Abstract
Background
Patients often have high expectations for recovery after critical illness, but the impact of these expectations on subsequent quality of life (QoL) after serious illnesses has not been evaluated empirically.
Research Question
Among adult survivors of acute respiratory failure (ARF), are met vs unmet expectations for health associated with self-reported QoL 6 months after discharge?
Study Design and Methods
This was a prospective longitudinal cohort study enrolling consecutive adult patients with ARF managed in ICUs at five academic medical centers. At hospital discharge, we evaluated participants’ expected health 6 months in the future via a visual analog scale (VAS; range, 0-100), with higher scores representing better expected health. At 6-month follow-up, perceived health was assessed using the EQ-5D VAS, and QoL was assessed using the World Health Organization Quality of Life Brief Version (WHOQOL-BREF) instrument. Participants’ health expectations were categorized as having been met when perceived health at 6 months was no more than eight points lower than their expectation at study enrollment. The primary analysis compared WHOQOL-BREF domain scores (range, 0-100) at 6 months after discharge in patients with met vs unmet health expectations using the nonparametric Mann-Whitney U test. Secondary analysis modeled WHOQOL-BREF domain scores using multivariate regression, and sensitivity analyses assessed QoL using EQ-5D-5L index values.
Results
In the primary analysis, QoL was significantly better among participants with met vs unmet health expectations across all domains of the WHOQOL-BREF: physical health (estimated difference in scores: median, 19 [interquartile range (IQR), 12-15]; P < .001), psychological health (median, 12 [IQR, 6-18]; P < .001), social relationships (median, 6 [IQR, 0-13]; P = .02), and environmental health (median, 12 [IQR, 6-13]; P < .001). In multivariate regression, the difference between expected and perceived health remained associated significantly with the physical health domain score.
Interpretation
Fulfillment of health expectations is associated with better QoL after ARF, suggesting a mechanism underpinning successful ICU recovery programs that incorporate normalization and expectation management.
Key Words: critical care outcomes, functional status, quality of life, respiratory distress syndrome, survivorship
Graphical Abstract
Take-home Points.
Study Question: Is there an association between health expectations and self-reported quality of life among adult survivors of acute respiratory failure?
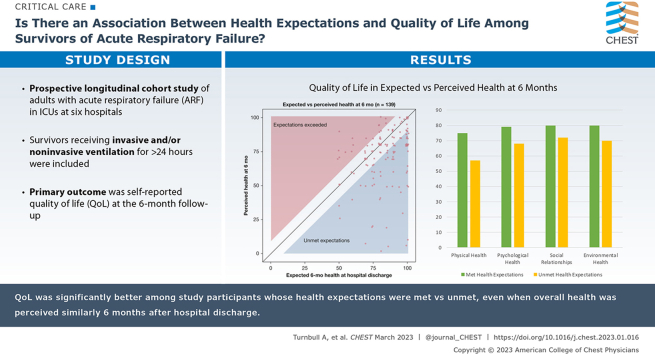
Results: Quality of life was significantly better among study participants whose health expectations were met vs unmet, even when overall health was perceived similarly 6 months after hospital discharge.
Interpretation: The observed association between expectation fulfillment and quality of life may explain the success of ICU recovery programs that incorporate normalization and expectation management.
As a result of clinical advances, most critically ill patients with acute respiratory failure (ARF) survive hospitalization.1 Declining ARF mortality rates have shifted attention to the long-term morbidities commonly experienced by the growing numbers of survivors.2, 3, 4, 5, 6, 7, 8 In response, critical care professional societies have identified improving long-term quality of life (QoL) outcomes for survivors of ARF as a key research priority.3,6,9, 10, 11
Patients with long ICU stays and their family members have high expectations for recovery after ARF and other critical illnesses.12,13 Pulmonary and critical care physicians cite families’ high expectations as a major barrier to discussing patients’ postdischarge outcomes during ICU family meetings.14 Expectation-disconfirmation theory postulates that expectations create a reference point from which individuals evaluate experiences.15,16 When our health status meets or exceeds our expectations, satisfaction with health is greater. The effects of expectation fulfillment after illness or injury has been evaluated primarily among surgical patients.17, 18, 19, 20 A systematic review reported that fulfillment of preoperative expectations is correlated with improved postoperative patient-reported outcomes and patient satisfaction.21 However, the impact of patients’ expectations for recovery on QoL after an unplanned, serious illnesses like ARF has not been evaluated previously.
Therefore, we designed a study to test whether met vs unmet expectations for health are associated with ARF survivors’ self-reported QoL 6 months after discharge. We hypothesized that perceiving overall health to be as expected or better than expected (vs worse than expected) at hospital discharge is associated with reporting better QoL.
Study Design and Methods
Study Population
The Observational Study of Expected ARF Recovery was a prospective longitudinal cohort study that enrolled consecutive adult patients with ARF managed in ICUs at six hospitals within five academic medical centers.22 To be eligible to participate, adult patients had to meet ≥ 1 of the following criteria and be discharged home alive: (1) mechanical ventilation via an endotracheal tube for ≥ 24 h, (2) noninvasive ventilation for ≥ 24 h that was not used for OSA or other stable indication, or (3) high-flow nasal cannula with Fio2 of ≥ 0.5 and flow rate of ≥ 30 L/min for ≥ 24 h consecutively. Exclusion criteria included (1) mechanical ventilation at baseline or solely for airway protection, (2) more than mild cognitive decline as determined using Information Questionnaire on Cognitive Decline in the Elderly screening,23,24 (3) life expectancy of < 6 months as per clinical judgment, (4) no fixed address, (5) inability to communicate by telephone in English, and (6) a neurological injury expected to prevent a return to consciousness. Around the time of hospital discharge or shortly after returning home, informed consent was obtained from patients with capacity. Institutional review boards at all participating sites agreed to rely on the Vanderbilt University Medical Center Institutional Review Board for this study.
Exposures: Met vs Unmet Expectations and the Health Expectations Gap
Participants’ expected overall health status at 6 months in the future was assessed at study enrollment over the phone using a visual analog scale (VAS) ranging from 0 to 100, with higher scores representing better expected health at 6 months after discharge. Participants were given the following prompt: “Please imagine yourself 6 months from now. Think about what you expect your life to be like. Please indicate on this scale how good or bad you expect your health to be in 6 months. The best health state you can imagine is marked 100 and the worst health state you can imagine is marked 0.”
This approach has been used in previous research on recovery expectations.25 Trained research staff then conducted follow-up assessments by phone at 6 months after hospital discharge. During the 6-month follow-up, perceived health was assessed using the EQ-5D VAS26 (e-Fig 1). During follow-up, participants were not reminded of the expectations they reported at enrollment. Only enrolled participants, not their surrogates or family members, were permitted to answer questions about expected overall health status and perceived health.
Participants’ health expectations were categorized as having been met when perceived health at 6 months was ≤ 8 points lower than expected at enrollment. Expectations were categorized as unmet when perceived health at 6 months was > 8 points lower than expected at enrollment. This 8-point cutoff was selected based on previous work identifying 8 points as a conservative minimal clinically important difference (MCID) for the EQ-5D VAS.27,28 Using this definition, participants whose overall self-rated health exceeded their expectations at hospital discharge also were categorized as having expectations that were met.
We defined a patient’s health expectation gap (HEG) as the difference between their self-reported perceived health at the 6-month follow-up and their expected overall health status at hospital discharge. The HEG was positive for participants whose perceived health at 6 months was greater than expected at hospital discharge and negative for participants whose perceived that their health was worse than expected, with a potential range of –100 to +100.
Primary Outcome: QoL
The primary outcome was QoL at the 6-month follow-up as assessed by the World Health Organization Quality of Life Brief Version (WHOQOL-BREF).29 The WHOQOL-BREF has a total of 26 items comprising four domains: physical health, psychological health, social relationships, and environmental health. Each item is scored from 1 to 5 on a five-point ordinal scale, with domain scores transformed to a 0-point to 100-point scale, with higher scores indicating better QoL (e-Table 1). The WHOQOL-BREF was chosen because it focuses on respondent perception and satisfaction with their lives (ie, QoL), as opposed to health-related QoL, which assesses daily functioning and participation.30 Patient surrogates and family members were not permitted to supply responses to questions in the WHOQOL-BREF. To enhance interpretability and comparisons with prior work, the EQ-5D-5L questionnaire also was administered at the 6-month follow-up as an assessment of self-reported health state.31,32 EQ-5D-5L utility scores were evaluated as a sensitivity analyses of the primary results using the WHOQOL-BREF.
Covariates Collected at Enrollment and Follow-up
We collected data on patient demographics, traits, severity of illness, and the home environment that were known or hypothesized to impact perceived health or QoL after critical illness. Participant age, sex, race, insurance status, admission diagnosis, surgical status, Acute Physiology and Chronic Health Evaluation II score, and the presence of ARDS and COVID-19 were collected from the electronic medical record. A standardized questionnaire was administered at enrollment to assess formal education, history of anxiety and depressive disorders, frailty via the Clinical Frailty Scale,33 and resilience via the Connor-Davidson Resilience Scale (CD-RISC-10).34,35 Residential address was used to determine the Area Deprivation Index36 and the median household income of the zip code. Functional status at follow-up was assessed by asking participants which activities of daily living (ADLs)37 and instrumental ADLs (IADLs)38 they could perform independently. Perceived social support was assessed at the 6-month follow-up using the Multidimensional Scale of Perceived Social Support (MSPSS).39,40
Statistical Analysis
Based on mortality after discharge and loss to follow-up in previous studies of ARF survivorship, we expected in-hospital mortality to be 10% and loss to follow-up of 15%. If expectations were achieved for 40% of survivors of ARF (as seen in other fields) and the two-tailed α value was .05, enrolling 180 participants would yield 80% power to detect a moderate effect size of ≥ 0.6 for expectation fulfillment on QoL.
Continuous data were summarized using medians and interquartile ranges (IQRs), and categorical data were summarized using numbers and percentages. The relationship between expected health status and perceived health at 6 months was visualized using histograms and a scatterplot. Scatterplots, Spearman correlation coefficients, and locally estimated scatterplot smoothing (LOESS) smoothers were used to visualize and explore the relationship between the WHOQOL-BREF’s four domain scores and exposures hypothesized to be associated with these outcomes. Our primary analysis compared QoL, measured using WHOQOL-BREF 6 months after ICU stay, in patients with met vs unmet health expectations using the nonparametric Mann-Whitney U test.
The adjusted association between the HEG and QoL also was modeled, with separate models fit for each WHOQOL-BREF domain score. To avoid overfitting, we decided a priori that all regression models would include age, sex, the number of IADLs and ADLs that participants reported performing independently at 6 months, MSPSS score, CD-RISC-10 score, and perceived health at 6 months. On visual inspection, the relationship between the HEG and WHOQOL-BREF score appeared to be nonlinear for some domains, so cubic regression splines for the basis of smooth functions of HEG were tested in generalized additive models and were incorporated if they improved fit for a domain score.41 Function parameters including the number and location of knots were chosen via a generalized cross-validation approach.42 Coefficients and fit metrics for final models were reported. To illustrate the relationship between HEG and QoL domain scores when smooth functions were included in models, we predicted and plotted WHOQOL-BREF physical health domain scores for a prototypical patient with identical characteristics (male patient who can perform all ADLs and IADLs at the 6-month follow-up, with median values for age, MSPSS score, CD-RISC-10 score, and perceived health) across a range of HEG values commonly observed in the study data.
As a sensitivity analysis, the primary analysis and modeling were repeated using the EQ-5D-5L utility score as the QoL outcome measure. All analyses were performed using R version 4.2.0 software (R Foundation for Statistical Computing). A two-sided P value of < .05 was treated as statistically significant.
Results
Among the 180 survivors of ARF enrolled, 11 patients (6%) died and nine patients (5%) were lost to follow-up before the 6-month assessment (e-Fig 2). The median age of enrollees was 53 years (IQR, 42-64 years), and 55% were male. One hundred fifteen participants (64%) were White, 52 participants (29%) were Black, three participants (2%) were Asian, one participant (1%) was multiracial, and race was not reported for nine participants (5%). Fifty-eight participants (32%) had completed a 4-year postsecondary degree, 114 participants (63%) had a primary ICU admission diagnosis of respiratory illness or cardiovascular disease, and 59 participants (33%) met the criteria for ARDS. Complete baseline characteristics are presented in Table 1.
Table 1.
Participant Demographics
| Participant Characteristic | No. (%) or Median (IQR) |
|---|---|
| No. of participants | 180 |
| Age, y | 53 (42-64) |
| Male sex | 99 (55) |
| Race | |
| Asian | 3 (2) |
| Black | 52 (29) |
| White | 115 (64) |
| Multiracial | 1 (1) |
| Unknown | 9 (5) |
| Completed educationa | |
| ≤ Eighth grade | 3 (2) |
| Some high school | 21 (12) |
| High school or GED | 42 (23) |
| Some college or 2-y degree | 41 (23) |
| 4-y degree | 40 (22) |
| > 4-y degree | 18 (10) |
| Median income of zip code, $ | 72,000 (52,000-94,000) |
| ADI national percentile | 39 (19-64) |
| Insurance status | |
| Private | 121 (67) |
| Medicare | 57 (32) |
| Medicaid | 30 (17) |
| Uninsured | 5 (3) |
| Clinical Frailty Scale score | 3 (2-4) |
| ICU admission diagnosis category | |
| Respiratory (including pneumonia) | 78 (43) |
| Cardiovascular | 36 (20) |
| GI | 15 (8) |
| Oncology | 9 (5) |
| Sepsis (excluding pneumonia) | 14 (8) |
| Trauma | 4 (2) |
| Other | 24 (13) |
| Surgical status | |
| Not surgical | 133 (74) |
| Elective | 29 (16) |
| Emergent | 18 (10) |
| Positive COVID-19 test results during admission | 35 (19) |
| ARDS | 59 (33) |
| APACHE II score | 20 (15-26) |
| Baseline history of depression | 59 (33) |
| Baseline history of anxiety disorder | 17 (9) |
| Length of hospital stay, d | 14 (10-23) |
| CD-RISC 10 resilience scoreb | 35 (30-39) |
| MSPSS social support scorec | 72 (61-81) |
| Significant other subscale | 6 (5-7) |
| Family subscale | 6 (6-7) |
| Friends subscale | 6 (5-7) |
Data are presented as No. (%) or median (IQR), unless otherwise indicated. ADI = Area Deprivation Index; APACHE II = Acute Physiology and Chronic Health Evaluation; CD-RISC = Connor-Davidson Resilience Scale; GED = General Educational Development Test; IQR = interquartile range; MSPSS = Multidimensional Scale of Perceived Social Support.
Missing for 15 participants.
Scores range from 0 to 40, with higher scores indicating greater resilience.
Scores range from 12 to 84, with higher scores indicating greater perceived social support.
Expected vs Perceived Health
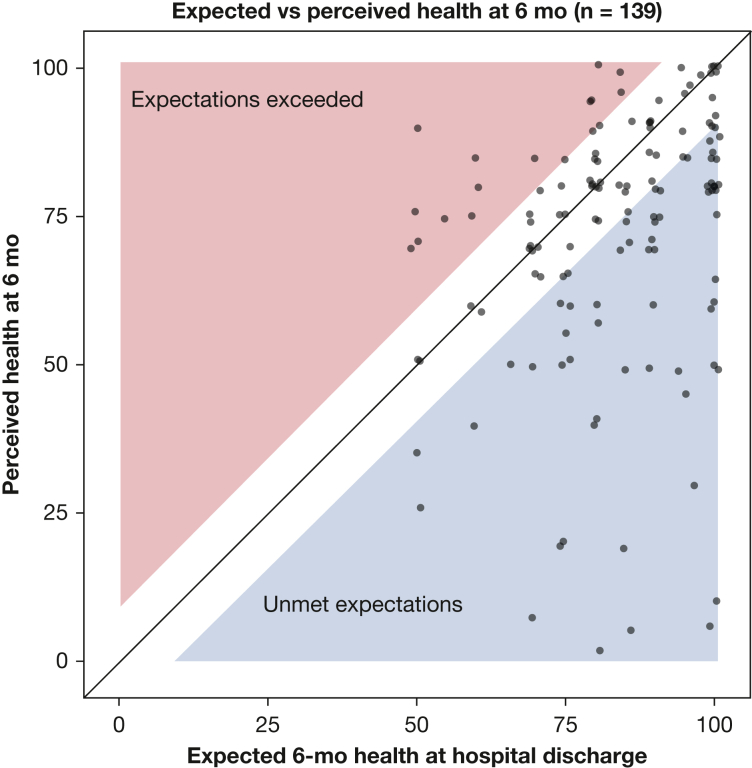
At hospital discharge, the median expectation for overall health using a VAS ranging from 0 to 100 was 85 (IQR, 75-95). At the 6-month follow-up, 139 participants contributed data on perceived health with a median value of 80 (IQR, 60-85). At follow-up, the perceived health of 70 participants met or exceeded their expectations (defined as being ≤ 8 points lower than expected), and expectations of 69 participants were unmet. The relationship between expected and perceived health is displayed in Figure 1. The HEG, defined as perceived minus expected health, showed a left-skewed distribution (e-Fig 3) and a median value of –8 (IQR, –20 to 0).
Figure 1.
Scatterplot showing expected vs perceived health 6 mo after acute respiratory failure. At enrollment near the time of hospital discharge, participants were asked to indicate how good or bad they expected their health to be after 6 mo using a visual analog scale (VAS) ranging from 0 to 100 (with higher score being better health). At the 6-mo follow-up, participants were asked about their perceived health using the EQ-5D VAS, that also ranges from 0 to 100. The unshaded area represents the minimal clinical important difference (MCID) for the VAS of 8 units. Participants in the unshaded area were classified as having their health expectations met. The blue region contains participants whose perceived health was worse than expected by more than the MCID, whereas the pink region contains participants whose perceived health was better than expected by more than the MCID. Points have been jittered ± 1 unit in the horizontal or vertical directions for clarity. Shaded areas have been shrunk by 1 unit to ensure that no point extends into a shaded region as a result of jittering.
Quality of Life
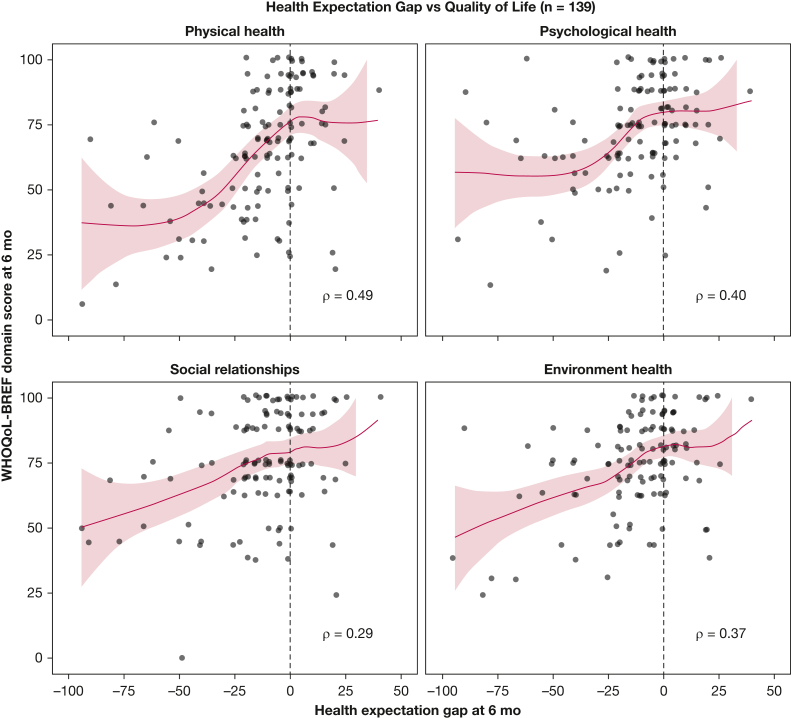
At follow-up, median QoL scores for the physical health, psychological health, social relationships, and environmental health domains of the WHOQOL-BREF instrument were 69 (IQR, 50-88), 75 (IQR, 63-88), 75 (IQR, 69-94), and 75 (IQR, 69-88), respectively. WHOQOL-BREF scores were significantly higher among participants with met vs unmet health expectations across each of the four domains (Table 2). The difference in QoL scores between participants with met vs unmet health expectations was greatest for the physical health domain (median, 75 vs 57; 95% CI for the estimated difference, 12-25; Mann-Whitney U test statistic, 3,473; P < .001) and smallest for the social relationships domain (median, 80 vs 72; 95% CI for the estimated difference, 0-13; Mann-Whitney U test statistic, 2,844; P = .02). In exploratory analysis (e-Figs 4-8), the number of IADLs and ADLs performed independently at follow-up was correlated strongly with the WHOQOL-BREF physical health domain score (Spearman’s correlation coefficient, r = 0.58), perceived social support at 6 months was correlated strongly with the social relationships and environmental health domain scores (r = 0.49 for both), and resilience was correlated most strongly with the psychological health domain score (r = 0.37). On visual inspection, the unadjusted relationship between HEG and QoL seemed to be nonlinear in the domains of physical and psychological health (Fig 2).
Table 2.
Quality of Life 6 Months After Discharge in Patients With vs Without Fully Achieved Expectations
| Variable | WHOQOL-BREF Domain Scores |
EQ-5D-5L Utility Score | |||
|---|---|---|---|---|---|
| Physical Health | Psychological Health | Social Relationships | Environmental Health | ||
| Met health expectations | 75 (68-94) | 79 (69-94) | 80 (69-94) | 80 (75-94) | 0.89 (0.82-1.0) |
| Unmet health expectations | 57 (44-75) | 68 (63-75) | 72 (63-75) | 70 (63-75) | 0.70 (0.60-0.84) |
| Estimated difference (95% CI)a | 19 (12-25) | 12 (6-18) | 6 (0-13) | 12 (6-13) | 0.17 (0.14-0.21) |
| Mann-Whitney U test, P valueb | 3,473, < .001 | 3,221, < .001 | 2,844, .02 | 3,159, < .001 | 3,626, < .001 |
Data are presented as median (interquartile range), unless otherwise indicated. WHOQOL-BREF = World Health Organization Quality of Life Brief Version.
Estimated via the Hodges Lehmann estimator for the difference between two populations.
The assumption of normality in the distribution of quality of life measures was rejected based on the Shapiro-Wilk normality test.
Figure 2.
Graphs showing the unadjusted association between participants’ health expectation gap (HEG), and World Health Organization Quality of Life Brief Version (WHOQOL-BREF) domain scores at the 6-mo follow-up. The HEG is defined as participants’ perceived health at 6 mo as measured using the EQ-5D visual analog scale (0-100 scale), minus their expected health (0-100 scale) at hospital discharge. When these two scores are identical, the HEG is 0. When expectations are exceeded, the HEG is positive. The WHOQOL-BREF comprises four domains, and each domain score is transformed to a 0 to 100 scale, with higher scores indicating better quality of life. Shaded areas depict 95% CIs. Spearman rank correlation coefficient is reported for each domain. Locally estimated scatterplot smoothing (LOESS) smoothers with α = .8 are displayed.
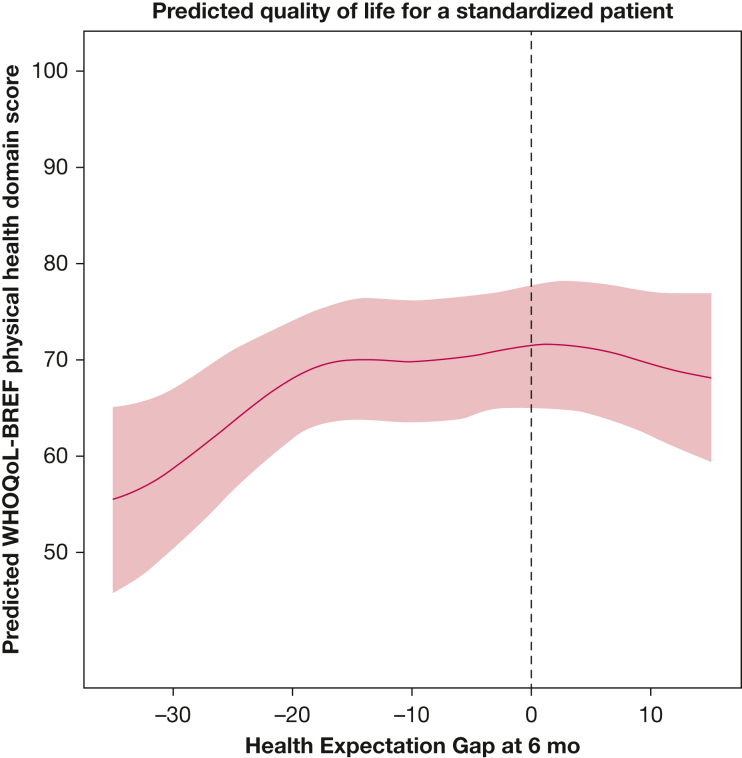
After adjusting for age, sex, number of IADLs and ADLs performed independently, MSPSS score, CD-RISC-10 score, and perceived health at follow-up, the association between HEG and the psychological health, social relationships, and environmental health domain scores were not statistically significant (e-Table 2). Including smoothed, nonlinear functions of HEG significantly improved fit when modeling the physical health domain scores, but made it difficult to summarize the association numerically. Thus, to illustrate the positive and statistically significant association between HEG and physical health domain score, we predicted physical health domain scores for a prototypical study participant (see Methods) with an ED-5D VAS score for perceived health of 65 across a range of HEG values commonly observed in the study (Fig 3). When the prototypical patient’s perceived health is substantially worse than expected (HEG, –35), his expected physical health domain score is 56 (IQR, 46-65), whereas when his health expectations are met (HEG, 0), his expected physical health domain score is 72 (IQR, 65-78).
Figure 3.
Graph showing the estimated World Health Organization Quality of Life Brief Version (WHOQOL-BREF) physical health domain scores across a range of health expectation gap (HEG) values for a standardized patient. The HEG, shown on the x-axis across a range of values commonly observed in this study, is defined as participants’ perceived health at 6 mo as measured using the EQ-5D visual analog scale (0-100 scale), minus their expected health (0-100 scale) at hospital discharge. The y-axis shows predicted WHOQOL-BREF score for the physical health domain. Predicted WHOQOL-BREF physical health domain scores (blue line) are for a prototypical patient in this cohort of survivors of acute respiratory failure (male patient who can perform all activities of daily living and instrumental activities of daily living at the 6-mo follow-up, with median values for age, resilience, and perceived social support scores), with an EQ-5D visual analog scale score for perceived health of 65 at follow-up. Estimates were obtained using the adjusted multivariate models incorporating cubic regression splines to model the nonlinear relationship between the HEG and the physical health domain scores of the WHOQOL-BREF instrument. Shaded areas indicate 95% CIs.
Sensitivity Analyses
Sensitivity analyses using EQ-5D-5L index scores as the QoL outcome measure largely were confirmatory. Median EQ-5D-5L index values were significantly higher among participants with met vs unmet health expectations (median, 0.89 vs 0.70; 95% CI for the estimated difference, 0.14-0.21; Mann-Whitney U test statistic, 3,626; P < .001) (Table 2). A moderate positive correlation was found between HEG and EQ-5D-5L utility score (r = 0.54) (e-Fig 9), although the association between and HEG and EQ-5D-5L utility score was linear and was not statistically significant in multivariate analyses (β = .002; P = .10) (e-Table 3).
Discussion
In this multicenter prospective cohort study of 180 adult survivors of ARF, having expectations for overall health that subsequently were met or exceeded was associated with reporting better QoL related to physical health during a 6-month follow-up. This association remained statistically significant after adjusting for other factors hypothesized to impact QoL, including age, sex, perceived health, perceived social support, resilience, and the number of IADLs and ADLs performed independently. Our findings suggest that 6 months after hospital discharge, two patients who perceive their health similarly (ie, same rating on the EQ-5D VAS), but who started with different expectations for recovery, would be expected to provide different responses to questions in the WHOQOL-BREF like: “How satisfied are you with your ability to perform your daily living activities?” and “How satisfied are you with your capacity for work?” and “Do you have enough energy for everyday life?”
Our findings are consistent with decades of health care marketing research,43, 44, 45 particularly in surgical fields,21 on the relationship between expectations and patient-reported outcomes and are explained best by assimilation-contrast theory.46,47 Like expectation-disconfirmation theory,15,16 assimilation-contrast theory posits that expectations serve as a reference point when a person evaluates a product, service, or experience. When a service or experience is close to their expectations, people assimilate the experience as coherent and acceptable. However, when an experience is so much better or worse than expected that it cannot be assimilated, the contrast, or difference between expectations and reality, affects how the experience is evaluated.
It is unclear how large the gap between expectations and perceptions of health can be before a survivor of ARF’s satisfaction with physical health is affected meaningfully. The difference between expected and perceived health, which we have termed the HEG, ranged substantially and included survivors whose perceived health was better than expected at hospital discharge. The apparent nonlinear relationship observed between HEG and QoL suggests that thresholds beyond which a larger or smaller value of the HEG does not matter much may exist. For example, perceiving current health as 60 points worse than expected may not be associated with a worse QoL than perceiving one’s health as 30 points worse than expected. On the other end of the scale, the relationship between HEG and QoL domain scores seem to flatten when expectations are close to being met. This may mean that exceeding expectations confers no additional positive association with QoL beyond meeting expectations, or it may reflect that as soon as expectations are met, some participants reported perfect QoL domain scores, creating a ceiling effect.
The difference between participants with met vs unmet expectations in psychological, social, and environmental domains of QoL scores was smaller than for the physical health domain and was not statistically significant in multivariate analyses. This is not surprising and can be interpreted to suggest that expectations about health are associated with satisfaction with physical health, but not with the feelings of ARF survivors about other QoL determinants like personal relationships, spirituality, physical safety, and money.
Reassuringly, EQ-5D-5L index values performed quite similarly to the WHOQOL-BREF physical health domain score in analyses. Unlike WHOQOL-BREF domain scores, which lack established MCID estimates, MCID estimates for EQ-5D-5L index values are available for a range of populations. MCID estimates for EQ-5D-5L index values for people with COPD and lung cancer range from 0.03 to 0.06.48,49 Using the estimate from the multivariate model of EQ-5D-5L index values, this corresponds to a 15-point to 30-point change in HEG, conferring a meaningful change in QoL.
Our study represents an initial step toward understanding the role of expectations in ARF survivorship. Clinical recommendations based on our current findings are premature. Nevertheless, trialists testing interventions after ICU stay may want to provide standardized information about recovery if QoL or satisfaction with health is an important trial outcome, particularly when masking is not possible. We also are encouraged to see experts on ICU survivorship thinking about the potential impact of expectations in the design and evaluation of clinical interventions. For example, qualitative interviews with patients discharged from the ICU in three countries identified normalization and expectation management as a key component of successful ICU recovery programs.50 Developing expectation management strategies to counter overly optimistic depictions of ICU outcomes in popular media also is a future research priority of the Intensive Care Society.51
We conducted a single prospective observational cohort study and cannot determine if the relationship between the expectations of ARF survivors and QoL is causal. This limitation is inevitable. A randomized trial of an intervention designed to shape expectations could estimate the effect of the intervention, but not the effect of expectation fulfillment. Thus, better understanding of the relationship between expectation fulfillment and QoL requires careful interpretation of observational data. The number of study participants who showed positive results for COVID-19 also was too small for a properly powered subgroup analysis. Given widespread public awareness of long COVID, we speculate that expectations about recovery may be different for this subpopulation of survivors of ARF. We encourage future studies of expectations in ARF survivorship to include prespecified subgroup analyses52,53 in their protocols for survivors of COVID-19. Finally, study participation was limited to patients being discharged to home, and our results may not be applicable to survivors with more care or rehabilitation needs.
Interpretation
Our multicenter prospective cohort study of adult survivors of ARF found that participants whose health expectations at hospital discharge were met or exceeded 6 months later reported greater QoL and satisfaction with physical health than survivors whose expectations were not met. This association remained even when survivors perceived their health similarly and reported being able to perform the same ADLs independently. Our findings are consistent with studies in other patient populations17, 18, 19, 20, 21 and suggest a mechanism underpinning successful ICU recovery programs that incorporate normalization and expectation management.50 As the number of ARF survivors continues to rise and ICU recovery clinics continue to expand, culturally appropriate interventions that ensure that those who survive an ICU stay and their families have access to information about common trajectories of survivorship may become a standard component of care. For that to happen, approaches to shaping expectations about ICU survivorship at both the individual and population level first will need to be designed and tested carefully.
Funding/Support
A. E. T. was supported by the National Heart, Lung, and Blood Institute [Grant K01HL141637]. This work also was supported by The Assistant Secretary of Defense for Health Affairs endorsed by the Department of Defense through the FY17 PRMRP Investigator-Initiated Research Award under award no. W81XWH-17-PRMRP-IIRA.
Financial/Nonfinancial Disclosures
None declared.
Acknowledgments
Author contributions: A. E. T. and D. M. N. contributed to conceptualization of the study. E. M. L. and V. D. D. supervised the study and performed data acquisition. A. E. T. performed the statistical analysis. A. E. T., S. M. B., and J. C. J. contributed to funding acquisition. D. M. N. and S. M. B. supervised the study. A. E. T. wrote the first draft of the manuscript. All authors read and approved the final manuscript.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
∗APICS-01 Study Team Collaborators: Elise Caraker, Sai Phani Sree Cherukuri, Naga Preethi Kadiri, Tejaswi Kalva, Mounica Koneru, Pooja Kota, Emma Maelian Lee, Mazin Ali Mahmoud, Albahi Malik, Roozbeh Nikooie, Darin Roberts, Sriharsha Singu, Parvaneh Vaziri, Katie Brown, Austin Daw, Mardee Merrill, Rilee Smith, Ellie Hirshberg, Jorie Butler, Benjamin Hoenig, Maria Karamourtopoulos, Margaret Hays, Rebecca Abel, Craig High, Emily Beck, Brent Armbruster, Darrin Applegate, Melissa Fergus, Naresh Kumar, Megan Roth, Susan Mogan (in memoriam), Rebecca Abel, Andre De Souza Licht, Isabel Londono, Julia Larson, Krystal Capers, Andrew Toksoz-Exley, Julia Crane, and Lauren Tsai.
Disclaimer: Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense.
Additional information: The analytic code associated with the tables and figures in this manuscript are publicly available from this site: https://github.com/aeturnbull/OSEAR_QoL_paper_share. The e-Figures and e-Tables are available online under “Supplementary Data.”
Contributor Information
Alison E. Turnbull, Email: turnbull@jhmi.edu.
Addressing Post-Intensive Care Syndrome-01 (APICS-01) Study Team:
Elise Caraker, Sai Phani Sree Cherukuri, Naga Preethi Kadiri, Tejaswi Kalva, Mounica Koneru, Pooja Kota, Emma Maelian Lee, Mazin Ali Mahmoud, Albahi Malik, Roozbeh Nikooie, Darin Roberts, Sriharsha Singu, Parvaneh Vaziri, Katie Brown, Austin Daw, Mardee Merrill, Rilee Smith, Ellie Hirshberg, Jorie Butler, Benjamin Hoenig, Maria Karamourtopoulos, Margaret Hays, Rebecca Abel, Craig High, Emily Beck, Brent Armbruster, Darrin Applegate, Melissa Fergus, Naresh Kumar, Megan Roth, Susan Mogan, Rebecca Abel, Andre De Souza Licht, Isabel Londono, Julia Larson, Krystal Capers, Andrew Toksoz-Exley, Julia Crane, and Lauren Tsai
Supplementary Data
References
- 1.Rubenfeld G.D., Caldwell E., Peabody E., et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353(16):1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 2.Cox C.E., Docherty S.L., Brandon D.H., et al. Surviving critical illness: acute respiratory distress syndrome as experienced by patients and their caregivers. Crit Care Med. 2009;37(10):2702–2708. doi: 10.1097/CCM.0b013e3181b6f64a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spragg R.G., Bernard G.R., Checkley W., et al. Beyond mortality: future clinical research in acute lung injury. Am J Respir Crit Care Med. 2010;181(10):1121–1127. doi: 10.1164/rccm.201001-0024WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zambon M., Vincent J.L. Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest. 2008;133(5):1120–1127. doi: 10.1378/chest.07-2134. [DOI] [PubMed] [Google Scholar]
- 5.Phua J., Badia J.R., Adhikari N.K.J., et al. Has mortality from acute respiratory distress syndrome decreased over time? A systematic review. Am J Respir Crit Care Med. 2009;179(3):220–227. doi: 10.1164/rccm.200805-722OC. [DOI] [PubMed] [Google Scholar]
- 6.Needham D.M., Davidson J., Cohen H., et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med. 2012;40(2):502–509. doi: 10.1097/CCM.0b013e318232da75. [DOI] [PubMed] [Google Scholar]
- 7.Hopkins R.O., Suchyta M.R., Kamdar B.B., Darowski E., Jackson J.C., Needham D.M. Instrumental activities of daily living after critical illness: a systematic review. Annals ATS. 2017;14(8):1332–1343. doi: 10.1513/AnnalsATS.201701-059SR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamdar B.B., Huang M., Dinglas V.D., et al. Joblessness and lost earnings after ARDS in a 1-year national multicenter study. Am J Respir Crit Care Med. 2017;196(8):1012–1020. doi: 10.1164/rccm.201611-2327OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lieu T.A., Au D., Krishnan J.A., et al. Comparative effectiveness research in lung diseases and sleep disorders: recommendations from the National Heart, Lung, and Blood Institute workshop. Am J Respir Crit Care Med. 2011;184(7):848–856. doi: 10.1164/rccm.201104-0634WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carson S.S., Goss C.H., Patel S.R., et al. An official American Thoracic Society research statement: comparative effectiveness research in pulmonary, critical care, and sleep medicine. Am J Respir Crit Care Med. 2013;188(10):1253–1261. doi: 10.1164/rccm.201310-1790ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deutschman C.S., Ahrens T., Cairns C.B., Sessler C.N., Parsons P.E. Critical Care Societies Collaborative/USCIITG Task Force on Critical Care Research. Multisociety task force for critical care research: key issues and recommendations. Am J Respir Crit Care Med. 2012;185(1):96–102. doi: 10.1164/rccm.201110-1848ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cox C.E., Martinu T., Sathy S.J., et al. Expectations and outcomes of prolonged mechanical ventilation. Crit Care Med. 2009;37(11):2888–2894. doi: 10.1097/CCM.0b013e3181ab86ed. quiz 2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamas D.J., Owens R.L., Nace R.N., et al. Opening the door: the experience of chronic critical illness in a long-term acute care hospital. Crit Care Med. 2017;45(4):e357–e362. doi: 10.1097/CCM.0000000000002094. [DOI] [PubMed] [Google Scholar]
- 14.Turnbull A.E., Davis W.E., Needham D.M., White D.B., Eakin M.N. Intensivist-reported facilitators and barriers to discussing post-discharge outcomes with intensive care unit surrogates. A qualitative study. Annals ATS. 2016;13(9):1546–1552. doi: 10.1513/AnnalsATS.201603-212OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oliver R.L. Effect of expectation and disconfirmation on postexposure product evaluations: an alternative interpretation. J Appl Psychol. 1977;62(4):480–486. [Google Scholar]
- 16.Thompson A.G., Suñol R. Expectations as determinants of patient satisfaction: concepts, theory and evidence. Int J Qual Health Care. 1995;7(2):127–141. doi: 10.1093/intqhc/7.2.127. [DOI] [PubMed] [Google Scholar]
- 17.de Tejada M.G.S., Escobar A., Herrera C., García L., Aizpuru F., Sarasqueta C. Patient expectations and health-related quality of life outcomes following total joint replacement. Value in Health. 2010;13(4):447–454. doi: 10.1111/j.1524-4733.2009.00685.x. [DOI] [PubMed] [Google Scholar]
- 18.Soroceanu A., Ching A., Abdu W., McGuire K. Relationship between preoperative expectations, satisfaction, and functional outcomes in patients undergoing lumbar and cervical spine surgery: a multicenter study. Spine. 2012;37(2):E103–E108. doi: 10.1097/BRS.0b013e3182245c1f. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton D.F., Lane J.V., Gaston P., et al. What determines patient satisfaction with surgery? A prospective cohort study of 4709 patients following total joint replacement. BMJ Open. 2013;3(4) doi: 10.1136/bmjopen-2012-002525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pihl K., Roos E.M., Nissen N., JøRgensen U., Schjerning J., Thorlund J.B. Over-optimistic patient expectations of recovery and leisure activities after arthroscopic meniscus surgery. Acta Orthopaedica. 2016;87(6):615–621. doi: 10.1080/17453674.2016.1228411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waljee J., McGlinn E.P., Sears E.D., Chung K.C. Patient expectations and patient-reported outcomes in surgery: a systematic review. Surgery. 2014;155(5):799–808. doi: 10.1016/j.surg.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Institutes of Health Clinical Center Observational Study of Expected ARF Recovery (OSEAR). NCT03797313. Clinical Trials.gov. National Institutes of Health. https://clinicaltrials.gov/ct2/show/NCT03797313 Updated December 15, 2021.
- 23.Pandharipande P.P., Girard T.D., Jackson J.C., et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jorm A.F. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med. 1994;24(1):145–153. doi: 10.1017/s003329170002691x. [DOI] [PubMed] [Google Scholar]
- 25.Aversa M., Chowdhury N.A., Tomlinson G., Singer L.G. Preoperative expectations for health-related quality of life after lung transplant. Clin Transplant. 2018;32(10) doi: 10.1111/ctr.13394. [DOI] [PubMed] [Google Scholar]
- 26.Herdman M., Gudex C., Lloyd A., et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual Life Res. 2011;20(10):1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zanini A., Aiello M., Adamo D., et al. Estimation of minimal clinically important difference in EQ-5D visual analog scale score after pulmonary rehabilitation in subjects with COPD. Respir Care. 2015;60(1):88–95. doi: 10.4187/respcare.03272. [DOI] [PubMed] [Google Scholar]
- 28.Nolan C.M., Longworth L., Lord J., et al. The EQ-5D-5L health status questionnaire in COPD: validity, responsiveness and minimum important difference. Thorax. 2016;71(6):493–500. doi: 10.1136/thoraxjnl-2015-207782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skevington S.M., Lotfy M., O’Connell K.A. The World Health Organization’s WHOQOL-BREF quality of life assessment: psychometric properties and results of the international field trial. A report from the WHOQOL Group. Qual Life Res. 2004;13(2):299–310. doi: 10.1023/B:QURE.0000018486.91360.00. [DOI] [PubMed] [Google Scholar]
- 30.Turnbull A.E., Hurley M.S., Oppenheim I.M., Hosey M.M., Parker A.M. Curb your enthusiasm: definitions, adaptation, and expectations for quality of life in ICU survivorship. Annals ATS. 2020;17(4):406–411. doi: 10.1513/AnnalsATS.201910-772IP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stolk E., Ludwig K., Rand K., van Hout B., Ramos-Goñi J.M. Overview, update, and lessons learned from the international EQ-5D-5L valuation work: version 2 of the EQ-5D-5L valuation protocol. Value in Health. 2019;22(1):23–30. doi: 10.1016/j.jval.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 32.Feng Y.S., Kohlmann T., Janssen M.F., Buchholz I. Psychometric properties of the EQ-5D-5L: a systematic review of the literature. Qual Life Res. 2021;30(3):647–673. doi: 10.1007/s11136-020-02688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rockwood K., Song X., MacKnight C., et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Connor K.M., Davidson J.R.T. Development of a new resilience scale: the Connor-Davidson Resilience Scale (CD-RISC) Depress Anxiety. 2003;18(2):76–82. doi: 10.1002/da.10113. [DOI] [PubMed] [Google Scholar]
- 35.Cosco T.D., Kaushal A., Richards M., Kuh D., Stafford M. Resilience measurement in later life: a systematic review and psychometric analysis. Health Qual Life Outcomes. 2016;14:16. doi: 10.1186/s12955-016-0418-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kind A.J.H., Buckingham W.R. Making neighborhood-disadvantage metrics accessible—the neighborhood atlas. N Engl J Med. 2018;378(26):2456–2458. doi: 10.1056/NEJMp1802313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katz S., Downs T.D., Cash H.R., Grotz R.C. Progress in development of the index of ADL. Gerontologist. 1970;10(1):20–30. doi: 10.1093/geront/10.1_part_1.20. [DOI] [PubMed] [Google Scholar]
- 38.Lawton M.P., Brody E.M. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–186. [PubMed] [Google Scholar]
- 39.Zimet G.D., Powell S.S., Farley G.K., Werkman S., Berkoff K.A. Psychometric characteristics of the Multidimensional Scale of Perceived Social Support. J Pers Assess. 1990;55(3-4):610–617. doi: 10.1080/00223891.1990.9674095. [DOI] [PubMed] [Google Scholar]
- 40.Shumaker S.C., Frazier S.K., Moser D.K., Chung M.L. Psychometric properties of the multidimensional scale of perceived social support in patients with heart failure. J Nurs Meas. 2017;25(1):90–102. doi: 10.1891/1061-3749.25.1.90. [DOI] [PubMed] [Google Scholar]
- 41.Wood S.N. Fast stable direct fitting and smoothness selection for generalized additive models. J R Stat Soc Series B Stat Methodol. 2008;70(3):495–518. [Google Scholar]
- 42.Wood S.N. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J R Stat Soc Series B Stat Methodol. 2011;73(1):3–36. [Google Scholar]
- 43.Calman K.C. Quality of life in cancer patients—an hypothesis. J Med Ethics. 1984;10(3):124–127. doi: 10.1136/jme.10.3.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ross C.K., Frommelt G., Hazelwood L., Chang R.W. The role of expectations in patient satisfaction with medical care. J Health Care Mark. 1987;7(4):16. [PubMed] [Google Scholar]
- 45.Carr A.J., Gibson B., Robinson P.G. Is quality of life determined by expectations or experience? BMJ. 2001;322(7296):1240–1243. doi: 10.1136/bmj.322.7296.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sherif M., Taub D., Hovland C. Assimilation and contrast effects of anchoring stimuli on judgments. J Exp Psychol. 1958;55:150–155. doi: 10.1037/h0048784. [DOI] [PubMed] [Google Scholar]
- 47.Sherif M., Hovland C.I. Yale University Press; 1961. Social Judgment: Assimilation and Contrast Effects in Communication and Attitude Change; p. xii, 218. [Google Scholar]
- 48.Pickard A.S., Neary M.P., Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes. 2007;5:70. doi: 10.1186/1477-7525-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bae E., Choi S.E., Lee H., Shin G., Kang D. Validity of EQ-5D utility index and minimal clinically important difference estimation among patients with chronic obstructive pulmonary disease. BMC Pulm Med. 2020;20(1):73. doi: 10.1186/s12890-020-1116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McPeake J., Boehm L.M., Hibbert E., et al. Key components of ICU recovery programs: what did patients report provided benefit? Crit Care Explor. 2020;2(4):e0088. doi: 10.1097/CCE.0000000000000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Intensive Care Society. Intensive care 2020 and beyond. Intensive Care Society website; 2020. https://www.ics.ac.uk/ICS/Guidelines/PDFs/Intensive_Care_2020_and_Beyond [Google Scholar]
- 52.Wang R., Lagakos S.W., Ware J.H., Hunter D.J., Drazen J.M. Statistics in medicine—reporting of subgroup analyses in clinical trials. N Engl J Med. 2007;357(21):2189–2194. doi: 10.1056/NEJMsr077003. [DOI] [PubMed] [Google Scholar]
- 53.Angus D.C., Chang C.C.H. Heterogeneity of treatment effect: estimating how the effects of interventions vary across individuals. JAMA. 2021;326(22):2312–2313. doi: 10.1001/jama.2021.20552. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.