Abstract
The pathogenesis of hematological tumors has not been fully elucidated. The academic community believes that genetic mutation abnormalities play a crucial role in the occurrence and development of hematological malignancies. Chronic neutrophilic leukemia (CNL) is a rare hematological tumor in the world. It is characterized by a Philadelphia chromosome BCR-ABL1-negative myeloproliferative tumor. It can be accompanied by mutations in various genes. Colony-stimulating factor 3 receptor (CSF3R) is a classic mutation in CNL and is included in the diagnostic criteria for CNL. This article described a 46-year-old male patient who came to the hospital with non-specific clinical manifestations such as unrelieved abdominal distension and edema of both lower extremities as the primary symptoms. The middle-aged male patient was provided with a peripheral a routine blood test. The biochemical tests revealed abnormalities. A bone marrow biopsy was performed to complete various tests such as bone marrow morphology, immunology, molecular biology, cytogenetics, and imaging. He was diagnosed with a rare chronic neutrophilic leukemia. After the diagnosis, the patient took ruxolitinib orally targeted therapy as prescribed by the doctor. Doctors regularly reviewed the peripheral blood examination and bone marrow status. The current condition is well controlled. CNL is extremely rare. The disease usually has non-specific clinical features and manifestations as the primary symptoms. These symptoms can easily be missed or lead to misdiagnosed ailments by clinicians. It is necessary to increase the awareness and vigilance of CNL.
Keywords: Chronic neutrophilic leukemia, CSF3R, Ruxolitinib, diagnostic biomarker, targeted therapy, prognosis
Introduction
CNL is a highly heterogeneous myeloproliferative disease that is rare worldwide. Most patients have splenomegaly and some have non-specific symptoms. CNL is different from chronic myeloid leukemia (CML) in that it does not have the Ph chromosome and BCR-ABL fusion gene [1]. It is difficult to diagnose CNL in the absence of specific typical symptoms and a bone marrow examination. The typical feature of CNL is the continuous proliferation of mature neutrophils in the bone marrow and the activating mutation of CSF3R gene. This is different from CML and myeloproliferative neoplasms (MPN) [2]. This article provided the clinical data of a CNL patient and reviewed relevant international academic literatures in recent years. This article is expected to provide a certain understanding and reference for the diagnosis and treatment of CNL in the global hematology community.
Clinical presentation
A 46-year-old male patient was admitted to the First Affiliated Hospital of Gannan Medical University for the first time on January 25, 2021 with the main complaint of “repeated abdominal distension for more than five months and abnormal white blood cells for one month”. The patient had abdominal distension and anorexia for no obvious cause since August 2020. He did not pay attention to the symptoms, until later, when he developed waist, hip, and left leg pain around December 2020. This was accompanied by limited movement, leading him to seek medical attention at a local private hospital. The routine blood test in the private hospital indicated: white blood cells 21.89 × 109/L, neutrophils 19.87 × 109/L, hemoglobin 98 g/L, and platelets 62 × 109/L. The patient was provided with anti-infection drugs and was discharged from the hospital. The patient’s symptoms of abdominal distension, anorexia, and pain in the waist, buttocks, and left leg gradually increased. This was accompanied by mild swelling of the left foot and severe limitation of activities. The patient went to the local municipal hospital for treatment. The routine blood test of the hospital showed: white blood cells 56.01 × 109/L, neutrophils 50.24 × 109/L, platelets 86 × 109/L, and hemoglobin 101 g/L. Taking into consideration that the patient was in critical condition, the municipal hospital did not carry out any clinical diagnosis and treatment plan. The patient was recommended to be transferred to a higher-level hospital for diagnosis and treatment. Then the patient was referred to our hospital (the First Affiliated Hospital of Gannan Medical University) for more treatment. The outpatient department of hematology in our hospital planned to diagnose the cause of “leukocytosis: chronic myelogenous leukemia?” The patient was admitted to the department of hematology for more diagnosis and treatment.
After detailed medical history inquiries were complete, we discovered that this middle-aged patient had a history of gout for more than 5 years. The spleen had been enlarged for more than 20 years (no attention or clinical diagnosis and treatment measures were taken). He had a history of smoking and drinking for more than 10 years. His other medical history, personal history, and family history were not significant. Bedides, we learned that the patient’s job was farming. This included long-term exposure to pollutants such as chemical fertilizers, dust, and the sun’s ultraviolet rays.
Routine physical examination of the patient after admission revealed the following: body temperature: 36.6°C, pulse rate: 120 beats/min, respiratory rate: 20 beats/min, and blood pressure: 117/73 mmHg. We found that the patient was conscious but appeared to be malnourished and anemic, with pale skin mucosa, no rash, no subcutaneous bleeding or ecchymosis, and no obvious enlargement of superficial lymph nodes. No obvious abnormality was found in the head, neck, and chest during the physical examination. The physical examination of the abdomen showed that the shape of the abdomen was bulging and the abdominal muscles were slightly tense. There was no tenderness and rebound tenderness, no abdominal mass was touched, and the liver was not palpated below rib edge. The spleen with hard texture can be palpated. The spleen was enlarged to below the level of the umbilicus, with III + 1 cm swelling and tenderness (+). Shifting dullness was negative, bowel sounds were normal, there was no percussion pain in the bilateral kidneys, and moderate edema of both lower extremities. Physical examination of the remaining organ systems was unremarkable.
Relevant examinations were completed after admission and the findings comprised the following: a routine blood test examination: white blood cells 26.36 × 109/L, hemoglobin 84 g/L, platelets 61 × 109/L, and neutrophil ratio 91%. The coagulation function analysis showed that the prothrombin time was 13.6 s, the fibrinogen was 3.11 g/L, and the activated partial thromboplastin time was 29.5 s. The blood biochemical examination showed albumin 36.8 g/L, globulin 19.9 g/L, lactate dehydrogenase 235 U/L, uric acid 828 μmol/L, and C-reactive protein 6.93 mg/L. The anemia test showed iron Protein 425 ng/ml, vitamin B12>2000.00 pg/ml, folic acid 1.89 ng/ml; ANA, ENA and ds-DNA were all negative. Routine electrocardiogram showed sinus tachycardia. The chest and whole abdomen CT scan (Figure 1A) showed that 1. Megalosplenia; 2. Multiple small stones in both kidneys; 3. Bilateral pleural thickening; 4. A small amount of pelvic fluid were present. We performed a bone marrow aspirate biopsy for the patient. The bone marrow results (Figure 2) indicated: 1. Increased neutrophil ratio; 2. Poor megakaryocyte maturation and thrombocytopenia; 3. Myeloproliferative disease: CML? We tested the bone marrow specimens of this patient by molecular biology techniques. The results showed that CSF3R gene exon 14 mutation (+), CSF3R gene exon 17 mutation (+), BCR-ABL, CALR, MPL-w515 gene were all negative. Cytogenetic test results (Figure 3) suggested that the chromosome was 46, XY [7].
Figure 1.

Abdominal CT showed megalosplenia. Note: (A) is the CT imaging signs of the patient upon his first admission; (B) is the CT imaging findings after treatment. The splenic reduction can be seen visually after treatment. The red arrows refer to the signs of the giant spleen on different CT sections of this patient. The red dashed line showed the position of the giant spleen in the abdominal cavity.
Figure 2.
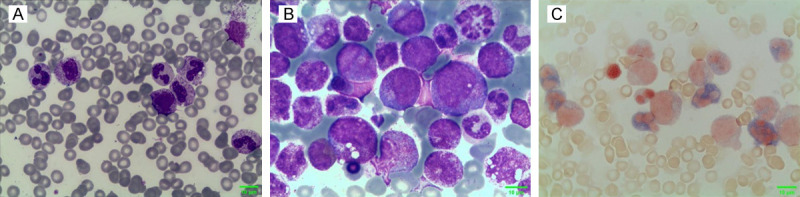
Cytochemical staining of morphology. A. Wright-Giemsa staining of peripheral blood × 1000; B. Wright-Giemsa staining of bone marrow × 1000; C. Neutrophil alkaline phosphatase (NAP) staining of bone marrow × 1000.
Figure 3.

The result of bone marrow cytogenetic test (chromosome) is 46, XY [7]. Note: (A, B) shows the metaphase phase of chromosomes, and (C) is the karyotype analysis diagram of chromosomes. The magnification multiples of these images are 10 ×, 40 × and 100 × respectively.
Based on the clinical manifestations and related examination results of the patient, we made a clear diagnosis for him: 1. Chronic neutrophilic leukemia; 2. Gout; 3. Renal insufficiency; 4. Gallstones. After treatment with sodium bicarbonate to alkalize the internal environment, hydroxyurea to reduce tumor burden, furosemide to reduce swelling, febuxostat to reduce uric acid, and oxycodone hydrochloride to relieve pain, symptoms of the patient improved significantly. He was discharged from the hospital. The family experienced economic factors and the patient was not provided with CNL treatment drugs for the first treatment. He received symptomatic and supportive treatment. The patient did not return to our hospital for treatment on time after discharge.
It was not until the patient developed symptoms such as increased abdominal pain, joint swelling, and pain throughout the body that he returned to the hospital for the second time on September 24, 2021. After admission, we provided relevant examinations for the patient: a routine blood examination showed white blood cells 15.68 × 109/L, hemoglobin 72 g/L, and platelets 62 × 109/L were normal. The coagulation function test showed prothrombin time 13.9 s, fibrinogen 3.05 g/L, activated partial thromboplastin time 31.7 s, prothrombin activity 68%, and D-dimer 1.33 mg/L. Blood biochemical tests showed albumin 31.7 g/L, globulin 19.5 g/L L, lactate dehydrogenase 266 U/L, C-reactive protein 7.63 mg/L, fasting blood glucose 2.31 mmol/L, potassium 3.91 mmol/L, and calcium 2.03 mmol/L were normal. A chest CT scan showed: 1. A small amount of bilateral pleural effusion, partial insufflation of the lower lobes of both lungs; 2. Enlarged heart, pericardial effusion; 3. Decreased cardiac cavity density, possible anemia; hepatosplenomegaly; 4. Thyroid nodules. Ultrasound of the digestive system, urinary system, and para-aortic lymph nodes showed that 1. The intrahepatic echo was denser and thicker; the portal vein was widened; 2. Gallbladder polyps; 3. Double renal cysts; 4. Splenomegaly (spleen long diameter 330 mm, thickness diameter 88 mm, 85 mm beyond the rib); 5. No obvious abnormality was found in the sonography of pancreas, bladder and prostate; 6. There was no dilation of the upper and lower segments of the bilateral ureters; 7. No obvious enlarged lymph nodes were found in the explorable range except for the abdominal aorta. We performed a bone marrow biopsy on the patient for the second time. The results showed that the bone marrow hyperplasia was extremely active, the myeloid lineage was significantly proliferated, blasts + promyelocytes accounted for 3.5%, teardrop-shaped red blood cells were seen, consider chronic neutrophilic leukemia. After the second admission, we provided the patient with a ruxolitinib targeted therapy for the primary disease of CNL. We administered cefotaxime, levofloxacin anti-infection, pain relief, and diuresis, to the patient. The patient’s symptoms of general discomfort improved significantly and the patient was discharged from our hospital. We provided Ruxolitinib 5 mg bid, Etoricoxib 60 mg qd, and Febuxostat 40 mg qd for the patient. This patient was instructed to regularly review blood routine and blood biochemical tests after discharge. After the second discharge of the patient, follow-up work was carried out in an orderly manner.
The patient returned to the hospital for the third time on January 8, 2022. He was re-examined for some tests: A routine blood examination showed white blood cells 41.07 × 109/L, hemoglobin 85 g/L, platelets 46 × 109/L, neutrophils 39.58 × 109/L, and the monocyte count was 0.03 × 109/L, were normal. There was no abnormality found in the coagulation analysis, blood biochemical examination. Re-examination of enhanced CT of the upper abdomen (Figure 1B) showed: 1. Megasplenomegaly, portal hypertension; low-density nodules in the spleen, consider cysts; 2. A little effusion in the abdominal cavity; 3. Multiple cysts in both kidneys, small stones in both kidneys. After admission, the patient underwent a series of treatments such as hydroxyurea to reduce white blood cells, treatment of anti-inflammatory and pain relief, Interleukin-11 promotes platelet production, and Ruxolitinib to treat the primary disease of CNL. After the above series of treatments, the patient’s symptoms of abdominal pain and bloating improved significantly. After discharge, we prescribed oral medicines for the patient (Febuxostat 40 mg qd, Etoricoxib 60 mg qd, Ruxolitinib 5 mg bid). The follow-up has progressed succesfully. The patient’s physical condition is acceptable, and the condition of CNL is well controlled.
Discussion
As early as one hundred years ago in 1920, Tuohy reported a case of an elderly woman with splenomegaly and polymorphonuclear neutrophilia, which may be the “rudiment” of CNL [3]. Tanzer creatively proposed the term of “chronic neutrophilic leukemia” in The Lancet in 1964 [4]. Chronic neutrophilic leukemia (CNL) is an extremely rare and highly heterogeneous myeloproliferative neoplasm (MPN) in clinical practice. The disease is dominated by case reports and a small number of case series studies. The incidence and epidemiological characteristics of CNL remain a mystery. The existing clinical studies believe that CNL is more common in middle-aged and elderly men. There is no significant regional or racial difference. The global incidence of CNL has been very low and has not shown an increasing trend [5].
CNL usually manifests as persistent mature neutrophils and leukocytosis in peripheral blood, bone marrow granulocyte hyperplasia, and significant liver and spleen enlargement [6]. Other non-specific clinical symptoms of CNL include anemia, fatigue, bleeding tendency, gout, and metabolic arthritis. Patients with CNL may even experience B symptoms of lymphoma, such as cutaneous pruritus, night sweats, and weight loss [7]. Some patients with CNL can be completely asymptomatic at the time of diagnosis. The only presentation may be an incidental finding of neutropenia [6]. The patient in this article was admitted to the hospital with the main symptoms of splenomegaly and abdominal distension, followed by pain and swelling in the waist, hip, and left leg, and limited mobility, indicating that the clinical manifestations of CNL are highly heterogeneous.
Due to the variable and non-specific clinical course of CNL patients, the causes of disease progression in CNL patients are usually refractory neutropenia, transformation to acute myeloid leukemia (AML), and progressively increase in organ enlargement (such as megalosplenia) [8]. Sudden intracranial hemorrhage, blast cell transformation in CNL patients, and side effects from clinical treatment strategies (such as chemotherapy or bone marrow transplantation) are the most common causes of death in CNL. The median time for CNL to transform into AML is 21 months, and the median survival time is only 23.5 months [6,7].
We were concerned that the patient presented in this study was admitted with non-specific symptoms such as abdominal distension and lower limb swelling and pain. This made the entire diagnosis and treatment process very tricky. Current studies [9-11] believe that most types of leukemia can infiltrate the liver and the spleen, resulting in excessive enlargement of the liver or spleen and affecting normal physiological functions. A significantly enlarged spleen can compress the inferior vena cava, resulting in venous return obstruction and cause lower limb swelling. A few studies [12] showed that cutaneous leukemia manifested by skin lesions was rarely seen clinically, CNL was included. The male patient indicated in this paper had swelling and pain in his lower limbs when he was admitted to hospital. His medical history indicated that he had severe gout in the past and had been taking oral medication extensively for it to keep it under control. Some academic studies have shown that gout can cause local swelling and pain in joints [13,14]. According to the above logical evidence-based thinking and reasoning, this strongly indicates that the swelling of the patient’s lower extremities was caused by the huge spleen pressing on the veins and other organs in the abdominal cavity. This made him feel unbearable abdominal distension and edema of the lower extremities caused by venous return obstruction. Severe gout made the edema of the lower extremities more complicated, making the patient feel obvious pain. It was not difficult to understand why the patient in this article was admitted to the hospital with unrelieved bloating, swelling, and pain in his lower extremities. The final diagnosis was chronic neutrophilic leukemia, a rare disease worldwide.
The clinical manifestations and characteristics of most patients are difficult to distinguish CNL from other malignant diseases. The positive mutation of colony-stimulating factor 3 receptor (CSF3R) gene is a typical and key molecular marker of the disease. BCR-ABL negative, and nearly 90% of CNL patients carry CSF3R mutations [15]. It is thought-provoking that the protagonist of this rare clinical case is a middle-aged male farmer, with a history of smoking and drinking for more than ten years, and long term exposured to chemical fertilizers and dust. Extended outdoor farming, caused long-term exposure to the sun’s ultraviolet rays. In this study, the issues of in-depth research in the academic community include the history of smoking and drinking, and environmental risk factors that can lead to the appearance and mutation of oncogenes including CSF3R. Recent studies had indicated that factors such as smoking, lack of physical exercise, high consumption of carbohydrates, factors including the use of household pesticides, proximity to highways, exposure to benzene and other chemical pollutants, and exposure to ionizing radiation contribute to the occurrence and development of leukemia [16,17]. Another study identified that craftsmen-associated occupations may contribute to leukemia [18]. Occupational exposure to hazardous chemicals by mothers during pregnancy contributes to childhood leukemia [19].
CSF3R mutations are of great significance in CNL. Mutant variants of CSF3R include point mutations of T618A and T618I, membrane-proximal mutations, and nonsense or frameshift mutations observed in the cytoplasmic tail of CSF3R [8,20]. There are two different types of CSF3R mutations that have been discovered. One is truncating mutations caused by Src family-TNK2 kinase dysregulation. The other is a membrane-proximal mutation that results in JAK family kinase dysregulation [21,22]. The two different types of CSF3R mutations described above have distinct clinical responses to tyrosine kinase inhibitors [22]. The former is sensitive to Dasatinib, and the latter appears to respond well to Ruxolitinib [23]. Fleischman used a bone marrow transplant mouse model to determine the ability of CSF3R T618I (the most common CSF3R mutation) to drive leukemia in CNL. He found that the CSF3R T618I mutation acts through the JAK-STAT signaling pathway [24]. Splenomegaly and agranulocytosis respond to treatment with the JAK inhibitor Ruxolitinib [8,25]. Studies suggest that CNL may be associated with genes such as SETBP1, ASXL1, TET2, and CALR [6,7]. The specific regulatory network and mechanism of action have not been unveiled. Further research is required [21].
In 2016, the WHO issued the diagnostic criteria for CNL, but it is frustrating that there is no international standard treatment plan and standard of care for the treatment of CNL. Many researchers have used hydroxyurea, interferon-α, or splenectomy as first-line treatments for the CNL patients, but the clinical effectiveness and remission rates are worrying [26,27]. CNL is a hematological malignancy. It is unknown if CNL patients can benefit from hematopoietic stem cell transplantation because there is a lack of sufficient data and consensus hematologists [5,8,28]. CNL is indeed a unique myeloproliferative tumor. As the driving oncogenic mutation gene of the disease, CSF3R deepens our understanding of the molecular pathogenesis of CNL. It provides diagnostic biomarkers and adds scientific and prospective significance to novel targeted therapies for CNL.
CNL is an extremely rare myeloproliferative tumor (MPN). Existing studies have shown that Ruxolitinib can inhibit the activation of JAK1/2 and CSF3R T618I mutations, reduce the malignant proliferation of CNL cells, and bring clinical benefits to CNL patients [29-31]. New studies have confirmed that, the efficacy of Ruxolitinib maintenance therapy in patients with CSF3R T618I mutant chronic neutrophil leukemia after allo hematopoietic stem cell transplantation is encouraging and may reduce the occurrence of chronic GVHD [32]. Some studies [33] reported that Ruxolitinib has good anti-inflammatory efficacy. This rapidly improves the condition of CNL and delays the progression of the disease. It rapidly improves the severe infection in patients with CNL, reduces the production of pro-inflammatory cytokines, and improves the function of neutrophils, to improve the systemic symptoms. The patient presented in this study told us that he did not want to undergo hematopoietic stem cell transplantation, despite our repeated recommendations that early hematopoietic stem cell transplantation would be more beneficial to him personally. After the diagnosis, he took Ruxolitinib for long-term treatment of the primary disease. His disease was well controlled and regular reexamination did not show disease progression or deterioration, suggesting that oral Ruxolitinib alone may benefit patients with CNL. Its long-term prognosis needs to be confirmed and clarified by more clinical data and longer follow-up time.
Acknowledgements
We would like to thank the Department of Medical Imaging and the laboratory of Hematology from the First Affiliated Hospital of Gannan Medical University who participated in this study. The study was supported by the Science and Technology Research Project of Education Department of Jiangxi Province (No. GJJ2201414); the Science and Technology Project of Jiangxi Provincial Administration of Traditional Chinese Medicine (No. 2022B072) and the Science and Technology Foundation of Jiangxi Provincial Health Commission (No. SKJP520201086-202130697).
Written informed consent was obtained from the patient’s famlily for the publication of this case report and the accompanying images.
Disclosure of conflict of interest
None.
References
- 1.Li YP, Chen N, Ye XM, Xia YS. Eighty-year-old man with rare chronic neutrophilic leukemia caused by CSF3R T618I mutation: a case report and review of literature. World J Clin Cases. 2020;8:6337–6345. doi: 10.12998/wjcc.v8.i24.6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elliott MA, Tefferi A. Chronic neutrophilic leukemia: 2018 update on diagnosis, molecular genetics and management. Am J Hematol. 2018;93:578–587. doi: 10.1002/ajh.24983. [DOI] [PubMed] [Google Scholar]
- 3.Tuohy EL. A case of splenomegaly with polymorphonuclear neutrophil hyperleukocytosis. Am J Med Sci. 1920;160:18–24. [Google Scholar]
- 4.Tanzer J, Harel P, Boiron M, Bernard J. Cytochemical and cytogenetic findings in a case of chronic neutriphilic leukaemia of mature cell type. Lancet. 1964;1:387–388. doi: 10.1016/s0140-6736(64)92142-7. [DOI] [PubMed] [Google Scholar]
- 5.Ruan GJ, Smith CJ, Day C, Harmsen WS, Zblewski DL, Alkhateeb H, Begna K, Al-Kali A, Litzow MR, Hogan W, Szuber N, Gangat N, Patnaik MS, Pardanani A, Elliott MA, Tefferi A, Go RS, Shah MV. A population-based study of chronic neutrophilic leukemia in the United States. Blood Cancer J. 2020;10:68. doi: 10.1038/s41408-020-0334-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szuber N, Tefferi A. Chronic neutrophilic leukemia: new science and new diagnostic criteria. Blood Cancer J. 2018;8:19. doi: 10.1038/s41408-018-0049-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menezes J, Cigudosa JC. Chronic neutrophilic leukemia: a clinical perspective. Onco Targets Ther. 2015;8:2383–2390. doi: 10.2147/OTT.S49688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szuber N, Elliott M, Tefferi A. Chronic neutrophilic leukemia: 2020 update on diagnosis, molecular genetics, prognosis, and management. Am J Hematol. 2020;95:212–224. doi: 10.1002/ajh.25688. [DOI] [PubMed] [Google Scholar]
- 9.Yi ES, Baek HJ, Ju HY, Kim SK, Lee JW, Cho B, Kim BK, Kang HJ, Kook H, Yang EJ, Lim YT, Ahn WK, Hahn SM, Park SK, Yoo ES, Yoo KH. Response to chemotherapy in juvenile myelomonocytic leukemia and its clinical implications for survival: a retrospective registry-based study of the Korean Pediatric Hematology-Oncology Group. Leuk Res. 2023;129:107070. doi: 10.1016/j.leukres.2023.107070. [DOI] [PubMed] [Google Scholar]
- 10.Cellini A, Scarmozzino F, Friziero A, Trimarco V, Dei Tos AP, Trentin L, Pizzi M, Visentin A. Persistent splenomegaly due to littoral cell angiomatosis in venetoclax-induced undetectable minimal residual disease of chronic lymphocytic leukemia. Ann Hematol. 2023;102:681–682. doi: 10.1007/s00277-022-05067-4. [DOI] [PubMed] [Google Scholar]
- 11.Haseyama Y, Takeda Y, Kumano K. Chronic myeloid leukemia presenting with marked eosinophilia. Rinsho Ketsueki. 2018;59:2594–2599. doi: 10.11406/rinketsu.59.2594. [DOI] [PubMed] [Google Scholar]
- 12.Zhou YB, Yao JF, Xu ZG, Wu RH. Case report: leukemia cutis as the first manifestation of chronic neutrophilic leukemia in a 6-year-old girl. Front Pediatr. 2022;10:972224. doi: 10.3389/fped.2022.972224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clebak KT, Morrison A, Croad JR. Gout: rapid evidence review. Am Fam Physician. 2020;102:533–538. [PubMed] [Google Scholar]
- 14.Qaseem A, Harris RP, Forciea MA Clinical Guidelines Committee of the American College of Physicians. Denberg TD, Barry MJ, Boyd C, Chow RD, Humphrey LL, Kansagara D, Vijan S, Wilt TJ. Management of acute and recurrent gout: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:58–68. doi: 10.7326/M16-0570. [DOI] [PubMed] [Google Scholar]
- 15.Yin B, Chen X, Gao F, Li J, Wang HW. Analysis of gene mutation characteristics in patients with chronic neutrophilic leukaemia. Hematology. 2019;24:538–543. doi: 10.1080/16078454.2019.1642554. [DOI] [PubMed] [Google Scholar]
- 16.Houot J, Marquant F, Goujon S, Faure L, Honoré C, Roth MH, Hémon D, Clavel J. Residential proximity to heavy-traffic roads, benzene exposure, and childhood leukemia-the GEOCAP study, 2002-2007. Am J Epidemiol. 2015;182:685–693. doi: 10.1093/aje/kwv111. [DOI] [PubMed] [Google Scholar]
- 17.Sermage-Faure C, Demoury C, Rudant J, Goujon-Bellec S, Guyot-Goubin A, Deschamps F, Hemon D, Clavel J. Childhood leukaemia close to high-voltage power lines--the Geocap study, 2002-2007. Br J Cancer. 2013;108:1899–1906. doi: 10.1038/bjc.2013.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delapierre B, Troussard X, Damaj G, Dejardin O, Tron L. Role of social status and social environment on net survival in patients with chronic lymphocytic leukemia: a high-resolution population-based study. Cancer Epidemiol. 2023;82:102292. doi: 10.1016/j.canep.2022.102292. [DOI] [PubMed] [Google Scholar]
- 19.Onyije FM, Olsson A, Erdmann F, Magnani C, Petridou E, Clavel J, Miligi L, Bonaventure A, Ferrante D, Piro S, Peters S, Vermeulen R, Kromhout H, Schüz J NARECHEM-ST Group. Parental occupational exposure to combustion products, metals, silica and asbestos and risk of childhood leukaemia: findings from the Childhood Cancer and Leukaemia International Consortium (CLIC) Environ Int. 2022;167:107409. doi: 10.1016/j.envint.2022.107409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spiciarich DR, Oh ST, Foley A, Hughes SB, Mauro MJ, Abdel-Wahab O, Press RD, Viner R, Thompson SL, Chen Q, Azadi P, Bertozzi CR, Maxson JE. A novel germline variant in CSF3R reduces N-glycosylation and exerts potent oncogenic effects in leukemia. Cancer Res. 2018;78:6762–6770. doi: 10.1158/0008-5472.CAN-18-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dwivedi P, Greis KD. Granulocyte colony-stimulating factor receptor signaling in severe congenital neutropenia, chronic neutrophilic leukemia, and related malignancies. Exp Hematol. 2017;46:9–20. doi: 10.1016/j.exphem.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maxson JE, Gotlib J, Pollyea DA, Fleischman AG, Agarwal A, Eide CA, Bottomly D, Wilmot B, McWeeney SK, Tognon CE, Pond JB, Collins RH, Goueli B, Oh ST, Deininger MW, Chang BH, Loriaux MM, Druker BJ, Tyner JW. Oncogenic CSF3R mutations in chronic neutrophilic leukemia and atypical CML. N Engl J Med. 2013;368:1781–1790. doi: 10.1056/NEJMoa1214514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mak KY, Au CH, Chan TL, Ma ESK, Chow EYD, Lin SY, Choi WWL. Next-generation sequencing panel for diagnosis and management of chronic neutrophilic leukaemia: a case report. Hong Kong Med J. 2019;25:248–250. doi: 10.12809/hkmj176959. [DOI] [PubMed] [Google Scholar]
- 24.Fleischman AG, Maxson JE, Luty SB, Agarwal A, Royer LR, Abel ML, MacManiman JD, Loriaux MM, Druker BJ, Tyner JW. The CSF3R T618I mutation causes a lethal neutrophilic neoplasia in mice that is responsive to therapeutic JAK inhibition. Blood. 2013;122:3628–3631. doi: 10.1182/blood-2013-06-509976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hinze A, Rinke J, Hochhaus A, Ernst T. Durable remission with ruxolitinib in a chronic neutrophilic leukemia patient harboring a truncation and membrane proximal CSF3R compound mutation. Ann Hematol. 2021;100:581–584. doi: 10.1007/s00277-020-04152-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yassin MA, Kohla S, Al-Sabbagh A, Soliman AT, Yousif A, Moustafa A, Battah AA, Nashwan A, Al-Dewik N. A case of chronic neutrophilic leukemia successfully treated with pegylated interferon alpha-2a. Clin Med Insights Case Rep. 2015;8:33–36. doi: 10.4137/CCRep.S22820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi J, Ni Y, Li J, Qiu H, Miao K. Concurrent chronic neutrophilic leukemia blast crisis and multiple myeloma: a case report and literature review. Oncol Lett. 2015;9:2208–2210. doi: 10.3892/ol.2015.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Itonaga H, Ota S, Ikeda T, Taji H, Amano I, Hasegawa Y, Ichinohe T, Fukuda T, Atsuta Y, Tanizawa A, Kondo T, Miyazaki Y. Allogeneic hematopoietic stem cell transplantation for the treatment of BCR-ABL1-negative atypical chronic myeloid leukemia and chronic neutrophil leukemia: a retrospective nationwide study in Japan. Leuk Res. 2018;75:50–57. doi: 10.1016/j.leukres.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Szuber N, Elliott M, Tefferi A. Chronic neutrophilic leukemia: 2022 update on diagnosis, genomic landscape, prognosis, and management. Am J Hematol. 2022;97:491–505. doi: 10.1002/ajh.26481. [DOI] [PubMed] [Google Scholar]
- 30.Venugopal S, Mascarenhas J. Chronic neutrophilic leukemia: current and future perspectives. Clin Lymphoma Myeloma Leuk. 2019;19:129–134. doi: 10.1016/j.clml.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 31.Dao KT, Gotlib J, Deininger MMN, Oh ST, Cortes JE, Collins RH Jr, Winton EF, Parker DR, Lee H, Reister A, Schultz, Savage S, Stevens, Brockett C, Subbiah N, Press RD, Raess PW, Cascio M, Dunlap J, Chen Y, Degnin C, Maxson JE, Tognon CE, Macey T, Druker BJ, Tyner JW. Efficacy of ruxolitinib in patients with chronic neutrophilic leukemia and atypical chronic myeloid leukemia. J. Clin. Oncol. 2020;38:1006–1018. doi: 10.1200/JCO.19.00895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ye P, Lin Q, Jin M, Gu X, Lu Y. Successful allogeneic stem cell transplantation with ruxolitinib maintenance therapy for CSF3R T618I mutant chronic neutrophilic leukemia. Turk J Haematol. 2023;40:73–74. doi: 10.4274/tjh.galenos.2022.2022.0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sahara N, Yokoyama K, Matsunaga T, Kitahara S, Fujii T, Kobayashi S, Yusa N, Shimizu E, Imoto S, Tojo A, Ohno N. Anti-inflammatory effects of ruxolitinib on chronic neutrophilic leukemia harboring CSF3R-T618I mutation with bilateral renal abscesses. Leuk Res Rep. 2022;18:100348. doi: 10.1016/j.lrr.2022.100348. [DOI] [PMC free article] [PubMed] [Google Scholar]


