|---|
| TI group (N = 25) |
TAU group (N = 26) |
p valuea | TI group (N = 25) |
TAU group (N = 26) |
p valueb | |
| Mean (SD) | Mean (SD) | n (%) | n (%) | |||
| 0 to 12 | 70.1 (19.2) (n = 20) |
67.0 (27.9) (n = 15) |
0.70 | 20 (92.0) | 15 (61.5) | 0.08 |
| 12 to 24 | 73.5 (20.1) (n = 17) |
69.4 (23.8) (n = 11) |
0.62 | 17 (76.0) | 11 (46.2) | 0.06 |
Abbreviations: SD: standard deviation; TAU: treatment as usual; TI: technology intervention.
P values were calculated using Student's t-test (for continuous data). A two-sided P-value ≤ 0.05 was deemed statistically significant.
P values were calculated using the chi-square test (for categorical data). A two-sided P-value ≤ 0.05 was deemed statistically significant.
4. Discussion
Expanding our results from a previous open trial that demonstrated the supplemental feasibility of the SoberDiary in assisting patients with recovery (You et al., 2017), this 24-week follow-up study further examined whether the SoberDiary system improved drinking outcomes, psychological symptoms, and self-efficacy using a randomized controlled design. We found that the drinking variables and craving, anxiety, and depression levels were comparable between the TI and TAU groups in the intervention phase (Phase 1, 12 weeks) and post-intervention phase (Phase II, 12 weeks). Notably, the TI group displayed greater self-efficacy in the refusal of alcohol drinking than the TAU group only in Phase II,
We found the addition of the SoberDiary system showed promising but inconclusive results for improving drinking and psychological outcomes (i.e., depression and anxiety) in either Phase I or II. The finding that technology-assisted intervention did not lead to a superior outcome compared with TAU was consistent with the literature (Gajecki et al., 2014; Hides et al., 2018; Witkiewitz et al., 2014). A recent review (Colbert et al., 2020) of 21 published papers demonstrated mixed results on the benefit of smartphone applications for managing alcohol problems. For youth, the applications did not show a significant advantage in reducing alcohol consumption (Gajecki et al., 2017, Gajecki et al., 2014; Hides et al., 2018; O'Donnell et al., 2019), while for adults, the benefit may seem promising but still inconclusive. Among the three studies aiming to examine the smartphone-based support system for assisting adult individuals with AD, only one study (Gustafson et al., 2014) (8-month intervention and 4-month follow-up) showed positive outcomes in reducing the risky drinking days, while the other two (Aharonovich et al., 2017; Mellentin et al., 2019) did not show the advantage on changing drinking behaviors (with treatment duration = 60 days and 6 months respectively). Because continuous care is essential for relapse prevention (McKay, 2005), future studies with a longer intervention than 12 weeks might be required to determine the potential benefit of technological intervention in alcohol recovery.
We found that SoberDiary might be associated with greater improvement in post-intervention drinking refusal self-efficacy than those in the TAU group. Self-efficacy, one of the core components of Bandura's Social Cognitive Theory (Bandura, 1986), has been identified as a significant factor in explaining why a treatment or care system can achieve efficacy. Its relationship with promoting behavioral change in relapse prevention has been seen in patients undergoing smoking cessation (Elshatarat et al., 2016), AD treatment (Burling et al., 1989), weight loss (Roach et al., 2003), and chronic disease self-management (Burling et al., 1989; Elshatarat et al., 2016; Holman and Lorig, 1992; Roach et al., 2003). Self-efficacy has been deemed as a strong predictor of treatment outcomes of a substance use disorder, including the quantity of use (Dolan et al., 2008; Kavanagh et al., 1996; Maisto et al., 2000; Sitharthan and Kavanagh, 1991), frequency-related outcomes (T.K. Greenfield et al., 2000), and abstinence (Adamson et al., 2009). In the context of AD, self-efficacy refers to the capability to resist or refuse drinking in high-risk situations (Ellickson and Hays, 1990; Oei and Burrow, 2000). Effective refusal self-efficacy is a key to abstaining from alcohol (Allsop et al., 2000; S.F. Greenfield et al., 2000; Long et al., 2000; Sitharthan and Kavanagh, 1991). One previous study using smartphone intervention to treat AD (Gustafson et al., 2014) has highlighted that competence, an essential construct in self-determination theory, is associated with a reduced number of risky drinking days. Although self-efficacy and competence are based on different theories, both are crucial dimensions contributing to an individual's adaptive functioning in behavioral modification (Sweet et al., 2012; Sweet et al., 2014). We suggest that our smartphone-based systems could enhance a patient's refusal self-efficacy, thereby promoting a better ability to adapt or cope with risky situations.
The reasons for the superior effect of self-efficacy in Phase II, not Phase I, of the intervention, are not entirely clear. One possibility might be that behavior change by psychological intervention in substance use disorder might be gradual and delayed. Several clinical studies, which aimed to evaluate the efficacy of behavioral treatment in substance use disorders, have repeatedly shown the delayed but consistent effect of behavioral change (Carroll et al., 1994; Goldstein et al., 1989; Hawkins et al., 1989; Rawson et al., 2002). For example, Rawson et al. recruited 120 participants with cocaine dependence (Rawson et al., 2002) who were randomly assigned into four groups, with each group receiving different behavioral treatment programs (cognitive-behavioral treatment [CBT] or contingency management, or both) for 16 weeks. They found that the treatment outcome in the CBT group was not superior to TAU when the intervention ended. Yet, the CBT participants showed substantial improvement in urinalysis and self-reported cocaine-used data at the 26-week and 52-week follow-ups. This observation suggests the cumulative effect of coping skills or behavioral modification, which further leads to an increased probability of relapse prevention. Nevertheless, it is important to consider that, given the multiple statistical tests conducted, we cannot dismiss the possibility of a chance finding. While the convergence of results from two different measures of refusal self-efficacy (AASE and DRSEQ) offers some level of confidence in our findings, we should treat these results as exploratory until further validation in future studies. Furthermore, it is worth noting that certain baseline differences, such as mean education years, may potentially influence these outcomes. In order to overcome this, we used the variables for adjustment in the analysis.
4.1. Strengths and limitations
This study has several strengths. First, we developed SoberDiary, which combines a Bluetooth breathalyzer as real-time input for feedback with a mobile self-help application as a thorough monitoring and intervention system for patients with AD. Our design tried to provide psychological treatment and access to a momentary feedback system, based on which we could adjust our intervention strategy accordingly. It also enhances the continuity of the treatment program, which is an unmet need among patients suffering from AD. Second, our study included a 12-week intervention phase and a 12-week post-intervention follow-up phase, allowing use in one way to elucidate the benefit of SoberDiary in assisting recovery and, in another, to understand how the changes proceeded after the discontinuation of the intervention.
Despite these strengths, we must interpret the results cautiously because of some limitations. First, our single-blinded randomization study design may influence the care administered to the TI and TAU groups. Participants assigned to the TI group carried a tangible system coupled with a breathalyzer, which might affect the treatment attitude of the physicians and study personnel. In addition, both groups underwent identical assessments, which may have produced an assessment effect (McCambridge and Kypri, 2011) through multiple contacts with the research team. On average, each participant received approximately 40 min per assessment session. The equal frequency of assessments between the TI and TAU groups in our study could unintentionally have functioned as a relapse prevention intervention, potentially obscuring the differences in outcomes between the two groups. Therefore, our results might only reflect the combining impact of SoberDiary implementation and regular assessments conducted throughout the follow-up period. Second, the study involved many self-report questionnaires, which do not capture a complete picture of each patient's drinking and could underestimate or overestimate drinking behavior due to recall bias. Third, this study was conducted in an addiction department of a psychiatric hospital. The greater severity of AD and associated poorer outcomes in this context limit the generalizability of our results to other populations with less severe drinking problems. Finally, the small sample size limited our ability to detect the potential efficacy of the SoberDiary system. Moreover, although not statistically significant, there were differences between groups in drinking outcomes at baseline, which might have affected our results by obscuring real differences in outcomes between the groups.
4.2. Conclusions
In conclusion, using our smartphone-based support system coupled with a Bluetooth breathalyzer (SoberDiary) for 12 weeks promoted self-efficacy of drinking refusal in the treatment of AD. Future studies are required to identify individuals who benefit the most from technology-assisted treatment programs, to determine whether this benefit persists longer than 24 weeks, and ultimately, to optimize the treatment of AD.
The following is the supplementary data related to this article.
Effects of treatment on outcomes of DRSEQ and AASE over time assessed by a mixed-modeling approach.
CRediT authorship contribution statement
Ming-Chyi Huang and Chuang-Wen You conceptualized and designed the study. Chuang-Wen You and Ming-Chyi Huang developed the SoberDiary system. Shu-Wei Liu, Hu-Ming Chang, and Ming-Chyi Huang conducted literature searches. Ming-Chyi Huang, Hu-Ming Chang, and Chuang-Wen You enrolled the patients. Su-Chen Fang conducted the statistical analyses. Shu-Wei Liu wrote the first draft of the Manuscript. All authors contributed to and approved the final manuscript.
Role of funding sources
This study was supported by grants from the Ministry of Science and Technology, Taiwan (109-2314-B-532-004; 110-2314-B-532-005-MY3); Taipei City Government (TPECH 11001-62-003 and 11101-62-029); and Taipei City Hospital (TPCH110-61; 111-56; 112-52), Taiwan.
Declaration of competing interest
All authors declare that they have no conflicts of interest.
References
- Adamson S.J., Sellman J.D., Frampton C.M. Patient predictors of alcohol treatment outcome: a systematic review. J. Subst. Abus. Treat. 2009;36(1):75–86. doi: 10.1016/j.jsat.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Aharonovich E., Stohl M., Cannizzaro D., Hasin D. HealthCall delivered via smartphone to reduce co-occurring drug and alcohol use in HIV-infected adults: a randomized pilot trial. J. Subst. Abus. Treat. 2017;83:15–26. doi: 10.1016/j.jsat.2017.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allsop S., Saunders B., Phillips M. The process of relapse in severely dependent male problem drinkers. Addiction. 2000;95(1):95–106. doi: 10.1046/j.1360-0443.2000.9519510.x. [DOI] [PubMed] [Google Scholar]
- Archibald L., Brunette M.F., Wallin D.J., Green A.I. Alcohol use disorder and schizophrenia or schizoaffective disorder. Alcohol Res. 2019;40(1) doi: 10.35946/arcr.v40.1.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandura A. Prentice-Hall; 1986. Social Foundations of Thought and Action: A Social Cognitive Theory.https://books.google.com.tw/books?id=HJhqAAAAMAAJ [Google Scholar]
- Bradizza C.M., Stasiewicz P.R., Paas N.D. Relapse to alcohol and drug use among individuals diagnosed with co-occurring mental health and substance use disorders: a review. Clin. Psychol. Rev. 2006;26(2):162–178. doi: 10.1016/j.cpr.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Bruffaerts R., Bonnewyn A., Demyttenaere K. Delays in seeking treatment for mental disorders in the Belgian general population. Soc. Psychiatry Psychiatr. Epidemiol. 2007;42(11):937–944. doi: 10.1007/s00127-007-0239-3. [DOI] [PubMed] [Google Scholar]
- Burling T.A., Reilly P.M., Moltzen J.O., Ziff D.C. Self-efficacy and relapse among inpatient drug and alcohol abusers: a predictor of outcome. J. Stud. Alcohol. 1989;50(4):354–360. doi: 10.15288/jsa.1989.50.354. [DOI] [PubMed] [Google Scholar]
- Carroll K.M., Rounsaville B.J., Nich C., Gordon L.T., Wirtz P.W., Gawin F. One-year follow-up of psychotherapy and pharmacotherapy for cocaine dependence. Delayed emergence of psychotherapy effects. Arch. Gen. Psychiatry. 1994;51(12):989–997. doi: 10.1001/archpsyc.1994.03950120061010. [DOI] [PubMed] [Google Scholar]
- Che H.-H., Lu M.-L., Chen H.-C., Chang S.-W., Lee Y.-J. Validation of the Chinese version of the Beck Anxiety Inventory. Formosan J. Med. 2006;10:447–454. doi: 10.6320/fjm.2006.10(4).05. [DOI] [Google Scholar]
- Cheng W.-J., Huang M.-C., Huang P.-S., Gau Y.-F., Chen C.-H. The Chinese version of the severity of alcohol dependence questionnaire: reliability and factor structure. Taiwan. J. Psychiatry. 2009;23(2):159–166. doi: 10.29478/TJP.200906.0008. [DOI] [Google Scholar]
- Colbert S., Thornton L., Richmond R. Smartphone apps for managing alcohol consumption: a literature review. Addict. Sci. Clin. Pract. 2020;15(1):17. doi: 10.1186/s13722-020-00190-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coriale G., Fiorentino D., De Rosa F., Solombrino S., Scalese B., Ciccarelli R., Attilia F., Vitali M., Musetti A., Fiore M., Ceccanti M. Treatment of alcohol use disorder from a psychological point of view. Riv. Psichiatr. 2018;53(3):141–148. doi: 10.1708/2925.29416. [DOI] [PubMed] [Google Scholar]
- DiClemente C.C., Carbonari J.P., Montgomery R.P., Hughes S.O. The alcohol abstinence self-efficacy scale. J. Stud. Alcohol. 1994;55(2):141–148. doi: 10.15288/jsa.1994.55.141. [DOI] [PubMed] [Google Scholar]
- Dolan S.L., Martin R.A., Rohsenow D.J. Self-efficacy for cocaine abstinence: pretreatment correlates and relationship to outcomes. Addict. Behav. 2008;33(5):675–688. doi: 10.1016/j.addbeh.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellickson P.L., Hays R.D. Beliefs about resistance self-efficacy and drug prevalence: do they really affect drug use? Int. J. Addict. 1990;25(11a):1353–1378. doi: 10.3109/10826089009068468. [DOI] [PubMed] [Google Scholar]
- Elshatarat R., Yacoub M., Khraim F., Saleh Z., Afaneh T. Self-efficacy in treating tobacco use: a review article. Proc. Singap. Healthc. 2016;25 doi: 10.1177/2010105816667137. [DOI] [Google Scholar]
- Fan A.Z., Chou S.P., Zhang H., Jung J., Grant B.F. Prevalence and correlates of past-year recovery from DSM-5 alcohol use disorder: results from National Epidemiologic Survey on Alcohol and Related Conditions-III. Alcohol. Clin. Exp. Res. 2019;43(11):2406–2420. doi: 10.1111/acer.14192. [DOI] [PubMed] [Google Scholar]
- Gajecki M., Berman A.H., Sinadinovic K., Rosendahl I., Andersson C. Mobile phone brief intervention applications for risky alcohol use among university students: a randomized controlled study. Addict Sci Clin Pract. 2014;9(1):11. doi: 10.1186/1940-0640-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajecki M., Andersson C., Rosendahl I., Sinadinovic K., Fredriksson M., Berman A.H. Skills training via smartphone app for university students with excessive alcohol consumption: a randomized controlled trial. Int J Behav Med. 2017;24(5):778–788. doi: 10.1007/s12529-016-9629-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein M.G., Niaura R., Follick M.J., Abrams D.B. Effects of behavioral skills training and schedule of nicotine gum administration on smoking cessation. Am. J. Psychiatry. 1989;146(1):56–60. doi: 10.1176/ajp.146.1.56. [DOI] [PubMed] [Google Scholar]
- Grant B.F., Goldstein R.B., Saha T.D., Chou S.P., Jung J., Zhang H., Pickering R.P., Ruan W.J., Smith S.M., Huang B., Hasin D.S. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72(8):757–766. doi: 10.1001/jamapsychiatry.2015.0584. (Aug) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield S.F., Hufford M.R., Vagge L.M., Muenz L.R., Costello M.E., Weiss R.D. The relationship of self-efficacy expectancies to relapse among alcohol dependent men and women: a prospective study. J. Stud. Alcohol. 2000;61(2):345–351. doi: 10.15288/jsa.2000.61.345. [DOI] [PubMed] [Google Scholar]
- Greenfield T.K., Midanik L.T., Rogers J.D. Effects of telephone versus face-to-face interview modes on reports of alcohol consumption. Addiction. 2000;95(2):277–284. doi: 10.1046/j.1360-0443.2000.95227714.x. [DOI] [PubMed] [Google Scholar]
- Gustafson D.H., McTavish F.M., Chih M.Y., Atwood A.K., Johnson R.A., Boyle M.G., Levy M.S., Driscoll H., Chisholm S.M., Dillenburg L., Isham A., Shah D. A smartphone application to support recovery from alcoholism: a randomized clinical trial. JAMA Psychiatry. 2014;71(5):566–572. doi: 10.1001/jamapsychiatry.2013.4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin D.S., Stinson F.S., Ogburn E., Grant B.F. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch. Gen. Psychiatry. 2007;64(7):830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Hawkins J.D., Catalano R.F., Jr., Gillmore M.R., Wells E.A. Skills training for drug abusers: generalization, maintenance, and effects on drug use. J. Consult. Clin. Psychol. 1989;57(4):559–563. doi: 10.1037//0022-006x.57.4.559. [DOI] [PubMed] [Google Scholar]
- Hides L., Quinn C., Cockshaw W., Stoyanov S., Zelenko O., Johnson D., Tjondronegoro D., Quek L.H., Kavanagh D.J. Efficacy and outcomes of a mobile app targeting alcohol use in young people. Addict. Behav. 2018;77:89–95. doi: 10.1016/j.addbeh.2017.09.020. [DOI] [PubMed] [Google Scholar]
- Holman H., Lorig K. Self-efficacy: Thought Control of Action. Vol. 1. 1992. Perceived self-efficacy in self-management of chronic disease; pp. 305–324. [Google Scholar]
- Kavanagh D.J., Sitharthan T., Sayer G.P. Prediction of results from correspondence treatment for controlled drinking. Addiction. 1996;91(10):1539–1545. [PubMed] [Google Scholar]
- Laaksonen E., Koski-Jännes A., Salaspuro M., Ahtinen H., Alho H. A randomized, multicentre, open-label, comparative trial of disulfiram, naltrexone and acamprosate in the treatment of alcohol dependence. Alcohol Alcohol. 2008;43(1):53–61. doi: 10.1093/alcalc/agm136. [DOI] [PubMed] [Google Scholar]
- Long C.G., Williams M., Midgley M., Hollin C.R. Within-program factors as predictors of drinking outcome following cognitive-behavioral treatment. Addict. Behav. 2000;25(4):573–578. doi: 10.1016/s0306-4603(99)00018-0. [DOI] [PubMed] [Google Scholar]
- Lu M.-L., Che H.H., Chang S., Shen W.W. Reliability and validity of the Chinese version of the Beck depression inventory-II. Taiwan. J. Psychiatry. 2002;16:301–310. [Google Scholar]
- Maisto S.A., Sobell L.C., Cooper A.M., Sobell M.B. Comparison of two techniques to obtain retrospective reports of drinking behavior from alcohol abusers. Addict. Behav. 1982;7(1):33–38. doi: 10.1016/0306-4603(82)90022-3. [DOI] [PubMed] [Google Scholar]
- Maisto S.A., Connors G.J., Zywiak W.H. Alcohol treatment, changes in coping skills, self-efficacy, and levels of alcohol use and related problems 1 year following treatment initiation. Psychol. Addict. Behav. 2000;14(3):257–266. doi: 10.1037//0893-164x.14.3.257. [DOI] [PubMed] [Google Scholar]
- McCambridge J., Kypri K. Can simply answering research questions change behaviour? Systematic review and meta analyses of brief alcohol intervention trials. PLoS One. 2011;6(10) doi: 10.1371/journal.pone.0023748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay J.R. Is there a case for extended interventions for alcohol and drug use disorders? Addiction. 2005;100(11):1594–1610. doi: 10.1111/j.1360-0443.2005.01208.x. [DOI] [PubMed] [Google Scholar]
- McKay J.R., Franklin T.R., Patapis N., Lynch K.G. Conceptual, methodological, and analytical issues in the study of relapse. Clin. Psychol. Rev. 2006;26(2):109–127. doi: 10.1016/j.cpr.2005.11.002. [DOI] [PubMed] [Google Scholar]
- McLellan A.T., Lewis D.C., O’Brien C.P., Kleber H.D. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. Jama. 2000;284(13):1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- McLellan A.T., McKay J.R., Forman R., Cacciola J., Kemp J. Reconsidering the evaluation of addiction treatment: from retrospective follow-up to concurrent recovery monitoring. Addiction. 2005;100(4):447–458. doi: 10.1111/j.1360-0443.2005.01012.x. [DOI] [PubMed] [Google Scholar]
- Mellentin A.I., Nielsen B., Nielsen A.S., Yu F., Mejldal A., Nielsen D.G., Stenager E. A mobile phone app featuring cue exposure therapy as aftercare for alcohol use disorders: an investigator-blinded randomized controlled trial. JMIR Mhealth Uhealth. 2019;7(8) doi: 10.2196/13793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M.M., Mendelson J., Gryczynski J., Carswell S.B., Schwartz R.P. A novel telehealth platform for alcohol use disorder treatment: preliminary evidence of reductions in drinking. Am. J. Drug Alcohol Abuse. 2020;46(3):297–303. doi: 10.1080/00952990.2019.1658197. [DOI] [PubMed] [Google Scholar]
- Morley K.C., Teesson M., Reid S.C., Sannibale C., Thomson C., Phung N., Weltman M., Bell J.R., Richardson K., Haber P.S. Naltrexone versus acamprosate in the treatment of alcohol dependence: a multi-centre, randomized, double-blind, placebo-controlled trial. Addiction. 2006;101(10):1451–1462. doi: 10.1111/j.1360-0443.2006.01555.x. [DOI] [PubMed] [Google Scholar]
- Muench F. The promises and pitfalls of digital technology in its application to alcohol treatment. Alcohol Res. 2014;36(1):131–142. [PMC free article] [PubMed] [Google Scholar]
- O’Donnell R., Richardson B., Fuller-Tyszkiewicz M., Staiger P.K. Delivering personalized protective behavioral drinking strategies via a smartphone intervention: a pilot study. Int. J. Behav. Med. 2019;26(4):401–414. doi: 10.1007/s12529-019-09789-0. [DOI] [PubMed] [Google Scholar]
- Oei T.P., Burrow T. Alcohol expectancy and drinking refusal self-efficacy: a test of specificity theory. Addict. Behav. 2000;25(4):499–507. doi: 10.1016/s0306-4603(99)00044-1. [DOI] [PubMed] [Google Scholar]
- Peacock A., Leung J., Larney S., Colledge S., Hickman M., Rehm J., Giovino G.A., West R., Hall W., Griffiths P., Ali R., Gowing L., Marsden J., Ferrari A.J., Grebely J., Farrell M., Degenhardt L. Global statistics on alcohol, tobacco and illicit drug use: 2017 status report. Addiction (Abingdon, England) 2018;113(10):1905–1926. doi: 10.1111/add.14234. [DOI] [PubMed] [Google Scholar]
- Rawson R.A., Huber A., McCann M., Shoptaw S., Farabee D., Reiber C., Ling W. A comparison of contingency management and cognitive-behavioral approaches during methadone maintenance treatment for cocaine dependence. Arch. Gen. Psychiatry. 2002;59(9):817–824. doi: 10.1001/archpsyc.59.9.817. [DOI] [PubMed] [Google Scholar]
- Rehm J. The risks associated with alcohol use and alcoholism. Alcohol Res. Health. 2011;34(2):135–143. [PMC free article] [PubMed] [Google Scholar]
- Richardson K., Baillie A., Reid S., Morley K., Teesson M., Sannibale C., Weltman M., Haber P. Do acamprosate or naltrexone have an effect on daily drinking by reducing craving for alcohol? Addiction. 2008;103(6):953–959. doi: 10.1111/j.1360-0443.2008.02215.x. [DOI] [PubMed] [Google Scholar]
- Roach J.B., Yadrick M.K., Johnson J.T., Boudreaux L.J., Forsythe W.A., Billon W. Using self-efficacy to predict weight loss among young adults. J. Am. Diet. Assoc. 2003;103(10):1357–1359. doi: 10.1016/S0002-8223(03)01072-1. [DOI] [PubMed] [Google Scholar]
- Roerecke M., Rehm J. Alcohol consumption, drinking patterns, and ischemic heart disease: a narrative review of meta-analyses and a systematic review and meta-analysis of the impact of heavy drinking occasions on risk for moderate drinkers. BMC Med. 2014;12:182. doi: 10.1186/s12916-014-0182-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner S., Leucht S., Lehert P., Soyka M. Acamprosate supports abstinence, naltrexone prevents excessive drinking: evidence from a meta-analysis with unreported outcomes. J. Psychopharmacol. 2008;22(1):11–23. doi: 10.1177/0269881107078308. [DOI] [PubMed] [Google Scholar]
- Sitharthan T., Kavanagh D.J. Role of self-efficacy in predicting outcomes from a programme for controlled drinking. Drug Alcohol Depend. 1991;27(1):87–94. doi: 10.1016/0376-8716(91)90091-c. [DOI] [PubMed] [Google Scholar]
- Sweet S.N., Fortier M.S., Strachan S.M., Blanchard C.M. Testing and integrating self-determination theory and self-efficacy theory in a physical activity context. Can. Psychol. 2012;53(4):319. doi: 10.1037/a0030280. [DOI] [Google Scholar]
- Sweet S.N., Fortier M.S., Strachan S.M., Blanchard C.M., Boulay P. Testing a longitudinal integrated self-efficacy and self-determination theory model for physical activity post-cardiac rehabilitation. Health Psychol. Res. 2014;2(1):1008. doi: 10.4081/hpr.2014.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K., Marlatt G.A. Relapse prevention for alcohol and drug problems: that was Zen, this is Tao. Am. Psychol. 2004;59(4):224–235. doi: 10.1037/0003-066x.59.4.224. [DOI] [PubMed] [Google Scholar]
- Witkiewitz K., Desai S.A., Bowen S., Leigh B.C., Kirouac M., Larimer M.E. Development and evaluation of a mobile intervention for heavy drinking and smoking among college students. Psychol. Addict. Behav. 2014;28(3):639–650. doi: 10.1037/a0034747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You C.W., Chen Y.C., Chen C.H., Lee C.H., Kuo P.H., Huang M.C., Chu H.H. Smartphone-based support system (SoberDiary) coupled with a bluetooth breathalyser for treatment-seeking alcohol-dependent patients. Addict. Behav. 2017;65:174–178. doi: 10.1016/j.addbeh.2016.10.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effects of treatment on outcomes of DRSEQ and AASE over time assessed by a mixed-modeling approach.