Abstract
Purpose
Multiple sclerosis (MS) is a chronic disease of the central nervous system that disproportionately affects women, with typical onset during reproductive age. Several disease-modifying therapies (DMTs) are FDA-approved to slow disease progression, but are not indicated for use during pregnancy. Our objective was to describe trends over time (2010–2019) in monthly point prevalence of DMT use among reproductive-age women, overall and by generic name.
Methods
This study used administrative claims data from the US during 2009–2019 to identify women age 15–45 with MS and continuous insurance coverage for ≥12 months. DMTs were identified using prescription fills and procedural claims for alemtuzumab, daclizumab, dimethyl fumarate, fingolimod, glatiramer acetate, interferon beta, mitoxantrone, natalizumab, ocrelizumab, and teriflunomide. Monthly prevalent use was defined as ≥1 days’ supply of a DMT in the month. Age- and region-standardized monthly prevalence was estimated nonparametrically.
Results
Among 42,281 reproductive-aged women over 818,179 person-months, DMT use increased from a minimum monthly prevalence of 49.3% (February 2011) to a maximum of 58.7% (April 2019). In 2010, prevalence of injectable DMTs was 43.1% compared to 2.5% for oral DMTs; by 2014, however, oral DMTs (26.5%) surpassed injectable DMTs (23.7%) as the most common route of administration. In the most recent data available (December 2019), the most common DMTs were dimethyl fumarate, glatiramer acetate, and fingolimod.
Conclusions
DMT use among reproductive-aged women has rapidly evolved during the past decade. Collaborative treatment decision making between women with MS and clinicians may help optimize MS care and improve DMT uptake during reproductive years.
Keywords: multiple sclerosis, disease-modifying therapies, women’s health, drug utilization, dimethyl fumarate, glatiramer acetate, fingolimod
Introduction
Multiple sclerosis (MS) is a chronic disease of the central nervous system. MS is more common in women than men and disease onset typically occurs during reproductive years.1 Relapsing-remitting MS, which constitutes 80–90% of new MS cases, is characterized by exacerbations of neurologic symptoms and worsening of neurologic function, followed by periods of symptom improvement.2 Approximately 10–15% of MS cases have a primary progressive onset, characterized by progressive worsening of neurologic function without periods of exacerbations.2
There is no cure for MS, and disease-modifying therapies (DMTs) are indicated to reduce the accumulation of brain lesions, slow disability, and prevent relapses. Several DMTs have been approved by the Food and Drug Administration (FDA) during the past decade. DMTs are currently available in injectable, infused, and oral forms. Guidelines advise starting DMTs early following the initial diagnosis of MS and continuous adherence and persistence to medications are associated with better outcomes.3 Choice of which treatment to initiate is generally made based on patient and provider preferences and considers efficacy, adherence, tolerability, costs, and potential drug interactions.3
No DMTs are approved for the treatment of MS during pregnancy and the safety profiles of DMTs during pregnancy remain largely unknown.4 Treatment guidelines advise women to discontinue DMTs prior to pregnancy unless there is high risk of relapse or disease progression.3 However, there are safety concerns related to withdrawing DMTs during pregnancy, including a potential for rebound in disease activity.4 Between one-fifth and one-third of women with MS deliver a child after disease onset.5
Recent studies on treatment utilization in patients with MS6–8 have not reported utilization in reproductive-aged women who have complex health concerns related to family planning. The objective of our study was to describe trends in the monthly prevalence of DMT use, overall and by type of DMT, among commercially-insured, reproductive-aged women with MS from 2010–2019.
Methods
Data source and study population
Our study utilized the IBM Watson Health MarketScan Commercial Claims and Encounters database from 2009–2019. For each calendar month between 2010 and 2019, we identified reproductive-aged women (15–44 years) who were continuously enrolled during the 12 months prior (baseline period; Supplemental Figure S1, Supplemental Figure S2). Women were required to have at least one inpatient or two outpatient diagnosis codes for MS (International Classification of Disease, Ninth Revision, Clinical Modification [ICD-9-CM]: 340; ICD-10-CM: G35) during the baseline period. Women contributed to all months where they met eligibility criteria.
Study variables
DMTs were identified using generic names in outpatient prescription claims and Healthcare Common Procedural Coding System codes in inpatient and outpatient services files (Supplemental Table S1). Fills with overlapping days’ supply were assumed to be taken sequentially. Days covered by DMTs identified using procedure codes were based on prescribing information (Supplemental Table S2, Supplemental Figure S3). The injectable DMTs considered included interferon beta (IFNβ-1a, IFNβ-1b, peginterferon beta 1a) and glatiramer acetate; infused DMTs included alemtuzumab, daclizumab, mitoxantrone, natalizumab, and ocrelizumab; and oral DMTs were dimethyl fumarate, fingolimod, and teriflunomide.
Baseline comorbidities, symptoms, medications, and healthcare utilization were assessed using diagnosis and procedure codes during the baseline period (Supplemental Table S1). Baseline MS relapses were identified using a validated claims-based algorithm.9,10 The algorithm defines relapses as either a hospitalization with a primary diagnosis of MS or an outpatient visit with a diagnosis of MS followed by a claim for corticosteroids within 7 days.
Statistical analysis
Baseline characteristics were summarized using descriptive statistics stratified by time period (2010–2013, 2014–2016, 2017–2019). The time periods were chosen based on FDA approvals for dimethyl fumarate (2013) and ocrelizumab (2017). We computed the monthly point prevalence as the number of women with at least 1 days’ supply of a DMT in the month, divided by the number of women meeting inclusion criteria in that month. We estimated 95% confidence intervals (CIs) using generalized estimating equations with an independent correlation structure to account for repeated observations within women. Prevalence estimates were standardized by age and region using direct standardization.11 The proportion of women in groups defined by age (15–24, 25–34, and 35–44 years) and region (South, North Central, Northeast, West) in December 2019 served as the standard population. We also assessed differences in monthly point prevalence of DMT use by age group during the period from 2017–2019. All analyses were performed using SAS Version 9.4 (SAS Institute, Cary, NC).
Results
Our study included 42,281 reproductive-aged women with MS who contributed 818,179 person-months to the study. The number of women in the study population in each month ranged from 5356 to 9126. Median age was stable over time at 38 years (Table 1). Twenty-five to thirty-three percent of women experienced at least one relapse during the baseline period, and the mean number of baseline relapses ranged from 0.4 to 0.5. Pain, mental illness or substance abuse, and fatigue were common. Baseline use of antidepressants, muscle spasm and anticholinergic medications, and corticosteroids was also high.
Table 1.
Demographic and clinical characteristics of reproductive-aged women with multiple sclerosis in the IBM Watson Health MarketScan Commercial Claims and Encounters database from 2010–2019, by time period
| Characteristic | 2010–2013 |
2014–2016 |
2017–2019 |
|||
|---|---|---|---|---|---|---|
| 358,817 PMs (23,672 women) |
251,589 PMs (17,567 women) |
207,773 PMs (13,725 women) |
||||
|
| ||||||
| Demographics | ||||||
|
| ||||||
| Age, mean ± SD | 36.4 | (6.1) | 36.4 | (6.2) | 36.4 | (6.2) |
| Median (IQR) | 38 | (33, 41) | 38 | (33, 42) | 38 | (33, 41) |
| Age categories, n (%) | ||||||
| 15–24 | 18,081 | (5.0) | 15,243 | (6.1) | 12,207 | (5.9) |
| 25–34 | 101,177 | (28.2) | 66,752 | (26.5) | 55,376 | (26.7) |
| 35–44 | 239,559 | (66.8) | 169,594 | (67.4) | 140,190 | (67.5) |
| Region, n (%) | ||||||
| South | 135,498 | (38.2) | 103,478 | (41.5) | 93,358 | (45.0) |
| North Central | 97,332 | (27.4) | 54,278 | (21.8) | 43,261 | (20.8) |
| Northeast | 66,874 | (18.8) | 51,719 | (20.8) | 42,646 | (20.5) |
| West | 55,335 | (15.6) | 39,667 | (15.9) | 28,311 | (13.6) |
| Unknown | 3,778 | 2,447 | 197 | |||
|
| ||||||
| Baseline relapses | ||||||
|
| ||||||
| Any baseline relapse, n (%) | 88,849 | (24.8) | 65,722 | (26.1) | 67,817 | (32.6) |
| Number of baseline relapses, mean (SD) | 0.4 | (0.8) | 0.4 | (0.8) | 0.5 | (0.9) |
| Median (IQR) | 0 | (0, 0) | 0 | (0, 1) | 0 | (0, 1) |
|
| ||||||
| Baseline comorbidities and symptoms | ||||||
|
| ||||||
| Pain, n (%) | 161,435 | (45.0) | 120,400 | (47.9) | 98,716 | (47.5) |
| Prior mental illness or substance use disorder, n (%) | 108,051 | (30.1) | 92,839 | (36.9) | 87,931 | (42.3) |
| Fatigue, n (%) | 88,597 | (24.7) | 73,583 | (29.2) | 64,707 | (31.1) |
| Thyroid disorder, n (%) | 45,217 | (12.6) | 35,273 | (14.0) | 31,068 | (15.0) |
| Hypertension, n (%) | 42,637 | (11.9) | 33,961 | (13.5) | 29,947 | (14.4) |
| Hyperlipidemia, n (%) | 38,181 | (10.6) | 26,725 | (10.6) | 21,796 | (10.5) |
| CCI, n (%) | ||||||
| 0 | 284,081 | (79.2) | 197,191 | (78.4) | 163,020 | (78.5) |
| 1 | 51,444 | (14.3) | 37,046 | (14.7) | 28,397 | (13.7) |
| 2 | 16,157 | (4.5) | 11,711 | (4.7) | 10,707 | (5.2) |
| 3+ | 7,135 | (2.0) | 5,641 | (2.2) | 5,649 | (2.7) |
| Conditions of the CCI, n (%) | ||||||
| COPD | 30,194 | (8.4) | 23,951 | (9.5) | 21,128 | (10.2) |
| Uncomplicated diabetes mellitus | 15,559 | (4.3) | 11,850 | (4.7) | 8,823 | (4.2) |
| Cerebrovascular disease | 17,256 | (4.8) | 10,839 | (4.3) | 6,936 | (3.3) |
| Cancer without metastases | 7,062 | (2.0) | 4,368 | (1.7) | 4,095 | (2.0) |
| Rheumatic disease | 6,692 | (1.9) | 4,762 | (1.9) | 3,961 | (1.9) |
| Hemiplegia | 4,503 | (1.3) | 3,522 | (1.4) | 3,227 | (1.6) |
| Diabetes mellitus with chronic complications | 2,462 | (0.7) | 2,676 | (1.1) | 4,146 | (2.0) |
| Peripheral vascular disease | 2,595 | (0.7) | 1,927 | (0.8) | 1,856 | (0.9) |
| Moderate to severe renal disease | 1,693 | (0.5) | 1,438 | (0.6) | 1,281 | (0.6) |
| Peptic ulcer disease | 1,531 | (0.4) | 993 | (0.4) | 989 | (0.5) |
| Congestive heart failure | 1,225 | (0.3) | 927 | (0.4) | 723 | (0.3) |
| Metastatic solid tumor | 769 | (0.2) | 580 | (0.2) | 610 | (0.3) |
| Prior myocardial infarction | 688 | (0.2) | 542 | (0.2) | 480 | (0.2) |
| Mild liver disease | 571 | (0.2) | 561 | (0.2) | 382 | (0.2) |
| Dementia | 331 | (0.1) | 266 | (0.1) | 296 | (0.1) |
| HIV/AIDS | 406 | (0.1) | 148 | (0.1) | 188 | (0.1) |
| Moderate to severe liver disease | 174 | (0.0) | 113 | (0.0) | 91 | (0.0) |
|
| ||||||
| Baseline drug use | ||||||
|
| ||||||
| Antidepressants | 153,586 | (42.8) | 104,754 | (41.6) | 85,671 | (41.2) |
| Muscle spasm and anticholinergic | 144,172 | (40.2) | 103,233 | (41.0) | 83,970 | (40.4) |
| Corticosteroid | 119,452 | (33.3) | 87,115 | (34.6) | 81,496 | (39.2) |
| Fatigue medication | 59,628 | (16.6) | 35,517 | (14.1) | 26,256 | (12.6) |
| Anti-hypertensives | 51,265 | (14.3) | 37,764 | (15.0) | 31,049 | (14.9) |
| Urinary continence treatment | 33,136 | (9.2) | 21,862 | (8.7) | 16,684 | (8.0) |
| Anti-hyperlipidemia | 15,071 | (4.2) | 10,262 | (4.1) | 6,603 | (3.2) |
| Dalfampridine | 11,845 | (3.3) | 8,152 | (3.2) | 6,516 | (3.1) |
|
| ||||||
| Baseline healthcare utilization | ||||||
|
| ||||||
| Any inpatient admission, n (%) | 53,356 | (14.9) | 33,263 | (13.2) | 27,279 | (13.1) |
| Any outpatient visit, n (%) | 356,845 | (99.5) | 250,346 | (99.5) | 206,558 | (99.4) |
| Number of outpatient visits, mean (SD) | 8.1 | (5.9) | 8.1 | (6.5) | 8.1 | (6.1) |
| Median (IQR) | 7 | (4, 10) | 7 | (4, 10) | 7 | (4, 11) |
| Any ER visit, n (%) | 108,433 | (30.2) | 72,640 | (28.9) | 60,007 | (28.9) |
| Number of ER visits, mean (SD) | 0.6 | (1.6) | 0.6 | (1.6) | 0.6 | (1.6) |
| Median (IQR) | 0 | (0, 1) | 0 | (0, 1) | 0 | (0, 1) |
Abbreviations: AIDS=acquired immunodeficiency syndrome; CCI=Charlson Comorbidity Index; COPD=chronic obstructive pulmonary disease; ER=emergency room; HIV=human immunodeficiency virus; IQR=interquartile range; PMs=person-months; SD=standard deviation.
The age- and region-standardized prevalence of any DMT use increased steadily over time, with a minimum prevalence of 49.3% (95% CI, 48.2–50.5%) in February 2011 and a maximum prevalence of 58.7% (95% CI, 57.6–60.1%) in April 2019 (Figure 1, Panel A). Oral DMTs were the most common type in later years, surpassing injectable DMTs in 2014 (Figure 1, Panel A). In the most recent years of data (2017–2019), utilization of DMTs increased with increasing age (Figure 1, Panel B), although the distribution of type of DMT was proportional across age groups. Utilization of DMTs by age for earlier years is provided in Supplemental Figure S4 and Supplemental Figure S5.
Figure 1. Prevalence of disease-modifying therapies from 2010–2019.
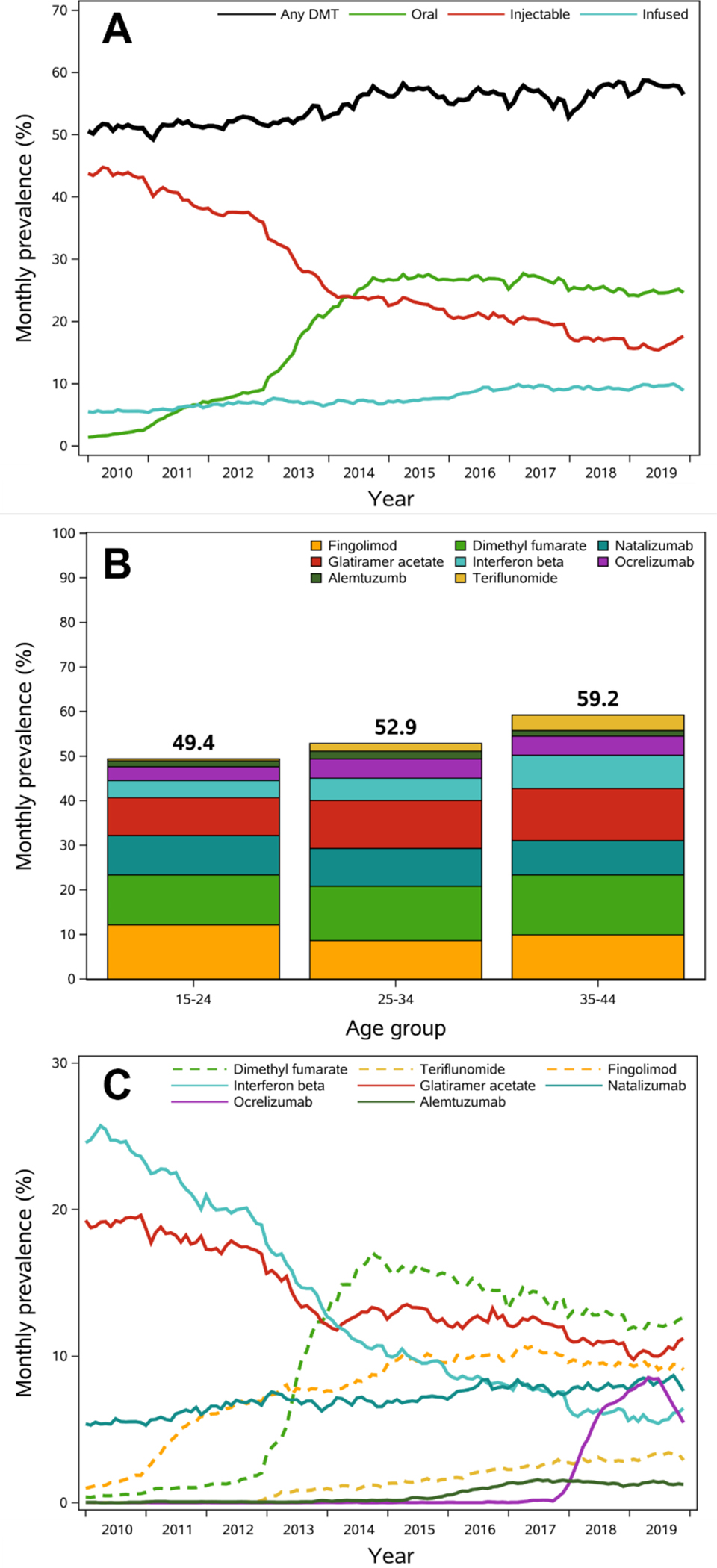
Abbreviations: DMT=disease modifying therapy.
A. Age- and region-standardized monthly prevalence of DMT use over time, overall and by route of administration.
B. Average monthly prevalence of DMT use during the period from 2017–2019 by age category. Daclizumab and mitoxantrone are not presented since prevalence was low across the entire study follow-up.
C. Age- and region-standardized monthly prevalence of DMT use over time, by generic name. Dashed lines represent oral DMTs, and solid lines represent infused or injected DMTs. Daclizumab and mitoxantrone are not presented since prevalence was low across the entire study follow-up (max monthly prevalence mitoxantrone: 0.1% in May 2010; daclizumab: 0.2% in September 2017).
Interferon beta was the most common DMT in 2010, with a peak monthly point prevalence of 25.7% in April 2010 that declined thereafter (Figure 1, Panel C). Prevalence of glatiramer acetate use declined steadily over time from a peak prevalence of 19.6% in December 2010 to a minimum of 9.8% in February 2019, but remained the second-most common DMT during later years. Dimethyl fumarate became the most common DMT in 2014, with a monthly prevalence of use after 2014 ranging from 11.8–17.1%. Use of fingolimod increased drastically after its approval in 2010, with a peak prevalence of 10.7% in April 2017. Fingolimod remained the third most common DMT in 2019. The prevalence of natalizumab use was stable across years, ranging from 5.3% in January 2011 to 8.7% in October 2019. Prevalence of ocrelizumab use increased sharply after its approval in 2017, peaking at 8.5% in May 2019. Use of alemtuzumab, teriflunomide, mitoxantrone, and daclizumab were low across all years.
Discussion
Our study is the first to describe trends in DMT use among reproductive-aged women with MS using contemporary US data. We found that during the past decade the prevalence of DMT use has ranged from 49.3–58.7% and that treatment patterns have rapidly changed. In recent years, dimethyl fumarate, glatiramer acetate, and fingolimod were the most common DMTs. Prevalence of ocrelizumab has seen the sharpest increase since its approval in 2017. Daclizumab use was low in all years, even prior to its withdrawal from the global market in March 2018.
There is strong evidence that DMTs are effective for reducing relapses and slowing the accumulation of brain lesions.3 Earlier initiation can improve outcomes and DMTs are recommended to be continued indefinitely once disease is controlled if no adverse events occur.3 Despite this, our study found that between 40–50% of women did not receive DMTs during each month. Some of this may be due to decisions to avoid DMTs during family planning. However, low utilization may also reflect suboptimal adherence and persistence, which have been reported in previous studies.3,7,12 We also found that the prevalence of DMT use was lower in younger age groups. Although evidence for the safety and effectiveness of certain DMTs for pediatric patients remains uncertain, patients <18 years of age are recommended to initiate treatment with a beta interferon or glatiramer acetate shortly after diagnosis.13
Reproductive-aged women have the highest incidence of MS and there remains a paucity of information on the safety of DMTs during pregnancy. In addition, due to the chronic nature of MS, treatment patterns in older populations differ than younger populations due to disease progression and subsequent discontinuations and treatment switches.14 Prior studies have reported high frequencies of DMT exposure during pregnancy for women with MS, particularly during the first trimester. In an analysis of the MarketScan database, 35% of women were exposed to DMTs during the 90 days prior to pregnancy and approximately a quarter were exposed during the first trimester.15 In the MSBase Registry, 42% of reported pregnancies were conceived while on a DMT.16 While minimal risks have been reported for the older injectable DMTs, evidence on newer agents remains limited.4,17 Future research on the safety of DMTs during pregnancy is vital, particularly for the DMTs that were found to have the highest utilization in our study.
Results of this study should be interpreted considering several limitations. First, our study was conducted in an employer-sponsored insurance claims database and women who are uninsured or covered by publicly-funded insurance are not represented. This population is not negligible, since many women with MS may be covered under federal insurance programs and 43% of births are covered under Medicaid.18 In addition, the high and variable costs of DMTs may drive utilization patterns in this study population of commercially-insured women, and may lead to different patterns in uninsured or publicly-insured women. The median annual wholesale acquisition costs for DMTs was $91,835 in 2020 and patients often face high out-of-pocket costs.19 Second, several new DMTs, such as ofatumumab, diroximel fumarate, siponimod, cladribine, ozanimod, and monomethyl fumarate have been approved for treatment of MS since 2019 and we were not able to capture them in our study. Third, outpatient prescription claims only capture fills and may not reflect actual consumption of medications. Alternatively, DMT use captured using procedure codes are likely very accurate. Finally, in order to evaluate monthly point prevalence, eligibility criteria for our cohort were applied at the person-month level to provide a set of serial snapshots of DMT use. In turn, this study cannot be used to evaluate duration of therapy, treatment discontinuation, and treatment switches at the individual level. The requirements for MS diagnosis codes during baseline may also have led to excluding some less severe cases of MS from our month-level denominator. However, women with MS tend to have high healthcare resource utilization,20 so we anticipate the number of women excluded based on these criteria to be small.
Our study summarized trends in utilization of DMTs among reproductive-aged women in a rapidly changing treatment landscape. Collaborative treatment discussions and decision making between women with MS and clinicians may help optimize MS care and improve DMT uptake during reproductive years. Finally, a greater understanding is needed about the safety of DMTs during pregnancy.
Supplementary Material
Key points.
Among a cohort of commercially insured women aged 15–45 years with multiple sclerosis in the US, the monthly point prevalence of disease-modifying therapy (DMT) use varied between 49.3–58.7% between 2010–2019.
Treatment patterns have rapidly evolved during this period with oral DMTs overtaking injectable DMTs as the most common formulation after 2014.
Dimethyl fumarate, glatiramer acetate, and fingolimod were the most common DMTs by the end of the study period (December 2019).
DMT use increased with increasing age, but utilization of specific DMTs was proportional across age groups.
Funding statement:
This project was supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through Grant Award Number UL1TR002489. Emilie Duchesneau is supported by the Cancer Care Quality Training Program at the University of North Carolina at Chapel Hill (grant T32CA116339). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of interest disclosure: ED and JL have received salary support from AbbVie, Inc. for unrelated work. GlaxoSmithKline (GSK), AbbVie, Boehringer Ingelheim, Takeda and UCB have collaborative agreements with the Center for Pharmacoepidemiology, Department of Epidemiology, University of North Carolina at Chapel Hill. MJF receives salary support as Director of the Center. MJF is a member of the Scientific Steering Committee (SSC) for a post-approval safety study funded by GSK. All compensation for services provided on the SSC is invoiced by and paid to UNC Chapel Hill. These companies do not review any research nor provide any input into the analysis of the drug classes being studied.
Ethics/IRB statement: This study was determined to be exempt by the Office of Human Research Ethics of the University of North Carolina at Chapel Hill (#19–1801).
Patient consent statement: This study uses data collected for administrated purposes and does not involve interacting with human participants or direct identifiers. No patient consent was needed.
Prior presentations: Preliminary results from this work were presented at the virtual International Conference on Pharmacoepidemiology and Therapeutic Risk Management (ICPE) All Access meeting, September 16–17, 2020.
References
- 1.Rankin K, Bove R. Caring for Women with Multiple Sclerosis Across the Lifespan. Curr Neurol Neurosci Rep. 2018;18(7):36. [DOI] [PubMed] [Google Scholar]
- 2.Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83(3):278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rae-Grant A, Day GS, Marrie RA, et al. Practice guideline recommendations summary: Disease-modifying therapies for adults with multiple sclerosis: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90(17):777–788. [DOI] [PubMed] [Google Scholar]
- 4.Alroughani R, Altintas A, Al Jumah M, et al. Pregnancy and the Use of Disease-Modifying Therapies in Patients with Multiple Sclerosis: Benefits versus Risks. Mult Scler Int. 2016;2016:1034912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Houtchens MK, Edwards NC, Schneider G, Stern K, Phillips AL. Pregnancy rates and outcomes in women with and without MS in the United States. Neurology. 2018;91(17):e1559–e1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cisternas M, Bartolome L, Gitar B, et al. Health care resource utilization and disease modifying treatment use in multiple sclerosis patients by age and insurance type. Curr Med Res Opin. 2021;37(4):597–604. [DOI] [PubMed] [Google Scholar]
- 7.Bowen J, Mehta R, Pelletier C, et al. Treatment Patterns Among Patients with Multiple Sclerosis Initiating Second-Line Disease-Modifying Therapy. Adv Ther. 2020;37(7):3163–3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freeman L, Kee A, Tian M, Mehta R. Evaluating Treatment Patterns, Relapses, Healthcare Resource Utilization, and Costs Associated with Disease-Modifying Treatments for Multiple Sclerosis in DMT-Naive Patients. Clinicoecon Outcomes Res. 2021;13:65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ollendorf DA, Jilinskaia E, Oleen-Burkey M. Clinical and economic impact of glatiramer acetate versus beta interferon therapy among patients with multiple sclerosis in a managed care population. J Manag Care Pharm. 2002;8(6):469–476. [DOI] [PubMed] [Google Scholar]
- 10.Chastek BJ, Oleen-Burkey M, Lopez-Bresnahan MV. Medical chart validation of an algorithm for identifying multiple sclerosis relapse in healthcare claims. J Med Econ. 2010;13(4):618–625. [DOI] [PubMed] [Google Scholar]
- 11.Greenland S, Rothman KJ. Introduction to Stratified Analysis. In: Rothman KJ, Greenland S, Lash TL, eds. Modern Epidemiology. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008:258–282. [Google Scholar]
- 12.Higuera L, Carlin CS, Anderson S. Adherence to Disease-Modifying Therapies for Multiple Sclerosis. J Manag Care Spec Pharm. 2016;22(12):1394–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chitnis T, Tenembaum S, Banwell B, et al. Consensus statement: evaluation of new and existing therapeutics for pediatric multiple sclerosis. Mult Scler. 2012;18(1):116–127. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Salter A, Jin S, et al. Disease-modifying therapy prescription patterns in people with multiple sclerosis by age. Ther Adv Neurol Disord. 2021;14:17562864211006499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacDonald SC, McElrath TF, Hernandez-Diaz S. Use and safety of disease-modifying therapy in pregnant women with multiple sclerosis. Pharmacoepidemiol Drug Saf. 2019;28(4):556–560. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen AL, Havrdova EK, Horakova D, et al. Incidence of pregnancy and disease-modifying therapy exposure trends in women with multiple sclerosis: A contemporary cohort study. Mult Scler Relat Disord. 2019;28:235–243. [DOI] [PubMed] [Google Scholar]
- 17.Canibano B, Deleu D, Mesraoua B, Melikyan G, Ibrahim F, Hanssens Y. Pregnancy-related issues in women with multiple sclerosis: an evidence-based review with practical recommendations. J Drug Assess. 2020;9(1):20–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medicaid and CHIP Payment and Access Commission. MACPAC Fact Sheet: Medicaid’s Role in Financing Maternity Care. https://www.macpac.gov/wp-content/uploads/2020/01/Medicaid’s-Role-in-Financing-Maternity-Care.pdf. Published 2020. Updated January 2020. Accessed 05 Mar 2021, 2020.
- 19.Hartung DM. Health economics of disease-modifying therapy for multiple sclerosis in the United States. Ther Adv Neurol Disord. 2021;14:1756286420987031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asche CV, Singer ME, Jhaveri M, Chung H, Miller A. All-cause health care utilization and costs associated with newly diagnosed multiple sclerosis in the United States. J Manag Care Pharm. 2010;16(9):703–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


