Summary
Background
Children in low and middle-income countries (LMICs) receive a staggering number of antibiotic prescriptions, many of which are inappropriate. We aimed to explore the proportion of antibiotic prescriptions from qualified sources of children under five who had a fever/cough in the two weeks prior to the survey in LMICs.
Methods
We used data from cross-sectional studies of the latest Demographic and Health Survey (DHS) datasets (n = 43,166) in 59 LMICs covering Sub-Saharan Africa, North Africa-West Asia-Europe, Central Asia, South & Southeast Asia, Oceania, and Latin America & the Caribbean regions. The study was conducted from March 2, 2020 to October 15, 2022. We only included the latest available surveys by country, and children under five who had taken antibiotics for fever/cough were included in the study. Finally, the outcome variable was classified into two distinct categories: those who had taken antibiotics from qualified sources and those who did not.
Findings
About three in four children (74.0%) received antibiotics from qualified sources. Tanzania (22.4%) and Malawi (99.9%) had the lowest and highest percentages of antibiotic prescriptions by qualified sources, respectively. Oceania had the highest percentage of qualified antibiotic prescriptions with 88.9% and Central Asia had the lowest percentage with 56.3%.
Interpretation
As unqualified sources of antibiotics for fever/cough in children under five were alarmingly high in some of the LMICs, the study emphasises the importance of nationwide efforts to regulate antibiotics prescriptions.
Funding
None.
Keywords: Antibiotics, LMICs, Qualified source, Standard DHS, Under-five children
Research in context.
Evidence before this study
The terms “child”, “children”, “antibiotic”, “antimicrobial”, “qualified”, “unqualified”, “low-income”, “developing”, “low income”, “low and middle income”, “Africa”, “Latin America”, “Asia”, “prescription” were used to search Google Scholar and PubMed for articles published between January 1, 2000 and March 1, 2022. There is no research on whether or not the antibiotics provided to young children in LMICs come from qualified sources.
Added value of this study
This is the first investigation into qualified sources of antibiotics for fever/cough in children under five. Our study included 59 LMICs, which results in a generalized result for antibiotic prescriptions from qualified sources. According to our research, eleven LMICs—Bangladesh, Chad, Comoros, Congo, Congo Democratic Republic, Côte d'Ivoire, Gabon, Kyrgyz Republic, Tajikistan, Tanzania, and Yemen—received more than 40% of their antibiotics from unqualified sources.
Implications of all the available evidence
Our research indicates that a significant number of antibiotics for fever/cough were coming from unqualified sources in eleven different LMICs. Also, getting antibiotics for fever/cough from unqualified sources was very common in the Central Asian region. The study sheds light on where children under five in LMICs get their prescriptions for antibiotics when they have a fever/cough. In order to prevent pharmacies from dispensing antibiotics recommended by unqualified sources, our study recommends implementing a more robust health infrastructure as well as various checks and balances.
Introduction
Antibiotics have been incredibly effective in enhancing health outcomes for children under five. Together with advancements in nutrition, clean water, sanitation, and vaccine coverage, antibiotics have contributed to a global decrease in children under five mortalities from 216 deaths per 1000 live births in 1950 to 39 in 2017 and a rise in male life expectancy from 48 years to 71 during the same period.1,2
However, antimicrobial resistance (AMR) is on the rise globally, posing a danger to the beneficial effects of antibiotics on health.3,4 It is a critical challenge to the successful treatment of an ever-increasing variety of diseases caused by viruses, bacteria, parasites, and fungi, according to a World Health Organization (WHO) global report.5 Therefore, it is urgent to address AMR, which kills 700,000 people annually worldwide.6 In addition to its impact on public health, AMR has also caused significant financial strain in low and middle-income countries (LMICs). Specifically, antimicrobial agents account for substantial healthcare expenses in developing countries, which may incur up to 40% of total healthcare costs. For example, antimicrobial agents consume 25–50% of India's, Bangladesh's, Thailand's, and Tanzania's government pharmaceutical expenditures.7 Antibiotic misuse and the emergence of AMR are inextricably connected.
There may be a link between primary care doctors' antibiotic prescriptions and AMR.8 Antibiotic usage was high in rural areas, especially in illnesses with a primarily viral aetiology, such as diarrhoea or URTI.8 The use of antibiotics has revealed that parents' lower socioeconomic class and education level may be major variables in their antibiotic misuse.9
Children in LMICs are administered an incredible number of antibiotics between birth and the age of five. The rate of antibiotic use in LMICs increased 39% between 2000 and 2015, while the amount of antibiotics consumed, measured in defined daily doses (DDD), increased 65% (21.1–34.8 billion DDDs) (11.3–15.7 DDDs per 1000 inhabitants per day).10 According to a study, around 57% of these doses appear to be inappropriate.11 Although data on antibiotic usage and the incidence of AMR in LMICs is more limited than in high-income countries, the current evidence shows that AMR is more prevalent in LMICs than in high-income countries.12 One of the factors that may contribute to this situation is the supply of antibiotics from unqualified sources, which can result in the overconsumption of antibiotics and unfinished doses, leading to a significant number of deaths in children.
Antimicrobial agents are sold without a prescription or medical supervision in many parts of the world. For example, according to a study in Thailand, antibiotics purchased through unqualified sources can account for a significant portion of antibiotics use.13 However, antimicrobial efficacy initiatives have mostly targeted hospitals or providers, omitting non-prescription antimicrobial usage.14 Healthcare professionals, especially those with little to no training in AMR and antibiotic use and those working in rural regions, demonstrated low awareness of the effects of antibiotics and AMR.
All sorts of healthcare providers should receive educational messages on the wise use of antibiotics and how they function as part of specific and targeted interventions to combat AMR in LMICs.15 A systematic review revealed that AMR and hospital-acquired infections can be reduced by interventions to prevent excessive antibiotic prescribing to hospital inpatients, while efforts to boost effective prescribing can improve clinical outcomes.16
To the best of our knowledge, there is no study on whether the antibiotics prescribed for children under five in LMICs originated from qualified sources or not. Accurate information about the sources of antibiotic prescription is crucial as prescriptions from unqualified sources burden LMICs with massive costs in the healthcare sector. Therefore, our study shed light on whether antibiotics are prescribed by qualified sources or not. Moreover, it will help policymakers and planners in LMICs take convenient and efficient steps regarding this crucial matter.
Methods
Data source & study design
We used data from cross-sectional studies of the latest standard Demographic and Health Survey (DHS) conducted in LMICs, funded by the U.S. Agency for International Development (USAID). DHS is a global survey that is conducted every three to five years in LMICs under the same protocol. The DHS survey includes women between the ages of 15 and 49 and children under five who reside in residential households. In some countries DHS includes male household members. The study time spanned from March 2, 2020 to October 15, 2022.
A two-stage stratified cluster sampling method is used in the survey. Selecting locations or clusters was the initial stage. The second stage involves systematically selecting households from each cluster or Enumeration Area (EA). The DHS program collects data for children under five on vaccination, childhood illness and newborn care information from their mother. To enable cross-country comparison, DHS surveys adhere to a set of standard operating protocols that include sampling, questionnaires, data collecting, cleaning, coding, and analysis. Oral and written consents were provided by women. DHS receives ethical approval for all surveys from the institutional review boards of ICF International and the ethics regulatory authorities of the nations in which the research is conducted. The detailed guidelines can be found at https://dhsprogram.com/.
Data harmonization
We downloaded the latest standard DHS data of 92 LMICs from the website https://dhsprogram.com/. Since our study involves only children under five, we used to “Children Recode (KR)” datasets to perform our analyses. 59 LMICs (Afghanistan, Albania, Angola, Bangladesh, Benin, Burkina Faso, Burundi, Cambodia, Cameroon, Chad, Comoros, Congo, Congo Democratic Republic, Cote d’Ivoire, Dominican Republic, Egypt, Eswatini, Ethiopia, Gabon, Gambia, Ghana, Guatemala, Guinea, Guyana, Haiti, Honduras, India, Indonesia, Jordan, Kenya, Kyrgyz Republic, Lesotho, Madagascar, Malawi, Maldives, Mali, Mauritania, Morocco, Mozambique, Myanmar, Namibia, Nepal, Nigeria, Papua New Guinea, Pakistan, Philippines, Rwanda, Sao Tome and Principe, Senegal, Sierra Leone, South Africa, Tajikistan, Tanzania, Timor-Leste, Togo, Uganda, Yemen, Zambia, Zimbabwe) were finally included from a possible of 92 countries in our study. These countries were chosen as they met our inclusion criteria and had up-to-date standard DHS data. For example, thirteen countries with Standard DHS datasets before 2000 (Botswana, Brazil, Ecuador, El Salvador, Kazakhstan, Mexico, Paraguay, Nigeria Ondo state, Sudan, Trinidad and Tobago, Uzbekistan, Thailand, and Tunisia) were excluded from the study as they may not represent the current state of antibiotic consumption. Moreover, eight countries (Sri Lanka, Samoa, Cape Verde, Eritrea, Turkmenistan, Lao People’s Democratic Republic, and Equatorial Guinea) were excluded due to their unavailability in the public domain. In addition, thirteen countries (Moldova, Nicaragua, Niger, Peru, Liberia, Armenia, Azerbaijan, Bolivia, Central African Republic, Ukraine, Vietnam, Turkey, and Colombia) were further eliminated because of insufficient data and relevant variables. The extent of non-response rate in each of the above countries is not reported. This is because, in some countries, the DHS survey reports are either publicly unavailable or not in English.
To analyse survey datasets some issues such as unequal unit selection probabilities should be considered. Sample weights help to eliminate bias that may arise from disproportionate sampling and impacts of non-response and are important in calculating standard errors. Thus, excluding weights from the study sometimes results in significantly biassed estimates.
In STATA, singleton was introduced to handle a single PSU in a stratum. Single PSU in stratum can occur because of various reasons such as missing data. This leads to numerous problems in analysing the data such as not being able to calculate standard errors. To handle singleton PSUs in each stratum, we evaluated three methods: singleton (certainty) where we treated every singleton unit as certainty units, singleton (scaled) where for each stratum we used the average of the variances from the strata with multiple sampling units as a scaling factor for singleton (certainty), and singleton (centred) where we centred the singleton PSUs at the grand mean. Of these three methods, we used singleton (scaled) method for our analysis. All levels of categorical explanatory variables were defined in a suitable manner for interpretation and ease of analysis. After extracting the study variables in each country dataset, we pooled them into one data set.17
Outcome variable
The outcome variable “Antibiotic Taken for Fever/Cough from Qualified Sources” is a dichotomous variable classified as “YES = 1/NO = 0.” We enquired if the children recently had a fever/cough. If the response is yes, we checked to see if they have had any medical treatment using antibiotics. Finally, we investigated whether the prescriptions were provided by qualified sources or not [Fig. 1]. We classified any government hospitals, private hospitals, clinics, NGOs, and public health sectors as qualified sources, whereas pharmacies, shops, churches, traditional practitioners, markets, drug sellers, friends/relatives, supermarkets, shops, and others were categorised as non-qualified. Although pharmacists are experts in medicine dispensing, they are not authorised to prescribe antibiotics in LMICs.18,19 Thus we categorised ‘pharmacy’ as unqualified sources of antibiotics.
Fig. 1.
Outcome extracting criteria.
Explanatory variables
Two types of explanatory variables were obtained from the standard DHS: level one (individual-level variables) and level two (community-level variables). Level one comprises the number of children under five, age in years, their sex, body mass index (BMI) of children under five, type of place of residence, wealth index, respondents' occupation, husband or partner's occupation, children having any type of vaccination (DHS survey only includes whether children ever received any type of vaccination), months of breastfeeding, delivery by caesarean section. Residence and country are variables at level two. For analysis and interpretation, these variables were recoded to make them relevant. Every variable was coded a priori by the available data and the most plausible causal pathways. Table 1 contains a detailed description of each variable.
Table 1.
Variables recoding procedures.
| Variables | Code at DHS dataset | Categories in DHS | Recoding procedure |
|---|---|---|---|
| Level one variables (Individual level variables) | |||
| Number of children under 5 | v137 | One | 1 = “one” |
| Two | 2 = “two” | ||
| Three | 3 = “three” | ||
| Four | 4 = “four” | ||
| Five or above | 5 = “five or above”. | ||
| Age of the child (In years) | hw1 | One year old | 1 = “min/12 months” |
| Two years old | 2 = “13/24 months” | ||
| Three years old | 3 = “25/36 months” | ||
| Four years old | 4 = “37/48 months” | ||
| Five years old | 5 = “49/60 months”. | ||
| Sex of the child | b4 | Male | 1 = “male” |
| Female | 2 = “female” | ||
| Body mass index (BMI) of children under five | This was deduced by code from the dataset. | 1 | 1 = “min–9.9” |
| 2 | 2 = “9.9–14.9” | ||
| 3 | 3 = “14.9–18.5” | ||
| 4 | 4 = “18.5–24.9” | ||
| 5 | 5 = “24.9–max”. | ||
| Wealth index | v190 | Poorest | 1 = “poorest” |
| Poorer | 2 = “poorer” | ||
| Middle | 3 = “middle” | ||
| Richer | 4 = “richer” | ||
| Richest | 5 = “richest” | ||
| Respondents’ occupation (grouped) | v705 | Did not work | 0 = “Did not work” |
| Agriculture | 1 = “Agriculture” | ||
| Manual labour | 2 = “Manual labour” | ||
| Professional/technical/managerial | 3 = “Professional/technical/managerial” | ||
| Others | 4 = “Others” | ||
| Partner/husband’s occupation (grouped) | v717 | Did not work | 0 = “Did not work” |
| Agriculture | 1 = “Agriculture” | ||
| Manual labour | 2 = “Manual labour” | ||
| Professional/technical/managerial | 3 = “Professional/technical/managerial” | ||
| Others | 4 = “Others” | ||
| Had fever in last two weeks | h22 | Yes | 1 = “Yes” |
| No | 0 = “No” | ||
| Antibiotic taken for fever | This was deducted by code from the dataset. | Yes | 1 = “Yes” |
| No | 0 = “No” | ||
| Months of breastfeeding | m5 | One year breastfed | 1 = “min/12 months” |
| Two years breastfed | 2 = “13/24 months” | ||
| Three years breastfed | 3 = “25/36 months” | ||
| Four years breastfed | 4 = “37/48 months” | ||
| Five years breastfed | 5 = “49/60 months” | ||
| Ever breastfed, not currently breastfed | 93 = “ever not currently breastfed” | ||
| Never breastfed | 94 = “never breastfed” | ||
| Inconsistent | 97 = “inconsistent” | ||
| Delivery by caesarean section | m17 | No | 1 = “Yes” |
| Yes | 0 = “No” | ||
| Ever had vaccination | h10 | No | 1 = “Yes” |
| Yes | 0 = “No” | ||
| Level two variables (Community variables) | |||
| Type of place of residence | v025 | Urban | 1 = “Urban” |
| Rural | 2 = “Rural” | ||
| Country code | v000 | This cell needs a hand | |
| Dependent variables | |||
| Antibiotics prescribed for fever by qualified doctors | This was deducted by code from the dataset. | Yes | 1 = “Yes” |
| No | 0 = “No” | ||
| Random effect variables | |||
| Sampling weight | v005 | For weighting the observation | |
| Primary sampling unit (PSU) | v021 | For sample selection | |
| Strata | v022 | For stratification | |
Statistical analysis
We calculated various descriptive statistics of qualified antibiotic prescriptions with respect to countries, continents, economic status, top and bottom ten countries in antibiotic consumption. All of the reported findings in tables and figures are based on weighted estimates to reflect the estimates of national figures, apart from the first paragraph of the ‘Results’ section where the actual overall numbers were summarised. All descriptive findings were accompanied with corresponding bar diagrams. The geospatial analysis was conducted through ArcGIS version 10.7.20 All statistical analyses were conducted in STATA version 14.21 All the graphs were produced by Microsoft Excel.
STROBE Statement—Checklist, outlining items to be included in reports of cross-sectional studies, was followed in this study [Supplementary Table S1].
Role of the funding source
There was no funding source for this study. We used publicly available datasets from DHS website, and all authors had full access to all the data in the study and accept responsibility for the decision to submit for publication. More than one author has verified the statistical analysis and results of the study and takes the full responsibility of the results.
Results
All of the findings reported below are based on the weighted estimates to reflect the population except the first paragraph of the country description.
Country description
We identified 43,166 children under five who had taken antibiotics for fever/cough two weeks prior to the survey. Then, we divided the children into two groups: children who obtained the antibiotic from a qualified source (74.0%) and those who did not. Among them 22,649 (52.6%) were males. The percentages of qualified antibiotic prescriptions for males and females were 74.7% and 73.4%, respectively.
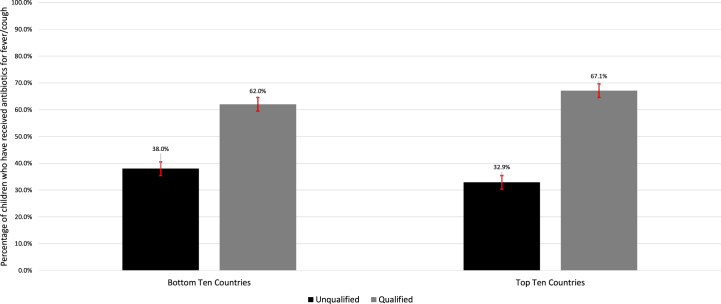
Among the selected countries, Cameroon (0.3%), Nigeria (4.3%), Mauritania (6.1%), Cambodia (6.8%), Bangladesh (7.0%), Mozambique (7.6%), Benin (15.3%), Mali (16.9%), Tanzania (18.2%), and Eswatini (19.0%) were the bottom ten in terms of consuming antibiotics for fever/cough [Table 2]. The above bottom ten countries in consuming antibiotics received 62.0% of their antibiotics from qualified sources [Fig. 2]. Moreover, Congo (68.7%), Egypt (65.5%), Tajikistan (61.8%), Honduras (52.1%), Gabon (51.1%), Albania (50.5%), Yemen (49.3%), Sao Tome and Principe (48.0%), Afghanistan (47.6%), and Namibia (46.3%) were the top ten in terms of consuming antibiotics [Table 2]. The above top ten countries in consuming antibiotics received 67.1% of their antibiotics from qualified sources [Fig. 2].
Table 2.
Weighted descriptive statistics of antibiotics for fever/cough from qualified and unqualified sources in 59 LMICs.
| Country | Had fever recently, weighted N (%) | Antibiotic taken for fever, weighted N (%) | Antibiotics prescribed from qualified sources, weighted N (%) | Antibiotics prescribed from unqualified sources, weighted N (%) |
|---|---|---|---|---|
| Afghanistan | 8682 (29.1) | 4040 (47.6) | 2977 (74.1) | 1043 (26.0) |
| Albania | 160.6 (6.4) | 81.1 (50.5) | 68.1 (84.0) | 13.0 (16.1) |
| Angola | 1829 (14.9) | 550.1 (30.1) | 462.9 (84.2) | 87.2 (15.8) |
| Bangladesh | 2697 (37.3) | 187.3 (7.0) | 44.3 (23.7) | 143 (76.3) |
| Benin | 2437 (19.6) | 372.9 (15.3) | 243.7 (65.4) | 129.1 (34.6) |
| Burkina Faso | 2868 (20.8) | 873.5 (30.7) | 781.8 (89.6) | 90.9 (10.4) |
| Burundi | 5033 (39.6) | 1118 (22.2) | 940.8 (84.2) | 177.2 (15.9) |
| Cambodia | 1953 (28.2) | 132.5 (6.8) | 125.5 (94.7) | 7.0 (5.3) |
| Cameroon | 1402 (15.6) | 4.7 (0.3) | 4.0 (86.4) | 0.6 (13.6) |
| Chad | 3949 (24.0) | 845.1 (22.0) | 400.5 (47.9) | 435.6 (52.1) |
| Comoros | 654.8 (22.0) | 170.4 (26.7) | 96.6 (56.7) | 73.8 (43.3) |
| Congo | 1840 (25.2) | 1257 (68.7) | 591.6 (47.1) | 664.1 (52.9) |
| Congo Democratic Republic | 4960 (29.9) | 1480 (30.2) | 681.5 (46.2) | 792.4 (53.8) |
| Cote d'Ivoire | 1584 (24.3) | 439 (28.4) | 249.2 (58.1) | 179.7 (41.9) |
| Dominican Republic | 747.7 (22.6) | 248.9 (33.4) | 195.5 (78.5) | 53.4 (21.5) |
| Egypt | 3870 (26.3) | 2531 (65.5) | 1985 (78.4) | 546.4 (21.6) |
| Eswatini | 676 (29.5) | 111.8 (19.0) | 101.5 (90.8) | 10.3 (9.2) |
| Ethiopia | 1470 (14.4) | 389.1 (26.5) | 304.6 (78.3) | 84.5 (21.7) |
| Gabon | 1120 (25.2) | 564.1 (51.1) | 326.3 (58.1) | 235.2 (41.9) |
| Gambia | 1102 (15.6) | 433.4 (39.4) | 307.8 (71.0) | 125.7 (29.0) |
| Ghana | 740.6 (14.2) | 187.1 (25.3) | 140.3 (75.0) | 46.8 (25.0) |
| Guatemala | 2835 (23.8) | 1077 (38.0) | 805.8 (74.8) | 271.1 (25.2) |
| Guinea | 1209 (17.3) | 232.1 (19.2) | 165 (71.1) | 67.1 (28.9) |
| Guyana | 359.3 (20.5) | 77.6 (22.1) | 61 (78.6) | 16.6 (21.4) |
| Haiti | 1789 (32.0) | 546.2 (30.5) | 405.1 (74.2) | 141.1 (25.8) |
| Honduras | 2235 (23.5) | 1160 (52.1) | 855.3 (73.8) | 303.6 (26.2) |
| India | 28,000 (13.2) | 6858 (24.5) | 5735 (83.6) | 1123 (16.4) |
| Indonesia | 5015 (31.5) | 1427 (28.6) | 1278 (89.6) | 148.7 (10.4) |
| Jordan | 1162 (12.9) | 459.1 (39.5) | 338.5 (73.7) | 120.6 (26.3) |
| Kenya | 4478 (24.9) | 1927 (43.6) | 1523 (79.5) | 391.7 (20.5) |
| Kyrgyz Republic | 203.7 (5.3) | 88 (45.3) | 52.7 (59.8) | 35.4 (40.2) |
| Lesotho | 398.7 (15.6) | 100.6 (25.3) | 74.7 (74.3) | 25.9 (25.7) |
| Madagascar | 1098 (9.4) | 430.1 (40.9) | 274.8 (64.0) | 154.9 (36.0) |
| Malawi | 4703 (29.2) | 1134 (24.1) | 916.8 (99.9) | 0.8 (0.1) |
| Maldives | 655.7 (24.6) | 285.4 (43.5) | 252.6 (88.5) | 32.8 (11.5) |
| Mali | 1507 (16.0) | 254.4 (16.9) | 179.2 (70.5) | 75.2 (29.5) |
| Mauritania | 1810 (16.6) | 111.2 (6.1) | 91.5 (82.3) | 19.7 (17.7) |
| Morocco | 1538 (27.3) | 427 (27.8) | 297.8 (69.8) | 129.2 (30.3) |
| Mozambique | 1439 (13.6) | 109.6 (7.6) | 105.5 (96.3) | 4.1 (3.7) |
| Myanmar | 636.3 (15.9) | 199.7 (31.5) | 149.4 (74.8) | 50.3 (25.2) |
| Namibia | 1026 (26.7) | 460.3 (46.3) | 312.6 (67.9) | 147.7 (32.1) |
| Nepal | 986.5 (21.6) | 345.5 (35.0) | 216.3 (62.6) | 129.3 (37.4) |
| Nigeria | 1739 (14.5) | 73.5 (4.3) | 65.33 (88.8) | 8.2 (11.2) |
| Pakistan | 3614 (37.7) | 1416 (39.2) | 1142 (80.6) | 274.5 (19.4) |
| Papua New Guinea | 1680 (20.1) | 308.9 (19.4) | 277.6 (89.9) | 31.1 (10.1) |
| Philippines | 1592 (16.8) | 352.7 (22.2) | 249.3 (70.7) | 103.5 (29.3) |
| Rwanda | 1472 (18.8) | 583.4 (39.6) | 482.2 (82.7) | 101.2 (17.4) |
| Sao Tome and Principe | 296.7 (17.4) | 135.9 (48.0) | 116.7 (85.9) | 19.2 (14.1) |
| Senegal | 2433 (23.2) | 636.2 (26.2) | 520 (81.7) | 116.2 (18.3) |
| Sierra Leone | 1443 (17.0) | 363.8 (25.2) | 278.5 (76.5) | 85.4 (23.5) |
| South Africa | 618.1 (21.2) | 180.5 (29.2) | 119.5 (66.2) | 61.0 (33.8) |
| Tajikistan | 572.5 (9.3) | 353.6 (61.8) | 196 (55.4) | 157.6 (44.6) |
| Tanzania | 1651 (18.3) | 299.8 (18.2) | 67.1 (22.4) | 232.7 (77.6) |
| Timor-Leste | 905.7 (13.2) | 206.7 (22.8) | 149.3 (72.3) | 57.3 (27.7) |
| Togo | 1345 (22.0) | 413.1 (30.9) | 291.6 (70.7) | 120.6 (29.3) |
| Uganda | 4689 (34.1) | 1360 (29.0) | 1029 (75.6) | 331.2 (24.4) |
| Yemen | 4774 (32.0) | 2290 (49.3) | 1186 (52.1) | 1091 (47.9) |
| Zambia | 1460 (16.2) | 499.1 (34.2) | 460.2 (92.2) | 39 (7.8) |
| Zimbabwe | 789.1 (14.0) | 268.5 (34.0) | 218.5 (81.4) | 50 (18.6) |
Due to missing information about sources of antibiotic consumption, the sum of qualified and unqualified sources does not sum up to 100% in some countries.22
Fig. 2.
Comparison between countries with the highest and lowest weighted antibiotics consumption rate. Legend: Here, the black colour represents the antibiotics from unqualified sources on the other hand the grey colour represents the antibiotics from qualified sources.
The countries that have the lowest percentage of antibiotic prescribed by qualified sources were Tanzania (22.4%), Bangladesh (23.7%), Congo Democratic Republic (46.2%), Congo (47.1%), Chad (47.9%), Yemen (52.1), Tajikistan (55.4), Comoros (56.7%), Côte d'Ivoire (58.1%), and Gabon (58.1%); the countries had the highest percentage of antibiotic prescribed by qualified sources were to Malawi (99.9%), Mozambique (96.3%), Cambodia (94.7%), Zambia (92.2%), Eswatini (90.8%), Papua New Guinea (89.9%), Burkina Faso (89.6%), Indonesia (89.6%), Nigeria (88.8%) and Maldives (88.5%) [Table 2] [Fig. 3].
Fig. 3.
The weighted percentage of children under five who had taken antibiotics from unqualified sources for fever/cough in lower and middle-income countries. Legend: Here, the darker shades of red indicating unqualified sources of antibiotics.
Descriptive statistics of socio-demographic variables
Table 3 shows the relationship between selected explanatory variables and antibiotics prescribed for fever/cough by qualified sources. In general, we found no sub-groups to be especially high or low risk-groups for taking antibiotics from qualified sources. Children living in rural areas received 75.0% of antibiotics from qualified sources, compared to urban areas with 72.2%. 78.8% of children whose parents received higher education went to qualified sources for antibiotics. However, there were 5.3% missing responses in this variable. The percentage of antibiotics received from qualified sources seems to decline as the number of children under five in the household increases (One; 76.8%, two; 73.1%, three; 69.1%, four; 69.7%). Conversely, the percentage increases with the year children under five were breastfed (One Year; 76.3%, Two Years; 77.2%, Three Years; 78.7%). However, the percentage of missing in this variable was 5.2%. Children who were delivered by caesarean sections received antibiotics from qualified sources 78.0% of the time. The percentage of missing in this variable was 0.4%.
Table 3.
Weighted descriptive statistics of socio-economic variables associated with prescriptions from qualified and unqualified sources.
| Variables | Category | Qualified, weighted N (%) | Unqualified, weighted N (%) | Missing percentage |
|---|---|---|---|---|
| Child’s age | One year old | 5274 (75.7) | 1691 (24.3) | 32.3 |
| Two years old | 5386 (73.8) | 1913 (26.2) | ||
| Three years old | 4458 (73.4) | 1615 (26.6) | ||
| Four years old | 3630 (72.0) | 1409 (28.0) | ||
| Five years old | 2742 (71.4) | 1100 (28.6) | ||
| Sex of the child | Male | 16,911.9 (74.7) | 5740 (25.3) | 0 |
| Female | 15,054.8 (73.4) | 5467 (26.6) | ||
| Type of place of residence | Urban | 10,686.8 (72.2) | 4121 (27.8) | 0 |
| Rural | 21,280.7 (75.0) | 7086 (25.0) | ||
| Mother’s highest educational level | No education | 8319 (74.3) | 2876 (25.7) | 5.3 |
| Primary | 8630 (74.1) | 3014 (25.9) | ||
| Secondary | 11,141.2 (76.1) | 3499 (23.9) | ||
| Higher | 2691 (78.8) | 726.1 (21.3) | ||
| Number of children under 5 in the household | One | 13,369.1 (76.8) | 4056 (23.3) | 0 |
| Two | 12,361.9 (73.5) | 4457 (26.5) | ||
| Three | 4143 (69.1) | 1852 (30.9) | ||
| Four | 1137 (69.7) | 494.5 (30.3) | ||
| Five or above | 913.7 (72.4) | 348.5 (27.6) | ||
| Partner’s/husband’s occupation (grouped) | Did not work | 678.8 (76.3) | 210.8 (23.7) | 22.2 |
| Agriculture | 6859 (71.7) | 2710 (28.3) | ||
| Manual labour | 7312 (71.7) | 2882 (28.3) | ||
| Professional/technical/managerial | 3058 (72.8) | 1145 (27.3) | ||
| Others | 6361 (72.7) | 2386 (27.3) | ||
| Respondents occupation (grouped) | Did not work | 13,253.5 (72.2) | 5093 (27.8) | 16.2 |
| Agriculture | 5155 (72.7) | 1937 (27.3) | ||
| Manual labour | 1654 (74.7) | 561.2 (25.3) | ||
| Professional/technical/managerial | 1414 (76.0) | 446.8 (24.3) | ||
| Others | 4721 (70.7) | 1954 (29.3) | ||
| Wealth index combined | Poorest | 6619 (73.70 | 2365 (26.3) | 0 |
| Poorer | 6398 (73.2) | 2343 (26.8) | ||
| Middle | 6571 (74.1) | 2296 (25.9) | ||
| Richer | 6687 (75.5) | 2174 (24.5) | ||
| Richest | 5692 (73.7) | 2029 (26.3) | ||
| Body mass index (BMI) of children under five | 1 | 157.2 (74.5) | 53.7 (25.5) | 36.1 |
| 2 | 6981 (74.2) | 2431 (25.8) | ||
| 3 | 11,184.3 (72.9) | 4173 (27.2) | ||
| 4 | 1805 (75.3) | 592.1 (24.7) | ||
| 5 | 152.7 (72.1) | 59.1 (27.9) | ||
| Delivery by caesarean section | No | 26,742.6 (73.1) | 9841 (26.9) | 0.4 |
| Yes | 5118 (80.0) | 1281 (20.0) | ||
| Months of breastfeeding | One year breastfed | 8178 (76.3) | 2534 (23.7) | 5.2 |
| Two years breastfed | 6675 (77.2) | 1973 (22.8) | ||
| Three years breastfed | 1706 (78.7) | 462.9 (21.3) | ||
| Four years breastfed | 409.8 (77.0) | 122.1 (23.0) | ||
| Five years breastfed | 157 (77.1) | 46.7 (22.9) | ||
| Ever breastfed, not currently breastfed | 12,123.8 (70.3) | 5122 (29.7) | ||
| Never breastfed | 990.1 (69.8) | 429 (30.2) | ||
| Inconsistent | 4.6 (90.1) | 0.5 (9.9) | ||
| Ever had vaccination | No | 779.5 (63.1) | 455.4 (36.9) | 72.6 |
| Yes | 7056 (66.6) | 3543 (33.4) |
The percentage of antibiotics taken for fever/cough from qualified sources for children aged one year, two years, three years, four years, and five years are 75.7%, 73.8%, 73.4%, 72.0%, and 71.4%, respectively. However, 32.3% of the variable was missing. According to their mothers' occupations—agricultural, manual labour, professional/technical/managerial, others, and mothers who did not work—children under the age of five received 72.2%, 72.7%, 74.7%, 76.0%, and 70.7% of their antibiotics from qualified sources, respectively. The missing percentage of this variable was 16.2%. On the other hand, the percentage of antibiotics taken from qualified sources for children under five on the basis of their mothers’ partner/husband’s occupation were 76.3% (agricultural), 71.7% (manual labour), 71.7% (professional/technical/managerial), 72.8% (others) and 72.7% whose mothers’ partner did not work. However, there were 22.2% missing response in this variable. The percentage of children taking antibiotics for fever/cough from qualified sources in terms of their BMI are 74.5% for <9.9, 74.2% for 9.9–14.9, 72.9% for 14–18.5, 75.3% for 18.5–24.9 and 72.1% for >24.9 of BMI index. In this variable, there were 36.1% missing responses. According to the wealth index of child’s family, the percentage of taking antibiotics for fever/cough is 73.7% for poorest, 73.2% for poorer, 74.1% for middle, 75.5% for richer and 73.7% for richest. For children who had vaccination at least once, the percentage who had taken antibiotics for fever/cough from qualified sources was 66.6%. In comparison, for children who never had vaccination the percentage who had taken antibiotics from qualified sources was 63.1%. However, there was 72.6% missing for this variable.
Stratified analysis
Country-wise comparison
In the least ten countries in terms of antibiotics consumption in LMICs, 62.0% of children with fever/cough received antibiotics from qualified sources. In contrast, in the highest ten countries, 67.1% of children with fever received antibiotics from qualified sources.
Continent wise comparison
From Fig. 4, comparing the continents, Oceania had the highest percentage of taking antibiotics from qualified sources (88.9%) whereas Central Asia had the lowest percentage (56.3%) of all continents. The other continents have the percentages of taking antibiotics from qualified sources as follows: Sub-Saharan Africa (71.4%), North Africa-West Asia-Europe 67.1%, South & Southeast Asia (79.8%) and Latin America & Caribbean (74.7%).
Fig. 4.
Comparison of weighted percentage of antibiotics sources across continents as defined by the World Bank. Legend: Here, the black colour represents the antibiotics from unqualified sources on the other hand the grey colour represents the antibiotics from qualified sources.
Country economic status
From Fig. 5, comparing the countries' socioeconomic status, the lower-middle-income countries had the highest rate (76.5%) of taking antibiotics from qualified sources, followed by upper-middle-income countries (72.2%) and lower-income countries (70.9%).
Fig. 5.
Comparison of weighted percentage of antibiotics sources across different income countries as defined by the World Bank. Legend: Here, the black colour represents the antibiotics from unqualified sources, on the other hand the grey colour represents the antibiotics from qualified sources.
Discussion
Antibiotic consumption in children under five has been well reported in LMICs.23 Antibiotic consumption comes with considerable health24 and economic7 burdens for LMICs. The sources from which people receive these antibiotics are of utmost importance as inappropriate use can lead to AMR.25
Our findings have revealed that antibiotic consumption for fever/cough among children is quite high across LMICs, which is in line with a previous study.23 Among the children receiving antibiotics, we found that one in four children receiving antibiotics from unqualified sources. Another study has also found similar percentages.26 Between the top ten and bottom ten LMICs in antibiotic usage, there appears to be a 5-percentage point variation (62% in bottom ten as opposed to 67.1% in top ten countries) in how those antibiotics were consumed. We have found that over 40% of antibiotics have been received from unqualified sources in eleven LMICs (Bangladesh, Chad, Comoros, Congo, Congo Democratic Republic, Côte d'Ivoire, Gabon, Kyrgyz Republic, Tajikistan, Tanzania, and Yemen). The percentages are particularly alarming for Tanzania and Bangladesh, with 77.6% and 76.3% prescriptions from unqualified sources, respectively. Among the countries in our study, Egypt, Albania, Gabon, Honduras and Tajikistan have very high (more than 50%) antibiotic consumption rate in children for fever/cough. Although these countries have a relatively high rate of prescription from qualified sources, the high consumption rate is detrimental to the health of the affected children.27 On the other hand, although Bangladesh has 76% of unqualified sources of antibiotics for fever/cough, only 7% of the children in Bangladesh received antibiotics at all for fever/cough. Therefore, despite having a relatively low rate of unqualified sources of antibiotics, the aforementioned five countries are in a worse position than countries such as Bangladesh.
A number of the countries (e.g., Uganda, Bangladesh etc.) in our study have inadequate access to medical treatment. This may prompt them to seek treatment from untrained providers like drug sellers or conventional healers.28, 29, 30 From the perspective of Bangladesh, there is a lack of trained healthcare professionals in Bangladesh, especially in rural regions. As a result, they could turn to unqualified healthcare providers like drug sellers or conventional healers. According to a study in Bangladesh, there is a high prevalence of using unqualified healthcare providers since there is little access to qualified medical professionals.31
Healthcare is quite expensive in many LMICs, which prompts individuals to look for less expensive options.32 For example, Bangladesh's medical expenses can be considerable, especially for those with limited resources. According to a study done in metropolitan Bangladesh, pharmacies sell antibiotics for less money than qualified healthcare professionals.31,33 Antibiotics can be more affordable from unqualified drug sellers than from qualified healthcare professionals. For instance, a study in Kyrgyzstan demonstrated that pharmacies sell antibiotics for less prices than qualified healthcare professionals.34 According to another study conducted in Tanzania, rural populations frequently turn to traditional healers since they have little access to modern medical care.35
The risks of improper use of antibiotics, such as the development of antibiotic resistance, may not be well recognised among LMICs. This may result in obtaining antibiotics from unqualified sources and using them inappropriately. For instance, a study in the Congo revealed that both patients and healthcare professionals were unaware of antibiotic resistance, and that over 70% of medicines were supplied incorrectly.35, 36, 37
The proportion of antibiotics received for fever/cough from qualified sources is influenced by a variety of socioeconomic variables. Children in rural areas have typically been receiving more prescriptions from qualified sources (about 3 percentage point) for antibiotics than those in urban areas. This is however not consistent across the LMICs. For example, in six countries (e.g., Chad, Congo Democratic Republic, Cote d’Ivoire, Eswatini, Haiti and Morocco), the percentage of prescriptions from qualified sources have been high in urban areas rather than in rural ones. There are a few reasons why antibiotic prescriptions by qualified sources are low in urban areas. Firstly, since people in urban areas are more educated and have basic knowledge about antibiotics for common childhood diseases,37,38 they buy antibiotics for their children without any doctor's consultation. Secondly, the abundance of pharmacies in urban areas compared to rural areas39 also leads to unqualified purchases of antibiotics. Finally, excessive queuing lines in urban government hospitals is also a contributing factor in people not going to government health workers for common and repetitive childhood diseases.40
Our findings indicate that the percentage of taking antibiotics from qualified sources drops as children grow older. Similar result was also shown in a study in China.41 We have further observed a decreasing pattern in prescriptions from qualified sources for antibiotics with the increase in the number of children in a household. When there have been correspondingly two, three, and four children, the proportion of receiving an antibiotic prescription from qualified sources decreased compared to one child. There could be a few reasons for the preceding results. Firstly, households with more than one child are more experienced in dealing with common childhood diseases, so they are more prone to use their experiences to buy antibiotics without a prescription rather than visiting a qualified doctor.42, 43, 44 Secondly, the number of children can weigh down economically on every household. As a result, households with more than one child look for cheaper and convenient sources for antibiotics and thus opt for unqualified sources.45
We found that the percentage of taking antibiotics from qualified sources for children increases with the mothers’ education level. Several studies also suggest that parents' higher education level significantly reduces taking antibiotics from unqualified sources.46,47
On the other hand, we have seen an upward trajectory in prescriptions from qualified sources with the increase in the years of breastfeeding. When children have been breastfed for correspondingly two, three, and four years, the proportion of receiving an antibiotic prescription from qualified sources increased considerably compared to those who never did. Mothers who have breastfed their child for a long time tend to take antibiotic prescriptions from a qualified source for the child more than others who breastfed for a short time or never did.
The children whose mothers had caesarean sections have received reasonably more (about 6 percentage point) prescriptions from qualified sources for antibiotics than those who did not. The primary reason is mothers who have had caesarean sections frequently require long-term care from qualified doctors and are more familiar with antibiotics. In such cases, children tend to receive more antibiotics from unqualified sources.42,43,48
Our study suggests that children in Malawi, Mozambique, Cambodia, Zambia, Eswatini, Papua New Guinea, Burkina Faso, Indonesia, Nigeria, and the Maldives have received a considerably high number of antibiotics for fever/cough from qualified sources. Of the six regions in which we divided our countries, Central Asia has the lowest percentage of antibiotics taken for fever/cough from qualified sources. Oceania, on the other hand, has the highest. Similar results have also been observed in a previous study.49 Lower-middle-income countries have the highest percentage of taking antibiotics from qualified sources compared to upper-middle-income and lower-income countries. Children in LMICs receive antibiotics from qualified sources more (about 3 percentage point) than lower-income countries.
Our study finds that antibiotic taking from unqualified sources is alarmingly high in some of the LMICs (Bangladesh, Chad, Comoros, Congo, Congo Democratic Republic, Côte d'Ivoire, Gabon, Kyrgyz Republic, Tajikistan, Tanzania, and Yemen). There is a need to strengthen the enforcement of laws and regulations to prevent the sale and distribution of antibiotics from unqualified sources in these countries. Additionally, there should be a greater focus on urban areas, where the proportion of antibiotics obtained from unqualified sources is higher than in rural areas. Targeted interventions, such as health education campaigns, free healthcare services, and improved access to qualified sources of antibiotics, should be implemented to support vulnerable populations, including households with multiple children and children with uneducated mothers. It is also essential to raise public awareness and understanding of appropriate antibiotic use through community outreach initiatives, public health campaigns, and school-based education programs. Finally, promoting rational antibiotic use by healthcare professionals, reducing over-the-counter access to antibiotics, and promoting alternative treatments can help reduce the demand for antibiotics from unqualified sources.
To our knowledge, this is the first study conducted on qualified sources of antibiotics for fever/cough in children under five. Using geospatial analysis, we provided a comprehensive picture of prescriptions from qualified sources in LMICs. Our study incorporates a large number of LMICs which gives a generalised result for prescriptions from qualified sources of antibiotics.
Our analysis has several limitations. Firstly, several countries were unavailable in the DHS website. Secondly, in the available datasets, some countries did not include some of our variables of interest. Thirdly, some DHS datasets had very low observations which led to some unexpected results. Fourthly, we observed that a few of our variables had large number of missing values which may affect the robustness of the estimates. For example, several socio-demographic variables (child’s age, BMI of children under five and ever had vaccination) had around 30% missing values. The variable “Ever had vaccination”, especially, had 72% missing but we kept the variable in our study as we believe this may be a contributing variable to the awareness and availability of qualified sources of antibiotics. The extent of these missing values could provide some ambiguity in interpreting the results of these variables. Further study can be done by imputing missing values to improve the estimates. Finally, we only examined the unqualified sources of antibiotics for one childhood disease: fever/cough. Further study is needed to illustrate a complete scenario of prescription patterns of antibiotics for other common childhood diseases such as diarrhoea, Acute Respiratory Infection (ARI), etc.
Our study shows that eleven countries were receiving a high amount of antibiotics for fever/cough from unqualified sources. The region of central Asia was also extremely prone to receiving antibiotics for fever from unqualified sources. Several variables contribute to the proportion of children under five who had received antibiotics for fever/cough from qualified sources which are the type of place of residence, the number of children under five in the household, years of breastfeeding, and mothers who had caesarean sections. The study puts the spotlight on the sources of antibiotic prescriptions for fever/cough in children under five in LMICs. Our study calls for more robust health infrastructures and implementing various checks and balances for pharmacies not to provide antibiotics suggested by unqualified sources. The researchers hope this study will draw the attention of the relevant authorities to this appalling situation and recommend providing need-oriented support services at an individual level. Finally, the executives who make public health policies must acknowledge the circumstances and take the necessary steps at the national level.
Contributors
MSH, MFI, PBA, and MR cleaned, compiled, analysed the dataset, and wrote the original draft. TSA performed geospatial analysis. TA and MABC gave feedback on data analysis and manuscript structure, reviewed, and edited the first draft. MJU conceptualised, commented, and supervised the study. All authors have verified the underlying data, the statistical analysis, and the results of the study and take full responsibility for the results. All authors had full access to all the data in the study, reviewed the study numerous times, and accept responsibility for the decision to submit it for publication.
Data sharing statement
The data are available on request to the corresponding author.
Declaration of interests
There is no conflict of interest.
Acknowledgements
We gratefully acknowledge the DHS Program for granting access to the LMICs datasets.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102055.
Appendix A. Supplementary data
References
- 1.GBD 2017 Mortality Collaborators Global, regional, and national age-sex-specific mortality and life expectancy, 1950–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1684–1735. doi: 10.1016/S0140-6736(18)31891-9. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(18)31891-9/fulltext [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burstein R., Henry N.J., Collison M.L., et al. Mapping 123 million neonatal, infant and child deaths between 2000 and 2017. Nature. 2019;574(7778):353–358. doi: 10.1038/s41586-019-1545-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laxminarayan R., Matsoso P., Pant S., et al. Access to effective antimicrobials: a worldwide challenge. Lancet. 2016;387(10014):168–175. doi: 10.1016/S0140-6736(15)00474-2. [DOI] [PubMed] [Google Scholar]
- 4.O’Neill J. Government of the United Kingdom; 2016. Tackling drug-resistant infections globally: final report and recommendations.https://apo.org.au/node/63983 [Google Scholar]
- 5.World Health Organization . World Health Organization; 2014. Antimicrobial resistance: global report on surveillance.https://apps.who.int/iris/handle/10665/112642 [Google Scholar]
- 6.Högberg L.D., Magiorakos A.P., Heuer O.E., Monnet D.L. Antimicrobial resistance surveillance in Europe: regional pooling of national data from a small number of sites can be misleading. Diagn Microbiol Infect Dis. 2014;80(1):90. doi: 10.1016/j.diagmicrobio.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 7.Di Martino M., Lallo A., Kirchmayer U., Davoli M., Fusco D. Prevalence of antibiotic prescription in pediatric outpatients in Italy: the role of local health districts and primary care physicians in determining variation. A multilevel design for healthcare decision support. BMC Public Health. 2017;17(1):886. doi: 10.1186/s12889-017-4905-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kolozsvári L.R., Kónya J., Paget J., et al. Patient-related factors, antibiotic prescribing and antimicrobial resistance of the commensal Staphylococcus aureus and Streptococcus pneumoniae in a healthy population - Hungarian results of the APRES study. BMC Infect Dis. 2019;19(1):253. doi: 10.1186/s12879-019-3889-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alvarez-Uria G., Zachariah S., Thomas D. High prescription of antimicrobials in a rural district hospital in India. Pharm Pract. 2014;12(2):384. doi: 10.4321/s1886-36552014000200003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klein E.Y., Tseng K.K., Pant S., Laxminarayan R. Tracking global trends in the effectiveness of antibiotic therapy using the drug resistance index. BMJ Glob Health. 2019;4(2) doi: 10.1136/bmjgh-2018-001315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu M., Zhao G., Stålsby Lundborg C., Zhu Y., Zhao Q., Xu B. Knowledge, attitudes, and practices of parents in rural China on the use of antibiotics in children: a cross-sectional study. BMC Infect Dis. 2014;14:112. doi: 10.1186/1471-2334-14-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murray C.J.L., Ikuta K.S., Sharara F., et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629–655. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lekagul A., Tangcharoensathien V., Mills A., Rushton J., Yeung S. How antibiotics are used in pig farming: a mixed-methods study of pig farmers, feed mills and veterinarians in Thailand. BMJ Glob Health. 2020;5(2) doi: 10.1136/bmjgh-2019-001918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belachew S.A., Hall L., Selvey L.A. Non-prescription dispensing of antibiotic agents among community drug retail outlets in Sub-Saharan African countries: a systematic review and meta-analysis. Antimicrob Resist Infect Control. 2021;10(1):13. doi: 10.1186/s13756-020-00880-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nahar P., Unicomb L., Lucas P.J., et al. What contributes to inappropriate antibiotic dispensing among qualified and unqualified healthcare providers in Bangladesh? A qualitative study. BMC Health Serv Res. 2020;20(1):656. doi: 10.1186/s12913-020-05512-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davey P., Brown E., Hartman G. In: The cochrane database of systematic reviews. The Cochrane Collaboration, editor. John Wiley & Sons, Ltd; 2002. Interventions to improve antibiotic prescribing practices for hospital inpatients. [DOI] [PubMed] [Google Scholar]
- 17.Croft T.N., Marshall A.M.J., Allen C.K., et al. Guide to DHS Statistics. United States Agency for International Development (USAID) ICF; Rockville, MD: 2018. [Google Scholar]
- 18.Samir N., Hassan M.Z., Biswas M.A.A.J., et al. Antibiotic use for febrile illness among under-5 children in Bangladesh: a nationally representative sample survey. Antibiotics. 2021;10(10):1153. doi: 10.3390/antibiotics10101153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aywak D., Jaguga C.D.P., Nkonge N.G., Kinuthia R., Ambale C., Awle I.A. Pharmacy practice in Kenya. Can J Hosp Pharm. 2017;70(6):456–462. doi: 10.4212/cjhp.v70i6.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ArcGIS Desktop Desktop GIS software suite. https://www.esri.com/en-us/arcgis/products/arcgis-desktop/overview
- 21.Statistical software for data science. Stata. https://www.stata.com/
- 22.Groves R.M., Fowler F.J. Survey methodology. John Wiley & Sons; 2011. [Google Scholar]
- 23.Fink G., D’Acremont V., Leslie H.H., Cohen J. Antibiotic exposure among children younger than 5 years in low-income and middle-income countries: a cross-sectional study of nationally representative facility-based and household-based surveys. Lancet Infect Dis. 2020;20(2):179–187. doi: 10.1016/S1473-3099(19)30572-9. [DOI] [PubMed] [Google Scholar]
- 24.Saha S.K., Promite S. Factors influencing clinician’s antibiotic prescribing behaviors (apb) in Bangladesh: an in–depth review using comb model. Open Access J Trans Med Res. 2017;1(4):91–95. doi: 10.15406/oajtmr.2017.01.00019. [DOI] [Google Scholar]
- 25.Hijazi K., Joshi C., Gould I.M. Challenges and opportunities for antimicrobial stewardship in resource-rich and resource-limited countries. Expert Rev Anti Infect Ther. 2019;17(8):621–634. doi: 10.1080/14787210.2019.1640602. [DOI] [PubMed] [Google Scholar]
- 26.Ocan M., Obuku E.A., Bwanga F., et al. Household antimicrobial self-medication: a systematic review and meta-analysis of the burden, risk factors and outcomes in developing countries. BMC Public Health. 2015;15(1):742. doi: 10.1186/s12889-015-2109-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brownlee S., Chalkidou K., Doust J., et al. Evidence for overuse of medical services around the world. Lancet. 2017;390(10090):156–168. doi: 10.1016/S0140-6736(16)32585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nuri N.N., Sarker M., Ahmed H.U., Hossain M.D., Beiersmann C., Jahn A. Pathways to care of patients with mental health problems in Bangladesh. Int J Ment Health Syst. 2018;12(1):39. doi: 10.1186/s13033-018-0218-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madinah N. Challenges and barriers to the health service delivery system in Uganda. IOSR J Nurs Health Sci. 2016;5(2):30–38. [Google Scholar]
- 30.Sudhinaraset M., Ingram M., Lofthouse H.K., Montagu D. What is the role of informal healthcare providers in developing countries? A systematic review. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0054978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan N.U.Z., Rasheed S., Sharmin T., et al. Experience of using mHealth to link village doctors with physicians: lessons from Chakaria, Bangladesh. BMC Med Inform Decis Mak. 2015;15:62. doi: 10.1186/s12911-015-0188-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakeena M.H.F., Bennett A.A., McLachlan A.J. Enhancing pharmacists’ role in developing countries to overcome the challenge of antimicrobial resistance: a narrative review. Antimicrob Resist Infect Control. 2018;7:63. doi: 10.1186/s13756-018-0351-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hossain M., Amran M. A cross-sectional pilot study on pharmacovigilance to improve the drug safety in Bangladesh. Biomed Pharmacol J. 2019;12:1039–1049. doi: 10.13005/bpj/1733. [DOI] [Google Scholar]
- 34.Waning B., Maddix J., Tripodis Y., Laing R., Leufkens H.G., Gokhale M. Towards equitable access to medicines for the rural poor: analyses of insurance claims reveal rural pharmacy initiative triggers price competition in Kyrgyzstan. Int J Equity Health. 2009;8:43. doi: 10.1186/1475-9276-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thriemer K., Katuala Y., Batoko B., Alworonga J.P. Antibiotic prescribing in DR Congo: a knowledge, attitude and practice survey among medical doctors and students. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0055495. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0055495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haenssgen M.J., Xayavong T., Charoenboon N., Warapikuptanun P., Khine Zaw Y. The consequences of AMR education and awareness raising: outputs, outcomes, and behavioural impacts of an antibiotic-related educational activity in Lao PDR. Antibiotics. 2018;7(4):95. doi: 10.3390/antibiotics7040095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Green D.L., Keenan K., Fredricks K.J., et al. The role of multidimensional poverty in antibiotic misuse: a mixed-methods study of self-medication and non-adherence in Kenya, Tanzania, and Uganda. Lancet Glob Health. 2023;11(1):e59–e68. doi: 10.1016/S2214-109X(22)00423-5. [DOI] [PubMed] [Google Scholar]
- 38.Widayati A., Suryawati S., de Crespigny C., Hiller J.E. Knowledge and beliefs about antibiotics among people in Yogyakarta City Indonesia: a cross sectional population-based survey. Antimicrob Resist Infect Control. 2012;1(1):38. doi: 10.1186/2047-2994-1-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Law M.R., Heard D., Fisher J., Douillard J., Muzika G., Sketris I.S. The geographic accessibility of pharmacies in Nova Scotia. Can Pharm J. 2013;146(1):39–46. doi: 10.1177/1715163512473062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lungu E.A., Biesma R., Chirwa M., Darker C. Healthcare seeking practices and barriers to accessing under-five child health services in urban slums in Malawi: a qualitative study. BMC Health Serv Res. 2016;16(1):410. doi: 10.1186/s12913-016-1678-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yuan J., Du W., Li Z., Deng Q., Ma G. Prevalence and risk factors of self-medication among the pediatric population in China: a national survey. Front Public Health. 2022;9 doi: 10.3389/fpubh.2021.770709. https://www.frontiersin.org/articles/10.3389/fpubh.2021.770709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jasim A. Parental self medication of antibiotics for children in Baghdad city. Int J Pharm Pharm Sci. 2014;6:485–489. [Google Scholar]
- 43.Paredes J.L., Navarro R., Watanabe T., et al. Knowledge, attitudes and practices of parents towards antibiotic use in rural communities in Peru: a cross-sectional multicentre study. BMC Public Health. 2022;22(1):459. doi: 10.1186/s12889-022-12855-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharif S.I., Masalmeh B.E.M., Awad H.M., Osama A., Abdulmqasood Y.A., Bugaighis L.M. Parents’ knowledge and attitude to self-medication of children with antibiotics. Arch Pharm Pract. 2015 https://archivepp.com/article/parents-knowledge-and-attitude-to-selfmedication-of-children-with-antibiotics [Google Scholar]
- 45.Do N.T.T., Vu H.T.L., Nguyen C.T.K., et al. Community-based antibiotic access and use in six low-income and middle-income countries: a mixed-method approach. Lancet Glob Health. 2021;9(5):e610–e619. doi: 10.1016/S2214-109X(21)00024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang L., Mendoza R., Costa M.M.G., et al. Antibiotic use in community-based pediatric outpatients in southern region of Brazil. J Trop Pediatr. 2005;51(5):304–309. doi: 10.1093/tropej/fmi022. [DOI] [PubMed] [Google Scholar]
- 47.Nyeko R., Otim F., Obiya E.M., Abala C. Pre-hospital exposures to antibiotics among children presenting with fever in northern Uganda: a facility-based cross-sectional study. BMC Pediatr. 2022;22(1):322. doi: 10.1186/s12887-022-03375-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Togoobaatar G., Ikeda N., Ali M., et al. Survey of non-prescribed use of antibiotics for children in an urban community in Mongolia. Bull World Health Organ. 2010;88(12):930–936. doi: 10.2471/BLT.10.079004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bert F., Previti C., Calabrese F., Scaioli G., Siliquini R. Antibiotics self medication among children: a systematic review. Antibiotics. 2022;11(11):1583. doi: 10.3390/antibiotics11111583. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.