Synopsis
Chemical defense is a crucial component of fitness in many organisms, yet the physiological regulation of defensive toxin synthesis is poorly understood, especially in vertebrates. Bufadienolides, the main defensive compounds of toads, are toxic to many predators and other natural enemies, and their synthesis can be upregulated by stressors, including predation risk, high conspecific density, and pollutants. Thus, higher toxin content may be the consequence of a general endocrine stress response in toads. Therefore, we hypothesized that bufadienolide synthesis may be stimulated by elevated levels of corticosterone (CORT), the main glucocorticoid hormone of amphibians, or by upstream regulators that stimulate CORT production. To test these alternatives, we treated common toad tadpoles with exogenous CORT (exoCORT) or metyrapone (MTP, a CORT-synthesis inhibitor that stimulates upstream regulators of CORT by negative feedback) in the presence or absence of predation cues for 2 or 6 days, and subsequently measured their CORT release rates and bufadienolide content. We found that CORT release rates were elevated by exoCORT, and to a lesser extent also by MTP, regardless of treatment length. Bufadienolide content was significantly decreased by treatment with exoCORT for 6 days but was unaffected by exposure to exoCORT for 2 days or to MTP for either 6 or 2 days. The presence or absence of predation cues affected neither CORT release rate nor bufadienolide content. Our results suggest that changes in bufadienolide synthesis in response to environmental challenges are not driven by CORT but may rather be regulated by upstream hormones of the stress response.
Introduction
Using toxic or noxious chemical compounds for protection from natural enemies, such as predators, pathogens, parasites, and competitors, is widespread in the animal kingdom (Blum 1981; Pawlik 1993; Brodie 2009; Casewell et al. 2013). Chemical defense is important from the perspective of ecology and life-history evolution, as chemically protected animals can have a longer life span (Hossie et al. 2013) and occupy a larger niche space (Arbuckle et al. 2013). Toxins can also influence the survival not only of the defended animals themselves (Hayes et al. 2009; Llewelyn et al. 2012), but also of other species. For instance, when consuming unusually toxic prey, predators can suffer high mortality and population declines, as shown by the infamous example of invasive cane toads (Rhinella marina) that endanger native fauna (Shine 2010). On the other hand, toxins can also change trophic interactions and community structure if predators learn to avoid the consumption of toxic animals and switch to alternative prey (Webb et al. 2008; Nelson et al. 2010, 2011). Furthermore, the presence of effective toxins may also induce co-evolutionary arms races between prey that produce them and predators that adapt to their consumption by evolving toxin resistance (Brodie and Brodie 1990; Ujvari et al. 2015). Despite the widespread occurrence of chemical defense and its importance for multiple aspects of biology, the physiological regulation of toxin synthesis remains poorly known in a plethora of species.
Toads (Bufonidae, Amphibia) produce skin secretions containing, among others, bufadienolides as defensive compounds, which can be effective against predators and possibly also against pathogens (Toledo and Jared 1995; Filho et al. 2005; Tempone et al. 2008; Barnhart et al. 2017; Üveges et al. 2019). Bufadienolides are potent inhibitors of Na+/K+-ATPase (Pierre and Xie 2006; Schoner and Scheiner-Bobis 2007; Lingrel 2010) and can cause serious symptoms upon ingestion, including nausea, convulsions, hypertension, cardiac arrhythmia, and even death (Toledo and Jared 1995; Chen and Huang 2013; Kamboj et al. 2013). The rate of bufadienolide synthesis in toads can be increased by various environmental factors such as predation risk (Hettyey et al. 2019), high conspecific density (Bókony et al. 2018; Üveges et al. 2021), reduced food availability (Üveges et al. 2017), and anthropogenic pollutants (Bókony et al. 2017). This high diversity of inducing stressors raises the question whether upregulated bufadienolide synthesis is a consequence or part of the generalized endocrine stress response.
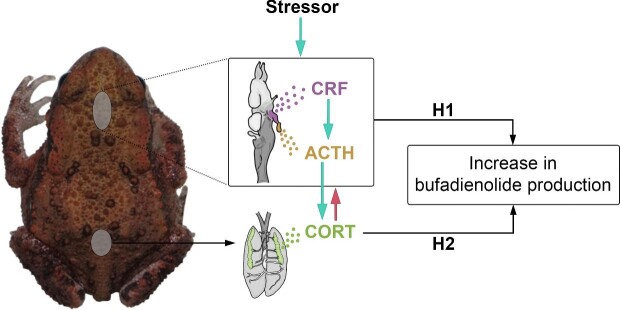
The endocrine stress response, which is highly conserved across vertebrates (Denver 2009; Godoy et al. 2018), serves to maintain homeostasis by allostasis and ensure survival by responding to environmental perturbance (McEwen 1998; McEwen and Wingfield 2003; Koolhaas et al. 2011). One integral part of this system is the hypothalamic–pituitary–adrenal (HPA, or hypothalamic–pituitary–interrenal [HPI]) axis (Fig. 1). The perception of environmental stressors triggers the discharge of corticotropin-releasing factor (CRF) from the hypothalamus, which in turn induces production of adrenocorticotropic hormone (ACTH) in the anterior pituitary gland. ACTH stimulates the adrenal glands (or interrenal glands in amphibians and fish; Denver 2009) to produce glucocorticoid hormones (GCs, notably cortisol and corticosterone [CORT]). GCs modulate a multitude of physiological processes, including metabolism and immune response, and facilitate recovery after stress by promoting energy uptake of cells and tissues via gluconeogenesis (Sterling and Eyer 1988; McEwen 1998, 2004; McEwen and Wingfield 2003; Denver 2009; Godoy et al. 2018). On the other hand, chronic stress might lead to long-term elevated levels of circulating GCs, resulting in adverse effects such as suppression of the immune system and cardiovascular, neurological, and metabolic disorders (Sterling and Eyer 1988; McEwen 1998, 2004; McEwen and Wingfield 2003; Denver 2009; Godoy et al. 2018). To keep GC levels in check, the HPA/HPI axis is under internal control by negative feedback loops. For instance, high levels of circulating GCs inhibit the release of CRF and ACTH, thus returning GC secretion to baseline levels (Gjerstad et al. 2018; Godoy et al. 2018; Herman et al. 2020).
Fig. 1.
Schematic representation of the HPI axis and potential physiological regulatory pathways of bufadienolide synthesis in common toads. Green arrows indicate upregulation, whereas a red arrow indicates negative feedback. Abbreviations: CRF, corticotropin-releasing factor; ACTH, adrenocorticotropic hormone; CORT, corticosterone; H1, hypothesis 1; H2, hypothesis 2. Photo of a juvenile toad by Nikolett Ujhegyi, used with permission.
Various environmental stressors that trigger the GC response, such as predation risk (Berger et al. 2007; Middlemis Maher et al. 2013), intraspecific competition (Glennemeier and Denver 2002a; Eraud et al. 2008; Braasch et al. 2014), and anthropogenic pollutants (Ye et al. 2015; Gabor et al. 2018; Monclús et al. 2018), also elevate the synthesis of bufadienolide toxins (Bókony et al. 2017, 2018; Üveges et al. 2017, 2021; Hettyey et al. 2019). Further similarities also suggest a physiological link between the two groups of compounds. Both CORT and bufadienolides are synthesized from the precursor cholesterol (Payne and Hales 2004; Fedorova et al. 2015), and the synthesis of cardiotonic steroids, the compound family bufadienolides belong to, has been suggested to be upregulated by ACTH in mammals (Vinge et al. 1993; Laredo et al. 1994, 1995; Li et al. 1994; Yamada et al. 1997; Sophocleous et al. 2003; Dostanic-Larson et al. 2005). However, the specific physiological regulatory mechanisms behind the synthesis of bufadienolides are still largely unknown (although see Fedorova et al. 1998; Dostanic-Larson et al. 2005), and explicit information about how GCs affect bufadienolide synthesis is, to our knowledge, entirely lacking.
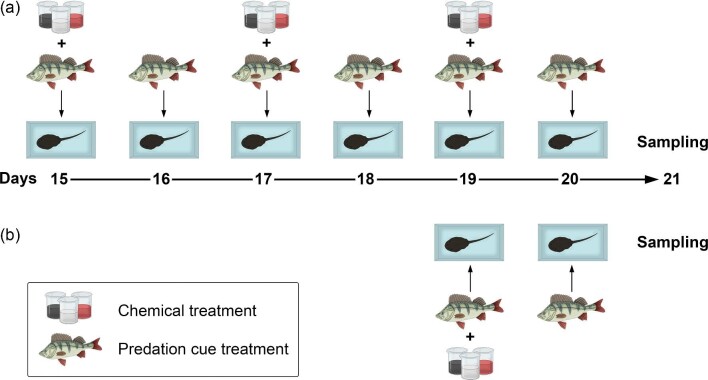
Given the information presented above, we hypothesized that the HPI axis may provide a key to understanding the proximate mechanism of what makes toads more (or less) toxic. Specifically, we formulated two alternative hypotheses (Fig. 1). According to hypothesis 1 (H1), increased CORT levels upregulate bufadienolide synthesis directly. Conversely, hypothesis 2 (H2) postulates that bufadienolide production is regulated by upstream hormones of the HPI axis, which simultaneously increase the levels of both CORT and bufadienolides (Fig. 1). To test these alternatives, we reared tadpoles of the common toad (Bufo bufo) in the laboratory and exposed them to treatments with exogenous CORT (exoCORT), metyrapone (MTP, a CORT synthesis inhibitor), or a solvent control. We predicted that treatment with exoCORT would lead to increased bufadienolide content if H1 was true, but to decreased bufadienolide content if H2 was true (due to negative feedback loops inhibiting expression of upstream regulators; Fig. 1). MTP is used to test HPA functionality by blocking CORT synthesis, which in turn can stimulate the secretion of CRF and ACTH (Gjerstad et al. 2018; Godoy et al. 2018; Herman et al. 2020). Thus, in toad tadpoles treated with MTP, we expected to observe a decrease in bufadienolide content if H1 was true, but an increase if H2 was true (Fig. 1). Because earlier results indicated that MTP may not reduce CORT in the absence of stressors (Gabor et al. 2018), we combined the chemical treatments with a predator treatment (presence or absence of predation cues; Fig. 2) since predator presence is a natural stressor highly relevant to chemical defense. To discriminate between the acute and chronic effects of CORT on bufadienolide synthesis, we applied the treatments for either 2 or 6 days (Fig. 2).
Fig. 2.
Experimental design applied to containers of tadpoles during the last week of the experiment (treatment period), beginning with day 15. (a) treatment length 6 days, (b) treatment length 2 days. Beakers represent changing the tadpoles’ rearing water with water containing exogenous chemicals (CORT, MTP) or the control solution. Perch represent the addition of reconstituted soft water (control tadpoles) or water from the fish's tank + tadpole homogenate. Icons of beakers used in the figure are licensed by Biorender.
Materials and Methods
Experimental procedures
We collected 50 common toad eggs from each of 8 clutches (egg strings) in April 2019 from a pond located in the Pilis Mountains, Hungary (Zánkói rét, 47.740515°N, 19.016276°E). We transported eggs to the laboratory at the Plant Protection Institute in Budapest. Eggs and embryos from each clutch were kept together in 1 L reconstituted soft water (RSW, 48 mg/L NaHCO3, 30 mg/L CaSO4 × 2H2O, 61 mg/L MgSO4 × 7H2O and 2 mg/L KCl dissolved in UV treated, reverse osmosis filtered, aerated tap water) in the laboratory until hatching, after which the water level was increased to 9 L (container size: 26.8 × 36.9 × 16.6 cm, width × length × height) until tadpoles reached the free-swimming stage (developmental stage 25, Gosner 1960). The air temperature in the laboratory was 20.1 ± 1.1°C, resulting in 19.0 ± 0.2°C water temperature in tadpole containers. The light:dark cycle was adjusted weekly to simulate natural changes in photoperiod.
We began the experiment when the tadpoles reached the free-swimming stage (day 1). We haphazardly selected 36 toad tadpoles from each clutch and placed them individually into 2-L plastic boxes (11.8 × 16.5 × 12.8 cm) containing 1 L RSW. We fed experimental tadpoles throughout the study twice a week ad libitum. To ensure that cholesterol, the precursor for both endogenous CORT and bufadienolides, was not a limiting factor for the tadpoles, we fed them with a 20:1 mixture of slightly boiled, chopped spinach and minced axolotl food (JBL NovoLotl M, fat content 12%). We changed the water of tadpoles twice a week for 2 weeks until the start of the treatment period (see below).
On day 15 of the experiment, we assigned the tadpoles randomly to treatments with exogenous chemicals combined with the simultaneous presence or absence of predation cues for either 6 or 2 days in a fully crossed design (see below for details, Fig. 2). We applied treatments during the third week of the experiment, when toxin production of common toad tadpoles reacts strongest to environmental change (Ujszegi et al. 2017; Üveges et al. 2017). We applied treatments only for relatively short periods because CORT inhibits the growth and development of tadpoles (Glennemeier and Denver 2002b), and we wanted to avoid inducing a strong effect on the formation of cells producing bufadienolides (Delfino et al. 1995). Experimental containers were arranged in a randomized block design with each block containing one individual from each treatment × clutch combination. We repeated each treatment × clutch combination three times (3 blocks). Therefore, the total number of experimental units was 288 toad tadpoles (8 clutches × 2 treatment lengths (6 or 2 days) × 3 chemical treatments (exoCORT, MTP, and control) × 2 predation cue treatments (cues present or absent) × 3 blocks). During the experiment, three tadpoles died for unknown reasons, reducing the final sample size to 285.
To manipulate the internal CORT levels of experimental tadpoles, we exposed them either to exoCORT or MTP (Sigma–Aldrich). As a solvent control treatment, the third group of the tadpoles received the same concentration of ethanol (EtOH) as their conspecifics in the exoCORT and MTP treatments. We did not use a control treatment with RSW only (without EtOH), because previous studies showed that the low concentration of ethanol used in our experiment does not affect the development and growth of tadpoles (Rohr et al. 2013; Young et al. 2020), including common toad larvae (Verebélyi 2017). Right before the start of treatments, we created stock solutions of CORT (cc. 25 mg/ml) and MTP (cc. 30 mg/ml) by dissolving them in 96% EtOH and stored these in the dark at 4°C until use. Similar to previous studies (Glennemeier and Denver 2002a, 2002b; Forsburg et al. 2019; Gabor et al. 2019), we diluted stock solutions to final concentrations of 125 nM exoCORT, 110 μM MTP, and 1 μL/L EtOH by adding them to RSW before each change of the rearing water of tadpoles. These concentrations of exoCORT and MTP increase and decrease, respectively, CORT in amphibian larvae, by approximately 50% (Glennemeier and Denver 2002a, 2002b; Forsburg et al. 2019; Gabor et al. 2019). We exposed tadpoles to exogenous chemicals by changing the water of experimental animals using these freshly prepared solutions on days 15, 17, and 19 (6-day treatment) or only on day 19 (2-day treatment, Fig. 2). This way, tadpoles were able to absorb exoCORT and MTP from their rearing water (Hayes and Wu 1995; Glennemeier and Denver 2002a, 2002b; Middlemis Maher et al. 2013; Gabor et al. 2018).
During the treatment period, half of the experimental tadpoles received cues indicating the presence of common perch (Perca fluviatilis), a predatory fish to which common toad larvae can respond with increased bufadienolide production (Hettyey et al. 2019). Predator cues were provided by six fish (body mass, mean ± SD: 39.28 ± 7.07 g) kept under laboratory conditions in 130 L aerated RSW, which was changed once a week. Fish were fed 3 times a week with 40 live agile frog (Rana dalmatina) tadpoles and Tubifex worms ad libitum during the first 2 weeks of the experiment. From day 14 until the termination of the experiment, we fed fish daily with 6.06 ± 0.38 g (mean ± SD) live agile frog tadpoles only. We fed fish with tadpoles to simulate a high risk of tadpole predation in general and to thereby elicit strong antipredator responses in toad larvae (Laurila et al. 1997; Schoeppner and Relyea 2005; Hettyey et al. 2015). We did not offer common toad tadpoles as food, because fishes often, but not always, avoid consuming them (Üveges et al. 2019), which would have made it difficult to obtain replicable quantities of chemical cues of predation risk. Agile frog tadpoles used for feeding fish were collected as eggs in a pond in the Pilis Mountains, Hungary (Jávor tó, 47.713646°N, 19.019953°E). Agile frog tadpoles and toad tadpoles used for the preparation of stimulus water (see below) were raised in the same way as focal tadpoles until developmental stage 25, after which they were reared in outdoor mesocosms. After the termination of the experiment, all remaining tadpoles were released to their pond of origin.
During the treatment period, we created stimulus water daily by homogenizing 276.33 ± 27.82 g (mean ± SD, days 15–18) and 487.55 ± 14.35 mg toad tadpoles (days 19–20) with a blender in ca. 50 mL RSW (Benard and Fordyce 2003; Hettyey et al. 2019). This resulted in the immediate death of tadpoles. We opted for this method, because we did not want chemicals (e.g., MS222) used for euthanizing tadpoles to affect our experimental animals (Achtymichuk et al. 2022). We then added this homogenate to 2 and 3.5 L water, respectively, taken from the fish tank 2–3 h after feeding the fish. This way, the concentration of chemical cues in the stimulus water was kept constant during the whole treatment period. We refilled the fish tank to the original volume with RSW after each stimulus-water preparation. We added the tadpole homogenate to ensure that experimental tadpoles were exposed to sufficiently high concentrations of conspecific prey-borne cues of predation to elicit strong antipredator responses (Laurila et al. 1997; Schoeppner and Relyea 2005; Hettyey et al. 2015). During the treatment period, we added 20 mL of stimulus water (predation cue treatment) or clean RSW (control treatment) to the water of focal tadpoles daily using a pipette. As a result, experimental tadpoles were exposed to chemical cues corresponding to 34.05 ± 1.15 mg/L fish (sources of kairomones, mean ± SD), 0.89 ± 0.01 mg/L agile frog tadpoles (chemical cues released by the fish digesting frog tadpoles and sources of alarm pheromones or “Schreckstoff,” von Frisch 1942), as well as to 2.66 ± 0.21 mg/L homogenized conspecifics (sources of chemical cues of conspecifics released by mechanical damage).
We terminated the experiment on day 21 after applying a noninvasive water-borne hormone sampling method that measures the amount of CORT released by tadpoles into the surrounding water (Gabor et al. 2013a; Baugh et al. 2018; Forsburg et al. 2019). This method provides a CORT measurement that is repeatable within individuals, correlates with plasma levels, and responds to ACTH challenge (Gabor et al. 2013a, 2016; Forsburg et al. 2019). All experimental tadpoles were transferred from their rearing containers to individual 0.5 L plastic cups containing 100 mL RSW in a random order between 09:00 and 10:00 am. To avoid transferring exogenous chemicals, we first poured the rearing water of experimental animals through a stretched-out aquarium net. Then, we used a dry piece of mosquito net (single-use for each animal) to transfer each tadpole one by one from the surface of the aquarium net into the plastic cups. After each tadpole spent 1 h in the cup, we poured the water into individual polypropylene (PP) containers and stored the water samples at –20°C until analysis. We then preserved each tadpole in a microcentrifuge tube containing HPLC (high-performance liquid chromatography) grade absolute methanol for chemical analysis of their toxin content, which resulted in the immediate death of tadpoles. The developmental stage of tadpoles (median: 35, range: 32–37, Gosner 1960) was determined by inspecting the preserved tadpoles under a stereomicroscope.
The study was approved by the Environment Protection and Nature Conservation Department of the Pest County Bureau of the Hungarian Government (PE-06/KTF/8060–1/2018, PE-06/KTF/8060–2/2018, PE-06/KTF/8060–3/2018, and PE/EA/295–7/2018,) as well as by the Ethics Committee of the Plant Protection Institute, Centre for Agricultural Research, Eötvös Loránd Research Network.
Preparation of hormone samples and analysis of CORT release rates
We extracted the CORT content of water samples using C18 solid phase extraction (SPE) columns (Sep-Pak Vac, 3cc/500mg, Waters Inc.) following an established protocol (Gabor et al. 2013a, 2016; Forsburg et al. 2019). We primed each SPE column with 4 mL HPLC grade abs. methanol and 4 mL reverse-osmosis filtered water. Samples were forced through SPE columns using Tygone tubing under a vacuum. We eluted samples with 4 mL HPLC grade absolute methanol into 5 mL PP microcentrifuge tubes. The eluted solution was kept at –20°C until samples were transferred into 15 mL PP centrifuge tubes and dried out by vacuum centrifugation (40°C, 1000 rpm, 100 Pa) and frozen again. Samples were analyzed using a competitive enzyme-linked immunosorbent assay (ELISA, Cayman Chemical Inc.) following the manufacturers’ protocol and based on the previously published methodology (Gabor et al. 2013a, 2016). We dissolved each sample in 12.5 μL of 96% EtOH, vortexed them for 5 min, and subsequently added 237.5 μL ELISA buffer to obtain a final volume of 250 μL. Samples obtained this way were homogenized on an orbital shaker for 45 min to promote dissolution of CORT in the ethanol–buffer solution before transferring them to plates. Two samples were lost during the preparation process, leaving us with 283 samples to be analyzed for CORT content. The ELISA color reaction was quantified at 412 nm wavelength using a microplate reader (Synergy HT, Bio-Tek Instruments).
Because we had no prior information on the range of waterborne CORT concentration in common toad samples, we prepared a dilution series from six samples to estimate the approximate range of sample concentrations in our study. To account for variation in the CORT concentrations of our samples, we then analyzed two dilutions of each sample that would potentially fit in the linear region of the standard curve of the CORT standard. For subsequent statistical analyses, we used CORT concentrations of the dilution with the best fit. CORT concentrations were corrected for dilution. Due to limited ELISA kit availability, we could not analyze samples of the same dilution in duplicate. Therefore, the intra-assay coefficient of variation (CV) could not be calculated. The inter-assay CV, calculated from concentrations of the CORT standard, was 9.4%.
Preparation of toxin samples and analysis of bufadienolide content
We prepared and analyzed bufadienolide samples following an established protocol (for a detailed description of methods, see the Supplementary material and Hettyey et al. 2019; Üveges et al. 2021). Briefly, we homogenized preserved tadpoles and dried the resulting homogenates, then we measured the dry mass of samples and re-dissolved them in 1 mL HPLC-grade absolute methanol. Finally, we filtered samples and analyzed the resulting solution using HPLC with diode-array detection and mass spectrometry (HPLC-DAD-MS). Bufadienolides were identified based on their characteristic peaks in the UV spectrum (Benard and Fordyce 2003; Hagman et al. 2009; Üveges et al. 2017, 2019; Bókony et al. 2018; Hettyey et al. 2019), by co-injection with selected bufadienolide standards and by additionally analyzing a bulk toxin sample from 49 juvenile common toads.
Statistical analysis
We considered a specific bufadienolide to be present if its signal-to-noise ratio was at least three in the chromatogram (Hettyey et al. 2019; Üveges et al. 2019, 2021). We estimated the quantity of each compound from the area values of MS chromatogram peaks based on the calibration curve of the marinobufotoxin standard. We subsequently summed these values to obtain estimates of the total bufadienolide quantity (TBQ) for each individual. This approach results in rough estimates of bufadienolide content, but because commercially available standards are lacking for most bufadienolide compounds, this is currently the best quantification method available (Benard and Fordyce 2003; Hagman et al. 2009; Üveges et al. 2021).
For data analysis, the CORT release rate for each tadpole was expressed as the total amount of CORT released per hour, divided by tadpole dry mass (pg/mg/h). Toxin content was expressed as mass-corrected TBQ (mcTBQ, ng/mg) by dividing the TBQ by tadpole dry mass. The response variables were adjusted to body mass to account for the potential allometric relationship between body mass and TBQ or CORT release rate (Fig. S1), and because body mass and developmental stage were highly correlated (Fig. S2).
All statistical analyses were run in R 4.0 (R Development Core Team 2017) using linear mixed-effects models. We log10-transformed both CORT release rate and mcTBQ to meet the model assumptions based on diagnostic residual plots. We used the “lmer” function of the “lme4” package (Bates et al. 2015) to analyze CORT release rate. We included treatment length (2 or 6 days), presence or absence of predation cues, and the type of exogenous chemicals (exoCORT, MTP, or EtOH) as fixed factors, and developmental stage centered to the mean as a covariate, as well as all two-way interactions between the fixed factors and their three-way interaction. We included clutch and block as crossed random factors. To analyze log10-transformed bufadienolide content, we used the same model structure as for CORT release rate, except that clutch was used as the sole random factor. Preliminary analysis of data indicated that the block had no effect on mcTBQ, therefore, it was omitted from this model. To account for differences in variances between the twelve treatment combinations and to improve model fit, we included the “weights” argument with the “varIdent” function in the mcTBQ model, which was run with the “lme” function of the “nlme” package (Pinheiro et al. 2017). We obtained P values from type-2 analysis-of-deviance (ANOVA) tables implemented in the “car” package (Fox and Weisberg 2019). We conducted post-hoc pairwise comparisons among treatment groups using linear contrasts implemented in the “emmeans” package (Lenth 2023). P values were corrected for multiple comparisons by using the false discovery rate (FDR) method (Benjamini and Hochberg 1995; Pike 2011). We present the results of pairwise comparisons using means ± 84% confidence intervals (CI), as suggested by Payton et al. (2003) for assessment of equality among groups (Fig. 3). Nonoverlapping CIs indicate significant differences between groups after correction for FDR. Additionally, we tested Pearson's product–moment correlation implemented in base R and partial correlation controlling for the developmental stage using the “ppcor” package (Kim 2015) between log10 transformed CORT release rate and log10 transformed mcTBQ.
Fig. 3.
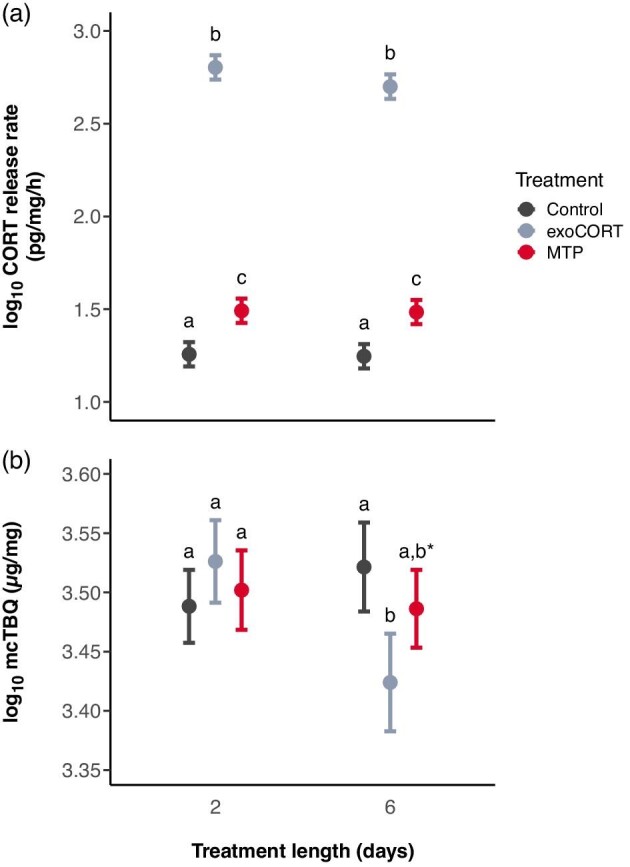
Effects of treatments (exoCORT: exogenous corticosterone, MTP: metyrapone) on corticosterone (CORT) release rate (a) and mass-corrected total bufadienolide content (mcTBQ, b) of toad tadpoles. Error bars represent estimated means and 84% confidence intervals (CI) calculated from linear mixed-effects models (N = 283). Nonoverlapping CIs indicate significant differences between groups after correction for false discovery rate. Letters above the error bars represent pairwise comparisons; groups not sharing any letter differ significantly (P < 0.05). An asterisk depicts a marginally non-significant difference (P = 0.071) between groups treated with exoCORT and MTP.
Results
The CORT release rate of tadpoles was significantly affected by exogenous chemicals (Table 1). Tadpoles exposed to exoCORT released significantly more CORT compared to the control and MTP-treated groups, while tadpoles in the MTP group exhibited intermediate CORT release rates, differing significantly from those in the control and exoCORT groups (Table 2, Fig. 3a). The effect of developmental stage on the CORT release rate was also significant: more developed tadpoles excreted lower amounts of CORT (Table 1, Fig. S3A). Treatment length, predation cues, and their interactions had no significant effect on CORT release rates (Table 1).
Table 1.
Treatment effects (type-2 analysis of deviance) on corticosterone (CORT) release rate and mass-corrected bufadienolide quantity (mcTBQ) of toad tadpoles.
| Response | Effect | χ 2 | df | P |
|---|---|---|---|---|
| CORT release rate | Developmental stage | 57.776 | 1 | <0.001 |
| Exogenous chemicals | 2674.305 | 2 | <0.001 | |
| Predation cues | 0.456 | 1 | 0.499 | |
| Treatment length | 2.365 | 1 | 0.124 | |
| Exogenous chemicals × predation cues | 3.024 | 2 | 0.221 | |
| Exogenous chemicals × treatment length | 3.079 | 2 | 0.215 | |
| Predation cues × treatment length | 0.193 | 1 | 0.661 | |
| Exogenous chemicals × predation cues × treatment length | 0.91 | 2 | 0.634 | |
| mcTBQ | Developmental stage | 272.422 | 1 | <0.001 |
| Exogenous chemicals | 0.559 | 2 | 0.756 | |
| Predation cues | 0.014 | 1 | 0.906 | |
| Treatment length | 1.257 | 1 | 0.262 | |
| Exogenous chemicals × predation cues | 0.071 | 2 | 0.965 | |
| Exogenous chemicals × treatment length | 10.327 | 2 | 0.006 | |
| Predation cues × treatment length | 2.28 | 1 | 0.131 | |
| Exogenous chemicals × predation cues × treatment length | 3.44 | 2 | 0.179 |
Significant effects are highlighted in bold.
Table 2.
Effects of exogenous chemicals on corticosterone (CORT) release rate and mass-corrected total bufadienolide quantity (mcTBQ) of toad tadpoles.
| Response | Treatment length (days) | Contrast | Estimate | SE | df | t | P |
|---|---|---|---|---|---|---|---|
| CORT release rate | 2 | exoCORT—MTP | –1.312 | 0.044 | 261 | –30.067 | <0.001 |
| 2 | EtOH—exoCORT | 1.546 | 0.044 | 261 | 35.423 | <0.001 | |
| 2 | EtOH—MTP | 0.234 | 0.044 | 261 | 5.376 | <0.001 | |
| 6 | exoCORT—MTP | –1.215 | 0.044 | 262 | –27.711 | <0.001 | |
| 6 | EtOH—exoCORT | 1.453 | 0.045 | 263 | 32.653 | <0.001 | |
| 6 | EtOH—MTP | 0.238 | 0.043 | 262 | 5.491 | <0.001 | |
| mcTBQ | 2 | exoCORT–—MTP | –0.024 | 0.028 | 264 | –0.855 | 0.590 |
| 2 | EtOH—exoCORT | 0.038 | 0.027 | 264 | 1.401 | 0.487 | |
| 2 | EtOH—MTP | 0.014 | 0.026 | 264 | 0.518 | 0.605 | |
| 6 | exoCORT—MTP | 0.062 | 0.031 | 264 | 1.991 | 0.071 | |
| 6 | EtOH—exoCORT | –0.097 | 0.034 | 264 | –2.898 | 0.012 | |
| 6 | EtOH—MTP | –0.035 | 0.030 | 264 | –1.194 | 0.233 |
Estimates of linear contrasts are differences between the mean values of tadpoles exposed to the solvent control (EtOH), exogenous CORT (exoCORT), or metyrapone (MTP). P values were corrected using the FDR method for each dependent variable. Significant differences are highlighted in bold.
mcTBQ of tadpoles was significantly affected by the interaction between treatment length and exposure to exogenous chemicals (Table 1). In the 6-day treatments, tadpoles exposed to exoCORT contained significantly lower mcTBQ than control tadpoles and marginally nonsignificantly lower mcTBQ than tadpoles exposed to MTP, while the toxin content of tadpoles in the control group and those exposed to MTP did not differ significantly from each other (Table 2, Fig. 3b). Conversely, in tadpoles treated for 2 days, mcTBQ did not differ among treatments. The developmental stage of tadpoles significantly influenced mcTBQ, where more developed tadpoles contained lower amounts of bufadienolides per unit mass (Table 1, Fig. S3B). Predator treatment and its interactions had no significant effect on mcTBQ (Table 1).
Mass-corrected bufadienolide quantity significantly and positively correlated with CORT release rate (r = 0.226, P < 0.001, Fig. S4). However, this relationship was driven by developmental stage (Fig. S3): when we controlled for this covariate the correlation was no longer significant (r = 0.049, P = 0.414).
Discussion
Treating tadpoles with exogenous CORT resulted in significantly increased CORT release rates, regardless of treatment length, and this effect was accompanied by a decrease in bufadienolide toxin content after 6 days. Based on these results, we can reject the hypothesis that the chemical defense of toad tadpoles is upregulated by increased CORT levels (H1, Fig. 1). The observed relationship between CORT release rate and bufadienolide content in our study is in concordance with previous findings indicating that exposure to the amphibian chytrid fungus (Batrachochytrium dendrobatidis [Bd]), an important amphibian pathogen, induces increased CORT levels in multiple amphibian species (e.g., Gabor et al. 2013b, 2015, 2018, but see Hammond et al. 2020), but decreases bufadienolide content of common toad tadpoles (Ujszegi et al. 2021; Kásler et al. 2022). We caution, however, against interpreting our result as CORT inhibiting bufadienolide synthesis, for the following reasons. First, multiple studies report that various environmental stressors (e.g., intraspecific competitors, predators, anthropogenic pollutants) known to increase CORT levels in animals (e.g., Glennemeier and Denver 2002a; Berger et al. 2007; Eraud et al. 2008; Middlemis Maher et al. 2013; Braasch et al. 2014; Ye et al. 2015; Gabor et al. 2018; Monclús et al. 2018) also lead to increased bufadienolide production in toads (Bókony et al. 2017, 2018; Hettyey et al. 2019; Üveges et al. 2021). Second, we found that individual variation in bufadienolide content was not correlated with variation in CORT release rate after controlling for the developmental stage. We propose instead that our results on the effects of exoCORT treatment indirectly support the second hypothesis of our study (H2, Fig. 1), that is, that bufadienolide synthesis is induced by upstream hormones of the HPI axis, such as ACTH (Fedorova et al. 1998; Dostanic-Larson et al. 2005). Although we did not measure ACTH in our experimental tadpoles, treating them with exoCORT most probably led to the suppression of ACTH discharge due to negative feedback (Gjerstad et al. 2018; Godoy et al. 2018; Herman et al. 2020), which may have ultimately resulted in decreased bufadienolide content. While our study cannot provide direct evidence for the role of upstream brain hormones in regulating bufadienolide production in toads (H2, Fig. 1), this remains a probable pathway, since previous studies suggested ACTH-induced synthesis of endogenous cardiotonic steroids (Vinge et al. 1993; Laredo et al. 1994, 1995; Li et al. 1994; Yamada et al. 1997; Sophocleous et al. 2003; Dostanic-Larson et al. 2005), including unidentified bufadienolide-like compounds (Fedorova et al. 1998), in mammals.
We expected that treatment with MTP would lead to decreased CORT release rates compared to control animals, as in previous studies on amphibians (Glennemeier and Denver 2002a, 2002b). However, we found instead that MTP slightly, albeit significantly, increased CORT release rates compared to control tadpoles, regardless of treatment length. Such inconsistent effects of MTP treatment have been reported previously (Glennemeier and Denver 2002a; Kulkarni et al. 2017; Gabor et al. 2018; Kennedy et al. 2020). There may be multiple, mutually nonexclusive explanations for the inability of MTP to reduce CORT. First, it is likely that the MTP concentration used in our study did not entirely impede CORT synthesis (Glennemeier and Denver 2002a, 2002b). Therefore, the elevated level of CORT observed in tadpoles treated with MTP may have simply been a consequence of compensatory ACTH release due to decreased CORT synthesis (Coppage et al. 1959; McEwen 2007). Second, MTP inhibits aldosterone (ALDO) synthesis by inhibiting the transformation of 11-deoxycorticosterone to CORT and then that of CORT to ALDO (Gower 1974). ALDO is a potent mineralocorticoid, regulating natriuresis and water retention (Liddle 1958), and decreased levels of this hormone lead, among others, to increased urine volume (Liddle 1958; Brismar et al. 1985; Costello-Boerrigter et al. 2003). Since in our study we measured CORT release rate of tadpoles, it is possible that the apparently increased CORT in response to MTP is a consequence of increased urine (and this way CORT) excretion due to the inhibition of ALDO synthesis.
We observed a decrease in bufadienolide content only after a 6-day exposure period to exoCORT. It is well known that systemic effects of GCs fundamentally differ when high levels are sustained only acutely versus chronically (Sterling and Eyer 1988; McEwen 1998, 2004; McEwen and Wingfield 2003; Denver 2009; Middlemis Maher et al. 2013; Godoy et al. 2018). Effects of acute exoCORT exposure, or spikes in innate CORT levels, are relatively quickly counteracted by other physiological processes, and the concentrations of CORT and upstream hormones return to baseline levels (McEwen 1998; McEwen and Wingfield 2003; Romero et al. 2009; McEwen et al. 2015). On the other hand, chronically high levels of CORT can induce emergency countermeasures or even pathological changes in a multitude of physiological processes (Sterling and Eyer 1988; McEwen 1998, 2004; McEwen and Wingfield 2003; Denver 2009; Godoy et al. 2018). Such effects of the 6-day exoCORT treatment might have led to a decrease in bufadienolide content by, for example, the breakdown of the acidic bile-acid pathway of steroidogenesis (Fedorova et al. 2015), or depletion of toxin reserves due to catabolism of toxin compounds for energy release.
Lastly, exposure to predation cues did not result in increased CORT release rates in experimental tadpoles. It is possible that cues on the presence of predators are relatively weak triggers of the endocrine stress response in common toad tadpoles. For instance, induced responses against predators in, for example, behavior and morphology are accompanied by increased CORT levels in amphibians (Middlemis Maher et al. 2013; Kulkarni and Gramapurohit 2017), but common toad tadpoles display less intense behavioral and morphological plasticity to the presence of predators compared to larvae of other amphibians (Laurila et al. 1998; Lardner 2000; Van Buskirk 2002). This is possibly due to the effectiveness of bufadienolides providing defense against various predator species even at non-induced levels (Üveges et al. 2019). Future studies similar to the current experiment should use a higher concentration of predator cues or other types of (possibly more effective) stressors to trigger the upregulation of CORT release rates in toad tadpoles.
Bufadienolides are emerging as important compounds for multiple fields of research. They are considered to be important ion-transport regulators and agents of cell signaling (Pierre and Xie 2006; Schoner and Scheiner-Bobis 2007; Lingrel 2010; Lichtstein et al. 2012); however, to the best of our knowledge, studies investigating the housekeeping aspect of bufadienolides are entirely lacking outside of mammals. Therefore, it would be worthwhile for future studies to also focus on nonmodel vertebrates. Investigating how bufadienolide production influences other physiological processes would also be welcome. For instance, bufadienolides can also inhibit steroidogenesis (Kau et al. 2012); therefore, changes in bufadienolide levels may also influence sex-hormone synthesis. The consequences of this interaction are currently not known, but could potentially impact individual fitness and thus population dynamics and survival in the long run (Bókony et al. 2019). A deeper understanding of the regulation of these compounds might also benefit native populations suffering from invasive toad species (Shine 2010; Licata et al. 2019), by, for example, potentially leading to methods for selective inhibition of bufadienolide synthesis, thereby facilitating the survival of toad predators (Phillips and Shine 2006; Greenlees et al. 2010; Caller and Brown 2013). Last but not least, research on how these housekeeping molecules became effective chemical weapons may also provide important insights into molecular evolution. Clearly, more research is needed to shed light on the background of phenotypically plastic bufadienolide synthesis, and its potential effects on the physiology of animals, as well as its ecological and evolutionary consequences.
Supplementary Material
Acknowledgments
We thank Dr. R. Capon for supplying the marinobufotoxin standard, Dr. Z. Bokor for supplying fish, Dr. E. Tóth for generously lending us the homogenizer, S. Orf, students and colleagues of the Lendület Evolutionary Ecology Research Group for their help during the experiment, K.Á. Hamow and M. Dernovics for their help in preparing samples for ELISA, and N. Ujhegyi for the photo of a juvenile common toad in Fig. 1.
Contributor Information
B Üveges, Department of Evolutionary Ecology, Plant Protection Institute, Centre for Agricultural Research, Eötvös Loránd Research Network, Herman Ottó út 15, 1022 Budapest, Hungary; Molecular Ecology and Evolution at Bangor, School of Natural Sciences, Bangor University, Environment Centre Wales, Bangor LL57 2UW, UK.
C Kalina, Department of Evolutionary Ecology, Plant Protection Institute, Centre for Agricultural Research, Eötvös Loránd Research Network, Herman Ottó út 15, 1022 Budapest, Hungary; Department of Ecology, Institute of Biology, University of Veterinary Medicine, István u. 2, 1078 Budapest, Hungary.
K Szabó, Division of Clinical Immunology, Department for Internal Medicine, Faculty of Medicine, University of Debrecen, Móricz Zsigmond út 22, 4032 Debrecen, Hungary.
Á M Móricz, Department of Pathophysiology, Plant Protection Institute, Centre for Agricultural Research, Eötvös Loránd Research Network, Herman Ottó út 15, 1022 Budapest, Hungary.
D Holly, Department of Evolutionary Ecology, Plant Protection Institute, Centre for Agricultural Research, Eötvös Loránd Research Network, Herman Ottó út 15, 1022 Budapest, Hungary.
C R Gabor, Department of Evolutionary Ecology, Plant Protection Institute, Centre for Agricultural Research, Eötvös Loránd Research Network, Herman Ottó út 15, 1022 Budapest, Hungary; Department of Biology, College of Science and Engineering, Texas State University, 601 University Dr., San Marcos, TX 78666, USA.
A Hettyey, Department of Evolutionary Ecology, Plant Protection Institute, Centre for Agricultural Research, Eötvös Loránd Research Network, Herman Ottó út 15, 1022 Budapest, Hungary.
V Bókony, Department of Evolutionary Ecology, Plant Protection Institute, Centre for Agricultural Research, Eötvös Loránd Research Network, Herman Ottó út 15, 1022 Budapest, Hungary; Department of Ecology, Institute of Biology, University of Veterinary Medicine, István u. 2, 1078 Budapest, Hungary.
Funding
This work was supported by the Lendület Programme of the Hungarian Academy of Sciences [grant number LP2012-24/2012], the National Research, Development and Innovation Office of Hungary [grant numbers K-115402 and 135016 and 2019-2.1.11-TÉT-2019-00026 and ÚNKP-21-5], the 2018 Szent-Györgyi Young Investigator Award of the New York Hungarian Scientific Society [B.Ü.], and the János Bolyai Scholarship of the Hungarian Academy of Sciences [A.H. and V.B.].
Data availability
Dataset of the study is available on the figshare digital repository at https://doi.org/10.6084/m9.figshare.15090339 (Üveges et al. 2023).
Conflict of interest
The authors declare no competing interests.
References
- Achtymichuk GH, Crane AL, Simko OM, Stevens HEF, Ferrari MCO. 2022. The choice of euthanasia techniques can affect experimental results in aquatic behavioural studies. Anim Behav 185: 1–8. [Google Scholar]
- Arbuckle K, Brockhurst M, Speed MP. 2013. Does chemical defence increase niche space? A phylogenetic comparative analysis of the Musteloidea. Evol Ecol 27: 863–81. [Google Scholar]
- Barnhart K, Forman ME, Umile TP, Kueneman J, McKenzie V, Salinas I, Minbiole KPC, Woodhams DC. 2017. Identification of bufadienolides from the boreal toad, Anaxyrus boreas, active against a fungal pathogen. Microb Ecol 74: 990–1000. [DOI] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker BM, Walker S. 2015. Fitting linear mixed-effects models using lme4. J Stat Softw 67: 1–48. [Google Scholar]
- Baugh AT, Bastien B, Still MB, Stowell N. 2018. Validation of water-borne steroid hormones in a tropical frog (Physalaemus pustulosus). Gen Comp Endocrinol 261: 67–80. [DOI] [PubMed] [Google Scholar]
- Benard MF, Fordyce JA. 2003. Are induced defenses costly? Consequences of predator-induced defenses in western toads, Bufo boreas. Ecology 84: 68–78. [Google Scholar]
- Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B 57: 289–300. [Google Scholar]
- Berger S, Wikelski M, Romero LM, Kalko EKV, Rödl T. 2007. Behavioral and physiological adjustments to new predators in an endemic island species, the Galápagos marine iguana. Horm Behav 52: 653–63. [DOI] [PubMed] [Google Scholar]
- Blum MS. 1981. Chemical defenses of Arthropods. New York (NY): Academic Press Inc. [Google Scholar]
- Bókony V, Mikó Z, Móricz ÁM, Krüzselyi D, Hettyey A. 2017. Chronic exposure to a glyphosate-based herbicide makes toad larvae more toxic. Proc R Soc Lond B Biol Sci 284: 20170493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bókony V, Üveges B, Móricz ÁM, Hettyey A. 2018. Competition induces increased toxin production in toad larvae without allelopathic effects on heterospecific tadpoles. Funct Ecol 32: 667–75. [Google Scholar]
- Bókony V, Üveges B, Verebélyi V, Ujhegyi N, Móricz ÁM. 2019. Toads phenotypically adjust their chemical defences to anthropogenic habitat change. Sci Rep 9: 3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braasch A, Becker PH, Groothuis TGG. 2014. Response of testosterone and corticosterone plasma levels to the challenge of sibling competition: a study in common terns. Gen Comp Endocrinol 204: 95–103. [DOI] [PubMed] [Google Scholar]
- Brismar K, Werner S, Thorén M, Wetterberg L. 1985. Metyrapone: an agent for melatonin as well as ACTH and cortisol secretion. J Endocrinol Invest 8: 91–5. [DOI] [PubMed] [Google Scholar]
- Brodie ED III. 2009. Toxins and venoms. Curr Biol 19: R931–5. [DOI] [PubMed] [Google Scholar]
- Brodie ED III, Brodie ED Jr. 1990. Tetrodotoxin resistance in garter snakes: an evolutionary response of predators to dangerous prey. Evolution 44: 651–9. [DOI] [PubMed] [Google Scholar]
- Caller G, Brown C. 2013. Evolutionary responses to invasion: cane toad sympatric fish show enhanced avoidance learning. PLoS One 8: e54909–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casewell NR, Wüster W, Vonk FJ, Harrison RA, Fry BG. 2013. Complex cocktails: the evolutionary novelty of venoms. Trends Ecol Evol 28: 219–29. [DOI] [PubMed] [Google Scholar]
- Chen L, Huang G-Z. 2013. Poisoning by toxic animals in China––18 autopsy case studies and a comprehensive literature review. Forensic Sci Int 232: e12–23. [DOI] [PubMed] [Google Scholar]
- Coppage WS Jr, Island D, Smith M, Liddle GW. 1959. Inhibition of aldosterone secretion and modification of electrolyte excretion in man by a chemical inhibitor of 11β-hydroxylation. J Clin Invest 38: 2101–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello-Boerrigter LC, Boerrigter G, Burnett JC. 2003. Revisiting salt and water retention: new diuretics, aquaretics, and natriuretics. Med Clin North Am 87: 475–91. [DOI] [PubMed] [Google Scholar]
- Delfino G, Brizzi R, Feri L. 1995. Chemical skin defence in Bufo bufo: an ultrastructural study during ontogenesis. Zool Anz 234: 101–11. [Google Scholar]
- Denver RJ. 2009. Structural and functional evolution of vertebrate neuroendocrine stress systems. Ann NY Acad Sci 1163: 1–16. [DOI] [PubMed] [Google Scholar]
- Dostanic-Larson I, Van Huysse JW, Lorenz JN, Lingrel JB. 2005. The highly conserved cardiac glycoside binding site of Na, K-ATPase plays a role in blood pressure regulation. Proc Natl Acad Sci 102: 15845–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eraud C, Trouvé C, Dano S, Chastel O, Faivre B. 2008. Competition for resources modulates cell-mediated immunity and stress hormone level in nestling collared doves (Streptopelia decaocto). Gen Comp Endocrinol 155: 542–51. [DOI] [PubMed] [Google Scholar]
- Fedorova OV, Anderson DE, Bagrov AY. 1998. Plasma marinobufagenin-like and ouabain-like immunoreactivity in adrenocorticotropin-treated rats. Am J Hypertens 11: 796–802. [DOI] [PubMed] [Google Scholar]
- Fedorova OV, Zernetkina VI, Shilova VY, Grigorova YN, Juhasz O, Wei W, Marshall CA, Lakatta EG, Bagrov AY. 2015. Synthesis of an endogenous steroidal Na pump inhibitor marinobufagenin, implicated in human cardiovascular diseases, is initiated by CYP27A1 via bile acid pathway. Circ Cardiovasc Genet 8: 736–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filho GAC, Schwartz CA, Resck IS, Murta MM, Lemos SS, Castro MS, Kyaw C, Pires OR Jr, Leite JR, Bloch C Jret al. 2005. Antimicrobial activity of the bufadienolides marinobufagin and telocinobufagin isolated as major components from skin secretion of the toad Bufo rubescens. Toxicon 45: 777–82. [DOI] [PubMed] [Google Scholar]
- Forsburg ZR, Goff CB, Perkins HR, Robicheaux JA, Almond GF, Gabor CR. 2019. Validation of water-borne cortisol and corticosterone in tadpoles: recovery rate from an acute stressor, repeatability, and evaluating rearing methods. Gen Comp Endocrinol 281: 145–52. [DOI] [PubMed] [Google Scholar]
- Fox J, Weisberg S. 2019. An R companion to applied regression. Thousand Oaks (CA): Sage. [Google Scholar]
- Gabor CR, Bosch J, Fries JN, Davis DR. 2013a. A non-invasive water-borne hormone assay for amphibians. Amphibia-Reptilia 34: 151–62. [Google Scholar]
- Gabor CR, Fisher MC, Bosch J. 2013b. A non-invasive stress assay shows that tadpole populations infected with Batrachochytrium dendrobatidis have elevated corticosterone levels. PLoS One 8: e56054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabor CR, Fisher MC, Bosch J. 2015. Elevated corticosterone levels and changes in amphibian behavior are associated with batrachochytrium dendrobatidis (Bd) infection and Bd lineage. PLoS One 10: e0122685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabor CR, Knutie SA, Roznik EA, Rohr JR. 2018. Are the adverse effects of stressors on amphibians mediated by their effects on stress hormones? Oecologia 186: 393–404. [DOI] [PubMed] [Google Scholar]
- Gabor CR, Perkins HR, Heitmann AT, Forsburg ZR, Aspbury AS. 2019. Roundup™ with corticosterone functions as an infodisruptor to antipredator response in tadpoles. Front Ecol Evol 7: 114. [Google Scholar]
- Gabor CR, Zabierek KC, Kim DS, da Barbiano LA, Mondelli MJ, Bendik NF, Davis DR. 2016. A non-invasive water-borne assay of stress hormones in aquatic salamanders. Copeia 104: 172–81. [Google Scholar]
- Gjerstad JK, Lightman SL, Spiga F. 2018. Role of glucocorticoid negative feedback in the regulation of HPA axis pulsatility. Stress 21: 403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennemeier KA, Denver RJ. 2002a. Role for corticoids in mediating the response of Rana pipiens tadpoles to intraspecific competition. J Exp Zool 292: 32–40. [DOI] [PubMed] [Google Scholar]
- Glennemeier KA, Denver RJ. 2002b. Small changes in whole-body corticosterone content affect larval Rana pipiens fitness components. Gen Comp Endocrinol 127: 16–25. [DOI] [PubMed] [Google Scholar]
- Godoy LD, Rossignoli MT, Delfino-Pereira P, Garcia-Cairasco N, de Lima Umeoka EH. 2018. A comprehensive overview on stress neurobiology: basic concepts and clinical implications. Front Behav Neurosci 12: 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosner KL. 1960. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16: 183–90. [Google Scholar]
- Gower DB. 1974. Modifiers of steroid-hormone metabolism: a review of their chemistry, biochemistry and clinical applications. J Steroid Biochem 5: 501–23. [DOI] [PubMed] [Google Scholar]
- Greenlees MJ, Phillips BL, Shine R. 2010. Adjusting to a toxic invader: native Australian frogs learn not to prey on cane toads. Behav Ecol 21: 966–71. [Google Scholar]
- Hagman M, Hayes RA, Capon RJ, Shine R. 2009. Alarm cues experienced by cane toad tadpoles affect post-metamorphic morphology and chemical defences. Funct Ecol 23: 126–32. [Google Scholar]
- Hammond TT, Blackwood PE, Shablin SA, Richards-Zawacki CL. 2020. Relationships between glucocorticoids and infection with batrachochytrium dendrobatidis in three amphibian species. Gen Comp Endocrinol 285: 113269. [DOI] [PubMed] [Google Scholar]
- Hayes RA, Crossland MR, Hagman M, Capon RJ, Shine R. 2009. Ontogenetic variation in the chemical defenses of cane toads (Bufo marinus): toxin profiles and effects on predators. J Chem Ecol 35: 391–9. [DOI] [PubMed] [Google Scholar]
- Hayes TB, Wu TH. 1995. Interdependence of corticosterone and thyroid hormones in toad larvae (Bufo boreas). II. Regulation of corticosterone and thyroid hormones. J Exp Zool 271: 103–11. [DOI] [PubMed] [Google Scholar]
- Herman JP, Nawreen N, Smail MA, Cotella EM. 2020. Brain mechanisms of HPA axis regulation: neurocircuitry and feedback in context Richard Kvetnansky lecture. Stress 23: 617–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettyey A, Tóth Z, Thonhauser KE, Frommen JG, Penn DJ, Van Buskirk J. 2015. The relative importance of prey-borne and predator-borne chemical cues for inducible antipredator responses in tadpoles. Oecologia 179: 699–710. [DOI] [PubMed] [Google Scholar]
- Hettyey A, Üveges B, Móricz ÁM, Drahos L, Capon RJ, Van Buskirk J, Tóth Z, Bókony V. 2019. Predator-induced changes in the chemical defence of a vertebrate. J Anim Ecol 88: 1925–35. [DOI] [PubMed] [Google Scholar]
- Hossie TJ, Hassall C, Knee W, Sherratt TN. 2013. Species with a chemical defence, but not chemical offence, live longer. J Evol Biol 26: 1598–602. [DOI] [PubMed] [Google Scholar]
- Kamboj A, Rathour A, Mandeep K. 2013. Bufadienolides and their medicinal utility: a review. Int J Pharm Pharm Sci 5: 20–7. [Google Scholar]
- Kásler A, Ujszegi J, Holly D, Üveges B, Móricz ÁM, Herczeg D, Hettyey A. 2022. Metamorphic common toads keep chytrid infection under control, but at a cost. J Zool 317: 159–69. [Google Scholar]
- Kau M-M, Wang J-R, Tsai S-C, Yu C-H, Wang PS. 2012. Inhibitory effect of bufalin and cinobufagin on steroidogenesis via the activation of ERK in human adrenocortical cells. Br J Pharmacol 165: 1868–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy CLM, Carter SD, Mifsud KR, Reul JMHM. 2020. Unexpected effects of metyrapone on corticosteroid receptor interaction with the genome and subsequent gene transcription in the hippocampus of male rats. J Neuroendocrinol 32: e12820. [DOI] [PubMed] [Google Scholar]
- Kim S. 2015. ppcor: an R package for a fast calculation to semi-partial correlation coefficients. Commun Stat Appl Methods 22: 665–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolhaas JM, Bartolomucci A, Buwalda B, de Boer SF, Flügge G, Korte SM, Meerlo P, Murison R, Olivier B, Palanza Pet al. 2011. Stress revisited: a critical evaluation of the stress concept. Neurosci Biobehav Rev 35: 1291–301. [DOI] [PubMed] [Google Scholar]
- Kulkarni PS, Gramapurohit NP. 2017. Effect of corticosterone on larval growth, antipredator behaviour and metamorphosis of Hylarana indica. Gen Comp Endocrinol 251: 21–9. [DOI] [PubMed] [Google Scholar]
- Kulkarni SS, Denver RJ, Gomez-Mestre I, Buchholz DR. 2017. Genetic accommodation via modified endocrine signalling explains phenotypic divergence among spadefoot toad species. Nat Commun 8: 993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lardner B. 2000. Morphological and life history responses to predators in larvae of seven anurans. Oikos 88: 169–80. [Google Scholar]
- Laredo J, Hamilton BP, Hamlyn JM. 1994. Ouabain is secreted by bovine adrenocortical cells. Endocrinology 135: 794–7. [DOI] [PubMed] [Google Scholar]
- Laredo J, Hamilton BP, Hamlyn JM. 1995. Secretion of endogenous ouabain from bovine adrenocortical cells: role of the zona glomerulosa and zona fasciculata. Biochem Biophys Res Commun 212: 487–93. [DOI] [PubMed] [Google Scholar]
- Laurila A, Kujasalo J, Ranta E. 1997. Different antipredator behaviour in two anuran tadpoles: effects of predator diet. Behav Ecol Sociobiol 40: 329–36. [Google Scholar]
- Laurila A, Kujasalo J, Ranta E. 1998. Predator-induced changes in life history in two anuran tadpoles: effects of predator diet. Oikos 83: 307–17. [Google Scholar]
- Lenth R. 2023. emmeans: estimated marginal means, aka least-squares means. R package version 1.8.4-1, ⟨https://CRAN.R-project.org/package=emmeans⟩, doi: https://doi.org/10.1080/00031305.1980.10483031.
- Li M, Wong K-S, Martin A, Whitworth JA. 1994. Adrenocorticotrophin-induced hypertension in rats: role of progesterone and digoxin-like substances. Am J Hypertens 7: 59–68. [DOI] [PubMed] [Google Scholar]
- Licata F, Ficetola GF, Freeman K, Mahasoa RH, Ravololonarivo V, Solofo Niaina Fidy JF, Koto-Jean AB, Nahavitatsara ER, Andreone F, Crottini A. 2019. Abundance, distribution and spread of the invasive Asian toad Duttaphrynus melanostictus in eastern Madagascar. Biol Invasions 21: 1615–26. [Google Scholar]
- Lichtstein D, Rosen H, Dvela M. 2012. Cardenolides and bufadienolides as hormones: what is missing? Am J Physiol Renal Physiol 302: F957–8. [DOI] [PubMed] [Google Scholar]
- Liddle GW. 1958. Aldosterone antagonists. Arch Intern Med 102: 998–1004. [DOI] [PubMed] [Google Scholar]
- Lingrel JB. 2010. The physiological significance of the cardiotonic steroid/ouabain-binding site of the Na,K-atpase. Annu Rev Physiol 72: 395–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewelyn J, Bell K, Schwarzkopf L, Alford RA, Shine R. 2012. Ontogenetic shifts in a prey's chemical defences influence feeding responses of a snake predator. Oecologia 169: 965–73. [DOI] [PubMed] [Google Scholar]
- McEwen BS. 1998. Stress, adaptation, and disease: allostasis and allostatic load. Ann NY Acad Sci 840: 33–44. [DOI] [PubMed] [Google Scholar]
- McEwen BS. 2004. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann NY Acad Sci 1032: 1–7. [DOI] [PubMed] [Google Scholar]
- McEwen BS. 2007. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev 87: 873–904. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Gray JD, Nasca C. 2015. Redefining neuroendocrinology: stress, sex and cognitive and emotional regulation. J Endocrinol 226: T67–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Wingfield JC. 2003. The concept of allostasis in biology and biomedicine. Horm Behav 43: 2–15. [DOI] [PubMed] [Google Scholar]
- Maher MJ, Warner EE, Denver RJ. 2013. Stress hormones mediate predator-induced phenotypic plasticity in amphibian tadpoles. Proc Royal Soc Lond B Biol Sci 280: 20123075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monclús L, Ballesteros-Cano R, De La Puente J, Lacorte S, Lopez-Bejar M. 2018. Influence of persistent organic pollutants on the endocrine stress response in free-living and captive red kites (Milvus milvus). Environ Pollut 242: 329–37. [DOI] [PubMed] [Google Scholar]
- Nelson DWM, Crossland MR, Shine R. 2010. Indirect ecological impacts of an invasive toad on predator–prey interactions among native species. Biol Invasions 12: 3363–9. [Google Scholar]
- Nelson DWM, Crossland MR, Shine R. 2011. Foraging responses of predators to novel toxic prey: effects of predator learning and relative prey abundance. Oikos 120: 152–8. [Google Scholar]
- Pawlik JR. 1993. Marine invertebrate chemical defenses. Chem Rev 93: 1911–22. [Google Scholar]
- Payne AH, Hales DB. 2004. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev 25: 947–70. [DOI] [PubMed] [Google Scholar]
- Payton ME, Greenstone MH, Schenker N. 2003. Overlapping confidence intervals or standard error intervals: what do they mean in terms of statistical significance? J Insect Sci 3: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips BL, Shine R. 2006. An invasive species induces rapid adaptive change in a native predator: cane toads and black snakes in Australia. Proc Royal Soc Lond B Biol Sci 273: 1545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre SV, Xie Z. 2006. The Na,K-atpase receptor complex. Cell Biochem Biophys 46: 303–16. [DOI] [PubMed] [Google Scholar]
- Pike N. 2011. Using false discovery rates for multiple comparisons in ecology and evolution. Methods Ecol Evol 2: 278–82. [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D; R Core Team . 2017. nlme: linear and nonlinear mixed effects models. Vienna: The R Project for Statistical Computing. [Google Scholar]
- R Development Core Team . 2017. A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Rohr JR, Raffel TR, Halstead NT, McMahon TA, Johnson SA, Boughton RK, Martin LB. 2013. Early-life exposure to a herbicide has enduring effects on pathogen-induced mortality. Proc Royal Soc Lond B Biol Sci 280: 20131502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero LM, Dickens MJ, Cyr NE. 2009. The reactive scope model—A new model integrating homeostasis, allostasis, and stress. Horm Behav 55: 375–89. [DOI] [PubMed] [Google Scholar]
- Schoeppner NM, Relyea RA. 2005. Damage, digestion, and defence: the roles of alarm cues and kairomones for inducing prey defences. Ecol Lett 8: 505–12. [DOI] [PubMed] [Google Scholar]
- Schoner W, Scheiner-Bobis G. 2007. Endogenous and exogenous cardiac glycosides and their mechanisms of action. Am J Cardiovasc Drugs 7: 173–89. [DOI] [PubMed] [Google Scholar]
- Shine R. 2010. The ecological impact of invasive cane toads (Bufo marinus) in Australia. Q Rev Biol 85: 253–91. [DOI] [PubMed] [Google Scholar]
- Sophocleous A, Elmatzoglou I, Souvatzoglou A. 2003. Circulating endogenous digitalis-like factor(s) (EDLF) in man is derived from the adrenals and its secretion is ACTH-dependent. J Endocrinol Invest 26: 668–74. [DOI] [PubMed] [Google Scholar]
- Sterling P, Eyer J. 1988. Allostasis: a new paradigm to explain arousal pathology. In: Fisher S., Reason J., editors. Handbook of life stress, cognition and health. John Wiley & Sons: New York, USA, p. 629–49. [Google Scholar]
- Tempone AG, Pimenta DC, Lebrun I, Sartorelli P, Taniwaki NN, de Andrade Jr HF, Antoniazzi MM, Jared C. 2008. Antileishmanial and antitrypanosomal activity of bufadienolides isolated from the toad Rhinella jimi parotoid macrogland secretion. Toxicon 52: 13–21. [DOI] [PubMed] [Google Scholar]
- Toledo RC, Jared C. 1995. Cutaneous granular glands and amphibian venoms. Comp Biochem Physiol A Physiol 111: 1–29. [Google Scholar]
- Ujszegi J, Ludányi K, Móricz ÁM, Krüzselyi D, Drahos L, Drexler T, Németh MZ, Vörös J, Garner TWJ, Hettyey A. 2021. Exposure to Batrachochytrium dendrobatidis affects chemical defences in two anuran amphibians, Rana dalmatina and Bufo bufo. BMC Ecol Evol 21: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujszegi J, Móricz ÁM, Krüzselyi D, Hettyey A. 2017. Skin toxin production of toads changes during early ontogeny but is not adjusted to the microbiota of the aquatic environment. Evol Ecol 31: 925–36. [Google Scholar]
- Ujvari B, Casewell NR, Sunagar K, Arbuckle K, Wüster W, Lo N, O'Meally D, Beckmann C, King GF, Deplazes Eet al. 2015. Widespread convergence in toxin resistance by predictable molecular evolution. Proc Natl Acad Sci 112: 11911–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Üveges B, Basson AC, Móricz ÁM, Bókony V, Hettyey A. 2021. Chemical defence effective against multiple enemies: does the response to conspecifics alleviate the response to predators? Funct Ecol 35: 2294–304. [Google Scholar]
- Üveges B, Fera G, Móricz ÁM, Krüzselyi D, Bókony V, Hettyey A. 2017. Age- and environment-dependent changes in chemical defences of larval and post-metamorphic toads. BMC Evol Biol 17: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Üveges B, Kalina C, Szabó K, Móricz ÁM, Holly D, Gabor CR, Hettyey A, Bókony V. 2023. Data: does stress make toads more toxic? An experimental study on the hormonal regulation of bufadienolide toxin synthesis. figshare, doi: 10.6084/m9.figshare.15090339. [DOI] [PMC free article] [PubMed]
- Üveges B, Szederkényi M, Mahr K, Móricz ÁM, Krüzselyi D, Bókony V, Hoi H, Hettyey A. 2019. Chemical defense of toad tadpoles under risk by four predator species. Ecol Evol 9: 6287–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Buskirk J. 2002. A comparative test of the adaptive plasticity hypothesis: relationships between habitat and phenotype in anuran larvae. Am Nat 160: 87–102. [DOI] [PubMed] [Google Scholar]
- Verebélyi V. 2017. Barna varangy (Bufo bufo) szaporodási sikerének és szennyezőanyag rezisztenciájának összehasonlítása különböző szennyezettségű élőhelytípusok között. Budapest: Institute of Biology, Department of Ecology, Unversity of Veterinary Medicine Budapest. [Google Scholar]
- Vinge E, Erfurth E-M, Odar-Cederlöf I. 1993. Immunoreactive endogenous digoxin-like substances: plasma levels are dependent on the hypothalamic-pituitary-adrenal axis for release and on kidney function for elimination. J Cardiovasc Pharmacol 22: S112–3. [PubMed] [Google Scholar]
- von Frisch K. 1942. Über einen schreckstoff der Fischhaut und seine biologische bedeutung. Z. Vergl. Physiol 29: 46–145. [Google Scholar]
- Webb JK, Brown GP, Child T, Greenlees MJ, Phillips BL, Shine R. 2008. A native dasyurid predator (common planigale, Planigale maculata) rapidly learns to avoid a toxic invader. Austral Ecol 33: 821–9. [Google Scholar]
- Yamada K, Goto A, Omata M. 1997. Adrenocorticotropin-induced hypertension in rats: role of ouabain-like compound. Am J Hypertens 10: 403–8. [PubMed] [Google Scholar]
- Ye L, Hu Z, Wang H, Zhu H, Dong Z, Jiang W, Zhao H, Li N, Mi W, Wang Wet al. 2015. Tris-(2,3-dibromopropyl) isocyanurate, a new emerging pollutant, impairs cognition and provokes depression-like behaviors in adult rats. PLoS One 10: e0140281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SD, Gavel MJ, Gutierrez-Villagomez JM, Forbes MR, Robinson SA. 2020. Assessment of sublethal ecotoxicity of solvents on larvae of a model native amphibian (Lithobates pipiens). J Appl Toxicol 40: 483–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Üveges B, Kalina C, Szabó K, Móricz ÁM, Holly D, Gabor CR, Hettyey A, Bókony V. 2023. Data: does stress make toads more toxic? An experimental study on the hormonal regulation of bufadienolide toxin synthesis. figshare, doi: 10.6084/m9.figshare.15090339. [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Dataset of the study is available on the figshare digital repository at https://doi.org/10.6084/m9.figshare.15090339 (Üveges et al. 2023).