Abstract
Objective
This study evaluated the effectiveness of moringa (Moringa oleifera) leaves decoction for removing a smear layer compared to sodium hypochlorite (NaOCl) and ethylenediaminetetraacetic acid (EDTA), as well as its antimicrobial activities.
Methods
The moringa leaves were extracted using hot water decoction at two different concentrations (2.5% and 5.0% w/v). A total of 30 extracted human single-rooted teeth were prepared to assess the smear layer removal efficacy. The presence of a smear layer in the middle third of the root canal was detected by confocal microscopy. Then the antibacterial effects were assessed against Enterococcus faecalis and Streptococcus mutans bacteria using the agar diffusion method.
Results
The 2.5% and 5.0% decoction were significantly more effective than 0.25% NaOCl in removing the smear layer (p < 0.05); however, no significant difference was observed compared to EDTA (p > 0.05). The in vitro antimicrobial assay showed that 5.0% decoction had higher antimicrobial activity against both of the test pathogens.
Conclusion
The findings of this study suggest that moringa leaves decoction can be considered an effective irrigant in endodontics.
Keywords: Antibacterial, Decoction, Irrigants, Moringa oleifera, Root canal, Smear layer
المخلص
أهداف البحث
هدفت الدراسة إلى تقييم فعالية مغلي أوراق الشجرة المعروفة بالمورينجا في إزالة الطبقة السميكة مقارنة بمادتين أخريين وكذلك الأنشطة المضادة للميكروبات.
طرق البحث
تم استخراج أوراق الشجرة باستخدام مغلي الماء الساخن بتركيزين مختلفين. تم تحضير ما مجموعه ثلاثين سنا بشريا مستخرجا ذو جذر واحد لتقييم فعالية إزالة الطبقة السميكة. تم حساب وجود الطبقة السميكة في الثلث الأوسط من قناة الجذر باستخدام الميكروسكوب الليزري الماسح الضوئي الحويصلي. ثم تم إجراء النشاط المضاد للبكتيريا على نوعين من البكتيريا باستخدام طريقة تشتت الأغار.
النتائج
وجدنا أن التراكيز 2,5% و 5% من المغلي أكثر فعالية بشكل ملحوظ في إزالة الطبقة السميكة، ومع ذلك، لم يتم ملاحظة فرق ملحوظ عند المقارنة مع المادتين الأخريين. أظهرت نتائج اختبار المضادات الحيوية المختبرية أن خمسة بالمائة من المغلي يظهر نشاطا مضادا للميكروبات أعلى ضد كلا المسارين التجريبيين.
الاستنتاجات
تشير هذه النتائج إلى أن مغلي أوراق الشجرة يمكن اعتباره محلولا فعالا في علاج الجذور.
الكلمات المفتاحية: مضاد للبكتيريا؛ مغلي؛ محاليل الغسيل؛ الشجرة المعروفة بالمورينجا؛ قناة الجذر؛ الطبقة السميكة.
Introduction
A variety of microorganisms in the root canal produce pulpal and periradicular infections. Root canal therapy aims to remove bacteria from the root canal and provide an environment conducive to tissue recovery. The success of endodontic treatment is determined by proper biomechanical preparation, irrigation, and root canal obturation.1 Irrigation is crucial during root canal therapy for teeth with complex interior structures. One of the gold standards in root canal irrigation is modification of the dentin substrate properties, and therefore, the interaction of dentin with root filling materials.2
The three irrigating substances that are most frequently used are chlorhexidine, ethylenediaminetetraacetic acid (EDTA), and sodium hypochlorite (NaOCl).3 Although NaOCl is the best irrigation solution since it can break down organic material, it also has drawbacks including toxicity and potentially irritating to periapical tissues, and having a disagreeable odour and taste.3,4
An ideal endodontic irrigant would have the following characteristics: the ability to completely remove the smear layer, antibacterial effects, and minimum toxic effects on the periapical tissue.5 The smear layer may prevent intracanal medicaments and sealants from reaching the dentinal tubules. EDTA 17.0% is effective for smear layer removal and as a bacteriostatic agent that chelates Ca2+ and Mg2+ cations to permeate the outer membrane of Gram-negative bacteria. Therefore, EDTA can reduce the dentin microhardness and react with calcium ions in dentine, causing calcium chelation and promoting dentine decalcification.6 While NaOCl 2.5% removes the smear layer in the third apical area incompletely, it has strong antibacterial effects.4 An alternative irrigant is needed to overcome this problem, that has antimicrobial activity and smear layer removal capacity without damaging the dentin. In the past decade, considerable efforts have been made to develop new irrigants from medicinal herbs to facilitate the removal of bacteria from the root canal system as well as to remove the smear layer.7
Moringa species are common plant herbs listed in ancient records because of their extraordinary nutritional and medicinal properties. Moringa oleifera is the most common moringa species. It contains a variety of phytochemical substances such as alkaloids, tannins, flavonoids, saponins, and triterpenoids, and has antimicrobial properties.8 Moringa leaves extract at 8% weight per volume (w/v) can inhibit the growth of Staphylococcus epidermidis with an inhibition zone around 14 mm.9
Methanolic extracts for moringa have antibacterial effects against Enterococcus faecalis after incubation for 24 and 48 h without any toxicity, using a low concentration.10 The aqueous extracts of moringa leaves have antimicrobial activity against Bacillus cereus, Escherichia coli, Salmonella typhi, and Staphylococcus aureus.11 Ethanol extract of moringa leaves exhibits a cariogenic biofilm formation due to Streptococcus mutans infection.12 According to Nugroho et al. (2021), the ethanol extract of moringa 5.0% is an alternative to root canal irrigant.13
The presence of isothiocyanates with their glucosinolate precursors is thought to have antimicrobial effects. The antibacterial effects of isothiocyanates are dose-dependent and mostly related to their reactivity with sulfhydryl groups. The antibacterial effects of isothiocyanates are dose-dependent and mostly related to their reactivity with sulfhydryl groups.14 The main advantage of moringa is its broad safety margin for human and animal consumption.15
In this study, we evaluated the antimicrobial activity of moringa leaves decoction against E. faecalis and S. mutans, and its effects on the smear layer using a confocal laser scanning microscope (CLSM).
Materials and Methods
Study design
This study was a laboratory experimental study that used a post-test only control group design and was conducted between January and March 2020.
Materials
The irrigation solutions used in this study are listed in Table 1.
Table 1.
Specifications of the irrigants used.
| Irrigant | Brand | Concentration (%) | Manufacturer Country |
|---|---|---|---|
| EDTA | Onemed | 17.0 | PT Jayamas Medica Industri, Indonesia |
| NaOCl | Onemed | 2.5 | PT Jayamas Medica Industri, Indonesia |
Preparation of plant decoction
The M. oleifera leaves used in this study were obtained from Toraja, South Sulawesi, Indonesia in January 2020. The plants were identified by Prof. Gemini Alam. Voucher specimens were deposited in Biological Laboratories, Sekolah Tinggi Ilmu Farmasi Makassar (2534B11). The leaves were harvested by hand, washed under running tap water, and drained. The samples underwent thermal drying for 48 h in an oven at 40 °C (Memmert, Buchenbach, Germany), and then were ground with a food grinder (Philips, Jakarta, Indonesia) to produce a fine powder. Decoction of M. oleifera 2.5% was made by weighing about 2.5 g M. oleifera dried leaves and placing them in distilled water (filled to reach 100 mL), while the water temperature was maintained at 90 °C (within ±2 °C) for 30 min. The mixture was filtered under hot conditions over a Buchner funnel, and hot water was directly poured on sample to reach 100 mL. The same procedure was conducted for M. oleifera 5.0%. The decoction was prepared in triplicate immediately before the experiment.
Phytochemical qualitative screening
Phytochemical qualitative analysis was determined using the following conventional procedures for the decoction.16
Test for tannin
Two millilitres of decoction was added to approximately 10 mL bromine water. The discoloration of bromine revealed the presence of tannins.16
Test for saponin
Five millilitres of decoction was added to a test tube, and a few drops of olive oil was mixed in. After vigorously homogenising, the appearance of foam showed the presence of saponins.16
Tests for flavonoid
A few magnesium ribbons and concentrated hydrogen chloride were combined with decoction and allowed to stand for a few minutes. The pink tint indicated the existence of flavonoids.16
Antimicrobial activity
The antimicrobial activity of moringa leaves decoction against pathogenic bacteria (E. faecalis ATCC 29212 and S. mutans ATCC 25175) was investigated. On Muller Hinton agar plates, the recently dissolved bacterial solution was spread out. One hundred microliters of each decoction were added to the wells, and the plate was incubated at 37 °C for 24 h. The positive control was NaOCl 2.5%. The zone of inhibition was recorded on each plate.
Specimen selection
In this investigation, the number of samples were calculated using Federer's formula:17 [t (r – 1) > 15], where t = number of treatments; r = number of replications. Thirty removed single-rooted human premolars teeth were used. A radiograph was taken of each tooth to establish the presence of a single canal. Internal resorption, fractures, root caries, curve canals, endodontic therapy, and calcification were all excluded. After removing the calculus and soft-tissue debris, the teeth were disinfected with 70% ethanol for 1 h before being preserved in saline solution until instrumentation.
Specimen preparation
The length of the teeth was standardised at 16 mm. The teeth were embellished using a safe-sided diamond disk attached to a low-speed handpiece with a water coolant. The working length was calculated by taking 1 mm away from the measurement that was taken. A ProTaper universal nickel-titanium rotary system was used to prepare the root canals.
Smear layer removal
Each tooth was divided into equal sections of the middle third with a diamond disk. Teeth were divided into the following five groups (n = 6) according to the irrigant used: Group 1, distilled water; Group 2, NaOCl 2.5%; Group 3, EDTA 17%; Group 4, moringa 2.5%; and Group 5, moringa 5.0%. After each file size, 5.0 mL irrigant solution was used to irrigate each group, and 5.0 mL distilled water was used for the final rinse. Images of each third of the canal were taken using a CLSM. Cleanliness was evaluated using criteria described by Tosco et al. (2023) (Table 2),18 and the results were tabulated. The smear layer was independently graded by two operators.
Table 2.
Smear layer evaluation criteria.18
| Score | Description |
|---|---|
| 1 | There is no smear layer, and all of the dentinal tubules are exposed. |
| 2 | Some dentinal tubules and a little bit of smear layer are open. |
| 3 | Only a few dentinal tubules are exposed due to a homogeneous smear film covering the root canal wall. |
| 4 | The complete root canal wall is covered by a homogeneous smear layer and there are no open dentinal tubules. |
| 5 | A heavy homogeneous smear layer is covering the complete root canal wall |
Statistical analyses
The decoction's zone of inhibition diameter was measured in triplicate. The mean and standard deviation were calculated. The Shapiro–Wilk test was used to evaluate the normality of data. One-way analysis of variance (ANOVA) was used to compare the mean zone of inhibition between groups, and Tukey's post hoc test was used to confirm the results. Kruskal–Wallis analysis was used to compare the smear layer removal efficacy among the five different groups, the Mann–Whitney U test was used for individual comparisons. P < 0.05 was considered statistically significant.
Results
Phytochemical screening
Phytochemical examination conducted on moringa leaves decoction revealed the presence of flavonoids, saponins, and tannins (Table 3). These phytochemical elements promote the bioactive activities in medicinal plants and are responsible for the antioxidant activity of the plant extract studied.
Table 3.
Phytochemical analysis for moringa based on the preliminary decoction leaves screening.
| Phytochemical compounds | Presence |
|---|---|
| Tannin | + |
| Saponin | + |
| Flavonoid | ++ |
Note: Absent = −, trace = +, highly present = ++.
Antimicrobial activity
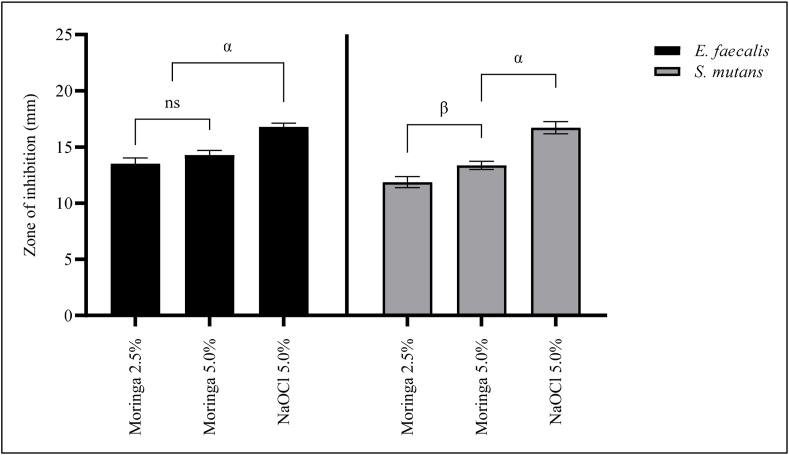
Based on the mean value zone of inhibition, the antibacterial activity of the moringa leaves decoction depended on the concentrations of the decoction bacteria used (Figure 1). At concentrations of 2.5% and 5.0% decoction, E. faecalis was non-significant (p > 0.05) or nearly similar on the mean zone of inhibition (12.70 ± 0.50 and 13.82 ± 0.42 mm, respectively). For S. mutans, the zones of inhibition of the decoction at 5% (11.87 ± 0.49 mm) were significantly different (p < 0.05) from those of 5.0% (13.37 ± 0.36 mm). However, NaOCl 5% was more effective against both E. faecalis (16.76 ± 0.32 mm) and S. mutans (16.72 ± 0.55 mm).
Figure 1.
Antimicrobial activity of moringa leaves decoction represented as the zone of inhibition mean (mm) for tested bacteria. Values are expressed as the mean ± standard deviation (n = 3); analysis was performed with one-way ANOVA followed by Tukey's test with post hoc multiple comparisons; α, compared to NaOCl 5.0%; β, compared to moringa 2.5%; ns, non-significant.
Smear layer removal efficiency
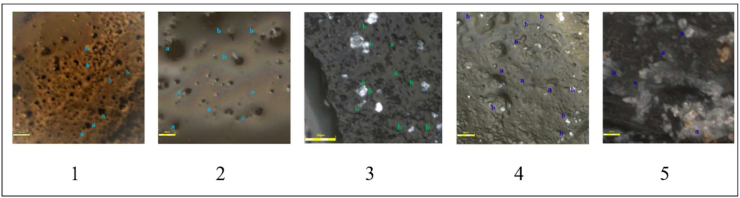
A comparison of the smear layer covering the middle third of the tooth between groups was performed (Figure 2). Regarding the smear layer score, moringa 2.5% and 5.0% had similar effectiveness (score of 1.83 ± 0.41). Both moringa 2.5% and 5.0% were more effective than NaOCl 2.5% and EDTA 17.0% (Table 4).
Figure 2.
Representative CLSM micrographs (x) in each group: (1) moringa 2.5%; (2) moringa 5.0%; (3) NaOCl 2.5%; (4) EDTA 17.0%; (5) distilled water. (a) Dentinal tubules without smear layer, (b) smear layer on the surface of dentinal tubules.
Table 4.
Mean ± standard deviation score of smear layer in the middle third of different groups, and the results of the Shapiro–Wilk and Kruskal–Wallis tests.
| Group | N | Mean | SD | Shapiro Wilk (P) | Kruskal–Wallis (p) |
|---|---|---|---|---|---|
| Moringa 2.5% | 6 | 1.83 | 0.41 | 0.000∗ | 0.001∗ |
| Moringa 5.0% | 6 | 1.83 | 0.41 | 0.000∗ | |
| NaOCl 2.5% | 6 | 2.33 | 0.52 | 0.000∗ | |
| EDTA 17.0% | 6 | 2.83 | 0.41 | 0.001∗ | |
| Distilled water | 6 | 4.83 | 041 | 0.000∗ |
Note: ∗ Statistically significant result (p < 0.05).
Generally, the Mann–Whitney U test showed a significant difference in the cleanliness of moringa decoction between different groups at each level of the smear layer (Table 5). In moringa 2.5% and 5.0%, there was no significant difference in the cleanliness of dentin (p = 1). Our results revealed a significant difference between the smear layers, both moringa (2.5% and 5.0%) and standard irrigant (NaOCl 2.5%), but more effective than NaOCl 2.5% to eliminate smear layer. This outcome suggests that moringa leaves decoction shows promise as an alternative irrigant.
Table 5.
Mann–Whitney test value to evaluate the difference between groups.
| Group | Moringa 2.5% | Moringa 5.0% | NaOCl 2.5% | EDTA 17.0% | Distilled water |
|---|---|---|---|---|---|
| Moringa 2.5% | |||||
| Moringa 5.0% | 1.000 | ||||
| NaOCl 2.5% | 0.001∗ | 0.001∗ | |||
| EDTA 17.0% | 0.092 | 0.092 | 0.019∗ | ||
| Distilled water | 0.002∗ | 0.002∗ | 0.001∗ | 0.001∗ |
Note: ∗ Statistically significant result (p < 0.05).
Discussion
Phytochemical screening confirmed the presence of compounds such as tannins, flavonoids, and saponins in moringa leaves decoction. These compounds are helpful for the treatment of infection in both preclinical and clinical studies.19,20 Chhikara et al. (2020) and Trigo et al. (2021) summarised the several bioactive compounds isolated and identified from moringa leaves. However, this report agreed with the studies by Chhikara et al. (2020), Enerijiofi et al. (2021), and Trigo et al. (2021), who also reported the presence of tannin, saponin, and flavonoid.21, 22, 23 Specifically, 2-octenoic acid and 1,2-epoxyhexadecane identified from the leaves water extract have shown antimicrobial activities.21 Thus, moringa leaves decoction containing this compound may be a potential source of bioactive compounds against pathogen bacteria. Besides killing the microbes, one of the properties of the irrigant solutions is eliminating the smear layer on the dentin.2 For this reason, we also evaluated the effectiveness of the moringa leaves for the removal of the smear layer compared to NaOCl and EDTA.
The smear layer consists of inorganic and organic components. The inorganic components are apatite particles, whereas the organic components include microorganisms and saliva.24 Generally, flavonoids decompose hydroxyapatite, releasing calcium ions (Ca2+) and hydrogen phosphate (HPO42−) soluble in water. As a result, demineralisation occurs.25 Saponin acts as emulsifiers to reduce the surface tension of the solution. Saponin consists of hydrophilic and hydrophobic groups. The hydrophilic group will bind to polar compounds from the organic smear layer, and hydrophobic groups will bind to non-polar compounds from the inorganic smear layer. Saponins also have distinctive physicochemical properties, namely foaming when soaked in the water. The chemical structure of saponins – consisting of glycosides (polar compounds) and triterpenes (non-polar compounds) – indicates that it belongs to a class of surfactants with detergent-like properties. This class of surfactants can dissolve polar and non-polar compounds.26,27
In contrast to moringa, NaOCl does not contain surfactant directly. However, the saponification reaction, in which sodium hypochlorite breaks down fatty acids and lipids to produce soap and glycerol, can demonstrate the dissolution of organic tissue.28 In addition, saponification reactions occur between NaOCl and root canal organic matter through neutralisation reactions and chlorination reactions. Amino acid neutralisation reactions occur when NaOCl neutralises amino acids into brine by removing hydroxyl ions, thereby lowering the pH. Chlorination is a reaction between hypochlorous acid contained in NaOCl solution in contact with organic matter, ending in a hydrolysis process.29,30 The findings in this study corroborate earlier reports from Khallaf et al. (2020), who reported that the leaves extracts of moringa showed the least amount of smear layer on canal wall.31 These results support the traditional usage of the plant extracts as a smear layer removal agent.32
Both moringa leaves decoction (2.5% and 5.0%) and EDTA showed a similar ability to remove the smear layer. EDTA 17.0% has a chelating effect. The chelating effect on EDTA occurred because at high pH (alkaline), excess hydroxyl ions will prolong the decomposition of hydroxyapatite and limit the number of calcium ions available. Thus, a negatively charged chelating agent will bind positively charged calcium ions from enamel or dentin.33,34 Several researchers have reported that the chelating effect of EDTA use causes erosion of root canal walls due to hyperdecalcification. Therefore, the EDTA solution can be applied for a shorter time and in smaller volumes to minimise erosion.35,36
Conclusion
Within the limitations of this study, the alternating the use of moringa leaves decoction showed significantly better ability to remove the smear layer dentinal tubules compared to NaOCl 2.5% and EDTA 17.0%. Therefore, we recommend the possible use of moringa leaves decoction as an alternative to irrigant solution. However, additional long-term clinical investigations are required to verify these findings and assess their applicability to treatment outcomes.
Source of funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
The research protocol was approved by the Human Ethics Review Committee of the Faculty of Dentistry, Hasanuddin University (No 0032/PL.09/KEPK FKG-RSGM UNHAS/2018) on October 2nd, 2018.
Authors’ contributions
YY, JJN, ACT, and LM conducted the research and collected the data. The study was designed and supervised by NN and MM, who also validated the data and evaluated the drafts. CAR, who also reviewed the article, organised, examined, and analysed the data. NN and LM collected, collated, and reviewed the article. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
Acknowledgments
We thank the Conservative Laboratory, Faculty of Dentistry, Hasanuddin University and Biological Pharmacy Laboratory, Sekolah Tinggi Ilmu Makassar, for providing laboratory facilities.
Footnotes
Peer review under responsibility of Taibah University.
References
- 1.Wong J., Manoil D., Näsman P., Belibasakis G.N., Neelakantan P. Microbiological aspects of root canal infections and disinfection strategies: an update review on the current knowledge and challenges. Front Oral Health. 2021;2 doi: 10.3389/froh.2021.672887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gomes B., Herrera D. Etiologic role of root canal infection in apical periodontitis and its relationship with clinical symptomatology. Braz Oral Res. 2018;32(suppl):e69. doi: 10.1590/1807-3107bor-2018.vol32.0069. [DOI] [PubMed] [Google Scholar]
- 3.Kucher M., Dannemann M., Modler N., Hannig C., Weber M.-T. Effects of endodontic irrigants on material and surface properties of biocompatible thermoplastics. Dent J. 2019;7(1):e26. doi: 10.3390/dj7010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abuhaimed T.S., Abou Neel E.A. Sodium hypochlorite irrigation and its effect on bond strength to dentin. BioMed Res Int. 2017 doi: 10.1155/2017/1930360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Topbaş C., Adıgüzel Ö. Endodontic irrigation solutions: a review. Int Dent Res. 2017;7:54–61. doi: 10.5577/intdentres.2017.vol7.no3.2. [DOI] [Google Scholar]
- 6.Umerska A., Strandh M., Cassisa V., Matougui N., Eveillard M., Saulnier P. Synergistic effect of combinations containing EDTA and the antimicrobial peptide AA230, an arenicin-3 derivative, on Gram-negative bacteria. Biomolecules. 2018;8(4):e122. doi: 10.3390/biom8040122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Almadi E.M., Almohaimede A.A. Natural products in endodontics. Saudi Med J. 2018;39(2):124–130. doi: 10.15537/smj.2018.2.21038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vergara-Jimenez M., Almatrafi M.M., Fernandez M.L. Bioactive components in Moringa oleifera leaves protect against chronic disease. Antioxidants. 2017;6(4):e91. doi: 10.3390/antiox6040091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ervianingsih Mursyid M., Annisa R.N., Zahran I., Langkong J., Kamaruddin I. Antimicrobial activity of moringa leaf (Moringa oleifera L.) extract against the growth of Staphylococcus epidermidis. IOP Conf Ser Earth Environ Sci. 2019;343 doi: 10.1088/1755-1315/343/1/012145. [DOI] [Google Scholar]
- 10.Arévalo-Híjar L., Aguilar-Luis M.Á., Caballero-García S., Gonzáles-Soto N., Del Valle-Mendoza J. Antibacterial and cytotoxic effects of Moringa oleifera (Moringa) and Azadirachta indica methanolic extracts against strains of Enterococcus faecalis. Int J Dent. 2018 doi: 10.1155/2018/1071676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al_husnan L.A., Alkahtani M.D.F. Impact of Moringa aqueous extract on pathogenic bacteria and fungi in vitro. Ann Agric Sci. 2016;61(2):247–250. doi: 10.1016/j.aoas.2016.06.003. [DOI] [Google Scholar]
- 12.Jwa S.-K. Efficacy of Moringa oleifera leaf extracts against cariogenic biofilm. Prev Nutr Food Sci. 2019;24(3):308–312. doi: 10.3746/pnf.2019.24.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nugroho J.J., Trilaksana A.C., Natsir N., Rovani C.A., Asrianti A., Hikmah N. Cytotoxicity of 5% ethanol extracts of Moringa oleifera leaf as an alternative of root canal irrigant to fibroblast BHK-21 cell culture. J Dentomaxillofac Sci. 2021;6(1):39–41. doi: 10.15562/jdmfs.v6i1.1105. [DOI] [Google Scholar]
- 14.van den Berg J., Kuipers S. The antibacterial action of Moringa oleifera: a systematic review. South Afr J Bot. 2022;151:224–233. doi: 10.1016/j.sajb.2022.09.034. [DOI] [Google Scholar]
- 15.Gopalakrishnan L., Doriya K., Kumar D.S. Moringa oleifera: a review on nutritive importance and its medicinal application. Food Sci Hum Wellness. 2016;5(2):49–56. doi: 10.1016/j.fshw.2016.04.001. [DOI] [Google Scholar]
- 16.Gul R., Jan S.U., Faridullah S., Sherani S., Jahan N. Preliminary phytochemical screening, quantitative analysis of alkaloids, and antioxidant activity of crude plant extracts from Ephedra intermedia indigenous to Balochistan. Sci World J. 2017 doi: 10.1155/2017/5873648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ihwah A., Deoranto P., Wijana S., Dewi I.A. Comparative study between Federer and Gomez method for number of replication in complete randomized design using simulation: study of areca palm (Areca catechu) as organic waste for producing handicraft paper. IOP Conf Ser Earth Environ Sci. 2018;131 doi: 10.1088/1755-1315/131/1/012049. [DOI] [Google Scholar]
- 18.Tosco V., Monterubbianesi R., Aranguren J., Memè L., Putignano A., Orsini G. Evaluation of the efficacy of different irrigation systems on the removal of root canal smear layer: a scanning electron microscopic study. Appl Sci. 2023;13(1):e149. doi: 10.3390/app13010149. [DOI] [Google Scholar]
- 19.Raji P., Samrot A.V., Keerthana D., Karishma S. Antibacterial activity of alkaloids, flavonoids, saponins and tannins mediated green synthesised silver nanoparticles against Pseudomonas aeruginosa and Bacillus subtilis. J Cluster Sci. 2019;30(4):881–895. doi: 10.1007/s10876-019-01547-2. [DOI] [Google Scholar]
- 20.Othman L., Sleiman A., Abdel-Massih R.M. Antimicrobial activity of polyphenols and alkaloids in middle eastern plants. Front Microbiol. 2019;10:e911. doi: 10.3389/fmicb.2019.00911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Enerijiofi K.E., Akapo F.H., Erhabor J.O. GC–MS analysis and antibacterial activities of Moringa oleifera leaf extracts on selected clinical bacterial isolates. Bull Natl Res Cent. 2021;45(1):e179. doi: 10.1186/s42269-021-00640-9. [DOI] [Google Scholar]
- 22.Chhikara N., Kaur A., Mann S., Garg M.K., Sofi S., Panghal A. Bioactive compounds, associated health benefits and safety considerations of Moringa oleifera L.: an updated review. Nutr Food Sci. 2020;51(2):255–277. doi: 10.1108/NFS-03-2020-0087. [DOI] [Google Scholar]
- 23.Trigo C., Castelló M.L., Ortolá M.D., García-Mares F.J., Desamparados Soriano M. Moringa oleifera: an unknown crop in developed countries with great potential for industry and adapted to climate change. Foods. 2021;10(1):e31. doi: 10.3390/foods10010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baruwa A.O., Martins J.N.R., Maravic T., Mazzitelli C., Mazzoni A., Ginjeira A. Effect of endodontic irrigating solutions on radicular dentine structure and matrix metalloproteinases; A comprehensive review. Dent J. 2022;10(12):e219. doi: 10.3390/dj10120219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolumban A., Moldovan M., Ța I.A., Chifor I., Cuc S., Bud M., et al. An evaluation of the demineralizing effects of various acidic solutions. Appl Sci. 2021;11(17) doi: 10.3390/app11178270. [DOI] [Google Scholar]
- 26.Liao Y., Li Z., Zhou Q., Sheng M., Qu Q., Shi Y., et al. Saponin surfactants used in drug delivery systems: a new application for natural medicine components. Int J Pharm. 2021;603 doi: 10.1016/j.ijpharm.2021.120709. [DOI] [PubMed] [Google Scholar]
- 27.Rajput G., Pandya N., Soni D., Vala H., Modi J. Interfacial behaviour of saponin based surfactant for potential application in cleaning: Grenzflächenverhalten eines Tensids auf Saponinbasis für potenzielle Anwendung in der Reinigung. Tenside Surfact Det. 2021;58(2):146–152. doi: 10.1515/tsd-2020-2319. [DOI] [Google Scholar]
- 28.Holland R., Gomes J.E.F., Cintra L.T.A., Queiroz Í O.A., Estrela C. Factors affecting the periapical healing process of endodontically treated teeth. J Appl Oral Sci. 2017;25(5):465–476. doi: 10.1590/1678-7757-2016-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernandes Zancan R., Hadis M., Burgess D., Zhang Z.J., Di Maio A., Tomson P., et al. A matched irrigation and obturation strategy for root canal therapy. Sci Rep. 2021;11 doi: 10.1038/s41598-021-83849-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palazzi F., Blasi A., Mohammadi Z., Del Fabbro M., Estrela C. Penetration of sodium hypochlorite modified with surfactants into root canal dentin. Braz Dent J. 2016;27(2):208–216. doi: 10.1590/0103-6440201600650. [DOI] [PubMed] [Google Scholar]
- 31.Khallaf M.E., Kataia E.M., Aly Y., Omar N., Mohamed M.A. Cleanliness efficacy and effect on dentin microhardness of a novel plant extract irrigant. Bull Natl Res Cent. 2020;44(1):e47. doi: 10.1186/s42269-020-00311-1. [DOI] [Google Scholar]
- 32.Subbiya A., Roopchander K., Mahalakshmi K., Padmavathy K., Vivekanandan P. Bactericidal and smear layer removal efficacy of herbal alternatives against Enterococcus faecalis dentinal biofilm – an ex-vivo study. Pesqui Bras Odontopediatria Clin Integr. 2022;20:e5475. doi: 10.1590/pboci.2020.076. [DOI] [Google Scholar]
- 33.Ballal N.V., Jain H., Rao S., Johnson A.D., Baeten J., Wolcott J.F. Evaluation of SmearOFF, maleic acid and two EDTA preparations in smear layer removal from root canal dentin. Acta Odontol Scand. 2019;77(1):28–32. doi: 10.1080/00016357.2018.1495842. [DOI] [PubMed] [Google Scholar]
- 34.Chawhuaveang D.D., Yu O.Y., Yin I.X., Lam W.Y.H., Chu C.H. Topical agents for nonrestorative management of dental erosion: a narrative review. Healthcare (Basel) 2022;10(8):e1413. doi: 10.3390/healthcare10081413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elbahary S., Haj-yahya S., Khawalid M., Tsesis I., Rosen E., Habashi W., et al. Effects of different irrigation protocols on dentin surfaces as revealed through quantitative 3D surface texture analysis. Sci Rep. 2020;10 doi: 10.1038/s41598-020-79003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kfir A., Goldenberg C., Metzger Z., Hülsmann M., Baxter S. Cleanliness and erosion of root canal walls after irrigation with a new HEDP-based solution vs. traditional sodium hypochlorite followed by EDTA. A scanning electron microscope study. Clin Oral Invest. 2020;24(10):3699–3706. doi: 10.1007/s00784-020-03249-w. [DOI] [PubMed] [Google Scholar]