Highlights
-
•
Erectile dysfunction (ED) persistent inability to attain, maintain penile erection.
-
•
Sildenafil citrate (ViagraⓇ), a potent selective phosphodiesterase-5 inhibitor.
-
•
ViagraⓇ has proven efficacy and safety as first line therapy in treatment of ED.
-
•
ViagraⓇ orodispersible film (ODF) benefits patients with swallowing difficulty.
-
•
ViagraⓇ ODF (with and without water) found bioequivalent to the film-coated tablet.
Key words: Bioequivalence, Erectile dysfunction, Film-coated tablets, Orodispersible film, Pharmacokinetic parameters, Sildenafil
Abstract
Background
Orodispersible film (ODF) formulation offers ease of use, convenience of administration, and other advantages, especially for patients who have difficulty in swallowing or are on liquid restriction compared with conventional oral formulations for the treatment of erectile dysfunction.
Objectives
These studies compared the bioequivalence of 50 mg sildenafil citrate ODF formulation (test drug) with the marketed 50 mg sildenafil citrate film-coated tablet (FCT) (ViagraⓇ; Pfizer, New York, NY) (reference drug), with and without water in 2 randomized cross-over studies.
Methods
Two randomized cross-over studies were conducted. The first study explored the bioequivalence of test drug administered with and without water compared with the reference drug with water. The second study investigated the bioequivalence of test drug, without water, compared with the reference drug with water. Forty-two and 80 healthy male volunteers were recruited in the first and second study, respectively. All volunteers fasted for 10 hours pre-dose. A 1-day washout period between doses was observed. Blood samples were collected at both before (up to 120 minutes before dosing) and after dosing (at different intervals up to 14 hours) stages. Statistical analyses on pharmacokinetic parameters were performed. Safety and tolerability for both the formulations were evaluated.
Results
In the first study, bioequivalence was demonstrated for sildenafil citrate ODF administered with water when compared with the ViagraⓇ FCT. The ratios of adjusted geometric means (90% confidence interval (CI)) were maximum plasma concentration: 1.02 (94.91–108.78) and area under the plasma concentration-time curve: 1.09 (104.49–113.21) for sildenafil citrate ODF administered with water vs ViagraⓇ FCT. These ratios were within the bioequivalence acceptance range of 80% to 125%, indicating that the bioequivalence criteria were met. The pharmacokinetic parameters for the second study also showed bioequivalence for sildenafil citrate ODF (without water) compared with ViagraⓇ FCT. The ratios of adjusted geometric means (90% CI) were maximum plasma concentration: 1.02 (95.47–109.36) and area under the plasma concentration-time curve: 1.06 (103.42–108.40) for sildenafil citrate ODF administered without water vs ViagraⓇ FCT. Adverse events in both the studies occurred at similar rates for the 2 formulations and were mild in intensity.
Conclusions
These results suggest that the new ODF formulation can be used interchangeably with the marketed FCT formulation. Sildenafil citrate ODF administered with and without water met bioequivalence criteria compared with ViagraⓇ FCT administered with water under fasted conditions in healthy adult male volunteers. The new ODF formulation can be used as a suitable alternative to the conventional oral solid dosage form.
Introduction
Erectile dysfunction (ED) is defined as the persistent inability to attain and maintain a penile erection sufficient to permit satisfactory sexual performance.1, 2, 3 Worldwide, ED has affected up to 150 million men and has been predicted to upsurge to 322 million cases by 2025. ED is most common in the United States, affecting 52% of men aged between 40 and 70 years and 70% of men older than age 70 years.4
Sildenafil citrate (ViagraⓇ; Pfizer Inc, New York) is a potent, competitive, and selective inhibitor of cyclic guanosine monophosphate-specific phosphodiesterase type 5. Due to its well-established safety and effective treatment profile, it has been recommended as the first-line therapy for ED treatment.5, 6, 7
Since approval in 1998, sildenafil citrate film-coated oral tablets (ViagraⓇ FCT) have been used in the treatment of ED and available at the doses of 25, 50, and 100 mg. However, the recommended dose for most patients is 50 mg to be taken approximately 1 hour before sexual activity. The dose may be increased to a maximum recommended dose of 100 mg or decreased to 25 mg based on efficacy and tolerability.8
ViagraⓇ FCT is rapidly absorbed (approximately 1 hour in the fasted state), the mean plasma clearance is 41 L/h and a mean absolute bioavailability of 41% (range 25%–63%). The mean steady-state sildenafil volume of distribution is 105 L, representative of high distribution into the tissues. It reaches peak plasma concentrations (CPEAK) within 30 to 120 minutes (median time = 60 minutes) and has a terminal t½ of 3 to 5 hours. Its pharmacokinetic parameters are dose-proportional from 25 to 100 mg, and it is predominantly eliminated via the cytochrome P450 3A4 enzymatic pathway.
The major active metabolite of sildenafil, N-desmethyl sildenafil, has roughly 50% the PDE-5 inhibitory potency and approximately 40% the plasma concentration as the parent. The tolerability and efficacy of sildenafil has been established in numerous placebo-controlled trials.8
A novel sildenafil orodispersible film (ODF) containing sildenafil citrate has been developed that disintegrates rapidly in the oral cavity, usually within few seconds, without the need for water. This provides a valuable alternative to solid oral forms (ie, tablets) for the treatment of ED. This formulation especially benefits patients who have difficulty in swallowing and who are on liquid restriction (eg, patients with renal impairment, endocrine system, and adrenal gland disorders).9, 10, 11, 12, 13
Furthermore, the ease of storage and portability (eg, availability in single-dosage packs or multi-unit film packaging) makes ODFs the preferred dose form over other solid dosage forms. They are also known to offer superior dosing accuracy, convenience, and rapid dissolution.
Two studies were conducted to demonstrate pharmacokinetic equivalence between sildenafil citrate ODF and ViagraⓇ FCT. The primary objectives of both the studies were to compare the rate (ie, peak plasma concentration [CPEAK]) and extent (area under the plasma concentration-time curve [AUCL]) of sildenafil absorption after single-dose administration of test and reference drug. The secondary end points were similar in both the studies and focused on identifying, monitoring, documenting, and categorizing the treatment-emergent adverse events (TEAEs), in the participants throughout the study.
Methods
Studies, protocols, and consent forms were approved by institutional review boards or ethics committees at the study sites, and studies were conducted in accordance with the principles of good clinical practice and the Declaration of Helsinki. All patients provided written informed consent before study participation.
Study design
The pharmacokinetic bio similarity between sildenafil citrate ODF and ViagraⓇ FCTs was investigated by conducting 2 randomized control trials, SILD-FMS-1001 and SILD-FMS-1002.
SILD-FMS-1001 was a single-dose, randomized, 3-period, 3-treatment cross-over study that explored the bioequivalence of sildenafil citrate ODF administered with and without water in comparison with ViagraⓇ FCTs. This study also monitored safety in the subjects by assessing adverse events (AEs) throughout the study period. The study was conducted in healthy adult male volunteers under fasting conditions. A total of 42 volunteers were enrolled in the study and allocated randomly to receive a single, oral dose of 50 mg (1 × 50 mg) of the test product, sildenafil citrate ODF, administered with or without water or the reference product, ViagraⓇ FCTs single 1 × 50 mg with a minimum 1-day washout period between the successive doses. The volunteers enrolled in all the 3 treatment groups were under fasting conditions overnight or for 10 hours.
-
○
Sildenafil citrate ODF 50 mg (test drug) with water: In this group, volunteers were allowed to pre-wet their mouth with 20 mL water, after which sildenafil citrate ODF 50 mg was placed directly on the tongue by the study investigator and allowed to disintegrate completely before administering 220 mL water.
-
○
Sildenafil citrate ODF 50 mg without water: The volunteers were allowed to pre-wet their mouth with 20 mL water following which sildenafil ODF 50 mg was placed directly on the tongue by the study investigator and allowed to disintegrate completely before the volunteer was allowed to swallow.
-
○
ViagraⓇ FCTs 50 mg with water (reference drug): This treatment group received the reference drug taken with 240 mL ±10 mL water.
SILD-FMS-1002 was a single-dose, randomized, 2-period, 2-treatment cross-over study that investigated the bioequivalence of sildenafil citrate ODF without water with ViagraⓇ FCTs. The study included 80 healthy adult male volunteers and allocated randomly to receive a single, oral dose of 50 mg (1 × 50 mg) of the test product, sildenafil citrate ODF, administered without water or the reference product, ViagraⓇ FCT single oral dose 1 × 50 mg, with a minimum 1-day washout period between successive doses under fasting conditions. In the treatment group with sildenafil citrate ODF without water condition, volunteers were instructed to swallow saliva at 20, 40, and 60 seconds after placement of ODF on tongue. In case the film did not dissolve within this time frame, volunteers were asked to wait until dissolution of the film was noted and or to be swallowed after 60 seconds of dosing. In the treatment group with ViagraⓇ FCT, the reference drug was administered to participants taken with 240 mL ±10 mL water.
Study subjects
Healthy volunteers were eligible to participate in the study if they satisfied the following inclusion criteria: aged 18 to 55 years, men, weighing at least 50 kg (110 lb), and with a body mass index ≤30.0 but ≥19.0. Also, subjects had no clinically relevant abnormalities related to blood pressure (BP), pulse rate (PR), respiratory rate (RR), and temperature and were willing to comprehend and sign the written informed consent form. The subjects were excluded if they had evidence or a history of clinically significant abnormal cardiovascular, hematological, hepatic, lipid and renal disease; history of or current hepatitis B or C, tuberculosis, human immune deficiency virus or syphilis infection; alcohol consumption within 48 hours; use of tobacco/nicotine-containing products within 1 year; history of use of any prescription or over-the-counter medications within 14 days; ingestion of any vitamins or herbal products within 7 days; use of drugs that induce or inhibit hepatic enzyme activity within 28 days; history or currently experiencing severe allergic reaction, anatomical deformation of the penis; and allergy or hypersensitivity to sildenafil.
The inclusion and exclusion criteria for both the studies were similar except that subjects between ages 18 and 45 years were included in the study SILD-FMS-1002.
Sample collection and assay
In both the studies SILD-FMS-1001 and SILD-FMS-1002, venous blood samples (6 mL) for the pharmacokinetic analysis of sildenafil citrate and its active metabolite N-desmethyl-sildenafil were collected in K2 EDTA tubes through indwelling catheter or direct venipuncture into a forearm vein.
The blood samples were collected at pre-dose (within 120 minutes before dosing) and at 0.083, 0.25, 0.50, 0.75, 1.00, 1.50, 2.0, 3.0, 4.0, 6.0, 8.0, 10, 12, and 14 hours post dose. BP, PR, temperature, and RR were measured at 2-, 4- and 14-hours after drug administration. All subjects were subjected to a post-study evaluation at study exit, including physical examination, vital signs (BP, PR, RR, and temperature), 12-lead electrocardiogram, and laboratory evaluation.
Pharmacokinetic analyses
Pharmacokinetic analyses were performed in a similar fashion for both SILD-FMS-1001 and SILD-FMS-1002 studies. The collected pharmacokinetic blood samples were centrifuged at 1900 g ±400 g for 10 minutes at 4°C to get plasma, and later stored in freezer at –70°C within 90 minutes after centrifugation in prelabeled polypropylene tubes. Plasma samples were assayed for sildenafil using 96-well solid phase extraction and validated through HPLC-MS/MS with a lower limit of quantification of 4 ng/mL. The calibration standard responses of assay were observed to be linear over the range of 4 to 1500 ng/mL. Samples were also assayed for N-desmethyl sildenafil using validated liquid-liquid extraction and HPLC-MS/MS with a lower limit of quantification of 1 ng/mL. The assay responses were linear from 1 to 375 ng/mL. All samples were analyzed at a bioanalytical laboratory in the United States (Mylan Pharmaceuticals Inc, Morgantown, West Virginia).
The pharmacokinetic parameters were determined for sildenafil and its metabolite using standard non-compartmental analysis. The maximum concentration (CPEAK [primary end point]) and the time at which it occurred relative to the administered dose (TPEAK) was determined from the observed plasma concentration-time profile over the sampling time interval. The elimination rate constant (KEL) was determined by linear regression of the terminal linear phase of the log plasma concentration-time profile.
AUCL is the sum of the linear trapezoidal estimation of the areas from the time of dosing to the time of the last quantifiable concentration. Area under the plasma concentration-time curve from zero to infinity (AUC∞) was calculated as: AUC∞ = AUCL + LQC/KEL where LQC is the last quantifiable concentration. The t½ was calculated as t½ = 0.693/KEL.
Patients were monitored for occurrence of any TEAEs, vital signs (BP and heart rate), general physical examination, laboratory parameters, and 12-lead electrocardiogram throughout the study period.
Statistical Analyses
A sample size of 39 subjects was required to provide 80% power with a true ratio between 95% and 105% and an intrasubject variability of 30% for the study SILD-FMS-1001 to conclude the bioequivalence between sildenafil citrate ODF with and without water and ViagraⓇ FCTs within the range of 80% to 125% for all parameters.
Whereas the sample size calculation for SILD-FMS-1002 study was assumed to have a true ratio between 87% and 115% and an intrasubject variability of 20%. A minimum of 72 subjects was required to conclude bioequivalence with approximately 80% power.
Considering the dropouts, noncompliance, and other such reasons, 42 subjects in the first study and 80 subjects in the second study were recruited.
The statistical analyses on pharmacokinetic parameters were performed using the General Linear Models Procedure (PROC GLM) of SAS Software (SAS Institute, Cary, North Carolina). The significance level was fixed at an alpha level of 0.05. The natural log-transformed parameters: LNAUCL and LNCPEAK were analyzed. The tests were performed to analyse for statistically significant differences in the pharmacokinetic parameters. Least squares means was used to determine the test to reference ratios of the pharmacokinetic parameters. The 90% CIs were constructed using the two 1-sided tests procedure.
The bioequivalence in first and second study was demonstrated, if the 90% CIs for the ratio of adjusted geometric means of test drug administered with and without water relative to the reference drug for sildenafil AUCL and CPEAK were within 80% to 125%. The relative bioavailability was estimated as the ratio of adjusted geometric means for sildenafil citrate ODF administered with and without water relative to the ViagraⓇ FCTs administered with water for sildenafil AUCL and CPEAK.
The pharmacokinetic parameters AUC∞, AUCL, CPEAK, TPEAK, t½, and KEL of sildenafil were summarized descriptively treatment-wise. For sildenafil AUCL, AUC∞, and CPEAK, individual subject parameters were plotted based on the treatment. In both the studies, mean sildenafil plasma concentration-time data were plotted against treatment and were represented on log-linear scales.
Results
In the SILD-FMS-1001 study, 42 subjects were enrolled, and 40 participants completed the study. Two subjects withdrew from the study due to personal reasons. The subjects included were White, Black or African American, Asian, American Indian or Alaska Native, Native Hawaiian or other Pacific Islander, and other races were recruited. All subjects were men between ages 18 and 55 years (mean age, 41.2 [9.46] years). The mean (SD) body mass index was 26.6 (2.69) (range, 19.6–30 kg/m2).
In the SILD-FMS-1002 study, 80 subjects were enrolled, and all subjects completed the study. The subjects who participated belonged to White, Black or African American, Asian, American Indian or Alaska Native, Native Hawaiian or other Pacific Islander, or other races. The subjects were men between ages 18 and 45 years (mean age, 31.2 [6.3] years). The mean (SD) body mass index was 23.90 (2.81) (range, 19.1–29.4 kg/m2).
Pharmacokinetics
Bioequivalence study of SILD-FMS-1001
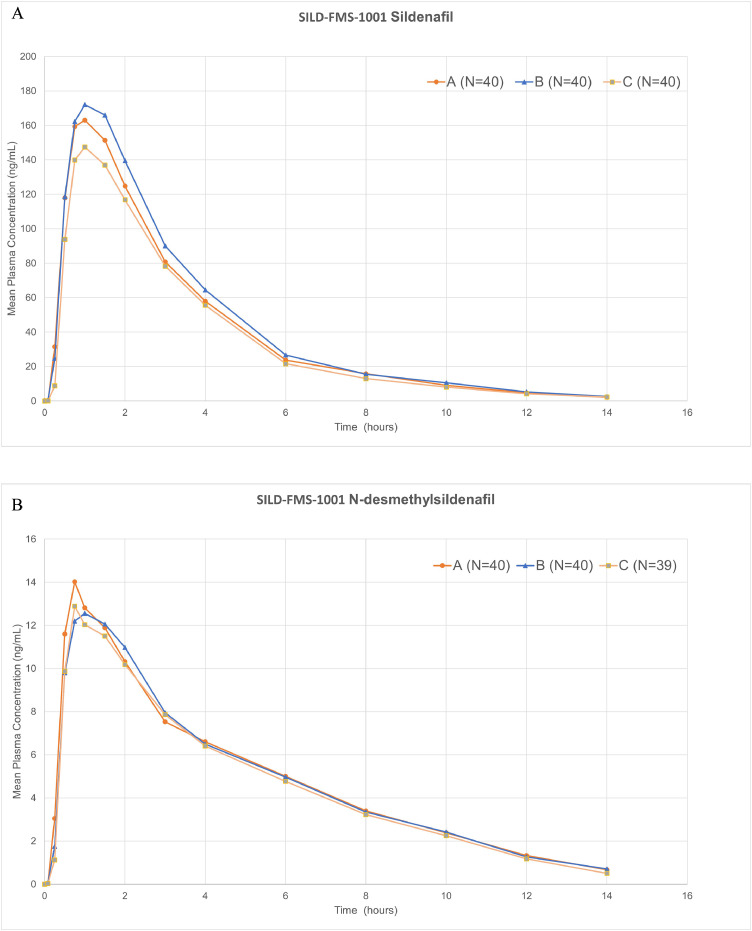
Following a single oral dose of 50 mg sildenafil citrate, the mean sildenafil plasma concentration–time profiles for all 3 treatments were principally overlapping. The corresponding pharmacokinetic parameters are provided in Table 1 and Table 2. The mean concentration–time profiles for sildenafil in first study have been illustrated in Fig. 1A. Systemic exposure (CPEAK and AUCL) of sildenafil and its metabolite were comparable across all the 3 treatments. TPEAK values were comparable across the treatments, with a range of 1.064 to 1.338 hour. Mean t½ was comparable in all the treatments with a mean value of approximately 2.6 hours. The variability for CPEAK and AUCL, based on arithmetic mean (%CV), was generally similar for all treatments (47%–57% and 38%–41%).
Table 1.
Descriptive summary of plasma sildenafil pharmacokinetic parameters after a single oral dose of 50 mg sildenafil citrate (SILD-FMS-1001).
| Parameter | Sildenafil citrate ODF with water (A) (n = 41)* |
Sildenafil citrate ODF without water (B) (n = 41)* |
ViagraⓇ† FCT (C) (n = 42)* |
...Ratio (A/C)* |
Ratio (B/C)‡,|| |
|---|---|---|---|---|---|
| CPEAK, ng/mL | 182.7 (46.72) | 201.3 (47.66) | 181.3 (57.40 | 1.02 (94.91–108.78) | 1.07 (91.26–125.95) |
| AUCL, ng/hr/mL | 578.3 (40.89) | 628.9 (40.05) | 525.3 (38.22) | 1.09 (104.49–113.21) | 1.12 (94.39–132.92) |
| AUC∞, ng/hr/mL | 606.2 (41.61)¶ | 668.0 (36.16)¶ | 547.4 (37.72) | – | – |
| KEL, h−1 | 0.2816 (31.11)¶ | 0.2929 (28.58)¶ | 0.2024 (34.11) | – | – |
| t½, h | 2.707 (31.35)¶ | 2.591 (33.21)¶ | 2.652 (34.04) | – | – |
| TPEAK, h | 1.044 (44.69) | 1.338 (66.19) | 1.188 (44.15) | – | – |
AUC... = area under the plasma concentration-time curve from zero to infinity; AUCL = area under the plasma concentration-time curve; CPEAK = maximum plasma concentration; FCT = film coated tablet; KEL = elimination rate constant; ODF = orally disintegrating film; TPEAK = time to CPEAK.
Values are presented as arithmetic mean (% coefficient of variation).
Pfizer Inc, New York, New York.
Ratio of geometric least-squares means (90% CI, used as natural log transformed parameter).
§Ratio (A/C) = e [LSMEAN of (LNA = Natural log transformed sildenafil citrate ODF with water – LNC = Natural log transformed Viagra FCT).
Ratio (B/C) = e [LSMEAN of (LNB = Natural log transformed sildenafil citrate ODF without water – LNC = Natural log transformed Viagra FCT)].
n=39.
Table 2.
Mean N-desmethyl-sildenafil plasma concentrations after single-dose administration of test and reference doses of sildenafil citrate 50 mg (SILD-FMS-1001).
| Parameter | Sildenafil citrate ODF with water (A) (n = 41)* |
Sildenafil citrate ODF without water (B) (n = 41)* |
ViagraⓇ† FCT (C) (n = 42)* |
Ratio (A/C)‡,§ |
Ratio (B/C)‡,|| |
|---|---|---|---|---|---|
| CPEAK, ng/mL | 15.85 (55.82) | 15.59 (43.86) | 15.95 (48.88)¶ | 0.96 (87.36–105.38) | 0.95 (81.78–111.19) |
| AUCL, ng/hr/mL | 67.15 (43.74) | 66.55 (42.00) | 63.84 (36.95)¶ | 1.01 (96.39–104.87) | 0.94 (75.58–117.37) |
| AUC∞, ng/hr/mL | 74.18 (40.26) | 75.58 (35.80)¶ | 70.30 (33.28)¶ | – | – |
| KEL, h−1 | 0.2076 (21.20) | 0.2121 (24.85)¶ | 0.2118 (19.70)¶ | – | – |
| t½, h | 3.492 (21.89) | 3.529 (34.62)¶ | 3.406 (20.81)¶ | – | – |
| TPEAK, h | 1.064 (49.56) | 1.269 (72.56) | 1.220 (52.16)¶ | – | – |
AUC... = area under the plasma concentration-time curve from zero to infinity; AUCL = area under the plasma concentration-time curve; CPEAK = maximum plasma concentration; FCT = film coated tablet; KEL = elimination rate constant; ODF = orally disintegrating film; TPEAK = time to CPEAK.
Values are presented as arithmetic mean (% coefficient of variation).
Pfizer Inc, New York, New York.
Ratio of geometric least-squares means (90% CI, used as natural log transformed parameter).
Ratio (A/C) = e [LSMEAN of (LNA = Natural log transformed sildenafil citrate ODF with water – LNC =Natural log transformed Viagra FCT).
Ratio (B/C) = e [LSMEAN of (LNB = Natural log transformed sildenafil citrate ODF without water – LNC = Natural log transformed Viagra FCT)].
n=39.
Figure 1.
(A) Mean plasma sildenafil citrate concentration-time profile following a single oral dose of 50 mg sildenafil citrate (SILD-FMS-1001). Mean sildenafil plasma concentration vs time profiles up to 24 hours after test and reference treatments. Treatment A: Sildenafil citrate 50 mg orodispersible film with water. Treatment B: Sildenafil citrate 50 mg orodispersible film without water; Treatment C: ViagraⓇ (Pfizer Inc, New York, New York) film-coated tablet with water. (B) Mean N-desmethyl sildenafil plasma concentrations (SILD-FMS-1001). Mean N-desmethyl sildenafil plasma concentration vs time profiles up to 24 hours after test and reference treatments. Treatment A: Sildenafil citrate 50 mg orodispersible film with water. Treatment B: Sildenafil citrate 50 mg orodispersible film without water; Treatment C: ViagraⓇ (Pfizer Inc, New York, New York) film-coated tablet with water.
The protocol-specified bioequivalence criteria were met for sildenafil citrate ODF given with water relative to ViagraⓇ FCTs with 90% CIs for the ratios of geometric means of sildenafil CPEAK and AUCL were entirely contained within the equivalence interval of 80% to 125% (Table 1).
In contrast, when sildenafil citrate ODF was given without water, the 90% CIs for the ratios of geometric means of sildenafil CPEAK (91.26%–125.95%) and AUCL (94.39%–132.92%) fell outside the upper limits of 125% due to an unanticipated increased variability observed with the without water administration.
The concentration–time profiles for the metabolite N-desmethyl sildenafil were generally similar for all 3 treatments and almost superimposable on each other (Fig. 1B). Consistent with sildenafil, the systemic exposure (CPEAK and AUCL) of N-desmethyl sildenafil was similar across all the 3 treatment groups. Arithmetic mean TPEAK values were comparable across the treatments, with a range of 1.064 to 1.269 hour. The t½ was similar across the treatments, with a mean value of approximately 3.5 hours and are summarized in Table 2.
Bioequivalence study of SILD-FMS-1002
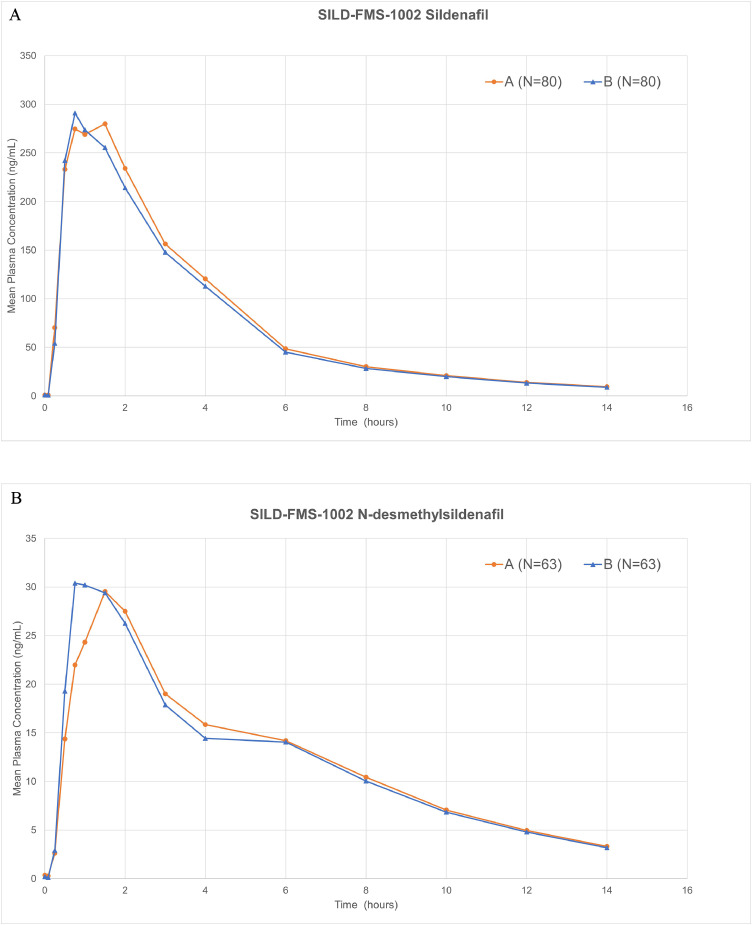
The mean plasma sildenafil pharmacokinetic parameters and the results of their statistical comparisons for the pharmacokinetic set (n = 80) are summarized in Table 3. The mean concentration–time profiles for sildenafil are shown in Fig. 2A. Specifically, the mean sildenafil plasma concentration–time profiles for both the treatments were nearly overlapping to each other. Systemic exposure (CPEAK and AUCL) of sildenafil and its metabolite were comparable across both the treatment groups. Mean TPEAK values were similar in sildenafil without water and ViagraⓇ FCTs group and mean t½ were nearly comparable in both the treatments. The variability for pharmacokinetic parameters (CPEAK: 39%–43%; AUCL: 31% vs 33%) based on %CV, was generally similar for all the treatments. The protocol-specified bioequivalence criteria were met for sildenafil citrate ODF given without water relative to ViagraⓇ FCTs because 90% CIs for the ratios of geometric means of sildenafil CPEAK and AUCL were confined within the equivalence interval of 80% to 125% (Table 3).
Table 3.
Descriptive summary of plasma sildenafil pharmacokinetic parameters after a single oral dose of 50 mg sildenafil citrate (SILD-FMS-1002).
| Parameter | Sildenafil citrate ODF without water (A) (n = 80)* |
ViagraⓇ† FCT (B) (n = 80)* |
Ratio A/B‡,§ |
|---|---|---|---|
| CPEAK, ng/mL | 382.3 (38.74) | 380.1 (43.26) | 1.02 (95.47–109.36) |
| AUCL, ng/hr/mL | 1131 (31.47) | 1074 (32.74) | 1.06 (103.42–108.40) |
| AUC∞, ng/hr/mL | 1182 (32.52) | 1123 (33.59) | – |
| KEL, h−1 | 0.2137 (18.63) | 0.2087 (18.84) | – |
| t½, h | 3.355 (18.50) | 3.433 (17.96) | – |
| TPEAK, h | 1.069 (44.51) | 1.050 (49.89) | – |
AUC... = area under the plasma concentration-time curve from zero to infinity; AUCL = area under the plasma concentration-time curve; CPEAK = maximum plasma concentration; FCT = film coated tablet; KEL = elimination rate constant; ODF = orally disintegrating film; TPEAK = time to CPEAK.
Values are presented as arithmetic mean (% coefficient of variation).
Pfizer Inc, New York, New York.
Ratio of geometric least-squares means (90% CI, used as natural log transformed parameter).
Ratio = e [LSMEAN of (LNA = Natural log transformed sildenafil citrate ODF with water–LNB = Natural log transformed sildenafil citrate ODF without water)].
Figure 2.
(A) Mean sildenafil plasma concentrations (SILD-FMS-1002). Mean sildenafil plasma concentration vs time profiles up to 24 hours after test and reference treatments. Treatment A: Sildenafil citrate 50 mg orodispersible film without water. Treatment B: ViagraⓇ (Pfizer Inc, New York, New York) film-coated tablet with water. (B) Mean N-desmethyl sildenafil plasma concentrations (SILD-FMS-1002). Mean N-desmethyl sildenafil plasma concentration vs time profiles up to 24 hours after test and reference treatments. Treatment A: Sildenafil citrate 50 mg orodispersible film without water; Treatment B: ViagraⓇ (Pfizer Inc, New York, New York) film-coated tablet with water.
Analogously, the concentration–time profiles for the metabolite N-desmethyl sildenafil were generally similar for the 2 treatments and fairly superimposable on each other (Fig. 2B). Congruent with sildenafil, CPEAK and AUCL of N-desmethyl sildenafil was akin between the 2 treatment groups. Arithmetic mean TPEAK values were comparable between the treatments, with mean values of 1.206 to 1.341 hour. Likewise, t½ was similar in the 2 treatment groups, with a mean value of approximately 3.7 hours (Table 4).
Table 4.
Mean N-desmethyl-sildenafil plasma concentrations after single-dose administration of test and reference doses of sildenafil citrate 50 mg (SILD-FMS-1002).
| Parameter | Sildenafil citrate ODF without water (A) (n = 80)* |
ViagraⓇ† FCT (B) (n = 80)* |
Ratio A/B‡,§ |
|---|---|---|---|
| CPEAK, ng/mL | 35.50 (32.84) | 37.91 (34.74) | 0.961 (90.81–101.30) |
| AUCL, ng/hr/mL | 173.5 (37.48) | 172.8 (38.66) | 1.01 (99.40–102.59) |
| AUC∞, ng/hr/mL | 192.9 (39.89) | 192.2 (41.87) | – |
| KEL, h−1 | 0.1942 (18.91) | 0.2002 (21.28) | – |
| t½, h | 3.725 (23.60) | 3.707 (36.39) | – |
| TPEAK, h | 1.341 (40.35) | 1.206 (46.27) | – |
AUC... = area under the plasma concentration-time curve from zero to infinity; AUCL = area under the plasma concentration-time curve; CPEAK = maximum plasma concentration; FCT = film coated tablet; KEL = elimination rate constant; ODF = orally disintegrating film; TPEAK = time to CPEAK.
Values are presented as arithmetic mean (% coefficient of variation).
Pfizer Inc, New York, New York.
Ratio of geometric least-squares means (90% CI, used as natural log transformed parameter).
Ratio = e [LSMEAN of (LNA = Natural log transformed sildenafil citrate ODF with water–LNB = Natural log transformed sildenafil citrate ODF without water)].
Safety
Sildenafil citrate ODF and ViagraⓇ FCT administered as a single dose were well tolerated by healthy adult men in both the studies. On the first study, the number of TEAE's reported after administration of sildenafil citrate ODF (with water), sildenafil citrate ODF (without water), and ViagraⓇ FCTs were comparable (8, 14, and 11 AEs, respectively). All AEs were of mild intensity and there were no discontinuations due to AEs. No serious AEs or deaths were reported during the study. The most frequent TEAE reported by the subjects after administration of sildenafil citrate ODF (with water) was headache (12.2%), and all events were related to the drug. Flushing was the most common drug related AE experienced in both sildenafil citrate ODF (without water), and ViagraⓇ FCTs in 14.6% and 11.9% of subjects, respectively. Based on system organ class (SOC) classification, the nervous system disorders system organ class SOC contained the most frequently reported AEs in subjects who received sildenafil citrate ODF (with water), whereas the most commonly reported AEs in subjects in the other 2 treatment groups were within the vascular disorders SOC system organ class.
In the study SILD-FMS-1002, a total of 12 AEs was experienced by the study participants. The AEs post administration of sildenafil citrate ODF without water, and ViagraⓇ FCTs were comparable (1 each). The remaining AEs (n = 10) were alterations in laboratory investigations that could not be definitively attributed to either of the treatment groups because clinical laboratory parameters were only evaluated at screening and study exit stage. No discontinuations, serious AEs, or deaths occurred during this study. All the AEs were considered mild. One subject who received sildenafil citrate ODF without water experienced an event of pain (1.25%), which was categorized within the general disorder and administration site condition SOC system organ class classification; this AE was considered to be drug-related. One subject receiving ViagraⓇ FCT reported headache (1.25%), which was considered as drug-related and categorized within the nervous system disorder SOC system organ class classification.
Discussion
Sildenafil citrate, with its proven efficacy and safety has been used in the treatment of ED for more than 2 decades. In addition to the presently marketed sildenafil FCT, the novel sildenafil ODF formulation offers an alternative option with a discrete and convenient method of administration with or without water for patients who have difficulty swallowing or are on liquid restrictions.
The first study demonstrated bioequivalence between the 2 formulations (sildenafil citrate ODF and ViagraⓇ FCT) when administered with water. However, the pharmacokinetic results between sildenafil without water and ViagraⓇ FCT with water were not found to be bioequivalent due to an unanticipated increased variability associated with the without water administration. To do further analysis, a second study, considering the increased intrasubject variability, was conducted to reassess the pharmacokinetic parameters and bioequivalence was established between the sildenafil without water and ViagraⓇ FCT with water.
The SILD-FMS-1001 study demonstrated that sildenafil citrate ODF given with water was bioequivalent to ViagraⓇ FCT given with water under fasting conditions as the 90% CI for the natural log-transformed LS Means ratio of CPEAK and AUCL for sildenafil were found to be between 80% and 125% satisfying the criteria for bioequivalence.
The pharmacokinetic data from N-desmethyl sildenafil, the active metabolite of sildenafil, was also similar between the 2 formulations, showing additional supportive evidence of comparable therapeutic outcome.
However, bioequivalence was not established between sildenafil citrate ODF without water and ViagraⓇ FCTs with water. The results were considered to be equivocal and another study SILD-FMS-1002 was planned in accordance with the observed increased intrasubject variance and conducted to reassess the bioequivalence of sildenafil citrate ODF without water and ViagraⓇ FCT with water.
The second study demonstrated that sildenafil citrate ODF dosed without water was found to be bioequivalent to ViagraⓇ FCT with water under fasting conditions. The 90% CIs for the natural log-transformed data for sildenafil fell within the acceptable bioequivalent range of 80% and 125%.
The pharmacokinetic data for N-desmethyl sildenafil, the active metabolite of sildenafil, was also similar between the 2 formulations taken with water, showing additional supportive evidence of comparable therapeutic outcome.
In addition, both the formulations shared a favorable safety profile with few AEs reported. Results here were similar in terms of reported AEs—mild, drug-related AEs were expected given the known safety profile of the drug and safety was a secondary end point for both studies.
Similar findings were found in a study conducted by Radicioni et al.13 Subjects were administered with a different sildenafil citrate 100 mg ODF (without water) and sildenafil citrate 100 mg FCTs (with water) and pharmacokinetic samples were investigated up to 24 hours post-administration. There was nearly superimposable mean plasma concentration–time profiles identified between the 2 treatment groups.13
A group of international experts from varied disciplines met to revise a Process of Care Model developed in 1999. This data-driven, evidence-based, update leveraged the extensive clinical expertise for effective management of ED. It provided guidance for primary care physicians on risk factors, comorbidities, PDE-5 inhibitors selection, dosing, sexual counseling, and patient education. The study highlighted that sildenafil citrate is an ideal and optimal choice of drug in patients with ED. It has been effectively used in patients with comorbidities, including spinal cord injury, severe hepatic injury, or renal insufficiency. It can also be used concomitantly in patients with arrythmias or on drugs that causes QT segment prolongation. It should be emphasized that effective management of ED may be achieved by counseling, understanding individualized needs, and creating a tailor-made treatment goal to reinstate patient and or couple sexual satisfaction.14
Recently, the Italian Society of Andrology and Sexual Medicine endorsed an expert task force from ten Italian societies related to the field of sexual medicine to facilitate guidelines on the diagnosis and management of ED with organic and psychosexual comorbidities. They reinstated that PDE-5 inhibitors remain the gold standard treatment for ED. They also emphasized stupendous benefits of sildenafil citrate orodispersible film. It does not require water for swallowing, offers enhanced bioavailability because it bypasses first-pass metabolism, and reduces incidence of AEs. The most remarkable advantage of sildenafil citrate ODF is that it respects the need for couple-privacy and improves patient sexual satisfaction.15
Ultimately, in addition to the presently available sildenafil FCTs, the novel ODF formulation of sildenafil offers an option for patients who have difficulty swallowing, as well as a convenient and discreet method of administration without the need for water.
Conclusions
The result of these studies demonstrates that the new sildenafil citrate ODF formulation administered with and without water is bioequivalent to ViagraⓇ FCT administered with water under fasted conditions in healthy male volunteers. Thus, the new ODF formulation is a safe and suitable alternative to the conventional oral solid dosage form.
Declaration of Competing Interest
A. Shaw, N. Summers, T. Hassan, T. Lawrence, S. Tarr, M. Liu, and S. Hackley are employees of Viatris Inc and hold stocks. S. Vignesh, T. Yan, and V. Daggumati are employees of Viatris Inc. J. Rondon is an employee of CPMI. The authors have indicated that they have no other conflicts of interest regarding the content of this article.
Acknowledgments
Funding for this study was provided by Viatris Inc.
Writing and editorial support was provided by Mamatha K. PhD and Shantha Kumar V. PhD from Viatris.
All authors substantially contributed to the manuscript. All authors contributed to the conceptualization or design of the work; the acquisition, analysis, or interpretation of data; drafting, editing, reviewing, and revising it critically for important intellectual content; for final approval of the version to be published; and agreeing to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- 1.Yaf FA, Jenkins L, Albersen M, Corona G, Isidori AM, Goldfarb S, et al. Erectile dysfunction. Nat Rev Dis Primers. 2016;2:16003. doi: 10.1038/nrdp.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersson KE. Mechanisms of penile erection and basis for pharmacological treatment of erectile dysfunction. Pharmacol Rev. 2011;63(4):811–859. doi: 10.1124/pr.111.004515. [DOI] [PubMed] [Google Scholar]
- 3.Gratzke C, Angulo J, Chitaley K, Dai YT, Kim NN, Paick JS, et al. Anatomy, physiology, and pathophysiology of erectile dysfunction. J Sex Med. 2010;7(1 Pt 2):445–475. doi: 10.1111/j.1743-6109.2009.01624.x. [DOI] [PubMed] [Google Scholar]
- 4.Artom N, et al. Prevalence of Erectile Dysfunction in a Cohort of Italian Hypertensive Subjects. Clinical and Experimental Hypertension. 2016;38(2):143. doi: 10.3109/10641963.2015.1060994. [DOI] [PubMed] [Google Scholar]
- 5.European Association of Urology. Guidelines on Male Sexual Dysfunction: Erectile Dysfunction and Prema-ture Ejaculation. http://uroweb.org/wp-content/uploads/14-Male-Sexual-Dysfunction_LR1.pdf. Accessed August 1, 2022.
- 6.Hackett G, Kell P, Ralph D, et al. British Society for Sexual Medicine guidelines on the management of erectile dysfunction. JSexMed. 2008;5(8):1841–1865. doi: 10.1111/j.1743-6109.2008.00773.x. [DOI] [PubMed] [Google Scholar]
- 7.Boolell M, Allen MJ, Ballard SA, et al. Sildenafil: an orally active type 5 cyclic GMP-specific phosphodiesterase inhibitor for the treatment of penile erectile dysfunction. Int J Impot Res. 1996;8(2):47–52. [PubMed] [Google Scholar]
- 8.Viagra 50 mg film-coated tablets - Summary of Product Characteristics (SmPC) - (emc). Medicines.org.uk [updated on 26 March 2022]. Available at: https://www.medicines.org.uk/emc/product/7980/smpc#gref Accessed August 1, 2022.
- 9.Scaglione F, Donde S, Hassan TA, et al. Phosphodiesterase type 5 inhibitors for the treatment of erectile dysfunction: Pharmacology and clinical impact of the sildenafil citrate orodispersible tablet formulation. Clin Ther. 2017;39:370–377. doi: 10.1016/j.clinthera.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Bala R, Pawar P, Khanna S, et al. Orally dissolving strips: A new approach to oral drug delivery system. Int J Pharm Investig. 2013;3:67–76. doi: 10.4103/2230-973X.114897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Efremov EA, Kasatonova EV, Melnik YI, et al. [PDE-5 inhibitors: patients’ preferences] Urologiia. 2017;3:120–126. [PubMed] [Google Scholar]
- 12.Karki S, Kim H, Na S-J, et al. Thin films as an emerging platform for drug delivery. Asian J Pharm Sci. 2016;11:559–574. [Google Scholar]
- 13.Radicioni M, Castiglioni C, Giori A, et al. Bioequivalence study of a new sildenafil 100 mg orodispersible film compared to the conventional film-coated 100 mg tablet administered to healthy male volunteers. Drug Des Devel Ther. 2017;11:1183–1192. doi: 10.2147/DDDT.S124034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mulhall JP, Giraldi A, Hackett G, et al. The 2018 Revision to the Process of Care Model for Management of Erectile Dysfunction. J Sex Med. 2018;15:1434e1445. doi: 10.1016/j.jsxm.2018.05.021. [DOI] [PubMed] [Google Scholar]
- 15.Corona G, Cucinotta D, Di Lorenzo G, et al. The Italian Society of Andrology and Sexual Medicine (SIAMS), along with ten other Italian Scientific Societies, guidelines on the diagnosis and management of erectile dysfunction. J. Endocrinol. Invest. 2023;25:1–34. doi: 10.1007/s40618-023-02015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]