Abstract
Background
Colorectal cancer screening plays a key role in mitigating morbidity and mortality associated with the disease. Regions such as the Eastern Mediterranean Region experience a particularly large burden of colorectal cancer. While trends have been described at the country level within the region, it is important to understand what barriers exist to colorectal cancer screening, so that more effective interventions can be conceptualized and implemented.
Methods
A scoping review was conducted by applying the Theoretical Domains Framework. The search strategy was conceptualized and implemented by searching two online databases (Scopus and PubMed) that identified papers published between 2000 and 2021 that were available in English and related to colorectal cancer screening in the Eastern Mediterranean Region. Duplicates were removed both automatically by EndNote and manually for those that remained by two members of the research team. Two data collection matrices, constructed according to the Theoretical Domains Framework, were used to extract data on multi-level barriers to screening as perceived by the at-risk population and providers.
Results
Barriers related to colorectal cancer screening were evident at the individual, public, provider, and health system levels. The most noted barriers among both matrices pertained to the domains of knowledge, emotion, environmental context and resources, and beliefs about consequences. At the individual level, knowledge was the most-cited barrier. At the provider and health system levels, knowledge and environmental context and resources were the most-cited barriers, respectively.
Conclusions
In understanding barriers at the individual, provider, and health system levels, more effective interventions can be developed to promote screening and early detection for colorectal cancer.
Keywords: Implementation science, colorectal cancer screening, theoretical domains framework, barriers, Eastern Mediterranean Region, scoping review
Highlight box.
Key findings
• The Knowledge domain emerged as the most-commonly reported barrier to screening in both the patient- and provider-oriented Frameworks. The complexity of screening uptake is further accentuated by way of this scoping review.
What is known and what is new?
• Barriers to colorectal cancer screening have been described previously at length, but the use of the novel Theoretical Domains Framework to sort these factors provides greater granularity to inform current and future screening practices.
What is the implication and what should change now?
• Now that a spectrum of barriers to colorectal cancer screening in the Eastern Mediterranean Region has been described in an aggregative manner, current programming and future initiatives should be refined to account for the multiplicity of factors that may contribute to lack of uptake of such measures.
Introduction
Colorectal cancer (CRC) is a malignancy that often starts as a polyp on the inner lining of the rectum or colon (1). While not all polyps turn into cancer, those that do can create a large burden for the affected individual. The burden of CRC is variable on the global scale, with some regions experiencing a sizable impact from the disease. The Eastern Mediterranean Region (EMR) is one of six World Health Organization (WHO) regions that includes 21 Member States, along with Palestine (Gaza Strip and West Bank) (2). CRC is the second most common cancer in the EMR (3,4) and its incidence is increasing in various countries in the region (5). According to 2020 Global Cancer Observatory (GLOBOCAN) data from the World Health Organization’s (WHO’s) International Agency for Research on Cancer (IARC), age-standardized incidence rates are as high as 18.6 per 100,000 in some populations of the EMR. Further GLOBOCAN 2020 data indicates 5-year prevalence (both sexes, all ages) to be as high as 39.1 per 100,000 within some contexts of the region (4).
In cancerous polyps, disease severity and outcomes can often be mitigated when polyps are detected at earlier stages of development. In these early stages of disease progression, treatments are likely to be more effective and less costly compared to more advanced stages (5). Screening for polyps in the colon and rectum, subsequently, is an effective means to detect CRC and reduce its morbidity, mortality, and cost. Several modalities for screening for CRC exist, including colonoscopy, flexible sigmoidoscopy, stool tests, and CT colonography (6,7).
The growing burden of CRC in the EMR requires a multipronged response that necessarily includes secondary prevention. To be effective, screening and early detection interventions need to be culturally tailored, evidence-based, and informed by theories of behavior and behavior change (8-11). While crucial for successful implementation, changing behavior at the individual level is not an easy task and is often predicated on a series of behaviors and contexts that occupy multiple levels, e.g., the patient, provider, and health system (12). For a behavior such as undergoing screening for colorectal cancer, its multi-level determinants need to be elucidated before interventions aimed at promoting screening are designed.
A novel framework to identify multi-tiered factors that influence behavior is the Theoretical Domains Framework (TDF), a consolidative approach to integrating theories of behavior change that is being used more widely and across a multitude of disciplines (12). The TDF is organized around 14 theoretical domains that serve as foci for assessing problems and barriers relating to implementation. While the TDF, a consolidation of 33 behavioral change theories, has now been used in a multitude of ways, it was originally created for implementation science, specifically for the means of identifying influences on behavior among health professionals (13,14). This original intention for the development of the TDF informs the aims of this paper, which are to: (I) identify barriers to recommending and undergoing colorectal cancer screening among health professionals and the public, respectively, and (II) provide a proof of concept for the utility of the TDF in conjunction with a scoping review to thoroughly identify such barriers from existing studies. We present this article in accordance with the PRISMA-ScR reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-510/rc).
Methods
Study selection
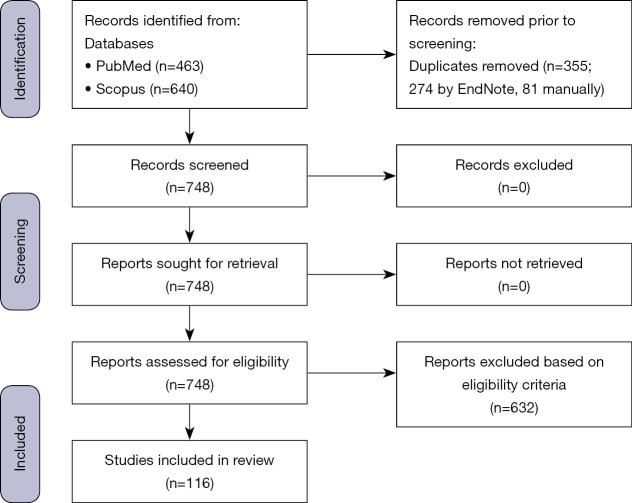
We conducted a scoping review of existing evidence on barriers to colorectal cancer screening in the Eastern Mediterranean Region. No restrictions were placed on the research approach (mixed methods, quantitative, or qualitative research) nor the study design (cross-sectional, cohort, etc.), so long as the paper met the following eligibility criteria: (I) the study setting was one of the countries part of the EMR, (II) the study timeframe was between 2000–2021, (III) the paper focused on CRC screening, and (IV) the paper focused on barriers or determinants of CRC screening. The identified articles were divided among two reviewers who independently determined eligibility. First, the title and abstract of each work was examined to decide if it was eligible or not. In cases where eligibility was questionable, the full paper was read to reach a determination. Such instances were also brought to the attention of the research supervisor to ensure proper designation of papers as to if it met inclusion criteria for this work. The flow chart of selected studies is included in Figure 1. The most recent search was executed on May 15, 2021.
Figure 1.
Flow chart of studies used in the scoping review.
Search strategy
A search strategy was built to identify literature pertaining to CRC screening in the EMR. The overarching search strategy, which was established a priori to data collection, was conducted through two outlets, PubMed and Scopus (Appendices 1 and 2). While terms such as ‘colorectal cancer’ and ’screening’ were used, related terms (as informed by the literature) were similarly included in the search strategy. Terms related to ‘colorectal cancer(s)’ included ‘intestinal neoplasms’, ‘bowel cancer(s)’, ‘rectal cancer(s)’, ‘rectum cancer(s)’, ‘colon cancer(s)’, ‘intestine cancer’, ‘intestinal cancer’, ‘colorectal neoplasms’, ‘colon neoplasms’, ‘rectum neoplasms’, ‘rectal neoplasms’, ‘intestines neoplasms’, and ‘bowel neoplasms’. Additionally, terms related to ’screening’ included ‘prevention and control’, ‘early detection’, ‘early detection of cancer’, ‘campaign’, ‘outreach’, and ‘guideline(s)’. Geographically, the search was limited to the EMR, so the name of each country was included in the search strategy, along with ‘EMRO’ (WHO Regional Office for the Eastern Mediterranean) and ‘Eastern Mediterranean’. In terms of temporality, literature from 2000–2021 was included, and as applicable, this time parameter was included in the text of the search equation (Scopus). In the case of PubMed, a built-in function was used to restrict the time parameters to the 2000–2021 timeframe.
Extraction of data
Informed by the TDF, two data extraction matrices were designed and used to collect and organize data from eligible studies. The first matrix captured the individual/ at-risk population perspective towards barriers and influences to ‘undergoing screening’, categorized according to the 14 domains of the TDF, and aggregated across the individual (self), healthcare provider, and health system levels (Table 1). The second matrix was oriented towards the provider’s perspective and targeted barriers for two separate behaviors, ‘recommending screening’ and ‘conducting screening’. The term ‘provider’ was used to refer to physicians, nurses, pharmacists, and other healthcare workers/providers. Similar to the first matrix, barriers identified by providers were organized around the 14 TDF domains and aggregated across the individual (patient), healthcare provider (self), and health system levels (15,23,26,27,42,48-52) (Table 2). The TDF classifies influencing factors according to 14 domains: knowledge, skills, social/professional role and identity, beliefs about capabilities, optimism, beliefs about consequences, reinforcement, intentions, goals, memory, attention and decision processes, environmental context and resources, social influences, emotion, and behavioral regulation. While each domain is complex, they can be defined simply while articulating associated constructs. For this work, the original definitions and constructs of each domain were used, as defined in the seminal work that validated the TDF’s utility in implementation research (12).
Table 1. Patient-identified barriers to colorectal cancer screening according to the theoretical domains framework in the EMR.
| Domain | Target behavior | Individual-level factors | Provider-level factors | Health system-level factors |
|---|---|---|---|---|
| Knowledge | Barriers to undergoing CRC screening | • Little knowledge of CRC symptoms (15) | • Low awareness and knowledge of CRC, risk factors, symptoms, and associated screening modalities among medical students (26) | • Lack of government awareness campaign (36) |
| • Lack of knowledge about CRC risk factors, the benefits of undergoing screening, and the overall importance of screening (16) | ||||
| • Unaware of CRC symptoms (17) | • Physicians with higher levels of education and qualifications are more likely to recommend CRC screening (35) | |||
| • Had not heard of CRC screening, unaware of different screening methods (18) | ||||
| • Inadequate knowledge of CRC risk factors (19) | ||||
| • Lack of knowledge regarding availability of fecal occult blood test (FOBT) (20) | ||||
| • Little understanding of the causes, symptoms, and screening methods for CRC (21) | ||||
| • Poor awareness of cancer symptoms and signs (22) | ||||
| • Lacking knowledge of CRC and providers (23) | ||||
| • Lack of knowledge regarding screening procedures (24) | ||||
| • Little knowledge of screening procedures (25) | ||||
| • Low awareness and knowledge of CRC, risk factors, symptoms, and associated screening modalities (26) | ||||
| • Lack of sufficient knowledge (27) | ||||
| • Low participant knowledge about colorectal cancer & 80.6% (377/468) of the participants stated that the most important reason for which they did not uptake FOBT (fecal occult blood test) was lack of knowledge (28) | ||||
| • Lack of awareness that CRC is a major cause of mortality (29) | ||||
| • Inadequate awareness of functional health literacy skills (FHLS), limited awareness regarding CRC testing and screening (30) | ||||
| • Lack of education beyond elementary school is a barrier to screening (31,32) | ||||
| • Higher knowledge associated with higher educational level, older age, and having family history of CRC (33) | ||||
| • Low literacy rates (34) | ||||
| Skills | Barriers to undergoing CRC screening | • Low literacy rates (34) | – | – |
| Social/professional role and identity | Barriers to undergoing CRC screening | • Women were more likely to uptake FOBT; individuals with higher levels of education are also more likely to undergo screening (28) | • Male primary healthcare physicians (PHPs) are less likely to recommend screening (35) | – |
| Beliefs about capabilities | Barriers to undergoing CRC screening | • Higher perceived self-efficacy leads to greater participation in CRC screening (28,37) | – | – |
| Beliefs about consequences | Barriers to undergoing CRC screening | • Oblivious to diagnosis is associated with a better quality of life which could lead to a lower likelihood of undergoing screening (31) | – | – |
| • Not at risk due to lack of symptoms, lack of family history of CRC, and having a healthy lifestyle (38) | ||||
| • Absence of clinical symptoms (39) | ||||
| • Patient did not have clinical symptoms or think screening was not needed (34) | ||||
| • Younger people are less likely to undergo screening, potentially due to the fact that they think they are at lower risk (28) | ||||
| • Absence of clinical symptoms (40) | ||||
| • Patient self-perception as immune to developing CRC (41) | ||||
| • Patient underestimation of CRC risk (33,42) | ||||
| • Are not feeling sick, so less reason to get screened (43) | ||||
| Optimism | Barriers to undergoing CRC screening | • Positive attitude towards FOBT uptake is a strong predictor towards screening (28) | – | – |
| Reinforcement | Barriers to undergoing CRC screening | • Lack of physician’s recommendation to undergo screening (31) • Non-acceptability of colonoscopy without sedation (44) |
• Lack of reminders by healthcare workers (38) • Lack of physician recommendation (20) • Lack of physician recommendation (34) • Not being recommended by their (patients’) doctor to get screening (43,45) |
– |
| Intentions | Barriers to undergoing CRC screening | • Low priority of health (21) | – | – |
| • Distrust of Western medicine (31) | ||||
| Goals | Barriers to undergoing CRC screening | – | – | – |
| Memory, attention, and decision processes | Barriers to undergoing CRC screening | – | • Lack of reminders by healthcare workers (38) | – |
| Environmental context and resources | Barriers to undergoing CRC screening | • Too busy to go to the doctor (17) | • Poor physician-patient relationships and overall distrust of physicians (21) | • Difficult to make an appointment, difficulty in arranging transport (17) |
| • Lack of time (38) | • Cost of tests, inadequate insurance coverage, and medical tariffs; mistrust in health care system (21) | |||
| • Time (46) | • Low socio-economic status, especially in rural areas; cost of test (34) | |||
| • Urban residents are more likely to be screened for CRC; distrust of Western medicine and religious objection (31) | • Low socio-economic status (47) | |||
| • Belief of religious protection against CRC (God’s control of fate and destiny) (33) | • Screening procedures are too expensive, lack of screening facilities (24) | |||
| • Cost (40,48) | ||||
| • Lack of government-level CRC screening programs and awareness campaigns (36) | ||||
| Social influences | Barriers to undergoing CRC screening | • Low social support (46) | – | – |
| Emotion | Barriers to undergoing CRC screening | • Fear of endoscopic procedures; weary of test being performed by a HCP that is not the same sex as the patient (15) | – | – |
| • Scared and embarrassed to undergo screening (17) | ||||
| • Embarrassed by the idea of a colonoscopy, fear of positive diagnosis of CRC (18) | ||||
| • Fear of undergoing screening and results (39) | ||||
| • Fear of painful colonoscopy procedures (20) | ||||
| • High degree of anxiety associated with cancer detection, as well as anticipated embarrassment from undergoing screening (21) | ||||
| • Fear of advanced CRC and the screening test (34) | ||||
| • Shyness and fear of screening results (23) | ||||
| • Fear of finding CRC, anxiety of screening procedures (25) | ||||
| • Bad feeling (46) | ||||
| • Fear of positive results and shame (40) | ||||
| • Patient fear of finding out they have cancer (42) | ||||
| • Finding the test to be embarrassing (31) | ||||
| • Fear of results (36) | ||||
| Behavioral regulation | Barriers to undergoing CRC screening | – | – | – |
EMR, Eastern Mediterranean Region; CRC, colorectal cancer.
Table 2. Provider-identified barriers to colorectal cancer screening according the theoretical domains framework in the EMR.
| Domain | Target | Patient-level Factors | Provider-level Factors | Health System-level Factors |
|---|---|---|---|---|
| Knowledge | Barriers to recommending CRC screening | Lack of CRC awareness and related screening modalities (49) | Unawareness of symptoms of CRC (15) | Inadequate training for laboratory technicians and providers (50) |
| Lack of public awareness, signs, and symptoms (23) | Belief that only high-risk patients should be screened (50) | |||
| Low awareness and knowledge of CRC, risk factors, symptoms, and associated screening modalities (26) | ||||
| Barriers to conducting CRC screening | Lack of sufficient knowledge (27) | Lack of sufficient knowledge (27) | Lack of hospital policy or protocols for cancer screening, shortage of trained HCPs (Health Care Providers) to conduct CRC screening or to follow up with invasive procedures, limited availability of screening services, and long waiting time for screening appointments (51) | |
| Lack of awareness of CRC tests (51) | HCPs (Health Care Providers) are not knowledgeable about CRC screening recommendations (42) | |||
| Poor knowledge of who should receive CRC screening and the frequency of screening (48) | ||||
| Skills | Barriers to recommending CRC screening | – | Inability to identify correct screening tests (52) | Inadequate training for laboratory technicians and providers (50) |
| Barriers to conducting CRC screening | – | – | – | |
| Social/professional role and identity | Barriers to recommending CRC screening | – | – | – |
| Barriers to conducting CRC screening | – | – | – | |
| Beliefs about capabilities | Barriers to recommending CRC screening | – | Lack of confidence in providers to perform and interpret screening test appropriately (50) | – |
| Barriers to conducting CRC screening | – | – | – | |
| Beliefs about consequences | Barriers to recommending CRC screening | – | – | – |
| Barriers to conducting CRC screening | – | – | – | |
| Optimism | Barriers to recommending CRC screening | – | – | – |
| Barriers to conducting CRC screening | – | – | – | |
| Reinforcement | Barriers to recommending CRC screening | – | Lack of emphasis on prevention (50) | – |
| Barriers to conducting CRC screening | – | – | – | |
| Intentions | Barriers to recommending CRC screening | – | Lack of emphasis on prevention (50) | – |
| Barriers to conducting CRC screening | – | – | – | |
| Goals | Barriers to recommending CRC screening | – | – | – |
| Barriers to conducting CRC screening | – | – | – | |
| Memory, attention, and decision processes | Barriers to recommending CRC screening | – | – | – |
| Barriers to conducting CRC screening | – | – | – | |
| Environmental context and resources | Barriers to recommending CRC screening | Socioeconomic status (50) | Inadequate training for providers (50) | Inadequate training for laboratory technicians, cost (50) |
| Barriers to conducting CRC screening | – | – | Shortage of specialized healthcare providers (49) | |
| Lack of hospital policy or protocols for cancer screening, shortage of trained HCPs (Health Care Providers) to conduct CRC screening or to follow up with invasive procedures, limited availability of screening services, and long waiting time for screening appointments (51) | ||||
| Social influences | Barriers to recommending CRC screening | – | – | – |
| Barriers to conducting CRC screening | – | – | – | |
| Emotion | Barriers to recommending CRC screening | Fear of painful procedures (23) | Fear (51) | – |
| Barriers to conducting CRC screening | – | – | – | |
| Behavioral regulation | Barriers to recommending CRC screening | – | – | – |
| Barriers to conducting CRC screening | – | – | – |
EMR, Eastern Mediterranean Region; CRC, colorectal cancer.
Knowledge was defined as awareness regarding the existence of something, and associated constructs include knowledge of task environment and procedural knowledge. Skills were defined as proficiency or ability that is acquired through practice. Associated constructs included competence, ability, practice, and skill assessment, among others. Social/professional role and identity was defined as a cogent set of personal qualities and behaviors of an individual that are displayed in a work or social setting. Related constructs included leadership, identity, organizational commitment, professional boundaries and confidence, and group identity. The beliefs about capabilities domain were defined as the acceptance of the reality, truth, or validity of a talent, ability, or facility that a person can put to use in a constructive way. Professional confidence, self-esteem, perceived behavioral control, and self-confidence were some of the constructs associated with this domain (12).
The optimism domain was defined as confidence that desired goals will be attained or that things will happen for the best, and constructs for this domain included identity, pessimism, and optimism. The beliefs about consequences domain were defined as the acceptance of the reality, truth, or validity about outcomes of a behavior in a given situation. Constructs associated with this domain were outcome expectancies, consequents, anticipated regret, and beliefs. Reinforcement was defined in the seminal work as the resulting increased probability of a response, due to the coordinating of a contingency or dependent relationship between the response and stimulus. Constructs for this domain included rewards, contingencies, sanctions, punishment, and incentives. Intentions were defined as the conscious decision to resolve to act in a particular way or to perform a certain behavior. Stability of intentions, stages of change model, and the transtheoretical model for stages of change were constructs associated with this domain (12).
The domain of goals was defined as mental representations of end states of outcomes that an individual desires to achieve. Constructs related to this domain include goals, target setting, implementation intention, and action planning. The memory, attention, and decision processes domain were defined as the ability to retain information, selectively focus on certain aspects of the environment, and choose between alternatives. Related constructs included tiredness, cognitive overload, attention, attention control, memory, and decision making. Environmental context and resources were defined as any circumstance of an individual’s environment or situation that modifies social competence, independence, adaptive behavior, and skills and abilities. Related constructs include environmental stressors, salient events and critical incidents, barriers and facilitators, organizational climate/culture, and resources. Social influences were defined as interpersonal processes that cause a change in thought, feeling, or behavior for an individual. Constructs such as modeling, group identity, social norms and social pressure, power, and intergroup conflict, among others, were associated with this domain (12).
The domain of emotion was defined as a complex pattern of reaction that involves behavioral, physiological, and experiential elements, in which an individual tries to deal with an event or matter that is personally significant. Fear, anxiety, affect (positive or negative), stress, and burn-out were all associated with emotion. Behavioral regulation, defined as anything seeking to manage or objectively change observed or measured actions, has several constructs associated with it, including action planning, breaking habit, and self-monitoring (12).
Data were extracted from the literature according to the 2 matrices. Data extraction was highly granular and included quotes and verbatim descriptions of identified barriers (Tables 1,2). The search strategy is summarized in Table 3.
Table 3. The search strategy summary.
| Items | Specification |
|---|---|
| Date of Search (specified to date, month and year) | 15 May 2021 |
| Databases and other sources searched | PubMed and Scopus |
| Search items used (including MeSH and free text search terms and filters) | Reference Search strategy, Lines 129−143; Appendices 1,2 |
| Timeframe | 1 January 2000−14 May 2021 |
| Inclusion and exclusion criteria (study type, language restrictions etc.) | Inclusion criteria: (I) the study setting was one of the countries part of the Eastern Mediterranean Region (as defined by the World Health Organization), (II) the study timeframe was between 2000-2021, (III) the paper focused on colorectal cancer screening, and (IV) the paper focused on barriers or determinants of colorectal cancer screening |
| No restrictions were placed on research approach (mixed methods, quantitative, or qualitative research), nor the study design (cross-sectional, cohort, etc.) | |
| Selection process (who conducted the selection, whether it was conducted, how consensus was obtained, etc.) | The study selection process was conducted by CH and NC, both of whom were supervised by GNF. Consensus was obtained by bringing studies in question to the group and deciding on their inclusion/exclusion by a majority vote |
| Any additional considerations, if applicable | N/A |
Data validation
Through the whole process, from study selection to data extraction and synthesis, several mechanisms were in place to promote validity and consistency. As noted above, two reviewers worked independently to extract data according to the TDF matrices and to determine the eligibility of papers that were yielded from the application of the search strategy. Each reviewer was in charge of a subset of the articles. Uncertainty about eligibility or categorization of extracted data was brought to the rest of the research team for discussion, with the research supervisor making a final determination. Additionally, the independent researchers cross-checked the data extraction of one another. Any discrepancies in applying the TDF matrix were brought for discussion by the full research team.
Synthesis of data
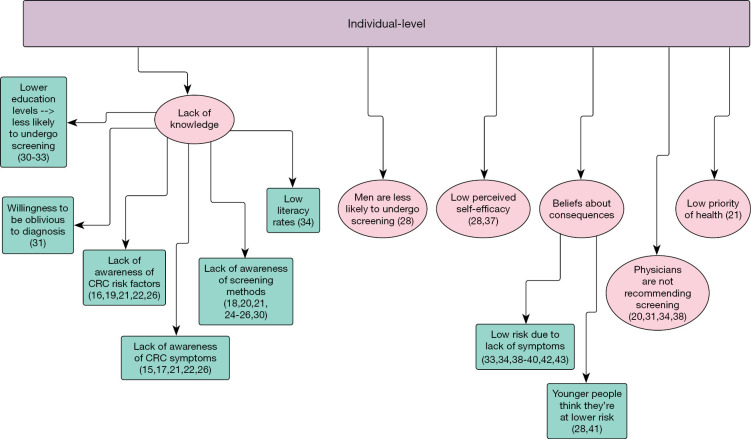
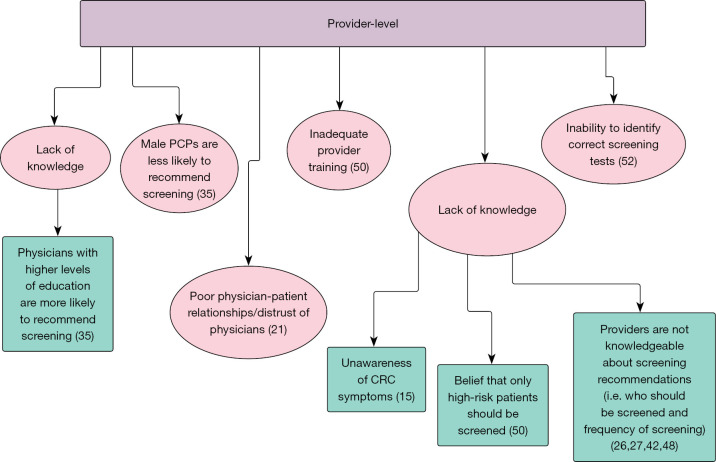
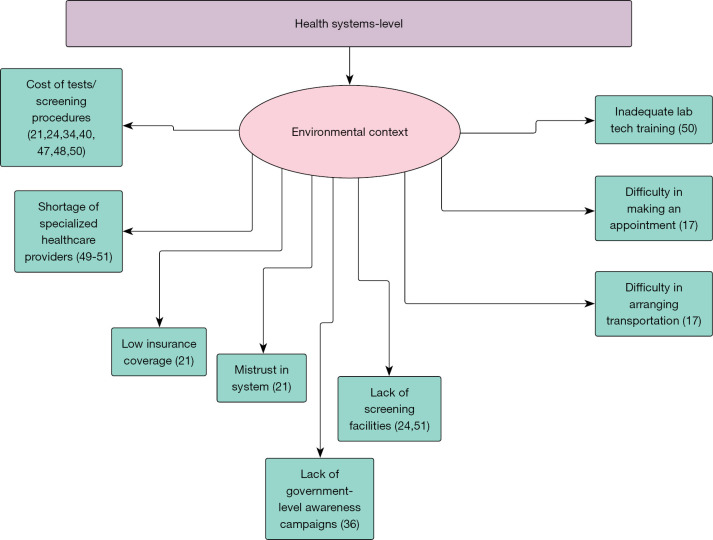
Extracted data, in granular verbatim form, were placed in the respective level (individual, provider, health system) and TDF domain of the applicable matrix. Domains not covered in the literature were designated as gaps in the literature and foundations for future work. In addition to granular data extraction, we further synthesized the data by collapsing identified factors that bear a similar meaning under common themes (Figures 2-4).
Figure 2.
Barriers to colorectal cancer screening in the Eastern Mediterranean Region: individual level (15-22,24-26,28,30-34,37-43).
Figure 3.
Barriers to colorectal cancer screening in the Eastern Mediterranean Region: provider level (15,21,26,27,35,42,48,50,52).
Figure 4.
Barriers to colorectal cancer screening in the Eastern Mediterranean Region: health systems level (17,21,24,34,36,40,47-51).
Results
Descriptive results
From 1,103 pieces of literature, 116 papers were eligible for data extraction (Figure 1). Studies were excluded for a variety of reasons, including not being in the English language, not primarily reporting on barriers, perceptions, etc., and not focusing on one of the populations of focus, among other reasons. Overall, the papers varied in their study location throughout the EMR (Table 4). Additionally, they varied in study type, and focused to different degrees on barriers to CRC screening as a primary aim. For the patient-oriented TDF data extraction (Table 1), factors related to 12 of the 14 TDF domains were identified, and the domain with the richest data was ‘Knowledge.’ For the provider-oriented TDF data extraction, factors that influenced barriers to recommending and conducting CRC screening were identified in 7 of 14 domains (Table 2). Yet again the ‘Knowledge’ domain was the domain with the most results (Tables 1,2).
Table 4. Distribution of studies by country and author(s).
| Country | Number of studies: Authors |
|---|---|
| United Arab Emirates | 2 studies: Al Abdouli, Al-Sharbatti |
| Iran | 42 studies: Baghianimoghadam, Besharati, Bidouei, Boogar, Chouhdari, Ghobadi Dashdebi, Gholampour, Jeihooni, Khani Jeihooni, Kharameh, Khashij, Mahdi, Maheri, Majidi, Mansour-Ghanaei, Maserat, Mirzaei, Mirzaei-Alavijeh, Montazeri, Movahedi, Mozafar Saadati, Nikbakht, Niya, Nopour, Pourhoseingholi, Qandian, Rahmati-Najarkolaei, Ramazani, Ramezani, Roshani, Sadeghei, Safaee, Safdari, Salimzadeh, Shiri, Sohrabi, Soodejani, Taghavi, Taheri-Kharameh, Tahmasebi, Valukalaie, Zali |
| Saudi Arabia | 18 studies: Al-Doghether, Al-Hajeili, Al-Thafar, Al-Zalabani, Aldiab, Alduraywish, Aljumah, Almadi, Almutairi, Althobaiti, Alyabsi, Galal, Gosadi, Imran, Khayyat, Mosli, Shah, Zubaidi |
| Lebanon | 2 studies: Telvizian, Tfaily |
| Palestine/Gaza Strip | 2 studies: Elshami, Qumseya |
| Jordan | 10 studies: Abuadas, Ahmad, Al-Jaberi, Alqudah, Mhaidat, Obeidat, Omran, Rababah, Shihab, Taha |
| Oman | 2 studies: Al-Azri, Muliira |
| Qatar | 2 studies: Al-Dahshan, Mahmoud |
| Bahrain | 1 study: Nasaif |
| Kuwait | 1 study: Saeed |
| Pakistan | 7 studies: Ahmed, Bhurgri, Hasan, Hussain, Khalid, Muhammad, Yousaf |
| Iraq | 1 study: Muhammed |
| Egypt | 2 studies: Brand Bateman, Zaher |
| Morocco | 1 study: Imad |
| Tunisia | 2 studies: Rejaibi, Rym |
Barriers to CRC screening according to domains of the TDF
Knowledge
Knowledge was the most identified domain in the literature in both the individual and provider matrices. From the public/individual perspective, barriers to undergo screening for CRC have knowledge-related factors at the public, provider, and health system levels. Regarding individual-level factors, poor knowledge/lack of awareness of CRC symptoms, risk factors, and screening modalities were the most commonly cited factors (15-29). Other factors such as, being unaware of the potential severity of CRC, having low functional health literacy skills (FHLS) and literacy rates, and education level were also factors that contributed to screening barriers at the individual level (30-34). Regarding provider-level factors, the public noted low awareness and knowledge of symptoms and risk factors for CRC among medical students, in addition to provider education level impacting screening (26,35). Health system-level factors included a lack of government awareness campaigns (36) (Table 1).
With the provider-oriented TDF, knowledge-related factors were noted at all levels: patient, provider, and health system. Providers noted a low level of public awareness of CRC, signs, symptoms, and screening tests (23,27,49,51). Among providers, it was noted that some believed that only those who are high-risk for CRC should be screened (50). In addition, a broader unfamiliarity with CRC screening modes, frequency, symptoms, and risk factors were noted in the literature (15,26,27,42,48). Factors at the health system and contextual levels were not directly identified in the literature, with the exception of a few. These factors include inadequate training for laboratory technicians and providers, a lack of hospital policy/procedures for screening, healthcare provider (HCP) shortages, long wait times, and acute availability of screening services (50,51) (Table 2).
Skills
Low literacy rates were the only noted barriers from the public perspective (34) (Table 1). Meanwhile, providers noted a low inability for them to identify the correct screening test for a patient (52). Additionally, inadequate training for laboratory technicians and providers was also noted (50) (Table 2). No other skill-related factors were identified in the literature.
Social/professional role and identity
From the public perspective, barriers to undergoing CRC screening, as they pertain to social/professional role and identity, included findings that women were more likely to undergo Fecal Occult Blood Test (FOBT) as compared to men, and that those who have a higher level of education are more likely to undergo screening for CRC (28) (Table 1). In terms of provider-level factors, findings that male primary care physicians are less likely to recommend CRC screening were noted in the literature (35). No factors were indicated in the literature for provider barriers to recommending and conducting CRC screening in relation to social/professional role and identity (Table 2).
Beliefs about capabilities
For the public, two studies found that more participation in CRC screening was influenced by higher perceived self-efficacy (28,37) (Table 1). For providers, they noted a lack of confidence to be able to perform and interpret screening tests in an appropriate manner (50) (Table 2).
Beliefs about consequences
The public/individual noted several individual-level factors related to beliefs about consequences. Many studies noted a poor estimation (often underestimation) of risk for CRC, often stemming from a lack of family history of CRC, having no clinical symptoms, being of young age, or perceived self-immunity (28,31,33,34,38-43). No factors were noted among providers or at the provider or health system levels from the view of the public/individual (Tables 1,2).
Optimism
One study indicated that a positive perception (in terms of self-efficacy) of FOBT uptake strongly predicts undergoing screening (28). No factors were noted among providers or at the provider or health system levels from the view of the public/individual (Tables 1,2).
Reinforcement
Several reinforcement-related factors were noted at the individual and provider levels from the view of the public. The first of these is a non-acceptability of having a colonoscopy performed without some form of sedation, as well as a lack of physician recommendation for screening (31,44). From this same view, but at the provider level, a lack of physician recommendation for screening and an absence of screening reminders by healthcare workers were factors noted in the literature (20,34,38,43,45). For providers, the only factor cited at any level was a lack of emphasis on prevention for providers (50) (Tables 1,2).
Intentions
The public noted a low priority for personal health and seeking health care. In turn, low priority for health and health care contributed to a low or overall lack of intention to seek health care services and valued personal health (21). The public also noted a distrust of Western medicine (31). Only a lack of emphasis on prevention among providers was extracted from the literature and from the provider perspective (50) (Tables 1,2).
Goals
No factors were extracted for ‘Goals’ for either TDF matrices (Tables 1,2).
Memory, attention, and decision processes
The only factor extracted for the memory, attention, and decision processes domain was a lack of reminders by healthcare workers (38) (Table 2).
Environmental context and resources
A plethora of factors at each level were extracted for both TDFs. Time restraints, religious objection, religious protection, urban residents being more likely to undergo screening, and a distrust of Western medicine were all extracted from the literature review at the individual level for the public (17,31,33,38,46). For provider-related factors, distrust of physicians and poor physician-patient relationships were noted as barriers to undergoing CRC screening (21). At the health system level, cost, a lack of screening facilities, difficulty in arranging transport to and from testing facilities, and low socioeconomic status were all found to be barriers to CRC screening (17,21,24,34,36,40,47,48) (Table 1).
When it comes to the provider perspective, providers noted socioeconomic status among some patients as an individual-level factor that is a barrier to recommending screening (50). For providers, they noted inadequate training as a barrier to recommending CRC screening (50). At the health system level, inadequate training for laboratory technicians was a barrier to recommending screening, and a lack of specialists, absence of hospital policy and protocols for cancer screenings, shortage of healthcare workers, acute availability of screening services, and long wait times were all indicated in the literature as barriers to conducting CRC screening (49-51) (Table 2).
Social influences
In the Social Influences domain in the public TDF, individual level factors included low social support while no factors were listed at any level for providers (46) (Tables 1,2).
Emotion
The Emotion domain was well-defined among the public at the individual level. Fear, as it relates to test results, undergoing screening, endoscopic procedures, and potential pain from screening are all factors that were commonly identified at this level (15,17,18,20,21,23,25,31,34,36,39,40,42,46). Additionally, anxiety, shyness, embarrassment/shame, weariness of screening being conducted by a provider of the opposite sex, and a ‘bad feeling’ were also described in the literature (15,17,18,21,23,25,31,46) (Table 1). Among providers, the only factor in the Emotion domain relating to recommending screening was the patients’ fear of painful procedures (23,51) (Table 2).
Behavioral regulation
No factors were extracted for ‘Behavioral Regulation’ for either TDF. An example of behavioral regulation includes using the Head-Toes-Knees-Shoulders (HTKS) to measure a child’s ability to integrate memory, attention, and inhibitory control tasks (53) (Tables 1,2).
Summary of barriers to CRC screening at the patient-, provider-, and health system levels
At the patient level, the domains of knowledge and emotion were the most-often cited barrier among both matrices. Factors related to knowledge and reinforcement were the most frequently encountered in the literature at the provider level, and for the health system level, the domain of environmental context and resources was the most populated as a result of the scoping review.
Discussion
This scoping review applied a methodical approach, the Theoretical Domains Framework, to answer two questions related to colorectal cancer screening in the EMR: (I) what are the barriers to undergoing screening from the perspective of the general public/population at risk? And (II) what are the barriers to recommending and conducting screening, regarded as two independent behaviors, from the perspective of healthcare providers? The work used an ecological approach to generate each of the two perspectives a comprehensive assessment of factors that influence screening aggregated according to the individual, provider, and health system levels. It also demonstrated the utility of the TDF in conjunction with a scoping review to thoroughly identify barriers to a certain behavior from existing studies.
Understanding behavioral influences at the individual, provider, and health system levels is crucial to successful and effective interventions. Data synthesized in this review provides a rich foundation for conceptualizing and implementing locally relevant and culturally appropriate screening programs throughout the EMR.
Multiple influences on behavior, coalescing with various TDF domains, were identified. For example, ‘Knowledge’ emerged as the richest domain and the most commonly reported influence on CRC screening-related behaviors across studies (Tables 1,2). Interestingly, both the individual and provider perspectives highlighted the multi-faceted impact of knowledge on CRC-related decision making. From the individual perspective, both personal knowledge about CRC (risk factors, symptoms, screening modalities, etc.) and perceived provider knowledge of the disease emerged as factors that influence screening (15-30). This was nicely complemented by the provider’s perspective, which identified limited knowledge of CRC-associated concepts (risk factors, screening modalities and frequencies, symptoms, etc.) at the provider level, as well as perceived patient knowledge, as barriers to recommending or conducting screening (15,23,26,27,42,48,50,51). The convergence of both perspectives around shortcomings in knowledge, among patients and providers alike, warrants prioritizing this domain in future interventions to promote screening.
Aside from knowledge, various TDF domains were linked to screening, including environmental context and resources, emotion, and beliefs about consequences (Tables 1,2). On the other hand, no data were identified for the domains of behavioral regulation, goals, and memory, attention, and decision processes. Investigating barriers that belong under these domains is warranted for a thorough accounting of influences on the decision-making process related to screening. Of note, some factors identified in the literature were cross-listed across multiple domains (i.e., lack of government awareness campaigns). This contributes to the factual basis of the complexity of factors that influence screening.
In general, only a small amount of data pertaining to health system level factors were identified through the scoping review. This is an indicator of the scarcity in research oriented towards assessing the capacity of the health system for cancer prevention and control. In comparing the amount of data in the two matrices, little amounts of data were extracted that was oriented from the perspective of the provider. This signals a need for additional research that targets providers.
The strength of this work is multifaceted. First, the utilization of the 14 domains of the well-validated TDF lends itself to a comprehensive assessment of barriers, as the TDF has been validated in various aspects. Subsequently, the use of the TDF in this work provides methodological strength. Third, the ecological component of the work, that is, exploring barriers at the public/at-risk population, provider, and health system levels, provides a multitude of levels to collect and further explore data. Finally, the dual perspective of patients and providers provides a means of validation of identified barriers. In all, these strengths help enable target interventions at a number of domains and levels, even among varying audiences.
Several weaknesses should be taken into account. First, only papers in English were included in the search strategy. This may have, subsequently, excluded papers that are valuable to this topic. Second, the 2000–2021 timeframe specified in the search strategy may not have been a wide enough time frame to gather important works. Additionally, because this work is not a scoping review, the quality of evidence was not critically appraised. Finally, the list of terms related to ‘colorectal cancer’ and ’screening’, while comprehensive, was not exhaustive. As such, the papers that used related terms that were not included in the search strategy were likely missed.
Conclusions
This application of the TDF to characterize determinants of undergoing CRC screening, as well as recommending it revealed that the primary barriers to CRC-related decision making pertain to the domains of knowledge at both the patient and provider levels, environmental context and resources at the health system and patient levels, and emotion at the patient level. Collectively, these domains were the most cited in the literature that was examined for this work. Each of these barriers offer a target around which theory-informed, culturally tailored interventions for CRC screening and early detection are designed and implemented.
Supplementary
The article’s supplementary files as
Acknowledgments
We would like to acknowledge the assistance of Hannah Rogers from the Woodruff Health Sciences Center Library at Emory University for her assistance in establishing the scope of the review. This work was presented as part of CH’s graduate school thesis defense.
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Reporting Checklist: The authors have completed the PRISMA-ScR reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-510/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-510/coif). The authors have no conflicts of interest to declare.
References
- 1.American Cancer Society. About Colorectal Cancer. American Cancer Society: ACS; [updated 2020 June 29; cited 2021 April 11]. Available online: https://www.cancer.org/cancer/colon-rectal-cancer/about/what-is-colorectal-cancer.html#written_by
- 2.Regional Office for the Eastern Mediterranean. About us. World Health Organization: WHO; [cited 2021 April 11]. Available online: http://www.emro.who.int/entity/about-us/index.html
- 3.Pourghazian N, Sankaranarayanan R, Alhomoud S, et al. Strengthening the early detection of common cancers in the Eastern Mediterranean Region. East Mediterr Health J 2019;25:767-8. 10.26719/2019.25.11.767 [DOI] [PubMed] [Google Scholar]
- 4.International Agency for Research on Cancer. Estimated age-standardized incidence and mortality rates (World) in 2020, both sexes, all ages. World Health Organization: Global Cancer Observatory; [cited 2021 April 11]. Available online: https://gco.iarc.fr/today/online-analysis-dual-bars-2?v=2018&mode=cancer&mode_population=regions&population=993&populations=993&key=asr&sex=0&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&nb_items=5&gr Accessed 15 Mar 2021.
- 5.Regional Office for the Eastern Mediterranean. Policy statement and recommended actions for early detection of colorectal cancer in the Eastern Mediterranean Region. World Health Organization: WHO; 2016 [cited 2021 April 11]. Available online: https://applications.emro.who.int/docs/Policy_statement_2016_en_19182.pdf?ua=1
- 6.Centers for Disease Control and Prevention. What is colorectal cancer? U.S. Department of Health & Human Services: CDC; 2022 February 17 [cited 2021 April 11]. Available online: https://www.cdc.gov/cancer/colorectal/basic_info/what-is-colorectal-cancer.htm
- 7.Centers for Disease Control and Prevention. Colorectal Cancer Screening Tests. U.S. Department of Health & Human Services: CDC; 2022 February 17 [cited 2021 April 11]. Available online: https://www.cdc.gov/cancer/colorectal/basic_info/screening/tests.htm
- 8.Taylor N, Conner M, Lawton R. The impact of theory on the effectiveness of worksite physical activity interventions: a meta-analysis and meta-regression. Health Psychol Rev 2011;6:33-73. 10.1080/17437199.2010.533441 [DOI] [Google Scholar]
- 9.Craig LE, McInnes E, Taylor N, et al. Identifying the barriers and enablers for a triage, treatment, and transfer clinical intervention to manage acute stroke patients in the emergency department: a systematic review using the theoretical domains framework (TDF). Implement Sci 2016;11:157. 10.1186/s13012-016-0524-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albarracín D, Gillette JC, Earl AN, et al. A test of major assumptions about behavior change: a comprehensive look at the effects of passive and active HIV-prevention interventions since the beginning of the epidemic. Psychol Bull 2005;131:856-97. 10.1037/0033-2909.131.6.856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noar SM, Zimmerman RS. Health Behavior Theory and cumulative knowledge regarding health behaviors: are we moving in the right direction? Health Educ Res 2005;20:275-90. 10.1093/her/cyg113 [DOI] [PubMed] [Google Scholar]
- 12.Cane J, O'Connor D, Michie S. Validation of the theoretical domains framework for use in behaviour change and implementation research. Implement Sci 2012;7:37. 10.1186/1748-5908-7-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atkins L, Francis J, Islam R, et al. A guide to using the Theoretical Domains Framework of behaviour change to investigate implementation problems. Implement Sci 2017;12:77. 10.1186/s13012-017-0605-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richardson M, Khouja CL, Sutcliffe K, et al. Using the theoretical domains framework and the behavioural change wheel in an overarching synthesis of systematic reviews. BMJ Open 2019;9:e024950. 10.1136/bmjopen-2018-024950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmed F. Barriers to colorectal cancer screening in the developing world: The view from Pakistan. World J Gastrointest Pharmacol Ther 2013;4:83-5. 10.4292/wjgpt.v4.i4.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al Abdouli L, Dalmook H, Akram Abdo M, et al. Colorectal Cancer Risk Awareness and Screening Uptake among Adults in the United Arab Emirates. Asian Pac J Cancer Prev 2018;19:2343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Azri M, Al-Kindi J, Al-Harthi T, et al. Awareness of Stomach and Colorectal Cancer Risk Factors, Symptoms and Time Taken to Seek Medical Help Among Public Attending Primary Care Setting in Muscat Governorate, Oman. J Cancer Educ 2019;34:423-34. 10.1007/s13187-017-1266-8 [DOI] [PubMed] [Google Scholar]
- 18.Al-Azri M, Al-Khatri S, Murthi Panchatcharam S. Attitudes toward and Knowledge of Colorectal Cancer Screening among an Omani Adult Population Attending a Teaching Hospital. Asian Pac J Cancer Prev 2020;21:3061-8. 10.31557/APJCP.2020.21.10.3061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Thafar AK, Al-Naim AF, Albges DS, et al. Knowledge Attitude and Practice of Colorectal Cancer among School Teachers in Al-Ahsa Saudi Arabia. Asian Pac J Cancer Prev 2017;18:2771-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alduraywish SA, Altamimi LA, Almajed AA, et al. Barriers of colorectal cancer screening test among adults in the Saudi Population: A Cross-Sectional study. Prev Med Rep 2020;20:101235. 10.1016/j.pmedr.2020.101235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Besharati F, Karimi-Shahanjarini A, Hazavehei SMM, et al. Socio-culturally informed views influencing Iranian adults’ decision about colorectal cancer screening: A Qualitative Study. Int J Cancer Manag 2018;11:e9546. 10.5812/ijcm.9546 [DOI] [Google Scholar]
- 22.Elshami M, Elshami A, Alshorbassi N, et al. Knowledge level of cancer symptoms and risk factors in the Gaza Strip: a cross-sectional study. BMC Public Health 2020;20:414. 10.1186/s12889-020-08553-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galal YS, Amin TT, Alarfaj AK, et al. Colon Cancer among Older Saudis: Awareness of Risk Factors and Early Signs, and Perceived Barriers to Screening. Asian Pac J Cancer Prev 2016;17:1837-46. 10.7314/APJCP.2016.17.4.1837 [DOI] [PubMed] [Google Scholar]
- 24.Hasan F, Mahmood Shah SM, Munaf M, et al. Barriers to Colorectal Cancer Screening in Pakistan. Cureus 2017;9:e1477. 10.7759/cureus.1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hussain I, Majeed A, Rasool MF, et al. Knowledge, attitude, preventive practices and perceived barriers to screening about colorectal cancer among university students of newly merged district, Kpk, Pakistan - A cross-sectional study. J Oncol Pharm Pract 2021;27:359-67. 10.1177/1078155220922598 [DOI] [PubMed] [Google Scholar]
- 26.Imran M, Sayedalamin Z, Alsulami SS, et al. Knowledge and Awareness of Colorectal Cancer among Undergraduate Students at King Abdulaziz University, Jeddah, Saudi Arabia: a Survey-Based Study. Asian Pac J Cancer Prev 2016;17:2479-83. [PubMed] [Google Scholar]
- 27.Mhaidat NM, Al-Husein BA, Alzoubi KH, et al. Knowledge and Awareness of Colorectal Cancer Early Warning Signs and Risk Factors among University Students in Jordan. J Cancer Educ 2018;33:448-56. 10.1007/s13187-016-1142-y [DOI] [PubMed] [Google Scholar]
- 28.Mirzaei-Alavijeh M, Schaafsma D, Karami-Matin B, et al. Socio-cognitive determinants of colorectal cancer screening uptake: An application of intervention mapping approach. Med J Islam Repub Iran 2019;33:80. 10.47176/mjiri.33.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Omran S, Barakat H, Muliira JK, et al. Assessment of Jordanian Patient's Colorectal Cancer Awareness and Preferences towards CRC Screening: Are Jordanians Ready to Embrace CRC Screening?. Asian Pac J Cancer Prev 2015;16:4229-35. 10.7314/APJCP.2015.16.10.4229 [DOI] [PubMed] [Google Scholar]
- 30.Almutairi KM, Alonazi WB, Alodhayani A, et al. A Cross-Sectional Assessment of Literacy and Awareness, Attitudes, and Beliefs About Colorectal Cancer and Its Screening in Riyadh Region. J Cancer Educ 2018;33:660-7. 10.1007/s13187-016-1129-8 [DOI] [PubMed] [Google Scholar]
- 31.Qumseya BJ, Tayem YI, Dasa OY, et al. Barriers to colorectal cancer screening in Palestine: a national study in a medically underserved population. Clin Gastroenterol Hepatol 2014;12:463-9. 10.1016/j.cgh.2013.08.051 [DOI] [PubMed] [Google Scholar]
- 32.Shah S, Wali M, Alhazmi M, et al. The assessment of awareness level regarding the risk factors and screening of colorectal cancer among the people of the northern border region - Kingdom of Saudi Arabia. Pakistan J Med Heal Sci 2019;13:716-9. [Google Scholar]
- 33.Taha H, Jaghbeer MA, Shteiwi M, et al. Knowledge and Perceptions about Colorectal Cancer in Jordan. Asian Pac J Cancer Prev 2015;16:8479-86. 10.7314/APJCP.2015.16.18.8479 [DOI] [PubMed] [Google Scholar]
- 34.Bidouei F, Abdolhosseini S, Jafarzadeh N, et al. Knowledge and perception toward colorectal cancer screening in east of Iran. Int J Health Policy Manag 2014;3:11-5. 10.15171/ijhpm.2014.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montazeri A, Tavoli A, Mohagheghi MA, et al. Disclosure of cancer diagnosis and quality of life in cancer patients: should it be the same everywhere? BMC Cancer 2009;9:39. 10.1186/1471-2407-9-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taha H, Al Jaghbeer M, Al-Sabbagh MQ, et al. Knowledge and Practices of Colorectal Cancer Early Detection Examinations in Jordan: A Cross Sectional Study. Asian Pac J Cancer Prev 2019;20:831-8. 10.31557/APJCP.2019.20.3.831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alqudah MAY, Al-Samman RM, Mukattash TL, et al. Knowledge and attitudes of pharmacists towards colorectal cancer health education in Jordan: A cross-sectional study. Int J Clin Pract 2021;75:e13986. 10.1111/ijcp.13986 [DOI] [PubMed] [Google Scholar]
- 38.Ramazani AA, Norozi E, AmirabadiZadeh H, et al. Predictors of Colorectal Cancer Screening Participation in Southern Khorasan (Iran). J Gastrointest Cancer 2021;52:187-91. 10.1007/s12029-020-00379-y [DOI] [PubMed] [Google Scholar]
- 39.Al-Dahshan A, Abushaikha S, Chehab M, et al. Perceived Barriers to Colorectal Cancer Screening among Eligible Adults in Qatar and the Associated Factors: A Cross- Sectional Study. Asian Pac J Cancer Prev 2021;22:45-51. 10.31557/APJCP.2021.22.1.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Hajeili M, Abdulwassi HK, Alshadadi F, et al. Assessing knowledge on preventive colorectal cancer screening in Saudi Arabia: A cross-sectional study. J Family Med Prim Care 2019;8:3140-6. 10.4103/jfmpc.jfmpc_508_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mozafar Saadati H, Khodamoradi F, Salehiniya H. Associated Factors of Survival Rate and Screening for Colorectal Cancer in Iran: a Systematic Review. J Gastrointest Cancer 2020;51:401-11. 10.1007/s12029-019-00275-0 [DOI] [PubMed] [Google Scholar]
- 42.Omran S, Barakat H, Muliira JK, et al. Knowledge, experiences, and barriers to colorectal cancer screening: a survey of health care providers working in primary care settings. J Cancer Educ 2015;30:53-61. 10.1007/s13187-014-0676-0 [DOI] [PubMed] [Google Scholar]
- 43.Omran S, Ismail AA. Knowledge and beliefs of Jordanians toward colorectal cancer screening. Cancer Nurs 2010;33:141-8. 10.1097/NCC.0b013e3181b823f3 [DOI] [PubMed] [Google Scholar]
- 44.Rejaibi S, Mahfoudh Mchirgui R, Ben Mansour N, et al. Colorectal cancer mass screening, Tunisia 2019. Evaluation of a pilot program in the Tunis region (Tunisia, 2019). Tunis Med 2021;99:158-67. [PubMed] [Google Scholar]
- 45.Salimzadeh H, Eftekhar H, Majdzadeh R, et al. More than half of senior residents in Tehran have never heard about colorectal cancer screening. Asian Pac J Cancer Prev 2011;12:2851-6. [PubMed] [Google Scholar]
- 46.Khani Jeihooni A, Kashfi SM, Shokri A, et al. Investigating Factors Associated with FOBT Screening for Colorectal Cancer Based on the Components of Health Belief Model and Social Support. Asian Pac J Cancer Prev 2017;18:2163-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chouhdari A, Yavari P, Pourhoseingholi MA, et al. Association Between Socioeconomic Status and Participation in Colonoscopy Screening Program in First Degree Relatives of Colorectal Cancer Patients. Iran J Cancer Prev 2016;9:e4809. 10.17795/ijcp-4809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hani Rabab M, Alhassan MA, Al-Motlaq MA. Knowledge of colorectal cancer screening guidelines and perceptions of barriers among nursing and medical students in a Jordanian university. Int J Cancer Res 2018;14:70-6. 10.3923/ijcr.2018.70.76 [DOI] [Google Scholar]
- 49.Althobaiti A, Jradi H. Knowledge, attitude, and perceived barriers regarding colorectal cancer screening practices and risk factors among medical students in Saudi Arabia. BMC Med Educ 2019;19:421. 10.1186/s12909-019-1857-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brand Bateman L, Khamess S, Abdelmoneim SE, et al. Designing an Effective Colorectal Cancer Screening Program in Egypt: A Qualitative Study of Perceptions of Egyptian Primary Care Physicians and Specialists. Oncologist 2020;25:e1525-31. 10.1634/theoncologist.2019-0687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muliira JK, D'Souza MS, Ahmed SM. Contrasts in Practices and Perceived Barriers to Colorectal Cancer Screening by Nurses and Physicians Working in Primary Care Settings in Oman. J Cancer Educ 2016;31:15-25. 10.1007/s13187-015-0806-3 [DOI] [PubMed] [Google Scholar]
- 52.Mosli M, Alnahdi Y, Alghamdi A, et al. Knowledge, attitude, and practices of primary health care physicians toward colorectal cancer screening. Saudi J Gastroenterol 2017;23:330-6. 10.4103/sjg.SJG_1_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wanless SB, McClelland MM, Acock AC, et al. Measuring behavioral regulation in four societies. Psychol Assess 2011;23:364-78. 10.1037/a0021768 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as