Abstract
Microbial biofilms are resilient, immune-evasive, often antibiotic-resistant health challenges, and increasingly the target for research into novel therapeutic strategies. We evaluated the effects of a nutraceutical enzyme and botanical blend (NEBB) on established biofilm. Five microbial strains with known implications in chronic human illnesses were tested: Candida albicans, Staphylococcus aureus, Staphylococcus simulans (coagulase-negative, penicillin-resistant), Borrelia burgdorferi, and Pseudomonas aeruginosa. The strains were allowed to form biofilm in vitro. Biofilm cultures were treated with NEBB containing enzymes targeted at lipids, proteins, and sugars, also containing the mucolytic compound N-acetyl cysteine, along with antimicrobial extracts from cranberry, berberine, rosemary, and peppermint. The post-treatment biofilm mass was evaluated by crystal-violet staining, and metabolic activity was measured using the MTT assay. Average biofilm mass and metabolic activity for NEBB-treated biofilms were compared to the average of untreated control cultures. Treatment of established biofilm with NEBB resulted in biofilm-disruption, involving significant reductions in biofilm mass and metabolic activity for Candida and both Staphylococcus species. For B. burgdorferi, we observed reduced biofilm mass, but the remaining residual biofilm showed a mild increase in metabolic activity, suggesting a shift from metabolically quiescent, treatment-resistant persister forms of B. burgdorferi to a more active form, potentially more recognizable by the host immune system. For P. aeruginosa, low doses of NEBB significantly reduced biofilm mass and metabolic activity while higher doses of NEBB increased biofilm mass and metabolic activity. The results suggest that targeted nutraceutical support may help disrupt biofilm communities, offering new facets for integrative combinational treatment strategies.
Keywords: Borrelia burgdorferi, drug-resistance, persister cells, Pseudomonas, Staphylococcus
Introduction
A microbial biofilm is a community of adherent microbial cellular forms with properties that help protect the microbial community from disruption by physical, chemical, or immunological attack. Microbial forms living in biofilms are morphologically and functionally distinct from those of free-floating (planktonic) forms of the same species. Biofilms have greater resistance to chemical, physical, and immunological insults than the planktonic forms from the same species [1]. Microbial biofilms pose a major medical and industrial challenge due to resistance to chemical treatments, antibiotics, and an ability to evade immune recognition [2]. As a result, there is a strong research focus on identifying methods to discourage biofilm initiation and formation, and to disrupt existing biofilms [3].
Microbial biofilms form on liquid/solid interfaces in nature, such as rocks and clay particles and decaying plant materials. Biofilms also form on metals and plastics, including medical devices and implants, causing device-related infections, which are associated with a large majority of hospital-acquired infections [4-6]. Biofilms also form on body surfaces, such as the mucosal membranes in the gut, bladder, eye, ear, and lung, as well as in chronic wounds [2]. Persistent biofilm infections can induce a hyper-inflammatory state in the host [7], and include many chronic inflammatory infections including gastrointestinal tract [8], urinary tract, otitis media, infective endocarditis, cystic fibrosis [9], and dental plaque [10].
Biofilms secrete a complex mucus polymer structure that plays a role in microbial adhesion, cell-to-cell interactions, antimicrobial resistance, and immune evasion [11, 12]. The structural framework of the biofilm matrix contains many types of polymers, including polysaccharides, proteins, lipids, bacterial cellulose, and extracellular DNA that offers structural and functional protection [13]. The organisms living within the biofilm need to be able to communicate with each other in a process called quorum sensing [14]. Biofilms may contain multiple species coexisting and collaborating.
The difficulty in treating biofilm infections with pharmaceutical antibiotics and antimicrobials has led to a search for new treatment approaches, including disruption of the protective biofilm matrix, disruption of biofilm adhesion to the substrate, disruption of intra-biofilm communication via quorum sensing, and altering the gene expression of the microbe to be unable to sustain the biofilm environment (Table 1) [6]. When the microbes are no longer able to maintain the biofilm environment, they may revert to a free planktonic state that is more vulnerable to antimicrobial treatments and more visible to the immune system [15].
Table 1.
Enzymes in the nutraceutical enzyme and botanical blend.
| Enzymes– 67 mg/oral dose | Target specificity | Disruption of biofilm |
|---|---|---|
| Lysosyme (from hen’s egg white) | Peptidoglycans in bacterial cell walls | [45, 46] |
| Serratiopeptidase | Proteins | [47, 48] |
| Beta-glucanase | Carbohydrates including fungal beta-glucans | [49, 50] |
| Lipase | Lipids | [26, 51] |
| Protease | Proteins | [27, 28] |
| Cellulase/ Hemicellulase | Bacterial cellulose | [52, 53] |
Enzymes that degrade biofilm polymers have been shown to inhibit new biofilm formation, detach existing biofilm colonies, and increase sensitivity of the biofilm to antimicrobial treatments [16], with the goal of reverting the microbial forms back to their planktonic state [6]. Combinations of antimicrobials that interfere with quorum sensing and adhesion with biofilm-disrupting agents offer additional strategies [17-21].
Based on the composition of biofilm matrices, enzymes are identified that can disrupt biofilm. Lysozyme is an enzyme that is naturally present in mucosal secretions and tissues of animals and humans as part of our innate immune system and able to disrupt bacterial biofilms [22, 23]. B-1,3-glucan is a vital component of fungal biofilms including Candida species, and glucanase enzymes can break down Candida biofilms and increase susceptibility to anti-fungals [24]. Enzyme cocktails including hemicellulases have been used on industrial scales to disrupt biofilms from biopolymer surfaces [25]. Lipase digests fats in the biofilm, making this enzyme important in breaking down both fungal and bacterial biofilms [26]. Many types of protease enzymes are helpful in breaking down the protein matrix and contribute to successful eradication of biofilms [27], leading to increased efficacy of antibiotics [28].
Biofilm formation requires a different gene expression profile than the free-floating microbial forms [29, 30]. Therefore, natural, or synthetic compounds that affect those aspects of microbial gene expression may discourage biofilm formation [31], and force microbes into the free-floating form that is more recognizable by the immune system. Examples include synthetic compounds [32], botanicals [33, 34], essential oils [35], secreted metabolites from beneficial probiotic bacteria [36], and bee venom [37].
Some herbs can inhibit the quorum sensing communication between microbes that contributes to the development of biofilms. Cranberry is well known for its use in preventing and treating urinary tract infections [38]. This is in part due to its ability to prevent and disassemble biofilms by multiple mechanisms including anti-adhesion, decreasing quorum sensing, and direct anti-microbial effects [39, 40]. Berberine, rosemary, and peppermint are other herbs that have been shown to be antimicrobial, anti-quorum sensing, and contributing to biofilm breakdown [41-43]. In addition to herbs, amino acids such as N-acetyl cysteine (NAC) are mucolytic and effective for eliminating bacterial biofilms [44].
We evaluated the biofilm-disrupting properties of a nutraceutical enzyme and botanical blend (NEBB) that contained a combination of enzymes and botanical antimicrobial extracts (Table 1). NEBB was tested for effects on disrupting established biofilms of five biofilm-forming microbial species, including one fungal species and four bacterial strains (Table 2). The purpose of this work was to conduct an initial screening for the effects of a consumable nutraceutical formulation, used by medical doctors to support the treatment of patients with severe chronic illnesses with suspected microbial biofilm involvement, which includes Candida and Staphylococcus subspecies. The types of patients who use this nutraceutical formulation under the supervision of doctors also include patients with chronic Lyme disease, i.e., infection by Borrelia burgdorferi.
Table 2.
Herbal ingredients in the nutraceutical enzyme and botanical blend.
| Herbal Ingredients – 905 mg/oral dose* | Types of anti-microbial effects |
|---|---|
| Cranberry (fruit) extract | Inhibition of bacterial biofilm formation [38, 39], inhibition of quorum-sensing [40] |
| Berberine | Growth inhibition [41, 54], inhibition of bacterial [55] and fungal [56] biofilm formation |
| Rosemary (leaf) extract | Inhibition of bacterial [57] and fungal [58] biofilm formation |
| Peppermint oil powder | Inhibition of bacterial biofilm formation [59], disruption of quorum sensing [60] |
| N-acetyl cysteine | Growth inhibition [44], Disrupts mucins [61] |
*This includes 300 mg of N-acetyl cysteine
Methods
Reagents
Bacterial culture media were purchased from Sigma-Aldrich Inc (USA): Nutrient Broth (Catalogue number 70122), Tryptic Soy Broth (Catalogue number T8907), and BSK medium with 6% rabbit serum (Catalogue number B8291). The 96-well culture plates were obtained from Thermo-Fisher Scientific (USA): CellStar (Greiner Bio-One, Catalogue number 655-180) for all microbes except Borrelia burgdorferi for which collagen-coated 96-well plates were used (Catalogue number 152038). Other reagents were Crystal Violet (Catalogue number V5265, Sigma-Aldrich and CyQUANT VyBrant MTT cell viability assay kit (Catalogue number V13154, Invitrogen, Thermo-Fisher Scientific).
Nutraceutical Enzyme and Botanical Blend
The nutraceutical enzyme and botanical blend (NEBB), BioDisrupt, was provided by the manufacturer, Researched Nutritionals, Los Olivos, CA, USA. The product is a powder that contains water-soluble enzymes (Table 1) and botanical extracts and N-acetyl Cysteine (Table 2).
In order to ensure the product was sterile, and would not introduce bacteria, yeast, or mold spores into the microbial cultures, the product was irradiated at 10 kGy. At NIS Labs, a sample of the powder was tested on Petrifilm culture plates to ensure there were no detectable aerobic bacteria, yeasts, or mold in the test product. Sterile emulsions were prepared from NEBB and introduced into the microbial biofilm cultures. Serial dilutions were tested across a broad dose range. Initial dose response testing revealed the ideal dose in biofilm cultures of this nutraceutical formulation, designed for human consumption, covered a range from 1 – 40 mg/ml, which is 10-fold higher than the dose range used for testing of antimicrobial effects of highly purified compounds.
Microbial Strains and Culture Methods
Five microbes, known for their ability to live in biofilms, were included in this testing (Table 3). The 5 microbial strains – 1 fungal and 4 bacterial – were purchased from the American Type Culture Collection. The recommended culture media for each strain was used, and cultures were performed under conditions that encourage biofilm formation. The testing for effects of the nutraceutical blend on biofilm disruption involved these steps: 1) Culture each microorganism to facilitate biofilm formation in flat-bottom 96-well culture plates, 2) Add NEBB and continue culture for 24 h, 3) Remove planktonic (free) forms including disrupted biofilm and forms released from biofilm and wash the remaining biofilm with physiological saline, 4) Evaluate the estimated mass and metabolic activity of the remaining biofilm in untreated versus treated cultures.
Table 3.
Microbial strains and culture media.
| Microbial strain | Culture medium (Duration, temperature) |
|---|---|
| Candida albicans (Robin) Berkhout (ATCC 10231) | Yeast malt broth (24 h, 37°C) |
| Staphylococcus aureus subsp. aureus Rosenbach (ATCC 6538) | Tryptic soy broth (24 h, 37°C) |
| Staphylococcus simulans Kloos and Schleifer (ATCC 11631)* | Nutrient broth (24 h, 37°C) |
| Pseudomonas aeruginosa (Schroeter) Migula (ATCC 9027) | Nutrient broth (24 h, 37°C) |
| Borrelia burgdorferi Strain B31 (ATCC 35210) | BSK-H complete medium (35 days, 33°C) |
*Coagulase-negative, penicillin-resistant.
Removal of Planktonic Forms
The treatment of established biofilm with NEBB and the resulting disruption of biofilm included release of planktonic forms into the culture supernatant and detachment of clumps of bacteria living in biofilm. In order to provide conclusive data on the effects on biofilms, following published methodology [62], planktonic forms had to be removed from the cultures before staining for biofilm mass and metabolic activity, The removal of planktonic forms and the addition of washing buffer was done using a very low speed to avoid mechanical removal of biofilm material. The removal of planktonic forms was performed using electronic 12-channel pipettes (Viaflo, Integra, USA), where the speed was set to “1” (the maximum speed is “10”). Phosphate-buffered saline was added, also using speed “1”, where the liquid was dispensed onto the sidewalls of each well to avoid disruption of biofilm by direct pipetting actions onto biofilm. For the cultures of Pseudomonas aeruginosa, this pipetting allowed scoring of slime formation, where “0” indicated no change in viscosity of the culture medium, and a score of “3” (300%) indicated that the entire culture medium had turned into a mucus plug.
Crystal Violet Staining for Biofilm Mass
The quantitative evaluation of biofilm mass for each microbial form was determined by crystal violet staining [63]. The saline was removed from each well and a 0.1% solution of Crystal Violet was added. The biofilm cultures were allowed to incubate with the crystal violet solution for a minimum of 10 min at room temperature, after which the culture plates were washed in distilled water. The distilled water was removed, and the stained biofilm plates allowed to air dry. The crystal violet was solubilized in 10% acetic acid for 15 min and the optical density measured by a plate-based spectrophotometer at 550 nanometers. The crystal violet staining was a measure for the relative mass of biofilm in each well. Untreated cultures served as a control for maximum biofilm formation. Blank wells without microbial forms served as negative controls. The percent-inhibition of biofilm formation was calculated for each dose of the test products.
Biofilm Metabolic Activity Using the MTT Assay
A second set of culture plates was washed in the same way as the plates used for Crystal Violet staining. The culture plates were tested for metabolic activity using the MTT assay, which involves a colorimetric reaction based on cellular metabolic activity [64]. The MTT assay has been used for testing of metabolic activity in multiple types of biofilm [65, 66]. In this bioassay, chemical reactions involved in cellular metabolic reactions where oxidoreductase enzymes reduce the tetrazolium dye 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to insoluble formazan crystals that are purple in color. The crystals are solubilized by addition of the detergent sodium dodecyl sulfate. The color development is measured by micro-plate-based spectrophotometry where the optical density is measured at 570 nanometers, using a PowerWave plate reader (BioTek Instruments, USA).
Statistical Analysis
Average and standard deviation for each data set was calculated using Microsoft Excel. Statistical analysis was performed using the 2-tailed, independent t-test. Statistical significance was set at p < 0.05, and a high level of significance at p < 0.01.
Results
Disruption of Established Microbial Biofilm
The treatment of established microbial biofilms in vitro with NEBB showed reduced biofilm. The types of observations varied between the different microbial species.
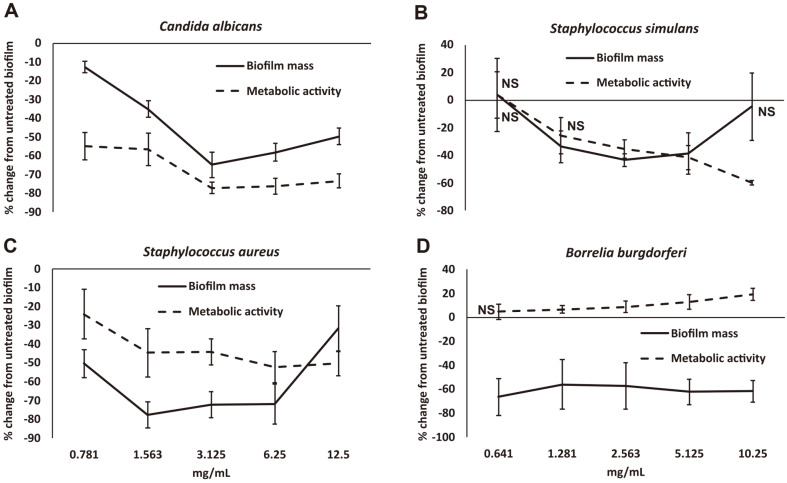
Candida albicans in culture rapidly formed robust biofilms. NEBB was capable of disrupting these established C. albicans biofilm within 24 h (Fig. 1A). The relative mass of biofilm was significantly reduced at all doses of NEBB. At the dose of 3.125 mg/ml, the biofilm mass was 60% reduced compared to untreated cultures. This was also reflected in the reduced metabolic activity in proportion to the reduced biofilm mass. At the mid-dose, the relative metabolic activity of the cultures was 75% reduced compared to untreated control cultures. The effect of NEBB was highly significant across all doses of the product.
Fig. 1. Disruption of established microbial biofilm from Candida albicans (A), Staphylococcus simulans (B), Staphylococcus aureus (C), and Borrelia burgdorferi (D) after treatment with a nutraceutical enzyme and botanical blend across a dose range of 0.8 – 12.5 mg/ml.
Data is shown as the average + standard deviation of nine repeats of each treatment dose, as the % change from untreated biofilm, where the untreated biofilm had gone through identical procedures for removal of planktonic forms, washing, and addition of fresh medium. Biofilm mass was quantified by crystal violet staining (solid lines), and the metabolic activity of the microbial biofilms was measured by the MTT assay (dashed lines). All data points were statistically significant when compared to the untreated control biofilm cultures, except where the data point is annotated by NS (not significant).
Established biofilm of the coagulase-negative, penicillin-resistant strain of S. simulans biofilm were also disrupted by NEBB at some doses. The relative mass of biofilm was significantly reduced at 1.281 – 5.125 mg/ml doses of NEBB. The dose response showed an unexpected increase in biofilm mass and metabolic activity at the lowest dose tested (Fig. 1B). It is possible that S. simulans sensed the effects of some ingredients even at this low dose and was able to respond by strengthening the biofilm as a protection.
The treatment of established biofilms of S. aureus resulted in rapid disruption of the biofilm, and this disruption was also associated with reduced metabolic activity (Fig. 1C). The reduced mass and metabolic activity of biofilm was significantly reduced at all doses of the product. For the reduced metabolic activity, the response showed a clear dose-dependent effect.
The effect of NEBB on mature biofilms from B. burgdorferi was more complex. The biofilms were established over a period of 5 weeks, leading to robust clusters of bacterial aggregates, where the morphology of the bacteria living in the biofilm showed dramatic changes from the free planktonic form of the spirochete. The biofilm formation and maturation were similar to published work from Sapi’s team [67], showing initial clustering of free planktonic forms, followed by disappearance of flagella, and increased encasement of the biofilm into hard structured aggregates adhering to the collagen-coated plastic surfaces. The clusters were connected by a dense network of spirochetal bridges, the relative metabolic activity was low, suggesting the bacteria adapted to the biofilm existence by converting into quiescent ‘persister’ cells [68]. When the B. burgdorferi biofilm was treated with NEBB for 24 h, there was a marked reduction in the biofilm mass, however, in contrast to the other bacterial forms, there was a mild increase in metabolic activity, suggesting that the bacterial forms returned into a more active state and were no longer as quiescent (Fig. 1D).
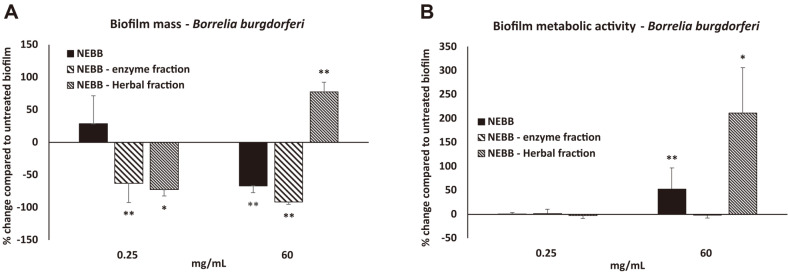
This prompted further testing of Borrelia biofilm cultures, to examine which fraction, the enzyme or the herbal, affected the metabolic activity the most (Fig. 2). Borrelia biofilm treated with the enzyme fraction showed reduced biomass (Fig. 2A) but the enzyme fraction had no effect on the metabolic activity (Fig. 2B). In contrast, the highest dose of the herbal fraction had a statistically significant increase in Borrelia biofilm metabolic activity (Fig. 2B).
Fig. 2. Effects on established bacterial biofilm from Borrelia burgdorferi on biofilm mass (A) and biofilm metabolic activity (B) after treatment with a nutraceutical enzyme and botanical blend (NEBB), compared to treatment with the NEBB enzyme fraction versus the NEBB herbal fraction.
Data is shown as the average ± standard deviation of nine repeats of each treatment dose, as the % change from untreated biofilm, where the untreated biofilm had gone through identical procedures for removal of planktonic forms, washing, and addition of fresh medium. Levels of statistical significance are shown on the graphs where changes compared to untreated biofilm is indicated by asterisks, where p < 0.10: (*), p < 0.05: * and p < 0.01: **.
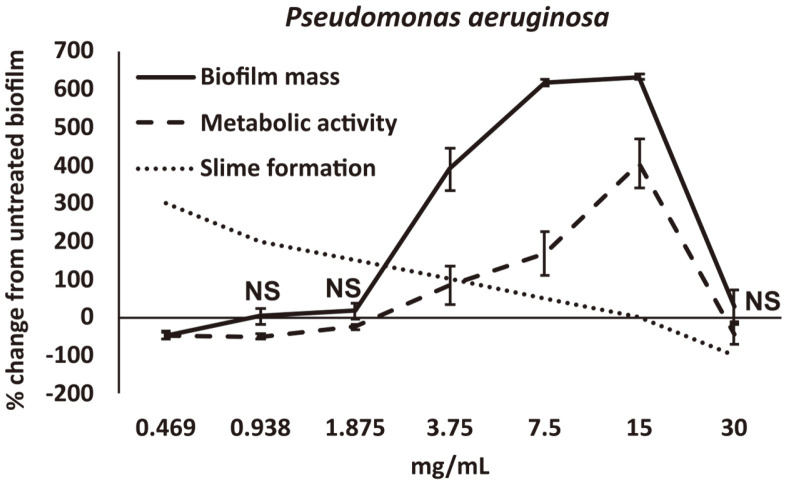
Established biofilms of P. aeruginosa showed complex responses to treatment with NEBB. At the higher doses, NEBB reduced both the biofilm mass and metabolic activity at 30 mg/mL, and the disruption was highly significant (p < 0.01). However, at lower doses, NEBB triggered a defensive response in P. aeruginosa to strengthen the biofilm; this was seen both for the relative biofilm mass and for the metabolic activity (Fig. 2). There was approximately 600% increase in relative biofilm mass and 300% increase in metabolic activity. This increase was highly significant.
Another observation associated with NEBB treatment was slime production. P. aeruginosa is notorious for producing slime, and when growing in liquid suspensions in culture flasks, will display veils of slimy material. In 96-well microplates, the entire volume of the liquid cultures in each well can turn into slime plugs, making pipetting a challenge. We noticed that at the lower doses of NEBB the slime formation was increased compared to untreated cultures, and also compared to higher doses of NEBB. The observation suggests that while at higher doses NEBB mildly inhibited both biofilm and slime formation, at lower doses the microbe made massive biofilms to protect itself from NEBB. At the lowest dose, P. aeruginosa did not make biofilm but increased slime production as another evasive tactic.
Discussion
The disruption of persistent immune-evasive and treatment-resistant microbial biofilms is a focus for research into novel types of treatments. Given today’s alarming problems with antibiotic-resistant bacteria, it is a desirable solution to evaluate non-pharmacological, non-antibiotic natural strategies [31].
Biofilms are the predominant form of existence of many microorganisms, including bacteria and simple fungi. The definition of biofilm involves a broad description of single-species, or multi-species, microbial community structures observed in both natural and laboratory environments. Treatment of microbial biofilms is recognized as an urgent need, in light of the multi-species communities inhabiting biofilm, and the resistance of such biofilms to conventional pharmaceutical treatments. Novel therapeutic strategies include combinations of enzymes targeted at the various matrix components surrounding adherent biofilm colonies, for a comprehensive approach to disrupting established biofilm [69]. Since many botanical compounds have also been associated with effects on biofilm survival and function, it was of interest to study the effect of a complex nutraceutical blend of enzymes and botanical extracts (NEBB), designed to break up established biofilm in the gut and tissue. NEBB was tested on established biofilms on 5 microbial species, selected based on their known ability to form biofilm, and the widely known association of these biofilms with chronic health problems.
We report here that established biofilms exposed to NEBB showed reduced biofilm mass when using crystal violet staining. The effects of NEBB on C. albicans and 2 species of Staphylococcus showed rapid disruption of biofilm, as seen by reduced biofilm mass; we suggest that the reduced metabolic activity in the cultures were in direct correlation to the reduced amount of biofilm. The reduction of S. aureus biofilm has multiple applications, since S. aureus biofilms are associated with multiple diseases, including sinus, ear, bone, heart, and non-healing wounds and infections in replacement joints. The reduction of C. albicans biofilm has direct implications for gut health since this microscopic yeast is known to be capable of forming biofilm along the intestinal mucosal barrier [70].
Pseudomonas aeruginosa is involved in severe acute and chronic infections, known to often involve other species such as commensal bacteria [71]. The effects of NEBB on P. aeruginosa involved a bi-phasic dose response where low doses of NEBB triggered significant reduction in biofilm, but higher doses triggered enhanced biofilm formation. This may possibly be due to evasive behavior by Pseudomonas exposed to high doses of NEBB. Further work should evaluate whether the biofilms showing enhanced biofilm formation were less virulent and inflammatory than untreated biofilms.
The spirochete B. burgdorferi is the causative agent of Lyme disease, a multisystemic disorder impacting primarily the skin, nervous system, and musculoskeletal functions, including Lyme arthritis. Immune cell-mediated clearance of B. burgdorferi infections depend in part on immune recognition and phagocytosis of free planktonic bacterial forms; this is hampered by bacterial biofilm formation [72]. We tested the effects of NEBB on B. burgdorferi and, in contrast to the other microbes tested, the reduction in biofilm was accompanied by an increase in metabolic activity. The observation that NEBB was able to change the metabolic state of B. burgdorferi and reduce the biofilm mass is clinically important. The stationary phase of persister cells with low metabolic activity [70] has been associated with more severe illness in a rodent model of Lyme arthritis [73], likely as a result of dysregulated hyper-inflammatory response that persists after the bacteria have either been cleared from the host [74], or taken on an obscure immune-resistant and antibiotic-resistant existence in various tissues in the form of quiescent biofilm [75].
The preliminary results reported here point to further directions for research, including biofilms associated with gut mucosa, as well as bacterial biofilms in tissue such as cartilage, and research involving intracellular biofilm-like colonies [76, 77]. This work, although novel and highly necessary, has limitations. Further research is needed involving multispecies biofilm, such as Borrelia/Candida or Borrelia/Staphylococcus co-cultures, and should include transcriptomics to evaluate changes to gene expression in co-cultures with or without treatment with NEBB, based on recent publications on multispecies biofilm [62, 78, 79]. In a clinical situation, biofilm will likely consist of multiple species, with an unknown combination of bacterial types, assisting the maintenance of the biofilm environment to protect itself from immune-mediated biofilm elimination. Our diagnostic methods are limited by tools available, and access to the deep tissue areas where biofilm may reside in quiescence avoiding detection [76].
We conclude that a targeted blend of botanical extracts and enzymes directed at biofilm matrix components is efficacious of disrupting established biofilm in vitro. This does not prove efficacy in a clinical situation. Further studies should establish whether NEBB disrupts for example Candida or Staphylococcus biofilm in the gut mucosa, as well as established biofilm in tissues. Work is in progress to evaluate the hyper-inflammatory effects of Borrelia biofilms and evaluate ways to reduce this inflammatory activity of the bacterial colonies (manuscript in preparation).
There is a great and urgent need for further research into complex biofilm communities. This is a well-known territory in geological sciences [80] but is in its infancy in medical science. Established biofilms may be more inflammatory and virulent than free planktonic forms, and further research should include proteomic evaluation of such stressors from complex multi-species biofilms.
Fig. 3. Disruption of established microbial biofilm from Pseudomonas aeruginosa after treatment with a nutraceutical enzyme and botanical blend across a dose range of 0.5 – 30 mg/ml.
Data is shown as the average ± standard deviation of a minimum of 3 repeats of each treatment dose, as the % change from untreated biofilm, where the untreated biofilm had gone through identical procedures for removal of planktonic forms, washing, and addition of fresh medium. Biofilm mass was quantified by crystal violet staining (solid lines), and the metabolic activity of the microbial biofilms was measured by the MTT assay (dashed lines). Slime formation was scored with “3” (300%) change indicating that each culture in the microtiter plate had turned to a mucus plug. All data points were statistically significant when compared to the untreated control biofilm cultures, except where the data point is annotated by NS (not significant).
Acknowledgments
The research was conducted at NIS Labs, an independent contract research organization that specializes in natural products research and testing. The study was sponsored by Researched Nutritionals LLC, the manufacturer of the nutraceutical blend tested in the study.
Footnotes
Authors Contributions
GSJ, DC, and DEH conceived of the questions to be tested. GSJ wrote the research protocol and designed the study and conducted the work presented here. GSJ performed the data analysis. GSJ, DC, and DEH wrote the manuscript. All co-authors participated in the final edit of the manuscript.
Conflict of Interest
The authors have no financial conflicts of interest to declare.
DEH is employed as the Director of Physician Education and Clinical Trials for the study sponsor, Researched Nutritionals, LLC.
References
- 1.Donlan RM. Biofilms: microbial life on surfaces. Emerg. Infect. Dis. 2002;8:881–890. doi: 10.3201/eid0809.020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 3.Rossiter SE, Fletcher MH, Wuest WM. Natural products as platforms to overcome antibiotic resistance. Chem. Rev. 2017;117:12415–12474. doi: 10.1021/acs.chemrev.7b00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arciola CR, Campoccia D, Montanaro L. Implant infections: adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018;7:397–409. doi: 10.1038/s41579-018-0019-y. [DOI] [PubMed] [Google Scholar]
- 5.Jamal M, Ahmad W, Andleeb S, Jalil F, Imran M, Asif NAwaz M, et al. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018;81:7–11. doi: 10.1016/j.jcma.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Høiby N, Bjarnsholt T, Moser C, Bassi GL, Coenye T, Donelli G, et al. ESCMID study group for biofilms and consulting external expert werner zimmerli. ESCMID guideline for the diagnosis and treatment of biofilm infections. Clin. Microbiol. Infect. Suppl. 2014;1:S1–25. doi: 10.1016/j.cmi.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 7.Moser C, Jensen PØ, Thomsen K, Kolpen M, Rybtke M, Lauland AS, et al. Immune responses to Pseudomonas aeruginosa biofilm infections. Front. Immunol. 2021;12:625597. doi: 10.3389/fimmu.2021.625597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Motta JP, Wallace JL, Buret AG, Deraison C, Vergnolle N. Gastrointestinal biofilms in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021;5:314–334. doi: 10.1038/s41575-020-00397-y. [DOI] [PubMed] [Google Scholar]
- 9.Moreau-Marquis S, Stanton BA, O'Toole GA. Pseudomonas aeruginosa biofilm formation in the cystic fibrosis airway. Pulm. Pharmacol. Ther. 2008;21:595–599. doi: 10.1016/j.pupt.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mirzaei R, Mohammadzadeh R, Alikhani MY, Shokri Moghadam M, Karampoor S, Kazemi S, et al. The biofilm-associated bacterial infections unrelated to indwelling devices. IUBMB Life. 2020;72:1271–1285. doi: 10.1002/iub.2266. [DOI] [PubMed] [Google Scholar]
- 11.Tolker-Nielsen T. Biofilm development. Microbiol. Spectr. 2015;3:MB-0001-2014. doi: 10.1128/microbiolspec.MB-0001-2014. [DOI] [PubMed] [Google Scholar]
- 12.Wolfaardt GM, Lawrence JR, Robarts RD, Caldwell SJ, Caldwell DE. Multicellular organization in a degradative biofilm community. Appl. Environ. Microbiol. 1994;60:434–446. doi: 10.1128/aem.60.2.434-446.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ciofu O, Tolker-Nielsen T. Antibiotic Tolerance and Resistance in Biofilms. In: Bjarnsholt T, Jensen P, Moser C, Høiby N, editors. Biofilm Infections. Springer; New York, NY: 2010. [Google Scholar]
- 14.Davies DG, Parsek MR, Pearson JP, Iglewski BH, Costerton JW, Greenberg EP. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science: a focus on anti-biofilm agents and their mechanisms of action. Virulence. 1998;9:522–554. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- 15.Rumbaugh KP, Sauer K. Biofilm dispersion. Nat. Rev. Microbiol. 2020;18:571–586. doi: 10.1038/s41579-020-0385-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaplan JB. Therapeutic potential of biofilm-dispersing enzymes. Int. J. Artif. Organs. 2009;32:545–54. doi: 10.1177/039139880903200903. [DOI] [PubMed] [Google Scholar]
- 17.Roy R, Tiwari M, Donelli G, Tiwari V. Strategies for combating bacterial biofilms: a focus on anti-biofilm agents and their mechanisms of action. Virulence. 2018;9:522–554. doi: 10.1080/21505594.2017.1313372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nobile CJ, Johnson AD. Candida albicans biofilms and human disease. Annu. Rev. Microbiol. 2015;69:71–92. doi: 10.1146/annurev-micro-091014-104330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gulati M, Nobile CJ. Candida albicans biofilms: development, regulation, and molecular mechanisms. Microbes Infect. 2016;18:310–321. doi: 10.1016/j.micinf.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Gara JP. Ica and beyond: biofilm mechanisms and regulation in Staphylococcus epidermidis and Staphylococcus aureus. FEMS Microbiol. Lett. 2007;270:179–188. doi: 10.1111/j.1574-6968.2007.00688.x. [DOI] [PubMed] [Google Scholar]
- 21.Lister JL, Horswill AR. Staphylococcus aureus biofilms: recent developments in biofilm dispersal. Front. Cell Infect. Microbiol. 2014;4:178. doi: 10.3389/fcimb.2014.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferraboschi P, Ciceri S, Grisenti P. Applications of lysozyme, an innate immune defense factor, as an alternative antibiotic. Antibiotics (Basel) 2021;10:1534. doi: 10.3390/antibiotics10121534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao X, Guo M, Zhang Z, Shen P, Yang Z, Zhang N. Baicalin promotes the bacteriostatic activity of lysozyme on S. aureus in mammary glands and neutrophilic granulocytes in mice. Oncotarget. 2017;8:19894–19901. doi: 10.18632/oncotarget.15193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan Y, Ma S, Leonhard M, Moser D, Schneider-Stickler B. β-1,3-glucanase disrupts biofilm formation and increases antifungal susceptibility of Candida albicans DAY185. Int. J. Biol. Macromol. 2018;108:942–946. doi: 10.1016/j.ijbiomac.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Kaur A, Soni SK, Vij S, Rishi P. Cocktail of carbohydrases from Aspergillus niger: an economical and eco-friendly option for biofilm clearance from biopolymer surfaces. AMB Express. 2021;11:22. doi: 10.1186/s13568-021-01183-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yassein AS, Hassan MM, Elamary RB. Prevalence of lipase producer Aspergillus niger in nuts and anti-biofilm efficacy of its crude lipase against some human pathogenic bacteria. Sci. Rep. 2021;11:7981. doi: 10.1038/s41598-021-87079-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elchinger PH, Delattre C, Faure S, Roy O, Badel S, Bernardi T, et al. Effect of proteases against biofilms of Staphylococcus aureus and Staphylococcus epidermidis. Lett. Appl. Microbiol. 2014;59:507–513. doi: 10.1111/lam.12305. [DOI] [PubMed] [Google Scholar]
- 28.Jadhav SB, Shah N, Rathi A, Rathi V, Rathi A. Serratiopeptidase: insights into the therapeutic applications. Biotechnol. Rep. 2020;28:e00544. doi: 10.1016/j.btre.2020.e00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sapi E, Theophilus PAS, Pham TV, Burugu D, Luecke DF. Effect of RpoN, RpoS and LuxS pathways on the biofilm formation and antibiotic sensitivity of Borrelia burgdorferi. Eur. J. Microbiol. Immunol. 2016;6:272–286. doi: 10.1556/1886.2016.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chai Y, Beauregard PB, Vlamakis H, Losick R, Kolter R. Galactose metabolism plays a crucial role in biofilm formation by Bacillus subtilis. mBio. 2012;3:e00184–12. doi: 10.1128/mBio.00184-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buommino E, Scognamiglio M, Donnarumma G, Fiorentino A, D'Abrosca B. Recent advances in natural product-based antibiofilm approaches to control infections. Mini Rev. Med. Chem. 2014;14:1169–1182. doi: 10.2174/1389557515666150101095853. [DOI] [PubMed] [Google Scholar]
- 32.Pierce CG, Chaturvedi AK, Lazzell AL, Powell AT, Saville SP, McHardy SF, et al. A novel small molecule inhibitor of Candida albicans biofilm formation, filamentation and virulence with low potential for the development of resistance. NPJ Biofilms Microbiomes. 2015;1 doi: 10.1038/npjbiofilms.2015.12. pii: 15012. Epub 2015 Aug 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farkash Y, Feldman M, Ginsburg I, Steinberg D, Shalish M. Green tea polyphenols and padma hepaten inhibit Candida albicans biofilm formation. Evid Based Complement. Alternat. Med. 2018;2018:1690747. doi: 10.1155/2018/1690747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goc A, Niedzwiecki A, Rath M. In vitro evaluation of antibacterial activity of phytochemicals and micronutrients against Borrelia burgdorferi and Borrelia garinii. J. Appl. Microbiol. 2015;119:1561–1572. doi: 10.1111/jam.12970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feng J, Zhang S, Shi W, Zubcevik N, Miklossy J, Zhang Y. Selective essential oils from spice or culinary herbs have high activity against stationary phase and biofilm Borrelia burgdorferi. Front. Med. 2017;4:169. doi: 10.3389/fmed.2017.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahdhi A, Leban N, Chakroun I, Bayar S, Mahdouani K, Majdoub H, Kouidhi B. Use of extracellular polysaccharides, secreted by Lactobacillus plantarum and Bacillus spp., as reducing indole production agents to control biofilm formation and efflux pumps inhibitor in Escherichia coli. Microb. Pathog. 2018;125:448–453. doi: 10.1016/j.micpath.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 37.Socarras KM, Theophilus PAS, Torres JP, Gupta K, Sapi E. Antimicrobial activity of bee venom and melittin against Borrelia burgdorferi. Antibiotics. 2017;6:31. doi: 10.3390/antibiotics6040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Howell AB, Botto H, Combescure C, Blanc-Potard AB, Gausa L, Matsumoto T, et al. Dosage effect on uropathogenic Escherichia coli anti-adhesion activity in urine following consumption of cranberry powder standardized for proanthocyanidin content: a multicentric randomized double blind study. BMC Infect. Dis. 2010;10:94. doi: 10.1186/1471-2334-10-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neto CC, Penndorf KA, Feldman M, Meron-Sudai S, Zakay-Rones Z, Steinberg D, et al. Characterization of non-dialyzable constituents from cranberry juice that inhibit adhesion, co-aggregation and biofilm formation by oral bacteria. Food Funct. 2017;8:1955–1965. doi: 10.1039/C7FO00109F. [DOI] [PubMed] [Google Scholar]
- 40.Maisuria VB, Yossef Lopwz-de Los Santos, Nathalie Tufenkji, Eric Deziel Cranberry-derived proanthocyanidins impair virulence and inhibit quorum sensing of Pseudomonas aeruginosa. Sci. Rep. 2016;6:30169. doi: 10.1038/srep30169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xie Y, Liu X, Zhou P. In vitro antifungal effects of berberine against Candida spp. in planktonic and biofilm conditions. Drug Des. Devel. Ther. 2020;14:87–101. doi: 10.2147/DDDT.S230857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosato A, Sblano S, Salvagno L, Carocci A, Clodoveo ML, Corbo F, et al. Anti-biofilm inhibitory synergistic effects of combinations of essential oils and antibiotics. Antibiotics. 2020;9:637. doi: 10.3390/antibiotics9100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sandasi M, Leonard CM, Viljoen AM. The in vitro antibiofilm activity of selected culinary herbs and medicinal plants against Listeria monocytogenes. Lett. Appl. Microbiol. 2010;50:30–35. doi: 10.1111/j.1472-765X.2009.02747.x. [DOI] [PubMed] [Google Scholar]
- 44.Quah SY, Wu S, Lui JN, Sum CP, Tan KS. N-acetylcysteine inhibits growth and eradicates biofilm of Enterococcus faecalis. J. Endod. 2012;38:81–85. doi: 10.1016/j.joen.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 45.Samaranayake YH, Cheung BP, Parahitiyawa N, Seneviratne CJ, Yau JY, Yeung KW, et al. Synergistic activity of lysozyme and antifungal agents against Candida albicans biofilms on denture acrylic surfaces. Arch. Oral Biol. 2009;54:115–126. doi: 10.1016/j.archoralbio.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 46.Matthes R, Jablonowski L, Holtfreter B, Pink C, Kocher T. Enzymatic biofilm destabilisation to support mechanical cleansing of inserted dental implant surfaces: an in-vitro pilot study. Odontology. 2021;109:780–791. doi: 10.1007/s10266-021-00599-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Longhi C, Scoarughi GL, Poggiali F, Cellini A, Carpentieri A, Seganti L, et al. Protease treatment affects both invasion ability and biofilm formation in Listeria monocytogenes. Microb. Pathog. 2008;45:45–52. doi: 10.1016/j.micpath.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 48.Katsipis G, Pantazaki AA. Serrapeptase impairs biofilm, wall, and phospho-homeostasis of resistant and susceptible Staphylococcus aureus. Appl. Microbiol. Biotechnol. 2023;107:1373–1389. doi: 10.1007/s00253-022-12356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tan Y, Leonhard M, Ma S, Moser D, Schneider-Stickler B. Dispersal of single and mixed non-albicans Candida species biofilms by β-1,3-glucanase in vitro. Microb. Pathog. 2017;113:342–347. doi: 10.1016/j.micpath.2017.10.057. [DOI] [PubMed] [Google Scholar]
- 50.Tan Y, Ma S, Ding T, Ludwig R, Lee J, Xu J. Enhancing the antibiofilm activity of β-1,3-glucanase-functionalized nanoparticles loaded with amphotericin B against Candida albicans biofilm. Front. Microbiol. 2022;13:815091. doi: 10.3389/fmicb.2022.815091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Palanichamy E, Repally A, Jha N, Venkatesan A. Haloalkaline lipase from Bacillus flexus PU2 efficiently inhibits biofilm formation of aquatic pathogen Vibrio parahaemolyticus. Probiotics Antimicrob. Proteins. 2022;14:664–674. doi: 10.1007/s12602-022-09908-6. [DOI] [PubMed] [Google Scholar]
- 52.Kamali E, Jamali A, Izanloo A, Ardebili A. In vitro activities of cellulase and ceftazidime, alone and in combination against Pseudomonas aeruginosa biofilms. BMC Microbiol. 2021;21:347. doi: 10.1186/s12866-021-02411-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deng Y, Wang SY. Sorption of cellulases in biofilm enhances cellulose degradation by Bacillus subtilis. Microorganisms. 2022;10:1505. doi: 10.3390/microorganisms10081505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang X, Yao X, Zhu Z, Tang T, Dai K, Sadovskaya I, et al. Effect of berberine on Staphylococcus epidermidis biofilm formation. Int.J. Antimicrob. Agents. 2009;34:60–66. doi: 10.1016/j.ijantimicag.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 55.Huang X, Zheng M, Yi Y, Patel A, Song Z, Li Y. Inhibition of berberine hydrochloride on Candida albicans biofilm formation. Biotechnol. Lett. 2020;42:2263–2269. doi: 10.1007/s10529-020-02938-6. [DOI] [PubMed] [Google Scholar]
- 56.da Silva AR, de Andrade Neto JB, da Silva CR, Campos Rde S, Costa Silva RA, Freitas DD, et al. Berberine antifungal activity in fluconazole-resistant pathogenic yeasts: action mechanism evaluated by flow cytometry and biofilm growth inhibition in Candida spp. Antimicrob Agents Chemother. 2016;60:3551–3557. doi: 10.1128/AAC.01846-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Oliveira JR, de Jesus D, de Oliveira LD. Rosmarinus officinalis L. (rosemary) extract decreases the biofilms viability of oral health interest. Braz. Dent. Sci. 2017;20:64–69. doi: 10.14295/bds.2017.v20i1.1317. [DOI] [Google Scholar]
- 58.Meccatti VM, Oliveira JR, Figueira LW, Lagareiro Netto AA, Zamarioli LS, Marcucci MC, et al. Rosmarinus officinalis L. (rosemary) extract has antibiofilm effect similar to the antifungal nystatin on Candida samples. An. Acad. Bras. Cienc. 2021;93:e20190366. doi: 10.1590/0001-3765202120190366. [DOI] [PubMed] [Google Scholar]
- 59.Agarwal V, Lal P, Pruthi V. Prevention of Candida albicans biofilm by plant oils. Mycopathol. 2008;165:13–19. doi: 10.1007/s11046-007-9077-9. [DOI] [PubMed] [Google Scholar]
- 60.Husain FM, Ahmad I, Khan MS, Ahmad E, Tahseen Q, Khan MS, et al. Sub-MICs of Mentha piperita essential oil and menthol inhibits AHL mediated quorum sensing and biofilm of Gram-negative bacteria. Front. Microbiol. 2015;6:420. doi: 10.3389/fmicb.2015.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pérez-Giraldo C, Rodríguez-Benito A, Morán FJ, Hurtado C, Blanco MT, Gómez-García AC. Influence of N-acetylcysteine on the formation of biofilm by Staphylococcus epidermidis. J. Antimicrob. Chemother. 1997;39:643–646. doi: 10.1093/jac/39.5.643. [DOI] [PubMed] [Google Scholar]
- 62.Kim YG, Lee JH, Park S, Lee J. The anticancer agent 3,3'-diindolylmethane inhibits multispecies biofilm formation by acnecausing bacteria and Candida albicans. Microbiol. Spectr. 2022;10:e0205621. doi: 10.1128/spectrum.02056-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grossman AB, Burgin DJ, Rice KC. Quantification of Staphylococcus aureus biofilm formation by crystal violet and confocal microscopy. Methods Mol. Biol. 2021;2341:69–78. doi: 10.1007/978-1-0716-1550-8_9. [DOI] [PubMed] [Google Scholar]
- 64.Kumar P, Nagarajan A, Uchil PD. Analysis of cell viability by the MTT assay. Cold Spring Harb. Protoc. 20182018 doi: 10.1101/pdb.prot095505. doi. 10.1101/pdb.prot095505. [DOI] [PubMed] [Google Scholar]
- 65.Li YY, Li BS, Liu WW, Cai Q, Wang HY, Liu YQ, et al. Effects of D-arginine on Porphyromonas gingivalis biofilm. J. Oral Sci. 2020;62:57–61. doi: 10.2334/josnusd.19-0075. [DOI] [PubMed] [Google Scholar]
- 66.Lee JH, Kim YG, Khadke SK, Yamano A, Watanabe A, Lee J. Inhibition of biofilm formation by Candida albicans and polymicrobial microorganisms by Nepodin via hyphal-growth suppression. ACS Infect. Dis. 2019;5:1177–1187. doi: 10.1021/acsinfecdis.9b00033. [DOI] [PubMed] [Google Scholar]
- 67.Sapi E, Bastian SL, Mpoy CM, Scott S, Rattelle A, Pabbati N, et al. Characterization of biofilm formation by Borrelia burgdorferi in vitro. PLoS One. 2012;7:e48277. doi: 10.1371/journal.pone.0048277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lewis K. Persister cells. Ann. Rev. Microbiol. 2010;64:357–372. doi: 10.1146/annurev.micro.112408.134306. [DOI] [PubMed] [Google Scholar]
- 69.Suresh MK, Biswas R, Biswas L. An update on recent developments in the prevention and treatment of Staphylococcus aureus biofilms. Int. J. Med. Microbiol. 2019;309:1–12. doi: 10.1016/j.ijmm.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 70.Dongari-Bagtzoglou A, Kashleva H, Dwivedi P, Diaz P, Vasilakos J. Characterization of mucosal Candida albicans biofilms. PLoS One. 2009;4:e7967. doi: 10.1371/journal.pone.0007967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thi MTT, Wibowo D, Rehm BHA. Pseudomonas eruginosa biofilms. Int. J. Mol. Sci. 2020;21:8671. doi: 10.3390/ijms21228671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roilides E, Simitsopoulou M, Katragkou A, Walsh TJ. How biofilms evade host defenses. Microbiol. Spectr. 2015;3 doi: 10.1128/microbiolspec.MB-0012-2014. doi: 10.1128/microbiolspec.MB-0012-2014. [DOI] [PubMed] [Google Scholar]
- 73.Feng J, Li T, Yee R, Yuan Y, Bai C, Cai M, et al. Stationary phase persister/biofilm microcolony of Borrelia burgdorferi causes more severe disease in a mouse model of Lyme arthritis: implications for understanding persistence, Post-treatment Lyme Disease Syndrome (PTLDS), and treatment failure. Discov. Med. 2019;27:125–138. [PubMed] [Google Scholar]
- 74.Lochhead RB, Strle K, Arvikar SL, Weis JJ, Steere AC. Lyme arthritis: linking infection, inflammation and autoimmunity. Nat. Rev. Rheumatol. 2021;17:449–461. doi: 10.1038/s41584-021-00648-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Berndtson K. Review of evidence for immune evasion and persistent infection in lyme disease. Int. J. Gen. Med. 2013;6:291–306. doi: 10.2147/IJGM.S44114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jensen GS, Benson KF.F. The blood as a diagnostic tool in chronic illness with obscure microbial involvement: a critical review. Int. J. Complement. Alt. Med. 2019;12:203–212. doi: 10.15406/ijcam.2019.12.00474. [DOI] [Google Scholar]
- 77.Benson KF, Jensen GS. Bacteria in blood from fibromyalgia patients include the Aquabacterium genus, producing metabolites with inflammatory properties in vitro. Results from a pilot study. Int. J. Complement. Alt. Med. 2019;12:232–239. doi: 10.15406/ijcam.2019.12.00479. [DOI] [Google Scholar]
- 78.Kim YG, Lee JH, Park S, Kim S, Lee J. Inhibition of polymicrobial biofilm formation by saw palmetto oil, lauric acid and myristic acid. Microb. Biotechnol. 2022;15:590–602. doi: 10.1111/1751-7915.13864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim YG, Lee JH, Park JG, Lee J. Inhibition of Candida albicans and Staphylococcus aureus biofilms by centipede oil and linoleic acid. Biofouling. 2020;36:126–137. doi: 10.1080/08927014.2020.1730333. [DOI] [PubMed] [Google Scholar]
- 80.Peter H, Ylla I, Gudasz C, Romaní AM, Sabater S, Tranvik LJ. Multifunctionality and diversity in bacterial biofilms. PLoS One. 2011;6:e23225. doi: 10.1371/journal.pone.0023225. [DOI] [PMC free article] [PubMed] [Google Scholar]