Abstract
Concurrent chemoradiotherapy (cCRT) has been predominantly used as the standard therapy for locally advanced or unresectable non-small cell lung cancer (NSCLC) patients with stage III disease. Based on the outstanding results of Phase III Pacific study, Programmed Death-Ligand 1 (PD-L1) inhibitor consolidation therapy after cCRT without progression disease (PD) has been recommended by National Comprehensive Cancer Network (NCCN) guideline as standard therapy for these patients. However, not all patients can tolerate a full course of cCRT due to the poor performance status, concurrent complications, or poor pulmonary function. Therefore, sequential chemoradiotherapy (sCRT) is often conducted for these selected patients who have been assessed as not suitable for cCRT. Moreover, not all patients are suitable for immunotherapy, especially for those with auto-immune disease or certain gene mutations associated with non-response of immunotherapy. Hence, we presented a case with both autoimmune disease and serine/threonine kinase 11 (STK11) mutation, who underwent angiogenesis inhibitor Endostar consolidation therapy after sCRT, and achieved a progression-free survival (PFS) more than 17 months and still in the process of follow-up. This case may offer an effective consolidation treatment for these patients with stage III disease unsuitable for immunotherapy. Further clinical trials are required to confirm this treatment option.
Keywords: Lung adenocarcinoma, stage III, sequential chemoradiotherapy, consolidation therapy, angiogenesis inhibitor
Introduction
Concurrent chemoradiotherapy (cCRT) has been predominantly used as the standard therapy for locally advanced or unresectable non-small cell lung cancer (NSCLC) patients with stage III disease [1]. In Pacific trail, Programmed Death-Ligand 1 (PD-L1) inhibitor Durvalumab was demonstrated to hold significant clinical benefit for patients with locally advanced/unresectable stage III NSCLC who experienced no disease progression (PD) after definitive cCRT [2]. However, not all the patients could tolerate the full course of cCRT, a considerable number of patients were administrated with sequential chemoradiotherapy (sCRT) in China. In Gemstone-301 trial, Programmed Death-1 (PD-L1) inhibitor Sugemalimab was also demonstrated to be superior to placebo in consolidation therapy after cCRT or sCRT [3].
While not all patients are suitable for immunotherapy, especially for those with auto-immune disease or certain gene mutations associated with non-response of immunotherapy. Angiogenesis inhibitor is a viable alternative in maintenance therapy, who has been wildly utilized in local advanced of metastatic NSCLCs. Folkman et al. first reported endostatin, a new protein with 20 kDa C-terminal fragment of collagen XVIII, specifically inhibiting endothelial proliferation and potently inhibits angiogenesis and tumor growth [4]. Endostar, a recombinant human endostatin (rh-endostatin), could downregulate the expression of vascular endothelial growth factor (VEGF) family and vascular endothelial growth factor receptor (VEGFR), which play an important role in the process of angiogenesis. It was approved by National Medical Products Administration (NMPA) of China for the combination treatment with Platinum-based chemotherapy of stage III/IV NSCLC. However, whether Endostar could improve the clinical outcome of NSCLC with stage III disease after cCRT or sCRT is still unknown. Hence, we presented a NSCLC patient with coexistence mutation of serine/threonine kinase 11 (STK11), tumor protein 53 (TP53) and ataxia telangiectasia-mutated gene (ATM), as well as a history of grade 3 hypertension and autoimmune disease psoriasis, who achieved durable disease control with Endostar consolidation therapy after sCRT.
Case presentation
On October 2020, a 66-year-old Chinese male ex-smoker who had a history of type 2 diabetes for 7 years, grade 3 hypertensions for 7 years and autoimmune disease psoriasis for 3 years, presented with pain and discomfort in the right upper shoulder for 2 weeks. Computed tomography (CT) scans of the chest revealed one mass in the right upper lobe and multiple enlarged mediastinal and cervical lymph nodes.
The patient was diagnosed with stage IIIC (cT3N3M0, according to 8th AJCC staging system) lung adenocarcinoma via needle biopsy of cervical lymph node and positron emission computerized tomography and computer tomography (PET-CT) (Figure 1). Next-generation sequencing (NGS) testing showed that this patient harbored STK11 mutation (p.D176Y, c.526G>T, 11.41%), TP53 mutation (p.M246V, c.736A>G, 8.81%) and ATM mutation (p.L2633, c.7898dupT, 9.23%) (Table 1).
Figure 1.
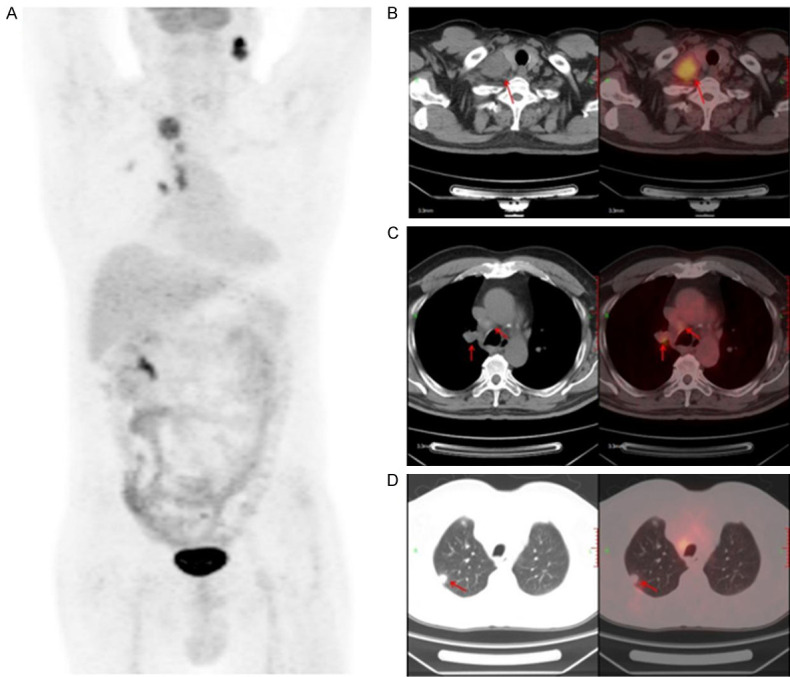
PET-CT scan of baseline lesions. Positron emission computerized tomography and computer tomography (PET-CT).
Table 1.
NGS test showed somatic mutation of tumor issue
| Gene | Variant | Exon | Transcript | Mutation abundance |
|---|---|---|---|---|
| STK11 | p.D176Y (c.526G>T) | Exon 4 | NM_000455.4 | 11.41% |
| TP53 | p.M246V (c.736A>G) | Exon 7 | NM_000546.5 | 8.81% |
| ATM | p.L2633Ffs*23 (c.7898dupT) | Exon 53 | NM_000051.3 | 9.23% |
| CDH9 | p.W453* (c.1358_1359delGGinsAA) | Exon 8 | NM_016279.3 | 7.05% |
| POLH | p.R167* (c.499C>T) | Exon 5 | NM_006502.2 | 0.61% |
| STAG2 | p.N236S (c.707A>G) | Exon 9 | NM_001042749.1 | 12.67% |
| NCOR1 | p.T1661I (c.4982C>T) | Exon 34 | NM_006311.3 | 9% |
| FAT2 | p.S1047L (c.3140C>T) | Exon 1 | NM_001447.2 | 8.09% |
| IL7R | p.S351F (c.1052C>T) | Exon 8 | NM_002185.3 | 5.19% |
| ASXL1 | p.L1408F (c.4224G>C) | Exon 13 | NM_015338.5 | 4.31% |
| CTNNB1 | p.S341R (c.1023C>G) | Exon 7 | NM_001904.3 | 4.19% |
| KMT2C | p.G315V (c.944G>T) | Exon 7 | NM_170606.2 | 1.08% |
The mutated codon cannot be translated into an amino acid.
Immunohistochemistry showed PD-L1 expression of TPS 1-2% using 22C3 antibody.
On October 23, 2020 and November 13, 2020, the patient was administrated with 2 cycle of PC regimen chemotherapy (Pemetrexed 500 mg/m2 and Carboplatin AUC = 5) with the consent from the patient. After the 2-month follow-up, the lesions achieved disease progression (PD) by Response Evaluation Criteria In Solid Tumors (RECIST) (version 1.1). Maximum diameter of cervical lymph nodes enlarged from 3.7 cm to 4.6 cm (Figure 2). On Dec 5, 2020, the patient was administrated with the first cycle of TP regimen chemotherapy (Albumin paclitaxel 260 mg/m2 day 1 and Cisplatin 75 mg/m2 day 1). Shortly, the patient received elective node irradiation (ENI) from Dec 21, 2020 to Feb 12, 2021 (Figure 3). Intensity modulated radiotherapy (IMRT) was adopted as the technique. Gross target volume (GTV) included the primary tumor located in the upper lobe of right lung, the metastatic tumor at the same lobe, and all the lymph nodes in the cervical and mediastinal region. The clinical target volume (CTV) covered all the GTV and the lymph node regions with observable metastatic lymph nodes, including supraclavicular, 2R, 4R, 4L, and subcarinal regions. The planning target volume (PTV) was defined as the CTV plus 0.5 cm margins (Figure 3). The total dose of PTV was 4785 cGy/29 f, and the dose of GTV was up to 5800 cGy/29 f using simultaneous integrated boost (SIB) technique. Dose constraints for normal tissues were required as follows: maximum dose for spinal cord should be less than 45 Gy; the mean lung dose was less than 12 Gy, and less than 25% of the lung volume received 20 Gy (V20<25%); and the mean cardiac dose was less than 30 Gy, with V30<40% and V40<30%. Sequentially, the patient received the second to the sixth cycles of TP regimen chemotherapy from Feb 18, 2021 to June 14, 2021. The assessment on July 1, 2021 was partial response (PR) after sCRT.
Figure 2.
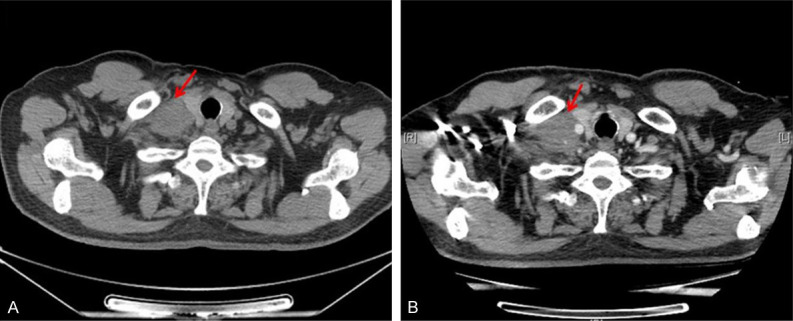
Radiological appraisal of the right cervical lymph node lesion progressed after 2 cycles of chemotherapy. A. Baseline before therapy. B. After 2 cycles of chemotherapy.
Figure 3.
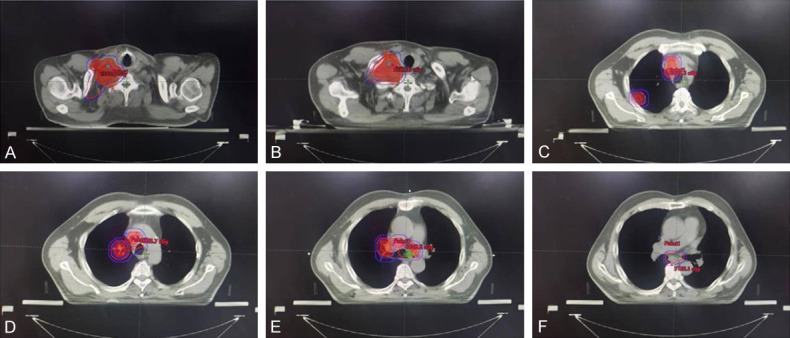
Target volume of radiotherapy. Inner dark red regions represented GTV. Outer red region represented CTV, and blue lines contoured the PTV for both GTV and CTV, respectively. A, B. Target volume at supraclavicular region. C. Target volume at thoracic inlet. GTV included mediastinal lymph nodes and metastatic tumor at the peripheral region of upper right lobe. D. Target volume at the level of aortic arch. E. Target volume at the level of tracheal carina. F. Target volume at subcarinal region. Gross target volume (GTV); clinical target volume (CTV); planning target volume (PTV).
As the treating mode in Gemstone 301 study or Pacific study, the subsequent PD-1 or PD-L1 inhibitor should be administrated in consolidation treatment for unresectable stage III NSCLCs after cCRT or sCRT. While this patient had a history of autoimmune disease psoriasis and immunotherapy non-response associated STK11 and TP53 mutations, immune checkpoint inhibitors (ICIs) might be not suitable for this patient, in case of the occurrence of uncontrolled immune-related adverse effects and immunotherapy super-progression. Considering rapid resistance to chemotherapy after the first 2 cycles, maintenance therapy was indispensable for him. Antiangiogenic consolidation therapy was a good recommendation according to locally advanced subgroup of the Beyond study [5]. Since this patient had a history of grade 3 hypertension, Bevacizumab was not suitable because of its adverse effect of exacerbating hypertension. Thus, during the 19 months of Endostar administration, the patient was recommended to receive 23 cycles consolidation therapy of Endostar with 210 mg continuous infusion for 72 hours per 21 days from July 8, 2021 to Feb 10, 2023. Imaging evaluation including enhanced chest CT, enhanced Brain magnetic resonance (MR), abdominal CT, pelvic CT and cervical ultrasound, was administrated per 3 cycles. Whole body bone imaging was reviewed twice a year. The evaluation was always stable disease (SD) with no new lesion or enlarged lesion detected during the follow-up (Figure 4). The date of the latest follow-up was Dec 19, 2022. The progression-free survival (PFS) of Endostar consolidation therapy has exceeded 17 months. Clinical course including treatment process and relevant assessment was shown in Figure 5.
Figure 4.
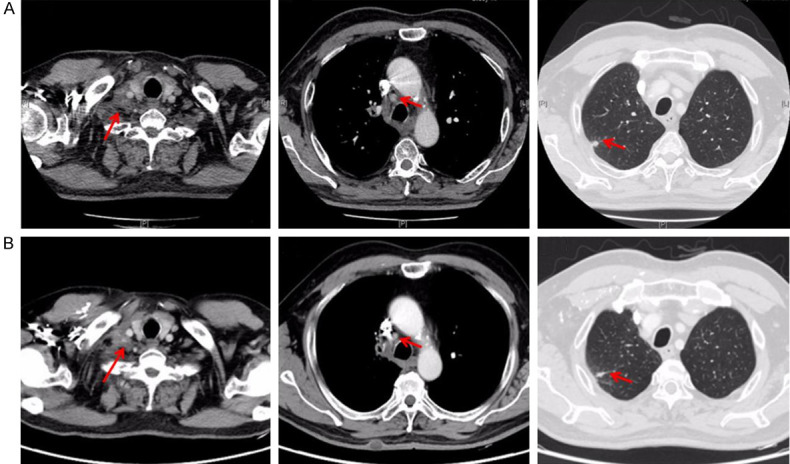
Radiological appraisal after Endostar consolidation treatment. A. Chest CT scan before Endostar consildation treatment on July 1, 2021. B. Chest CT scan after 17 months follow-up of Endostar consildation treatment on Dec 19, 2022.
Figure 5.
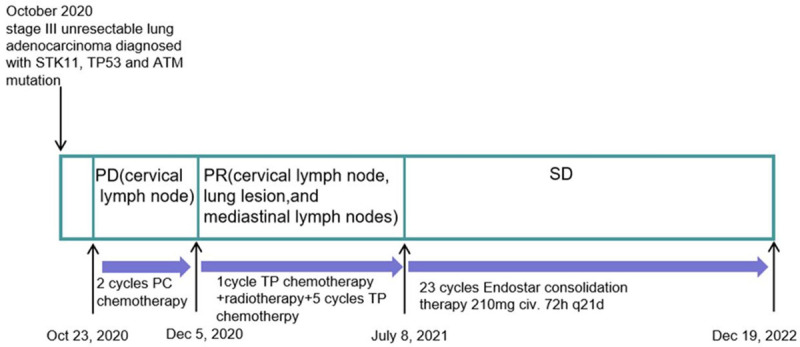
Flowchart of treatment.
Discussion
Stage III NSCLC is a heterogeneous disease, generally categorized into resectable and unresectable disease subgroups. Platinum-based doublet chemotherapy is the main treating modality for local advanced or unresectable stage III NSCLCs in both concurrent and sequential settings combined with radiotherapy. The phase III Pacific trail compared Durvalumab with placebo in unresectable, stage III NSCLC patents with no PD after cCRT. Recently, 5-year survival outcome of Durvalumab consolidation therapy revealed that Durvalumab significantly improved PFS (16.9 months vs. 5.6 moths, HR 0.55, 95% CI: 0.45-0.68) and OS (47.5 months vs. 29.1 moths, HR 0.72, 95% CI: 0.59-0.89) [2]. Based on aforementioned results, Pacific modality has been recommended by NCCN guideline as standard therapy for unresectable NSCLC with stage III disease. However, not all patients could tolerate full course of cCRT due to the poor performance status, concurrent complications, or poor pulmonary function. Therefore, sCRT is often conducted for these selected patients who have been assessed as not suitable for cCRT in China. Therefore, the phase 3 Gemstone-301 trail of PD-1 inhibitor Sugemalimab enrolled the patients who does not have PD after cCRT or sCRT. PFS assessed by independent review committee was significantly longer with Sugemalimab than that with placebo (median 9.0 months vs. 5.8 months; HR 0.64, 95% CI: 0.48-0.85, P = 0.0026) [6]. As for this patient, sCRT was adopted, and the planned dose to GTV was tailored to 5800 cGy (a little lower than RTOG 0617 study [1]) based on the comparatively large target volume and his co-morbidities.
Although ICIs therapy or combination therapies have been widely used in patients with NSCLC, there are still many unsolved problems about ICIs therapy, such as the predictive bio-markers and resistance mechanisms. NSCLC patients with STK11 mutation in primary tumor were reported to be associated with immunosuppression of the tumor microenvironment, and thus have a worse response than those with STK11 wild-type. STK11 mutation might be a primary underlying molecular mechanism of resistance to ICIs, even probably leading to hyper-progressive disease [7,8]. It has been reported that STK11 and KEAP1 mutations confer worse outcomes to immunotherapy among patients with KRAS mutation but not among KRAS wild-type NSCLCs [9]. Additionally, this study did not take TP53 mutation status into consideration.
TP53 is a tumor suppressor gene, which directs the synthesis of tumor protein p53 (or p53). This protein can act as a tumor suppressor and prevent tumor cells from uncontrolled growth, proliferation and division. The mutant TP53 protein acquires oncogenic properties and is capable of promoting cell invasion, metastasis, proliferation and division [10]. It was widely demonstrated that concurrent TP53 mutations could predict poor outcome of EGFR-TKI and ALK-TKI treatment in advanced NSCLCs [11-13]. Studies reported that patients with co-occurring of TP53 and KRAS mutations showed remarkable clinical benefit to PD-1 inhibitors [14,15]. Concurrent mutations in TP53 and STK11 were shown to confer poor survival in the KRAS mutated lung adenocarcinoma [16], while the influence on clinical response to ICIs is still unclear when TP53 mutation combining with STK11 mutation, without KRAS mutation. The patient in this case report harbored TP53 and STK11 mutations with wild-type KRAS. In case of immune hyper-progression, we were very cautious about the administration of immunotherapy.
ATM, one of DNA damage response and repair genes, whose alteration has been identified as independently associated with response to PD-1/PD-L1 blockade in patients with metastatic urothelial carcinoma [17]. Two other Phase II trials found that Ipilimumab/nivolumab and pembrolizumab were effective in patients with metastatic refractory prostate cancer carrying DNA repair related genes (BRCA1/2, ATM, MSH6, FANCM, FANCA, POLH) variants [18,19]. However, the relationship between ATM and immunotherapy response is still unclear in lung cancer.
Data on the safety of ICIs in patients with concurrent autoimmune diseases are limited. Almost all prospective randomized trials of ICI have excluded patients with pre-existing autoimmune diseases. IrAE or severe flare of pre-existing autoimmune diseases occurred occasionally in these patients [20]. After carefully evaluated the safety and efficacy, this patient was exempted from the administration of ICIs.
Since immunotherapy is not suitable, antiangiogenic therapy is qualified for consolidation therapy after cCRT or sCRT. Considering bevacizumab may aggravate hypertension, Endostar was applied in consolidation therapy in this patient. A phase II trial HELPER study indicated that the median times of PFS and OS were 13.3 months and 34.7 months in Endostar and cCRT combination treatment [21]. It has been reported that Endostar infusion throughout the peri-radiotherapy period enhanced radiosensitivity and showed better survival outcomes in locally advanced NSCLCs. Moreover, this treating modality also showed fewer adverse events in terms of radiation-related pulmonary disease [22]. A meta-analysis reviewed 10 studies and 716 patients revealed that Endostar combined with cCRT significantly improved ORR (RR: 1.263, 95% CI: 1.137-1.403, P<0.001), DCR (RR: 1.274, 95% CI: 1.124-1.444, P<0.001) and one-year survival (RR = 1.113, 95% CI: 1.006-1.231, P = 0.038) compared with cCRT [23]. Based on this evidence, we assumed that Endostar may be effective in consolidation therapy after cCRT or sCRT. Surprisingly, the PFS of Endostar consolidation therapy for this patient has exceeded for 17 months, longer than the median PFS of both Gemstone-301 study and Pacific study.
Conclusions
This case may offer an effective consolidation treatment for these patients with stage III disease not suitable for ICIs therapy. Therefore, further clinical trials are required to expand treatment options for unresectable stage III NSCLC patients.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81801734 to X.Y., 81802271 to N.A.), the Natural Science Foundation of Shandong Province, China (ZR2019QH003 to X.Y.) and by Qilu Health Outstanding Young Talents Training Project (to N.A. No. 3843).
Disclosure of conflict of interest
None.
References
- 1.Bradley JD, Paulus R, Komaki R, Masters G, Blumenschein G, Schild S, Bogart J, Hu C, Forster K, Magliocco A, Kavadi V, Garces YI, Narayan S, Iyengar P, Robinson C, Wynn RB, Koprowski C, Meng J, Beitler J, Gaur R, Curran W Jr, Choy H. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16:187–199. doi: 10.1016/S1470-2045(14)71207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spigel DR, Faivre-Finn C, Gray JE, Vicente D, Planchard D, Paz-Ares L, Vansteenkiste JF, Garassino MC, Hui R, Quantin X, Rimner A, Wu YL, Ozguroglu M, Lee KH, Kato T, de Wit M, Kurata T, Reck M, Cho BC, Senan S, Naidoo J, Mann H, Newton M, Thiyagarajah P, Antonia SJ. Five-year survival outcomes from the PACIFIC trial: durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. J. Clin. Oncol. 2022;40:1301–1311. doi: 10.1200/JCO.21.01308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou Q, Chen M, Wu G, Chang JH, Jiang O, Cui JW, Han G, Lin Q, Fang J, Chen GY, Wu YL. GEMSTONE-301: a phase III clinical trial of CS1001 as consolidation therapy in patients with locally advanced/unresectable (stage III) non-small cell lung cancer (NSCLC) who did not have disease progression after prior concurrent/sequential chemoradiotherapy. Transl Lung Cancer Res. 2020;9:2008–2015. doi: 10.21037/tlcr-20-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 5.Zhou C, Wu YL, Chen G, Liu X, Zhu Y, Lu S, Feng J, He J, Han B, Wang J, Jiang G, Hu C, Zhang H, Cheng G, Song X, Lu Y, Pan H, Zheng W, Yin AY. BEYOND: a randomized, double-blind, placebo-controlled, multicenter, phase III study of first-line carboplatin/paclitaxel plus bevacizumab or placebo in Chinese patients with advanced or recurrent nonsquamous non-small-cell lung cancer. J. Clin. Oncol. 2015;33:2197–2204. doi: 10.1200/JCO.2014.59.4424. [DOI] [PubMed] [Google Scholar]
- 6.Zhou Q, Chen M, Jiang O, Pan Y, Hu D, Lin Q, Wu G, Cui J, Chang J, Cheng Y, Huang C, Liu A, Yang N, Gong Y, Zhu C, Ma Z, Fang J, Chen G, Zhao J, Shi A, Lin Y, Li G, Liu Y, Wang D, Wu R, Xu X, Shi J, Liu Z, Cui N, Wang J, Wang Q, Zhang R, Yang J, Wu YL. Sugemalimab versus placebo after concurrent or sequential chemoradiotherapy in patients with locally advanced, unresectable, stage III non-small-cell lung cancer in China (GEMSTONE-301): interim results of a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2022;23:209–219. doi: 10.1016/S1470-2045(21)00630-6. [DOI] [PubMed] [Google Scholar]
- 7.Skoulidis F, Goldberg ME, Greenawalt DM, Hellmann MD, Awad MM, Gainor JF, Schrock AB, Hartmaier RJ, Trabucco SE, Gay L, Ali SM, Elvin JA, Singal G, Ross JS, Fabrizio D, Szabo PM, Chang H, Sasson A, Srinivasan S, Kirov S, Szustakowski J, Vitazka P, Edwards R, Bufill JA, Sharma N, Ou SI, Peled N, Spigel DR, Rizvi H, Aguilar EJ, Carter BW, Erasmus J, Halpenny DF, Plodkowski AJ, Long NM, Nishino M, Denning WL, Galan-Cobo A, Hamdi H, Hirz T, Tong P, Wang J, Rodriguez-Canales J, Villalobos PA, Parra ER, Kalhor N, Sholl LM, Sauter JL, Jungbluth AA, Mino-Kenudson M, Azimi R, Elamin YY, Zhang J, Leonardi GC, Jiang F, Wong KK, Lee JJ, Papadimitrakopoulou VA, Wistuba II, Miller VA, Frampton GM, Wolchok JD, Shaw AT, Janne PA, Stephens PJ, Rudin CM, Geese WJ, Albacker LA, Heymach JV. STK11/LKB1 mutations and PD-1 inhibitor resistance in KRAS-mutant lung adenocarcinoma. Cancer Discov. 2018;8:822–835. doi: 10.1158/2159-8290.CD-18-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Long Y, Tang Y, Cai C, Yu M, Zhang M, Chen R, Huang M. The influence of STK11 mutation on acquired resistance to immunotherapy in advanced non-small cell lung cancer with Lynch syndrome: a case report and literature review. Ann Palliat Med. 2021;10:7088–7094. doi: 10.21037/apm-20-1639. [DOI] [PubMed] [Google Scholar]
- 9.Ricciuti B, Arbour KC, Lin JJ, Vajdi A, Vokes N, Hong L, Zhang J, Tolstorukov MY, Li YY, Spurr LF, Cherniack AD, Recondo G, Lamberti G, Wang X, Venkatraman D, Alessi JV, Vaz VR, Rizvi H, Egger J, Plodkowski AJ, Khosrowjerdi S, Digumarthy S, Park H, Vaz N, Nishino M, Sholl LM, Barbie D, Altan M, Heymach JV, Skoulidis F, Gainor JF, Hellmann MD, Awad MM. Diminished efficacy of programmed death-(ligand)1 inhibition in STK11- and KEAP1-mutant lung adenocarcinoma is affected by KRAS mutation status. J Thorac Oncol. 2022;17:399–410. doi: 10.1016/j.jtho.2021.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muller PA, Vousden KH. p53 mutations in cancer. Nat Cell Biol. 2013;15:2–8. doi: 10.1038/ncb2641. [DOI] [PubMed] [Google Scholar]
- 11.Hou H, Qin K, Liang Y, Zhang C, Liu D, Jiang H, Liu K, Zhu J, Lv H, Li T, Zhang X. Concurrent TP53 mutations predict poor outcomes of EGFR-TKI treatments in Chinese patients with advanced NSCLC. Cancer Manag Res. 2019;11:5665–5675. doi: 10.2147/CMAR.S201513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li XM, Li WF, Lin JT, Yan HH, Tu HY, Chen HJ, Wang BC, Wang Z, Zhou Q, Zhang XC, Su J, Chen RL, Wu YL, Yang JJ. Predictive and prognostic potential of TP53 in patients with advanced non-small-cell lung cancer treated with EGFR-TKI: analysis of a phase III randomized clinical trial (CTONG 0901) Clin Lung Cancer. 2021;22:100–109. e3. doi: 10.1016/j.cllc.2020.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Tanimoto A, Matsumoto S, Takeuchi S, Arai S, Fukuda K, Nishiyama A, Yoh K, Ikeda T, Furuya N, Nishino K, Ohe Y, Goto K, Yano S. Proteasome inhibition overcomes ALK-TKI resistance in ALK-rearranged/TP53-mutant NSCLC via Noxa expression. Clin Cancer Res. 2021;27:1410–1420. doi: 10.1158/1078-0432.CCR-20-2853. [DOI] [PubMed] [Google Scholar]
- 14.Dong ZY, Zhong WZ, Zhang XC, Su J, Xie Z, Liu SY, Tu HY, Chen HJ, Sun YL, Zhou Q, Yang JJ, Yang XN, Lin JX, Yan HH, Zhai HR, Yan LX, Liao RQ, Wu SP, Wu YL. Potential predictive value of TP53 and KRAS mutation status for response to PD-1 blockade immunotherapy in lung adenocarcinoma. Clin Cancer Res. 2017;23:3012–3024. doi: 10.1158/1078-0432.CCR-16-2554. [DOI] [PubMed] [Google Scholar]
- 15.Gu M, Xu T, Chang P. KRAS/LKB1 and KRAS/TP53 co-mutations create divergent immune signatures in lung adenocarcinomas. Ther Adv Med Oncol. 2021;13:17588359211006950. doi: 10.1177/17588359211006950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.La Fleur L, Falk-Sorqvist E, Smeds P, Berglund A, Sundstrom M, Mattsson JS, Branden E, Koyi H, Isaksson J, Brunnstrom H, Nilsson M, Micke P, Moens L, Botling J. Mutation patterns in a population-based non-small cell lung cancer cohort and prognostic impact of concomitant mutations in KRAS and TP53 or STK11. Lung Cancer. 2019;130:50–58. doi: 10.1016/j.lungcan.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Teo MY, Seier K, Ostrovnaya I, Regazzi AM, Kania BE, Moran MM, Cipolla CK, Bluth MJ, Chaim J, Al-Ahmadie H, Snyder A, Carlo MI, Solit DB, Berger MF, Funt S, Wolchok JD, Iyer G, Bajorin DF, Callahan MK, Rosenberg JE. Alterations in DNA damage response and repair genes as potential marker of clinical benefit from PD-1/PD-L1 blockade in advanced urothelial cancers. J. Clin. Oncol. 2018;36:1685–1694. doi: 10.1200/JCO.2017.75.7740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antonarakis ES, Piulats JM, Gross-Goupil M, Goh J, Ojamaa K, Hoimes CJ, Vaishampayan U, Berger R, Sezer A, Alanko T, de Wit R, Li C, Omlin A, Procopio G, Fukasawa S, Tabata KI, Park SH, Feyerabend S, Drake CG, Wu H, Qiu P, Kim J, Poehlein C, de Bono JS. Pembrolizumab for treatment-refractory metastatic castration-resistant prostate cancer: multicohort, open-label phase II KEYNOTE-199 study. J. Clin. Oncol. 2020;38:395–405. doi: 10.1200/JCO.19.01638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shenderov E, Boudadi K, Fu W, Wang H, Sullivan R, Jordan A, Dowling D, Harb R, Schonhoft J, Jendrisak A, Carducci MA, Eisenberger MA, Eshleman JR, Luo J, Drake CG, Pardoll DM, Antonarakis ES. Nivolumab plus ipilimumab, with or without enzalutamide, in AR-V7-expressing metastatic castration-resistant prostate cancer: a phase-2 nonrandomized clinical trial. Prostate. 2021;81:326–338. doi: 10.1002/pros.24110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fountzilas E, Lampaki S, Koliou GA, Koumarianou A, Levva S, Vagionas A, Christopoulou A, Laloysis A, Psyrri A, Binas I, Mountzios G, Kentepozidis N, Kotsakis A, Saloustros E, Boutis A, Nikolaidi A, Fountzilas G, Georgoulias V, Chrysanthidis M, Kotteas E, Vo H, Tsiatas M, Res E, Linardou H, Daoussis D, Bompolaki I, Andreadou A, Papaxoinis G, Spyratos D, Gogas H, Syrigos KN, Bafaloukos D. Real-world safety and efficacy data of immunotherapy in patients with cancer and autoimmune disease: the experience of the Hellenic Cooperative Oncology Group. Cancer Immunol Immunother. 2022;71:327–337. doi: 10.1007/s00262-021-02985-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhai Y, Ma H, Hui Z, Zhao L, Li D, Liang J, Wang X, Xu L, Chen B, Tang Y, Wu R, Xu Y, Pang Q, Chen M, Wang L. HELPER study: a phase II trial of continuous infusion of endostar combined with concurrent etoposide plus cisplatin and radiotherapy for treatment of unresectable stage III non-small-cell lung cancer. Radiother Oncol. 2019;131:27–34. doi: 10.1016/j.radonc.2018.10.032. [DOI] [PubMed] [Google Scholar]
- 22.Zhu J, Chen G, Niu K, Feng Y, Xie L, Qin S, Wang Z, Li J, Lang S, Zhuo W, Chen Z, Sun J. Efficacy and safety of recombinant human endostatin during peri-radiotherapy period in advanced non-small-cell lung cancer. Future Oncol. 2022;18:1077–1087. doi: 10.2217/fon-2021-1239. [DOI] [PubMed] [Google Scholar]
- 23.Yuan M, Zhai Y, Men Y, Wang J, Deng L, Wang W, Bao Y, Yang X, Sun S, Ma Z, Liu Y, Wang J, Zhu H, Hui Z. Endostar (rh-endostatin) improves efficacy of concurrent chemoradiotherapy for locally advanced non-small cell lung cancer: a systematic review and meta-analysis. Thorac Cancer. 2021;12:3208–3215. doi: 10.1111/1759-7714.14188. [DOI] [PMC free article] [PubMed] [Google Scholar]


