Abstract
Background: Infectious mononucleosis (IM) is characterized by pharyngitis, cervical lymphadenopathy, fatigue and fever. IM is most commonly seen in primary Epstein-Barr virus (EBV) infection, with higher occurrence in children. Objective: To explore the value of gamma globulin combined with acyclovir for IM children and their impact on immune function. Methods: This prospective randomized controlled study recruited 111 children under 14 years old with IM from Anhui Provincial Children’s Hospital during March 2019 and March 2022. Among them, 11 children dropped out, and 100 eligible children were randomized 1:1 into a control group and a study group. The control group received acyclovir, and the study group received additional gamma globulin. The baseline data, clinical efficacy, immune function, and adverse reactions were collected and compared. Results: The study group had a shorter antipyretic time, lymph node reduction time, pharyngitis improvement time, and hospital stay compared to the control group (P < 0.05). The study group yielded lower levels of total white blood cell count, alanine aminotransferase, and creatine kinase-MB than the control group (P < 0.05). After treatment, the levels of CD3+ and CD8+ were lower, and the levels of CD4+, CD4+/CD8+, IgA, and IgG were higher in the study group than those in the control group (all P < 0.05). The incidence of adverse reactions between the two groups was comparable (14.00% vs. 24.00%). The positive rates of EBV-specific antibody and nuclear antigen in the study group were lower than those in the control group (P < 0.05). Conclusion: The combined treatment of gamma globulin and acyclovir is a promising alternative for patients with IM compared to acyclovir alone. This combined regimen shortens the duration of clinical manifestations in children, promotes the recovery of laboratory indices, improves clinical efficacy, and enhances immune function. Furthermore, its safety profile is acceptable, warranting its further promotion.
Keywords: Gamma globulin, acyclovir, infectious mononucleosis in children, application value, immune function
Introduction
Infectious mononucleosis (IM) is a clinical syndrome typically caused by Epstein-Barr virus (EBV), which is a lymphocytic virus and a member of the gamma-herpesvirus family [1]. It affects approximately 90% of the population worldwide, and the majority have no identifiable disease [2]. IM is characterized by enlarged lymph nodes, angina pectoris, fever, and sore throat with atypical peripheral large lymphocytes. Due to the severity and duration of the acute disease, as well as the potential for cancer and autoimmune disease-associated complications, IM poses a major health concern [3,4]. Children are more susceptible to IM compared to adults, and IM is known to be associated with a higher risk of chronic active EBV infection-related malignant diseases, as well as possible multi-system damage if not promptly treated [5].
Clinically, IM is a benign, self-limiting disease that requires only symptomatic treatment. Nonetheless, the course of this disease might be prolonged and lead to decreased productivity in study or work [6]. In addition to symptomatic treatment, the use of antiviral drugs in the treatment of EBV-IM has been widely recognized in clinical practice, but with controversial results [7]. Acyclovir, a nucleoside broad-spectrum antiviral drug, is a synthetic purine nucleoside analogue, that is mainly used in various infections caused by herpes simplex virus, including herpes zoster, varicella and other herpes viruses [8]. In recent years, immunological studies have shown that, in addition to direct damage to the body, EBV can also cause immune system dysfunction and damage [9]. Injection of γ-globulin, as a passive immunotherapy, can prevent viral venereal diseases such as infectious hepatitis and measles, with a favourable efficacy profile [10].
Both acyclovir and gamma globulin have been used in the treatment of diseases associated with viral infections, including IM [11]. However, the clinical efficacy of the combination of gamma globulin and acyclovir in children with IM has been only sporadically reported. Accordingly, this study aimed to analyze and explore the efficacy and safety of gamma globulin plus acyclovir in children with IM.
Materials and methods
Study design
This prospective randomized controlled study utilized a 1:1 grouping ratio, with clinical efficacy as the primary outcome. After reviewing the relevant literature, the control group was expected to have an overall response rate of 70%, with a minimum detectable difference of 20. The study group was expected to have a total effective rate of 90%. A two-sided test was conducted with a significance level of 0.05 and an expected power level of 0.60. The R language pwr package was used to calculate the sample size. The w (power effect) was calculated to be 0.25, and the total sample size was 78 cases. Considering potential sample attrition, a total sample size of 100 cases was planned.
A total of 111 children with IM diagnosed and treated in Anhui Provincial Children’s Hospital from March 2019 to March 2022 were recruited in this study. Among them, 11 children dropped out, and 100 eligible children who met the inclusion criteria were included. All children were randomized at a 1:1 ratio to either a control group receiving acyclovir or a study group receiving gamma globulin plus acyclovir. This study was approved by the Ethics Committee of the Anhui Provincial Children’s Hospital. Parents of all the included children signed consent forms voluntarily. The CONSORT diagram is shown in Figure 1.
Figure 1.
CONSORT diagram.
Diagnostic criteria
1) The diagnosis of IM was based on the presence of fever for more than 5 days, along with clinical manifestations such as angina, hepatosplenomegaly, tonsillitis, and lymphadenopathy, with any 3 or more of the above features being present [12]; 2) On admission, the white blood cells (WBCs) in the peripheral blood smear were found to be elevated or normal, with abnormal lymphocytes > 0.10 or total lymphocytes > 10 × 109/L; 3) Positive serum anisotropic agglutination test was identified within 1 week after admission; 4) EBV antibody testing was performed to confirm the diagnosis (positive VCA.IgM in the acute phase, and a fourfold or greater increase in VCA.I titer in both sera).
Inclusion and exclusion criteria
Inclusion criteria: 1) children with age < 14 years old; 2) children with clinical manifestations such as fever, tonsil inflammation, and lymphadenopathy; 3) children with positive serum heterophilic antibody; 4) children with no previous allergic history to the drugs used in this study.
Exclusion criteria: 1) children with incomplete clinical data or loss to follow-up; 2) children with malignant tumour or hemophagocytic syndrome; 3) children with a history of antiviral drug treatment; 4) children with severe organ dysfunction.
Treatment measures
Control group
After admission, the control group received 5 mg acyclovir (approval number: H20020013, Ranbaxy Laboratories Limited) by intravenous infusion, diluted in 50 mL 0.9% sodium chloride injection (approval number: H19983149, Shanghai Baxter medical supplies Co., LTD.) twice a day (or 10 mg of acyclovir diluted in 100 mL of 0.9% sodium chloride injection in two doses) for two weeks.
Study group
The study group received additional gamma globulin (approval number: S19994004, Shanxi Kangbao Biological Products Co., LTD.) by intravenous injection, 400 mg/kg, once a day for 5 days.
Main outcomes
Clinical efficacy
After treatment for two weeks, efficacy was assessed according to the Criteria for Diagnosis and Efficacy of Hematological Diseases and classified as effective (body temperature dropped to 37.5°C within 3 days, clinical symptoms relieved within 2 weeks), improved (body temperature dropped to 37.5°C within 5 days, clinical symptoms relieved within 3 weeks), and ineffective (body temperature was still higher than 37.5°C after 5 days, symptoms not relieved within 3 weeks). Overall response rate = (effective + improved) cases/total number of cases × 100%.
EBV
The EBV-specific antibody and EBV nuclear antigen positive rates of the children after treatment were recorded and compared between the two groups.
Secondary outcomes
Clinical indicators
After treatment, clinical indicators including fever reduction time, lymph node normalization time, pharyngitis improvement time, and length of hospital stay, were recorded.
Laboratory recovery indicators
The laboratory recovery indicators were observed and recorded before and after treatment, including the total WBC count, alanine aminotransferase (ALT), and creatine kinase-MB (CK-MB) levels.
Immune function
In the morning before and after treatment, 2-5 ml venous blood was collected from the children and centrifuged for 15 min at 3000 r/min. The supernatant was isolated for EDTA anticoagulation. Then, CD3+, CD4+ and CD8+ were determined using ELISA kits, and CD4+/CD8+ was calculated. A biochemical analyzer (Beckman Coulter, USA) and its matching kit (immunoturbidimetric method) were employed to detect the content of serum immunoglobulin IgA and IgG.
Adverse reactions
The incidence of adverse reactions after treatment was recorded, including diarrhoea, nausea, rash, thrombocytopenia, and liver function impairment. The total incidence of adverse reactions = the number of cases with adverse reactions/total number of cases × 100%.
Statistical analysis
Statistical analysis was performed with SAS, version 9.4 (SAS Institute, Cary, NC). All analyses were conducted with the significance level set at 2-sided P < 0.05. GraphPad Prism 8 was used for graphics plotting. If the measured data conformed to a normal distribution, the mean ± standard deviation was used to describe the data. The comparison between groups was performed by independent sample t-test, and intragroup before-after comparison was performed by paired sample t test. The counted data were expressed as frequency (percent), and the comparison between groups was performed by chi-square test.
Results
Baseline characteristics
Baseline characteristics including sex, age, body mass index, course of disease, and clinical symptoms were comparable between the two groups (all P > 0.05). The distribution of the baseline characteristics was homogeneous (Table 1).
Table 1.
Comparison of the baseline data
| Control group | Study group | t/χ2 | P | ||
|---|---|---|---|---|---|
| n | 50 | 50 | - | - | |
| Sex (Male/female) | 32/18 | 35/15 | 0.407 | 0.523 | |
| Age (x̅±s, years) | 4.48±2.17 | 4.23±2.28 | 0.562 | 0.575 | |
| Body mass index (x̅±s, kg/m2) | 21.25±1.58 | 21.47±1.29 | 0.763 | 0.447 | |
| Course of disease (x̅±s, d) | 10.18±5.14 | 10.02±5.33 | 0.153 | 0.879 | |
| Clinical symptoms | High fever | 11 | 12 | 0.056 | 0.812 |
| Moderate-low fever | 39 | 35 | 0.468 | 0.494 | |
| Angina | 25 | 27 | 0.160 | 0.689 | |
| Hepatomegaly | 26 | 24 | 0.160 | 0.689 | |
| Spleen enlargement | 24 | 25 | 0.040 | 0.841 | |
Clinical indices
Overall, the combined treatment of gamma globulin plus acyclovir was associated with shortened fever reduction time, lymph node normalization time, pharyngitis improvement time and hospital stay compared to acyclovir alone (P < 0.05) (Table 2).
Table 2.
Comparison of clinical-related indicators (x̅±s, d)
| Control group | Study group | t | P | |
|---|---|---|---|---|
| n | 50 | 50 | - | - |
| Antifebrile time | 7.14±1.15 | 2.58±1.01 | 21.067 | < 0.001 |
| Lymph node reduction time | 9.28±1.85 | 5.54±1.45 | 11.251 | < 0.001 |
| Pharyngitis improvement time | 8.41±2.02 | 4.15±1.41 | 12.228 | < 0.001 |
| Hospital stays | 14.98±2.58 | 8.14±1.88 | 15.151 | < 0.001 |
Laboratory indices
There was no significant difference in laboratory indices between the two groups before treatment (P > 0.05). After treatment, gamma globulin plus acyclovir led to lower levels of total WBC count, ALT, and CK-MB than acyclovir alone (P < 0.05) (Table 3).
Table 3.
Comparison of laboratory recovery indexes (x̅±s)
| Control group | Study group | t | P | ||
|---|---|---|---|---|---|
| n | - | 50 | 50 | - | - |
| Before | Total White blood cells (109/L) | 17.45±5.15 | 17.58±5.23 | 0.125 | 0.901 |
| Alanine transaminase (IU/L) | 158.41±29.14 | 158.97±29.45 | 0.096 | 0.924 | |
| Creatine Kinase-MB (IU/L) | 28.15±4.98 | 28.31±4.77 | 0.164 | 0.870 | |
| After | Total White blood cells (109/L) | 11.98±4.25* | 10.05±4.13* | 2.303 | 0.023 |
| Alanine transaminase (IU/L) | 63.87±9.15* | 57.14±9.28* | 3.652 | < 0.001 | |
| Creatine Kinase-MB (IU/L) | 22.41±4.14* | 15.17±4.65* | 8.223 | < 0.001 |
indicates P < 0.05 compared before treatment.
Clinical efficacy
Gamma globulin plus acyclovir exhibited a higher clinical efficacy than acyclovir alone (96.00% vs. 76.00%) (P < 0.05) (Figure 2).
Figure 2.
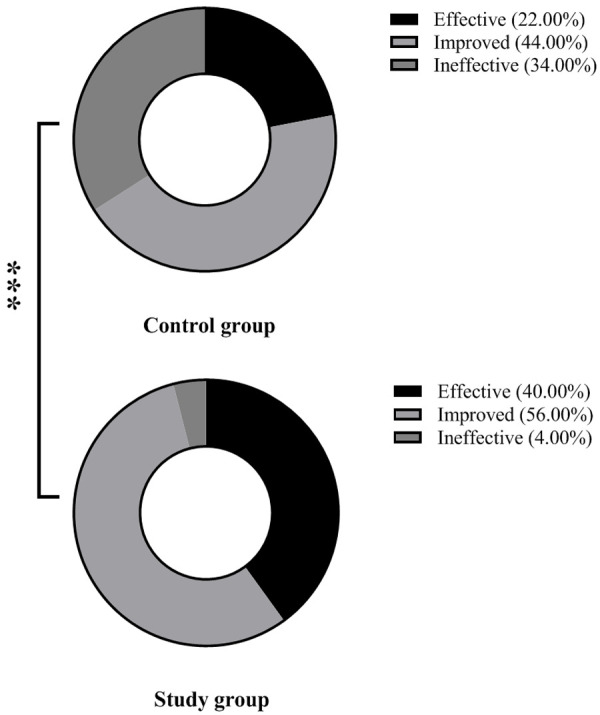
Comparison of clinical efficacy. Note: *** means P < 0.001.
Immune function
Before treatment, there was no significant difference in the level of T lymphocyte populations between the two groups (all P > 0.05). After treatment, the levels of CD3+ and CD8+ were lower, and the levels of CD4+, CD4+/CD8+ were higher in the study group than in the control group (all P < 0.001) (Table 4).
Table 4.
Comparison of T lymphocytes (x̅±s)
| Control group | Study group | t | P | ||
|---|---|---|---|---|---|
| n | - | 50 | 50 | - | - |
| Before | CD3+ | 79.52±6.98 | 79.81±6.54 | 0.214 | 0.831 |
| CD4+ | 23.41±7.02 | 23.78±6.94 | 0.265 | 0.792 | |
| CD8+ | 36.48±7.11 | 36.39±7.36 | 0.062 | 0.951 | |
| CD4+/CD8+ | 0.69±0.65 | 0.69±0.59 | 0.000 | 1.000 | |
| After | CD3+ | 66.98±8.41* | 60.41±7.14* | 4.211 | < 0.001 |
| CD4+ | 28.48±7.41* | 34.48±5.48* | 4.603 | < 0.001 | |
| CD8+ | 32.78±7.45* | 27.59±6.14* | 3.801 | < 0.001 | |
| CD4+/CD8+ | 0.88±0.51* | 1.21±0.31* | 3.910 | < 0.001 |
indicates P < 0.05 compared before treatment.
Before treatment, there was no significant difference in the immunoglobulin level between the two groups (all P > 0.05). After treatment, the levels of IgA and IgG were higher in the study group than in the control group (all P < 0.05) (Table 5).
Table 5.
Comparison of immunoglobulins between the two groups (x̅±s, g/L)
| Control group | Study group | t | P | ||
|---|---|---|---|---|---|
| n | - | 50 | 50 | - | - |
| Before | IgA | 0.91±0.21 | 0.92±0.25 | 0.217 | 0.829 |
| IgG | 8.52±2.11 | 8.49±2.30 | 0.068 | 0.946 | |
| After | IgA | 1.02±0.23* | 1.19±0.28* | 3.317 | 0.001 |
| IgG | 10.23±1.59* | 11.14±2.32* | 2.288 | 0.024 |
indicates P < 0.05 compared before treatment.
Adverse reactions
In the control group, there were 4 cases of diarrhea, 6 cases of nausea, 5 cases of rashes, 2 cases of thrombocytopenia, and 2 cases of liver function damage, while in the study group these were 1, 2, 0, 1 and 0 respectively. The combined treatment of gamma globulin plus acyclovir was associated with a lower incidence of adverse reactions (42.00% vs. 8.00%) (P < 0.001) (Table 6).
Table 6.
Comparison of adverse reactions (n)
| Control group | Study group | χ2 | P | |
|---|---|---|---|---|
| n | 50 | 50 | ||
| Diarrhoea | 2 | 4 | 0.709 | 0.340 |
| Nausea | 1 | 2 | 0.344 | 0.558 |
| Rashes | 1 | 2 | 0.344 | 0.558 |
| Thrombocytopenia | 2 | 1 | 0.344 | 0.558 |
| Kidney dysfunction | 1 | 3 | 1.042 | 0.307 |
| Total incidence | 7 | 12 | 1.614 | 0.202 |
EBV
In the control group, there were 24 cases (48.00%) with EBV-specific antibodies and 47 cases (94.00%) with positive nuclear antigens. In the study group, there were 8 cases (16.00%) with EBV-specific antibodies and 32 cases (64.00%) with positive nuclear antigens. The positive rates of EBV-specific antibody and nuclear antigen in the study group were all lower than in the control group (P < 0.001) (Table 7).
Table 7.
Comparison of positive rates of Epstein-Barr virus-specific antibodies and nuclear antigens (n)
| Control group | Study group | χ2 | P | |
|---|---|---|---|---|
| n | 50 | 50 | ||
| Specific antibody | 24 (48.00%) | 8 (16.00%) | 11.765 | < 0.001 |
| Nuclear antigen positive | 47 (94.00%) | 32 (64.00%) | 13.562 | < 0.001 |
Discussion
IM is a lymphoproliferative disorder that is more common in preschool and school-age children whose immune system is not yet fully developed. It is triggered majorly by EBV, one of the herpes viruses that can damage multiple organs throughout the body and is transmitted through the respiratory tract [13]. In the early stage, blood routine examination may not be conclusive, resulting in a high misdiagnosis rate [14]. Notoriously, without timely treatment, IM can progress rapidly, causing significant harm to patients, particularly in children during their critical growth stages, and may even lead to disability or death [15]. Despite EBV being self-limited, the course is long, and there are no specific drugs currently available for IM [16]. Symptomatic treatment is the preferred option to improve children’s autoimmune function, and antiviral intervention is supplemented to enhance the overall outcome. Nevertheless, the effectiveness and differences among antiviral drugs in the treatment of children with IM remain controversial, with some arguing for a dismal effectiveness of antiviral drugs alone [17].
The primary outcomes of this study were the clinical efficacy, the immune function before and after treatment, and the incidence of adverse reactions. The study group exhibited higher clinical efficacy, shorter fever reduction time, lymph node normalization time, pharyngitis improvement time and length of hospital stay, as well as lower WBC count, ALT and CK-MB levels after treatment than the control group. These results suggest that both intervention methods were effective for children with IM, but gamma globulin plus acyclovir demonstrated superior efficacy. Acyclovir a nucleoside analogue drug that acts against DNA viruses by inhibiting viral DNA polymerase synthesis during the viral replication phase. It binds to a DNA chain of growing replication, interrupting its extension and reducing the viral replication [18]. Furthermore, acyclovir possesses the function of suppressing and inactivating virus replication, making it widely used for various viruses, including herpes simplex virus, and demonstrating good resistance [19].
A previous randomized controlled trial found that after 1 week of acyclovir treatment, the clinical symptoms were significantly improved [20]. Clinical studies have suggested that intravenous injection of gamma globulin has the functions of immune regulation and immune replacement [14], which are conducive to the treatment of immune diseases, and can rapidly enhance the body’s resistance to disease, thereby shortening the recovery time of patients [21]. Therefore, the combination of the two drugs in this study complemented each other by improving the immune function of children and enhancing the antiviral effect of acyclovir, thereby significantly improving the clinical efficacy and shortening the recovery process, which is consistent with previous research results. To our knowledge, the lesions in children with IM can involve multiple systems, and the immune dysfunction can lead to a sharp increase in peripheral blood lymphocytes in the acute phase [22]. In the acute phase of EBV infection, there is a rapid proliferation of CD cells with cytotoxic features. These cells play a crucial role in killing toxic cells in body fluids and lymph nodes, inhibiting their abnormal proliferation, and participating in the regulation and control of cellular immune response. They also secrete various cytokines to eliminate toxic cells [23]. EBV infection involves multiple organs, and the impact on immune function is significant. EBV viral infection is accompanied by a significant proliferation of virus-specific T cells, which is the body’s defense mechanism against viral replication and cell transformation. The EBV virus can bind to surface receptors, infect B cells, and undergo extensive replication. As a result, T cells undergo selective clonal expansion, leading to an increase in specific CD8+ levels. These CD8+ T cells play a crucial role in killing B cells and causing immune disorders [24,25].
This study measured the T lymphocytes and immunoglobulin levels of patients, and the results showed better T lymphocyte and immunoglobulin levels after treatment. Gamma globulin contains a diverse array of antibodies required by human serum. It is particularly rich in IgG, and also contains a small amount of IgA, IgM, and Th2 cytokines [26]. It neutralizes a variety of pathogenic antigens and inflammatory cytokines, making immune complexes easily engulfed and cleared by phagocytes [27]. It can be injected into the body by static drops for 2-3 weeks, which enhances the body’s immunity, neutralizes pathogens, activates complement, promotes phagocytosis, regulates immune response, and reverses the immune damage from IM [28]. Evidence has suggested that immune system dysfunction may be implicated in children presenting with symptoms such as fever, angina, lymphoid tissue enlargement in the liver and spleen, and superficial lymph node enlargement. Additionally, there is an association between the use of acyclovir and lowered WBC count, neutropenia, or agranulocytosis [29]. By recovering the function of the human immune system, the patient’s resistance to the virus will be enhanced, and more specific antibodies will be produced. Gamma globulin can promote the increase of immune globulins, inhibit T cell activity, neutralize superantigens, further correct delayed apoptosis of activated T cells, and produce specific antibodies to eliminate pathogenic bacteria and block immune inflammation [30]. In addition, gamma globulin is rich in autoantibodies and polyclonal antibodies, which can neutralize inflammatory cells and antigen production, and bind immune complex antigens to change their molecular weight, so as to facilitate phagocytosis and clearance [31]. The results of this study showed that the positive rates of EBV-specific antibodies and nuclear antigens in children in the study group were lower than those in the control group. This is because gamma globulin in children can form immune complexes consisting of antigens and antibodies. This can stimulate the production of messenger RNA of thymidine phosphorylase protein, which in turn inhibits the enzymes required by the virus, leading to a reduction in the rate of virus proliferation. This facilitates the subsequent use of interferon drugs to effectively kill the virus and resist its replication. As a result, gamma globulin can contribute to reducing the long-term recurrence rate of the virus [32,33]. Encouragingly, the combination of the two is a safe and reliable therapy for the treatment of children with IM.
Inevitably, the results in the current study might be limited by the lack of a larger sample size and long-term follow-up. Ongoing studies with larger sample size and long-term follow-up are required to validate the conclusions in the present study.
Conclusion
The combined treatment of gamma globulin plus acyclovir is a promising alternative for children with IM. This combined regimen shortens the time until relief of clinical manifestations, promotes the recovery of laboratory indices, boosts the clinical efficacy, and enhances immune function. Furthermore, its safety profile is acceptable, warranting further promotion of this treatment approach.
Disclosure of conflict of interest
None.
References
- 1.Womack J, Jimenez M. Common questions about infectious mononucleosis. Am Fam Physician. 2015;91:372–376. [PubMed] [Google Scholar]
- 2.Naughton P, Healy M, Enright F, Lucey B. Infectious mononucleosis: diagnosis and clinical interpretation. Br J Biomed Sci. 2021;78:107–116. doi: 10.1080/09674845.2021.1903683. [DOI] [PubMed] [Google Scholar]
- 3.Rostgaard K, Balfour HH Jr, Jarrett R, Erikstrup C, Pedersen O, Ullum H, Nielsen LP, Voldstedlund M, Hjalgrim H. Primary Epstein-Barr virus infection with and without infectious mononucleosis. PLoS One. 2019;14:e0226436. doi: 10.1371/journal.pone.0226436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fugl A, Andersen CL. Epstein-Barr virus and its association with disease - a review of relevance to general practice. BMC Fam Pract. 2019;20:62. doi: 10.1186/s12875-019-0954-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sylvester JE, Buchanan BK, Silva TW. Infectious mononucleosis: rapid evidence review. Am Fam Physician. 2023;107:71–78. [PubMed] [Google Scholar]
- 6.Ebell MH, Call M, Shinholser J, Gardner J. Does this patient have infectious mononucleosis? The rational clinical examination systematic review. JAMA. 2016;315:1502–1509. doi: 10.1001/jama.2016.2111. [DOI] [PubMed] [Google Scholar]
- 7.De Paor M, O’Brien K, Fahey T, Smith SM. Antiviral agents for infectious mononucleosis (glandular fever) Cochrane Database Syst Rev. 2016;12:CD011487. doi: 10.1002/14651858.CD011487.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang S, Zhu Y, Jin Y, Sun H, Wang W, Zhan L. Difference between acyclovir and ganciclovir in the treatment of children with Epstein-Barr virus-associated infectious mononucleosis. Evid Based Complement Alternat Med. 2021;2021:8996934. doi: 10.1155/2021/8996934. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Middeldorp JM. Epstein-Barr virus-specific humoral immune responses in health and disease. Curr Top Microbiol Immunol. 2015;391:289–323. doi: 10.1007/978-3-319-22834-1_10. [DOI] [PubMed] [Google Scholar]
- 10.Ouedraogo DE, Makinson A, Vendrell JP, Casanova ML, Nagot N, Cezar R, Bollore K, Al Taaba Y, Foulongne V, Badiou S, Viljoen J, Reynes J, Van de Perre P, Tuaillon E. Pivotal role of HIV and EBV replication in the long-term persistence of monoclonal gammopathy in patients on antiretroviral therapy. Blood. 2013;122:3030–3033. doi: 10.1182/blood-2012-12-470393. [DOI] [PubMed] [Google Scholar]
- 11.Lu BY, Kojima L, Huang MS, Friedmann AM, Ferry JA, Weinstein HJ. Facial manifestations of Epstein-Barr virus-related lymphoproliferative disease in childhood acute lymphoblastic leukemia in remission: two atypical presentations. Pediatr Blood Cancer. 2016;63:2042–2045. doi: 10.1002/pbc.26102. [DOI] [PubMed] [Google Scholar]
- 12.Shi T, Huang L, Luo L, Yu Q, Tian J. Diagnostic value of serological and molecular biological tests for infectious mononucleosis by EBV in different age stages and course of the disease. J Med Virol. 2021;93:3824–3834. doi: 10.1002/jmv.26558. [DOI] [PubMed] [Google Scholar]
- 13.Styczynski J, van der Velden W, Fox CP, Engelhard D, de la Camara R, Cordonnier C, Ljungman P Sixth European Conference on Infections in Leukemia, a joint venture of the Infectious Diseases Working Party of the European Society of Blood and Marrow Transplantation (EBMT-IDWP), the Infectious Diseases Group of the European Organization for Research and Treatment of Cancer (EORTC-IDG), the International Immunocompromised Host Society (ICHS) and the European Leukemia Net (ELN) Management of Epstein-Barr virus infections and post-transplant lymphoproliferative disorders in patients after allogeneic hematopoietic stem cell transplantation: sixth European Conference on Infections in Leukemia (ECIL-6) guidelines. Haematologica. 2016;101:803–811. doi: 10.3324/haematol.2016.144428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magurano F, Baggieri M, Marchi A, Bucci P, Rezza G, Nicoletti L. Mumps clinical diagnostic uncertainty. Eur J Public Health. 2018;28:119–123. doi: 10.1093/eurpub/ckx067. [DOI] [PubMed] [Google Scholar]
- 15.Hu H, Deng H, Bi J, Xu Y, Li S, Xie Y, Sun X, Wang D, Li X, Ouyang W, Hu B, Zhang Y, Tang H, Fang C, Zhang H, Guo L, Wang C, Wang T, Yang F, Jiang T, Xie Z, Liu G. Clinical characteristics and effectiveness of antiviral agents in hospitalized children with infectious mononucleosis in China: a multicenter retrospective study. Pediatr Investig. 2021;5:188–194. doi: 10.1002/ped4.12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X, Akinyemi IA, You JK, Rezaei MA, Li C, McIntosh MT, Del Poeta M, Bhaduri-McIntosh S. A mechanism-based targeted screen to identify Epstein-Barr virus-directed antiviral agents. J Virol. 2020;94:e01179-20. doi: 10.1128/JVI.01179-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cauldwell K, Williams R. Unusual presentation of Epstein-Barr virus hepatitis treated successfully with valganciclovir. J Med Virol. 2014;86:484–486. doi: 10.1002/jmv.23766. [DOI] [PubMed] [Google Scholar]
- 18.Kausar S, Said Khan F, Ishaq Mujeeb Ur Rehman M, Akram M, Riaz M, Rasool G, Hamid Khan A, Saleem I, Shamim S, Malik A. A review: mechanism of action of antiviral drugs. Int J Immunopathol Pharmacol. 2021;35:20587384211002621. doi: 10.1177/20587384211002621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dai SS, Zhou K. Clinical effect of recombinant human interferon α1b adjuvant therapy in infectious mononucleosis: a prospective randomized controlled trial. Zhongguo Dang Dai Er Ke Za Zhi. 2020;22:953–957. doi: 10.7499/j.issn.1008-8830.2002204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bollard CM, Cohen JI. How I treat T-cell chronic active Epstein-Barr virus disease. Blood. 2018;131:2899–2905. doi: 10.1182/blood-2018-03-785931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vouloumanou EK, Rafailidis PI, Falagas ME. Current diagnosis and management of infectious mononucleosis. Curr Opin Hematol. 2012;19:14–20. doi: 10.1097/MOH.0b013e32834daa08. [DOI] [PubMed] [Google Scholar]
- 22.Sakamoto Y, Mariya Y, Kubo K. Quantification of Epstein-Barr virus DNA is helpful for evaluation of chronic active Epstein-Barr virus infection. Tohoku J Exp Med. 2012;227:307–311. doi: 10.1620/tjem.227.307. [DOI] [PubMed] [Google Scholar]
- 23.Lion T. Adenovirus infections in immunocompetent and immunocompromised patients. Clin Microbiol Rev. 2014;27:441–462. doi: 10.1128/CMR.00116-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei A, Li Z, Ma H, Zhang L, Zhang J, He L, Lian H, Zhang Q, Chen S, Xu J, Wang D, Liu W, Zhang R, Wang T. Clinical analysis of chronic active Epstein-Barr virus infection involving the gastrointestinal tract. Pediatr Infect Dis J. 2023;42:13–19. doi: 10.1097/INF.0000000000003734. [DOI] [PubMed] [Google Scholar]
- 25.Gottschalk S, Rooney CM. Adoptive T-cell immunotherapy. Curr Top Microbiol Immunol. 2015;391:427–454. doi: 10.1007/978-3-319-22834-1_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen ZG, Li M, Ji JZ, Chen H, Chen YF, Chen FH. Significance of changes of T lymphocytes subsets in children with infectious mononucleosis and the effects of different interventions. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2009;23:118–120. [PubMed] [Google Scholar]
- 27.Chen L, Chen X, Yao W, Wei X, Jiang Y, Guan J, Liu X, Xie Y, Lu H, Qian J, Zhang Z, Wu L, Lin X. Dynamic distribution and clinical value of peripheral lymphocyte subsets in children with infectious mononucleosis. Indian J Pediatr. 2021;88:113–119. doi: 10.1007/s12098-020-03319-7. [DOI] [PubMed] [Google Scholar]
- 28.Sulik A, Oldak E, Kroten A, Lipska A, Radziwon P. Epstein-Barr virus effect on frequency of functionally distinct T cell subsets in children with infectious mononucleosis. Adv Med Sci. 2014;59:227–231. doi: 10.1016/j.advms.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Gao LW, Xie ZD, Liu YY, Wang Y, Shen KL. Epidemiologic and clinical characteristics of infectious mononucleosis associated with Epstein-Barr virus infection in children in Beijing, China. World J Pediatr. 2011;7:45–49. doi: 10.1007/s12519-011-0244-1. [DOI] [PubMed] [Google Scholar]
- 30.Wang Q, Wang ZF, Cao M, Wang ZY. Changes of FoxP3, CD4(+)CD25(+) regulatory T cells, TLR2 and TLR9 in children with infectious mononucleosis. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2013;21:469–473. doi: 10.7534/j.issn.1009-2137.2013.02.044. [DOI] [PubMed] [Google Scholar]
- 31.Ohga S, Ishimura M, Yoshimoto G, Miyamoto T, Takada H, Tanaka T, Ohshima K, Ogawa Y, Imadome K, Abe Y, Akashi K, Hara T. Clonal origin of Epstein-Barr virus (EBV)-infected T/NK-cell subpopulations in EBV-positive T/NK-cell lymphoproliferative disorders of childhood. J Clin Virol. 2011;51:31–37. doi: 10.1016/j.jcv.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 32.Niazi B, Ali S, Elias S, Sciarra M. Protein loss enteropathy as an initial presentation of gastric Epstein-Barr virus lymphoma. Case Rep Gastrointest Med. 2022;2022:5143760. doi: 10.1155/2022/5143760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abrahamovych U, Abrahamovych O, Guta S, Tsyhanyk L, Romaniuk O. The peculiarities of average values of the indices of non-virus laboratory markers of active epstein-barr virus infection in patients with systemic lupus erythematosus. Georgian Med News. 2020:126–130. [PubMed] [Google Scholar]


