Abstract
Objective: To explore the validity of the neutrophil-to-lymphocyte ratio (NLR) combined with the platelet-to-lymphocyte ratio (PLR) in predicting the short-term prognosis of acute myocardial infarction (AMI). Methods: We collected the data from a total of 3,246 clinical AMI patients hospitalized in the Second Affiliated Hospital of Dalian Medical University from December 2015 to December 2021. All patients underwent routine blood examination within 2 hours of admission. Outcome was defined as all-cause mortality during hospitalization. A total of 94 pairs of patients were generated by propensity score matching (PSM), and a combined NLR-based and PLR-based indicators was constructed according to receiver operating characteristic (ROC) curves and multivariate logistic regression analysis. Results: We finally generated 94 pairs of patients by PSM, and analyzed NLR and PLR in those patients using ROC curves, and converted NLR (optimal cut-off = 5.094) and PLR (optimal cut-off = 165.413) into binary variables according to optimal cut-offs, defined as NLR grouping (5.094 vs. > 5.094, ≤ 5.094 = 0, > 5.094 = 1) and PLR grouping (165.413 vs. > 165.413, ≤ 165.413 = 0, > 165.413 = 1). We constructed a combined indicator (NLR grouping + PLR grouping) based on the results of multivariate logistic regression. Combined indicator has four conditions [Y1 = 0.887 (NLR grouping = 0; PLR grouping = 0); Y2 = 0.949 (NLR grouping = 0; PLR grouping = 1); Y3 = 0.972 (NLR grouping = 1; PLR grouping = 0); and Y4 = 0.988 (NLR grouping = 1; PLR grouping = 1)]. Univariate logistic regression showed that the risk of in-hospital death was significantly increased when the combined indicator of patients was in Y3 (OR = 4.968, 95% CI 2.215-11.141, P < 0.0001) and Y4 (OR = 10.473, 95% CI 4.610-23.793, P < 0.0001). Combined indicator constructed by NLR grouping and PLR grouping can better predict the risk of in-hospital mortality in AMI patients and help clinical cardiologists to more finely care for and treat these high-risk groups to improve their short-term prognostic outcomes.
Keywords: Combined predictive value, acute myocardial infarction, propensity score-matched, neutrophil to lymphocyte, platelet-to-lymphocyte ratio
Introduction
AMI is considered a common and critical emergency event in cardiology departments. With improvements in living standards and modern lifestyles, its incidence continues to increase each year. In previous studies, AMI has generally been characterized by the presence of plaque instability and vulnerability. Acute events caused by unstable plaque rupture are considered to be the main cause of death in patients with coronary artery disease when stimulated by certain factors [1]. Vulnerable plaques are characterized in pathology by the presence of thin-cap fibroatheroma (TCFA ≤ 65 um), large lipid pools, vascular inflammation (macrophage/monocyte infiltration), intimal erosion with plaque rupture and bleeding, and platelet aggregation [2,3].
Studies have shown that inflammation is an important mechanism underlying vulnerable plaque rupture, and most patients with AMI have accompanying hypertension, hypercholesterolemia, and other underlying diseases, leading to an overall inflammatory state of the human body [4-10] and in turn aggravating the continuous deterioration of AMI. Therefore, there is an urgent need to identify new inflammatory markers that can accurately reflect the current inflammatory status of AMI patients to better assess their prognosis. In previous studies, the neutrophil-lymphocyte ratio and platelet-lymphocyte ratio have each attracted much attention because they are convenient to obtain and can reflect the systemic inflammatory state. Platelets, neutrophils and lymphocytes are affected by a variety of physiological conditions; in contrast, PLR and NLR are more stable [11,12]. NLR and PLR have each been shown to be associated with risk stratification and poor prognosis in patients with ACS in several studies [13-15]. However, few studies have investigated the short-term prognostic effect of NLR combined with PLR as a combined predictor in patients with AMI.
The aim of this study was to construct a new indicator by combining NLR with PLR through a logistic regression model to better help clinical cardiologists identify the probability of their in-hospital mortality risk in AMI patients early on admission.
Methods
General data
This was a single-center, observational, retrospective study of 3,246 patients with AMI admitted to the Department of Cardiology of the Second Hospital of Dalian Medical University from December 2015 to December 2021, their demographic information, clinical characteristics, medical history, laboratory tests, treatment, and outcome at their admission and during hospitalization were extracted from the electronic medical record system. Among the patients, a total of 115 AMI patients who died during hospitalization were finally identified and enrolled as the study subjects in the in-hospital death group (n = 94) and the non-death group (n = 94) by PSM. The baseline clinical data of patients before and after matching are detailed in Table 1. This study is an observational study, in which patients informed consent can be exempted and ethical requirements in the Declaration of Helsinki have been met, and the study was approved by the Ethics Committee of the Second Hospital of Dalian Medical University (No: 2023046).
Table 1.
Baseline characteristics of subjects before and after matching
| Variables | Before Matching | After Matching | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| With Death (n = 115) | Without Death (n = 3,131) | P-value | With Death (n = 94) | Without Death (n = 94) | P-value | |
| Male, n (%) | 64 (55.65%) | 2116 (67.60%) | 0.009 | 53 (56.38%) | 48 (51.06) | 0.465 |
| Age (years) | 77.46 ± 11.48 | 66.14 ± 12.39 | < 0.001 | 76.61 ± 11.98 | 76.88 ± 9.04 | 0.029 |
| BMI (kg/m2) | 23.95 ± 3.10 | 25.51 ± 3.55 | < 0.001 | 23.93 ± 3.25 | 24.73 ± 3.91 | 0.018 |
| SBP (mmHg) | 131.75 ± 31.75 | 139.61 ± 24.91 | 0.006 | 134.17 ± 30.24 | 141.96 ± 26.85 | 0.213 |
| Previous stroke, n (%) | 13 (11.30) | 131 (4.18%) | < 0.001 | 11 (11.70%) | 15 (15.95) | 0.398 |
| Blood Pressure class, n (%) | 0.544 | 0.628 | ||||
| Normal | 37 (32.20%) | 923 (29.50%) | 31 (32.97%) | 34 (36.17%) | ||
| I | 17 (14.80%) | 578 (18.50%) | 21 (22.34%) | 15 (15.95%) | ||
| II | 10 (8.70%) | 355 (11.30%) | 9 (9.57%) | 7 (7.44%) | ||
| III | 51 (44.30%) | 1275 (40.70%) | 33 (35.10%) | 38 (40.42%) | ||
| HF, n (%) | 107 (93.00%) | 1725 (55.10%) | < 0.001 | 86 (91.48%) | 86 (91.48%) | / |
| Diabetes, n (%) | 59 (51.30%) | 1309 (41.80%) | 0.044 | 49 (52.12%) | 38 (40.42%) | 0.108 |
| HbA1c (%) | 6.79 ± 1.55 | 6.88 ± 1.64 | 0.58 | 6.83 ± 1.59 | 6.71 ± 1.60 | 0.724 |
| Acute coronary syndrome, n (%) | 0.051 | 0.463 | ||||
| NSTEMI | 74 (64.30%) | 2274 (72.60%) | 66 | 61 | ||
| STEMI | 41 (35.70%) | 857 (27.40) | 28 | 33 | ||
| Blood routine examination | ||||||
| Na (mmol/L) | 138.26 ± 5.78 | 139.86 ± 3.14 | < 0.005 | 138.46 ± 6.07 | 138.62 ± 3.55 | < 0.001 |
| K (mmol/L) | 4.15 ± 0.67 | 3.92 ± 0.46 | < 0.001 | 4.13 ± 0.69 | 3.99 ± 0.54 | 0.019 |
| PLT (109/L) | 215.15 ± 85.03 | 214.13 ± 60.52 | 0.89 | 212.48 ± 68.35 | 203.52 ± 60.22 | 0.169 |
| Neutrophils (109/L) | 9.10 ± 4.01 | 5.63 ± 2.54 | < 0.001 | 8.76 ± 3.81 | 5.98 ± 2.75 | 0.001 |
| Lymphocytes (109/L) | 1.23 ± 0.58 | 1.64 ± 0.61 | < 0.001 | 1.21 ± 0.62 | 1.45 ± 0.54 | 0.167 |
| eGFR (mL/min/1.73 m2) | < 0.001 | 0.487 | ||||
| > 60/n (%) | 40 (34.80%) | 2291 (73.20%) | 35 (37.23%) | 43 (45.74%) | ||
| 30-60/n (%) | 39 (33.90%) | 316 (10.10%) | 29 (30.85%) | 26 (27.65%) | ||
| < 30/n (%) | 36 (31.30%) | 524 (16.70%) | 30 (31.91%) | 25 (26.59%) | ||
| Blood biochemistry | ||||||
| TG (mmol/L) | 1.17 [0.92-1.69] | 1.43 [1.04-2.05] | < 0.001 | 1.17 [0.90-1.76] | 1.16 [0.95-1.77] | 0.847 |
| LDLC (mmol/L) | 2.27 [1.79-3.09] | 2.45 [1.87-3.04] | 0.323 | 2.23 [1.74-3.02] | 2.38 [1.84-3.14] | 0.402 |
| Apolipoprotein ratio | 1.37 [1.04-1.67] | 1.37 [1.13-1.71] | 0.177 | 1.36 [1.02-1.68] | 1.39 [1.16-1.77] | 0.178 |
| ALT (U/L) | 32.00 [16.10-81.28] | 24.13 [6.23-38.10] | < 0.001 | 29.72 [14.86-73.94] | 26.92 [17.04-47.25] | 0.441 |
| AST (U/L) | 81.91 [27.32-234.86] | 28.10 [19.45-62.56] | < 0.001 | 68.17 [23.99-198.17] | 43.12 [21.21-142.08] | 0.155 |
| CTNI (ug/L) | 5.52 [0.77-16.72] | 1.76 [0.82-3.23] | < 0.001 | 2.99 [0.62-8.53] | 3.23 [0.85-7.60] | 0.940 |
| In-Hospital treatment | ||||||
| Aspirin, n (%) | 98 (85.20%) | 2971 (94.90%) | < 0.001 | 79 (84.04%) | 82 (87.23%) | 0.533 |
| Statins, n (%) | 105 (91.30%) | 3084 (98.50%) | < 0.001 | 86 (89.36%) | 92 (97.87%) | 0.051 |
| ACEI, n (%) | 28 (24.30%) | 1556 (49.70%) | < 0.001 | 23 (24.46%) | 44 (46.80%) | 0.001 |
| Lymphocyte-based inflammatory indices | ||||||
| NLR | 7.36 [4.71-12.18] | 3.19 [2.29-4.78] | < 0.001 | 7.47 [4.79-11.76] | 3.62 [2.64-5.60] | < 0.001 |
| PLR | 170.83 [122.32-258.82] | 132.06 [103.39-170.72] | < 0.001 | 190.35 [121.98-268.53] | 142.47 [110.48-167.35] | < 0.001 |
Note: If the continuous data fitted a normal distribution, it was described by X ± SD; otherwise, it was described by the median and quartiles (25%, 75%). BMI, Body mass index; HF, heart failure; HbA1c, glycated hemoglobin; TG, triglyceride; LDLC, low-density lipoprotein cholesterol; Apolipoprotein ratio, apolipoprotein A-to-B ratio; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CTNI, cardiac troponin I; ACEI, angiotensin-converting enzyme inhibitors; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio.
The inclusion criteria were: (1) Age ≥ 18 years. (2) ST-segment elevation myocardial infarction (STEMI): patients with dynamic changes in myocardial injury markers (troponin) levels exceeding the upper limit of normal levels by 99%, and patients with myocardial ischemia. Patients with left sternal pain lasting for more than 30 min that was not relieved by nitric acid and other drugs, patients with arcuate ST segment elevation in ECG (newly arcuate ST segment elevation in leads V1-V3, amplitude ≥ 0.2 mV, or ST segment elevation in other leads and amplitude ≥ 0.1 mV), or new changes in left bundle branch block, pathological Q waves in corresponding leads of ECG (showing Q waves ≥ 30 ms in more than two adjacent leads, depth at least 1 mm), and patients with new loss of viable myocardium or abnormal regional ventricular wall motion diagnosed by imaging. (3) Non-ST segment elevation myocardial infarction: patients with angina pectoris lasting for more than 20 min and pain above grade 3, and patients with positive myocardial injury markers14.
Exclusion criteria: (1) Incomplete medical history data in the electronic medical record system. (2) Patients with severe diseases (such as advanced malignant tumors) that had a life expectancy of less than half a year. (3) Patients with hematological diseases. (4) Patients who had recently received chemotherapy and/or radiotherapy. (5) Patients with a history of trauma surgery or blood transfusion within the past 30 days.
Data collection and processing
The demographic information, clinical characteristics, medical history, laboratory tests, treatments, and results of patients were extracted from the electronic medical record system at admission and during hospitalization. The initial laboratory test results following admission were extracted, including routine blood tests, biochemical tests of liver and kidney function, and coagulation tests. Drug therapy during hospitalization was recorded, including commonly used lipid-lowering therapy and antithrombotic therapy. The outcome was defined as all-cause mortality during hospitalization. The data were collated by experienced physicians and statisticians.
Propensity score matching
Before performing PSM, we first identified seven risk factors associated with in-hospital mortality in AMI patients by multivariate logistic regression analysis, including age, liver function (alanine aminotransferase), renal function (eGFR class), presence of heart failure, history of stroke, and cardiac troponin I levels (CTNI). We calculated propensity scores for all patients based on these seven variables and then performed 1- to -1 matching, for a total of 94 pairs of patients successfully matched.
Statistical analysis
Data were processed by SPSS 23.0, MedCalc 15.0, and R 4.2.1. For categorical variables, the data were described as the frequency or percentage. For continuous variables, if they conformed to the normal distribution, the data were presented as the mean ± standard deviation; otherwise, data were presented as quartiles [median (quartile 25%, 75%)]. If continuous data satisfied normality, comparisons between two groups or among multiple groups were analyzed by the t-test or ANOVA analysis; otherwise, the non-parametric test was used. Fisher’s exact test or the Chi-square test was used for the comparison of categorical variables.
ROC curves were used to analyze the optimal cut-off values, and the areas under the ROC curves constructed by different indicators were compared using the DELONG method.
NLR grouping, PLR grouping, and other risk factors that may still contribute to in-hospital mortality in AMI patients were included in multivariate logistic regression. Combined indicators were constructed according to the coefficients of NLR grouping and PLR grouping in multivariate logistic regression models, and predicted values were calculated according to logistic equation: ŷ = 1/[1 + exp.(-xβ)] [16]. All confidence intervals, significance tests, and resulting P-values were two-sided at an alpha level of 0.05 [17-19].
Results
Comparison of baseline data before and after matching
Before matching, we first identified the risk factors leading to in-hospital death in AMI patients by multivariate logistic regression analysis, including age, liver function (alanine aminotransferase), renal function (eGFR class), presence of heart failure, history of stroke, and cardiac troponin I levels (CTNI), and the results of multivariate regression analysis are detailed in Supplementary Table 1. Even if some of these variables were not statistically significant (P > 0.05), they served as one of the matching variables if they were already recognized as the main cause of in-hospital death in AMI patients. We calculated the propensity score of patients according to these seven variables, then matched them according to a ratio of 1- to -1, and finally a total of 94 pairs of patients were successfully matched, and the baseline data of patients before and after matching are detailed in Table 1. The matched results showed that there was no significant difference between the two groups in the above 7 variables (P > 0.05). From the results of routine blood examination, the mean neutrophil level in the death group (8.76 ± 3.81) was higher than that in the non-death group (5.98 ± 2.75), and the difference was statistically significant (P = 0.001), although the mean platelet level in the death group (212.48 ± 68.35) was also higher than that in the non-death group (203.52 ± 60.22), the difference was not statistically significant (P = 0.169). However, patients in the death group had lower lymphocyte counts than patients in the non-death group, but the difference was not statistically significant (P = 0.167). In addition, NLR and PLR were higher in patients who died than in patients who did not die [7.47 (4.79-11.76) vs. 3.62 (2.64-5.60), P < 0.001]; [190.35 (121.98-268.53) vs. 142.47 (110.48-167.35), P < 0.001].
The optimal cutoff value of NLR and PLR
We calculated the optimal cutoff values of NLR and PLR by receiver operating characteristic curve (ROC) and converted them into categorical variables (NLR grouping, PLR grouping) according to their respective optimal cutoff values, which were done to avoid the influence of interactive effects when constructing combined indicators (NLR + PLR). The results showed that the optimal cutoff values for NLR and PLR were 5.094 and 165.413, respectively. If NLR > 5.094, this defined the high NLR grouping, otherwise it was categorized as low NLR grouping. Similarly, high PLR was defined if PLR > 165.413, and vice versa low PLR. The ROC analysis results showed that the AUC of NLR grouping was 0.718, while the AUC of PLR grouping was 0.665, as shown in Figure 1. In addition, we also compared the ability of NLR grouping and PLR grouping to predict the risk of death in patients with NSTEMI and STEMI, respectively, and the results showed that there was no significant difference in the AUC between NLR grouping and PLR grouping between NSTEMI and STEMI, as shown in Figure 2.
Figure 1.
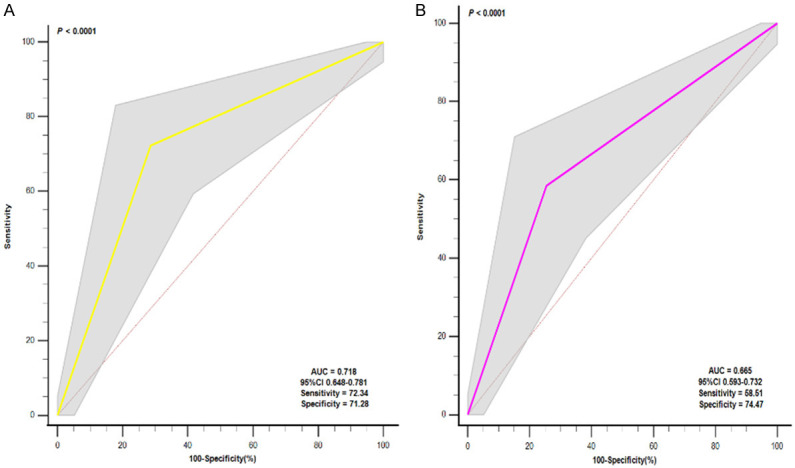
Receiver operating characteristic (ROC) curve analysis of Neutrophil-to-Lymphocyte Ratio (NLR) grouping and Platelet-to-Lymphocyte Ratio (PLR) grouping. A. ROC curve analysis of NLR grouping; B. ROC curve analysis of PLR grouping.
Figure 2.
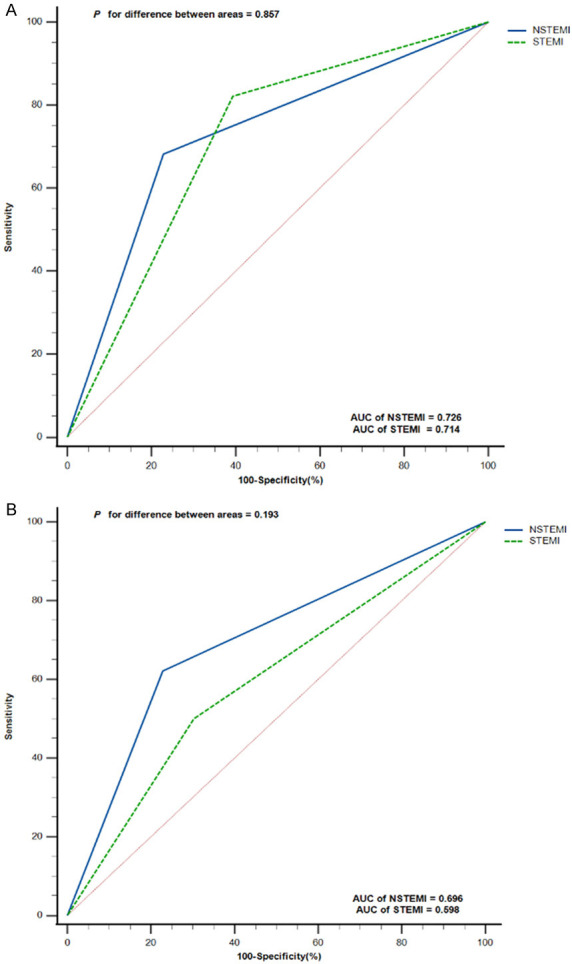
Receiver operating characteristic (ROC) Curve Comparison of Neutrophil-to-Lymphocyte Ratio (NLR) grouping and Platelet-to-Lymphocyte Ratio (PLR) grouping in NSTEMI and STEMI patients. A. ROC curve analysis and comparison of NLR grouping in NSTEMI and STEMI; B. ROC curve analysis and comparison of PLR grouping in NSTEMI and STEMI. NSTEMI, non-ST segment elevation myocardial infarction; STEMI, ST segment elevation myocardial infarction.
Multivariate logistic regression analysis of risk of in-hospital mortality in patients with AMI
We have performed a multivariate logistic regression analysis to correct for confounding before performing PSM. Among matched patients, we included NLR grouping (NLR ≤ 5.094 vs. NLR > 5.094) and PLR grouping (PLR ≤ 165.413 vs. PLR > 165.413) as risk factors in regression analysis to identify their association with the risk of in-hospital death in AMI patients. In addition, we included factors such as body mass index, systolic blood pressure, alanine aminotransferase, low-density lipoprotein cholesterol, and in-hospital medication (Aspirin use, Statins use) in order to correct for residual confounding. The results of multivariate logistic regression analysis showed that patients with NLR > 5.094 had a 4.469-fold higher risk of in-hospital death than patients with NLR ≤ 5.094 (95% CI 2.069-9.653, P < 0.001); patients with PLR > 165.413 had a 2.41-fold higher risk of in-hospital death than patients with PLR ≤ 165.413 (95% CI 1.155-5.029, P = 0.019), as shown in Table 2.
Table 2.
Multivariate logistic regression analysis of risk of in-hospital mortality in patients with AMI after propensity score matching
| β | OR | 95% CI | P-value | |
|---|---|---|---|---|
| NLR grouping | 1.497 | 4.469 | 2.069-9.653 | < 0.001 |
| PLR grouping | 0.879 | 2.410 | 1.155-5.029 | 0.019 |
| BMI | -0.063 | 0.939 | 0.855-1.032 | 0.189 |
| SBP | -0.004 | 0.996 | 0.984-1.009 | 0.582 |
| AST | 0.002 | 1.002 | 0.999-1.005 | 0.150 |
| CKMB | -0.004 | 0.996 | 0.991-1.000 | 0.049 |
| LDL-C | -0.083 | 0.920 | 0.643-1.317 | 0.649 |
| Aspirin use | 0.149 | 1.160 | 0.397-3.393 | 0.786 |
| Statins use | -1.251 | 0.286 | 0.038-2.177 | 0.227 |
Note: NLR grouping (NLR ≤ 5.094 = 0; NLR > 165.413 = 1, NLR ≤ 5.094 as reference); PLR grouping (PLR ≤ 165.413 = 0; PLR > 165.413 = 1, PLR ≤ 165.413 as reference). NLR, neutrophil-lymphocyte ratio; PLR, platelet-lymphocyte ratio; BMI, body mass index; SBP, systolic blood press; AST, aspartate aminotransferase; CK-MB, creatine kinase-MB; LDL-C, low-density lipoprotein cholesterol.
Combined indicator (NLR + PLR) based on multivariate logistic regression model
We combined NLR grouping and PLR grouping to construct a new combined indicator to evaluate the risk of in-hospital mortality in AMI patients based on the results of multivariate logistic regression. Individual patient risk scores were calculated using the following formula: xβ = 2.062 + (1.497 × NLR grouping (5.094 vs. > 5.094, ≤ 5.094 = 0, > 5.094 = 1)) + (0.879 × PLR grouping (165.413 vs. > 165.413, ≤ 165.413 = 0, > 165.413 = 1)). The probability of in-hospital death in AMI patients was calculated using the following formula: (Y = 1/[1 + exp.(-xβ)]): = 1/[1 + exp.(-(2.062 + 1.497* NLR grouping (5.094 vs. > 5.094, ≤ 5.094 = 0, > 5.094 = 1) + 0.879* PLR grouping (165.413 vs. > 165.413, ≤ 165.413 = 0, > 165.413 = 1)))].
Association between combined indicator and risk of in-hospital mortality in patients with AMI and ROC analysis
According to the formula, there were four conditions in calculating the risk of in-hospital death, and we divided them into four groups: Y1 = 0.887 (NLR grouping = 0; PLR grouping = 0); Y2 = 0.949 (NLR grouping = 0; PLR grouping = 1); Y3 = 0.972 (NLR grouping = 1; PLR grouping = 0); and Y4 = 0.988 (NLR grouping = 1; PLR grouping = 1). Univariate logistic regression analysis showed that when AMI patients were in Y3 grouping, the risk of death was 4.968 times higher than that in Y1 group (95% CI 2.215-11.141, P < 0.0001); when AMI patients were in Y4 grouping, the risk of death was 10.473 times higher than that in Y1 grouping (95% CI 4.610-23.793, P < 0.0001), as detailed in Table 3. ROC was used to analyze the combined indicator, and the results showed that the AUC of the combined indicator was 0.740 (95% CI 0.671-0.801, Sensitivity = 80.85%, Specificity = 59.57%, P < 0.0001), as shown in Figure 3. Similarly, analysis of the predictive effect of the combined indicator in patients with NSTEMI and STEMI showed that there was no significant difference in the AUC of the combined indicator in patients with NSTEMI or STEMI (P > 0.05), as detailed in Figure 4. We used the DELONG method to compare the AUC of NLR grouping, PLR grouping and combined indicator, and the results showed that the AUC of combined indicator was significantly higher than that of NLR grouping and PLR grouping, and the difference was statistically significant (P < 0.05), as shown in Table 4.
Table 3.
Univariate logistic regression analysis of combined indicator and risk of in-hospital mortality in patients with AMI
| Variables | β | OR | 95% CI | P-value |
|---|---|---|---|---|
| Y1 (REF) | / | / | / | / |
| Y2 | 1.070 | 2.914 | 0.689-12.321 | 0.146 |
| Y3 | 1.603 | 4.968 | 2.215-11.141 | < 0.0001 |
| Y4 | 2.349 | 10.473 | 4.610-23.793 | < 0.0001 |
Note: Y1 = 0.887 (NLR grouping = 0; PLR grouping = 0); Y2 = 0.949 (NLR grouping = 0; PLR grouping = 1); Y3 = 0.972 (NLR grouping = 1; PLR grouping = 0); Y4 = 0.988 (NLR grouping = 1; PLR grouping = 1). NLR grouping (NLR ≤ 5.094 = 0; NLR > 5.094 = 1, NLR ≤ 5.094 as reference); PLR grouping (PLR ≤ 165.413 = 0; PLR > 165.413 = 1, PLR ≤ 165.413 as reference). NLR, neutrophil-lymphocyte ratio; PLR, platelet-lymphocyte ratio.
Figure 3.
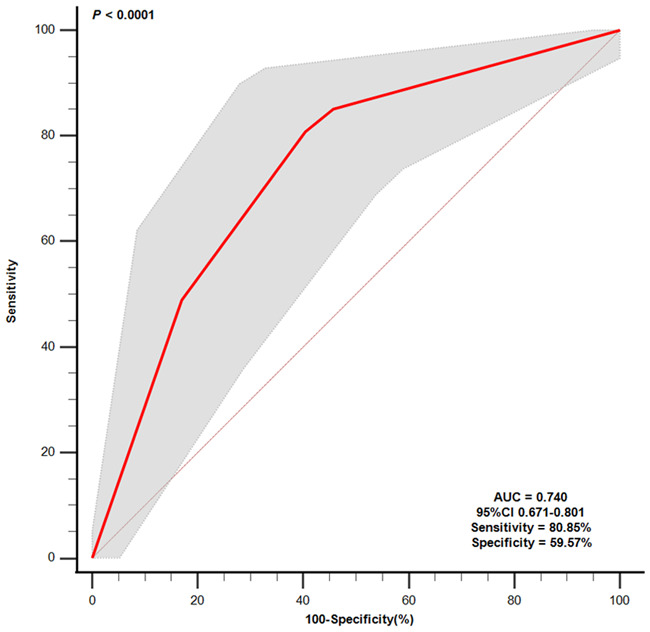
Receiver operating characteristic (ROC) curve analysis of Combined indicators. NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-lymphocyte ratio; NLR + PLR, NLR combined with PLR.
Figure 4.
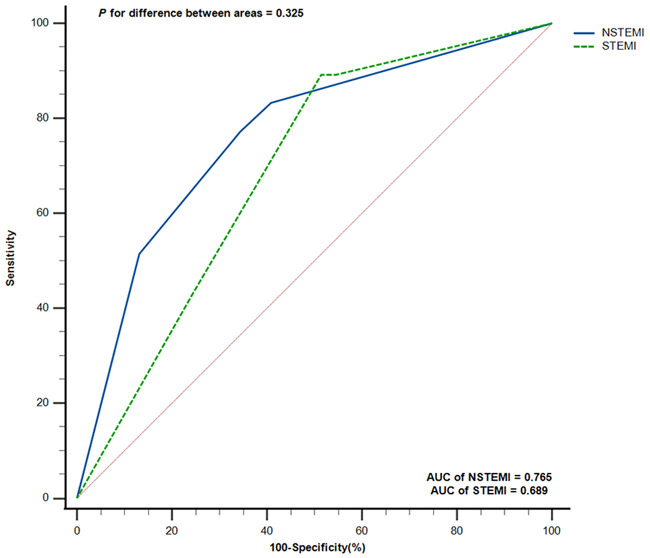
Receiver operating characteristic (ROC) Curve Comparison of Combined indicators in NSTEMI and STEMI patients. NSTEMI, non-ST segment elevation myocardial infarction; STEMI, ST segment elevation myocardial infarction.
Table 4.
AUC of combined indicator compared to NLR grouping and PLR grouping
| Variables | Difference between areas | Standard Error | 95% CI | P-value |
|---|---|---|---|---|
| NLR grouping vs. PLR grouping | 0.047 | 0.038 | -0.026-0.123 | 0.208 |
| Combined indicator vs. PLR grouping | 0.085 | 0.025 | 0.035-0.135 | < 0.001 |
| Combined indicator vs. NLR grouping | 0.037 | 0.017 | 0.003-0.071 | 0.030 |
Note: NLR grouping (NLR ≤ 5.094 = 0; NLR > 5.094 = 1, NLR ≤ 5.094 as reference); PLR grouping (PLR ≤ 165.413 = 0; PLR > 165.413 = 1, PLR ≤ 165.413 as reference); Combined index: NLR + PLR. NLR, neutrophil-lymphocyte ratio; PLR, platelet-lymphocyte ratio.
Clinical application of combined indicators in predicting the risk of in-hospital mortality in patients with AMI
We defined the Y1 and Y2 groups in the combined measure as the low mortality risk group, while the Y3 and Y4 groups were defined as the high mortality risk group. The results showed that 56 (75.68%) of 74 patients in Y1 and Y2 groups were correctly identified, and no in-hospital deaths occurred; a total of 114 people in Y3 and Y4 groups, 76 (66.67%) of whom were correctly identified, died during hospitalization.
Discussion
In this retrospective study of 3,246 patients with acute myocardial infarction, we evaluated the predictive value of NLR combined with PLR for the risk of in-hospital death in patients with AMI based on propensity score matching. By developing and deriving the logistic regression model, we constructed a new joint indicator (NLR grouping + PLR grouping). Our results showed that the AUC of the combined index was significantly higher than the AUC of NLR and PLR, and this difference was statistically significant (P < 0.05). According to the different results of NLR grouping and PLR grouping, we divided the combined predictors into four groups, namely Y1 (NLR grouping = 0, PLR grouping = 0), Y2 (NLR grouping = 0, PLR grouping = 1), Y3 (NLR grouping = 1, PLR grouping = 0) and Y4 (NLR grouping = 1, PLR grouping = 1). We found that if AMI patients were in the Y3 and Y4 groups, their risk of in-hospital mortality would be greatly increased.
The inflammatory response plays an important role in the development of coronary heart disease. Inflammation causes vascular injury through immune cells [17], the most important damage cells are neutrophils, when the human body becomes inflamed, neutrophils can be activated and then release a large number of reactive oxygen species, while producing extracellular traps (NETs) [17], although these products can kill pathogens to some extent, they also exacerbate damage to the vessel wall and promote thrombosis [18]. Platelets are an important component of coronary plaque formation, and regardless of their role in the general inflammatory response, platelets are always closely associated with endothelial activation and coordination [19]. Increased platelet activation can lead to destructive inflammatory reactions and prothrombotic states [20]. Unlike neutrophils and platelets, lymphocytes are in most cases thought to be associated with anti-vascular arteriosclerosis [21]. Lower lymphocyte counts are associated with worse cardiovascular outcomes. Decreased lymphocyte counts in humans during some cardiovascular critical events, such as AMI, may be associated with stress response inhibiting lymphocyte proliferation and differentiation, as well as lymphocyte redistribution [22,23]. In our study, patients in the death group had higher mean neutrophil and platelet counts, while lymphocyte counts were lower than in the non-death group.
NLR can systematically reflect and accurately reflect the degree of body inflammation and stress [13], and PLR also reflects platelet activation and prothrombotic state [20]. NLR and PLR are more accurate and objective lymphocytes than neutrophils, platelets, and lymphocytes alone in predicting outcomes in patients with AMI because they always combine two indicators that reflect the body’s inflammatory status. Studies have shown that NLR is of great value in predicting adverse outcome events in cardiovascular patients, for example, NLR > 2.83 can predict the risk of major adverse cardiovascular events (MACEs) [14], and NLR > 5.509 can predict a positive correlation with the risk of in-hospital death in AMI patients [13]. Li et al. showed that high PLR was associated with in-hospital mortality risk in elderly patients with AMI [20], but some studies suggested that PLR was weaker than NLR in predicting adverse cardiovascular outcomes, and PLR alone was not recommended to predict cardiovascular outcomes, and it was recommended to be combined with other indicators [14]. At present, only one study investigated the correlation between NLR combined with PLR and the prognosis of AMI patients, and its results showed that the higher NLR-PLR, the greater the risk of major adverse cardiovascular and cerebrovascular events (MACCEs) in patients [24]. However, no study has investigated the effect of NLR combined with PLR on the short-term prognosis of AMI patients. Unlike previous studies, what we call the combination of NLR and PLR is not a simple numerical combination, but a new formula is constructed to calculate the combined index of NLR and PLR through a multivariate logistic regression model after fine matching and correction for multiple confounders. The results showed that this new combined index could better identify the risk of death of AMI during hospitalization (the recognition rate of dead patients was 66.67%, and the recognition rate of non-dead patients was 75.68%), and it could also be directly applied in the clinical diagnosis and treatment process, and the risk stratification could be performed by only a simple routine blood examination of patients. It is worth noting that, unlike traditional interventional means, this measure provides a reference for clinicians to hospitalized AMI patients at high risk of death, allowing clinicians to pay more attention to such patients without additional burden and harm to the patients themselves even if they do not experience death events.
Limitations
Our study had the following limitations: this is a retrospective study conducted in a single center, and it is necessary to conduct a prospective study in a multi-center to evaluate the effectiveness of the combined indicators. Second, we failed to follow the long-term prognosis of these patients, which limits the predictive value of NLR combined with PLR for long-term mortality risk in elderly AMI patients. Finally, retrospective studies were unable to identify a causal relationship between NLR, PLR, and risk of in-hospital mortality in patients with AMI.
Conclusions
We constructed a new combined indicator to evaluate the risk of in-hospital death in AMI patients by combining NLR grouping and PLR grouping. We found that when calculated by combined indicators, if AMI patients were in Y3 (NLR grouping = 1, PLR grouping = 0) and Y4 (NLR grouping = 1, PLR grouping = 1) groups, then they had a higher risk of in-hospital death. This noninvasive and easily available new combined indicator provided a reference for cardiology clinicians to give a short-term prognosis for AMI patients, and could carry out more refined care and risk stratification for patients accordingly.
Acknowledgements
This study was funded by the National Key R&D Program (grant number 2020YFC2004701) and Liaoning Provincial “Selecting the Best Candidates by Opening Competition Mechanism” Science and Technology Program (grant number 2022JH1/10400004).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Pasterkamp G, den Ruijter HM, Libby P. Temporal shifts in clinical presentation and underlying mechanisms of atherosclerotic disease. Nat Rev Cardiol. 2017;14:21–9. doi: 10.1038/nrcardio.2016.166. [DOI] [PubMed] [Google Scholar]
- 2.Stone GW, Mintz GS, Virmani R. Vulnerable plaques, vulnerable patients, and intravascular imaging. J Am Coll Cardiol. 2018;72:2022–2026. doi: 10.1016/j.jacc.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Arbab-Zadeh A, Fuster V. The myth of the “vulnerable plaque”: transitioning from a focus on individual lesions to atherosclerotic disease burden for coronary artery disease risk assessment. J Am Coll Cardiol. 2015;65:846–855. doi: 10.1016/j.jacc.2014.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ginsberg HN, Packard CJ, Chapman MJ, Boren J, Aguilar-Salinas CA, Averna M, Ference BA, Gaudet D, Hegele RA, Kersten S, Lewis GF, Lichtenstein AH, Moulin P, Nordestgaard BG, Remaley AT, Staels B, Stroes ESG, Taskinen MR, Tokgözoğlu LS, Tybjaerg-Hansen A, Stock JK, Catapano AL. Triglyceride-rich lipoproteins and their remnants: metabolic insights, role in atherosclerotic cardiovascular disease, and emerging therapeutic strategies-a consensus statement from the European Atherosclerosis Society. Eur Heart J. 2021;42:4791–4806. doi: 10.1093/eurheartj/ehab551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Musher DM, Abers MS, Corrales-Medina VF. Acute infection and myocardial infarction. N Engl J Med. 2019;380:171–176. doi: 10.1056/NEJMra1808137. [DOI] [PubMed] [Google Scholar]
- 6.Skoog T, Dichtl W, Boquist S, Skoglund-Andersson C, Karpe F, Tang R, Bond MG, de Faire U, Nilsson J, Eriksson P, Hamsten A. Plasma tumour necrosis factor-alpha and early carotid atherosclerosis in healthy middle-aged men. Eur Heart J. 2002;23:376–83. doi: 10.1053/euhj.2001.2805. [DOI] [PubMed] [Google Scholar]
- 7.Webb RJ, Mazidi M, Lip GYH, Kengne AP, Banach M, Davies IG. The role of adiposity, diet and inflammation on the discordance between LDL-C and apolipoprotein B. Nutr Metab Cardiovasc Dis. 2022;32:605–615. doi: 10.1016/j.numecd.2021.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Raposeiras-Roubin S, Rossello X, Oliva B, Fernandez-Friera L, Mendiguren JM, Andres V, Bueno H, Sanz J, Martínez de Vega V, Abu-Assi E, Iñiguez A, Fernández-Ortiz A, Ibáñez B, Fuster V. Triglycerides and residual atherosclerotic risk. J Am Coll Cardiol. 2021;77:3031–3041. doi: 10.1016/j.jacc.2021.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mikolajczyk TP, Szczepaniak P, Vidler F, Maffia P, Graham GJ, Guzik TJ. Role of inflammatory chemokines in hypertension. Pharmacol Ther. 2021;223:107799. doi: 10.1016/j.pharmthera.2020.107799. [DOI] [PubMed] [Google Scholar]
- 10.Harrison DG, Marvar PJ, Titze JM. Vascular inflammatory cells in hypertension. Front Physiol. 2012;3:128. doi: 10.3389/fphys.2012.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheng J, Liu N, He F, Cheng C, Shen S, Sun Y. Changes in the neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios before and after percutaneous coronary intervention and their impact on the prognosis of patients with acute coronary syndrome. Clinics (Sao Paulo) 2021;76:e2580. doi: 10.6061/clinics/2021/e2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kounis NG, Koniari I, Plotas P, Soufras GD, Tsigkas G, Davlouros P, Hahalis G. Inflammation, thrombosis, and platelet-to-lymphocyte ratio in acute coronary syndromes. Angiology. 2021;72:6–8. doi: 10.1177/0003319720946213. [DOI] [PubMed] [Google Scholar]
- 13.Ji Z, Liu G, Guo J, Zhang R, Su Y, Carvalho A, Qu Y, Zuo W, Yao Y, Lin J, Ma G. The neutrophil-to-lymphocyte ratio is an important indicator predicting in-hospital death in AMI patients. Front Cardiovasc Med. 2021;8:706852. doi: 10.3389/fcvm.2021.706852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Q, Ma X, Shao Q, Yang Z, Wang Y, Gao F, Zhou Y, Yang L, Wang Z. Prognostic impact of multiple lymphocyte-based inflammatory indices in acute coronary syndrome patients. Front Cardiovasc Med. 2022;9:811790. doi: 10.3389/fcvm.2022.811790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haybar H, Pezeshki SMS, Saki N. Evaluation of complete blood count parameters in cardiovascular diseases: an early indicator of prognosis? Exp Mol Pathol. 2019;110:104267. doi: 10.1016/j.yexmp.2019.104267. [DOI] [PubMed] [Google Scholar]
- 16.Zhao F, Zhou Y, Ge PF, Huang CJ, Yu Y, Li J, Sun YG, Meng YC, Xu JX, Jiang T, Zhang ZX, Sun JP, Wang W. A prediction model for lymph node metastases using pathologic features in patients intraoperatively diagnosed as stage I non-small cell lung cancer. BMC Cancer. 2017;17:267. doi: 10.1186/s12885-017-3273-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gómez-Moreno D, Adrover JM, Hidalgo A. Neutrophils as effectors of vascular inflammation. Eur J Clin Invest. 2018;48(Suppl 2):e12940. doi: 10.1111/eci.12940. [DOI] [PubMed] [Google Scholar]
- 18.Döring Y, Soehnlein O, Weber C. Neutrophil extracellular traps in atherosclerosis and atherothrombosis. Circ Res. 2017;120:736–43. doi: 10.1161/CIRCRESAHA.116.309692. [DOI] [PubMed] [Google Scholar]
- 19.Nikolsky E, Grines CL, Cox DA, Garcia E, Tcheng JE, Sadeghi M, Mehran R, Lansky AJ, Na Y, Stone GW. Impact of baseline platelet count in patients undergoing primary percutaneous coronary intervention in acute myocardial infarction (from the CADILLAC trial) Am J Cardiol. 2007;99:1055–61. doi: 10.1016/j.amjcard.2006.11.066. [DOI] [PubMed] [Google Scholar]
- 20.Li L, Ma Y, Geng XB, Tan Z, Wang JH, Cui C, Wang HL, Shang XM. Platelet-to-lymphocyte ratio relates to poor prognosis in elderly patients with acute myocardial infarction. Aging Clin Exp Res. 2021;33:619–624. doi: 10.1007/s40520-020-01555-7. [DOI] [PubMed] [Google Scholar]
- 21.Wigren M, Nilsson J, Kolbus D. Lymphocytes in atherosclerosis. Clin Chim Acta. 2012;413:1562–8. doi: 10.1016/j.cca.2012.04.031. [DOI] [PubMed] [Google Scholar]
- 22.Mooren FC, Blöming D, Lechtermann A, Lerch MM, Völker K. Lymphocyte apoptosis after exhaustive and moderate exercise. J Appl Physiol (1985) 2002;93:147–53. doi: 10.1152/japplphysiol.01262.2001. [DOI] [PubMed] [Google Scholar]
- 23.Bergquist J, Tarkowski A, Ewing A, Ekman R. Catecholaminergic suppression of immunocompetent cells. Immunol Today. 1998;19:562–7. doi: 10.1016/s0167-5699(98)01367-x. [DOI] [PubMed] [Google Scholar]
- 24.Liu J, Ao W, Zhou J, Luo P, Wang Q, Xiang D. The correlation between PLR-NLR and prognosis in acute myocardial infarction. Am J Transl Res. 2021;13:4892–4899. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


