Abstract
White Sutton Syndrome is a rare autosomal dominant disorder resulting from a de novo mutation of Pogo Transposable Element Derived with Zinc Finger domain gene. The phenotype is characterized by a wide spectrum of cognitive dysfunction and developmental delays. Hearing loss is frequently mentioned as one of the symptoms of this rare disease, but details are usually scant. We report a case of a male child affected by White Sutton Syndrome and sensorineural hearing loss, with audiological findings of an auditory neuropathy spectrum disorder, a dysfunction of the auditory pathway with preserved cochlear outer hair cell function. Up to date, the present case is the first description of hearing loss due to an auditory neuropathy spectrum disorder in White Sutton Syndrome. A comprehensive audiological assessment is therefore mandatory in all White Sutton Syndrome patients in order to recognize a possible auditory neuropathy disorder and then avoid misdiagnosis, or erroneous clinical management.
Keywords: Auditory brainstem evoked responses, Deafness, Hearing aids, Hearing loss
Introduction
The White Sutton Syndrome (WHSUS) is a rare autosomal dominant disorder resulting from a de novo mutation of Pogo Transposable Element Derived with Zinc Finger (POGZ) domain gene. According to the literature, the WHSUS phenotype is characterized by a wide spectrum of cognitive dysfunction, developmental delays, hypotonia, and autism spectrum disorder. Hearing loss is often described, but usually, details are not provided.
As far as we know, we describe the first WHSUS case with hearing loss due to an auditory neuropathy spectrum disorder reported in the literature, with the aim to share our experience and potential new clinical insights on this rare condition.
Case Presentation
We report a case of a male child affected by WHSUS, diagnosed after genetic counseling and WES-trio investigation. The patient, a first-twin pre-term baby (30 weeks and 3 days), was conceived through in vitro fertilization. As a newborn, he was treated for respiratory distress, hypokalemia, and jaundice. The higher level of bilirubinemia was 242 mmol/L in the fifth day of life. It was the unique value exceeding the limit to start phototherapy (200 mmol/L), as suggested by the NHS (National Institute for Health and Clinical Excellence) reference values for neonates of 30 weeks of gestation. He required short treatment (24 hours) with phototherapy with a rapid and stable decrease of values (155 mmol/L after 6 hours of treatment). Bilirubinemia values were checked from the first day of life on a regular basis (twice in the first days and daily as the values were stable after the treatment). Blood exchange was not required.
Hypospadias, ambiguous genitals, hemorrhage of the germinative matrix, anemia, recurrent bacterial conjunctivitis, ostium secundum atrial septal defect (ASD), retinopathy, and moderate broncho-pulmonary dysplasia were his other clinical features. At 3 months of corrected age, he developed central apneas, for which he still needs non-invasive ventilation during sleep.
At birth, the audiological assessment revealed bilateral failure of transiently evoked otoacoustic emissions (TEOAEs) and automatic auditory brain responses (ABRs). The clinical ABR, to evaluate the hearing threshold, showed a wave V undetectable at the maximum intensity of stimuli.
At 4 months of correct age, the audiological assessment disclosed a bilateral PASS (defined when 3 out of 4 bands in the speech frequency range pass the test) at the TEOAEs test (performed at 80 dB SPL of intensity, with the right ear the stimulus stability of 91% and with an artifact rate of 12%, while in the left ear the stimulus stability was 98% with the artifact rate of 3%).
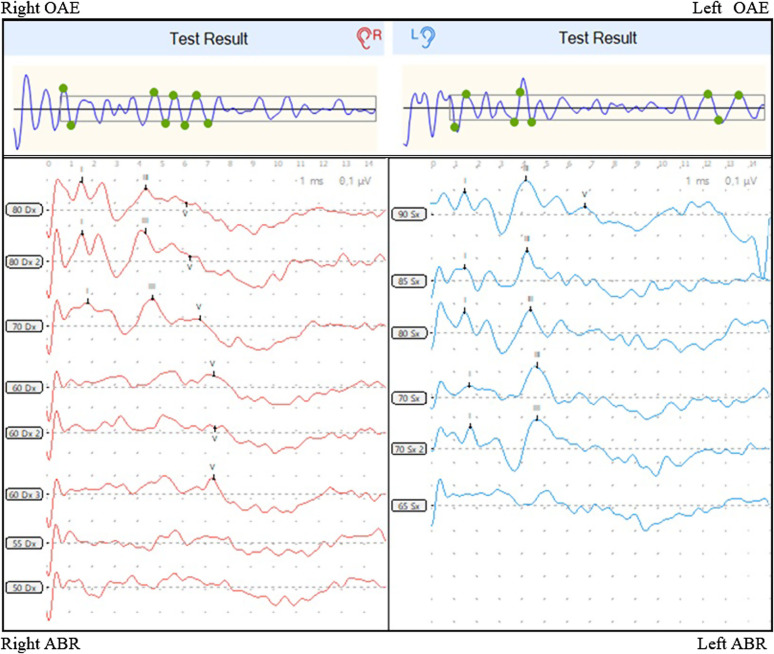
At the clinical (threshold) ABR, the hearing threshold was found at 60 dB nHL on the right side and at 90 dB nHL on the left side (see Figure 1). Tympanogram was type A bilaterally.
Figure 1.
Audiological assessment at 4 months, disclosing bilateral TEOAEs PASS, with hearing threshold at 60 dB nHL on the right and at 90 dB nHL on the left, at the clinical ABR. There were no signs of conductive hearing loss examining the ABR trace, and the tympanogram was type A bilaterally. ABRs, automatic auditory brain responses; TEOAEs, transiently evoked otoacoustic emissions.
At the Auditory Steady-State Responses, hearing threshold was 20 dB nHL at 2 kHz and 25 dB nHL at 4 kHz on the right side and 35 dB nHL at 2 kHz and 20 dB nHL at 4 kHz on the left side.
These audiological findings were further confirmed at 5, 10, and 12 months of correct age. Behavioral tests did not show reliable results, so far.
Cerebral magnetic resonance imaging was performed at 7 months of correct age, disclosing brachycephaly, enlargement of the subarachnoid spaces along the frontotemporal convexity, hypoplasia of the corpus callosum, and apparent normal cochlear nerve pattern.
We have decided to prescribe bilateral hearing aids, which the little patient is now wearing since 3 months. Speech therapy is performed weekly. Unfortunately, so far, no concrete progressions in language development have been noticed.
The parents of the patient provided consent for the publication of this case report.
Discussion
The prevalence of WHSUS is reported as <1/1 000 000, with a total of approximately 117 cases identified worldwide. (1) Studies investigating genotype–phenotype association are currently scant. Because of the rarity of this syndrome, the description of its clinical manifestations mainly arises from single case reports or small case series. Alongside cognitive, developmental, and behavioral dysfunction, other additional features commonly reported include seizures, refractive errors, strabismus, hearing loss, sleep disturbance (particularly sleep apnea), feeding and gastrointestinal problems, mild genital abnormalities in males, and urinary tract involvement in both males and females. Speech delay is reported to occur in up to 88% of subjects with POGZ mutation. While hearing impairment is frequently reported, details of audiological investigations are not provided. In a recent review, the rate of WHSUS patients affected by sensorineural hearing impairment was estimated at around 54% of cases.1
The audiological findings, in the reported case, define the clinical picture of an auditory neuropathy spectrum disorder, a specific defect due to a dysfunction of the auditory pathway at different levels (i.e., cochlear, neural, or central), with preserved cochlear outer hair cell function. At the instrumental evaluation, this condition is characterized by normal OAEs and altered ABR, as per the definition of the British Association of Audiovestibular Physicians, the British Society of Audiology, and the Guidelines Development Conference at NHS Como.2–4
Our patient presented also other risk factors for auditory neuropathy spectrum disorder, such as hyper-bilirubinemia. However, the possibility of bilirubinaemia-related sequelae, such as kernicterus and auditory defects, can be considered when there is a need for blood exchange, clinical symptoms caused by hyperbilirubinemia, and specific findings at neuroimaging. In the present case, the patient did not present any of these conditions; furthermore, the pathognomonic findings of hyper-bilirubinemia (bilirubin deposition in the basal ganglia and cranial nerves) were not detectable at the cerebral MRI. Besides, his neuroimaging findings (brachycephaly, enlargement of the subarachnoid spaces along the frontotemporal convexity, hypoplasia of the corpus callosum) were consistent with those of other children affected by WHSUS and may be peculiar signs of the disease, even if according to the literature, it is still difficult to delineate a comprehensive neuroimaging definition of WHSUS,5,6 since approximately less than 120 cases have been reported worldwide, so far.
The use of hearing aids in auditory neuropathy is still controversial. While early amplification is recommended for children with cochlear sensorineural hearing loss, it is difficult to estimate the degree of auditory deficiency, related to the neural processing disorder, in babies and infants with auditory neuropathy. Moreover, in these subjects, the presence of other co-morbidities can have a negative impact on hearing rehabilitation as well as speech development, such as in the presented case. However, there is some evidence that children wearing hearing aids may report better scores on speech recognition tests; unfortunately, unless behavioral responses can be investigated, the results are difficult to assess in early childhood. As suggested by the American Academy of Audiology—Pediatric Amplification guidelines,7 since hearing aids are easily accessible, non-invasive, and can be removed at any time if bothersome, a trial may be worthwhile, despite the uncertainty of the outcomes.
Conclusion
To the best of our knowledge, the present is the first reported case of hearing loss due to an auditory neuropathy spectrum disorder in WHSUS. A comprehensive audiological assessment is therefore mandatory in WHSUS patients in order to recognize a possible auditory neuropathy disorder and then avoid misdiagnosis or erroneous clinical management.
Footnotes
Informed Consent: Verbal informed consent was obtained from the parents who agreed to take part in the study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – V.F., L.B.M.; Design – V.F., L.B.M.; Supervision – A.C., S.B., E.B.; Resources – L.N.; Materials – L.N., L.B.M., E.B., S.B., V.F., A.C.; Data Collection and/or Processing – L.N., A.C.; Analysis and/or Interpretation – A.C., V.F.; Literature Search – V.F., L.B.M., E.B., S.B.; Writing – V.F., L.B.M.; Critical Review – A.C., E.B., S.B.
Declaration of Interests: The authors have no conflict of interest to declare.
Funding: The authors declared that this study has received no financial support.
References
- 1. Nagy D, Verheyen S, Wigby KM, et al. Genotype-phenotype comparison in POGZ-elated neurodevelopmental disorders by using clinical scoring. Genes (Basel). 2022;13(1):154. ( 10.3390/genes13010154) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guidelines for Aetiological Investigation into Auditory Neuropathy Spectrum Disorder in Children and Young Adults July 2018 Produced by British Association of Audiovestibular Physicians. Available at: https://www.baap.org.uk/uploads/1/1/9/7/119752718/guidelines_for_ansd_final_version.pdf. Accessed September 1, 2022. [Google Scholar]
- 3.Available at: https://www.thebsa.org.uk/wp-content/uploads/2019/01/OD104-85-Recommended-Procedure-Assessment-and-Management-of-ANSD-in-Young-Infants.pdf. Accessed September 1, 2022. [Google Scholar]
- 4. Guidelines for Identification and Management of Infants and Young Children with Auditory Neuropathy Spectrum Disorder Guidelines Development Conference at NHS. Como, Italy; 2008. Available at: https://www.childrenscolorado.org/globalassets/departments/earnose-throat/ansd-monograph.pdf. Accessed September 1, 2022. [Google Scholar]
- 5. Ye Y, Cho MT, Retterer K, et al. De novo POGZ mutations are associated with neurodevelopmental disorders and microcephaly. Cold Spring Harb Mol Case Stud. 2015;1(1):a000455. ( 10.1101/mcs.a000455) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dentici ML, Niceta M, Pantaleoni F, et al. Expanding the phenotypic spectrum of truncating POGZ mutations: association with CNS malformations, skeletal abnormalities, and distinctive facial dysmorphism. Am J Med Genet A. 2017;173(7):1965 1969. ( 10.1002/ajmg.a.38255) [DOI] [PubMed] [Google Scholar]
- 7. American Academy of Audiology clinical practice guidelines on pediatric amplification. http://www.audiology.org/resources/documentlibrary/Documents/PediatricAmplificationGuidelines.pdf. Accessed September 1, 2022. [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a