INTRODUCTION
The advent of modern cancer therapy has considerably improved the outcomes of oncology patients and, for the first time, has introduced survivorship as a theme in the management of patients with cancer. However, both traditional and novel chemotherapies are associated with cardiotoxicity. Increasingly, patients with cancer are older, have cardiovascular comorbidities, have prior exposure to anticancer therapies, and/or have received combinations of agents. These factors can predispose to cardiotoxic effects of chemotherapies and contribute to a growing prevalence of patients with cardiac diseases related to cancer treatments. Cardio-oncology services are emerging internationally to guide optimal management of this challenging patient population.
The more common anticancer therapies and their respective cardiotoxicities are outlined in Table 1. This review focuses on cardiomyopathy associated with anthracycline chemotherapies and HER-2 targeted therapies, which together constitute a major subset of referrals to cardio-oncology clinics. Cardiomyopathy in the setting of these agents can range from asymptomatic reversible myocardial injury to irreversible, symptomatic congestive heart failure. Surveillance and management strategies used to identify and treat adverse cardiac effects for these chemotherapies are reviewed. The challenges facing cardio-oncologists are also explored, including the lack of consensus guidelines to direct clinical practice.
Table 1.
Commonly prescribed anticancer agents with summary of respective cardiotoxicity profiles
| Class | Commonly Prescribed Agents | Trade Name | Incidence of Cardiotoxicitya (%) (Incidence of Left Ventricular Dysfuntion -%) | Reversibility |
|---|---|---|---|---|
| Anthracyclines | Doxorubicin Epirubicin | Adriamycin Ellence | Dose dependent; up to 26b | + |
| HER-2 targeted therapies | ||||
| A. Monoclonal Antibodies | Trastuzumab | Herceptin | 4.5c (up to 30% with asymptomatic cardiomyopathy) | +++ |
| B. TKIs | Lapatinib | Tykerb | 1.5–2.2g | Uncertain |
| VSP inhibitors | ||||
| A. Monoclonal Antibodies | Bevacizumab | Avastin | 2.2h | Uncertain |
| B. TKIs | Sorafenib Sunitinib |
Nexavar Sutent |
2.1d 4.1e |
+++ |
| Other Tyrosine Kinase Inhibitors (TKIs) | Imatinib | Gleevec | 1.7f | Uncertain |
Abbreviations: HER2, human epidermal growth factor receptor 2; TKI, tyrosine kinase inhibitor; VSP, vascular endothelial growth factor signaling pathway.
Note: Definitions of cardiotoxicity used by sources vary, limiting direct comparisons of incidence between agents. Reversibility indicates the percentage of patients who have cardiomyopathy reversibility after cessation of drug treatment and/or initiation of cardioprotective medications. “+” denotes least likely to reverse and “++++” most reversible. We additionally suggest changing the class grades as follow: anthracyclines “+”, Her-2 receptor targeted therapies “+++” and TKIs under VSP inhibitors (see below for clarification of table 1) “+++”.
Jensen. Semin Oncol. 2006;33:S15–21.
Suter, Procter M, van Veldhuisen DJ, et al. J Clin Oncol. 2007:25:3859–65.
Escudier, Eisen T, Stadler WM, et al. N Engl J Med. 2007;356(2):125.
Richards, Je Y, Schutz FA, et al. J Clin Oncol. 2011;29(25):3450.
Atallah, Durand JB, Kantarjian H, et al. Blood. 2007;110(4):1233.
Perez, Koehler M, Byrne J, et al. Mayo Clin Proc. 2008;83(6):679.
Brana Choueiri TK, Mayer EL, Je Y, et al. J Clin Oncol. 2011;29(6):632–8.
ANTHRACYCLINE-INDUCED CARDIOMYOPATHY
Anthracyclines serve as effective chemotherapy for the treatment of breast cancer, acute leukemia, Hodgkin and non-Hodgkin lymphoma, and sarcomas—all of which are cancer types that can occur in young people and are potentially curable. Doxorubicin (Adriamycin) and daunorubicin (daunomycin) are glycoside antibiotics and were the original anthracyclines isolated from the pigment-producing bacterium Streptomyces peucetius in the 1960s. The precise mechanisms of action of anthracyclines are still debated but likely involve tumor DNA intercalation, DNA binding and alkylation, increased DNA damage via the generation of reactive oxygen species, inhibition of topoisomerase II, and induction of apoptosis. Anthracyclines, and specifically doxorubicin, remain an important cornerstone of chemotherapy for a vast variety of cancers and are increasingly being used in older patient populations.
In initial trials with doxorubicin and daunorubicin, toxicities were noted in tissues with a high mitotic rate, such as the bone marrow, gastrointestinal system, and hair follicles. Accordingly, many patients suffered from nausea, vomiting, myelosuppression, and alopecia2; but these toxicities were generally reversible or responsive to drug dose adjustments. It later came as a surprise when further studies with doxorubicin indicated that the major limitation of use was cardiac toxicity. Clinicians now widely recognize that anthracyclines pose a significant cardiac risk, which can manifest as a reversible early (or acute) cardiac toxicity and/or a later (chronic) presentation of cardiomyopathy with clinical heart failure and associated mortality. More recently, it has become evident that myocardial injury can occur at the time of acute treatment with doxorubicin and that, conversely, doxorubicin-induced chronic cardiomyopathy is potentially reversible.
Acute Cardiotoxicity
Acutely, anthracycline treatment can cause tachycardia, hypotension, electrocardiographic changes, arrhythmias, and a myocarditis-pericarditis syndrome. In rare cases, myocardial infarction or even sudden cardiac death can occur within hours following anthracycline treatment. Nonetheless, acute cardiovascular complications are largely asymptomatic and are generally thought to be reversible.3 In a study of almost 1700 patients with non-Hodgkin lymphoma, 55 patients developed acute cardiotoxicity requiring a cardiology referral, including 5 cases of acute heart failure and 1 case of myocardial infarction.4 Older patients, who are more likely to have coexisting cardiovascular disease, seem to be predisposed to the acute cardiotoxicities associated with doxorubicin.5 In a study evaluating the use of liposomal doxorubicin in an older patient cohort (mean age of 72 years), 1 out of every 5 patients developed acute cardiotoxicity, including tachycardia, atrial fibrillation, and cardiomyopathy.6
Although acute cardiotoxicity associated with anthracyclines has been traditionally thought to be reversible, recent studies have highlighted the potential for irreversible myocardial injury. At the time of infusion-based administration of anthracyclines, elevation of serum troponins have been detected.7 Furthermore, myocardial biopsies obtained within hours after anthracycline exposure have demonstrated evidence of myocardial tissue injury and cell death.8 Evidence of anthracycline-induced myocyte damage has also been seen in children in whom troponin elevations have been correlated with later left ventricular remodeling on echocardiography.9 More studies are needed to determine whether acute cardiotoxicity after anthracycline exposure predicts future cardiomyopathy or heart failure.
Chronic Cardiotoxicity
Chronic cardiovascular toxicities associated with anthracyclines are a cause for even greater concern. Cardiomyopathy and clinical heart failure are the main dose-limiting side effects of anthracyclines. Anthracycline-induced cardiomyopathy can occur within the first year and up to a decade after completion of therapy. A recent meta-analysis showed that the use of an anthracycline-based chemotherapy was associated with a significant increased risk of both clinical and subclinical cardiotoxicity over use of a non-anthracycline-based chemotherapy.10 Initial studies suggested an incidence of 2.2% of developing clinical congestive heart failure after doxorubicin treatment.11 A significantly higher percentage of patients have evidence of subclinical heart failure. Fifty-seven percent of children exposed to doxorubicin had echocardiographic evidence of cardiac dysfunction, although only 10% had clinical heart failure.12 In a separate study of adult survivors of childhood cancer, most of whom were treated with anthracyclines, 27% of the patients had echocardiographic evidence of cardiac dysfunction.13
Several risk factors predispose patients to anthracycline-induced cardiomyopathy. The strongest predictor for cardiac dysfunction is the cumulative dose of anthracyclines administered.14 In the case of doxorubicin, general recommendations dictate the cumulative dose of doxorubicin not to exceed 450 mg/m2 in adults. However, there is variability seen among patients, with some developing heart failure at a cumulative dose of 300 mg/m2. Other risk factors predisposing to anthracyclines-induced cardiomyopathy include age extremes, concomitant chemotherapy and radiation, and a history of cardiovascular disease. Although preexisting cardiac history or cardiac risk factors may potentiate the development of doxorubicin-induced heart failure in older patients, it is less clear why children are at an increased risk of developing anthracycline-induced cardiomyopathy, where a younger age is associated with echocardiographic evidence of cardiac dysfunction.15
Pathogenesis
Most proposed models for anthracycline-induced cardiomyopathy suggest progressive myocardial cell death following successive anthracycline exposure.16 Although earlier stages of anthracycline-induced cardiac damage may be undetectable via standard cardiac imaging, cumulative exposure to anthracyclines eventually exceeds a threshold of myocardial damage that manifests initially as overt structural cardiac changes (eg, myocardial dilatation, cardiomyopathy) and ultimately as frank heart failure. Acute exposure to anthracyclines leads to myocardial damage, as evidenced by elevated cardiac serum biomarkers and histologic changes on myocardial biopsy, including mitochondrial swelling and chromatin contraction, consistent with apoptosis.16
Less clear are the upstream pathways leading to myocardial cell death. The presumed mechanism of action of anthracyclines is interference with DNA replication in rapidly dividing cells, such as cancer cells. Consistent with this hypothesis, some side effects of anthracyclines are seen in organs with high turnover, such as bone marrow and hair follicles. It is, therefore, surprising that the heart (an organ with limited regenerative capacity) is the major organ of toxicity. The proposed mechanism of cardiotoxicity is the generation of reactive oxygen species by various mechanisms including redox cycling, iron complexation, and electron transport chain uncoupling. Accordingly, overexpression of free of radical scavengers, or co-treatment with strong antioxidants, protects against anthracyclines-induced cardiomyopathy in animal models. However, these preclinical studies have not translated to human clinical trials, indicating that oxidative stress may not be the sole mechanism of toxicity.17
Aside from standard cardioprotective medications, the only compound that has been found to be consistently protective in anthracycline-mediated cardiomyopathy has been dexrazoxane, an iron chelator that can attenuate iron-catalyzed hydroxyl radical formation. Dexrazoxane prevents or reduces cardiac injury, as reflected by elevations in troponin T and long-term echocardiographic measures of cardiac remodeling in children treated with leukemia.18,19 More studies are needed to assess clinical end points such as incident heart failure or cardiac death. Notably, the use of dexrazoxane has met some resistance in the adult oncology community due to a perceived risk of secondary malignant formation.
HER2 TARGETED THERAPY
The human epidermal growth factor receptors (EGF receptors or ErbB) are a family of transmembrane receptor tyrosine kinases involved in the regulation of cell growth and cell survival, with important roles in tumor genesis and growth.20,21 HER2, one of the 4 members of this family, is overexpressed in about 20% to 30% of breast cancers and is often associated with a more aggressive tumor phenotype associated with a poorer prognosis.22–25 HER2 overexpression in cancer cells leads to continuous stimulation of downstream signaling pathways and uncontrolled cell proliferation.26`
Trastuzumab (Herceptin), a humanized monoclonal antibody against the extracellular domain of the HER2 protein that can inhibit proliferation of malignant cells, is approved for the treatment of both metastatic and early stage breast cancer with HER2 overexpression.22 A meta-analysis of 5 randomized controlled trials comparing chemotherapy with and without trastuzumab in 13 493 women with HER2-positive breast cancer reported a 34% lower relative risk of mortality, a 36% lower relative risk of locoregional recurrence, and a 40% lower relative risk of distant recurrence among patients receiving trastuzumab.1 Lapatinib is another Food and Drug Administration–approved second-line agent for use with capecitabine for HER2-positive metastatic breast cancer, or for use in combination with letrozole for hormone receptor–positive disease.27 Unlike trastuzumab, lapatinib is an oral small tyrosine kinase inhibitor (TKI) that competes with ATP for binding to the ATP binding pocket of kinases HER1 and HER2, blocking phosphorylation and activation of both receptors.28 Phase III trials showed that adjuvant lapatinib improves outcomes in patients with advanced breast cancer overexpressing HER2.29,30 More recently, pertuzumab, a monoclonal antibody that inhibits HER2 dimerization and activation, as well as the antibody-drug composite, trastuzumab emtansine (also known as T-DM1), have shown efficacy in patients with HER2-positive breast cancer. These novel therapies illustrate an expanding arsenal of HER2-targeted therapies that are being used, often in combination, for HER2-positive breast cancer.
Cardiotoxicity
Trastuzumab can cause an asymptomatic decline in cardiac function as well as symptomatic congestive heart failure. In early clinical trials, trastuzumab was given concomitantly with other chemotherapies for breast cancer treatment, resulting in a variable incidence of heart failure. For example, patients treated with trastuzumab and along with concurrent anthracycline and cyclophosphamide had an incidence of symptomatic cardiac dysfunction of 27%, with the incidence decreasing to 13% in the setting of paclitaxel and trastuzumab combination therapy.22 In subsequent trials, trastuzumab was given sequentially following other chemotherapies, resulting in a significant decrease in the incidence of cardiomyopathy. In these studies, the incidence of severe symptomatic (New York Heart Association class III or IV) heart failure in patients treated with chemotherapy and adjuvant trastuzumab was found to range from 0.5% to 3.7% compared with 0% up to 0.7% among patients treated with chemotherapy alone.1 The incidence of asymptomatic reductions in LVEF was higher and resulted in the discontinuation of trastuzumab in approximately 14% of patients in one study.31 In contrast, in a pooled analysis of 44 clinical trials, only 1.6% of 3689 patients treated with lapatinib experienced a 20% or more decrease in LVEF relative to baseline and most were asymptomatic.32
Cardiac dysfunction caused by trastuzumab differs from anthracycline-induced cardiomyopathy in that it is not dose dependent; it can occur acutely, even after the first exposure; and, it is largely reversible on treatment withdrawal. These differences have led to the subclassification of chemotherapy-associated cardiomyopathies into type I (anthracycline-like, which presumably involves myocyte death) and type II cardiomyopathy (trastuzumab-like, where there is reversibility of myocardial impairment).33 These subtypes are not mutually exclusive and may coexist in patients treated with contemporary multi-agent treatment regimens.
Pathophysiology
The pathophysiological mechanisms underlying trastuzumab-induced cardiotoxicity are not fully understood. The activation of HER2/HER4 heterodimers by neuregulin1 (NRG1), a growth factor produced by cardiac endothelial cells, stimulates many important downstream signaling pathways that promote cardiomyocyte growth and survival.34–37 By specifically blocking HER2, trastuzumab treatment appears to compromise the ability of myocytes to withstand or recover from stressors, such as anthracycline-induced damage. Mitochondrial dysfunction and disruption of ATP production have also been postulated as a possible mechanism for trastuzumab-associated cardiotoxicity.38,39 The relatively lower incidence of cardiotoxicity observed with lapatinib, which also blocks the NRG/ErbB pathway, is puzzling. A few hypotheses have been proposed. As a monoclonal antibody, trastuzumab may mediate antibody-dependent cell cytotoxicity and complement-dependent cytotoxicity, thus augmenting cardiotoxicity.28,40 Furthermore, differential inhibition or activation of downstream signaling pathways by lapatinib versus trastuzumab may also explain observed discrepancies in outcomes.41
DIAGNOSIS
Maintaining an index of suspicion for cardiotoxicity in patients receiving cancer therapies is fundamental for timely diagnosis. A review of symptoms and clinical examination at regular intervals before, during, and after chemotherapy are necessary. However, cardiotoxicity is often subclinical until a certain threshold of injury is exceeded, limiting the sensitivity of symptoms and signs of heart failure in early diagnosis of cardiac injury. Therefore, cardiac imaging and cardiac biomarkers are complementary in the diagnosis and screening of patients with possible chemotherapy-induced cardiotoxicity.
Cardiac Imaging
Transthoracic echocardiography
In current clinical practice, transthoracic echocardiography (TTE) and multi-gated radionuclide angiography (MUGA) are the most commonly used modalities for non-invasive baseline and serial assessment of LVEF in patients receiving chemotherapeutic agents. TTE provides a qualitative and quantitative assessment of LVEF, in addition to useful information regarding valvular function, pericardial processes, and diastolic function. It should be noted that TTE image quality and interpretation can be limited in patients with obesity, chronic obstructive pulmonary disease, and musculoskeletal deformities. For such cases, contrast-enhanced echocardiography can improve endocardial definition allowing for accurate and reproducible assessments of LVEF.42,43 The diagnosis of cardiotoxicity relies on the detection of often subtle differences in LVEF, underscoring the need for echocardiogram reports to detail any quality issues that might compromise the reliability of results.
MUGA
MUGA assessments of LV systolic and diastolic function are commonly used. MUGA provides a well-established, simple, reproducible, and accurate measurement of LV function. Oncologists’ familiarity with this technique and interpreting results likely contribute to the pervasive presence of MUGA in contemporary surveillance strategies. Unlike TTE, MUGA exposes patients to radiation and fails to provide additional information on valvular and pericardial processes, which are not infrequent complications of cancer and cancer therapies. Surveillance schedules have been suggested for monitoring patients with serial MUGAs for doxorubicin-induced cardiotoxicity; monitoring LVEF in accordance with these protocols has been associated with a fourfold reduction in the incidence of doxorubicin-associated heart failure.44
Novel imaging techniques
Echocardiography: myocardial strain and tissue Doppler imaging
Deterioration in LV systolic function to the point that it is manifest as a noticeable change in LVEF is a relatively late and potentially irreversible finding. More sensitive imaging strategies that may identify cardiotoxicity at an earlier, modifiable stage in the disease process are under review. Interest has focused particularly on the contemporary echocardiographic techniques of tissue Doppler and myocardial strain imaging.
Reductions in LV diastolic function may precede LV systolic function in chemotherapy-induced cardiotoxicity (Fig. 1).45,46 Several small and largely single-center studies have reported observing utility in assessing serial changes in radionuclide angiography or TTE-derived measurements of LV diastolic function in the effort to detect subclinical cardiotoxicity.1,2,47,48 However, serial echocardiographic assessment of diastolic parameters failed to predict cardiotoxicity among 43 patients receiving anthracycline and trastuzumab as the treatment of breast cancer.49 Such small, single-center studies with inconsistent findings highlight the need for larger multicenter prospective studies to determine the role for serial quantification of diastolic function.
Fig. 1.


(A) Pulsed-wave (PW) analysis of mitral annular velocity at the septal wall is obtained with tissue Doppler imaging (TDI). Med E′ Vel (medial early myocardial velocity) of 4.19 cm/s is lower than the reference range of normal (12.2 ± 2.3cm/s) for this 46-year-old female patient with preserved LVEF receiving doxorubicin. (B) PW of the mitral annulus (lateral wall) obtained by TDI from the same patient. Lat E′ Vel (lateral early myocardial velocity) of 4.97 cm/s is also lower than the reference range of normal for this patient (16.1 ± 2.3 cm/s). These findings confirm the presence of impaired left ventricular relaxation (diastolic dysfunction), which may be an earlier marker of cardiotoxicity compared to overt changes in LV systolic function.
Longitudinal myocardial strain echocardiography has also been reported in several small studies as more sensitive than conventional LVEF assessment in the early detection of LV cardiotoxicity (Fig. 2).50,51 An early decrease (>10%) in peak systolic myocardial longitudinal strain predicted the later occurrence of cardiotoxicity among 43 patients receiving anthracyclines and trastuzumab with a sensitivity and specificity of 78% and 79%, respectively.49 Among 81 women treated with anthracyclines followed by taxanes and trastuzumab, abnormalities of peak systolic longitudinal myocardial strain measured after completion of anthracycline therapy predicted subsequent cardiotoxicity.7 Eighteen (51%) out of 35 patients undergoing trastuzumab therapy for breast cancer demonstrated significant reductions in longitudinal strain, identifying preclinical myocardial dysfunction before standard echocardiographic measures, such as LVEF.52
Fig. 2.
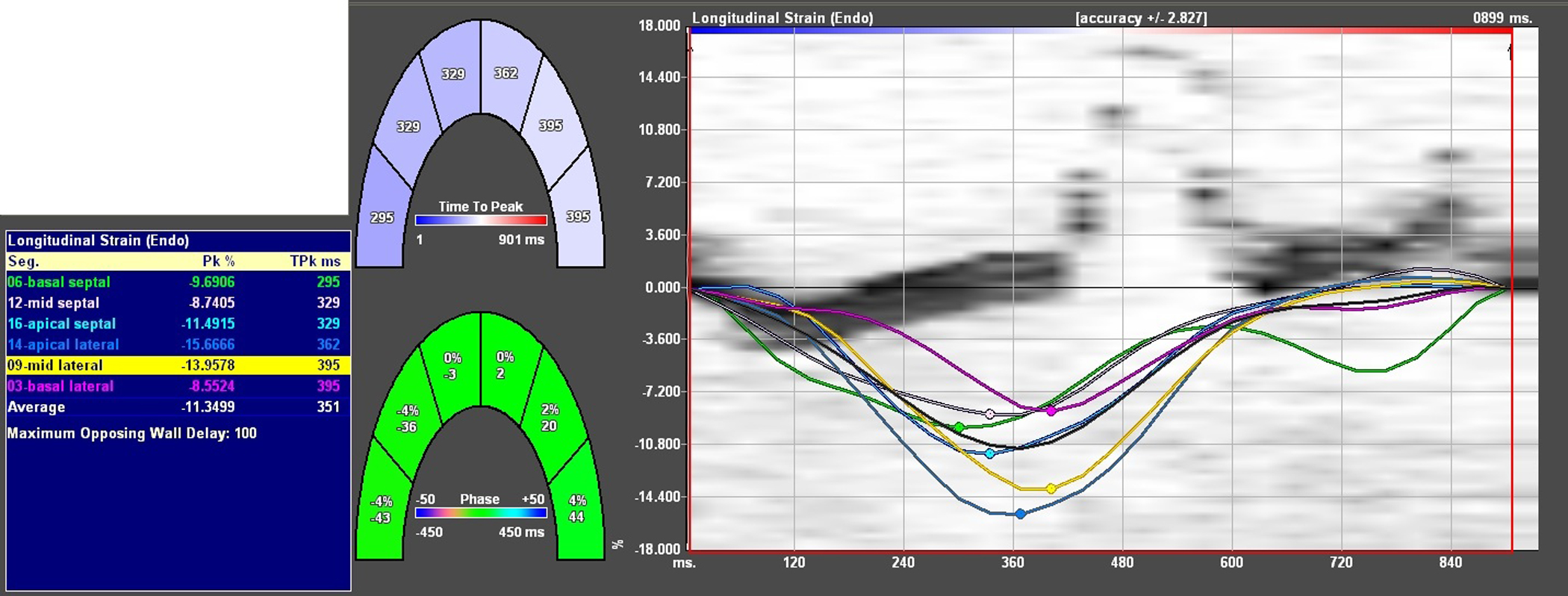
Longitudinal strain analysis performed on the apical 4-chamber view. The 7 curves represent longitudinal myocardial strain (y axis) over a single cardiac cycle (x axis) for each of 6 LV segments (basal septum [green], midseptal [white], apical septal [light blue], apical lateral [dark blue], midlateral [yellow], basal lateral [pink]) and a gray curve that represents the average (global) longitudinal strain of these 6 segments. The numerical values for peak longitudinal strain for each of these segments are outlined in the adjacent table. In a study of 240 healthy volunteers, mean (±SD) overall global longitudinal strain was −18.6 ± 5.1%. The strain analysis in this figure was performed on a 46-year-old woman with normal LVEF who recently completed anthracycline chemotherapy; a mean peak systolic longitudinal myocardial strain of −11.4% is abnormal and is associated with increased risk of subsequent cardiomyopathy, indicating the need for surveillance assessment of LVEF. (Data from Marwick TH, Leano RL, Brown J, et al. Myocardial Strain Measurement With 2-Dimensional Speckle-Tracking Echocardiography. J Am Coll Cardiol Img 2009;2:80–4.)
Serial real-time 3-dimensional TTE (RT3DTTE) assessment of LV end-diastolic volumes (LVEDVs) has been shown to strongly correlate with measurements of cardiac magnetic resonance imaging (CMR) and/or MUGA-derived measurements of LVEDV in a cohort of 50 female patients with breast cancer who received trastuzumab after doxorubicin.53 As imaging technology advances and experience with acquisition and post-processing accumulates, RT3DTE may assume an important role as a reliable and reproducible alternative to 2-dimensional TTE assessment of LV volumes. The additive value of dobutamine or exercise stress echocardiography in the early detection and surveillance of cardiotoxicity is uncertain, with inconsistent results from small single-center studies.54–56
Nuclear imaging techniques
Nuclear imaging techniques that detect myocardial injury at an early stage preceding the development of overt LV systolic dysfunction are under investigation. Such techniques include 123I-labeled meta-iodobenzylguanidine (MIBG) scintigraphy (decreased 123I-MIBG uptake in cases of doxorubicin-induced cardiomyopathy), cardiac sympathetic neuronal imaging using positron-emission tomography (PET), and 111In-antimyosin scintigraphy (111In-antimyosin binds to intracellular myosin of injured myocytes).57 It will be necessary to standardize acquisition protocols and to clarify the role and utility of these techniques through clinical studies before their application in practice.
CMR
CMR allows very accurate and reproducible assessment of ventricular volumes and estimation of LVEF. T2-weighted imaging with fat suppression can detect myocardial edema that may be a feature of acute myocardial inflammation and injury secondary to carditoxicity58; the role of this technique in the diagnosis of cardiotoxicity and the clinical significance of myocardial edema in this context requires evaluation in prospective studies. The pattern and significance of late gadolinium enhancement (LGE) in patients with cardiotoxicity also requires investigation. Subepicardial linear LGE of the lateral segments of the LV has been described in patients with trastuzumab-induced cardiomyopathy.59 Relatively limited access, cost, and time required for acquisition and post-processing limit the practical use of CMR technology in routine surveillance for cardiotoxicity. However, CMR may be more practically employed in the single-use evaluation of patients with manifest LV dysfunction following exposure to chemotherapy. In such patients, CMR can assist in evaluating for alternative or contributing disease processes such as ischemic heart disease and infiltrative disorders.
Appropriate use criteria
Concerns about potential overutilization of various cardiac imaging modalities led to the development of appropriate use criteria for each modality by the various academic bodies, such as the American College of Cardiology and American Heart Association. The use of TTE or MUGA is considered appropriate in the baseline and serial evaluation of patients undergoing therapy with cardiotoxic agents.60,61 CMR is considered appropriate in the evaluation of LV function in patients with technically challenging echocardiograms and/or in the evaluation of cardiomyopathies caused by cardiotoxic therapies.62
Cardiac Biomarkers
Elevated troponin or NT-pro-brain natriuretic peptide (BNP) in the early post-exposure period can identify an at-risk subgroup that may benefit from increased frequency of cardiac function testing.7,63–66 Elevated ultrasensitive troponin I assays exceeding 30 pg/mL at the completion of anthracycline therapy were predictive of subsequent cardiotoxicity in a study of 81 patients with breast cancer.7 Persistently elevated NT-pro-BNP assays in the early aftermath of high-dose chemotherapy were strongly associated with downstream cardiac dysfunction among 52 patients treated with high-dose chemotherapy.66 In clinical practice, it is likely that the utility of biomarkers will prove most helpful as an adjunct to cardiac imaging in the intensified surveillance of patients predisposed to cardiotoxicity for the detection of earlier subclinical toxicity, directing more timely interventions and surveillance schedules.
MANAGEMENT
The management of chemotherapy-induced cardiomyopathy requires a multidisciplinary approach with input from both the oncology and cardiology teams. Benefits of continuing chemotherapy and/or modifying treatment regimens must be weighed against the risk of irreversible cardiovascular outcomes. The development of cardio-oncology programs will help to deliver experience and expertise in the management of this growing and challenging patient population.
Surveillance
Cardiac imaging–based surveillance of LV function during treatment with agents other than trastuzumab remains controversial. In the case of trastuzumab, the timing of surveillance cardiac function tests generally adheres to pretreatment baseline studies and then quarterly studies for the duration of chemotherapy.49,67,68 The frequency of studies following the completion of chemotherapy varies without consensus. The protocol followed in the authors’ institution for patients receiving trastuzumab is outlined in Fig. 3. There are currently no published consensus guidelines on the prospective surveillance and management of cardiotoxic effects of cancer therapies in adults.69
Fig. 3.

Local institutional algorithm for cardiac surveillance of patients receiving trastuzumab with or without prior exposure to chemotherapy. a Requires consensus of cardiologist and oncologist following risk-benefit analysis. ACC, American College of Cardiology; AHA, American Heart Association; HF, Heart Failure; EF, Ejection Fraction.
The concept of a standard surveillance schedule is arbitrary. Individual susceptibility to cardiotoxicity and the cardiotoxic potential of all anticancer treatments are not uniform. Thus, a reasonable approach is to tailor surveillance schedules according to patient susceptibility and the agent involved. Patients require pretreatment screening for characteristics associated with increased risk of cardiotoxicity. Identifying high-risk patients should prompt intensified surveillance of cardiac function.70 High-risk features that are largely relevant in the context of exposure to any potentially cardiotoxic drug treatment are outlined in Box 1.
Box 1. General risk factors that predispose to cardiotoxic effects of anti-cancer drugs.
| Very young (applies to anthracyclines) or old age at exposure Combination of anti-cancer agents Prior exposure to anti-cancer agents Radiation therapy that included the heart or part of the heart in the irradiated volume Cumulative dose (applies to anthracyclines) Baseline LV dysfunction Cardiovascular risk factors (eg, hypertension, diabetes mellitus) Elevated troponin and/or NT-pro-BNP assays early following exposure |
Pharmacotherapy
In the context of established LV systolic impairment that is presumed secondary to anticancer therapy, heart failure therapies, including beta-blockers and angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers, are introduced and up-titrated according to contemporary guidelines for the management of heart failure.71 Studies of heart failure treatments specifically in patients with chemotherapy-induced cardiomyopathy are few. The addition of enalapril, with or without carvedilol, was studied in a single-center prospective study of 201 patients with anthracycline-induced cardiomyopathy (LVEF ≤45%), and was associated with some improvement in LV function in 55% of patients.72 In the absence of specific guidelines, standard pharmacotherapy of heart failure should also be initiated in the setting of trastuzumab-induced cardiomyopathy. Given the preclinical and clinical evidence in favor of dexrazoxane, prophylactic use should be considered in high-risk patients being treated with anthracyclines. The optimal heart failure regimen for chemotherapy-induced cardiotoxicity requires evaluation through dedicated clinical trials.
Balancing Cancer and Cardiovascular Outcomes
The main challenge of cardio-oncology is to balance cancer and cardiovascular outcomes. An overall aim is to avoid compromising the efficacy of anti-cancer therapy while minimizing the risk of cardiac side effects. The evidence base to achieve such goals is deficient and consensus is lacking. This deficiency emphasizes the need to include cardiac outcomes in the design of trials for anti-cancer therapies, extend follow-up periods to detect late cardiotoxicity, and determine optimal cost-effective surveillance strategies and schedules for the early detection of cardiotoxicity. The clinical significance of abnormalities in ventricular strain and diastolic measurements, myocardial edema on T2-weighted magnetic resonance imaging, and abnormalities on nuclear functional imaging need to be evaluated by prospective trials. Overinterpreting the clinical significance of such findings may prompt modifications to anti-cancer therapies at the cost of efficacy. On the other hand, such findings may present an opportunity for early intervention that may reduce the risk of downstream, irreversible cardiac morbidity.
Recommendations
There are certain key concepts that are clinically applied at the authors’ institution. All patients undergoing therapy with a potentially cardiotoxic agent require a baseline pretreatment study of cardiac function. Although the need is apparent, cardiac testing pretreatment is underutilized. For example, less than 30% of 3779 women with metastatic breast cancer receiving trastuzumab between 2001 and 2010 had pretreatment cardiac function testing.73 The authors encourage pretreatment screening of all patients for the high-risk features outlined in Box 1 and advocate surveillance for specific high-risk cohorts. Serial TTE with the measurement of LV systolic and diastolic parameters, in addition to myocardial strain and strain rate imaging, and direct comparison with equivalent measures on prior studies is the contemporary default surveillance strategy in the authors’ institution, maintaining a low threshold for using ultrasound contrast agents as needed.
It is important to remember that LVEF measured by the differing modalities of MUGA, TTE, and CMR are not interchangeable.74 Therefore, the same modality is used throughout surveillance for reliable interval comparisons.
For patients with established LV systolic dysfunction, additional cardiac testing is considered on an individual patient basis. Stress imaging can help exclude ischemia as an alternative cause of or contributor to LV impairment. Several stress-imaging modalities are useful in this context and selection will be governed by local availability and expertise. Stress CMR offers the advantage of evaluating for ischemia while also providing useful additional information, including accurate quantification of LV volumes and information on the pattern of any myocardial edema or LGE that may be present. However, patients with contraindications to magnetic resonance imaging (MRI) (eg, pacemaker) or significant renal dysfunction precluding administration of gadolinium are not suitable for this technique. Stress myocardial perfusion imaging with single-photon emission computed tomography or PET-computed tomography (CT) are alternatives for evaluating for ischemia; PET-CT offers the advantages of lower radiation exposure and the ability to quantify coronary flow reserve.
SUMMARY
The pharmacologic armamentarium used in the treatment of all stages of cancer has evolved in recent decades, and ongoing oncology clinical trials promise further progress. As such, drug regimens used in the treatment of cancer are increasingly complex with combinations of agents with differing, and sometimes synergistic, cardiotoxic potential.
Clinical trials of chemotherapeutic approaches have been criticized for inconsistent definitions of cardiotoxicity, variable cardiac surveillance strategies and schedules, and failure to monitor diastolic function.75 Furthermore, trials may fail to include cardiac endpoints in the study design, and follow-up durations are often insufficient to detect late cardiotoxicity. These factors contribute to uncertainty of the true incidence of cardiotoxicity for many anticancer agents. The prevalence of chemotherapy-related cardiac disease is increasing and management demands a multidisciplinary approach from cardiologists and oncologists.
Pretreatment identification of predisposing risk factors and the assessment of cardiac function before and at intervals during and after therapy with cardiotoxic agents are necessary. In clinical practice, surveillance is largely performed using TTE or MUGA. Imaging strategies that detect cardiac injury before overt LV systolic dysfunction provide an opportunity for early intervention and improved cardiac outcomes. Appropriately designed clinical trials are needed to clarify optimal surveillance strategies and schedules, the cost-effectiveness of various approaches, and the appropriate management based on imaging findings.
EPILOGUE
The patient presented in the case vignette was immediately initiated on an ACE inhibitor and a beta-blocker, with up-titration of each to the maximum tolerated doses. Cardiovascular risk factor screening did not identify any additional contributing or alternative cause for cardiomyopathy. Cardiac MRI was negative for stress-induced perfusion abnormalities, demonstrating a nonspecific, nondilated cardiomyopathy with an LVEF of 41%. A diagnosis of anthracycline-associated cardiomyopathy was made.
Repeat TTE performed 4 weeks after the initiation of heart failure therapy confirmed an interval improvement in LVEF from 45% to 50%. Given the compelling survival advantage offered by trastuzumab for the patient’s cancer type, the multidisciplinary consensus was to commence trastuzumab and continue heart failure therapies concurrently.
Surveillance TTE was performed every 3 weeks before each trastuzumab dose; a troponin assay and NT-pro-BNP were drawn after each dose. At 3 months, LVEF remained stable at 50% in the absence of symptoms, and all biomarkers since trastuzumab initiation were within normal limits. Intervals between surveillance echocardiograms were extended to every 2 months for the remainder of trastuzumab therapy, and post-dose biomarker surveillance was discontinued. Trastuzumab therapy was then continued for 1 year without event. The patient currently remains free of recurrent cancer 2 years following the completion of trastuzumab, continues on ACE inhibitor and beta-blocker therapy, and undergoes a routine surveillance TTE annually. The most recent TTE confirms a recovery of LVEF to 60% (Video 2).
Supplementary Material
Video 1: Non-dilated, globally hypokinetic left ventricle and mild dilatation of the right ventricle.
Video 2: Normal left ventricular function available at http://www.medical.theclinics.com/
KEY POINTS.
The prevalence of chemotherapy-related cardiac disease is increasing and management requires a multidisciplinary approach from cardiologists and oncologists.
Pretreatment identification of predisposing risk factors and assessment of cardiac function before and at intervals during and after therapy with cardiotoxic agents are necessary.
In clinical practice, surveillance is largely performed using transthoracic echocardiography or multi-gated radionuclide angiography. Imaging strategies that detect cardiac injury before overt left ventricular systolic dysfunction provide an opportunity for early intervention and improved cardiac outcomes.
CASE VIGNETTE.
A 41-year-old woman with no previous cardiac history was diagnosed with stage II invasive ductal carcinoma of the right breast. Pathology confirmed that the tumor was estrogen receptor negative, progesterone receptor negative, and human epidermal receptor (HER)-2 positive. Initial chemotherapy consisted of doxorubicin, to a cumulative dose of 240 mg/m2, and adjuvant cyclophosphamide. Transthoracic echocardiography on completion of this regimen revealed left ventricular systolic dysfunction, with an estimated left ventricular ejection fraction (LVEF) of 40% to 45% (Video 1), whereas pretreatment LVEF had been normal at 60%. Her oncologist had planned to commence adjuvant trastuzumab, a monoclonal antibody targeting HER2, because a meta-analysis of randomized controlled trials suggested that adjuvant trastuzumab would offer this patient a 34% lower relative risk of mortality, a 36% lower relative risk of locoregional recurrence, and 40% lower relative risk of distant recurrence compared with chemotherapy without trastuzumab.1 She was referred to the cardio-oncology clinic for further evaluation and management of her cardiomyopathy and for assessment of cardiac suitability for trastuzumab therapy.
ACKNOWLEDGMENTS
JM is supported by an NIH Career Development Award (K08), Watkins Discovery Award Program and Cardiovascular Leadership Council Investigator Award (both by Brigham and Women’s Hospital).
Footnotes
SUPPLEMENTARY DATA
Supplementary data related to this article can be found online at: http://dx.doi.org/10.1016/j.mcna.2012.07.008.
REFERENCES
- 1.Dahabreh IJ, Linardou H, Siannis F, et al. Trastuzumab in the adjuvant treatment of early-stage breast cancer: a systematic review and meta-analysis of randomized controlled trials. Oncologist 2008;13(6):620–30. [DOI] [PubMed] [Google Scholar]
- 2.Bonadonna G, Monfardini S, De Lena M, et al. Phase I and preliminary phase II evaluation of Adriamycin (NSC 123127). Cancer Res 1970;30(10):2572–82. [PubMed] [Google Scholar]
- 3.Von Hoff DD, Rozencweig M, Layard M, et al. Daunomycin-induced cardiotoxicity in children and adults. A review of 110 cases. Am J Med 1977;62(2):200–8. [DOI] [PubMed] [Google Scholar]
- 4.Wojnowski L, Kulle B, Schirmer M, et al. NAD(P)H oxidase and multidrug resistance protein genetic polymorphisms are associated with doxorubicin-induced cardiotoxicity. Circulation 2005;112(24):3754–62. [DOI] [PubMed] [Google Scholar]
- 5.Tirelli U, Errante D, Van Glabbeke M, et al. CHOP is the standard regimen in patients > or = 5 70 years of age with intermediate-grade and high-grade non-Hodgkin’s lymphoma: results of a randomized study of the European Organization for Research and Treatment of Cancer Lymphoma Cooperative Study Group. J Clin Oncol 1998;16(1):27–34. [DOI] [PubMed] [Google Scholar]
- 6.Luminari S, Montanini A, Caballero D, et al. Nonpegylated liposomal doxorubicin (MyocetTM) combination (R-COMP) chemotherapy in elderly patients with diffuse large B-cell lymphoma (DLBCL): results from the phase II EUR018 trial. Ann Oncol 2010;21(7):1492–9. [DOI] [PubMed] [Google Scholar]
- 7.Sawaya H, Sebag IA, Plana JC, et al. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes and trastuzumab. Circ Cardiovasc Imaging 2012. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Unverferth DV, Fertel RH, Talley RL, et al. The effect of first-dose doxorubicin on the cyclic nucleotide levels of the human myocardium. Toxicol Appl Pharmacol 1981;60(1):151–4. [DOI] [PubMed] [Google Scholar]
- 9.Lipshultz SE, Miller TL, Scully RE, et al. Changes in cardiac biomarkers during doxorubicin treatment of pediatric patients with high-risk acute lymphoblastic leukemia: associations with long-term echocardiographic outcomes. J Clin Oncol 2012;30(10):1042–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith LA, Cornelius VR, Plummer CJ, et al. Cardiotoxicity of anthracycline agents for the treatment of cancer: systematic review and meta-analysis of randomised controlled trials. BMC Cancer 2010;10:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Von Hoff DD, Layard MW, Basa P, et al. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med 1979;91(5):710–7. [DOI] [PubMed] [Google Scholar]
- 12.Lipshultz SE, Colan SD, Gelber RD, et al. Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. N Engl J Med 1991;324(12):808–15. [DOI] [PubMed] [Google Scholar]
- 13.van der Pal HJ, van Dalen EC, Hauptmann M, et al. Cardiac function in 5-year survivors of childhood cancer: a long-term follow-up study. Arch Intern Med 2010;170(14):1247–55. [DOI] [PubMed] [Google Scholar]
- 14.Lefrak EA, Pitha J, Rosenheim S, et al. A clinicopathologic analysis of Adriamycin cardiotoxicity. Cancer 1973;32(2):302–14. [DOI] [PubMed] [Google Scholar]
- 15.Lipshultz SE, Lipsitz SR, Mone SM, et al. Female sex and drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N Engl J Med 1995;332(26):1738–43. [DOI] [PubMed] [Google Scholar]
- 16.Sawyer DB, Peng X, Chen B, et al. Mechanisms of anthracycline cardiac injury: can we identify strategies for cardioprotection? Prog Cardiovasc Dis 2010;53(2):105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gianni L, Herman EH, Lipshultz SE, et al. Anthracycline cardiotoxicity: from bench to bedside. J Clin Oncol 2008;26(22):3777–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lipshultz SE, Scully RE, Lipsitz SR, et al. Assessment of dexrazoxane as a cardioprotectant in doxorubicin-treated children with high-risk acute lymphoblastic leukaemia: long-term follow-up of a prospective, randomised, multicentre trial. Lancet Oncol 2010;11(10):950–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lipshultz SE, Rifai N, Dalton VM, et al. The effect of dexrazoxane on myocardial injury in doxorubicin-treated children with acute lymphoblastic leukemia. N Engl J Med 2004;351(2):145–53. [DOI] [PubMed] [Google Scholar]
- 20.Fuller SJ, Sivarajah K, Sugden PH. ErbB receptors, their ligands, and the consequences of their activation and inhibition in the myocardium. J Mol Cell Cardiol 2008;44(5):831–54. [DOI] [PubMed] [Google Scholar]
- 21.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer 2005;5(5):341–54. [DOI] [PubMed] [Google Scholar]
- 22.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344(11):783–92. [DOI] [PubMed] [Google Scholar]
- 23.Nabholtz JM, Reese DM, Lindsay MA, et al. HER2-positive breast cancer: update on Breast Cancer International Research Group trials. Clin Breast Cancer 2002;3(Suppl 2):S75–9. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura S, Ando M, Masuda N, et al. Randomized phase II study of primary systemic chemotherapy and trastuzumab for operable HER2 positive breast cancer. Clin Breast Cancer 2012;12(1):49–56. [DOI] [PubMed] [Google Scholar]
- 25.Borg A, Baldetorp B, Ferno M, et al. ERBB2 amplification in breast cancer with a high rate of proliferation. Oncogene 1991;6(1):137–43. [PubMed] [Google Scholar]
- 26.Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987;235(4785):177–82. [DOI] [PubMed] [Google Scholar]
- 27.Rana P, Sridhar SS. Efficacy and tolerability of lapatinib in the management of breast cancer. Breast Cancer (Auckl) 2012;6:67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen MH, Kerkela R, Force T. Mechanisms of cardiac dysfunction associated with tyrosine kinase inhibitor cancer therapeutics. Circulation 2008;118(1):84–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 2006;355(26):2733–43. [DOI] [PubMed] [Google Scholar]
- 30.Johnston S, Pippen J Jr, Pivot X, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol 2009;27(33):5538–46. [DOI] [PubMed] [Google Scholar]
- 31.Telli ML, Hunt SA, Carlson RW, et al. Trastuzumab-related cardiotoxicity: calling into question the concept of reversibility. J Clin Oncol 2007;25(23):3525–33. [DOI] [PubMed] [Google Scholar]
- 32.Perez EA, Koehler M, Byrne J, et al. Cardiac safety of lapatinib: pooled analysis of 3689 patients enrolled in clinical trials. Mayo Clin Proc 2008;83(6):679–86. [DOI] [PubMed] [Google Scholar]
- 33.Ewer MS, Lippman SM. Type II chemotherapy-related cardiac dysfunction: time to recognize a new entity. J Clin Oncol 2005;23(13):2900–2. [DOI] [PubMed] [Google Scholar]
- 34.Baliga RR, Pimental DR, Zhao YY, et al. NRG-1-induced cardiomyocyte hypertrophy. Role of PI-3-kinase, p70(S6K), and MEK-MAPK-RSK. Am J Physiol 1999;277(5 Pt 2):H2026–37. [DOI] [PubMed] [Google Scholar]
- 35.Kuramochi Y, Guo X, Sawyer DB. Neuregulin activates erbB2-dependent src/FAK signaling and cytoskeletal remodeling in isolated adult rat cardiac myocytes. J Mol Cell Cardiol 2006;41(2):228–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fukazawa R, Miller TA, Kuramochi Y, et al. Neuregulin-1 protects ventricular myocytes from anthracycline-induced apoptosis via erbB4-dependent activation of PI3-kinase/Akt. J Mol Cell Cardiol 2003;35(12):1473–9. [DOI] [PubMed] [Google Scholar]
- 37.De Keulenaer GW, Doggen K, Lemmens K. The vulnerability of the heart as a pluricellular paracrine organ: lessons from unexpected triggers of heart failure in targeted ErbB2 anticancer therapy. Circ Res 2010;106(1):35–46. [DOI] [PubMed] [Google Scholar]
- 38.Grazette LP, Boecker W, Matsui T, et al. Inhibition of ErbB2 causes mitochondrial dysfunction in cardiomyocytes: implications for Herceptin-induced cardiomyopathy. J Am Coll Cardiol 2004;44(11):2231–8. [DOI] [PubMed] [Google Scholar]
- 39.Gordon LI, Burke MA, Singh AT, et al. Blockade of the erbB2 receptor induces cardiomyocyte death through mitochondrial and reactive oxygen species-dependent pathways. J Biol Chem 2009;284(4):2080–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Imai K, Takaoka A. Comparing antibody and small-molecule therapies for cancer. Nat Rev Cancer 2006;6(9):714–27. [DOI] [PubMed] [Google Scholar]
- 41.Spector NL, Yarden Y, Smith B, et al. Activation of AMP-activated protein kinase by human EGF receptor 2/EGF receptor tyrosine kinase inhibitor protects cardiac cells. Proc Natl Acad Sci U S A 2007;104(25):10607–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nayyar S, Magalski A, Khumri TM, et al. Contrast administration reduces interobserver variability in determination of left ventricular ejection fraction in patients with left ventricular dysfunction and good baseline endocardial border delineation. Am J Cardiol 2006;98(8):1110–4. [DOI] [PubMed] [Google Scholar]
- 43.Kurt M, Shaikh KA, Peterson L, et al. Impact of contrast echocardiography on evaluation of ventricular function and clinical management in a large prospective cohort. J Am Coll Cardiol 2009;53(9):802–10. [DOI] [PubMed] [Google Scholar]
- 44.Schwartz RG, McKenzie WB, Alexander J, et al. Congestive heart failure and left ventricular dysfunction complicating doxorubicin therapy. Seven-year experience using serial radionuclide angiocardiography. Am J Med 1987;82(6):1109–18. [DOI] [PubMed] [Google Scholar]
- 45.Patel CD, Balakrishnan VB, Kumar L, et al. Does left ventricular diastolic function deteriorate earlier than left ventricular systolic function in anthracycline cardiotoxicity? Hell J Nucl Med 2010;13(3):233–7. [PubMed] [Google Scholar]
- 46.Di Lisi D, Bonura F, Macaione F, et al. Chemotherapy-induced cardiotoxicity: role of the conventional echocardiography and the tissue Doppler. Minerva Cardioangiol 2011;59(4):301–8. [PubMed] [Google Scholar]
- 47.Radulescu D, Pripon S, Radulescu LI, et al. Left ventricular diastolic performance in breast cancer survivors treated with anthracyclines. Acta Cardiol 2008;63(1):27–32. [DOI] [PubMed] [Google Scholar]
- 48.Pudil R, Horacek JM, Strasova A, et al. Monitoring of the very early changes of left ventricular diastolic function in patients with acute leukemia treated with anthraxcyclines. Exp Oncol 2008;30(2):160–2. [PubMed] [Google Scholar]
- 49.Sawaya H, Sebag IA, Plana JC, et al. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol 2011;107(9):1375–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Al-Biltagi M, Abd Rab Elrasoul Tolba O, El-Shanshory MR, et al. Strain echocardiography in early detection of doxorubicin-induced left ventricular dysfunction in children with acute lymphoblastic leukemia. ISRN Pediatr 2012;2012:870549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stoodley PW, Richards DA, Hui R, et al. Two-dimensional myocardial strain imaging detects changes in left ventricular systolic function immediately after anthracycline chemotherapy. Eur J Echocardiogr 2011;12(12):945–52. [DOI] [PubMed] [Google Scholar]
- 52.Hare JL, Brown JK, Leano R, et al. Use of myocardial deformation imaging to detect preclinical myocardial dysfunction before conventional measures in patients undergoing breast cancer treatment with trastuzumab. Am Heart J 2009;158(2):294–301. [DOI] [PubMed] [Google Scholar]
- 53.Walker J, Bhullar N, Fallah-Rad N, et al. Role of three-dimensional echocardiography in breast cancer: comparison with two-dimensional echocardiography, multiple-gated acquisition scans, and cardiac magnetic resonance imaging. J Clin Oncol 2010;28(21):3429–36. [DOI] [PubMed] [Google Scholar]
- 54.Hamada H, Ohkubo T, Maeda M, et al. Evaluation of cardiac reserved function by high-dose dobutamine-stress echocardiography in asymptomatic anthracycline-treated survivors of childhood cancer. Pediatr Int 2006;48(3):313–20. [DOI] [PubMed] [Google Scholar]
- 55.Lanzarini L, Bossi G, Laudisa ML, et al. Lack of clinically significant cardiac dysfunction during intermediate dobutamine doses in long-term childhood cancer survivors exposed to anthracyclines. Am Heart J 2000;140(2):315–23. [DOI] [PubMed] [Google Scholar]
- 56.Sieswerda E, Kremer LC, Vidmar S, et al. Exercise echocardiography in asymptomatic survivors of childhood cancer treated with anthracyclines: a prospective follow-up study. Pediatr Blood Cancer 2010;54(4):579–84. [DOI] [PubMed] [Google Scholar]
- 57.de Geus-Oei LF, Mavinkurve-Groothuis AM, Bellersen L, et al. Scintigraphic techniques for early detection of cancer treatment-induced cardiotoxicity. J Nucl Med 2011;52(4):560–71. [DOI] [PubMed] [Google Scholar]
- 58.Oberholzer K, Kunz RP, Dittrich M, et al. [Anthracycline-induced cardiotoxicity: cardiac MRI after treatment for childhood cancer]. Rofo 2004;176(9):1245–50 [in German]. [DOI] [PubMed] [Google Scholar]
- 59.Fallah-Rad N, Lytwyn M, Fang T, et al. Delayed contrast enhancement cardiac magnetic resonance imaging in trastuzumab induced cardiomyopathy. J Cardiovasc Magn Reson 2008;10:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Douglas PS, Khandheria B, Stainback RF, et al. ACCF/ASE/ACEP/ASNC/SCAI/SCCT/SCMR 2007 appropriateness criteria for transthoracic and transesophageal echocardiography: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American Society of Echocardiography, American College of Emergency Physicians, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and the Society for Cardiovascular Magnetic Resonance endorsed by the American College of Chest Physicians and the Society of Critical Care Medicine. J Am Coll Cardiol 2007;50(2):187–204. [DOI] [PubMed] [Google Scholar]
- 61.Hendel RC, Berman DS, Di Carli MF, et al. ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 appropriate use criteria for cardiac radionuclide imaging: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the American Society of Nuclear Cardiology, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, and the Society of Nuclear Medicine. Circulation 2009;119(22):e561–87. [DOI] [PubMed] [Google Scholar]
- 62.Hendel RC, Patel MR, Kramer CM, et al. ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American College of Radiology, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, American Society of Nuclear Cardiology, North American Society for Cardiac Imaging, Society for Cardiovascular Angiography and Interventions, and Society of Interventional Radiology. J Am Coll Cardiol 2006;48(7):1475–97. [DOI] [PubMed] [Google Scholar]
- 63.Cardinale D, Sandri MT, Martinoni A, et al. Left ventricular dysfunction predicted by early troponin I release after high-dose chemotherapy. J Am Coll Cardiol 2000;36(2):517–22. [DOI] [PubMed] [Google Scholar]
- 64.Cardinale D, Sandri MT, Martinoni A, et al. Myocardial injury revealed by plasma-troponin I in breast cancer treated with high-dose chemotherapy. Ann Oncol 2002;13(5):710–5. [DOI] [PubMed] [Google Scholar]
- 65.Cardinale D, Sandri MT, Colombo A, et al. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation 2004;109(22):2749–54. [DOI] [PubMed] [Google Scholar]
- 66.Sandri MT, Salvatici M, Cardinale D, et al. N-terminal pro-B-type natriuretic peptide after high-dose chemotherapy: a marker predictive of cardiac dysfunction? Clin Chem 2005;51(8):1405–10. [DOI] [PubMed] [Google Scholar]
- 67.Cochet A, Quilichini G, Dygai-Cochet I, et al. Baseline diastolic dysfunction as a predictive factor of trastuzumab-mediated cardiotoxicity after adjuvant anthracycline therapy in breast cancer. Breast Cancer Res Treat 2011;130(3):845–54. [DOI] [PubMed] [Google Scholar]
- 68.Fallah-Rad N, Walker JR, Wassef A, et al. The utility of cardiac biomarkers, tissue velocity and strain imaging, and cardiac magnetic resonance imaging in predicting early left ventricular dysfunction in patients with human epidermal growth factor receptor II-positive breast cancer treated with adjuvant trastuzumab therapy. J Am Coll Cardiol 2011;57(22):2263–70. [DOI] [PubMed] [Google Scholar]
- 69.Schmitz KH, Prosnitz RG, Schwartz AL, et al. Prospective surveillance and management of cardiac toxicity and health in breast cancer survivors. Cancer 2012;118(Suppl 8):2270–6. [DOI] [PubMed] [Google Scholar]
- 70.Aapro M, Bernard-Marty C, Brain EG, et al. Anthracycline cardiotoxicity in the elderly cancer patient: a SIOG expert position paper. Ann Oncol 2011;22(2):257–67. [DOI] [PubMed] [Google Scholar]
- 71.Hunt SA, Abraham WT, Chin MH, et al. 2009 Focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in collaboration with the International Society for Heart and Lung Transplantation. J Am Coll Cardiol 2009;53(15):e1–90. [DOI] [PubMed] [Google Scholar]
- 72.Cardinale D, Colombo A, Lamantia G, et al. Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol 2010;55(3):213–20. [DOI] [PubMed] [Google Scholar]
- 73.Lu CY, Srasuebkul P, Drew AK, et al. Positive spillover effects of prescribing requirements: increased cardiac testing in patients treated with trastuzumab for HER2+ metastatic breast cancer. Intern Med J 2011. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 74.Bellenger NG, Burgess MI, Ray SG, et al. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance; are they interchangeable? Eur Heart J 2000;21(16):1387–96. [DOI] [PubMed] [Google Scholar]
- 75.Verma S, Ewer MS. Is cardiotoxicity being adequately assessed in current trials ofcytotoxic and targeted agents in breast cancer? Ann Oncol 2011;22(5):1011–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1: Non-dilated, globally hypokinetic left ventricle and mild dilatation of the right ventricle.
Video 2: Normal left ventricular function available at http://www.medical.theclinics.com/


