Abstract
Background: Skin toxicity of varying severity occurs mostly during various courses of chemotherapy. In clinical trials and practice, we have found that both nab-paclitaxel and paclitaxel cause side effects such as rash and pruritus. To further clarify the incidence of rash and pruritus in both, we conducted the present study by a systematic evaluation, the results of which can be used to guide clinical dosing choices. Methods: An electrical search was performed on randomized controlled research trials of nab-paclitaxel and paclitaxel for the treatment of malignancies. The necessary data were extracted, integrated, and analyzed from the included studies by systematic evaluation and meta-analysis, depending on the study design. Further subgroup analyses were performed to explore the incidence of rash and pruritus in nab-paclitaxel and paclitaxel. Results: Eleven studies with a total of 971 patients with malignancy were included. Four studies were application of single-agent nab-paclitaxel compared with paclitaxel, and seven studies were comparative chemotherapy drug combinations. The incidence of rash was higher in all grades of nab-paclitaxel than that in paclitaxel (OR=1.39, CI 95% [1.18-1.62]); the incidence of rash was higher in lower grades of paclitaxel than that in solvent-based paclitaxel (OR=1.31, CI 95% [1.11-1.53]); the incidence of rash was higher in all grades in the single-agent application comparison. The incidence of rash was higher in nab-paclitaxel than that in paclitaxel (OR=1.81, CI 95% [1.26-2.59]); there was no significant difference in the incidence of pruritus between nab-paclitaxel and paclitaxel (OR=1.19, CI 95% [0.88-1.61]). Conclusion: In comparison with paclitaxel, nab-paclitaxel significantly increased the risk of a teething rash. There was a significant risk correlation between nab-paclitaxel and teething rash. Early prevention, identification, and treatment of rash could significantly improve patient’s quality of life and optimize their clinical survival.
Keywords: Paclitaxel, nab-paclitaxel, rash, pruritus, META analysis
Introduction
In 2020, the number of new cancer cases in the world was about 19.29 million, and the growth of cancer will seriously threaten human life and health [1]. Paclitaxel is a kind of chemotherapy drug widely used for cancer treatment. At present, it plays an important role in chemotherapy drugs for breast cancer [2], lung cancer [3], bladder cancer [4], gastric cancer [5], ovarian cancer [6], and other cancers. Paclitaxel is believed to induce mitotic arrest and apoptosis of cancer cells [7]. The specific mechanism of action is to promote microtubule aggregation through tubulin dimer and inhibit microtubule depolymerization to stabilize the microtubule system, thus damaging the mitotic process, leading to cell arrest in G2 or M phase, and ultimately leading to mitotic arrest and apoptosis [8]. However, because of its poor solubility, paclitaxel needs to be stored in absolute ethanol and polyoxyethylene castor oil. The disadvantage of this storage method is that an obvious hypersensitivity reaction is easy to occur after paclitaxel enters the human body [9]. To solve the problem of hypersensitivity caused by paclitaxel, researchers wrapped hydrophobic paclitaxel in human serum albumin nanoparticles, namely nab-paclitaxel [10]. Nab-paclitaxel not only does not require pretreatment, but also enhances the endothelial transport of paclitaxel, increases the concentration of paclitaxel, and better kills tumor cells. In the analysis of different research results, it was found that in the neoadjuvant treatment of breast cancer, compared with paclitaxel, nab-paclitaxel improved the pathological response rate (pCR) and event-free survival rate (EFS) [11,12]. However, the clinical application of nab-paclitaxel still causes some minor or serious side effects [13-15]. Some studies [16] have shown that nab-paclitaxel causes fewer and more serious side effects than paclitaxel, but other studies [17] have shown contrary findings. In our clinical work, we found that both paclitaxel and nab-paclitaxel can cause skin damage, mainly manifested as rash and pruritus, which is a common adverse event in chemotherapy drugs. Skin toxicity rarely causes serious consequences [18]. More serious is that the drug is reduced or even stopped because of the severity of the rash and/or pruritus [19]. In the worst case, death may occur. In the same way, severe rash and pruritus will bring serious psychological burdens to patients, reduce their quality of life, and affect treatment compliance. To further clarify the occurrence of rash and pruritus side effects of paclitaxel and nab-paclitaxel, the META analysis protocol was used to compare the incidence of rash and pruritus between them, which provides a basis for clinical treatment and side effect management.
Methods
Registration
This study is prospectively registered in the PROSPERO Systematic Evaluation Database, No. CRD42021265808.
Search strategy
As of December 2020, we systematically searched the relevant databases: Pubmed, Embase, and Cochrane Library. The search strategy included: paclitaxel, nab-paclitaxel, docetaxel, and other relevant keywords and Mesh terms. A final check was made to ensure that no additional studies were missed. The above process was performed independently by two participants.
Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) Study type: a randomized controlled trial in cancer patients; (2) Study population: patients received paclitaxel-based chemotherapy for all malignancies; (3) Control group: patients received paclitaxel, including paclitaxel and docetaxel; (4) Outcome indicators: number of rashes, low-grade rashes, and pruritus occurring. Ethics committee approval was not required for this study because the meta-analysis was conducted as a secondary statistical study with no direct relationship to the subjects.
The exclusion criteria were as follows: (1) literature related to reviews, conferences, meta-analyses, case reports, animal experiments, and studies that did not match; (2) literature without primary outcome indicators.
Data extraction and quality assessment
Two investigators (Hong-Bo Li and Zhi-Yong Wang) independently read the relevant literature and extracted the data. In case of any disagreement, a third investigator (Wen-Hui Wang) was asked. Whenever possible, the original authors were contacted for additions after missing literature data were identified. In the literature screening process, the title and abstract were screened in the first step to eliminate irrelevant literature; the full text was screened in the second step to determine whether it could be included in this paper. The following information was included for each eligible study: first author, year of publication, country of publication, trial design, sample size, gender, method of medication administration, and outcome indicators.
Literature quality assessment
The Cochrane Risk Assessment Tool was applied to assess the risk of bias in randomized controlled studies to determine if there was an impact on the results. The evaluation phase was assessed independently by two investigators (Hong-Bo Li and Zhi-Yong Wang) and finally compared and charted, with a third investigator (Wen-Hui Wang) requested to assess and negotiate the decision in case of a dispute. The quality of the included studies was evaluated by the Cochrane Collaboration Risk of Bias Tool which assessed the trials from six aspects. Each aspect was ranked as “high risk”, “unclear risk” or “low risk”.
Evaluation indicators
Number of rashes, number of low-grade rashes, number of pruritus.
Statistical analysis
The final included literature was analyzed using Revman 5.3 software, and a random-effects model was used to test for heterogeneity when I2 > 50%, and the odds ratio (OR) and 95% confidence interval (CI) were applied to dichotomous variables.
Study results
Process of literature selection and description of qualified studies
The flow diagram (Figure 1) showed detailed literature search steps. Initially, 1855 relevant literature was screened from 3 databases. 249 duplicate publications were removed using endnote X9 software, leaving 1606 remaining; 1534 publications were excluded from the initial screening by title and abstract reading, leaving 72 remaining; 11 publications were finally included in this study after reading the full text according to the inclusion and exclusion criteria.
Figure 1.
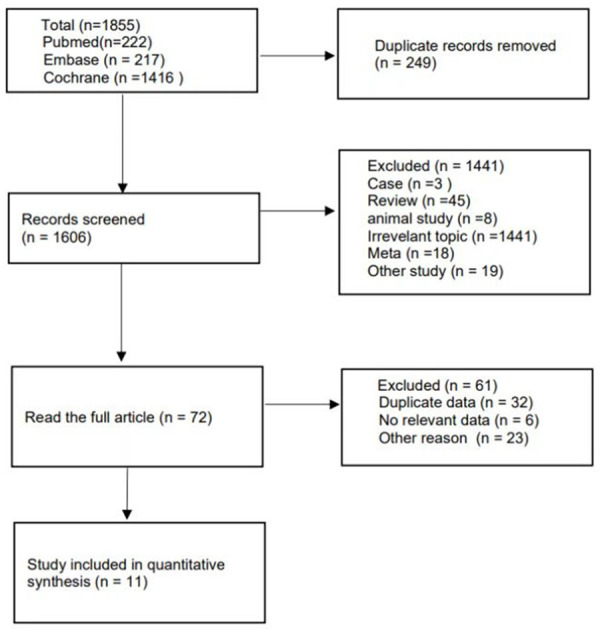
Flow chart of the literature search and selection process in the meta-analysis.
Inclusion of literature
Eleven studies were included as randomized controlled trials (RCT) with a total of 971 patients, 555 patients in the nab-paclitaxel treatment group and 416 patients in the paclitaxel group. There were 7 studies [20-25] on breast cancer, 2 studies on non-small cell lung cancer, 1 study [26] on gastric cancer, and 1 study on uroepithelial cancer. 4 studies [20,24-26] were single-agent nab-paclitaxel compared with paclitaxel, and 4 studies were comparative studies of chemotherapeutic drug combinations. 7 studies [20-26] have been published and the remaining 4 experimental studies are closed and unpublished, with data available in the Cochrane Library search. The main characteristics of the included studies in the meta-analysis are shown in Table 1.
Table 1.
Main characteristics of the included studies
| Author | Year | Gender | Age (y) | Type | Experiment | Events (n) | Control | Events (n) | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| John Pippen | 2006 | F: 197 (100%) | 51.2±9.23 | RCT | AC-albumin-bound paclitaxel + bevacizumab | 28/98 | AC-paclitaxel + bevacizumab | 22/99 | AEs |
| William J. Gradishar | 2006 | F: 150 (100%) | 53.9±10.05 | RCT | Nab-paclitaxel | 5/76 | Docetaxel | 4/74 | ORR |
| NCT00540514 | 2007 | F: 263 (25%) | 59.6±9.33 | RCT | albumin-bound paclitaxel + Carboplatin | 50/514 | paclitaxel + Carboplatin | 43/524 | ORR |
| M: 789 (75%) | |||||||||
| NCT02033993 | 2014 | F: 55 (27.6%) | 67 (24-88) | RCT | Nab-paclitaxel + Platinum | 15/99 | Paclitaxel + Platinum | 20/100 | PFS |
| M: 144 (72.4%) | |||||||||
| NCT02367794 | 2015 | F: 186 (18.2%) | 64.6 (8.6) | RCT | Atezolizumab + Nab-paclitaxel + Carboplatin | 48/334 | Tezolizumab + paclitaxel + Carboplatin | 54/332 | PFS/OS |
| M: 835 (81.8%) | |||||||||
| NCT00785291 | 2008 | F: 788 (98.6%) | NR | RCT | Nab-paclitaxel + bevacizumab | 85/264 | paclitaxel + bevacizumab | 31/272 | PFS |
| M: 11 (1.4%) | |||||||||
| Liang Huang | 2015 | F: 120 (100%) | 49 (29-66) | RCT | Nab-paclitaxel + Carboplatin | 11/30 | Paclitaxel + Carboplatin | 23/90 | pCR |
| Kenji Tamaura | 2017 | F: 200 (100%) | NR | RCT | Nab-paclitaxel | 61/100 | paclitaxel | 50/100 | PFS |
| Zhong-Zhen GUAN | 2009 | F: 210 (100%) | 50 (24-70) | RCT | Nab-paclitaxel | 28/104 | paclitaxel | 10/106 | ORR |
| Kohei Shitara | 2017 | F: 129 (27.0%) | NR | RCT | Nab-paclitaxel | 22/241 | paclitaxel | 16/243 | OS |
| M: 354 (73.0%) | |||||||||
| Michael Untch | 2016 | F: 1206 (100%) | NR | RCT | Nab-paclitaxel-AC/HP | 202/605 | paclitaxel-AC/HP | 143/601 | pCR |
Note: F = female; M = male; NR = not report; RCT: randomized controlled trial; A: epirubicin; C: cyclophosphamide; H: trastuzumab; P: pertuzumab; AEs: adverse events; ORR: overall response rate; PFS: progression-free survival; OS: overall survival; pCR: pathologic complete response.
Incidence of all levels of rash
A total of 971 patients were included in 11 studies, 555 patients in the nab-paclitaxel treatment group and 416 patients in the paclitaxel group. Analysis of the overall rash incidence results showed that nab-paclitaxel rash occurred significantly higher compared to paclitaxel (OR=1.55, CI 95% [1.34-1.80]). See Figure 2. Figure 3 shows the risk of bias in included trials summary. Figure 4 shows the risk of bias in included trials graph. Figure 5 is the funnel plot showing that there was high publication bias. Because I2 > 50%, after excluding 1 literature with I2 < 50% after performing heterogeneity analysis using a random-effects model, the remaining 10 literature studies [20-26] with a total of 855 patients, 470 patients in the nab-paclitaxel treatment group and 385 patients in the paclitaxel group, showed a significantly higher occurrence of nab-paclitaxel rash (OR=1.39, CI 95% [1.18-1.62]). See Figure 6. Figure 7 is the funnel plot showing that there was low publication bias (I2 < 50%).
Figure 2.
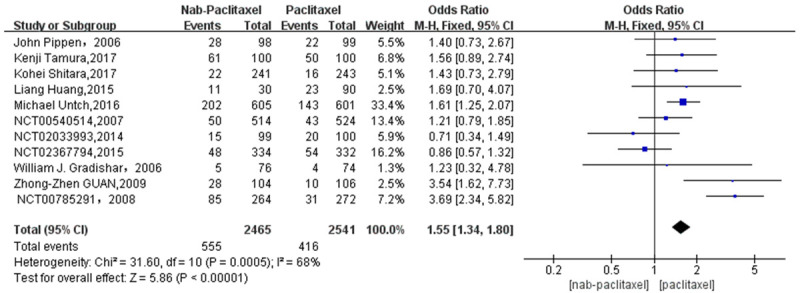
Overall rash incidence results (11 studies).
Figure 3.
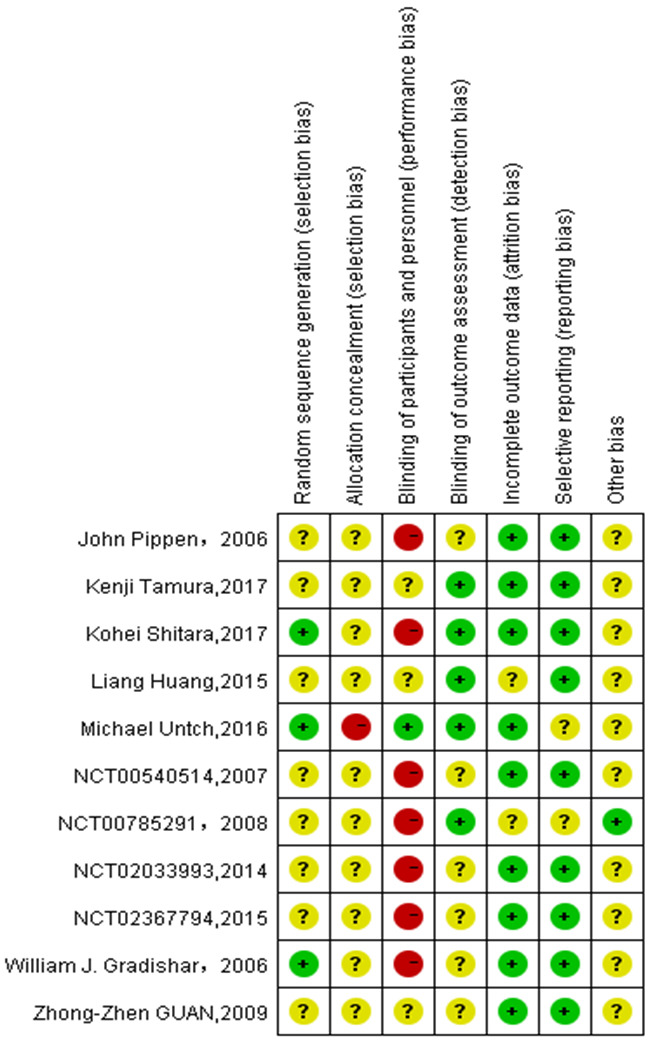
Risk of bias of included trials summary.
Figure 4.
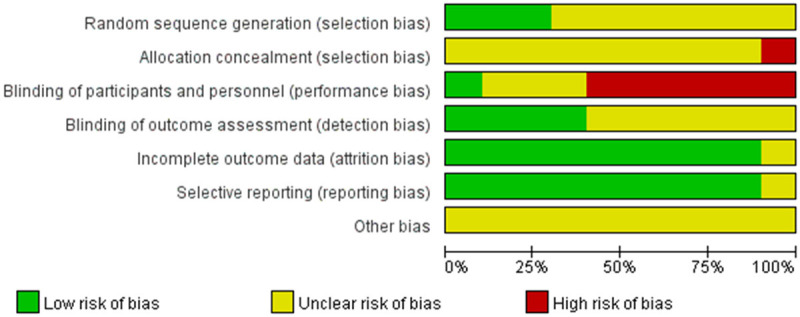
Risk of bias of included trials graph.
Figure 5.
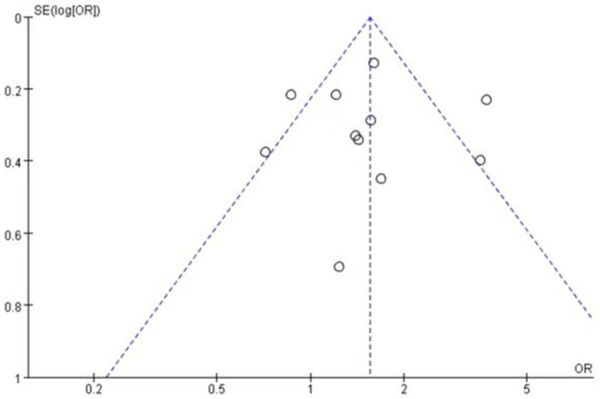
The funnel plot showed that there was high publication bias.
Figure 6.
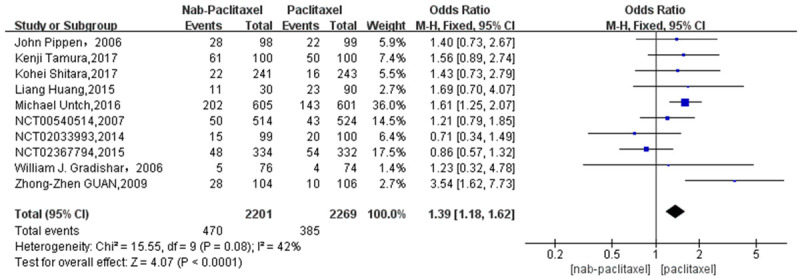
Overall rash incidence results (10 studies).
Figure 7.
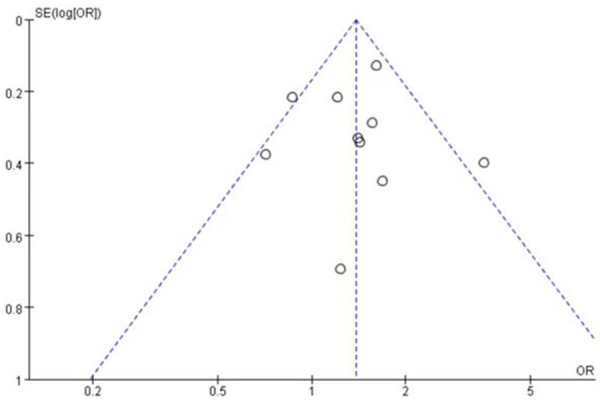
The funnel plot showed that there was low publication bias.
Incidence of low-grade rash
A total of 9 studies [20-24,26] involving 802 patients were studied regarding the incidence of low-grade rash. There were 432 patients in the nab-paclitaxel treatment group and 370 patients in the paclitaxel group. Analysis of low-grade rash incidence results showed that the incidence of low-grade rash was significantly higher in nab-paclitaxel group compared to that in paclitaxel group (OR=1.31, CI 95% [1.11-1.53]). See Figure 8. Figure 9 is the funnel plot showing that there was low publication bias (I2 < 50%).
Figure 8.
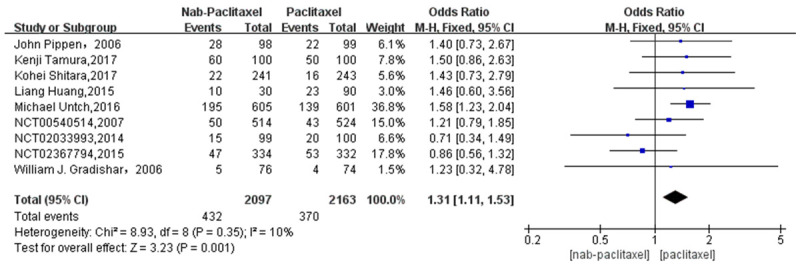
Odds ratio of Nab-paclitaxel to paclitaxel in the treatment of low-grade rash.
Figure 9.
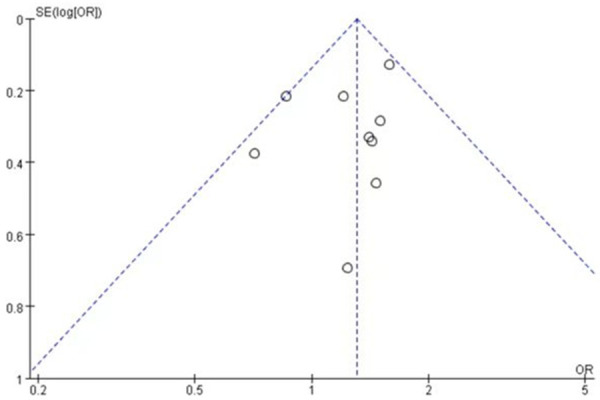
The funnel plot showed that there was low publication bias.
A total of 4 studies [20,24-26] involving 196 patients studied the incidence of rash regarding the comparison of single-agent nab-paclitaxel against paclitaxel. There were 116 patients in the nab-paclitaxel treatment group and 80 patients in the paclitaxel group. Analysis of the single-agent rash incidence results showed that the application of single-agent nab-paclitaxel had a significantly higher rash incidence compared to single-agent paclitaxel (OR=1.81, CI 95% [1.26-2.59]). See Figure 10. Figure 11 is the funnel plot showing that there was low publication bias (I2 < 50%).
Figure 10.
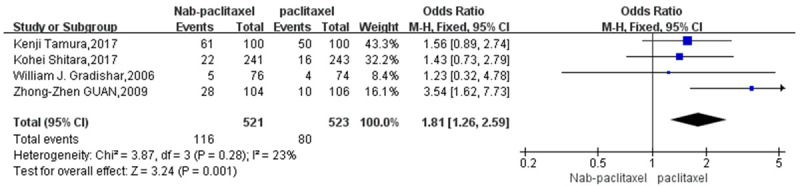
Odds ratio of Nab-paclitaxel to paclitaxel in the treatment of single-agent rash.
Figure 11.
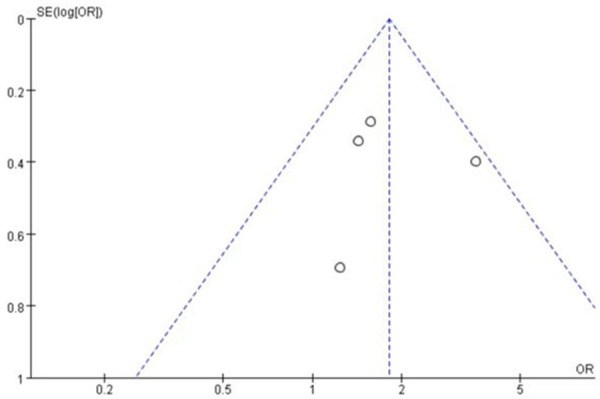
The funnel plot showed that there was low publication bias.
Incidence of pruritus
A total of 7 studies [22,24-26] involving 185 patients were studied regarding the incidence of pruritus. There were 100 patients in the nab-paclitaxel treatment group and 85 patients in the paclitaxel group. Analysis of the overall results regarding pruritus incidence showed no significant difference in the incidence of pruritus between nab-paclitaxel and paclitaxel groups (OR=1.19, CI 95% [0.88-1.61]). See Figure 12. Figure 13 is the funnel plot showing that there was low publication bias (I2 < 50%).
Figure 12.
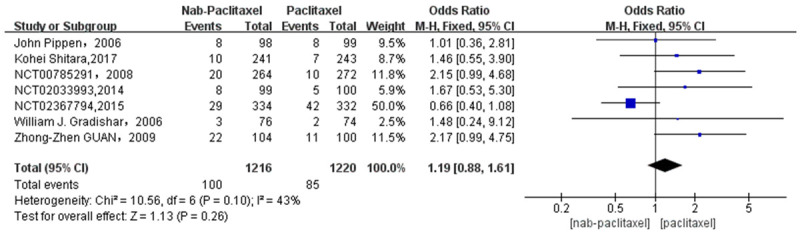
Odds ratio of Nab-paclitaxel to paclitaxel in the treatment of pruritus.
Figure 13.
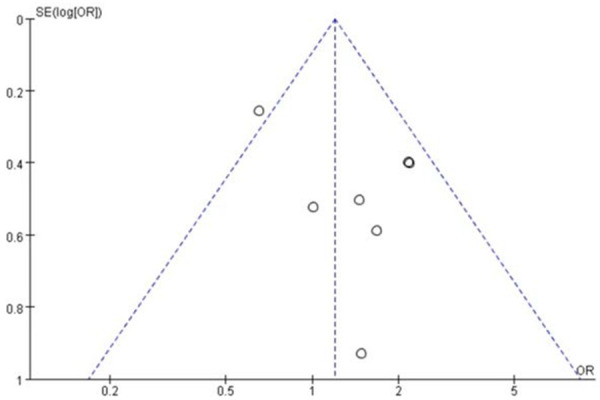
The funnel plot showed that there was low publication bias.
Discussion
In 2020, the data released by the International Agency for Research on Cancer (IARC) shows that nearly 10 million people will die of cancer in the world in 2020, exceeding the population of Hungary. Cancer has always been one of the most serious threats to human survival. In the past decade, chemotherapy has played an important and irreplaceable role in cancer treatment. Chemotherapy can kill local lymph nodes and subclinical occult metastasis in distant organs, but the killing effect of “the enemy is me” will also bring some side effects to the body, and serious side effects may even lead to patient death [27]. Paclitaxel, as one of the important chemotherapeutic drugs, has played an indispensable role in the development of chemotherapeutic drugs in recent decades. nab-paclitaxel has certain advantages over paclitaxel, such as no pretreatment before use, increased paclitaxel concentration in tissues [28], and a strong targeting effect [29]. The advantages of nab-paclitaxel also cause some side effects. We focus on skin toxicity, especially the incidence of skin rash and pruritus. According to the drug design of nab-paclitaxel, allergic reactions such as rash and pruritus will be alleviated. However, in clinical practice, it is still shown in some literature that the incidence of rash in patients using nab-paclitaxel is higher than that of paclitaxel. The most common skin rashes are mostly benign maculopapular, and Stevens-Johnson syndrome, toxic epidermal neurosis and other serious side effects can be seen occasionally. The most serious side effects will lead to patient death [30].
This analysis includes 11 studies, including 7 published literature and 4 completed studies. In this META analysis, we found that in patients with solid malignant tumors, the incidence of rash in patients treated with nab-paclitaxel was significantly higher than that in patients treated with paclitaxel. This is consistent with the results of the latest META analysis, which mainly compares the efficacy and safety of nab-paclitaxel and paclitaxel. It is found that the incidence of rash and pruritus of nab-paclitaxel is higher than that of paclitaxel. In this study, the incidence of low-grade rash also has statistically significant data; however, there is no significant difference in the incidence of grade rash ≥ 3. In 11 studies of all grade rash studies, there is significant heterogeneity because I2 > 50%. We found that, after excluding the corresponding studies one by one, and the study number NCT00785291, I2 changed from 68% to 42%, OR=1.39, CI 95% [1.18-1.62]. After applying the fixed effect model, it was clear that the incidence of nab-paclitaxel rash was still higher than that of paclitaxel. In the study of the incidence rate of skin rashes below grade 3 (< grade 3), the same fixed effect model was applied, and the OR=1.31, CI 95% [1.11-1.53] was obtained. However, in the study of grade rash incidence rate ≥ 3, there is no statistical significance because of the small number of events. To avoid the interference of the combined use of chemotherapy drugs on the study of rash and pruritus, we further extracted a single dose of nab-paclitaxel and paclitaxel for statistics and concluded that the incidence of a rash of a single dose of nab-paclitaxel was significantly higher than that of a single dose of paclitaxel. Similarly, in the META analysis of nab-paclitaxel and paclitaxel for neoadjuvant treatment of breast cancer, it was also found that the incidence of a rash of nab-paclitaxel was high [31]. However, in another META analysis of neoadjuvant therapy for breast cancer, there was no statistical significance between the incidence of rash caused by nab-paclitaxel and traditional paclitaxel [32]. We found that the number of articles included in the two studies was different, and different results were obtained. Our literature included in this paper is high-quality literature. In addition, unfortunately, we have not found a clear mechanism for the rash caused by nab-paclitaxel. Clinicians and some literature have considered that the skin reaction caused by nab-paclitaxel is mediated by an immune mechanism, because the occurrence of skin rash caused by nab-paclitaxel suggests sensitization and specific immune memory, rather than direct drug toxicity [33]. When the drug causes adverse skin reactions, the expression level of inflammatory mediators will be increased by skin infiltrating CD8+ T lymphocytes and NKp46+ cells [34]. However, based on the study and research of relevant literature, the author believes that according to the drug mechanism of paclitaxel, the occurrence of skin toxicity caused by paclitaxel should also consider the causes of paclitaxel’ toxicity, not limited to the immune direction. On the one hand, paclitaxel chemotherapy has a direct cytotoxic effect on basal keratinocytes [35]. It can induce mitochondrial dysfunction of basal epidermal keratinocytes and reduce the repair ability of basal epidermal keratinocytes, leading to rash [36]. On the other hand, after the skin barrier function is damaged, paclitaxel can lead to increased water loss through the epidermis, resulting in low skin water content entering the cuticle, resulting in dry skin and pruritus [37].
The rash usually occurs in warm parts of the body, such as wrinkles, but fortunately, it occurs in the places where the body contacts or rubs with other objects, such as under the pads, underwear friction, etc. [38]. For the above rash-prone areas, it is considered that the rapid renewal of a large number of capillaries and keratinocytes, the abundance of endocrine glands in some areas, the increased drug accumulation per unit area due to more secretion of various chemotherapy drugs, and repeated rubbing or trauma in these areas [39,40]. The occurrence of rash and pruritus usually does not lead to the death of patients, especially when the rash ≤ grade 2 it does not affect the survival time of patients, and only requires relative treatment [41]. However, considering different individual differences, combined with the actual clinical work, it was found that the occurrence of rash had brought more negative psychological effects to patients, and even giving up the chemotherapy program. However, this indirect effect was not reflected in the clinical statistics. Giving up chemotherapy because it affects the quality of life of patients has an indirect impact on the survival time of patients. It is reported that the patient died due to a severe rash, but we have not found relevant information [30].
In this META analysis study involving breast cancer, gastric cancer, non-small cell carcinoma, and uroepithelial cancer, the total incidence of cancer rash was 15.90%, and the incidence of rash for nab-paclitaxel for breast cancer was 33.07%, for non-small cell lung cancer was 11.56%, for uroepithelial cancer was 15.15%, and for gastric cancer was 9.13%. We did not find direct evidence that the difference in the type of cancer affects the incidence of rash. However, it is unreasonable to draw conclusions based on the data in this paper, while the data from the articles included in this paper are more biased. Also, it remains unclear whether gender, ethnicity, dose size, and regimen type affect rash occurrence. Some studies have demonstrated a higher incidence of rash in the analyzed Asian population [42].
In the seven studies examining nab-paclitaxel and paclitaxel regarding pruritic side effects, we applied a fixed-effects model and came up with an OR=1.19, CI 95% [0.88-1.61], P=0.26, which did not show statistical significance. This demonstrates that the incidence of pruritus with nab-paclitaxel approximates the incidence with paclitaxel. Further exploring the incidence of > 3-grade pruritus is not addressed here because the data are less statistically significant.
The present study was first conducted based on the good efficacy of nab-paclitaxel in chemotherapy, and after finding the high incidence of rash with the application of nab-paclitaxel, clinicians were advised to choose the effective chemotherapy drugs for treatment according to the different economic conditions of patients and their acceptance of drug side effects. The study also has some limitations: (1) The sample size of some of the included clinical studies was small, lacking large-scale data support, and some of the randomized controlled studies were non-double-blind trials, which led to a large bias in the risk assessment of the literature; (2) The paclitaxel treatment group involved two drugs, paclitaxel, and docetaxel, which are similar in terms of drug principles, but whether they affect the incidence of rash and pruritus in the study is not sure; (3) In the included literature, we are not sure whether the application of the combination of chemotherapeutic agents affects the incidence of side effects. Although our study showed a significantly higher incidence of rash with single-agent nab-paclitaxel application than with paclitaxel, the interfering nature of the other drugs in combination is unknown to us.
Conclusion
In conclusion, compared to nab-paclitaxel, rash incidence with paclitaxel was significantly higher, both in all grades of rash and in lower grades. Also, single chemotherapeutic drug application showed a high rash incidence with nab-paclitaxel. In addition, our study did not find any statistical difference in the incidence of pruritus between nab-paclitaxel and paclitaxel. We hope that the above study will serve as a guide for clinicians that other factors should be taken into account when selecting appropriate chemotherapeutic agents. Early intervention and timely treatment should be provided in case of side effects to avoid the serious problem of patients giving up chemotherapy because of the occurrence of low-grade side effects, which can affect patient prognosis.
Disclosure of conflict of interest
None.
References
- 1.Erratum: global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2020;70:313. doi: 10.3322/caac.21609. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg SM, O’Neill A, Sepucha K, Miller KD, Dang CT, Northfelt DW, Sledge GW, Schneider BP, Partridge AH. Quality of life following receipt of adjuvant chemotherapy with and without bevacizumab in patients with lymph node-positive and high-risk lymph node-negative breast cancer. JAMA Netw Open. 2022;5:e220254. doi: 10.1001/jamanetworkopen.2022.0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sugawara S, Lee JS, Kang JH, Kim HR, Inui N, Hida T, Lee KH, Yoshida T, Tanaka H, Yang CT, Nishio M, Ohe Y, Tamura T, Yamamoto N, Yu CJ, Akamatsu H, Namba Y, Sumiyoshi N, Nakagawa K. Nivolumab with carboplatin, paclitaxel, and bevacizumab for first-line treatment of advanced nonsquamous non-small-cell lung cancer. Ann Oncol. 2021;32:1137–1147. doi: 10.1016/j.annonc.2021.06.004. [DOI] [PubMed] [Google Scholar]
- 4.van der Heijden MS, Loriot Y, Durán I, Ravaud A, Retz M, Vogelzang NJ, Nelson B, Wang J, Shen X, Powles T. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma: a long-term overall survival and safety update from the phase 3 IMvigor211 clinical trial. Eur Urol. 2021;80:7–11. doi: 10.1016/j.eururo.2021.03.024. [DOI] [PubMed] [Google Scholar]
- 5.Tsuburaya A, Yoshida K, Kobayashi M, Yoshino S, Takahashi M, Takiguchi N, Tanabe K, Takahashi N, Imamura H, Tatsumoto N, Hara A, Nishikawa K, Fukushima R, Nozaki I, Kojima H, Miyashita Y, Oba K, Buyse M, Morita S, Sakamoto J. Sequential paclitaxel followed by tegafur and uracil (UFT) or S-1 versus UFT or S-1 monotherapy as adjuvant chemotherapy for T4a/b gastric cancer (SAMIT): a phase 3 factorial randomised controlled trial. Lancet Oncol. 2014;15:886–93. doi: 10.1016/S1470-2045(14)70025-7. [DOI] [PubMed] [Google Scholar]
- 6.Gershenson DM, Miller A, Brady WE, Paul J, Carty K, Rodgers W, Millan D, Coleman RL, Moore KN, Banerjee S, Connolly K, Secord AA, O’Malley DM, Dorigo O, Gaillard S, Gabra H, Slomovitz B, Hanjani P, Farley J, Churchman M, Ewing A, Hollis RL, Herrington CS, Huang HQ, Wenzel L, Gourley C. Trametinib versus standard of care in patients with recurrent low-grade serous ovarian cancer (GOG 281/LOGS): an international, randomised, open-label, multicentre, phase 2/3 trial. Lancet. 2022;399:541–553. doi: 10.1016/S0140-6736(21)02175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4:253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 8.Chen Q, Xu S, Liu S, Wang Y, Liu G. Emerging nanomedicines of paclitaxel for cancer treatment. J Control Release. 2022;342:280–294. doi: 10.1016/j.jconrel.2022.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Lenz HJ. Management and preparedness for infusion and hypersensitivity reactions. Oncologist. 2007;12:601–609. doi: 10.1634/theoncologist.12-5-601. [DOI] [PubMed] [Google Scholar]
- 10.Hudis CA. The current state of adjuvant therapy for breast cancer: focus on paclitaxel. Semin Oncol. 1999;26(Suppl 2):1–5. [PubMed] [Google Scholar]
- 11.Liu M, Liu S, Yang L, Wang S. Comparison between nab-paclitaxel and solvent-based taxanes as neoadjuvant therapy in breast cancer: a systematic review and meta-analysis. BMC Cancer. 2021;21:118. doi: 10.1186/s12885-021-07831-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zong Y, Wu J, Shen K. Nanoparticle albumin-bound paclitaxel as neoadjuvant chemotherapy of breast cancer: a systematic review and meta-analysis. Oncotarget. 2017;8:17360–17372. doi: 10.18632/oncotarget.14477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caiado J, Picard M. Diagnostic tools for hypersensitivity to platinum drugs and taxanes: skin testing, specific IgE, and mast cell/basophil mediators. Curr Allergy Asthma Rep. 2014;14:451. doi: 10.1007/s11882-014-0451-7. [DOI] [PubMed] [Google Scholar]
- 14.de Leon MC, Bolla S, Greene B, Hutchinson L, Del Priore G. Successful treatment with nab-paclitaxel after hypersensitivity reaction to paclitaxel and docetaxel. Gynecol Oncol Case Rep. 2013;5:70–71. doi: 10.1016/j.gynor.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elzoghby AO, Samy WM, Elgindy NA. Albumin-based nanoparticles as potential controlled release drug delivery systems. J Control Release. 2012;157:168–182. doi: 10.1016/j.jconrel.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 16.Lee H, Park S, Kang JE, Lee HM, Kim SA, Rhie SJ. Efficacy and safety of nanoparticle-albumin-bound paclitaxel compared with solvent-based taxanes for metastatic breast cancer: a meta-analysis. Sci Rep. 2020;10:530. doi: 10.1038/s41598-019-57380-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan H, Hu J, Liu S. Efficacy and safety of nanoparticle albumin-bound paclitaxel in non-small cell lung cancer: a systematic review and meta-analysis. Artif Cells Nanomed Biotechnol. 2019;47:268–277. doi: 10.1080/21691401.2018.1552595. [DOI] [PubMed] [Google Scholar]
- 18.Salloum A, Habre M, Chebl JA, Chebl KA, Atallah C, Medawar G, Kourie HR. Dermatological adverse events associated with immune checkpoint inhibitor-based combinations of anticancer therapies: a systematic review. Immunotherapy. 2022;14:489–503. doi: 10.2217/imt-2021-0244. [DOI] [PubMed] [Google Scholar]
- 19.Loureiro A, Azoia NG, Gomes AC, Cavaco-Paulo A. Albumin-based nanodevices as drug carriers. Curr Pharm Des. 2016;22:1371–90. doi: 10.2174/1381612822666160125114900. [DOI] [PubMed] [Google Scholar]
- 20.Tamura K, Inoue K, Masuda N, Takao S, Kashiwaba M, Tokuda Y, Iwata H, Yamamoto N, Aogi K, Saeki T, Nakayama T, Sato N, Toyama T, Ishida T, Arioka H, Saito M, Ohno S, Yamauchi H, Yamada K, Watanabe J, Ishiguro H, Fujiwara Y. Randomized phase II study of nab-paclitaxel as first-line chemotherapy in patients with HER2-negative metastatic breast cancer. Cancer Sci. 2017;108:987–994. doi: 10.1111/cas.13221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang L, Chen S, Yao L, Liu G, Wu J, Shao Z. Phase II trial of weekly nab-paclitaxel and carboplatin treatment with or without trastuzumab as nonanthracycline neoadjuvant chemotherapy for locally advanced breast cancer. Int J Nanomedicine. 2015;10:1969–1975. doi: 10.2147/IJN.S77000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pippen J, Paul D, Vukelja S, Clawson A, Iglesias J. Dose-dense doxorubicin and cyclophosphamide followed by dose-dense albumin-bound paclitaxel plus bevacizumab is safe as adjuvant therapy in patients with early stage breast cancer. Breast Cancer Res Treat. 2011;130:825–831. doi: 10.1007/s10549-011-1678-9. [DOI] [PubMed] [Google Scholar]
- 23.Untch M, Jackisch C, Schneeweiss A, Conrad B, Aktas B, Denkert C, Eidtmann H, Wiebringhaus H, Kümmel S, Hilfrich J, Warm M, Paepke S, Just M, Hanusch C, Hackmann J, Blohmer JU, Clemens M, Darb-Esfahani S, Schmitt WD, Dan Costa S, Gerber B, Engels K, Nekljudova V, Loibl S, von Minckwitz G German Breast Group (GBG); Arbeitsgemeinschaft Gynäkologische Onkologie-Breast (AGO-B) Investigators. Nab-paclitaxel versus solvent-based paclitaxel in neoadjuvant chemotherapy for early breast cancer (GeparSepto-GBG 69): a randomised, phase 3 trial. Lancet Oncol. 2016;17:345–356. doi: 10.1016/S1470-2045(15)00542-2. [DOI] [PubMed] [Google Scholar]
- 24.Gradishar WJ, Krasnojon D, Cheporov S, Makhson AN, Manikhas GM, Clawson A, Bhar P, McGuire JR, Iglesias J. Phase II trial of nab-paclitaxel compared with docetaxel as first-line chemotherapy in patients with metastatic breast cancer: final analysis of overall survival. Clin Breast Cancer. 2012;12:313–321. doi: 10.1016/j.clbc.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 25.Guan ZZ, Li QL, Feng F, Jiang Z, Shen Z, Yu S, Fen J, Huang J, Yao Z, Bhar P. Superior efficacy of a Cremophor-free albumin-bound paclitaxel compared with solvent-based paclitaxel in Chinese patients with metastatic breast cancer. Asia Pac J Clin Oncol. 2009;5:165–174. [Google Scholar]
- 26.Shitara K, Takashima A, Fujitani K, Koeda K, Hara H, Nakayama N, Hironaka S, Nishikawa K, Makari Y, Amagai K, Ueda S, Yoshida K, Shimodaira H, Nishina T, Tsuda M, Kurokawa Y, Tamura T, Sasaki Y, Morita S, Koizumi W. Nab-paclitaxel versus solvent-based paclitaxel in patients with previously treated advanced gastric cancer (ABSOLUTE): an open-label, randomised, non-inferiority, phase 3 trial. Lancet Gastroenterol Hepatol. 2017;2:277–287. doi: 10.1016/S2468-1253(16)30219-9. [DOI] [PubMed] [Google Scholar]
- 27.Taylor C, Meisel J, Kalinsky K. Are we closer to being able to select patients with node-positive hormone receptor-positive breast cancer who can safely omit chemotherapy? Ther Adv Med Oncol. 2022;14:17588359221084769. doi: 10.1177/17588359221084769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen N, Brachmann C, Liu X, Pierce DW, Dey J, Kerwin WS, Li Y, Zhou S, Hou S, Carleton M, Klinghoffer RA, Palmisano M, Chopra R. Albumin-bound nanoparticle (nab) paclitaxel exhibits enhanced paclitaxel tissue distribution and tumor penetration. Cancer Chemother Pharmacol. 2015;76:699–712. doi: 10.1007/s00280-015-2833-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Jong WH, Borm PJ. Drug delivery and nanoparticles: applications and hazards. Int J Nanomedicine. 2008;3:133–149. doi: 10.2147/ijn.s596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Abraxane Prescribing Information (2014) (Accessed January 3, 2014, at http://abraxane.com/downloads/Abraxane_PrescribingInformation.pdf.)
- 31.Li Y, Lu X, Lin Q, Li W. Is nab-paclitaxel better than conventional taxanes as neoadjuvant therapy for breast cancer? A meta-analysis. J Int Med Res. 2020;48:300060520943473. doi: 10.1177/0300060520943473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu M, Liu S, Yang L, Wang S. Comparison between nab-paclitaxel and solvent-based taxanes as neoadjuvant therapy in breast cancer: a systematic review and meta-analysis. BMC Cancer. 2021;21:118. doi: 10.1186/s12885-021-07831-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roujeau JC. Immune mechanisms in drug allergy. Allergol Int. 2006;55:27–33. doi: 10.2332/allergolint.55.27. [DOI] [PubMed] [Google Scholar]
- 34.Schlapbach C, Zawodniak A, Irla N, Adam J, Hunger RE, Yerly D, Pichler WJ, Yawalkar N. NKp46+ cells express granulysin in multiple cutaneous adverse drug reactions. Allergy. 2011;66:1469–76. doi: 10.1111/j.1398-9995.2011.02677.x. [DOI] [PubMed] [Google Scholar]
- 35.Stravodimou A, Voutsadakis IA. Hand and foot syndrome associated with docetaxel treatment. Acta Oncol. 2012;51:554–6. doi: 10.3109/0284186X.2011.636755. [DOI] [PubMed] [Google Scholar]
- 36.Cirrincione AM, Pellegrini AD, Dominy JR, Benjamin ME, Utkina-Sosunova I, Lotti F, Jergova S, Sagen J, Rieger S. Paclitaxel-induced peripheral neuropathy is caused by epidermal ROS and mitochondrial damage through conserved MMP-13 activation. Sci Rep. 2020;10:3970. doi: 10.1038/s41598-020-60990-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fabbrocini G, Cameli N, Romano MC, Mariano M, Panariello L, Bianca D, Monfrecola G. Chemotherapy and skin reactions. J Exp Clin Cancer Res. 2012;31:50. doi: 10.1186/1756-9966-31-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sibaud V, Leboeuf NR, Roche H, Belum VR, Gladieff L, Deslandres M, Montastruc M, Eche A, Vigarios E, Dalenc F, Lacouture ME. Dermatological adverse events with taxane chemotherapy. Eur J Dermatol. 2016;26:427–443. doi: 10.1684/ejd.2016.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corazza M, Minghetti S, Borghi A, Virgili A, Ballardini P. Hand-foot syndrome caused by docetaxel with no recurrence after switch to paclitaxel, a different taxane. Int J Dermatol. 2014;53:e180–2. doi: 10.1111/j.1365-4632.2012.05782.x. [DOI] [PubMed] [Google Scholar]
- 40.Harris CS, Wang D, Carulli A. Docetaxel-associated palmar-plantar erythrodysesthesia: a case report and review of the literature. J Oncol Pharm Pract. 2014;20:73–80. doi: 10.1177/1078155213475466. [DOI] [PubMed] [Google Scholar]
- 41.Ianoşi SL, Batani A, Ilie MA, Tampa M, Georgescu SR, Zurac S, Boda D, Ianosi NG, Neagoe D, Calina D, Tutunaru C, Constantin C. Non-invasive imaging techniques for the in vivo diagnosis of Bowen’s disease: three case reports. Oncol Lett. 2019;17:4094–4101. doi: 10.3892/ol.2019.10079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takashima S, Kiyoto S, Takahashi M, Hara F, Aogi K, Ohsumi S, Mukai R, Fujita Y. Clinical experience with nanoparticle albumin-bound paclitaxel, a novel taxane anticancer agent, and management of adverse events in females with breast cancer. Oncol Lett. 2015;9:1822–1826. doi: 10.3892/ol.2015.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]


