Abstract
Background
Lung cancer screening (LCS) with low-dose CT (LDCT) imaging was recommended in 2013, making approximately 8 million Americans eligible for LCS. The demographic characteristics and outcomes of individuals screened in the United States have not been reported at the population level.
Research Question
What are the outcomes among people screened and entered in the American College of Radiology’s Lung Cancer Screening Registry compared with those of trial participants?
Study Design and Methods
This was a cohort study of individuals undergoing baseline LDCT imaging for LCS between 2015 and 2019. Predictors of adherence to annual screening were computed. LDCT scan interpretations by Lung Imaging Reporting and Data System (Lung-RADS) score, cancer detection rates (CDRs), and stage at diagnosis were compared with National Lung Cancer Screening Trial data.
Results
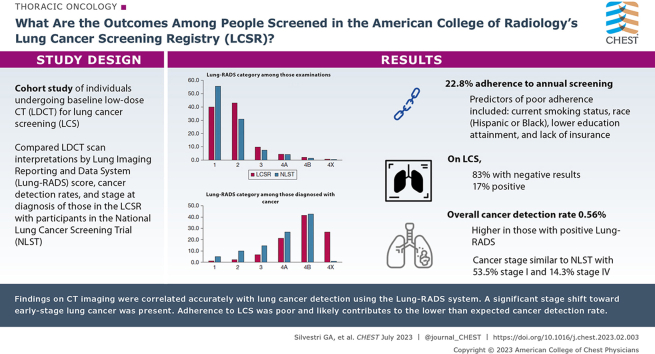
Adherence was 22.3%, and predictors of poor adherence included current smoking status and Hispanic or Black race. On baseline screening, 83% of patients showed negative results and 17% showed positive screening results. The overall CDR was 0.56%. The percentage of people with cancer detected at baseline was higher in the positive Lung-RADS categories at 0.4% for Lung-RADS category 3, 2.6% for Lung-RADS category 4A, 11.1% for Lung-RADS category 4B, and 19.9% for Lung-RADS category 4X. The cancer stage distribution was similar to that observed in the National Lung Cancer Screening Trial, with 53.5% of patients receiving a diagnosis of stage I cancer and 14.3% with stage IV cancer. Underreporting into the registry may have occurred.
Interpretation
This study revealed both the positive aspects of CT scan screening for lung cancer and the challenges that remain. Findings on CT imaging were correlated accurately with lung cancer detection using the Lung-RADS system. A significant stage shift toward early-stage lung cancer was present. Adherence to LCS was poor and likely contributes to the lower than expected cancer detection rate, all of which will impact the outcomes of patients undergoing screening for lung cancer.
Key Words: adherence, lung cancer, outcomes, screening
Graphical Abstract
FOR EDITORIAL COMMENT, SEE PAGE 18
Take-home Points.
Study Question: What are the predictors of adherence, radiographic findings, cancer detection rate, and stage at diagnosis, of the first 1 million patients screened whose results are reported in the American College of Radiology Lung Cancer Screening Registry?
Results: Adherence to annual screening was 22.3%, and predictors of poor adherence included current smoking status, Hispanic or Black race, lower education attainment, and lack of insurance. On baseline screening, 82.6% of those screened showed negative results (Lung Imaging Reporting and Data System [Lung-RADS] categories 1 or 2), and 17.3% showed positive screening results (Lung-RADS categories 3 or 4). Among individuals with negative baseline screening results, fewer than 1 in 3,330 harbored detected cancer. The overall cancer detection rate was 0.56%. The percentage of people with cancer detected at baseline was higher in the positive Lung-RADS categories at 0.4% for Lung-RADS category 3, 2.6% for Lung-RADS category 4A, 11.1% for Lung-RADS category 4B, and 19.9% for Lung-RADS category 4X. The cancer stage distribution was similar to that observed in the National Lung Screening Trial, with 53.5% receiving a diagnosis of stage I cancer and 14.3% receiving a diagnosis of stage IV cancer.
Interpretation: This study revealed both the positive aspects of CT scan screening for lung cancer and the challenges in implementing screening nationally. Reassuringly, most patients met criteria to be screened. Findings on CT imaging were correlated accurately with lung cancer detection using the Lung-RADS system. A significant stage shift toward early-stage lung cancer was found. However, adherence to lung cancer screening was poor, was more likely to occur in the underserved, and likely contributes to the lower than expected cancer detection rate, all of which will impact the outcomes of patients undergoing screening for lung cancer.
After the results of the National Lung Screening Trial (NLST)1 were published, the United States Preventive Services Task Force (USPSTF) issued a grade B recommendation for screening with annual low-dose CT (LDCT) imaging of the chest in those at high risk as defined by age and smoking history.2 As part of the Patient Protection and Affordable Care Act provisions, the USPSTF decision meant that commercial payors were required to cover lung cancer screening (LCS).3 In 2015, the Centers for Medicare and Medicaid Services (CMS) made LCS a covered benefit4 and mandated that facilities report data to a CMS-approved registry. Nationwide data on the performance of LCS are available from a single source, whereas they are not for other commonly screened-for cancers. The American College of Radiology’s Lung Cancer Screening Registry (LCSR) is the only CMS-approved registry collecting data nationwide.5,6
An estimated 8 million people in the United States are eligible for LCS as defined by the 2013 USPSTF recommendations, hereafter referred to as the eligible population.7 With the change in eligibility criteria for LCS by the 2021 USPSTF guidelines, an estimated additional 6.5 million people are eligible for LCS.8 Although many studies have been published from single-center institutions focusing on various aspects of the LCS continuum, few have examined a nationwide sample.9 The ACR and the National Lung Cancer Roundtable at the American Cancer Society previously published the demographics, eligibility, and overall adherence of the first 1 million people screened and entered into the ACR registry.10
To achieve the 1.1% cancer detection rate and the 20% relative risk reduction in lung cancer mortality documented in the NLST, 95% of participants adhered to the annual screening protocols. Adherence is critical for realizing the promise of LCS, and the cancer detection rate is an important outcome when we think about whether the USPSTF recommendations include the most efficient criteria for identifying individuals at high risk who will benefit most from LCS. Herein, we present further analyses from this cohort to highlight outcomes including radiographic findings, predictors of adherence, cancer detection rate, and stage at diagnosis.
Study Design and Methods
Data on individuals undergoing baseline chest LDCT imaging for LCS from 2015 through 2019 were obtained from 3,625 facilities reporting to the LCSR.11 The LCSR collects data on: (1) patient characteristics (age, sex, race, ethnicity, and smoking status); (2) examination information, including Lung Imaging Reporting and Data System (Lung-RADS) category; and (3) cancer diagnosis and stage. Some data elements are required and others are optional, as noted in e-Table 1.
We were interested in adherence and outcomes among people who were eligible to receive screening according to the 2013 USPSTF recommendations (aged 55-80 years, current tobacco use with ≥ 30 pack-years of smoking or prior tobacco use with ≥ 30 pack-years of smoking and quit within the past 15 years).2 Between 2015 and 2019, 1,203,364 individuals received LDCT scan screening. Screened individuals who were missing data on smoking history (n = 17,513), did not meet the 2013 USPSTF criteria (n = 110,008), had signs or symptoms of cancer (n = 11,109), or were missing a primary care provider order (n = 26,759) were excluded (e-Fig 1). At baseline, 1,052,591 individuals were screened who were included in the analyses.
Descriptive statistics on screened participants’ smoking history and sociodemographic factors were compiled. Adherence to screening also was assessed, and it was computed at three time points: T1, T2, and T3. T1 was defined as the proportion of eligible patients undergoing a subsequent screening within 11 to 15 months after baseline. This time frame was selected to match the NLST definition of adherence.1 T2 was defined as undergoing an examination at 16 to 24 months. T3 was defined as undergoing an examination at ≥ 24 months. For screening adherence calculations, only those who were eligible for another examination were included in the denominator. For example, those with a diagnosis of cancer (n = 6,123) or who showed positive Lung-RADS (≥ 3) findings at the baseline examination (n = 104,358) or who no longer met the USPSTF definition at the anticipated year of follow-up (n = 16,157), those who underwent the baseline screening in 2019 (n = 345,376), or both were excluded from the T1 calculations, leaving 571,264 people available for analyses (e-Fig 1). Three hundred seven thousand twelve people were included in T2 (16-24 months) calculations, and 128,609 people were included in T3 (≥ 24 months) calculations. Those who showed positive screening results, defined as having Lung-RADS categories 3 or 4 findings, were not included in adherence calculations because these individuals are recommended to undergo more aggressive follow-up testing and were not considered in the formula for adherence to annual screening. Multivariate generalized estimating equations were used to estimate adjusted ORs (aORs) and 95% CIs of predictors of annual adherence; these models accounted for the correlation of patients within the same facilities.12 Variables in models were selected a priori based on previous lung and other cancer screening studies.13, 14, 15 Indicators for missing observations were included in the models.
Outcomes among individuals screened in the LCSR also were examined. First, we examined the LDCT scan interpretations by Lung-RADS score, categorized as 1 (negative, no noncalcified lung nodules), 2 (negative, nodules benign in appearance), 3 (probably benign), 4 (4A, suspicious and 4B, very suspicious), and 4X, a category used by radiologists to upscore the risk of cancer because of additional nodule features or findings not included in the Lung-RADS classifiers. Second, cancer detection rates were computed by dividing the number of cancers by the number of individuals with screening examinations, similar to previous studies.16 Third, the stage distribution at diagnosis was calculated for both baseline and subsequent annual screenings. The LCSR’s cancer detection rate was compared with NLST results for both baseline and subsequent annual screenings. Prevalence ratios (PR) compared the proportion of cancers of stage I, II, and so forth with cancers in the LCSR and NLST (PR = proportion in LSCR / proportion in NLST). The PR’s 95% CIs were computed with the Delta method.17
To understand better the potential biases of missing data, we examined characteristics, adherence, and outcomes among the 2,355 facilities with ≥ 70% completeness of race and insurance data and 536 facilities with ≥ 70% completeness of race, insurance, and education data. Analyses were conducted in SAS version 9.4 software (SAS Institute). The Medical University of South Carolina Institutional Review Board approved this study.
Results
Descriptive Statistics
Among 1,052,591 individuals who were screened, 90.8% met the 2013 USPSTF eligibility criteria, and most (83.1%) were screened from 2017 through 2019. Among those meeting USPSTF criteria, slightly more than one-half were male (51.7%), 55 to 64 years of age (53.0%), and 61.4% currently used tobacco (Table 1). Among those with known race or ethnicity data, 91.6% were White, 7.4% were Black, 0.9% were Asian, and 0.1% were Native Hawaiian or Pacific Islanders. Among those with known insurance data, 49.3% were privately insured, 42.7% had Medicare insurance, 6.9% had Medicaid insurance, and 1.2% were uninsured. Distributions of known values generally were similar when restricting the analyses to facilities with > 70% completeness of race, insurance, ethnicity, and education (e-Table 2).
Table 1.
Sociodemographic Features and Socioeconomic Status Among Patients Screened in the Lung Cancer Screening Registry
| Variable | No. | % |
|---|---|---|
| Total | 1,052,591 | 100.0 |
| Sex | ||
| Male | 544,482 | 51.7 |
| Female | 505,318 | 48.0 |
| Missing, unknown, or not reported | 2,791 | 0.3 |
| Age group, y | ||
| 55-59 | 232,307 | 22.1 |
| 60-64 | 272,487 | 25.9 |
| 65-69 | 188,161 | 17.9 |
| 70-74 | 191,680 | 18.2 |
| 75-80 | 67,956 | 6.5 |
| Race | ||
| Asian | 4,385 | 0.4 |
| Black | 37,111 | 3.5 |
| Hawaiian native/Pacific Islander | 658 | 0.1 |
| White | 461,593 | 43.9 |
| Unknown, other, missing, or not reported | 548,844 | 52.1 |
| Hispanic origin | ||
| No | 400,737 | 38.1 |
| Yes | 8,807 | 0.8 |
| Not reported, missing, or unknown | 643,047 | 61.1 |
| Education | ||
| < High school | 10,743 | 1.0 |
| High school | 34,150 | 3.2 |
| Some college | 24,758 | 2.4 |
| College or more | 13,613 | 1.3 |
| Unknown, declined to answer, missing, unspecified | 969,327 | 92.09 |
| Health insurance type | ||
| Medicare | 224,405 | 21.3 |
| Medicaid | 36,216 | 3.4 |
| Private insurance | 259,154 | 24.6 |
| Self-pay or uninsured | 6,328 | 0.6 |
| Unknown, other, or missing | 526,488 | 50.0 |
| Smoking history | ||
| Current, 30+ pack-y | 645,875 | 61.4 |
| Former, 30+ pack-y, quit ≤ 15 y | 406,700 | 38.6 |
| Examination year of first occurrence | ||
| 2015 | 35,575 | 3.4 |
| 2016 | 141,673 | 13.5 |
| 2017 | 225,413 | 21.4 |
| 2018 | 304,554 | 28.9 |
| 2019 | 345,376 | 32.8 |
| Region | ||
| Northeast | 264,819 | 25.2 |
| Midwest | 315,702 | 30.0 |
| South | 363,509 | 34.5 |
| West | 107,502 | 10.2 |
Adherence to Repeat Annual Screening
Among individuals with baseline examinations between 2015 and 2018, 127,194 of the 570,302 people who were eligible (22.3%) underwent a follow-up examination at T1 (11-15 months). Adherence ranged from 20% to 30% in all age, smoking status, sex, race or ethnicity, and education groups, but was particularly low among individuals who currently smoked, were Hispanic or Black, or had completed a lower level of education. It varied widely by insurance, from 9.7% among the uninsured or self-payers to 23.5% among those with Medicare insurance (Table 2). In adjusted analyses, Black people (aOR, 0.84; 95% CI, 0.76-0.92), Hispanic people (aOR, 0.73; 95% CI, 0.63-0.85), and Asian people (aOR, 0.79; 95% CI, 0.64-0.99) were significantly less likely than White people to undergo at least one subsequent examination. Adherence also was less common in individuals residing in the western (aOR, 0.71; 95% CI, 0.52-0.96) or southern (aOR, 0.65; 95% CI, 0.53-0.79) parts of the United States compared with those residing in the northeastern United States. Adherence rates were not significantly different by health insurance or educational status (Table 2). Adherence patterns generally were similar when restricted to facilities with ≥ 70% completeness of race and insurance data (e-Table 3). If the repeat annual screening was extended to 24 months, an additional 11.7% were considered adherent, and an additional 5.9% more were considered adherent after 24 months (e-Table 4).
Table 2.
Proportion and aORs of Adherence to a Follow-up Annual Examination (11-15 Mo) in the LCSR 2015-2018a
| Characteristic | Follow-up Annual Examination Between 11 and 15 Months (T1), % | aOR | 95% CI |
|---|---|---|---|
| Total | 22.3 | . . . | . . . |
| Sex | |||
| Male | 22.2 | 1.00 | … |
| Female | 22.4 | 1.01 | 1.00-1.02 |
| Age group, y | |||
| 55-59 | 20.2 | 1.00 | . . . |
| 60-64 | 21.7 | 1.06 | 1.05-1.08 |
| 65-69 | 24.2 | 1.19 | 1.16-1.21 |
| 70-77 | 23.2 | 1.13 | 1.11-1.16 |
| Smoking history | |||
| Former | 24.7 | 1.17 | 1.15-1.18 |
| Current | 20.8 | 1.00 | . . . |
| Race | |||
| White | 24.2 | 1.00 | . . . |
| Black | 19.4 | 0.84 | 0.76-0.92 |
| Asian | 18.3 | 0.79 | 0.64-0.99 |
| Hawaiian native/Pacific Islander | 19.6 | 0.91 | 0.71-1.61 |
| Other | 20.2 | 0.89 | 0.76-1.03 |
| Missing race/ethnicity | 20.9 | 0.92 | 0.83-1.02 |
| Hispanic origin | |||
| No | 24.2 | 1.00 | . . . |
| Yes | 16.6 | 0.73 | 0.63-0.85 |
| Missing | 21.1 | 0.92 | 0.82-1.19 |
| Education | |||
| < High school | 27.1 | 0.86 | 0.76-1.20 |
| High school or GED | 29.0 | 0.89 | 0.81-1.16 |
| Some college | 30.5 | 0.93 | 0.86-1.13 |
| College or more | 32.2 | 1.00 | . . . |
| Missing education | 22.6 | 0.73 | 0.64-1.23 |
| Health insurance | |||
| Uninsured (55-64 y) | 9.7 | 0.44 | 0.37-1.32 |
| Medicaid (55-64 y) | 18.8 | 0.83 | 0.76-1.14 |
| Private (55-64 y) | 21.0 | 0.88 | 0.84-1.07 |
| Medicare (65-80 y) | 23.5 | 1.00 | . . . |
| Missing | |||
| 55-64 y | 19.7 | 0.89 | 0.82-1.14 |
| 65-80 y | 22.7 | 1.01 | 0.93-1.13 |
| Baseline scan year | |||
| 2015 | 24.9 | 1.00 | . . . |
| 2016 | 23.0 | 0.86 | 0.74-1.00 |
| 2017 | 23.4 | 0.88 | 0.75-1.03 |
| 2018 | 20.9 | 0.77 | 0.65-0.90 |
| Geographic region | |||
| Northeast | 25.2 | 1.00 | . . . |
| Midwest | 24.2 | 0.83 | 0.69-1.01 |
| South | 19.3 | 0.65 | 0.53-0.79 |
| West | 19.0 | 0.71 | 0.52-0.96 |
aOR = adjusted OR; GED = Graduate Educational Development; LCSR = Lung Cancer Screening Registry.
Annual examination was defined as people receiving a follow-up examination within 11-15 mo of the initial examination. If a second examination occurred < 11 mo after baseline, it was assumed to be a part of the workup of screening-detected abnormalities. Analyses were limited to individuals with Lung Imaging Reporting and Data System categories 1 and 2 at baseline examination. Models were adjusted for sex, age, race, smoking status, ethnicity, education, insurance type, geographic region, year, and facility category. Models account for correlation among patients within facilities.
Outcomes of Individuals Screened
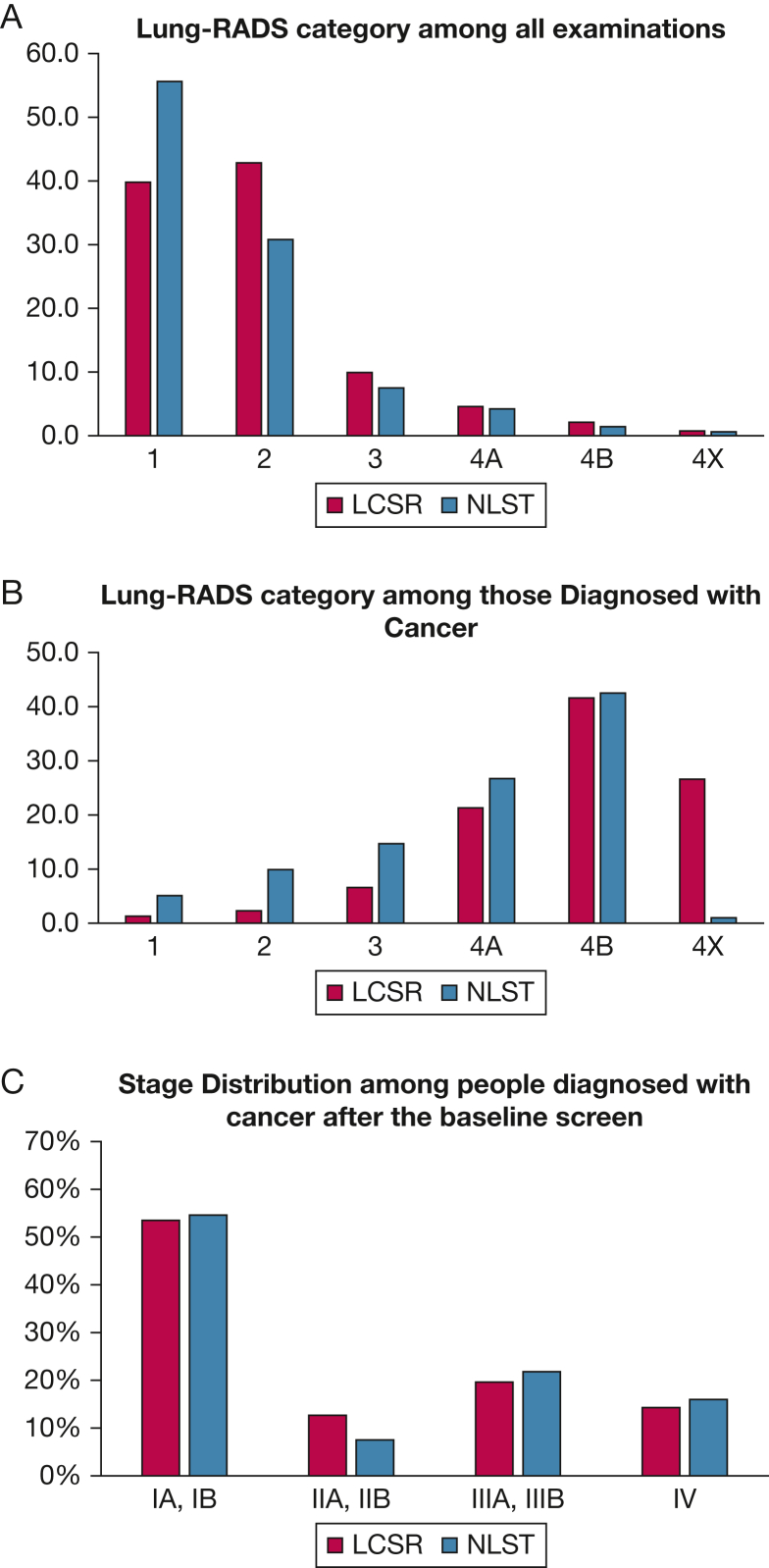
On baseline screening in individuals meeting USPSTF criteria, 82.6% showed negative screening results (Lung-RADS category 1, 39.8%; Lung-RADS category 2, 42.8%), and 17.3% showed positive screening results (Lung-RADS category 3, 9.9%; Lung-RADS category 4, 7.4%) (Fig 1, Table 3). In comparison, on repeat annual screening, nearly 90% of individuals screened showed negative results (Lung-RADS category 1, 35.6%; Lung-RADS category 2, 54.3%) and 9.7% showed positive results (Lung-RADS category 3, 5.2%; Lung-RADS category 4, 4.5%).
Figure 1.
A-C, Bar graphs showing Lung-RADS category distribution among all examinations (A) and those with a cancer diagnosis (B) and stage distribution (C) at baseline and annual examinations and follow-up. LCSR = Lung Cancer Screening Registry; Lung-RADS = Lung Imaging Reporting and Data System; NLST = National Lung Cancer Screening Trial.
Table 3.
Lung Cancer Screening Outcomes Among Baseline and Annual Examinations in the LCSR 2015-2019 and NLST
| Variable | Baseline Examination (T0) |
Annual Examination (T1) |
NLSTa |
|||
|---|---|---|---|---|---|---|
| No. | Percentage | No. | Percentage | No. | Percentage | |
| Lung-RADS category among all examinations | 1,052,591 | . . . | 209,611 | . . . | 26,455 | . . . |
| 1, Negative | 418,417 | 39.8 | 74,570 | 35.6 | 14,709 | 55.6 |
| 2, Benign appearance | 450,452 | 42.8 | 113,807 | 54.3 | 8,145 | 30.8 |
| 3, Probably benign | 104,358 | 9.9 | 10,869 | 5.2 | 1,697 | 7.5 |
| 4A, Probably suspicious | 48,043 | 4.6 | 5,841 | 2.8 | 1,397 | 4.2 |
| 4B, Suspicious, solid nodule | 22,137 | 2.1 | 3,536 | 1.7 | 358 | 1.4 |
| 4X, category 3 or 4 with additional features | 7,872 | 0.7 | 783 | 0.4 | 149 | 0.6 |
| Unknown | 8 | . . . | 5 | . . . | . . . | . . . |
| 0, Incomplete examinations | 1,304 | 0.1 | 200 | 0.1 | . . . | . . . |
| CDR | CDR | CDR | ||||
|---|---|---|---|---|---|---|
| Cancer diagnosis | ||||||
| Diagnoses of cancer | 5,882 | 0.56 | 721 | 0.3440 | 297 | 1.13 |
| 1, Negative | 77 | 0.0184 | 15 | 0.0201 | 15 | 0.102 |
| 2, Benign appearance | 134 | 0.0297 | 34 | 0.0299 | 29 | 0.356 |
| 3, Probably benign | 389 | 0.3728 | 46 | 0.4232 | 43 | 2.534 |
| 4A, Probably suspicious | 1,254 | 2.6102 | 139 | 2.3797 | 78 | 5.583 |
| 4B, Suspicious, solid nodule | 2,448 | 11.0584 | 357 | 10.0962 | 124 | 34.637 |
| 4X, category 3 or 4 with additional features | 1,564 | 19.8679 | 128 | 16.3474 | 3 | 2.013 |
| Percentage | Percentage | Percentage | ||||
|---|---|---|---|---|---|---|
| Lung-RADS category among those with a cancer diagnosis | ||||||
| 1, Negative | 77 | 1.3 | 15 | 2.1 | 15 | 5.1 |
| 2, Benign appearance | 134 | 2.3 | 34 | 4.7 | 29 | 9.9 |
| 3, Probably benign | 389 | 6.6 | 46 | 6.4 | 43 | 14.7 |
| 4A, Probably suspicious | 1,254 | 21.4 | 139 | 19.3 | 78 | 26.7 |
| 4B, Suspicious, solid nodule | 2,448 | 41.7 | 357 | 49.5 | 124 | 42.5 |
| 4X, category 3 or 4 with additional features | 1,564 | 26.7 | 128 | 17.8 | 3 | 1.0 |
| 0, Incomplete examination | 16 | . . . | 2 | . . . | . . . | . . . |
Cancer detection rates = (No. of cancers/No. of examinations) × 100. CDR = cancer detection rate; LCSR = Lung Cancer Screening Registry; NLST = National Lung Cancer Screening Trial.
From Pinsky et al.18
Subsequent to the 1,052,591 baseline examinations, 5,882 lung cancers were diagnosed, for a cancer detection rate of 0.56%, approximately one-half that reported in the NLST (Table 3). Among the 209,611 repeat annual screenings, 721 cancers were diagnosed, for a cancer detection rate of 0.34%, which is 70% less cancers than detected in the first year of follow-up in the NLST (PR, 0.30; 95% CI, 0.29, 0.34). Among 868,869 individuals with negative baseline screening results, cancer was detected in 211 people (0.2%) (Table 3). The percentage of people with cancer that was detected at baseline was higher in the positive Lung-RADS categories at 0.4% for Lung-RADS category 3, 2.6% for Lung-RADS category 4A, 11.1% for Lung-RADS category 4B, and 19.9% for Lung-RADS category 4X. Of cancers detected after a baseline screening, < 4% (211 of 5,882) occurred with negative screening results (Lung-RADS categories 1 or 2). A higher percentage was found with each increase in Lung-RADS score, with 6.6% for Lung-RADS category 3, 21% for Lung-RADS category 4A, and 41.6% for Lung-RADS category 4B (e-Table 5). Approximately 26.7% of cancers detected were classified as Lung-RADS category 4X (Fig 1A-1B, Table 3). A similar pattern was observed for cancers detected at annual repeat screenings.
Cancer stage was not documented in 26.0% of the cancers detected, and at baseline, 39.5% were stage I, 9.4% were stage II, 14.5% were stage III, and 10.5% were stage IV. Among cancers with known stage, the LCSR distribution was similar to that of the NLST, with 53.5% diagnosed at stage I, 12.7% diagnosed at stage II, 19.6% diagnosed at stage III, and 14.3% diagnosed at stage IV (Fig 1C, Table 4). Among cancers detected at repeat annual screening, 59.8% were diagnosed at stage I; the annual screening stage distribution was similar to the NLST annual repeat screening examination results (Table 4).
Table 4.
| Stage | Baseline (T0) |
Annual (T1) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LCSR |
NLST |
LCSR vs NLST |
LCSR |
NLST |
LCSR vs NLST |
|||||||||
| No. | Percentage | No. | Percentage | PR | 95% CI | No. | Percentage | No. | Percentage | PR | 95% CI | |||
| IA, IB | 2,326 | 53.5 | 160 | 54.6 | 0.98 | 0.85 | 1.13 | 307 | 59.8 | 108 | 59.0 | 1.01 | 0.85 | 1.22 |
| IIA, IIB | 551 | 12.7 | 22 | 7.5 | 1.69 | 0.38 | 7.47 | 61 | 11.9 | 18 | 9.8 | 1.21 | 0.26 | 5.77 |
| IIIA, IIIB | 850 | 19.6 | 64 | 21.8 | 0.90 | 0.55 | 1.46 | 84 | 16.4 | 33 | 18.0 | 0.91 | 0.38 | 2.18 |
| IV | 620 | 14.3 | 47 | 16.0 | 0.89 | 0.45 | 1.77 | 61 | 11.9 | 24 | 13.1 | 0.91 | 0.26 | 3.12 |
| Unknowna | 1,529 | … | 4 | … | … | … | … | 208 | … | 3 | … | … | … | … |
| Total | 5,882 | … | 297 | … | … | … | … | 513 | … | 186 | … | … | … | … |
| Total known | 4,347 | … | 293 | … | … | … | … | 721 | … | 183 | … | … | … | … |
LCSR = Lung Cancer Screening Registry; NLST = National Lung Cancer Screening Trial; PR = prevalence ratio.
NLST data are from Aberle et al. (2011)1.
In the LCSR, an additional six and two cancers were diagnosed at stage 0 at baseline and annually, respectively.
SE using the Delta method-derived formula: SE log(p1 / p2) = [(1 – p1) / (p1 × n1) + (1 – p2) / (p2 × n2)]0.5; CI = exp[ln(p1 / p2) ± 1.96 SE], where p1 is the observed proportion, n1 is number of individuals in the LCSR, p2 is the expected proportion, and n2 is the expected number of individuals in the NLST.
Discussion
Although LCS was endorsed by the USPSTF in 2013, few reports on national outcomes exist. This study provided important and robust insight into outcomes from LCS in the first 1 million people screened and catalogued in the ACR LCSR. Annual adherence to screening is unacceptably low overall, especially among individuals who currently smoke or are Hispanic or Black. Lung-RADS was effective at differentiating the LDCT scan examinations with abnormalities that were more or less likely to be cancer, with a higher percentage of cancers detected with each increase in Lung-RADS score. Finally, most screen-detected cancers were at an early stage and showed a similar distribution of cancer stages to the NLST.1 The percentage of screened individuals who were identified as having cancer at the baseline and annual repeat scannings was lower than that observed in the NLST, which may reflect underreporting in the registry or may be the result of poor adherence to annual screening. It is also possible that patients with screening-detected abnormalities were not receiving appropriate diagnostic testing and will demonstrate advanced cancer later with additional years of follow-up.
Adherence to successive annual screening was poor in this cohort, even when a more liberal definition was applied. The NLST, the Veterans Health Administration data, and data from a large cohort of patients across the United States documented 95%, 63%, and 48% adherence rates, respectively.1,19,20 In this study, factors significantly associated with poor adherence included current smoking, negative baseline screening results, minority population, and residing in the western or southern United States. Similar factors were found to impact veterans’ adherence to LCS and included those with lower income, negative baseline scanning results, and Black race.19 One multicenter cohort study of patients undergoing screening at five US health care systems also found that among people with negative baseline screening examination results, Black race was associated with reduced adherence, but only in those screened in a decentralized program.21 A centralized approach to LCS, although not always feasible, has been shown to improve adherence as compared with traditional decentralized screening conducted by primary care providers.21,22 Adherence must be emphasized, and interventions must be designed and tested to improve adherence to achieve optimal mortality benefit from LCS.
In this study, the percentage of cancers according to Lung-RADS category was similar to what was found in a prior study that applied Lung-RADS to the NLST data set for both baseline and annual repeat screenings.18 In that study, the cancer detection rate was very low (< 0.1%) in individuals with negative screening results (defined as Lung-RADS categories 1 and 2) and increased incrementally from approximately 0.4% for Lung-RADS category 3 to approximately 11% to 13% for Lung-RADS category 4B and 19.9% for Lung-RADS category 4X findings.
Although the percentage of cancer diagnoses with localized disease was improved when compared with that of historical control participants, the cancer detection rate after baseline screening of 0.56% was about one-half or less of baseline rates in both the NLST (1.1%) and the Veterans Health Administration demonstration project (1.5%) and lower than what was observed among men in the Dutch Belgian randomised lung cancer screening (NELSON) trial (0.9%).1,23,24 As noted already, a potential explanation for this finding is underreporting to the LCSR of cancers detected outside the screening facility, a possibility that warrants further investigation, as does the possibility of screening being embraced by relatively healthy first-adopters.
The demographic characteristics of individuals screened in the LCSR have been reported previously and highlight the fact that we are screening more people who currently smoke, more women, and more older patients than expected.10 Additional important differences compared with those eligible include education and insurance status. Individuals who were screened were better educated and more likely to have health insurance than the eligible population, consistent with use of other cancer screening tests and what was seen in NLST participants.25 The new USPSTF criteria have lowered the age for eligibility to reach more individuals at risk; however, uptake may remain poor because younger individuals with lower socioeconomic status may be less likely to be insured as opposed to their Medicare counterparts, who enjoy uniform health care coverage. One study estimated that of those eligible for screening, more than one-half of people 50 to 64 years of age are uninsured or have Medicaid, which does not always cover LCS.26 This, too, may contribute to racial disparities because many racial and ethnic populations are more likely to be underinsured or uninsured.27 Insurance status along with additional barriers to accessing preventive health care28,29 will continue to contribute to meager uptake of and adherence to LCS.26,30 Nationally, approximately 13% of eligible individuals have ever been screened, and 6% of eligible individuals had been screened in the previous year, with heterogeneity across states ranging from a high of nearly 20% in Massachusetts and 15% in Kentucky to a low of 2% in Nevada, California, and Wyoming.31 Those with a heavy smoking history tend to reside in areas of lower socioeconomic status and face multiple barriers accessing preventive health care,28,29 which likely contributes to screening uptake.
Interpretation
This study has several limitations. First, LCS underreporting may have occurred, and Veterans Health Administration and Department of Defense facilities are not required to report data; thus, generalizability of results to the population receiving care at these facilities may be limited. In addition, not all fields in the LCSR are mandatory, and underreporting of some data elements is likely. Additional efforts to better capture race or ethnicity, education, and insurance data are needed to improve the inferences that can be drawn from the LCSR, which is uniquely positioned to address critical questions on the social determinants of LCS implementation. Furthermore, we were able to examine adherence and outcomes only among people who received screening, and the low proportion of people who were Asian and Hispanic (representing < 5% of those screened) in our study, may reflect disparities in receipt of screening. Despite these limitations, our analyses of adherence and use according to race or ethnicity, education, and insurance coverage were in the expected direction and were consistent with findings from previous studies.32 The large and nationally representative data are the strength of this study.
The first 1 million screening examinations for lung cancer in the United States submitted to the American College of Radiology LCSR shed light on the demographic features of those being screened, the performance of Lung-RADS classification, lung cancer stage distribution, and factors associated with adherence. We are encouraged that the existing Lung-RADS classification system seems to be well calibrated to estimate the probability of cancer and that a high proportion of cancers detected are at an early stage. The data presented here highlight continued challenges to both uptake of and adherence to LCS. These findings confirm some of the previous work published in the screening literature (eg, overall adherence, confirmation that Lung-RADS classification works, and stage shift favoring early-stage disease) and further buttress them with the largest number of patients ever assembled and reported on the subject. Additionally, the fact that these screenings were from a nationwide population of differing health care settings and outside the auspices of a research trial make the results widely generalizable. The current study not only supports previous work, but also expands our current knowledge as it relates to the cancer detection rate, which is lower than previously reported, and adds to our knowledge of the predictors of poor adherence to screening. To understand the challenges of better implementing LCS nationally, studies that link this registry data with other databases, including Medicare and the Surveillance End Epidemiology Results cancer registry, are needed. They could confirm or refute the findings herein and aid with other analyses, such as the type of testing and treatment undertaken in patients with screening-detected abnormalities, as well as other related outcomes of interest such as 5-year survivorship. Unfortunately, the new CMS guidelines no longer require data submission to the LCSR, and this may be our last chance to have truly nationally representative data on screening, although it should be noted that for now, most institutions continue to contribute data to the registry voluntarily. In summary, multilevel interventions that include policy, health system, provider, and patient education are needed both to improve uptake and to reduce the disparities associated with screening for lung cancer. Because the new USPSTF criteria increase the number of individuals eligible, the lung cancer community should leverage this experience to work toward improving delivery, uptake, and adherence to target those who stand to benefit the most.
Funding/Support
The authors have reported to CHEST that no funding was received for this study.
Financial/Nonfinancial Disclosures
None declared.
Acknowledgments
Author contributions: G. S. served as principal author. G. S. and S. F. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis and intepretation and the writing of the manuscript. L. G., N. T., J. B., M. G., E. K., P. M., M. P. R., P. D. R., L. R., M. S., and R. S. contributed substantially to the study design, data analysis and interpretation, and the writing of the manuscript.
Other contributions: The authors thank Bruce Johnson, MD, for his thoughtful review and insightful comments, which strengthened this article.
Disclaimer: The views expressed hereing are those of the authors only and do not represent any official position of the National Cancer Institute or National Institutes of Health.
Additional information: The e-Figure and e-Tables are available online under “Supplementary Data.”
Footnotes
P. R. is currently at the Division of Pulmonary and Critical Care, University of Rochester Medical Center (Rochester, NY).
Supplementary Data
References
- 1.National Lung Screening Trial Research Team. Aberle D.R., Adams A.M., et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moyer V.A., United States Preventive Services Task Force Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330–338. doi: 10.7326/M13-2771. [DOI] [PubMed] [Google Scholar]
- 3.Koh H.K., Sebelius K.G. Promoting prevention through the Affordable Care Act. N Engl J Med. 2010;363(14):1296–1299. doi: 10.1056/NEJMp1008560. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Medicare and Medicaid Services Decision memo for screening for lung cancer with low dose computed tomography (LDCT) (CAG-00439N). 2015. Centers for Medicare and Medicaid Services website. https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274
- 5.He D., McInerney M., Mellor J. Physician responses to rising local unemployment rates: healthcare provision to Medicare and privately insured patients. J Health Econ. 2015;40:97–108. doi: 10.1016/j.jhealeco.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 6.American College of Radiology Lung Cancer Screening Registry. 2022. https://www.acr.org/Practice-Management-Quality-Informatics/Registries/Lung-Cancer-Screening-Registry American College of Radiology website.
- 7.Landy R., Young C.D., Skarzynski M., et al. Using prediction models to reduce persistent racial and ethnic disparities in the draft 2020 USPSTF lung cancer screening guidelines. J Natl Cancer Inst. 2021;113(11):1590–1594. doi: 10.1093/jnci/djaa211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Medicare and Medicaid Services Screening for lung cancer with low dose computed tomography (LDCT) decision memo. 2021. https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=Y&ncaid=304& Centers for Medicare and Medicaid Services website.
- 9.Mazzone P.J., Silvestri G.A., Patel S., et al. Screening for lung cancer: CHEST guideline and expert panel report. Chest. 2018;153(4):954–985. doi: 10.1016/j.chest.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 10.Silvestri G.A., Goldman L., Burleson J., et al. Characteristics of Persons Screened for Lung Cancer in the United States. Ann Int Med. 2022;175(11):1501–1505. doi: 10.7326/M22-1325. [DOI] [PubMed] [Google Scholar]
- 11.National Center for Health Statistics, Centers for Disease Control and Prevention 2015 national health interview survey. 2015. https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm Centers for Disease Control and Prevention website.
- 12.Hanley J.A., Negassa A., Edwardes M.D., Forrester J.E. Statistical analysis of correlated data using generalized estimating equations: an orientation. Am J Epidemiol. 2003;157(4):364–375. doi: 10.1093/aje/kwf215. [DOI] [PubMed] [Google Scholar]
- 13.Tanner N.T., Brasher P.B., Wojciechowski B., et al. Screening adherence in the Veterans Administration Lung Cancer Screening Demonstration Project. Chest. 2020;158(4):1742–1752. doi: 10.1016/j.chest.2020.04.063. [DOI] [PubMed] [Google Scholar]
- 14.Hubbard R.A., O'Meara E.S., Henderson L.M., et al. Multilevel factors associated with long-term adherence to screening mammography in older women in the U.S. Prev Med. 2016;89:169–177. doi: 10.1016/j.ypmed.2016.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Vlugt M., Grobbee E.J., Bossuyt P.M., et al. Adherence to colorectal cancer screening: four rounds of faecal immunochemical test-based screening. Br J Cancer. 2017;116(1):44–49. doi: 10.1038/bjc.2016.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tammemagi M.C., Ruparel M., Tremblay A., et al. USPSTF2013 versus PLCOm2012 lung cancer screening eligibility criteria (International Lung Screening Trial): interim analysis of a prospective cohort study. Lancet Oncol. 2022;23(1):138–148. doi: 10.1016/S1470-2045(21)00590-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernandez M.A.F. Delta method in epidemiology: an applied and reproducible tutorial. 2020. https://migariane.github.io/DeltaMethodEpiTutorial.nb.html github website.
- 18.Pinsky P.F., Gierada D.S., Black W., et al. Performance of Lung-RADS in the National Lung Screening Trial: a retrospective assessment. Ann Intern Med. 2015;162(7):485–491. doi: 10.7326/M14-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Núñez E.R., Caverly T.J., Zhang S., et al. Adherence to follow-up testing recommendations in US veterans screened for lung cancer, 2015-2019. JAMA Netw Open. 2021;4(7) doi: 10.1001/jamanetworkopen.2021.16233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith H.B., Schneider E., Tanner N.T. An evaluation of annual adherence to lung cancer screening in a large national cohort. Am J Prev Med. 2022;63(2):e59–e64. doi: 10.1016/j.amepre.2022.01.016. [DOI] [PubMed] [Google Scholar]
- 21.Kim R.Y., Rendle K.A., Mitra N., et al. Racial disparities in adherence to annual lung cancer screening and recommended follow-up care: a multicenter cohort study. Ann Am Thorac Soc. 2022;19(9):1561–1569. doi: 10.1513/AnnalsATS.202111-1253OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith H.B., Ward R., Frazier C., Angotti J., Tanner N.T. Guideline-recommended lung cancer screening adherence is superior with a centralized approach. Chest. 2022;161(3):818–825. doi: 10.1016/j.chest.2021.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Kinsinger L.S., Anderson C., Kim J., et al. Implementation of lung cancer screening in the Veterans Health Administration. JAMA Intern Med. 2017;177(3):399–406. doi: 10.1001/jamainternmed.2016.9022. [DOI] [PubMed] [Google Scholar]
- 24.de Koning H.J., van der Aalst C.M., de Jong P.A., et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382(6):503–513. doi: 10.1056/NEJMoa1911793. [DOI] [PubMed] [Google Scholar]
- 25.Goding Sauer A., Siegel R.L., Jemal A., Fedewa S.A. Current prevalence of major cancer risk factors and screening test use in the United States: disparities by education and race/ethnicity. Cancer Epidemiol Biomarkers Prev. 2019;28(4):629–642. doi: 10.1158/1055-9965.EPI-18-1169. [DOI] [PubMed] [Google Scholar]
- 26.Jemal A., Fedewa S.A. Lung cancer screening with low-dose computed tomography in the United States—2010 to 2015. JAMA Oncol. 2017;3(9):1278–1281. doi: 10.1001/jamaoncol.2016.6416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lozier J.W., Fedewa S.A., Smith R.A., Silvestri G.A. Lung cancer screening eligibility and screening patterns among black and white adults in the United States. JAMA Netw Open. 2021;4(10) doi: 10.1001/jamanetworkopen.2021.30350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller E.A., Pinsky P.F. Healthcare access, utilization, and preventive health behaviors by eligibility for lung cancer screening. J Cancer Educ. 2021;36(2):330–337. doi: 10.1007/s13187-019-01634-y. [DOI] [PubMed] [Google Scholar]
- 29.Silvestri G.A., Nietert P.J., Zoller J., Carter C., Bradford D. Attitudes towards screening for lung cancer among smokers and their non-smoking counterparts. Thorax. 2007;62(2):126–130. doi: 10.1136/thx.2005.056036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richards T.B., Soman A., Thomas C.C., et al. Screening for lung cancer—10 states, 2017. MMWR Morb Mortal Wkly Rep. 2020;69(8):201–206. doi: 10.15585/mmwr.mm6908a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fedewa S.A., Bandi P., Smith R.A., Silvestri G.A., Jemal A. Lung cancer screening rates during the COVID-19 pandemic. Chest. 2022;161(2):586–589. doi: 10.1016/j.chest.2021.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopez-Olivo M.A., Maki K.G., Choi N.J., et al. Patient adherence to screening for lung cancer in the US: a systematic review and meta-analysis. JAMA Netw Open. 2020;3(11) doi: 10.1001/jamanetworkopen.2020.25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.