Abstract
The Keap1-Nrf2 pathway has been established as a therapeutic target for Alzheimer's disease (AD). Directly inhibiting the protein-protein interaction (PPI) between Keap1 and Nrf2 has been reported as an effective strategy for treating AD. Our group has validated this in an AD mouse model for the first time using the inhibitor 1,4-diaminonaphthalene NXPZ-2 with high concentrations. In the present study, we reported a new phosphodiester containing diaminonaphthalene compound, POZL, designed to target the PPI interface using a structure-based design strategy to combat oxidative stress in AD pathogenesis. Our crystallographic verification confirms that POZL shows potent Keap1-Nrf2 inhibition. Remarkably, POZL showed its high in vivo anti-AD efficacy at a much lower dosage compared to NXPZ-2 in the transgenic APP/PS1 AD mouse model. POZL treatment in the transgenic mice could effectively ameliorate learning and memory dysfunction by promoting the Nrf2 nuclear translocation. As a result, the oxidative stress and AD biomarker expression such as BACE1 and hyperphosphorylation of Tau were significantly reduced, and the synaptic function was recovered. HE and Nissl staining confirmed that POZL improved brain tissue pathological changes by enhancing neuron quantity and function. Furthermore, it was confirmed that POZL could effectively reverse Aβ-caused synaptic damage by activating Nrf2 in primary cultured cortical neurons. Collectively, our findings demonstrated that the phosphodiester diaminonaphthalene Keap1-Nrf2 PPI inhibitor could be regarded as a promising preclinical candidate of AD.
Keywords: Keap1-Nrf2, Protein-protein interaction, Inhibitor, Alzheimer's disease, Phosphodiester, Diaminonaphthalene
Graphical abstract
Highlights
-
•
POZL was developed by utilizing a phosphodiester moiety and crystallized with Keap1.
-
•
POZL ameliorated learning and memory dysfunction in transgenic APP/PS1 AD mice.
-
•
POZL effectively reversed Aβ caused synaptic damage by activating Nrf2 in neurons and AD mice.
1. Introduction
As an aging-related progressive neurodegenerative disease, more than 6.5 million people (age ≥65) are currently living or suffering with Alzheimer's disease (AD). According to U.S. data, the prevalence of AD is predicted to grow to 13.8 million by the year 2060 [1]. It is one of the major healthcare burdens jeopardizing patients and their families. Beyond that, AD cannot be clinically healed so far. As a 'time bomb' of the accelerated aging society, there is an urgent solution for AD therapy.
Symptomatically, AD is characterized by memory, language, and thinking problems [1]. Histopathologically, it is characterized by the overproduction/aggregation of beta-amyloid (Aβ) plaque, Tau-containing neurofibrillary tangles, neurite and synapse loss, and neuroinflammation, along with neurodegeneration and brain atrophy [2,3]. However, the exact mechanism of AD remains unidentified. Several hypotheses have been proposed to explain AD pathogenesis, such as mutations in amyloid precursor protein (APP), apolipoprotein E (ApoE) and presenilin (PS1, PS2) genes. These genes contribute to APP synthesis and proteolysis leading to the overproduction and aggregation of Aβ and the hyperphosphorylation of Tau protein [4,5]. Besides, the mitochondrial dysfunction, endoplasmic reticulum (ER) stress, and oxidative stress are also involved in progressive neuronal death and dysfunction associated with AD [[6], [7], [8]]. Among these, oxidative stress is recognized to be one of the earlier events and intermingled with all the AD pathogenic processes, causing neuronal dysfunction in AD [6,7,9,10]. It has been reported that cellular antioxidant capacity is diminished, and free radical generation is increased in both human and animal AD brains [11,12]. Therefore, combating oxidative stress by targeting associated proteins is a logically viable strategy for developing effective therapeutic approaches.
Nuclear factor erythroid 2-related factor 2 (Nrf2) is one of the critical transcription factors to protect cells from oxidative/electrophilic stress [13,14]. In the physiologically normal condition, Nrf2 binds to the Kelch-like ECH-associated protein 1 (Keap1), resulting in an inactive and degradable state of Nrf2. Upon stress condition by free radicals or electrophiles, Nrf2 releases from the Keap1-Nrf2 complex, translocates to the nucleus and binds the antioxidant response element (ARE). The transcription and expression of phase II detoxifying enzymes and antioxidants are then performed [14]. Therefore, abnormality in Nrf2 signaling results in intolerance to oxidative stress that is proposed to be involved in the pathogenesis of AD [[15], [16], [17]]. It is demonstrated that the Nrf2 expression in hippocampus is decreased in APPswe/PS1dE9 (APP/PS1) transgenic mice [18]. Spatial learning is improved by injection of lentiviral-vectored Nrf2 into the hippocampus of AD [19]. In the brain tissues of AD patients, mRNA, and protein levels of Nrf2, and the nuclear Nrf2 content are decreased compared with the normal brains [20]. Thus, genetic and pharmacological activation of Nrf2 expression could be the efficient strategies for the AD treatment.
To date, many covalent and non-covalent small molecules have been developed to activate Nrf2 [[21], [22], [23], [24]]. First, upon oxidative stress, Keap1 could be covalently modified at the cysteine residues by the electrophiles/chemicals. The modification leads to the conformational changes of Keap1, then Nrf2 is released, thus, allowing the accumulation and subsequent Nrf2 nuclear translocation for cell protection [17,25,26]. Accordingly, the electrophilic agents/compounds, such as sulforaphane (SFN), dimethyl fumarate (DMF), 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid-methylamide (CDDO-MA) and their derivatives, have been discovered to effectively activate Nrf2 and have beneficial effects in AD models [[27], [28], [29], [30]]. However, the covalent modification associated off-target cytotoxicity should be noted [25,31,32]. For instance, a phase III trial of the Nrf2 activator bardoxolone methyl (CDDO-Me) was terminated because of the cardiovascular events [33]. With this factor in mind, the direct inhibition of protein-protein interaction (PPI) between Keap1 and Nrf2 via a non-covalent binding is then alternatively developed to activate Nrf2. In the last 10 years, several classes of PPI inhibitors bearing 1,4-diaminonaphthalenes, 1,4-diphenyl-1,2,3-triazoles, tetrahydroisoquinolines, indoline derivatives, and phenylpropanoic acids, have been disclosed and exhibited marked activity [[23], [24], [25], [26],32,[34], [35], [36]].
NXPZ-2 (Fig. 1), an 1,4-diaminonaphthalene compound, was designed as a non-covalent Keap1-Nrf2 inhibitor by our group [37]. It has been confirmed the beneficial effect in an Aβ1-42-induced AD mouse model by activating Nrf2 for the first time. However, there are some problems unsolved for NXPZ-2, and its further development as a preclinical candidate is limited. First, the highly hydrophobic compound with a symmetric-like property possesses very low solubility, leading to a high in vivo dose of NXPZ-2 (210 mg/kg). Second, efficacy of the PPI inhibitor in transgenic AD models has not been tested. In this study, we designed and synthesized a new phosphodiester containing diaminonaphthalene compound, POZL, based on the crystal complex of Keap1 and NXPZ-2 (PDB code: 7XM2) via a structure-based strategy. The in vivo activity with a lower dosage and the anti-oxidative stress mechanism in the APP/PS1 transgenic mice were also evaluated.
Fig. 1.
The chemical structure of NXPZ-2 and the crystal complex with Keap1 (PDB code: 7XM2).
2. Materials and methods
2.1. Chemistry
The synthesis of POZL is presented in Scheme 1. The synthesis and characterizations of the other analogues could be found in Scheme S1, Supporting information. All solvents and starting materials, including anhydrous solvents and chemicals, were purchased from commercial vendors, and used without any further purification. TLC analysis was carried out on silica gel plates GF254 (Qingdao Haiyang Chemical, China). Column chromatography was carried out on silica gel 300∼400 mesh. Nuclear magnetic resonance (1H NMR, 13C NMR) spectra were recorded in DMSO-d6 on a Bruker Avance 400 spectrometer (Bruker Company, Germany). Chemical shifts (δ values) and coupling constants (J values) are given in ppm and Hz, respectively, using tetramethylsilane (TMS) as an internal standard. High-resolution mass spectrometer data were acquired using a quadrupole time-of-flight micro mass spectrometer. The final structures were fully characterized by 1H NMR, 13C NMR, and HRMS. The compound was purified to >95%, as determined by high-performance liquid chromatography (HPLC) analysis, prior to use in biological evaluation.
Scheme 1.
Reagents and conditions: (a) diethylphosphoric acetic acid, HATU, pyridine, 85 °C, 3h, 87%; (b) 2-bromoacetamide, K2CO3, KI, dry DMF, 60 °C, overnight, 71%.
To a solution of compound N1 (2.4 g, 4.96 mmol) [37,38] in pyridine (15 mL), HATU (3.77g, 1.04 mmol), and diethylphosphoacetic acid (1.19 mL, 7.44 mmol) were added at room temperature (Scheme 1). The reaction was continued in 85°C for 3 h and TLC showed the reaction was completed (DCM/MeOH = 30:1, Rf = 0.4). The mixture was diluted with EtOAc (50 mL) and washed with water (30 mL × 2). The organic layer was dried by Na2SO4, filtered, and the solvent was removed under reduced pressure, dried to give the crude compound N2 (2.86 g, 4.32 mmol, 87% yield) as a white solid, which was directly used in the next step. To a solution of compound 2 (1.26 g, 1.9 mmol) in dry DMF (8 mL), K2CO3 (1.05 g, 7.6 mmol), KI (157.7 mg, 0.95 mmol), and 2-bromoacetamide (919 mg, 1.05 mmol) were added at 50 °C overnight. TLC showed the reaction was completed (DCM/MeOH = 10:1, Rf = 0.2). Water (10 mL) was added to the reaction mixture, and diluted with ethyl acetate (50 mL) and washed with saturated brine (30 mL × 2). The organic layer was dried by Na2SO4, filtered, and the solvent was removed under reduced pressure. The crude residue was purified by flash column chromatography (DCM /MeOH = 20:1) on silica gel to afford the desired compound POZL (1.05 g, 0.18 mmol, 71.3% yield) as a white solid, mp: 191 - 192 °C. 1H NMR (400 MHz, DMSO-d6) δ 1.23 - 1.28 (m, 6H), 3.16 (dd, J = 21.64 Hz, J = 12.72 Hz, 2H), 3.86 (d, J = 22.00 Hz, 3H), 4.03 - 4.13 (m, 4H), 4.16 - 4.32 (m, 4H), 6.87 (dd, J = 18.80 Hz, J = 7.92 Hz, 1H), 6.95 - 7.07 (m, 4H), 7.13 (dt, J = 9.04 Hz, J = 2.96 Hz, 1H), 7.31 (d, J = 19.20 Hz, 2H), 7.54 - 7.64 (m, 6H), 7.72 (d, J = 8.92 Hz, 1H), 7.81 (d, J = 8.92 Hz, 1H), 8.16 - 8.23 (m, 1H), 8.28 - 8.33 (m, 1H), 10.57 (d, J = 29.80 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 168.8, 168.7, 163.8, 162.9,143.1, 143.0, 137.1, 136.8, 133.2, 133.0, 132.1, 131.5, 130.2, 130.0, 129.4, 129.1, 129.0, 126.5, 126.0, 125.8, 124.8, 124.7, 118.5, 118.4, 114.2, 114.2, 62.0, 61.9, 55.9, 55.7, 54.0, 36.7, 35.4, 16.3, 16.2. 31P NMR (202 MHz, DMSO-d6) δ 23.02. HRMS (ESI, positive) m/z calcd for C33H38N5O11PS2 [M+H]+: 776.1827; found 776.1842, HPLC analysis: retention time = 7.5 min; peak area, >95% (210, 254nm).
2.2. Biology
2.2.1. Fluorescent anisotropy assay
The equilibrium dissociation constants KD for POZL was determined using the fluorescence anisotropy assay with FITC−βAla−DEETGEF−OH as the fluorescence probe. The binding affinity of the probe was determined as KD1 in our previous studies [37,39,40]. The fluorescence anisotropy was measured on SpectraMax M5 microplate reader with 485 nm excitation and 535 nm emission after 60 min incubation at room temperature. Three independent experiments were performed.
2.2.2. X-ray crystallography of Keap1-POZL
Genes coding Keap1 Kelch domain (residues 322-609, E540A, E542A) was subcloned into pET28a with a N-terminal his tag. The plasmid was transformed into E. coli. Arctic ExpressTM(DE3) and induced using 0.25 mM β-d-thiogalactopyranoside at A600nm∼0.8. After 20h at 20°C, the cells were collected, homogenized in buffer containing 20 mM Tris-HCl (pH 8.0) and 500 mM NaCl, and lysed using a French press with two passes at 15000 p.s.i. Cell debris was removed by centrifugation. Then, the supernatant was loaded onto a Ni2+−NTA affinity column (Smart Lifesciences). After competitive wash with 20 mM Tris-HCl (pH 8.0), 25 mM imidazole, the protein was eluted from the column using 20 mM Tris-HCl (pH 8.0), 250 mM imidozale. After concentration, the protein was loaded onto a Superdex 200 column (Cytiva) equilibrated with gel filtration buffer (20 mM Tris (pH 8.0), 500 mM NaCl). The fractions of the protein peaks were collected and concentrated. The protein was incubated with the compound at a molar ration of 1:10 on ice for 30 min, and the sample was then set up for crystallization with the sitting-drop vapor diffusion method at 20 °C. Keap1-POZL crystals grew in the reservoir solution contained 4.0 M ammonium acetate, 0.1 M Tris-HCl, pH 8.8. Using mother liquor supplemented with 30% (v/v) glycerol as cryo-protectant, the crystals were frozen in liquid nitrogen before data collection. X-ray diffraction data were collected at BL10U2 and BL19U1 beamline of Shanghai Synchrotron Radiation Facility and processed using program XDS [41,42]. The structure was solved through molecular replacement using Phaser-MR in program PHENIX [43]. The structure refinement was carried out using PHENIX, and manual model building with Coot [44]. All the crystallographic information is summarized in Table S1.
2.2.3. The APP/PS1 AD mouse model and the treatment
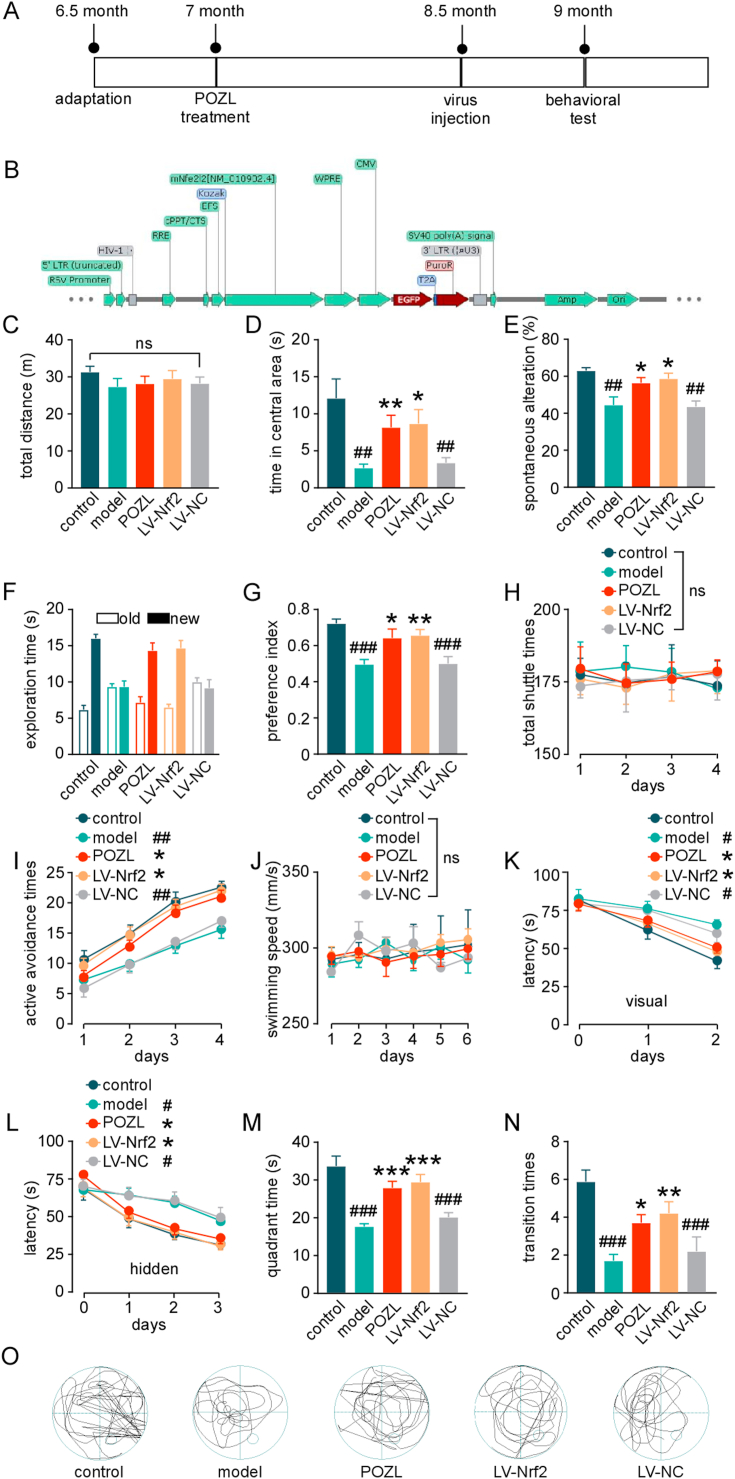
C57BL/6J mice and APP/PS1 mice were obtained from Shanghai Model Organisms Center, Inc. All animal procedures were approved by the Animal Care and Use Committees at China Pharmaceutical University (Nanjing, China), conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication, eighth Edition, 2011). In brief, forty-five male APP/PS1 mice aged 7 months were randomized into 3 groups, age-matched with non-transgene expressed wild type littermates (Fig. 3A): (1) control (C57BL/6J mice, solution, 3% Tween-80 + 0.5% CMC-Na +96.5% NS), (2) model (APP/PS1 mice, solution, 3% Tween-80 + 0.5% CMC-Na +96.5% NS), (3) POZL (APP/PS1 mice, POZL, 40 mg/kg, i.g., once daily for 60 days), (4) LV-Nrf2 (APP/PS1 mice, virus, LV-EFS > mNfe2l2-CMV > eGFP/T2A/Puro, 109 TU/ml, i.c.v.), and (5) LV-NC (APP/PS1 mice, LV-CMV > eGFP/T2A/Puro, 109 TU/ml, i.c.v, Fig. 3B). Green fluorescent protein (GFP)‐tagged lentiviral plasmids carrying a full‐length mouse Nrf2 cDNA or without the Nrf2 cDNA were purchased from Cyagen Biosciences (Fig. 3B). For positive control group, after 1.25% avertin (i.p., 250 mg/kg) anesthesia, the animals were set on the stereotaxic instrument. The bilateral hippocampus and cortex were located (anteroposterior = -2mm, mediolateral = 1.5 mm, and dorsoventral = 1.5 mm from Bregma), with reference to the Mice Brain in Stereotaxic Coordinates. Virus (5 μL) was injected into the bilateral hippocampus, using Hamilton micro-syringe. The injection lasted for 2 min, and the needle was left in for an additional 2 min. Fourteen days post-surgery, behavioral experiments were performed.
Fig. 3.
Effect of POZL on cognitive function in the transgenic APP/PS1 AD mouse model. (A) Temporal schematic diagram of the experimental procedures. (B) Vector map of Nrf2 over-expressed lentivirus. (C-D) Autonomic capacity test of mice using open field test, the effects of surgery and POZL (40 mg/kg) to the mice are shown as the total distance of the mice (C), anxiety-like behavior of mice using open field test shown as the central distance(D). (E) Spatial memory test of mice using Y-maze. (F-G) Exploration ability of mice using novel objective recognition. (F) Exploration time for new and old objects. old, exploration time of old object; new, exploration time of new object. (G) Preference index for new and old objects. (H) Learning and memory test of mice using active avoidance test. The total shuttle times (H), and the learning and memory are assessed by active avoidance times (I). (J-O) Spatial learning test of mice using Morris water maze. The swimming speed spatial during 6 days (J), the learning capacity are assessed by the Latency during the place navigation test with visual (K) and hidden platform (L), and the quadrant time(M) and transition times (N) during the probe trial. The trajectory on the sixth day of the place navigation test (O). *P < 0.05, **P < 0.01, ***P<0.001 vs model. #P < 0.05, ##P<0.01, ###P<0.001 vs control. Data are mean ± SEM (n = 15).
2.2.4. RNA sequencing
RNA samples from the hippocampus and cortex of the wide-type mice, APP/PS1 mice, and APP/PS1 mice treated with POZL (40 mg/kg, i.g., once daily for 60 days) were extracted separately from randomly selected individual samples. Three biological replicates were used for each group. Total RNA was extracted using the RNeasy Plus Kit (Meiji, Shanghai, China) and RNA concentration was determined using the NanoDrop 2000 (Thermo Fisher Scientific) according to the manufacturer's instructions. RNA-Seq, data generation, and normalization were performed by Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China).
2.2.5. Behavioral experiments
All behavioral tests were conducted during the light cycle with the mice aged 9 months. Before testing, mice were placed in the behavioral room for at least 30 min habituation. The investigator was blinded to the mice grouping during the behavioral experiment. The open-field test, active avoidance test, Morris water maze (MWM) test, novel object recognition (NOR), and Y maze were utilized following our previous protocols [37].
2.2.6. Histology
Organs were fixed with formalin and embedded in paraffin. 6-μm paraffin sections were cut and stained with hematoxylin and eosin (HE) [45]. For Nissl staining, 4-μm paraffin sections of the brains were cut, and performed according to the manufacturer's manual (Beijing Leagene Biotech, Beijing, China).
2.2.7. Biochemical assay
The anti-oxidative factors, including superoxide dismutase (SOD), and glutathione (GSSH), in serum, cortex and hippocampus were assayed using kits obtained from Jiancheng Bioengineering Co., Nanjing, Jiangsu, China, following the instructions. GSSH was further analyzed by LC-ESI-MS/MS assay [46].
2.2.8. Primary cortical neuron culture and cell viability
The primary cortical neuron culture was conducted as previously described [47]. Briefly, cerebral cortex tissues were isolated from mice on the 19th to 21st day of embryogenesis, and dissociated with 0.25% trypsin for 10 min at 37°C. Cells were pelleted by centrifugation at 500 rpm for 5 min. The dissociated cells were then plated in a 96-well plate pre-coated with poly-l-lysine (50 μg/ml) at a density of 1 × 106 cells/well. Complete culture medium was changed after 4 h, and the neurons were cultured for 7 days for the following experiments. Neurons were stimulated with Aβ (5 μM) for 24 h and then exposed to POZL (20 μM) or NXPZ-2 (20 μM) for 24 h. For the cell viability, neurons were exposed to POZL (ranged from 0 to 100 μM) for 24 h. CCK-8 (100 μL) solution was added to each well and incubated for 1 h, and the absorbance was measured at 470 nm. Cytotoxicity was represented as the cell viability rate.
2.2.9. Western blotting
Tissues obtained from the hippocampus and frontal cortex or primary cultured cortical neurons were placed in RIPA lysis buffer, containing a protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN, USA). Cytoplasmic and nuclear proteins were extracted from fresh tissues, following the kit instructions (Invent Biotechnologies. Inc., sc-003). Protein samples (30 μg) were separated with SDS-PAGE and transferred onto polyvinylidene difluoride (PVDF) membranes (Amersham Biosciences, Piscataway, NJ, USA). The membrane was blocked with blocking buffer (TBS, 0.1% Tween-20, 5% non-fat milk or 2% BSA), for 2 h at room temperature, and incubated with primary antibodies against Nrf2 (1:1000, Abcam, Cambridge, MA, USA), Keap1 (1:1000, Abcam, Cambridge, MA, USA), Aβ (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA, USA), Tau and p-Tau (ser396) (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA, USA), PSD95 (1:1000, Proteintech, Wuhan Sanying, Hubei, P.R.C), HO-1 (1:1000, Proteintech, Wuhan Sanying, Hubei, P.R.C), NQO1(1:1000, Proteintech, Wuhan Sanying, Hubei, P.R.C), GPX4 (1:1000, Proteintech, Wuhan Sanying, Hubei, P.R.C), NDP52 (1:1000, Proteintech, Wuhan Sanying, Hubei, P.R.C), BACE1 (1:1000, Proteintech, Wuhan Sanying, Hubei, P.R.C), β-actin (1:5000, Santa Cruz Biotechnology, Santa Cruz, CA, USA), Lamin B (1:1000, Abcam, Cambridge, MA, USA) and GAPDH (1:5000, Santa Cruz Biotechnology, Santa Cruz, CA, USA), at 4 °C overnight. The membrane was then incubated with horseradish peroxidase-conjugated goat anti-rabbit (1:10000, Abcam, Cambridge, MA, USA) and anti-mouse IgG (1:5000, Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 60 min and overnight at room temperature, respectively. The protein bands were visualized using an ECL kit (Applygen Technologies Inc, Beijing, China), and scanned by an enhanced chemiluminescence detection system (4200 Chemiluminescent Imaging System, Shanghai Tanon Science & Technology Co. Ltd, China). Relative protein expression levels were quantified by optical density analysis (Quantity-One software, Bio Rad Gel Doc 1000, Milan, Italy), and normalized to GAPDH or β-actin.
2.2.10. Electrophoretic mobility shift assay (EMSA)
To test the binding activity of Nrf2 protein with ARE, EMSA was performed using an EMSA Kit (Beyotime, Nanjing, China). Briefly, nuclear proteins were extracted from mouse brain using a cytoplasmic/nuclear protein extraction kit (KeyGene BioTECH, Nanjing, China). The nuclear extracts were incubated with labeled probes for 20 min at room temperature. ARE probe sequences are as follows: 5′-ACTGAGGGTGACTCAGCAAAATC-3′, 3′-TGACTCCCACTGAGTCGTT TTAG-5′. Unlabeled probe was used for competitive hybridization. The binding reactions (20 μL) were electrophoresed in 6.5% native polyacrylamide gel, and then electrotransferred onto a nylon membrane. The bands were visualized using Omega Lum G imaging system (Aplegen, San Francisco, CA, USA)
2.2.11. Immunofluorescence
For the confirmation, the cortical neurons were fixed with 4% paraformaldehyde for 10 min, then treated with 0.3% triton-X 100 solution for 15 min, blocked with 10% goat serum for 45 min. Then, the microtubule-associated protein-2 (MAP-2, 1:500, Santa Cruz Biotechnology, Santa Cruz, CA, USA) monoclonal antibody was added at 4 °C overnight. FITC-conjugated goat anti-rabbit IgG (1:200) was added in the next day, and the mixture was incubated at 37 °C for 1 h, washed for 3 times with PBS, stained with Hoechst for 1 min. DCFH-DA staining for analysis of ROS level were followed our previous study [40]. Detection of JC-1 mitochondrial membrane potential is performed by kit (Abcam, Cambridge, MA, USA) following instruction. The images were captured under fluorescence microscope (Leica, Wetzlar, Hesse-Darmstadt, Germany).
2.2.12. Patch clamp
The primary cortical neurons were seeded on slides treated with poly-l-lysine and cultured for 7 days. The cells were then stimulated with Aβ (5 μM) for 24 h. POZL (20 μM) were given to treat the neurons for another 24 h. After treatment, the cultured neurons were transferred to a chamber perfused with the extracellular solution and contained (in mM): NaCl, 117; KCl, 4.7; MgCl2, 1.2; NaH2PO4, 1.2; NaHCO3, 25; CaCl2, 2.5; d-glucose 10 (pH = 7.3). The recording electrodes of 3–7 MΩ were filled with solutions containing (in mM): K-gluconate, 140; MgCl2, 2; CaCl2, 2; Mg-ATP, 2; Na-GTP, 0.2; EGTA, 5; HEPES, 10 (pH = 7.4). The recorded current signals were amplified by axopatch-700B amplifier, and converted into digital signals by Axon DigiData1440A converter, filtered (1 KHz), collected and observed by Clamp10.4 software. In addition, 1 μM of TTX (block action potential) and 100 mM picrotoxin (blocking GABA receptors) were added to the extracellular solution for mEPSC recordings. All experiments were conducted at 27-28 °C.
2.2.13. Data analyses
Statistical analyses were performed using SPSS software (v13.0, SPSS Inc, Chicago, IL, USA). The quantitative data are expressed as means ± SEM. Statistical analysis was performed using one-way ANOVA, followed by the post hoc Bonferroni test, or General linear model with repeated measures, followed by Bonferroni test. P < 0.05 was considered statistically significant.
3. Results
3.1. Design of the unsymmetric diaminonaphthalene analogues discovers the potent phosphodiester compound POZL
Based on the crystal complex of Keap1 Kelch domain and NXPZ-2 that has been first determined by our group (PDB code: 7XM2, Fig. 1), the sulfones are observed to be critical to generate hydrogen bonds with surrounding residues of Keap1. The two symmetric aromatic rings form π-π packing interaction and are exposed to solvent. This side has been confirmed to be suitable for further chemical modification to decrease the symmetric property to address the low solubility recently [48]. Thus, in this study, different substitutions were introduced on one side of the sulfones or directly changed the benzosulfamide moiety. The newly designed compounds would not change the main interactions with the Keap1 binding pocket and the binding affinity with Keap1 protein was determined (Table 1). As a result, these unsymmetric bisulfonamides showed slightly lower activity than that of NXPZ-2, but still in the same order of magnitudes. Compound A2 with a 2-naphthalene, A4 with a 2-thiophene, A6 with a morpholine, A7 with a substituted methylthiazol, A8 with a methyl-1H-imidazole, A9 with a methyl-1H-pyrazole, A10 with a pentamethyl-2,3-dihydrobenzofuran, A11 with a dihydrobenzofuran had the Keap1-Nrf2 binding affinity with the KD values of 1304, 1109, 2066, 1433, 1153, 1143, 1275 nM, respectively. The phosphodiester compound POZL showed the best potency in this new series of analogues with the KD value of 940 nM. Compound A1 with a 1- naphthalene, A3 with a biphenyl, A5 with a chloropyridine, A12 with a 3,5-dimethylisoxazole, A13 with a methylbenzo[b]thiophene did not show apparent activity at 10 μM.
Table 1.
The Keap1-Nrf2 binding affinity of the unsymmetric bisulfonamides determined by the fluorescence anisotropy assay.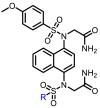 .
.
| Entry | R | KD (nM)a | Entry | R | KD (nM) |
|---|---|---|---|---|---|
| A1 |  |
>10000 | A7 | 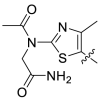 |
1433 |
| A2 | 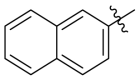 |
1304 | A8 | 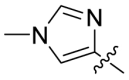 |
1153 |
| A3 | 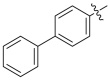 |
>10000 | A9 |  |
1143 |
| A4 | 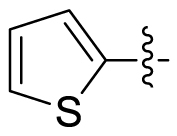 |
1109 | A10 | 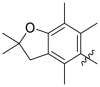 |
3270 |
| A5 | 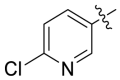 |
>10000 | A11 | 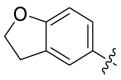 |
1275 |
| A6 | 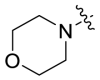 |
2066 | A12 | 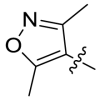 |
>10000 |
| POZL |  |
940 | A13 | 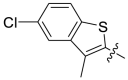 |
>10000 |
| NXPZ-2 |  |
230 |
Duplicated experiment and the average data.
3.2. Crystallographic study obtains the crystal complex of the potent phosphodiester compound POZL with Keap1 Kelch domain
To understand the molecular details of the interactions between Keap1 and the inhibitor, we determined a 2.7 Å resolution crystal structure of human Keap1 Kelch domain (amino acids 322-609) in complex with POZL (Fig. 2A&Table S1). Clear electron density is observed for most of NXPZ-2 part of POZL (Fig. 2F). This compound is built in two ways with about 50% occupancy for each conformation because NXPZ-2 part is symmetric and the density of adding group is very weak. Structure analysis result shows that the POZL binds Keap1 in the same pocket as previous compounds (Fig. 2C). There are many strong interactions between Keap1 and POZL which is consistent with the high affinity measured (Kd = 940 nM, Fig. 2B). Residues Arg 363, Arg 380, Asn 414, Arg 415, Ser 508, Ser 555 and Ser 602 form hydrogen bonds with POZL. Besides that, Tyr334, Tyr 525, Tyr 572 and Phe 577 residues interact with aromatic ring of the compound through π-π packing and Arg 415 forms a π-cation interaction with double aromatic ring region of the compound (Fig. 2D&E). Because our compound is very similar to NXPZ-2, we superimposed structures of Keap1-POZL and NXPZ-2 (Fig. 2E). Structure comparison shows that all the residues are involved in the Keap1-compound interactions and compounds are superposed very well. The group adding to NXPZ-2 is very flexible and only weak density can be observed (Fig. 2F).
Fig. 2.
(A) Chemical structure of POZL. (B) The binding affinity with Keap1 determined by a fluorescence anisotropy assay. (C-F) Structure of the Keap1-POZL complex (PDB code: 7XOT). (C) Overall structure of Keap1 with POZL. Keap1 is shown in gray ribbon and surface model with residues involved in protein- POZL interaction shown in green stick model. Compound is shown in blue stick model. (D) Interaction between Keap1 and POZL. Residues of Keap1 interacting with POZL is labeled and shown in green stick model. Hydrogen bond is shown with black dash. (E) Superimposition between structures of POZL and NXPZ-2 in complex with Keap1. Keap1-POZL is shown in green with Keap1-NXPZ-2 structure shown in gray. Interaction residues are shown in stick model with corresponding color. (F) Omit Fo-Fc electron density at 2.7 Å resolution for POZL, contoured at 3σ. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.3. POZL treatment improves the cognitive function in a transgenic APP/PS1 AD mouse model
The basic pharmacokinetic (PK) parameters of POZL in the male Han Wistar rat via single intravenous or oral treatment at a dose of 28 mg/kg (solution, 3% Tween-80 + 0.5% CMC-Na +96.5% NS) were determined. The results showed that the oral bioavailability was not very high but still acceptable in this compound series (F = 4.0119 ± 1.0067%) (Table S2). Besides, the male AD mice, established by injection of Aβ1-42 (410 pmol in 5 μL) into the bilateral hippocampus, were used to study the compound permeability in the brain. With single oral treatment at a dose of 40 mg/kg, animals were euthanized and brains were collected at different time point. 5 mice at each time point were used for the preliminary study of POZL on blood-brain barrier permeability. After a single treatment, the concentration of POZL in the brains continuously increased over time, reaching a peak with a concentration of 347.2 ± 160.3 ng/ml at 30 min. Subsequently, the concentration was decreased and reached 1.584 ± 0.2759 ng/ml at 6 h (Fig. S1). This result suggested that POZL can enter the central nervous system by passing through the blood-brain barrier. Thus, we assumed that the compound concentration in the brains can maintain for several hours and almost disappears within 6 h. The number of animals and significant individual differences might be the reasons for affecting the results.
The behavioral experiments were used to evaluate the effect of POZL treatment on a transgenic APP/PS1 mouse model. The mice were treated by POZL daily from 7- to 9-month-old before the behavioral test (Fig. 3A&B). In the open field experiment, the total observed distance in 5 min was not significantly different among the control, model, the intracerebroventricular (i.c.v.) injection of Nrf2 over-expressed lentivirus and POZL administration (40 mg/kg) groups (P > 0.05, Fig. 3C). The result suggested no influence on spontaneous activity of the POZL treatment or Nrf2 overexpression. Compared with the model group, the central distance of mice in the POZL administration group and the virus-treated group significantly increased (P<0.01, Fig. 3D). This result indicated that both POZL and Nrf2 overexpression can improve anxiety, behavior, and exploratory ability of AD mice. The learning and memory functions were evaluated by the classical Y maze test, active avoidance test, novel object recognition (NOR) test, and Morris water maze (MWM) test. The model mice showed statistically decreased spontaneous alternation, while it was increased in POZL-treated mice and Nrf2 lentivirus injected mice (P<0.05, P<0.05, Fig. 3E). In the NOR experiment, Nrf2 lentivirus injected mice and POZL-treated mice could prolong the exploration time for new objects (P<0.05, P<0.05, Fig. 3F) and the preference index was significantly increased (P<0.01, P<0.001, Fig. 3G). The result revealed that POZL could significantly improve AD mice's ability to explore novel objects. The active avoidance test showed the similar number of the total shuttle times among all groups (P>0.05, Fig. 3H) and a significantly increased number of active avoidance times in the virus group and POZL group (P<0.05, P<0.05, Fig. 3I), suggesting POZL can significantly enhance the ability of AD mice to actively avoid unfavorable stimuli as the virus. The classic behavioral experiment (MWM test) was performed to investigate the POZL treatment effects on spatial learning and memory (Fig. 3J-O). During the six day's experiment, there were no differences of the swimming speed between all groups (Fig. 3J), suggesting the normal athletic ability between each group. In the positioning navigation experiment, the latency of the model group was significantly longer than that of the control group, both in the visible-platform test (days 1–2) and hidden-platform test (days 3–5) (P<0.05, Fig. 3K&L). Compared with the control group, the percentage of the time spent in the target quadrant and number of platform crossing was significantly lower (P<0.05, P<0.01), indicating that the learning ability of the 9-month-old APP/PS1 AD mice was severely impaired by the treatment. With the POZL treatment or Nrf2 virus injection, the latency of the mice was significantly shortened (P<0.05, P<0.05, Fig. 3K&L), and the percentage of quadrant time and the transition times were significantly increased (P<0.01, P<0.01, Fig. 3M&N). The typical trajectory on the sixth day of the place navigation test was shown in Fig. 3O. All the above behavioral experiment results demonstrated that POZL improved the spatial learning and memory functions of AD mice.
3.4. POZL treatment rescues the brain structure damages of APP/PS1 AD mice with no obvious toxicity
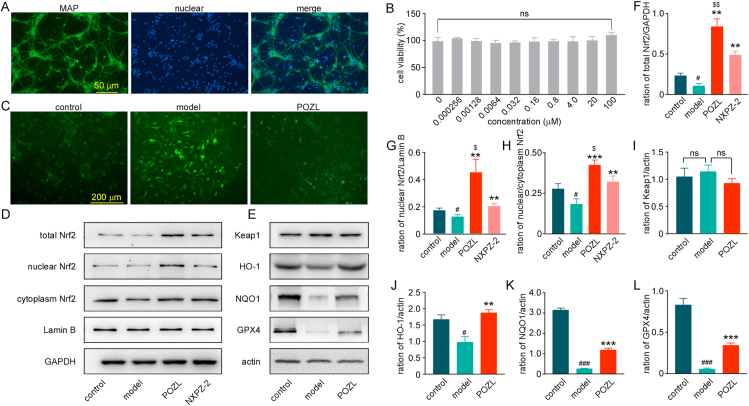
HE staining was performed to analyze brain cortex and hippocampus morphological changes of mice. The results showed that the hippocampus cell numbers were decreased, and morphology damage was demonstrated in the AD mouse model (Fig. 4A). However, cell number and morphology in the POZL-treated group were restored to different degrees. After treatment, the pyramidal cells were neatly arranged with a complete structure, without obvious cell swelling and increased number. Furthermore, Nissl staining, representing the neural characteristic structure, was used to reflect the neural state. Normally, Nissl bodies are big and abundant, indicating an active and strong neuronal protein synthesis. Conversely, when nerve cells are damaged, Nissl body numbers are reduced or even disappeared. The results showed that in CA1, CA3 and DG regions, dead neuron numbers in the POZL-treated or control groups were significantly lower than those in the AD model group (Fig. 4B and 4C). These data indicate that Nrf2 activation leads to improvement of the damaged hippocampal structure. Additionally, 2-month treatment of POZL showed no obvious effect on heart, liver, lung, spleen, and kidney of mice, indicating the safety profile and low toxicity of POZL (Fig. 4D), which was consistent with the in vitro cell viability results (see Fig. 8B).
Fig. 4.
Effect of POZL (40 mg/kg) on the organ structure. (A) HE staining of the brains. Scale bar = 100 μm. (B) Nissl staining of the brains. Scale bar = 100 μm. (C) Quantitative statistics of the Nissl staining in the CA1, CA3 and DG region, showed by the number of positive staining cells. (D) HE staining of the main organs, including heart, liver, lung, spleen, and kidney. Scale bar = 100 μm **P < 0.01, ***P<0.001 vs model. ##P < 0.01, ###P < 0.001 vs control. Data are mean ± SEM (n = 5).
Fig. 8.
Effect of POZL (20 μM) on the Keap1- Nrf2 pathway in the primary cultured neurons. (A) Identification of neurons stained with MAP-2 antibody (green) and Hoechst (blue). Scale bar = 50 μm. (B) Cell viability of POZL. (C) Immunofluorescence of primary cultured neurons under different culture conditions, stained with DCFH-DA (green). Scale bar = 200 μm. (D, F-H) The protein expression levels of total Nrf2, nuclear Nrf2, and cytoplasm Nrf2 at different culture conditions. (E, I-L) The protein expression levels of Keap1, HO-1, NQO, and GPX4 at different culture conditions. **P<0.01, ***P<0.001 vs model, #P<0.05, ###P<0.001 vs control. $ P<0.05, $$ P<0.01 vs NXPZ-2. Data are mean ± SEM (n = 3). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.5. POZL treatment decreases oxidative stress by activating Keap1-Nrf2 pathway in APP/PS1 AD mice
To identify the protective mechanism of POZL on the cognitive dysfunction, RNA-sequencing were performed on the hippocampus and cortex from the wide-type, APP/PS1, and POZL-treated APP/PS1 mice. Remarkably, many of Nrf2-targeted genes, such as NQO1, SOD, GPX4, were regulated in the hippocampus (Fig. 5A) and cortex (Fig. 5B) of AD mice, suggesting that the Nrf2 signaling pathway may play an essential role in the progression of AD. In addition, the GSH, an endogenous antioxidant, and SOD, a free radical scavenger [49], have been tested by a LC-ESI-MS/MS assay or kits for reflecting the state of oxidative stress. With the treatment of POZL or Nrf2 lentivirus, the activities of SOD (Fig. 5C–E) and GSH (Fig. 5F–K) were significantly recovered in the plasma, hippocampus, and frontal cortex, compared to the APP/PS1 model mice, indicating the decreasing of oxidative stress in vivo.
Fig. 5.
Effect of POZL (40 mg/kg) on the expression level of anti-oxidative proteins. The Heatmap of the expression of the common Nrf2 target genes in differentially expressed genes (DEGs) from hippocampus (A) and cortex (B) of the mice. (C-H) The concentrations of GSH and SOD in the serum (C, F), hippocampus (D, G) and frontal cortex (E, H) with kits. (I-K) The concentrations of GSH in the serum (I), hippocampus (J) and frontal cortex (K) with a LC-ESI-MS/MS assay. *P<0.05, **P<0.01, ***P<0.001 vs model. ##P<0.01, ###P<0.001 vs control. Data are mean ± SEM (n = 6–8).
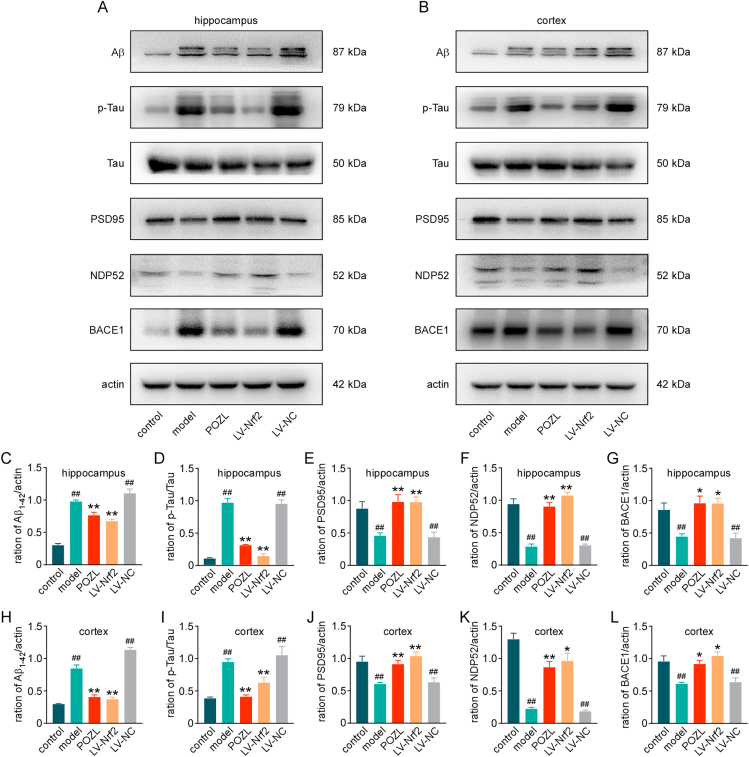
To characterize whether the protective effects of POZL on APP/PS1 mice was Keap1-Nrf2 mediated mechanism, the hippocampus and frontal cortex Nrf2 levels were determined (Fig. 6). Total Nrf2 expressions were decreased in both hippocampus (Fig. 6A and 6E) and frontal cortex (Fig. 6B and 6M) of AD mice, while POZL or Nrf2-overexpression increased the total Nrf2 expression levels. As expected, the Keap1 levels were recovered similar to those of the control groups (P> 0.05, NS, Fig. 6A, 6B, 6F, 6 N). Additionally, Nrf2-ARE binding was further assessed by electrophoretic mobility shift assay (EMSA). The results indicated that POZL treatment significantly increased Nrf2-ARE binding in both hippocampus and frontal cortex (Fig. 6C&D), suggesting the regulation of the downstream genes. As a transcription factor sensitive to redox, Nrf2 can regulate many detoxifying and antioxidant defense gene expressions, such as HO-1, NQO-1 and GPX4, to protect from oxidative stress [17]. POZL and Nrf2 lentivirus treatment significantly increased the protein expressions of HO-1, NQO-1 and GPX4 compared to the model group (Fig. 6G–I, 6O-Q), as well as the mRNA expression levels of these molecules (Fig. S2). Then, the Nrf2 cytoplasmic and nuclear proteins were extracted from fresh tissues clearly. As a result, both the nucleus protein levels of Nrf2 and the ratio of nuclear to cytoplasm Nrf2 level was markedly increased in POZL treatment group (Fig. 6A–B, Fig. 6J-L, 6R-T). The result identified Nrf2 nuclear translocation and activation of Nrf2-targeted genes by POZL treatment. What's more, POZL treatment also increased the expression levels of total Nrf2, and nuclear Nrf2 in the hippocampus and cortex in normal mice (Figs. S3A–B).
Fig. 6.
Effect of POZL (40 mg/kg) on the Keap1- Nrf2 pathway in the brain of mice. The protein expression levels of Nrf2, Keap1, HO-1, NQO, and GPX4 in the hippocampus (A, E-I) and frontal cortex (B, M-Q). (A, B, J-L, R-T) The Nrf2 nuclear translocation effect of POZL. The protein expression levels of nuclear Nrf2, cytoplasm Nrf2, Lamin B and GAPDH in the hippocampus (A, J-L) and frontal cortex (B, R-T). (C-D) Effects of POZL on the Nrf2-ARE binding affinity determined by an electrophoretic mobility shift assay (EMSA) in the hippocampus and cortex. *P<0.05, **P<0.01, ***P<0.001 vs model. #P<0.05, ##P<0.01 vs control. Data are mean ± SEM (n = 3).
3.6. POZL treatment decreases AD biomarkers in APP/PS1 AD mice
As the cognitive function improved and the Keap1-Nrf2 pathway activated in mice after POZL treatment, we tested the changes on AD biomarkers. Western blot results showed that both in the hippocampus and frontal cortex, Aβ and p-Tau levels were significantly decreased with POZL treatment and virus-induced Nrf2 overexpression (P<0.01, Fig. 7A–D, 7H, 7I). PSD95, a biomarker of synaptic plasticity, was markedly decreased in AD mice (P<0.01, Fig. 7A, 7B, 7E, 7J), suggesting improvement of synaptic function. The β-site APP-cleaving enzyme 1 (BACE1), an important β-secretase, has been recognized as a biomarker for AD diagnosis [50]. In transgenic APP/PS1 mice, abnormal (increased) expression of BACE1 in the brain can be detected from 6 months of age [51]. Nuclear dot protein 52 (NDP52), strongly induced by Nrf2, was scarcely detected in the hippocampal tissues of Nrf2 knockout mice [52]. Moreover, human NDP52 also decreases sarkosyl-insoluble tau in human AD cerebral cortex. In the present study, the significant increase of BACE1 level was confirmed, and the NDP52 level was significantly decreased in the 9-month APP/PS1 AD mice compared to the control normal mice (P<0.01, Fig. 7A, 7B, 7F, 7K). The NDP52 level was recovered remarkably after the Nrf2 lentivirus injection and a much higher improvement of NDP52 was observed in POZL treatment group (P<0.05, P<0.01, Fig. 7A, 7B, 7I, 7J). With 2-month daily treatment of POZL, the BACE1 level was markedly decreased as well as the Nrf2 lentivirus injection (P<0.05, Fig. 7A, 7B, 7G, 7L). Consequently, POZL not only ameliorated the cognitive dysfunction, but also reduced the expression level of AD biomarkers in mice.
Fig. 7.
Effect of POZL (40 mg/kg) on the expression level of AD marker proteins. The expression level of Aβ, Tau, p-Tau and PSD95, NDP52, and BACE1 in the hippocampus (A, C-G) and frontal cortex (B, H-L). *P<0.05, **P<0.01 vs model. ##P<0.01 vs control. Data are mean ± SEM (n = 3).
3.7. POZL treatment decreases oxidative stress by activating Keap1-Nrf2-ARE pathway in primary cultured cortical neurons
We further confirmed the mechanism in the primary cultured cortical neurons. First, the confirmation of primary cultured cortical neurons showed that the primary cultured cells were MAP-2-labeled green neuron cells. The purity of the neurons could reach higher than 90% after randomly selection (Fig. 8A). Then, with these pure neurons, the toxicity of POZL on primary cultured cortical neurons was evaluated. Neurons were viable at different POZL doses, even up to 100 μM (Fig. 8B), indicating no obvious cytotoxicity.
The oxidative stress, resulting in the excess reactive oxygen species (ROS), occupies a key role in the AD pathological mechanism [53]. It is confirmed that the Aβ1-42 (5 μM) treated primary cortical neurons significantly increased the ROS production, while POZL treatment reduced the production (Fig. 8C). To identify the mechanism, the changes of Keap1-Nrf2 pathway in primary cultured neurons were tested. Western blotting assay showed that the total Nrf2 and nuclear Nrf2 expression levels as well as ratio of nuclear Nrf2 level to cytoplasm Nrf2 were increased in POZL and NXPZ-2 treated neurons (Fig. 8D, 8F, 8G, 8H), indicating that the Nrf2 protein level was recovered by POZL or NXPZ-2 treatment as the in vivo assay. Moreover, under the same concentration, the total Nrf2, nuclear Nrf2 expression levels and Nrf2 translocation ratio were higher in POZL-treated groups than NXPZ-2 treated group (Fig. 8F-8H), suggesting that the higher potency of POZL than NXPZ-2. The Keap1 level of POZL treated cells was similar to that of the control group (Fig. 8E, 8I, P> 0.05, NS). For the Nrf2 downstream phase II enzymes HO-1, NQO1, and GPX4, the expression levels were significantly downregulated in Aβ1-42 treated model group compared to control group, and rescued by POZL treatment (Fig. 8E, 8J-L). All these data showed that POZL activated Keap1-Nrf2 pathway in primary cultured neurons, consistent with the results in transgenic AD model mice.
3.8. POZL treatment reduces the damage to primary cortical neurons by decreasing Nrf2-related oxidative stress
Besides ROS, the mitochondrial localization assay also revealed that Aβ1-42 could result in the loss of mitochondrial membrane potential (MMP) consistent with the increased intracellular ROS content as the carbonyl cyanide 3-chlorophenylhydrazone (CCCP, Fig. 9A). POZL treatment reduced oxidative stress damage, which further protected mitochondrial function, revealed by the increased MMP. We tested expression levels of AD biomarkers and found that POZL significantly decreased the p-Tau and BACE1 expression levels (Fig. 9B–D), as well as increased the NDP52 and PSD95 expressions (Fig. 9B, 9E-F), suggesting improvement of neurons damage. To further analyze the neuron synapse function, the electrophysiology of neurons was recorded under different conditions. Representative traces of micro excitatory postsynaptic currents (mEPSCs) recordings of the control, Aβ stimulation (5 μM, model) and POZL treatment group were shown in Fig. 9G. The Aβ stimulation for 24 h could reduce the mEPSC frequency (Fig. 9I), but not mEPSC amplitude (Fig. 9J) in primary neurons, indicating that synaptic plasticity was disrupted. With the POZL treatment, the damage caused by Aβ could be significantly reversed by increasing the mEPSC frequency but not amplitude. Therefore, POZL protected neuron function in vitro.
Fig. 9.
Effect of POZL (20 μM) on the function of primary cultured neurons. (A) Mitochondrial membrane potential under different culture conditions. (B-F) The expression levels of AD biomarkers, including Aβ, Tau, p-Tau and PSD95, NDP52, and BACE1 in the primary cultured neurons. (G) Spontaneous mEPSC were recorded in whole-cell patch-clamp configuration at a holding potential of -60 mV, in the presence of 500 nM TTX and 50 μM PTX. (H) Representative traces of mEPSC recordings. (I) Cumulative frequency distribution of mEPSC frequency. (J) Cumulative frequency distribution of mEPSC amplitude. *P<0.05, **P<0.01, ***P<0.001 vs model. ##P<0.01 ###P<0.001 vs control. Data are mean ± SEM (n = 3).
4. Discussion
For the design of these compounds, decreasing the symmetric property of the diaminonaphthalene is a strategy to improve the druggablilty, such as the development of Bicyclol [54]. Introducing different substitutions on one side of the sulfones obtained the unsymmetric diaminonaphthalenes, which has been confirmed as an efficient strategy for improve the in vivo property [38,48]. However, these compounds showed 3∼5-fold decreased activity compared with the parent compound NXPZ-2, probably due to the higher steric hindrance of these substitutions compared with the small amino group. Among them, POZL with a phosphodiester group on the amino side, exhibited the best Keap1-Nrf2 potency with a KD of 940 nM that should be acceptable for further biological evaluation. Besides, introducing the phosphate group into molecules is the classic drug design strategy to regulate the distribution coefficient and improve the drug-like property, which is suitable to address the drawbacks of NXPZ-2 [[55], [56], [57], [58]]. The phosphodiester has also been utilized in anti-AD compounds recently, showing promising activity in inhibiting Aβ aggregation [59]. The crystal complex of Keap1 with POZL (PDB code: 7XOT) further confirmed the binding mode between protein and compound similar to that of Keap1_NXPZ-2 complex (PDB code: 7XM2). The phosphodiester group is located at the solvent exposed site as expected (Fig. 2), validating our hypothesis.
The in vivo efficacy of NXPZ-2 has been for the first time validated in an Aβ1-42 oligomer intracerebroventricularly injected AD pathology-like mouse model and the administration dose of NXPZ-2 was extremely high to 210 mg/kg [37]. Nevertheless, this high dose reflected the low in vivo toxicity for the long-term administration. Unsymmetric optimizations of NXPZ-2 led to piperazinyl- or isothiocyanate- containing naphthalenesulfonamide compounds which showed satisfactory in vivo activity at much lower doses (20 mg/kg) for acute inflammations [38,48]. However, the anti-AD efficacy has not been evaluated in the study. Especially, the efficacy in the transgenic AD models of this scaffold has not been determined yet. Herein, in the APP/PS1 transgenic mice, POZL with a much lower dosage (40 mg/kg vs 210 mg/kg of NXPZ-2) showed significantly ameliorated learning and memory dysfunction for a two-month treatment. Meanwhile, a higher level of Nrf2 was activated in POZL treated primary cultured neurons compared with the same concentration of NXPZ-2, suggesting a higher potency of POZL. The toxicity was extremely low based on the mouse behavior and the cytotoxicity evaluation on primary cortical neurons. This is consistent with the detoxification property of the Keap1-Nrf2 inhibitors or Nrf2 activators to induce the phase II cytoprotective enzymes [60]. What's more, the pharmacokinetic profile and brain penetration ability of the naphthalenesulfonamide have been preliminarily investigated. To analyze the compound concentration in the blood, blood samples were collected continuously from each animal at different time points after a single treatment. Differently, to analyze the compound concentration in the brain, each animal was euthanized at specific time points after receiving a single treatment to collect brain samples. This method was used instead of continuous sampling in each animal. The results showed that POZL had a relatively short half-life when administered intravenously. The oral bioavailability of POZL was deemed acceptable. Although an increasing trend in compound concentration followed by a decline was demonstrated in brain, it is important to note that there were significant individual differences in brain permeability. These differences might be attributed to the limitations of the utilized experimental technique and the insufficient number of animals used in the study. Further in-depth and comprehensive studies with more animals would be conducted in the future, to address these limitations and provide an exact understanding of the compound's absorption, distribution, metabolism, and excretion. The result also supported that continuous administration for 2 to 3 months activated the Keap1-Nrf2 pathway persistently, and finally improved the cognitive impairment in APP/PS1 mice. The result suggested daily administration and the requirement of future structural modification and transformation or preparation technology improvement on POZL to elevate its bioavailability and blood-brain barrier permeability.
There have been already reported many Nrf2 activators in different activation mechanisms, such as sulforaphane (SFN), dimethyl fumarate (DMF), CDDO-methyl ester (CDDO-Me) and so on [17]. SFN is easily to covalently bind to cysteine residues in Keap1 protein, resulting in a conformational change of Keap1 and increased release of Nrf2 protein into the nucleus [61]. As an approved drug for the treatment of relapsing multiple sclerosis, DMF modifies the reactive cysteines in the BTB domain of Keap1 and thus activates Nrf2 transcription function [62]. CDDO-Me can interact with the methylcysteine residue of Keap1, inhibit the ubiquitination of Nrf2, increase the translocation of Nrf2 into the nucleus and combine with ARE to up-regulate the transcription of antioxidant genes [63]. Omaveloxolone (SKYCLARYS™) is an orally active, small molecule semi-synthetic triterpenoid drug that activated Nrf2 pathway by blocking the ubiquitination and degradation of Nrf2 [64]. In 2023, Omaveloxolone was approved for the treatment of Friedreich's ataxia in adults and adolescents aged ≥16 in USA, suggesting more considerations on the safety and efficacy of Nrf2 activators [65,66]. POZL acts in a different way on the Keap1-Nrf2 protein interaction interface, blocks their interaction, dissociates the interaction and activates Nrf2, which effectively avoids carcinogenesis and other safety problems caused by covalent binding [67,68]. As mentioned before, the in vitro cell viability results and the nearly 3-month in vivo treatment of POZL showed no obvious effect on the main organs of mice, indicating its safety profile and low toxicity in long-term medication.
For the mechanism, oxidative stress occurs in the early stage of AD pathology and is a common feature of many central nervous system diseases [17]. Studies have shown that oxidative stress may be one of the critical causes of AD. During the development of AD, Aβ plaque deposition and neurofibrillary tangles caused by hyperphosphorylation of tau protein are the two main features. Due to the presence of oxidative substances such as ROS, intracellular proteins undergo structural changes and accelerate Aβ plaque deposition and tau hyperphosphorylation. It has been reported that gene induction of Nrf2 ameliorates cognitive impairment in the App knock-in AD model mice by suppressing oxidative stress and neuroinflammation, suggesting that Nrf2 is an important therapeutic target of AD [69]. Here, Nrf2 gene induction by injection of over-expressed lentivirus in bilateral hippocampus of APP/PS1 mice was used as the positive control. After POZL treatment or Nrf2 gene induction in APP/PS1 mice, both total Nrf2 and Nrf2 translocation levels were increased in the frontal cortex and hippocampus, as well as the expression level of the downstream anti-oxidative genes, such as HO-1, NQO-1 and GPX-4. The downstream genes were reported to induce anti-oxidative stress related genes to protect neurons from the AD associated oxidative damage [7]. With the POZL treatment, the degree of oxidative stress (GSH, SOD) was decreased. In addition, few neurons with abnormal morphology were detected in both AD patient and animal brains [70]. The HE staining and Nissl staining both demonstrated that POZL treatment restored the cell number and morphology in the hippocampus of the transgenic mice. Much more live neurons in the POZL groups were observed than in the untreated AD groups. For AD biomarker expressions, Aβ and hyperphosphorylation of Tau were decreased after POZL treatment. BACE1 and NDP52 were also changed in neurons with POZL treatment. In the Aβ pathogenic hypothesis, Aβ plaques are secreted out of cells by APP through the action of cleavage enzyme, and then combined with many insoluble substances to form plaque-like precipitates. The cleavage enzyme BACE1 is the rate-limiting enzyme of this biological process. The expression of BACE1 has been implicated in the activity of Nrf2, and studies have shown that Nrf2 binds to anti-oxidative stress elements and inhibits the transcription of BACE1 [71]. Our results revealed that POZL treatment increased the expression and nuclear translocation of Nrf2, and further promoted the binding of Nrf2 with ARE element to represses the expression of BACE1. The decreased BACE1 expression level inhibited Aβ production to reduce Aβ induced neuron toxicity, which improved the cognitive deficits in AD mice. NDP52 expression is also regulated by Nrf2, belonging to the regulatory protein of autophagy process, whose gene promoter region contains three antioxidant response elements [52]. POZL activated Nrf2 translocation to increase NDP52 expression and further promote the degradation of hyperphosphorylation Tau.
The mechanism to activate Nrf2 and reduce oxidative stress was further confirmed in the primary cultured cortical neurons. As shown above, POZL ameliorated Aβ1-42 induced primary cultured neurons damage by activating Nrf2 expression and translocation to induce anti-oxidative enzyme transcription and reduce ROS production. Consistent with in vivo experiments, NDP52 expression increased and BACE1 expression decreased in POZL treated neurons, resulting in decreased expression of hyperphosphorylation Tau. As a result, a lower level of oxidative stress leads to recovery of MMP and synaptic plasticity. In a word, POZL activated anti-oxidative system through inhibiting the protein-protein interaction between Keap1 and Nrf2, to reduce the level of Aβ and hyperphosphorylation Tau, and protect neuron mitochondrial function and synaptic function, which further ameliorated the AD mice cognitive function.
5. Conclusion
In conclusion, we provided a novel unsymmetric diaminonaphthalene analogue POZL containing a phosphodiester group as a new Keap1-Nrf2 PPI inhibitor. This compound could be orally active to improve cognitive dysfunction and inhibit oxidative stress in the transgenic APP/PS1 AD mouse model. This study further demonstrated that Keap1-Nrf2 pathway is a viable therapeutic target of AD, and direct inhibition of the Keap1-Nrf2 PPI is a potential drug development strategy for AD treatment. Further optimizations by medicinal chemistry and drug design will be utilized to develop more efficient and phosphodiester diaminonaphthalene Keap1-Nrf2 PPI inhibitors.
Accession codes
Authors will release the atomic coordinates upon article publication. PDB code for POZL with Keap1: 7XOT.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was funded by grants from the National Natural Science Foundation of China (82022065and 81703520), the National Key R&D Program of China (2021YFA1302200); Shanghai “Shuguang” Project (21SG38), and the Key Research and Development Program of Ningxia (2019BFG02017). We thank Prof. Ke Xu at Tongji University and the staff members at SSRF BL10U2 and BL19U1 for their technical assistance in X-ray diffraction data collection.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2023.102793.
Contributor Information
Ling He, Email: heling92@hotmail.com.
Chunlin Zhuang, Email: zclnathan@163.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.2022 Alzheimer's disease facts and figures. Alzheimers Dement. 2022;18(4):700–789. doi: 10.1002/alz.12638. [DOI] [PubMed] [Google Scholar]
- 2.Selkoe D.J., Hardy J. The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol. Med. 2016;8(6):595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Strooper B., Karran E. The cellular phase of Alzheimer's disease. Cell. 2016;164(4):603–615. doi: 10.1016/j.cell.2015.12.056. [DOI] [PubMed] [Google Scholar]
- 4.Mazanetz M.P., Fischer P.M. Untangling tau hyperphosphorylation in drug design for neurodegenerative diseases. Nat. Rev. Drug Discov. 2007;6(6):464–479. doi: 10.1038/nrd2111. [DOI] [PubMed] [Google Scholar]
- 5.Hoogmartens J., Cacace R., Van Broeckhoven C. Insight into the genetic etiology of Alzheimer's disease: a comprehensive review of the role of rare variants. Alzheimers Dement (Amst) 2021;13(1) doi: 10.1002/dad2.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gella A., Durany N. Oxidative stress in Alzheimer disease. Cell Adhes. Migrat. 2009;3(1):88–93. doi: 10.4161/cam.3.1.7402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butterfield D.A., Boyd-Kimball D. Oxidative stress, amyloid-beta peptide, and altered key molecular pathways in the pathogenesis and progression of Alzheimer's disease. J Alzheimers Dis. 2018;62(3):1345–1367. doi: 10.3233/JAD-170543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X., Wang W., Li L., Perry G., Lee H.G., Zhu X. Oxidative stress and mitochondrial dysfunction in Alzheimer's disease. Biochim. Biophys. Acta. 2014;1842(8):1240–1247. doi: 10.1016/j.bbadis.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheignon C., Tomas M., Bonnefont-Rousselot D., Faller P., Hureau C., Collin F. Oxidative stress and the amyloid beta peptide in Alzheimer's disease. Redox Biol. 2018;14:450–464. doi: 10.1016/j.redox.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tramutola A., Lanzillotta C., Perluigi M., Butterfield D.A. Oxidative stress, protein modification and Alzheimer disease. Brain Res. Bull. 2017;133:88–96. doi: 10.1016/j.brainresbull.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Misrani A., Tabassum S., Yang L. Mitochondrial dysfunction and oxidative stress in Alzheimer's disease. Front. Aging Neurosci. 2021;13 doi: 10.3389/fnagi.2021.617588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jurcau A., Simion A. Oxidative stress in the pathogenesis of Alzheimer's disease and cerebrovascular disease with therapeutic implications. CNS Neurol. Disord.: Drug Targets. 2020;19(2):94–108. doi: 10.2174/1871527319666200303121016. [DOI] [PubMed] [Google Scholar]
- 13.Sies H., Jones D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020;21(7):363–383. doi: 10.1038/s41580-020-0230-3. [DOI] [PubMed] [Google Scholar]
- 14.Cuadrado A., Rojo A.I., Wells G., Hayes J.D., Cousin S.P., Rumsey W.L., Attucks O.C., Franklin S., Levonen A.L., Kensler T.W., Dinkova-Kostova A.T. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat. Rev. Drug Discov. 2019;18(4):295–317. doi: 10.1038/s41573-018-0008-x. [DOI] [PubMed] [Google Scholar]
- 15.Ren P., Chen J., Li B., Zhang M., Yang B., Guo X., Chen Z., Cheng H., Wang P., Wang S., Wang N., Zhang G., Wu X., Ma D., Guan D., Zhao R. Nrf2 ablation promotes Alzheimer's disease-like pathology in APP/PS1 transgenic mice: the role of neuroinflammation and oxidative stress. Oxid. Med. Cell. Longev. 2020;2020 doi: 10.1155/2020/3050971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang T., Zhang F. Targeting transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2) for the intervention of vascular cognitive impairment and dementia. Arterioscler. Thromb. Vasc. Biol. 2021;41(1):97–116. doi: 10.1161/ATVBAHA.120.314804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qu Z., Sun J., Zhang W., Yu J., Zhuang C. Transcription factor NRF2 as a promising therapeutic target for Alzheimer's disease. Free Radic. Biol. Med. 2020;159:87–102. doi: 10.1016/j.freeradbiomed.2020.06.028. [DOI] [PubMed] [Google Scholar]
- 18.Ramsey C.P., Glass C.A., Montgomery M.B., Lindl K.A., Ritson G.P., Chia L.A., Hamilton R.L., Chu C.T., Jordan-Sciutto K.L. Expression of Nrf2 in neurodegenerative diseases. J. Neuropathol. Exp. Neurol. 2007;66(1):75–85. doi: 10.1097/nen.0b013e31802d6da9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanninen K., Heikkinen R., Malm T., Rolova T., Kuhmonen S., Leinonen H., Yla-Herttuala S., Tanila H., Levonen A.L., Koistinaho M., Koistinaho J. Intrahippocampal injection of a lentiviral vector expressing Nrf2 improves spatial learning in a mouse model of Alzheimer's disease. Proc. Natl. Acad. Sci. U. S. A. 2009;106(38):16505–16510. doi: 10.1073/pnas.0908397106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kerr F., Sofola-Adesakin O., Ivanov D.K., Gatliff J., Gomez Perez-Nievas B., Bertrand H.C., Martinez P., Callard R., Snoeren I., Cocheme H.M., Adcott J., Khericha M., Castillo-Quan J.I., Wells G., Noble W., Thornton J., Partridge L. Direct Keap1-Nrf2 disruption as a potential therapeutic target for Alzheimer's disease. PLoS Genet. 2017;13(3) doi: 10.1371/journal.pgen.1006593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scott D.E., Bayly A.R., Abell C., Skidmore J. Small molecules, big targets: drug discovery faces the protein-protein interaction challenge. Nat. Rev. Drug Discov. 2016;15(8):533–550. doi: 10.1038/nrd.2016.29. [DOI] [PubMed] [Google Scholar]
- 22.Wilson A.J., Kerns J.K., Callahan J.F., Moody C.J. Keap calm, and carry on covalently. J. Med. Chem. 2013;56(19):7463–7476. doi: 10.1021/jm400224q. [DOI] [PubMed] [Google Scholar]
- 23.Pallesen J.S., Narayanan D., Tran K.T., Solbak S.M.O., Marseglia G., Sorensen L.M.E., Hoj L.J., Munafo F., Carmona R.M.C., Garcia A.D., Desu H.L., Brambilla R., Johansen T.N., Popowicz G.M., Sattler M., Gajhede M., Bach A. Deconstructing noncovalent Kelch-like ECH-associated protein 1 (Keap1) inhibitors into fragments to reconstruct new potent compounds. J. Med. Chem. 2021;64(8):4623–4661. doi: 10.1021/acs.jmedchem.0c02094. [DOI] [PubMed] [Google Scholar]
- 24.Mou Y., Wen S., Li Y.X., Gao X.X., Zhang X., Jiang Z.Y. Recent progress in Keap1-Nrf2 protein-protein interaction inhibitors. Eur. J. Med. Chem. 2020;202 doi: 10.1016/j.ejmech.2020.112532. [DOI] [PubMed] [Google Scholar]
- 25.Lu M.C., Ji J.A., Jiang Z.Y., You Q.D. The Keap1-Nrf2-ARE pathway as a potential preventive and therapeutic target: an update. Med. Res. Rev. 2016;36(5):924–963. doi: 10.1002/med.21396. [DOI] [PubMed] [Google Scholar]
- 26.Zhuang C., Wu Z., Xing C., Miao Z. Small molecules inhibiting Keap1-Nrf2 protein-protein interactions: a novel approach to activate Nrf2 function. Medchemcomm. 2017;8(2):286–294. doi: 10.1039/c6md00500d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McBean G.J., Lopez M.G., Wallner F.K. Redox-based therapeutics in neurodegenerative disease. Br. J. Pharmacol. 2017;174(12):1750–1770. doi: 10.1111/bph.13551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee C., Park G.H., Lee S.R., Jang J.H. Attenuation of beta-amyloid-induced oxidative cell death by sulforaphane via activation of NF-E2-related factor 2. Oxid. Med. Cell. Longev. 2013;2013 doi: 10.1155/2013/313510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campolo M., Casili G., Lanza M., Filippone A., Paterniti I., Cuzzocrea S., Esposito E. Multiple mechanisms of dimethyl fumarate in amyloid beta-induced neurotoxicity in human neuronal cells. J. Cell Mol. Med. 2018;22(2):1081–1094. doi: 10.1111/jcmm.13358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dumont M., Wille E., Calingasan N.Y., Tampellini D., Williams C., Gouras G.K., Liby K., Sporn M., Nathan C., Flint Beal M., Lin M.T. Triterpenoid CDDO-methylamide improves memory and decreases amyloid plaques in a transgenic mouse model of Alzheimer's disease. J. Neurochem. 2009;109(2):502–512. doi: 10.1111/j.1471-4159.2009.05970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prestera T., Holtzclaw W.D., Zhang Y., Talalay P. Chemical and molecular regulation of enzymes that detoxify carcinogens. Proc. Natl. Acad. Sci. U. S. A. 1993;90(7):2965–2969. doi: 10.1073/pnas.90.7.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Magesh S., Chen Y., Hu L. Small molecule modulators of Keap1-Nrf2-ARE pathway as potential preventive and therapeutic agents. Med. Res. Rev. 2012;32(4):687–726. doi: 10.1002/med.21257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Zeeuw D., Akizawa T., Audhya P., Bakris G.L., Chin M., Christ-Schmidt H., Goldsberry A., Houser M., Krauth M., Lambers Heerspink H.J., McMurray J.J., Meyer C.J., Parving H.H., Remuzzi G., Toto R.D., Vaziri N.D., Wanner C., Wittes J., Wrolstad D., Chertow G.M., Investigators B.T. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N. Engl. J. Med. 2013;369(26):2492–2503. doi: 10.1056/NEJMoa1306033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang Z.Y., Lu M.C., You Q.D. Discovery and development of Kelch-like ECH-associated protein 1. Nuclear factor erythroid 2-related factor 2 (KEAP1:NRF2) protein-protein interaction inhibitors: achievements, challenges, and future directions. J. Med. Chem. 2016;59(24):10837–10858. doi: 10.1021/acs.jmedchem.6b00586. [DOI] [PubMed] [Google Scholar]
- 35.Zhuang C., Miao Z., Sheng C., Zhang W. Updated research and applications of small molecule inhibitors of Keap1-Nrf2 protein-protein interaction: a review. Curr. Med. Chem. 2014;21(16):1861–1870. doi: 10.2174/0929867321666140217104648. [DOI] [PubMed] [Google Scholar]
- 36.Zhuang C., Narayanapillai S., Zhang W., Sham Y.Y., Xing C. Rapid identification of Keap1-Nrf2 small-molecule inhibitors through structure-based virtual screening and hit-based substructure search. J. Med. Chem. 2014;57(3):1121–1126. doi: 10.1021/jm4017174. [DOI] [PubMed] [Google Scholar]
- 37.Sun Y., Huang J., Chen Y., Shang H., Zhang W., Yu J., He L., Xing C., Zhuang C. Direct inhibition of Keap1-Nrf2 Protein-Protein interaction as a potential therapeutic strategy for Alzheimer's disease. Bioorg. Chem. 2020;103 doi: 10.1016/j.bioorg.2020.104172. [DOI] [PubMed] [Google Scholar]
- 38.Zhang L., Xu L., Chen H., Zhang W., Xing C., Qu Z., Yu J., Zhuang C. Structure-based molecular hybridization design of Keap1-Nrf2 inhibitors as novel protective agents of acute lung injury. Eur. J. Med. Chem. 2021;222 doi: 10.1016/j.ejmech.2021.113599. [DOI] [PubMed] [Google Scholar]
- 39.Zhuang C., Narayanapillai S., Zhang W., Sham Y.Y., Xing C. Rapid identification of Keap1-Nrf2 small-molecule inhibitors through structure-based virtual screening and hit-based substructure search. J. Med. Chem. 2014;57(3):1121–1126. doi: 10.1021/jm4017174. [DOI] [PubMed] [Google Scholar]
- 40.Meng N., Tang H., Zhang H., Jiang C., Su L., Min X., Zhang W., Zhang H., Miao Z., Zhang W., Zhuang C. Fragment-growing guided design of Keap1-Nrf2 protein-protein interaction inhibitors for targeting myocarditis. Free Radic. Biol. Med. 2018;117:228–237. doi: 10.1016/j.freeradbiomed.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 41.Kabsch W. Integration, scaling, space-group assignment and post-refinement. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 2):133–144. doi: 10.1107/S0907444909047374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kabsch W. Xds. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 2):125–132. doi: 10.1107/S0907444909047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adams P.D., Grosse-Kunstleve R.W., Hung L.W., Ioerger T.R., McCoy A.J., Moriarty N.W., Read R.J., Sacchettini J.C., Sauter N.K., Terwilliger T.C. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr D Biol Crystallogr. 2002;58(Pt 11):1948–1954. doi: 10.1107/s0907444902016657. [DOI] [PubMed] [Google Scholar]
- 44.Emsley P., Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60(Pt 12 Pt 1):2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 45.Zhu J., Xin M., Xu C., He Y., Zhang W., Wang Z., Zhuang C. Ligand-based substituent-anchoring design of selective receptor-interacting protein kinase 1 necroptosis inhibitors for ulcerative colitis therapy. Acta Pharm. Sin. B. 2021;11(10):3193–3205. doi: 10.1016/j.apsb.2021.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen B., Chen C., Hu K., Li X., Kang D., Li H., Zhu Z., Yin X., Xu Y., Shen J., Guo H., Xie L., Wang G., Liang Y. Activated charcoal significantly improved the reliability of methods for quantitative analysis of endogenous substances in biological specimens: glutathione and cysteine as cases. J. Chromatogr., B: Anal. Technol. Biomed. Life Sci. 2018;1095:241–250. doi: 10.1016/j.jchromb.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 47.Sciarretta C., Minichiello L. The preparation of primary cortical neuron cultures and a practical application using immunofluorescent cytochemistry. Methods Mol. Biol. 2010;633:221–231. doi: 10.1007/978-1-59745-019-5_16. [DOI] [PubMed] [Google Scholar]
- 48.Liu G., Hou R., Xu L., Zhang X., Yan J., Xing C., Xu K., Zhuang C. Crystallography-Guided optimizations of the Keap1-Nrf2 inhibitors on the solvent exposed region: from symmetric to asymmetric naphthalenesulfonamides. J. Med. Chem. 2022;65(12):8289–8302. doi: 10.1021/acs.jmedchem.2c00170. [DOI] [PubMed] [Google Scholar]
- 49.Wang H., Wei W., Lan X., Liu N., Li Y., Ma H., Sun T., Peng X., Zhuang C., Yu J. Neuroprotective effect of Swertiamain on cerebral Ischemia/reperfusion injury by inducing the Nrf2 protective pathway. ACS Chem. Neurosci. 2019;10(5):2276–2286. doi: 10.1021/acschemneuro.8b00605. [DOI] [PubMed] [Google Scholar]
- 50.Decourt B., Sabbagh M.N. BACE1 as a potential biomarker for Alzheimer's disease. J Alzheimers Dis. 2011;24(Suppl 2):53–59. doi: 10.3233/JAD-2011-110017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li L., Luo J., Chen D., Tong J.B., Zeng L.P., Cao Y.Q., Xiang J., Luo X.G., Shi J.M., Wang H., Huang J.F. BACE1 in the retina: a sensitive biomarker for monitoring early pathological changes in Alzheimer's disease. Neural Regen Res. 2016;11(3):447–453. doi: 10.4103/1673-5374.179057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jo C., Gundemir S., Pritchard S., Jin Y.N., Rahman I., Johnson G.V. Nrf2 reduces levels of phosphorylated tau protein by inducing autophagy adaptor protein NDP52. Nat. Commun. 2014;5:3496. doi: 10.1038/ncomms4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martin E., Boucher C., Fontaine B., Delarasse C. Distinct inflammatory phenotypes of microglia and monocyte-derived macrophages in Alzheimer's disease models: effects of aging and amyloid pathology. Aging Cell. 2017;16(1):27–38. doi: 10.1111/acel.12522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu G.T. Bicyclol: a novel drug for treating chronic viral hepatitis B and C. Med. Chem. 2009;5(1):29–43. doi: 10.2174/157340609787049316. [DOI] [PubMed] [Google Scholar]
- 55.Ji X., Wang J., Zhang L., Zhao L.X., Jiang H.L., Liu H. [Application of phosphates and phosphonates prodrugs in drug research and development] Yao Xue Xue Bao. 2013;48(5):621–634. [PubMed] [Google Scholar]
- 56.Hecker S.J., Erion M.D. Prodrugs of phosphates and phosphonates. J. Med. Chem. 2008;51(8):2328–2345. doi: 10.1021/jm701260b. [DOI] [PubMed] [Google Scholar]
- 57.Mucha A., Kafarski P., Berlicki L. Remarkable potential of the alpha-aminophosphonate/phosphinate structural motif in medicinal chemistry. J. Med. Chem. 2011;54(17):5955–5980. doi: 10.1021/jm200587f. [DOI] [PubMed] [Google Scholar]
- 58.Huang X., Huang R., Gou S., Wang Z., Wang H. Anticancer platinum(IV) prodrugs containing monoaminophosphonate ester as a targeting group inhibit matrix metalloproteinases and reverse multidrug resistance. Bioconjugate Chem. 2017;28(4):1305–1323. doi: 10.1021/acs.bioconjchem.7b00117. [DOI] [PubMed] [Google Scholar]
- 59.Romanucci V., Giordano M., De Tommaso G., Iuliano M., Bernini R., Clemente M., Garcia-Vinuales S., Milardi D., Zarrelli A., Di Fabio G. Synthesis of new tyrosol-based phosphodiester derivatives: effect on amyloid beta aggregation and metal chelation ability. ChemMedChem. 2021;16(7):1172–1183. doi: 10.1002/cmdc.202000807. [DOI] [PubMed] [Google Scholar]
- 60.Johnson D.A., Johnson J.A. Nrf2--a therapeutic target for the treatment of neurodegenerative diseases. Free Radic. Biol. Med. 2015;88(Pt B):253–267. doi: 10.1016/j.freeradbiomed.2015.07.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Horie Y., Suzuki T., Inoue J., Iso T., Wells G., Moore T.W., Mizushima T., Dinkova-Kostova A.T., Kasai T., Kamei T., Koshiba S., Yamamoto M. Molecular basis for the disruption of Keap1-Nrf2 interaction via Hinge & Latch mechanism. Commun Biol. 2021;4(1):576. doi: 10.1038/s42003-021-02100-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Unni S., Deshmukh P., Krishnappa G., Kommu P., Padmanabhan B. Structural insights into the multiple binding modes of Dimethyl Fumarate (DMF) and its analogs to the Kelch domain of Keap1. FEBS J. 2021;288(5):1599–1613. doi: 10.1111/febs.15485. [DOI] [PubMed] [Google Scholar]
- 63.Wang Y.Y., Yang Y.X., Zhe H., He Z.X., Zhou S.F. Bardoxolone methyl (CDDO-Me) as a therapeutic agent: an update on its pharmacokinetic and pharmacodynamic properties. Drug Des. Dev. Ther. 2014;8:2075–2088. doi: 10.2147/DDDT.S68872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lynch D.R., Chin M.P., Boesch S., Delatycki M.B., Giunti P., Goldsberry A., Hoyle J.C., Mariotti C., Mathews K.D., Nachbauer W., O'Grady M., Perlman S., Subramony S.H., Wilmot G., Zesiewicz T., Meyer C.J. Efficacy of Omaveloxolone in Friedreich's ataxia: delayed-start analysis of the MOXIe extension. Mov. Disord. 2023;38(2):313–320. doi: 10.1002/mds.29286. [DOI] [PubMed] [Google Scholar]
- 65.Lynch D.R., Chin M.P., Delatycki M.B., Subramony S.H., Corti M., Hoyle J.C., Boesch S., Nachbauer W., Mariotti C., Mathews K.D., Giunti P., Wilmot G., Zesiewicz T., Perlman S., Goldsberry A., O'Grady M., Meyer C.J. Safety and efficacy of Omaveloxolone in Friedreich ataxia (MOXIe study) Ann. Neurol. 2021;89(2):212–225. doi: 10.1002/ana.25934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee A. Omaveloxolone: first approval. Drugs. 2023;83(8):725–729. doi: 10.1007/s40265-023-01874-9. [DOI] [PubMed] [Google Scholar]
- 67.El-Deek H.E.M., Ahmed A.M., Mohammed R.A.A. Aberration of Nrf2-Bach1 pathway in colorectal carcinoma; role in carcinogenesis and tumor progression. Ann. Diagn. Pathol. 2019;38:138–144. doi: 10.1016/j.anndiagpath.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 68.Sporn M.B., Liby K.T. NRF2 and cancer: the good, the bad and the importance of context. Nat. Rev. Cancer. 2012;12(8):564–571. doi: 10.1038/nrc3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Uruno A., Matsumaru D., Ryoke R., Saito R., Kadoguchi S., Saigusa D., Saito T., Saido T.C., Kawashima R., Yamamoto M. Nrf2 suppresses oxidative stress and inflammation in App knock-in Alzheimer's disease model mice. Mol. Cell Biol. 2020;40(6) doi: 10.1128/MCB.00467-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Blennerhassett R., Lillo P., Halliday G.M., Hodges J.R., Kril J.J. Distribution of pathology in frontal variant Alzheimer's disease. J Alzheimers Dis. 2014;39(1):63–70. doi: 10.3233/JAD-131241. [DOI] [PubMed] [Google Scholar]
- 71.Bahn G., Park J.S., Yun U.J., Lee Y.J., Choi Y., Park J.S., Baek S.H., Choi B.Y., Cho Y.S., Kim H.K., Han J., Sul J.H., Baik S.H., Lim J., Wakabayashi N., Bae S.H., Han J.W., Arumugam T.V., Mattson M.P., Jo D.G. NRF2/ARE pathway negatively regulates BACE1 expression and ameliorates cognitive deficits in mouse Alzheimer's models. Proc. Natl. Acad. Sci. U. S. A. 2019;116(25):12516–12523. doi: 10.1073/pnas.1819541116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.