Abstract
BACKGROUND
: Side effects occurring after COVID-19 vaccination can include vertigo and dizziness. Despite its high incidence, few studies to date have assessed dizziness/vertigo after vaccination. The present study investigated the incidence of dizziness/vertigo after COVID-19 vaccination in South Korea.
Methods:
Adverse reactions to COVID-19 vaccination reported to the Korea Disease Control and Prevention Agency from February 26, 2021, to July 31, 2022 (week 74) were analyzed. The incidence rates of dizziness/vertigo in subjects vaccinated with 5 COVID-19 vaccines, AZD1222 (AstraZeneca), BNT162b2 (Pfizer-BioNTech), JNJ-78436735 (Janssen), mRNA-1273 (Moderna), and NVX-CoV2373 (Novavax), were determined.
Results:
A total of 126 725 952 doses of COVID-19 vaccine were administered, with 473 755 suspected adverse reactions (374 per 100 000 vaccinations) reported. Vertigo/dizziness was reported after the administration of 68 759 doses, or 54.3 per 100 000 vaccinations, making it the third most common adverse reaction after headache and muscle pain.
Conclusion:
Dizziness/vertigo was generally a mild adverse reaction after COVID-19 vaccination, but it was the third most common adverse reaction in Korea. Studies are necessary to clarify the causal relationship between vaccination and dizziness/vertigo and to prepare subjects for this possible adverse reaction.
Keywords: COVID-19 vaccination, Side effect, Vertigo, Dizziness
Introduction
The coronavirus disease-2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), prompted an unprecedented effort to develop and disseminate vaccines worldwide. Although these vaccines have been shown to be safe and effective, they have given rise to various unexpected side effects. Although most of these adverse effects have been mild, some have been severe. Because their benefits in protecting individuals from COVID-19 generally outweigh any possible side effects of COVID-19 vaccines, vaccination is recommended.1,2
Dizziness/vertigo is one of the most common side effects following COVID-19 vaccination; however, to date, little is known about the incidence and intensity of dizziness/vertigo after COVID-19 vaccination. The present study therefore evaluated the nationwide incidence and severity of dizziness/vertigo following COVID-19 vaccination in South Korea.
Methods
The records of the Korea Disease Control and Prevention Agency were reviewed to determine the number of doses of COVID-19 vaccine administered nationwide in Korea from February 26, 2021, to July 31, 2022 (week 74), as well as the numbers and types of adverse reactions following vaccination.3 Adverse reactions were defined as undesirable and unintended signs, symptoms, or diseases occurring after vaccination, without having a causal relationship with the vaccine. Data were recorded on the number of people who received 5 COVID-19 vaccines, AZD1222 (AstraZeneca), BNT162b2 (Pfizer-BioNTech), JNJ-78436735 (Janssen), mRNA-1273 (Moderna), and NVX-CoV2373 (Novavax), and on their adverse reactions. Four of these vaccines, AZD1222, BNT162b2, mRNA-1273, and NVX-CoV2373, were administered in a 2-dose regimen, whereas JNJ-78436735 was administered as a single dose.3 All information on cases with adverse events was updated weekly and was available at the website https://ncv.kdca.go.kr/eng/. Vaccination data, including vaccine type, epidemiological data, symptom onset dates, symptoms, and complications, were obtained from the Korea Centers for Disease Control and Prevention.4
Adverse events were expressed as numbers per 100 000 inoculations and stratified by vaccine type. The study protocol was approved by the Institutional Review Board of Department of Obstetrics and Gynecology, St. Vincent’s Hospital, The Catholic University of Korea, Suwon, Korea, which waived the requirement for informed consent because of the retrospective nature of this study (IRB No. 2022-06-042).
Results
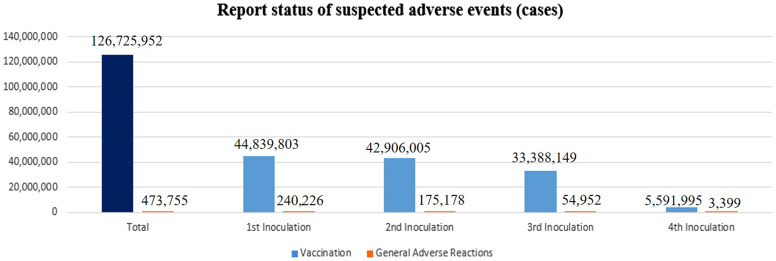
From February 26, 2021, to July 31, 2022, 125 309 520 doses of COVID-19 vaccine were administered, with 473 775 suspected adverse reactions (374 per 100 000) reported. The numbers and rates of adverse reactions decreased as the number of doses increased, with 240 226 suspected adverse reactions (536 per 100 000) reported after first doses, 175 178 (408 per 100 000) after second doses, 54 952 (165 per 100 000) after third doses, and 3399 (61 per 100 000) after fourth doses (Figure 1).
Figure 1.
Numbers of suspected adverse reactions to COVID-19 vaccination.
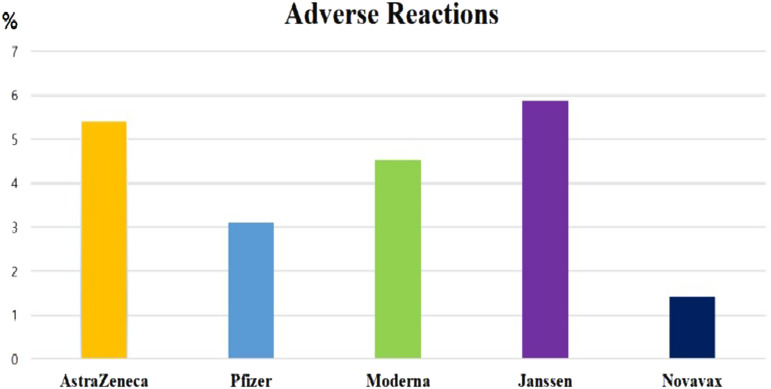
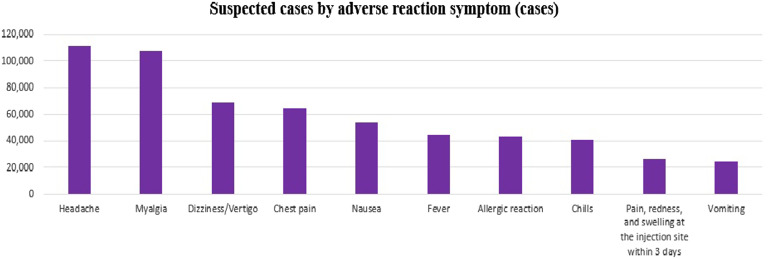
Of the COVID-19 vaccine doses administered, 62.7% were the Pfizer vaccine, 19.6% were the Moderna vaccine, 16.1% were the AstraZeneca vaccine, 1.2% were the Janssen vaccine, and 0.5% were the Novavax vaccine. Rates of adverse reactions per 100 000 doses of these 5 vaccines were 305, 452, 541, 587, and 142, respectively (Figure 2). Dizziness/vertigo was reported following the administration of 68 759 doses of vaccine (54.3 per 100 000), making dizziness/vertigo the third most common suspected adverse reaction, after headache and muscle pain (Figure 3).
Figure 2.
Rates of suspected adverse reactions to each type of COVID-19 vaccine.
Figure 3.
Rates of each type of adverse reaction to vaccines.Rates were calculated based on information reported by medical institutions of suspected adverse reactions after vaccination against COVID-19. The diagnostic accuracy of symptoms or the causal relationships between vaccination and adverse reactions were not determined.
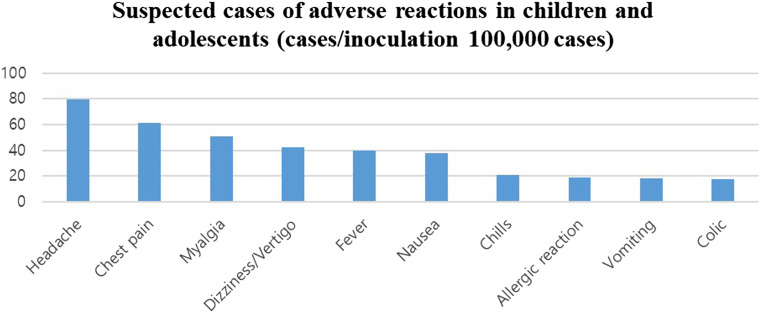
In children and adolescents, dizziness/vertigo was reported after administration of 2859 doses of vaccine (42.6 per 100 000), making dizziness/vertigo the fourth most frequent adverse event in this age group, after headache, chest pain, and muscle pain (Figure 4).
Figure 4.
Rates of each type of adverse reaction to vaccines in children and adolescents. Rates were calculated based on information reported by medical institutions of suspected adverse reactions after vaccination against COVID-19. The diagnostic accuracy of symptoms and the causal relationships between vaccination and adverse reactions were not determined.
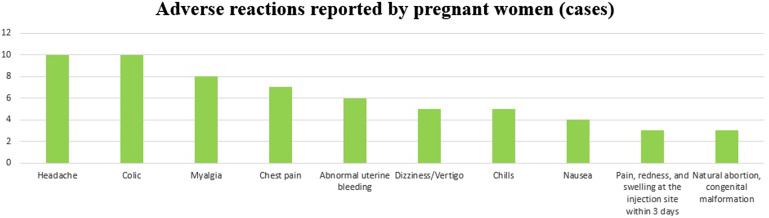
Fifty adverse reactions to vaccine were reported in pregnant women, including 1 death due to a severe adverse reaction, 2 spontaneous abortions, and 1 congenital malformation. Dizziness/vertigo was observed in 5 pregnant women after vaccination, making it the sixth most common adverse reaction, after headache, abdominal pain, muscle pain, chest pain, and abnormal uterine bleeding (Figure 5).
Figure 5.
Rates of each type of adverse reaction to vaccines in pregnant women.Rates were calculated based on information reported by medical institutions of suspected adverse reactions after vaccination against COVID-19. The diagnostic accuracy of symptoms and the causal relationships between vaccination and adverse reactions were not determined.
Discussion
According to the World Health Organization (WHO), the number of confirmed cases of COVID-19 worldwide has exceeded 585 950 085, and 6 425 422 persons have died of this disease.5 This respiratory syndrome is caused by infection with the SARS-CoV-2 RNA virus, which enters the body via aerosol, blood, and the nervous system. Three months after the first case was reported in December 2019, the WHO declared a global pandemic. Although fatality rates vary greatly by country and age, the global fatality rate is about 2.1%. Elderly patients, immunocompromised patients, and patients with underlying diseases are particularly susceptible to severe infection and death.
Aerosol transmission involves the movement of SARS-CoV-2 from one cell to another. Although the pathophysiology of audiovestibular disorders due to COVID-19 is unknown, several potential mechanisms have been suggested. First, SARS-CoV-2 can induce neuroinflammation, resulting in brainstem damage or dysfunction and leading to cranial nerve paralysis and motor deficits as well as defects in the vestibular system.6,7 Second, virus can invade the inner ear or vestibular cochlear nerve, giving rise to cochleitis and/or neuritis leading to vertigo, tinnitus, and hearing loss.8 Third, because the cochlea and semicircular canals are highly susceptible to ischemia due to their lack of an ancillary blood supply, SARS-CoV-2 infection of these structures can give rise to vascular disorders. Various cardiovascular symptoms, including coagulation abnormalities, have been reported in patients with COVID-19, with the sequelae of these symptoms leading to thrombosis or hypoxia in the inner ear.9-11 Fourth, viruses can damage the blood–labyrinth barrier and invade inner ear structures through infected and activated monocytes that attack the vasculature. Viral deoxygenation of red blood cells can also lead to hypoxia and further damage to the inner ear.7,12 Fifth, antibodies or T cells directed against viruses can misidentify inner ear antigens as viruses and cause accidental damage to the inner ear.8 Sixth, sequelae of immune-mediated disorders, such as excessive production of proinflammatory cytokines, can negatively affect the audiovestibular system.13-15 Finally, excess reactive oxygen species and some natural and artificial chemicals can stimulate inflammatory processes, inducing the synthesis and secretion of proinflammatory cytokines, such as interleukin (IL)-6 and IL-1β, and tumor necrosis factor-alpha. Inflammation and oxidative stress are closely related to pathophysiological processes and are closely linked to each other.7,16
As of August 13, 2022, 12 355 390 461 vaccine doses have been administered worldwide.5 Vaccination against COVID-19 is a safe way to train the body's immune system to acquire immunity and prevent viral infection. In addition to reducing the risk of infection, vaccination can prevent the progression to serious illness or death. The COVID-19 vaccine can protect high-risk individuals from contracting COVID-19, such as medical staff, elderly patients, and patients with underlying medical conditions, and those at high risk of developing severe illness when infected.17,18
Adverse events after vaccination against COVID-19 refer to all unintended symptoms that occurred after vaccination. In the data analyzed in the present study, the rate of dizziness/vertigo after COVID-19 vaccine was 54.3 per 100 000, making dizziness/vertigo the third most reported adverse event, after headache and muscle pain. The onset of symptoms of dizziness/vertigo has been reported to occur from 1 to 42 days after COVID-19 vaccination.19
Audiovestibular adverse events following immunization (AEFI) with COVID-19 vaccine are very rare, whereas the rates of sudden sensorineural hearing loss, tinnitus, and vertigo have been reported to have increased over time. The pathophysiology of audiovestibular disorders, including dizziness/vertigo, occurring after COVID-19 vaccination is unclear, but several potential mechanisms have been suggested. First, mRNA vaccines can induce self-adjuvanting activity, allowing mRNA to act as antigen and adjuvant simultaneously. This may result in the induction of an autoimmune response and the production of type I interferon, resulting in strong T and B cell responses and activation of autoreactive lymphocytes.20 Second, antibodies to SARS-CoV-2 spike protein may cross-react with inner ear antigens due to molecular mimicry, resulting in the development of AEFI.21,22 Third, viral antigen–antibody complexes can cause hypersensitivity reactions, leading to local inflammation that damages microvessels in the inner ear.23 Finally, because SARS-CoV-2 spike protein is an effective activator of the alternative complement pathway, it can damage endothelium and induce thrombus formation by enhancing platelet aggregation.24,25
Future analyses of dizziness/vertigo following COVID-19 vaccination should determine whether dizziness is due to peripheral vertigo, central vertigo, or vertigo caused by other diseases. Otoneurological evaluation, along with a dizziness test, of 33 patients with “acute vertigo” after COVID-19 vaccination showed that 16 (48.5%) had symptoms of objective vertigo, 16 (48.5%) had subjective vertigo, 14 (42.4%) had vertigo, and 3 (9.1%) had dizziness. On bedside examination, 7 (21.2%) and 9 (27.3%) patients had horizontal and rotatory nystagmus, respectively, and 17 (51.5%) had vertical or oblique nystagmus. Although the patient sample was small, these results suggested that 51.5% of those with “acute vertigo” after COVID-19 vaccination did not have peripheral vestibular dysfunction.15
It is also necessary to determine whether dizziness/vertigo occurring after COVID-19 vaccination was due to the vaccine itself or to exacerbation of existing conditions. A study of 30 subjects with otologic conditions after COVID-19 vaccination showed that 6 (20%) had Meniere's disease, 2 (6.7%) had autoimmune inner ear disease (AIED), and 3 (10%) had a history of simultaneous treatment for Meniere's disease and AIED.26
It is also important to analyze vertigo and dizziness separately. Most previous studies of dizziness/vertigo have been based on self-reported questionnaires without detailed history taking and examinations for dizziness/vertigo and most reported the combined prevalence of vertigo and dizziness. Because dizziness is commonly reported in COVID-19 patients and because it is not of vestibular origin, it is necessary to separately study vertigo of pure vestibular origin. Moreover, it is important to accurately determine the causes of dizziness/vertigo. Because anxiety and stress can also trigger vertigo attacks, vertigo is not likely a side effect of COVID-19 vaccination. Prospective, large-scale studies that include accurate diagnoses of dizziness and vertigo are needed to determine the causal relationship of the vaccine with dizziness/vertigo. The present study had several limitations. First, this study included only numerical data on subject reports of dizziness/vertigo after vaccination, but there is no information on telephone interviews, bedside examinations, questionnaires, the side effects incidence shown for decades of age in the studied population, objective test results, and accurate diagnoses of dizziness/vertigo. Second, because immunity is usually acquired 2 or more weeks after vaccination, it is possible to become infected with COVID-19 immediately after vaccination. Therefore, the number of subjects with dizziness/vertigo after vaccination injection may include subjects with dizziness/vertigo due to COVID-19 or another upper respiratory tract infection, suggesting that, in these subjects, dizziness/vertigo was not a side effect of the vaccine. Third, this study combined vertigo and dizziness as a side effect of COVID-19 vaccination. Future studies are needed to separately evaluate rates of vertigo of vestibular origin and dizziness of non-vestibular origin. Fourth, because the reported rates of adverse effects were influenced by various factors, such as subject age at inoculation, gender, and awareness of vaccines, it was not possible to analyze the incidence of dizziness/vertigo related to each vaccine or the time of occurrence after inoculation.
Conclusion
Dizziness/vertigo was generally a mild adverse reaction after COVID-19 vaccination, but it was the third most common adverse reaction in Korea. Studies are necessary to clarify the causal relationship between vaccination and dizziness/vertigo and to prepare subjects for this possible adverse reaction.
Footnotes
Ethics Committee Approval: Ethical committee approval was received from the Institutional Review Board of Department of Obstetrics and Gynecology, St. Vincent’s Hospital, The Catholic University of Korea University (IRB No. 2022-06-042).
Informed Consent: N/A.
Peer-review: Externally peer reviewed.
Author Contribution: Concept – S.G.Y.; Design – S.S.K., S.H.K.; Supervision – Y.S.C., S.G.Y.; Funding – S.G.Y., J.S.L.; Materials – K.H.P.; Data Collection and/or Processing – S.H.K., Y.J.L.; Analysis and/or Interpretation – D.K.Y., D.C.P.; Literature Review – H.S.R.; Writing – S.H.K., D.K.Y.; Critical Review – S.H.K., S.G.Y.
Acknowledgments: This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (NRF-2018R1A6A1A03025124) (NRF-2019R1F1A1049878). The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the report. Data regarding the number of people vaccinated with COVID-19 vaccines and the adverse reactions that followed were collected from the Korea Disease Control and Prevention Agency on August 13, 2021.
Declaration of Interests: The authors declare that they have no competing interest.
Funding: This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (NRF-2018R1A6A1A03025124) (NRF-2019R1F1A1049878). This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HV22C0233).
References
- 1. Anderson RM, Vegvari C, Truscott J, Collyer BS. Challenges in creating herd immunity to SARS-CoV-2 infection by mass vaccination. Lancet. 2020;396(10263):1614 1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dodd RH, Pickles K, Nickel B, et al. Concerns and motivations about COVID-19 vaccination. Lancet Infect Dis. 2021;21(2):161 163. ( 10.1016/S1473-3099(20)30926-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee DS, Kim JW, Lee KL, Jung YJ, Kang HW. Adverse events following COVID-19 vaccination in South Korea between February 28 and August 21, 2021: A nationwide observational study. Int J Infect Dis. 2022;118:173 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baek K-R, Lee Y-K. Adverse Reactions after Weekly COVID-19 Vaccination. Korea Disease Control and Prevention Agency. Corona 19 Vaccination Response Promotion Team. Safety Vaccination Management Team:1-31:2022. [Google Scholar]
- 5. World Health Organization. WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int Accessed August 2022. [Google Scholar]
- 6. Benghanem S, Mazeraud A, Azabou E, et al. Brainstem dysfunction in critically ill patients. Crit Care. 2020;24(1):5. ( 10.1186/s13054-019-2718-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jafari Z, Kolb BE, Mohajerani MH. Hearing loss, tinnitus, and dizziness in COVID-19: A systematic review and meta-analysis. Can J Neurol Sci. 2022;49(2):184 195. ( 10.1017/cjn.2021.63) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lang B, Hintze J, Conlon B. Coronavirus disease 2019 and sudden sensorineural hearing loss. J Laryngol Otol. 2020;1:1 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chandrasekhar SS, Tsai Do BS, Schwartz SR, et al. Clinical practice guideline: sudden hearing loss (update). Otolaryngol Head Neck Surg. 2019. August;161(1):S1 S45. [DOI] [PubMed] [Google Scholar]
- 10. Whittaker A, Anson M, Harky A. Neurological manifestations of COVID-19: A systematic review and current update. Acta Neurol Scand. 2020;142(1):14 22. ( 10.1111/ane.13266) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kwenandar F, Valeriani Japar K, Damya V, et al. Coronavirus disease 2019 and cardiovascular system: A narrative review. Int J Cardiol Heart Vasc. 2020;29:1 00557. ( 10.1016/j.ijcha.2020.100557) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: Tissue distribution, Host-Virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11(7):995 998. ( 10.1021/acschemneuro.0c00122) [DOI] [PubMed] [Google Scholar]
- 13. Cure E, Cumhur Cure M. Comment on “Hearing loss and COVID-19: A note”. Am J Otolaryngol. 2020;41(4):102513. ( 10.1016/j.amjoto.2020.102513) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Almufarrij I, Munro KJ. One year on: an updated systematic review of SARS-CoV-2, COVID-19 and audio-vestibular symptoms. Int J Audiol. 2021;60(12):935 945. ( 10.1080/14992027.2021.1896793) [DOI] [PubMed] [Google Scholar]
- 15. Wichova H, Miller ME, Derebery MJ. Otologic manifestations after COVID-19 vaccination: the house ear clinic experience. Otol Neurotol. 2021;42(9):e1213-e1218. ( 10.1097/MAO.0000000000003275) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Delgado-Roche L, Mesta F. Oxidative stress as key player in severe acute respiratory syndrome coronavirus (SARS-CoV) infection. Arch Med Res. 2020;51(5):384 387. ( 10.1016/j.arcmed.2020.04.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Center for Disease Control and Prevention (USCDC). Interim clinical considerations for use of COVID-19 vaccines currently authorized in the United States [internet] Accessed 2021 May 12. https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html. Atlanta (GA): USCDC, c2021. [Google Scholar]
- 18. Corona 19 vaccination response promotion group. Guidelines for the Corona Virus-19 Vaccination Project – for hospital-level or higher medical institutions (self-vaccination) [internet]. Cheongju (KR): Korea Disease Control and Prevention Agency, c2021. Accessed 2021 May 12. https://ncv.kdca.go.kr/board.es?mid=a12101000000&bid=0031&act=view&list_no=263&t ag=&nPage=1. [Google Scholar]
- 19. Pisani D, Gioacchini FM, Viola P, et al. Audiol res. Audiovestibular disorders after COVID-19 vaccine: is there an association?. Audiol Res. 2022;12(3):212 223. ( 10.3390/audiolres12030024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oldstone MB. Molecular mimicry: its evolution from concept to mechanism as a cause of autoimmune diseases. Monoclon Antibodies Immunodiagn Immunother. 2014;33(3):158 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vojdani A, Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2020;217:108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kanduc D, Shoenfeld Y. Molecular mimicry between SARS-CoV-2 spike glycoprotein and mammalian proteomes: implications for the vaccine. Immunol Res. 2020;68(5):310 313. ( 10.1007/s12026-020-09152-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen X, Fu YY, Zhang TY. Role of viral infection in sudden hearing loss. J Int Med Res. 2019;47(7):2865 2872. ( 10.1177/0300060519847860) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Montano D. Frequency and associations of adverse reactions of COVID-19 vaccines reported to pharmacovigilance systems in the European Union and the United States. Front Public Health. 2021;9:756633. ( 10.3389/fpubh.2021.756633) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sessa M, Kragholm K, Hviid A, Andersen M. Thromboembolic events in younger women exposed to Pfizer-BioNTech or Moderna COVID-19 vaccines. Expert Opin Drug Saf. 2021;20(11):1451 1453. ( 10.1080/14740338.2021.1955101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Di Mauro P, La Mantia I, Cocuzza S, et al. Acute vertigo after COVID-19 vaccination: case series and literature review. Front Med (Lausanne). 2022;6(8):790931. [DOI] [PMC free article] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a