Abstract
Opioids are effective analgesics but can cause harm. Opioid stewardship is key to ensuring that opioids are used effectively and safely. There is no agreed set of quality indicators relating to the use of opioids perioperatively. This work is part of the Yorkshire Cancer Research Bowel Cancer Quality Improvement programme and aims to develop useful quality indicators for the improvement of care and patient outcomes at all stages of the perioperative journey.
A rapid review was performed to identify original research and reviews in which quality indicators for perioperative opioid use are described. A data tool was developed to enable reliable and reproducible extraction of opioid quality indicators.
A review of 628 abstracts and 118 full-text publications was undertaken. Opioid quality indicators were identified from 47 full-text publications. In total, 128 structure, process and outcome quality indicators were extracted. Duplicates were merged, with the final extraction of 24 discrete indicators. These indicators are based on five topics: patient education, clinician education, pre-operative optimization, procedure, and patient-specific prescribing and de-prescribing and opioid-related adverse drug events.
The quality indicators are presented as a toolkit to contribute to practical opioid stewardship. Process indicators were most commonly identified and contribute most to quality improvement. Fewer quality indicators relating to intraoperative and immediate recovery stages of the patient journey were identified. An expert clinician panel will be convened to agree which of the quality indicators identified will be most valuable in our region for the management of patients undergoing surgery for bowel cancer.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13741-023-00312-4.
Keywords: Analgesics, Opioids, Opioid stewardship, Quality indicators, Colorectal cancer surgery
Background
Inappropriate opioid prescribing is an internationally recognized threat to population health and a pressing challenge for healthcare services (Kiang et al. 2020; Curtis et al. 2019; Degenhardt et al. 2019). The North American ‘opioid crisis’ continues, with an ongoing rise in opioid-related mortality, initially due to prescription opioids and more recently illicit heroin and fentanyl use (Berterame et al. 2016). Despite increased awareness of risks and opioid abuse, prescription opioid use remains historically high in both North America and Europe (Jani et al. 2020; Schieber et al. 2019; Verhamme and Bohnen 2019; Lancet 2022). Inappropriate prescribing following surgery is increasingly recognized as a contributor to the problem. Opioids are effective analgesics for managing acute pain following surgical trauma (Small and Laycock 2020) and were increasingly used in longer and higher doses following the publication of guidelines on post-operative pain management (Ballantyne et al. 2016). However, opioids also have significant adverse effects, including sedation, constipation, nausea, and confusion. Long-term use can lead to tolerance, dependence, hyperalgesia, addiction and increased mortality (Colvin et al. 2019). There is increasing attention being paid to the role of postoperative opioids in slowing recovery from surgery and contributing to long-term opioid use (Glare et al. 2019; Levy et al. 2021; Daliya et al. 2021). Enhanced recovery after surgery (ERAS) programmes often include the provision of multi-modal analgesia to promote faster recovery with fewer complications. Whilst short-term opioid use provides effective relief from acute pain following surgery, it is increasingly recognized that the perioperative period is a time when longer-term opioid usage may begin. Effective opioid stewardship in the perioperative period is therefore of critical importance.
Improving opioid stewardship in the peri- and post-operative management of patients with bowel cancer has the potential to improve recovery, lead to faster discharge, improve outcomes and most importantly, prevent patient harm. A requirement for an effective opioid stewardship program is the ability to measure the appropriateness of opioid use.
Health care quality indicators are a type of performance measure (Stelfox and Straus 2013) that evaluate aspects of quality of care, without which the monitoring of healthcare quality is impossible (Mainz 2003; Arah et al. 2006). Quality indicators are used to measure the variability in the quality of care, identify potential areas for improvement and can be used to feedback on performance to healthcare teams to change clinical practice. They should be relevant, actionable, reliable, show room for improvement and data collection should be feasible (Ivers et al. 2012; Kelley and Hurst 2006; Fabian and Geppert 2011). Donabedian’s framework (Donabedian 1988) describes quality as a function of three domains: structure, process and outcome. The structure is defined by the attributes of the setting in which care is provided, process by the input of the practitioners working in that system and outcome by the change in health status of the patient.
No quality indicators for perioperative opioid use are currently described in the National Institute for Health and Care Excellence Standards and Indicators library (Standards and Indicators | NICE (accessed 22nd March 2022). A rapid review was performed to identify quality indicators for perioperative opioid stewardship for patients undergoing abdominal surgery for bowel cancer. This is a form of knowledge synthesis that streamlines the process of conducting a traditional systematic review to produce evidence in a rapid resource-efficient manner (Hamel et al. 2021) and has been chosen to allow timely evidence synthesis to inform decision-making (Haby et al. 2016).
The objective of this rapid review was to identify and extract potential quality indicators from the best available evidence on perioperative opioid use in patients undergoing major abdominal surgery for bowel cancer. This approach to the development of actionable quality indicators has been described and applied effectively in other clinical settings (Kallen et al. 2018).
Methods
Cochrane rapid review methods were followed (Garrity et al. 2021). A systematic literature search of Medline was performed and included all articles available to April 2021. Systematic reviews and primary studies were sought. The types of participants were not restricted and could be individuals, organizations or systems. Search terms are shown in Table 1 and include terms and truncations for quality indicators, opioids, surgery (with potential limitation to colorectal cancer surgery) and development. The search was limited to studies of adult subjects and studies published in English. A manual search was conducted of the reference lists of the selected papers. Searches were conducted between the 1st and the 25th August 2021 and supplementary searches of reference lists were conducted in December 2021. The National Quality Measures Clearinghouse project (2022) was also reviewed for relevant content.
Table 1.
MEDLINE search strategy
| Quality indicator | AND | Opioids | AND | Abdominal surgery/bowel cancer surgery |
|---|---|---|---|---|
|
1. Quality indicator [Mesh] OR 2. Quality criterion OR 3. Quality measure* OR 4. Performance indicator OR 5. Performance measure OR 6. Outcome measure OR 7. Outcome indicator OR 8. Audit OR 9. Outcome assessment [Mesh] OR 10. Process assessment [Mesh] |
1. Analgesics, Opioid [Mesh] OR 2. Opioid* OR 3. Stewardship [tw] OR 4. Appropriate opioid use [tw] OR 5. Opioid use |
1. Colonic neoplasms [Mesh] OR 2. Colorectal neoplasms [Mesh] OR 3. Intestinal neoplasms [Mesh] OR 4. Bowel cancer OR 5. Laparoscopy [Mesh] OR 6. Digestive system surgical procedures [Mesh] OR 7. Colectomy [Mesh] OR 7. Bowel cancer surgery OR 9. Abdominal surgery |
*Truncation symbol = different words/terms can be searched for (singular/plural/conjugations)
Limited to English language and adults
The initial search identified 588 abstracts. These results were imported into Rayyan (http://rayyan.qcri.org/) (Ouzzani et al. 2016) a free web tool used to facilitate the screening and selection of studies for systematic and scoping reviews.
Three members of the project team (MA, KP, DY) screened all titles and abstracts for inclusion. Duplicate studies, case reports, editorials, non-English language studies, quality improvement not concerning opioid use, abdominal or colorectal surgery, or performance measures and quality improvement in specific subgroups of patients were excluded. Where a decision on inclusion could not be reached, two further team members (CT, SH) reviewed the titles and abstracts. All studies included at this stage underwent full-text review, undertaken by two members of the project team (CT, SH). MA, KP, and DY accessed the full-text articles of all included papers, which were uploaded and accessed using Rayyan. The extraction of quality indicators from the full texts was undertaken by CT and SH. All those included were papers from which quality indicators could be extracted. A quality indicator extraction tool was developed in advance of data extraction, with potential indicators categorized to the stage of perioperative care they relate to (Supplementary materials 1) to enable reproducible results. Finally, a full list of potential indicators was composed, in which indicators were rephrased where needed and duplicate indicators removed. Reporting has been guided by the PRISMA extension for scoping reviews (PRISMA-ScR) (Extension and for Scoping Reviews (PRISMA-ScR): Checklist and Explanation | The EQUATOR Network (equator-network.org) (accessed 22nd March 2022).
Results
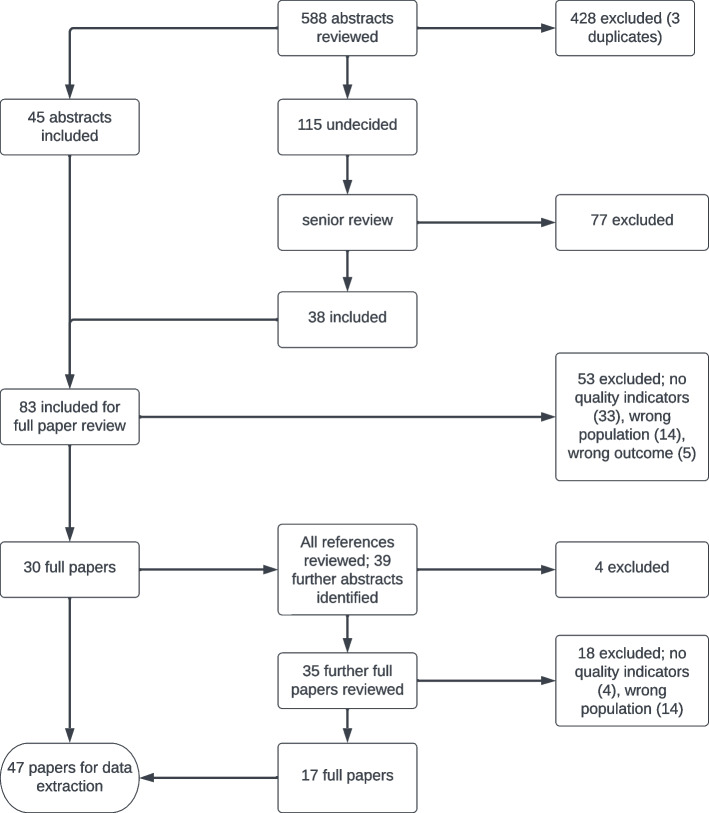
Five hundred eighty-eight publications were identified by the literature search. Three duplicates and a further 425 abstracts were removed as they did not meet inclusion criteria. Eighty-three of these papers were included for full-text review, of which 53 were excluded because they reported on another outcome or population, or because the paper did not include quality indicators. Thirty papers were included, the references of which were reviewed. A further 35 full-text papers were reviewed, of which 17 were included. The study selection flowchart (Fig. 1) details this process. In total, 47 papers were identified from which quality indicators were extracted. Review of the quality indicators clearinghouse did not yield results.
Fig. 1.
Study selection flowchart
The characteristics (study design and numbers of participants) of the included papers are shown in Supplementary Materials 2.
One hundred twenty-eight quality indicators from 47 papers were extracted, with some papers describing several indicators. See Supplementary materials 1 for full details of all raw extracted quality indicators. Duplicates were removed, leading to the identification of 24 discrete indicators. Table 2 shows the numbers of discrete quality indicators identified at each stage of the patient journey.
Table 2.
Numbers of discrete quality indicators identified at each stage of the patient journey
| Stage of patient journey | Number of quality indicators | Number of papers | Number of distinct quality indicators |
|---|---|---|---|
| Pre-operative | 26 | 19 | 7 |
| Intra-operative | 13 | 12 | 1 |
| Recovery | 5 | 13 | 2 |
| Post-operative | 19 | 17 | 4 |
| Discharge | 43 | 17 | 6 |
| Follow up | 22 | 13 | 4 |
Instruments for collecting data on quality indicators, and structural, process, and outcome indicators were collated. These are grouped according to stage in the perioperative journey and are shown in Table 3.
Table 3.
Structural, process, and outcome quality indicators across the patient perioperative journey
| First author, year of publication | Brief topic of quality indicator | Instruments for collecting data on quality indicators | Structural/process quality indicators | Outcome quality indicators | |
|---|---|---|---|---|---|
| Preoperative |
(Bardiau et al. 1999) |
Patient education | Presence of preoperative patient education materials on perioperative pain and pain management, risks of opioids | ||
|
(Bongiovanni et al. 2020) (Hopkins et al. 2020) |
Staff education | Presence of multi-professional education materials for staff on opioid stewardship and need for multimodal analgesia | |||
|
(Brat et al. 2018) (Cron et al. 2017) (Fields et al. 2019) (Gan et al. 2020) (Hilliard et al. 2018) (Truong et al. 2019) |
Preoperative identification and optimization for patients with opioid tolerance | Opioid tolerant definition: if opioids used for more than 7 days in the 60 days prior to surgery/any opioid use in 12/12 prior to surgery, any opioid medication on admission meds list |
System to identify preoperative opioid use in elective population Specialist pain referral pathway to enable opioid weaning and perioperative analgesic planning for preoperative optimization in opioid tolerant population |
||
|
(Brummett et al. 2017) (Clarke et al. 2014) (Fields et al. 2019) (Jiang et al. 2017) (Lee et al. 2017) (Macintyre et al. 2014) (Stafford et al. 2018) |
Identification of patients at greatest risk of persistent postoperative opioid use (PPOU) | Opioid Risk Tool (ORT), Screener for Opioid Assessment and Patients with Pain (SOAPP) and Brief Risk Interview (BRI) may be of use in acute pain setting |
Presence of screening tool to identify risk factors for persistent postoperative opioid use (PPOU) defined as use of opioids at 90–180 days postoperatively Identified prevalence of PPOU risk |
||
| (Minkowitz et al. 2014) | Identification of patients at risk of opioid-related adverse drug events (ORADEs) | Presence of screening tool to identify preoperatively those at greater risk of postoperative opioid-related adverse drug events (ORADEs) | |||
| (Felling et al. 2018) (Yap et al. 2020) | Use of multimodal analgesia | Presence of protocol to reduce perioperative opioid use with preoperative multimodal analgesia | |||
| (Lee et al. 2017) (Macintyre et al. 2014) | Concept of ‘universal precautions’ in the use of perioperative opioids | Adoption of ‘universal precautions’ when initiating perioperative opioids | |||
| Intraoperative |
(Bardiau et al. 1999) (Brandal et al. 2017) (Cheung et al. 2009) (Felling et al. 2018) (Keller et al. 2019) (Mujukian et al. 2020), (Neuman et al. 2019) (Stafford et al. 2018) (Thiele et al. 2015) (Truong et al. 2019) (Wick et al. 2017) (Yap et al. 2020) |
Use of multimodal analgesia |
Presence of opioid-sparing protocol for intra operative use including minimally invasive surgery, regional blocks and multimodal analgesia Adherence to intra-operative opioid sparing protocol |
||
| Recovery | (Fields et al. 2019) | Identification of patients at greatest risk of PPOU | Presence of review/re-screen with new risk factors for PPOU including formation of a stoma | ||
|
(Bardiau et al. 1999) (Brandal et al. 2017) (Cheung et al. 2009) (Felling et al. 2018) (Keller et al. 2019) (Mujukian et al. 2020) (Neuman et al. 2019) (Stafford et al. 2018) (Thiele et al. 2015) (Truong et al. 2019) (Wick et al. 2017) (Yap et al. 2020) |
Use of multimodal analgesia |
Presence of opioid-sparing protocol for recovery/immediate postoperative use including regional blocks and multimodal analgesia Adherence to recovery/immediate postoperative opioid sparing protocol |
|||
| Postoperative | (Bardiau et al. 1999) (Brandal et al. 2017) | Access to acute pain service |
Availability of an acute pain service Delivery of a daily pain review |
||
| (Bardiau et al. 1999) (Brandal et al. 2017) (Cheung et al. 2009) (Felling et al. 2018) (Gan et al. 2015) (Keller et al. 2019) (Mujukian et al. 2020) (Neuman et al. 2019) (Stafford et al. 2018) (Thiele et al. 2015) (Truong et al. 2019) (Wick et al. 2017) (Yap et al. 2020) | Use of multimodal analgesia |
Presence of opioid-sparing protocol for postoperative use including regional blocks and multimodal analgesia Adherence to postoperative opioid sparing protocol |
Rate of postoperative ileus | ||
|
(Keller et al. 2019) (Kessler et al. 2013) (Lee et al. 2010) (Oderda et al. 2013) (Tsui et al. 1996) |
Presence of ORADEs |
Scoring of frequency, severity, and distress of opioid-related side effects as 0 to 60 on the Perioperative Opioid-related Symptom Distress scale |
Presence of review for ORADEs | Rate of ORADEs, severity of ORADEs detected, impact of ORADEs on length of stay | |
|
(Greco et al. 2014) |
Protocolized opioid prescribing in hospital |
Procedure-specific protocol for use of in-hospital opioids, promoting avoidance of long acting opioids Electronic clinical quality measure (eCQM) to assess potentially inappropriate high dose postoperative opioid prescribing practices, e.g., an average daily dose ≥ 90 MME for the duration of postoperative opioid prescription in preoperatively opioid naïve patients |
|||
| Discharge | (Brandal et al. 2017) (Bromberg et al. 2021) (Chen et al. 2018) (Fields et al. 2019) (Fujii et al. 2018) (Hill et al. 2017) (Hill et al. 2018) (Hopkins et al. 2020) (Lee et al. 2017) (Macintyre et al. 2014) (Neuman et al. 2019) (Pruitt et al. 2020) (Thiels et al. 2017) (Wang et al. 2021) (Wick et al. 2017) | Protocolized opioid prescribing on discharge | Procedure-specific MME centiles to reduce inter-prescriber variation |
Presence of a patient group specific guideline or algorithm for discharge opioid prescribing, opioid use in 24 h prior to discharge to guide opioids prescribed on discharge aiming at prescribing the lowest dose opioid possible for the shortest duration Procedure specific post op prescribing guidelines to provide enough doses to cover 75% of patients Procedure specific prescribing limits built into electronic patient record Procedure-specific mean discharge MME prescribed |
Total milligram of morphine equivalents (MME) consumed during 24 h prior to discharge Opioid present on hospital discharge prescription Frequency of slow-release opioids prescribed on discharge Frequency of immediate-release opioids prescribed on discharge Non-opioid adjuvant analgesia present on discharge prescription |
|
(Brandal et al. 2017) (Wang et al. 2021) |
Review of inpatient opioid use | Presence of recording tool for opioids used during inpatient stay |
Total milligram of morphine equivalents (MME) consumed during hospital stay Procedure specific mean daily inpatient MME used |
||
| (Fields et al. 2019) (Hoang et al. 2020) | Identification of patients at greatest risk of PPOU | Use > 90th centile MME opioids, or equivalent of over 50 5 mg oxycodone prescribed at discharge as risk factor/flag for PPOU | |||
| (Hopkins et al. 2020) (Macintyre et al. 2014) | Opioid de-escalation and tapering |
Presence of a de-escalation plan for opioids prescribed on discharge Use of ‘reverse pain ladder’ to guide de-escalation Pain management plan and tapering strategies clearly communicated to primary care team in a timely manner |
|||
| (Bartels et al. 2016) (Fujii et al. 2018) (Lee et al. 2017) (Hill et al. 2017) (Macintyre et al. 2014) (Neuman et al. 2019) | Patient education |
Provision of patient education on safe storage and disposal of unused opioids and avoidance of opioid diversion Opioid-specific discharge advice, e.g., do not drive for up to 4 weeks until opioid dose is stable |
|||
| (Macintyre et al. 2014) | Identification of patients at risk of ORADEs | Identify those at risk of ORADEs when prescribing opioids for use at home. Male, obese, over 65, greater comorbidities, pre-op opioid use, concurrent sedative medication use. | |||
| Follow up | (Agarwal et al. 2021) (Bartels et al. 2016) (Bromberg et al. 2021) (Howard et al. 2019) (Meyer et al. 2021) (Pruitt et al. 2020) (Roughead et al. 2019) | Review of opioids prescribed v used | MME prescribed and consumed | Presence of process to assess opioids prescribed v opioids used following surgical procedures to allow tailoring of opioid prescriptions to need for a patient group/specific procedure reduce unused opioid in the community | Post op prescription considered to have been given if opioids dispensed between 2–7 days following discharge |
| (Brat et al. 2018) (Clarke et al. 2014) (Fields et al. 2019) (Hill et al. 2018) (Pullman et al. 2021) (Roughead et al. 2019) | Identification of patients at greatest risk of or with PPOU | In primary care, detection of opioid misuse/PPOU after discharge, defined as at least one of the ICD-9 diagnosis code of opioid dependence, abuse, or overdose | Hospital analgesic policies include strategies to support post-discharge assessment and follow-up of patients at risk of becoming chronic opioid users |
New or repeat opioid prescriptions within 30 days of discharge Use of higher dosage of opioids at any time (> 50–60 MME) PPOU: ongoing opioid use at 90–180 days post-discharge Incidence of opioid-related re-admissions Time to opioid cessation: a period without an opioid prescription equivalent to three times the estimated supply duration in preoperatively opioid naïve patients |
|
| (Pruitt et al. 2020) | Staff education | Staff education: prescribers sent quarterly reports on their prescribing v guidelines | |||
| (Macintyre et al. 2014) | Management of those with PPOU | Presence of plan/protocol if opioid abuse or misuse is detected |
The quality indicators identified which could be grouped into five topics: patient education, staff education, preoperative patient optimization, patient and procedure-specific prescribing and deprescribing and opioid-related adverse drug events (ORADEs) and are shown in Table 4. Full details of the quality indicator topics are shown in Supplementary materials 3.
Table 4.
Full list of proposed quality indicators
| Theme | Proposed quality indicators |
|---|---|
| Patient education | The site provides and delivers patient education materials in the preoperative period which cover expectations of perioperative pain and pain management options including the risks and benefits of opioids |
| The site provides and delivers patient education materials at discharge which cover the provision of patient education on safe storage and disposal of unused opioids in the community, the requirement to avoid opioid diversion, and opioid specific discharge advice, e.g., DVLA requirements | |
| Staff education | The site provides and delivers multi-professional education materials on opioid stewardship |
| The site provides and delivers multi-professional education materials on the provision of multimodal analgesia at all stages of the patient journey starting in the preoperative setting | |
| Percentage of prescribers who receive regular reports comparing their prescribing to hospital guidelines | |
| The site provides and delivers educational materials on the need for a clear discharge pain management plan and tapering strategy | |
| Preoperative patient optimization | The presence of a system to identify opioid tolerance preoperatively, defined as opioids used for 7 days or fewer in the 60 days prior to surgery. |
| The provision of a specialist pain service and referral pathway to enable opioid weaning and patient-specific analgesic planning for preoperative optimization for patients with opioid tolerance | |
| The site uses a preoperative screening tool to identify patients with risk factors for persistent postoperative opioid use (PPOU) | |
| Patient and procedure-specific prescribing and deprescribing | The site has an acute pain service with the ability to provide a daily pain review |
| The electronic record is used as a means to detect or highlight potentially inappropriate high-dose postoperative opioid prescriptions | |
| Review takes place to evaluate the procedure-specific mean daily inpatient MME used | |
| Use of higher dosage of opioids (> 50–60 MME per day) at any time during the perioperative journey is used as a flag for further review | |
| The site has a perioperative analgesia protocol which includes regional blocks and multimodal analgesia | |
| The presence of procedure-specific protocols for use of in-patient opioids specifically promoting the avoidance of long-acting opioids | |
| The presence of a review postoperatively seeking new risk factors for PPOU identified including, e.g., formation of a stoma | |
| The percentage of those who are still using opioids at 90–180 days postoperatively (where the denominator is patients undergoing major surgery for bowel cancer) | |
| The use of protocolized opioid prescribing for hospital discharge: | |
| The site has a system to guide prescribing | |
| The site has a system to allow the review of the procedure-specific mean discharge opioids prescribed for a particular patient group | |
| The site has a patient group-specific guideline or algorithm to guide discharge opioid prescribing | |
| The electronic record is used to enable procedure-specific prescribing limits | |
| Procedure-specific postoperative prescribing guidelines are used to provide enough doses at discharge to cover 75% of patients (where the denominator is all patients undergoing that procedure) | |
|
The site has a system in place to allow the discharge pain management plan and tapering strategy to be clearly communicated to primary care team in a timely manner The opioid requirement, e.g., total consumed during the 24 h prior to discharge is used as a guide for opioids prescribed on discharge | |
| The presence of a review process for opioid prescription at discharge, where the denominator is all patients discharged having had a major surgery for bowel cancer: | |
| The frequency of any opioids prescribed on hospital discharge | |
| The frequency of slow-release opioid prescription on discharge | |
| The frequency of immediate-release opioid prescription on discharge | |
| The frequency of non-opioid adjuvant analgesia prescription on discharge | |
| The presence of a protocol to guide de-escalation plan for opioids prescribed on discharge | |
| Protocolized use of the ‘reverse pain ladder’ to guide de-escalation | |
| Pain management plan and tapering strategy clearly communicated to the primary care team in a timely manner | |
| The presence of a process to assess opioids prescribed versus opioids actually used following surgical procedures to allow tailoring of opioid prescriptions to need for a patient group/specific procedure | |
| The presence of patient screening for risk of PPOU at discharge | |
| Follow up for patients at greatest risk of persistent postoperative opioid use | |
| The presence of a system to detect new or repeat opioid prescriptions given within 30 days of discharge | |
| The presence of a protocol or clear plan to follow if opioid abuse or misuse is detected | |
| Opioid-related adverse drug events (ORADEs) | The site uses a preoperative screening tool to identify patients at greatest risk of postoperative opioid-related adverse drug events (ORADEs). Documented risk factors are those who are male, obese, over 65, with comorbidities, a history of preoperative opioid use and those concurrently using sedative medication. |
| The site has a system in place to detect ORADEs among postoperative inpatients | |
| There is a system in place to detect ORADEs in the community setting following discharge |
Discussion
Opioids are highly effective analgesics but can cause harm and there is now increasing concern about their perioperative use. A number of contributing problems have been identified. Opioid tolerance preoperatively is a risk factor for poorer outcome (Cron et al. 2017; Gan et al. 2020; Gan et al. 2015; Kessler et al. 2013). When opioids are used by either opioid-naïve or opioid tolerant patients, they are put at risk of opioid-related adverse drug events (ORADEs) (Macintyre et al. 2014; Minkowitz et al. 2014; Keller et al. 2019; Kessler et al. 2013; Oderda et al. 2013), and opioid use is associated with postoperative complications and increased length of stay (Cron et al. 2017; Gan et al. 2020; Gan et al. 2015). Additionally, the perioperative period has been identified as a period of risk for the development of chronic opioid use (Lee et al. 2017; Brummett et al. 2017; Clarke et al. 2014; Macintyre et al. 2014; Roughead et al. 2019; Srivastava et al. 2021). At discharge, opioid prescriptions in excess of requirements are widely reported (Neuman et al. 2019; Bromberg et al. 2021; Hill et al. 2017; Hill et al. 2018; Pruitt et al. 2020; Bartels et al. 2016; Agarwal et al. 2021; Howard et al. 2019; Meyer et al. 2021) and opioids initially used for short-term pain relief can become part of repeat prescriptions following hospital discharge. Poor practice around safe storage and disposal of opioids following discharge contributes to increased opioid in the community with the potential for opioid diversion (Fujii et al. 2018; Hill et al. 2017; Bartels et al. 2016). These factors contribute to the development of persistent postoperative opioid use (PPOU) with the increased potential for ORADEs in the community following discharge.
Effective opioid stewardship is therefore an important part of the provision of opioids in the perioperative period, and a need to improve has been identified (Srivastava et al. 2021). Quality indicators are used to monitor and improve quality in healthcare (Stelfox and Straus 2013; Mainz 2003; Fabian and Geppert 2011; Donabedian 1988; Rademakers et al. 2011). Good quality indicators are based on the best available evidence, should be highly specific and sensitive, with the integration of best clinical evidence, clinical expertise, and patient values. No quality indicators for perioperative opioid stewardship currently exist. The review of the supporting evidence base is required to enable the development of a practical set of reliable quality indicators (Stelfox and Straus 2013).
Extracted quality indicators
Our review identified indicators relating to five key topics during the perioperative patient journey. These five topics are patient education, staff education, preoperative patient optimization, patient and procedure-specific prescribing and deprescribing and opioid-related adverse drug events. All five topics include structure, process, and outcome quality indicators (Table 4).
Definitions and comparisons
Varying definitions used in the literature have emerged from this review and consideration of these when discussing quality indicators is useful. Persistent perioperative opioid use is frequently described as the ongoing use of opioids at 90–180 days postoperatively (Fields et al. 2019; Lee et al. 2017; Clarke et al. 2014; Roughead et al. 2019; Pullman et al. 2021). Opioid tolerance is variably described as being present if a patient has used opioids for more than 7 days in the 60 days prior to surgery, any opioid use in 12 months prior to surgery or any opioid on the admission medication list (Fields et al. 2019; Brat et al. 2018; Cron et al. 2017; Gan et al. 2020; Hilliard et al. 2018; Truong et al. 2019). Milligrams of morphine equivalents (MME) or oral morphine equivalents (OME) are the most widely used methods to describe and compare opioid use. When reviewing postoperative patients in the community, the postoperative prescription can be considered to have been used if the prescribed opioids are dispensed between 2 and 7 days following discharge (Roughead et al. 2019). The detection of opioid misuse or PPOU after discharge is defined as at least one of the ICD-9 diagnosis code of opioid dependence, abuse or overdose (Brat et al. 2018). When reviewing the time to opioid cessation, a suggested definition is a period without an opioid prescription equivalent to three times the estimated supply duration in preoperatively opioid naïve patients (Roughead et al. 2019).
Data collection tools
Instruments to collect data for quality indicators are also reported although none have been specifically developed for postoperative opioid use. The Opioid Risk Tool (ORT), Screener for Opioid Assessment and Patients with Pain (SOAPP), and Brief Risk Interview (BRI) have been proposed for use in perioperative practice when screening patients preoperatively for risk of PPOU (Macintyre et al. 2014). The frequency, severity, and distress caused by opioid-related side effects can be scored as 0 to 60 on the Perioperative Opioid-related Symptom Distress scale and has been reported as a tool to assess ORADEs (Lee et al. 2010).
Addressing the gaps
More process quality indicators than structure or outcome quality indicators are described in the literature. However, the factors which are reported to make the greatest difference to a patient’s assessment of healthcare quality are process-related and process quality indicators are especially useful to consider when quality improvement is desired (Rademakers et al. 2011). Fewer quality indicators concern the intraoperative and immediate recovery period. The impact of specific changes in practice on long-term outcomes remains unclear, and our rapid review of quality indicators will enable rigorous studies of the implementation and impact of interventions to improve opiate stewardship in the perioperative period.
Algorithms and electronic systems
The screening of patients for potential opioid tolerance, future likelihood of PPOU, and patient-group-specific prescribing with limits on the type, dose, and duration of opioid prescription may be best undertaken with the use of algorithms and the development in machine learning (Ellis et al. 2019). Electronic records and prescribing (which are already well-embedded in primary care) are now used increasingly in hospital clinical practice and this may present a good opportunity to develop patient-or patient-group-specific guidelines for opioid prescribing with limits and alerts if there is deviation from agreed protocols.
Limitations
Limitations of this work include those relating to rapid review methodology. This is a relatively recently developed form of knowledge synthesis, and while valid (Garrity et al. 2021), is less comprehensive than a systematic review. Most of the studies included are retrospective cohort studies, and most originate using data from patients in a different healthcare systems (often from the USA). The characteristics of papers are reported (Supplementary Materials 2) but an assessment of risk of bias was not undertaken. This work has been done to drive improvement in outcomes for patients undergoing bowel cancer and this may limit its applicability to a wider perioperative population.
Conclusion and future work
The concept of ‘universal precautions’ have been suggested as being applicable to the prescribing and administration of opioids in the perioperative period (Lee et al. 2017; Macintyre et al. 2014) and encompass strategies at each stage of a patient’s perioperative journey to ensure that the lowest dosage, shortest acting opioids are used for the shortest possible time, while ensuring good analgesia and patient satisfaction. This will be used as an underpinning principle for our ongoing work.
This project forms part of the wider YCRBCIP program for use in the improvement of outcomes for patients with bowel cancer undergoing surgery. We have identified a set of quality indicators which may help to improve quality of care for patients undergoing major abdominal surgery for bowel cancer who receive perioperative opioids. We will now integrate the extracted quality indicators with clinician expertise and patient values to develop a more concise toolkit which providers in our region can use to benchmark and improve quality in the use of perioperative opioids for patients with bowel cancer.
Supplementary Information
Additional file 1: Supplementary materials 1. Extracted quality indicators
Additional file 2: Supplementary materials 2. Characteristics of papers
Additional file 3: Supplementary materials 3. Quality indicators and themes
Acknowledgements
Not applicable
Authors’ contributions
SA performed the literature search. MA, KP, and DY reviewed all abstracts. CT and SJH read all full texts. CT and SJH extracted all indicators. CT wrote the manuscript. All authors read and approved the final manuscript.
Funding
The Yorkshire Cancer Research Bowel Cancer Improvement Programme is funded by Yorkshire Cancer Research (YCR) Grant L394. YCR had no direct involvement in the design of the study, collection, analysis, interpretation of data or in writing the manuscript.
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Agarwal A, Lee D, Ali Z, et al. Patient-reported opioid consumption and pain intensity after common orthopedic and urologic surgical procedures with use of an automated text messaging system. JAMA. 2021;4:e213243. doi: 10.1001/jamanetworkopen.2021.3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arah OA, Westert GP, Hurst J, Klazinga NS. A conceptual framework for the OECD Health Care Quality Indicators Project. Int J Qual Health Care. 2006;18:5–13. doi: 10.1093/intqhc/mzl024. [DOI] [PubMed] [Google Scholar]
- Ballantyne JC, Kalso E, Stannard C. WHO analgesic ladder: a good concept gone astray. Br Med J. 2016;352:i20. doi: 10.1136/bmj.i20. [DOI] [PubMed] [Google Scholar]
- Bardiau F, Braeckman M, Seidel L, Albert A, Boogaerts J. Effectiveness of an acute pain service inception in a general hospital. J Clin Anesth. 1999;11:583–9. doi: 10.1016/S0952-8180(99)00101-4. [DOI] [PubMed] [Google Scholar]
- Bartels K, Mayes L, Dingmann C, Bullard K, Hopfer C, Binswanger I. Opioid use and storage patterns by patients after hospital discharge following surgery. PLOS ONE. 2016;11:e0147972. doi: 10.1371/journal.pone.0147972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berterame S, Erthal J, Thomas J, Fellner S, Vosse B, Clare P, et al. Use of and barriers to access to opioid analgesics: a worldwide, regional, and national study. The Lancet. 2016;387:1644–56. doi: 10.1016/S0140-6736(16)00161-6. [DOI] [PubMed] [Google Scholar]
- Bongiovanni T, Hansen K, Lancaster E, O’Sullivan P, Hirose K, Wick E. Adopting best practices in post-operative analgesia prescribing in a safety-net hospital: Residents as a conduit to change. Am J Surg. 2020;219:299–303. doi: 10.1016/j.amjsurg.2019.12.023. [DOI] [PubMed] [Google Scholar]
- Brandal D, Keller MS, Lee C, et al. Impact of enhanced recovery after surgery and opioid-free anesthesia on opioid prescriptions at discharge from the hospital: a historical-prospective study. Anesth Analg. 2017;125:1784–92. doi: 10.1213/ANE.0000000000002510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brat G, Agniel D, Beam A, et al. Postsurgical prescriptions for opioid naive patients and association with overdose and misuse: a retrospective cohort study. Br Med J. 2018;360:j5790. [DOI] [PMC free article] [PubMed]
- Bromberg W, Emanuel T, Zeller V, et al. Assessment of post-operative opioid prescribing practices in a community hospital ambulatory surgical center. J Opioid Manag. 2021;17:241–249. doi: 10.5055/jom.2021.0634. [DOI] [PubMed] [Google Scholar]
- Brummett C, Waljee J, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. J Am Med Assoc Surg. 2017;152:e170504. doi: 10.1001/jamasurg.2017.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Marcantonio A, Tornetta P. Correlation between 24-hour predischarge opioid use and amount of opioids prescribed at hospital discharge. J Am Med Assoc Surg. 2018;153:e174859. doi: 10.1001/jamasurg.2017.4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung C, Ying C, Lee L, Tsang S, Tsui S, Irwin M. An audit of postoperative intravenous patient-controlled analgesia with morphine: evolution over the last decade. Eur J Pain. 2009;13:464–71. doi: 10.1016/j.ejpain.2008.05.013. [DOI] [PubMed] [Google Scholar]
- Clarke H, Soneji N, Ko D, Yun L, Wijeysundera D. Rates and risk factors for prolonged opioid use after major surgery: population based cohort study. Br Med J. 2014;348:g1251. doi: 10.1136/bmj.g1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin LA, Bull F, Hales TG. Perioperative opioid analgesia—when is enough too much? A review of opioid-induced tolerance and hyperalgesia. The Lancet. 2019;393:1558–68. doi: 10.1016/S0140-6736(19)30430-1. [DOI] [PubMed] [Google Scholar]
- Cron D, Engelsbe M, Bolton C, et al. Preoperative opioid use is independently associated with increased costs and worse outcomes after major abdominal surgery. Ann Surg. 2017;265:695–701. doi: 10.1097/SLA.0000000000001901. [DOI] [PubMed] [Google Scholar]
- Curtis HJ, Croker R, Walker AJ, Richards GC, Quinlan J, Goldacre B. Opioid prescribing trends and geographical variation in England, 1998–2018: a retrospective database study. The Lancet Psychiatry. 2019;6:140–50. doi: 10.1016/S2215-0366(18)30471-1. [DOI] [PubMed] [Google Scholar]
- Daliya P, Adiamah A, Roslan F, et al. Opioid prescription at postoperative discharge: a retrospective observational cohort study. Anaesthesia. 2021;76:1367–76. doi: 10.1111/anae.15460. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Grebely J, Stone J, Hickman M, Vickerman P, Marshall BDL, et al. Global patterns of opioid use and dependence: harms to populations, interventions, and future action. The Lancet. 2019;394:1560–79. doi: 10.1016/S0140-6736(19)32229-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donabedian A. The quality of care. How can it be assessed? JAMA. 1988;260:1743–8. doi: 10.1001/jama.1988.03410120089033. [DOI] [PubMed] [Google Scholar]
- Ellis R, Wang Z, Genes N, Ma’ Ayan A. Predicting opioid dependence from electronic health records with machine learning. BioData Mining. 2019;12:3. doi: 10.1186/s13040-019-0193-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian L, Geppert J. Quality indicator measure development, implementation, maintenance, and retirement summary. Rockville, MD. Agency for Healthcare Research and Quality, 2011.
- Felling D, Jackson M, Ferraro J, et al. Liposomal bupivacaine transversus abdominis plane block versus epidural analgesia in a colon and rectal surgery enhanced recovery pathway: a randomized clinical trial. Dis Colon Rectum. 2018;61:1196–1204. doi: 10.1097/DCR.0000000000001211. [DOI] [PubMed] [Google Scholar]
- Fields A, Cavallaro P, Correll D, et al. Predictors of prolonged opioid use following colectomy. Dis Colon Rectum. 2019;62:1117–23. doi: 10.1097/DCR.0000000000001429. [DOI] [PubMed] [Google Scholar]
- Fujii M, Hodges A, Russell R, et al. Post-discharge opioid prescribing and use after common surgical procedures. J Am Coll Surg. 2018;226:1004–1012. doi: 10.1016/j.jamcollsurg.2018.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan T, Robinson S, Oderda G, Scranton R, Pepin J, Ramamoorthy S. Impact of postsurgical opioid use and ileus on economic outcomes in gastrointestinal surgeries. Curr Med Res Opin. 2015;31:677–86. doi: 10.1185/03007995.2015.1005833. [DOI] [PubMed] [Google Scholar]
- Gan T, Jackson N, Castle J. A retrospective review: patient-reported preoperative prescription opioid, sedative, or antidepressant use is associated with worse outcomes in colorectal surgery. Dis Colon Rectum. 2020;63:965–73. doi: 10.1097/DCR.0000000000001655. [DOI] [PubMed] [Google Scholar]
- Garrity C, Gartlehner G, Nussbaumer-Streit B, et al. Cochrane Rapid Reviews Methods Group offers evidence-informed guidance to conduct rapid reviews. J Clin Epidemiol. 2021;130:13–22. doi: 10.1016/j.jclinepi.2020.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glare P, Aubrey KR, Myles PS. Transition from acute to chronic pain after surgery. The Lancet. 2019;393:1537–46. doi: 10.1016/S0140-6736(19)30352-6. [DOI] [PubMed] [Google Scholar]
- Greco M, Capretti G, Beretta L, Gemma M, Pecorelli N, Braga M. Enhanced recovery program in colorectal surgery: a meta-analysis of randomized controlled trials. World J Surg. 2014;38:1531–41. doi: 10.1007/s00268-013-2416-8. [DOI] [PubMed] [Google Scholar]
- Haby M, Chapman E, Clark R, Barreto J, Reveiz L, Lavis J. What are the best methodologies for rapid reviews of the research evidence for evidence-informed decision making in health policy and practice: a rapid review. Health Res Policy Syst. 2016;14:83. doi: 10.1186/s12961-016-0155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel C, Michaud A, Thuku M, et al. Defining rapid reviews: a systematic scoping review and thematic analysis of definitions and defining characteristics of rapid reviews. J Clin Epidemiol. 2021;129:74–85. doi: 10.1016/j.jclinepi.2020.09.041. [DOI] [PubMed] [Google Scholar]
- Hill MV, McMahon ML, Stucke RS, Barth RJ., Jr Wide variation and excessive dosage of opioid prescriptions for common general surgical procedures. Ann Surg. 2017;265:709–714. doi: 10.1097/SLA.0000000000001993. [DOI] [PubMed] [Google Scholar]
- Hill MV, Stucke RS, McMahon ML, Beeman JL, Barth RJ., Jr An educational intervention decreases opioid prescribing after general surgical operations. Ann Surg. 2018;267:468–72. doi: 10.1097/SLA.0000000000002198. [DOI] [PubMed] [Google Scholar]
- Hilliard P, Waljee J, Moser S, et al. Prevalence of preoperative opioid use and characteristics associated with opioid use among patients presenting for surgery. J Am Med Assoc Surg. 2018;153:929–37. doi: 10.1001/jamasurg.2018.2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang S, Vemuru S, Hassinger T, Friel C, Hedrick T. An unintended consequence of a new opioid legislation. Dis Colon Rectum. 2020;63:389–96. doi: 10.1097/DCR.0000000000001554. [DOI] [PubMed] [Google Scholar]
- Hopkins R, Bui T, Konstantatos A, et al. Educating junior doctors and pharmacists to reduce discharge prescribing of opioids for surgical patients: a cluster randomised controlled trial. Med J Aust. 2020;213:417–23. doi: 10.5694/mja2.50812. [DOI] [PubMed] [Google Scholar]
- Howard R, Fry B, Gunaseelan V, et al. Association of opioid prescribing with opioid consumption after surgery in Michigan. J Am Med Assoc Surg. 2019;154:e184234. doi: 10.1001/jamasurg.2018.4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivers N, Jamtvedt G, Flottorp S et al. Audit and feedback: effects on professional practice and healthcare outcomes. The Cochrane Library 2012; 6: CD000259–CD000259 [DOI] [PMC free article] [PubMed]
- Jani M, Birlie Yimer B, Sheppard T, Lunt M, Dixon WG. Time trends and prescribing patterns of opioid drugs in UK primary care patients with non-cancer pain: a retrospective cohort study. PLOS Med. 2020;17:e1003270. doi: 10.1371/journal.pmed.1003270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Orton M, Feng R. Chronic Opioid Usage in Surgical Patients in a Large Academic Center. Ann Surg. 2017;265:722–27. doi: 10.1097/SLA.0000000000001780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallen M, Roos-Blom M, Dongelmans D, et al. Development of actionable quality indicators and an action implementation toolbox for appropriate antibiotic use at intensive care units: a modified-RAND Delphi study PLOS ONE. 2018;13:e0207991. doi: 10.1371/journal.pone.0207991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller D, Zhang J, Chand M. Opioid-free colorectal surgery: a method to improve patient & financial outcomes in surgery. Surg Endosc. 2019;33:1959–66. doi: 10.1007/s00464-018-6477-5. [DOI] [PubMed] [Google Scholar]
- Kessler ER, Shah M, Gruschkus SK, Raju A. Cost and quality implications of opioid-based postsurgical pain control using administrative claims data from a large health system: opioid-related adverse events and their impact on clinical and economic outcomes. Pharmacotherapy. 2013;33:383–91. doi: 10.1002/phar.1223. [DOI] [PubMed] [Google Scholar]
- Kelley, E, Hurst J. Health care quality indicators project: Conceptual Framework Paper. OECD Health Working Papers 2006; 23.
- Kiang MV, Humphreys K, Cullen MR, Basu S. Opioid prescribing patterns among medical providers in the United States, 2003–17: a retrospective, observational study. Br Med J. 2020;368: l6968. doi: 10.1136/bmj.l6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancet The. Managing the opioid crisis in North America and beyond. The Lancet. 2022;399:495. doi: 10.1016/S0140-6736(22)00200-8. [DOI] [PubMed] [Google Scholar]
- Lee A, Chan SK, Chen PP, Gin T, Lau AS, Chiu CH. The costs and benefits of extending the role of the acute pain service on clinical outcomes after major elective surgery. Anesth Analg. 2010;111:1042–50. doi: 10.1213/ANE.0b013e3181ed1317. [DOI] [PubMed] [Google Scholar]
- Lee JS, Hu HM, Edelman AL, et al. New persistent opioid use among patients with cancer after curative-intent surgery. J Clin Oncol. 2017;35:4042–49. doi: 10.1200/JCO.2017.74.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy N, Quinlan J, El-Boghdadly K, Fawcett WJ, Agarwal V, Bastable RB, et al. An international multidisciplinary consensus statement on the prevention of opioid-related harm in adult surgical patients. Anaesthesia. 2021;76:520–36. doi: 10.1111/anae.15262. [DOI] [PubMed] [Google Scholar]
- Macintyre PE, Huxtable CA, Flint SL, Dobbin MD. Costs and consequences: a review of discharge opioid prescribing for ongoing management of acute pain. Anaesth Intensive Care. 2014;42:558–74. doi: 10.1177/0310057X1404200504. [DOI] [PubMed] [Google Scholar]
- Mainz J. Defining and classifying clinical indicators for quality improvement. Int J Qual Health Care. 2003;15:523–530. doi: 10.1093/intqhc/mzg081. [DOI] [PubMed] [Google Scholar]
- Meyer DC, Hill SS, McDade JA, et al. Opioid consumption patterns after anorectal operations: development of an institutional prescribing guideline. Dis Colon Rectum. 2021;64:103–111. doi: 10.1097/DCR.0000000000001680. [DOI] [PubMed] [Google Scholar]
- Minkowitz HS, Gruschkus SK, Shah M, Raju A. Adverse drug events among patients receiving postsurgical opioids in a large health system: risk factors and outcomes. Am J Health Syst Pharm. 2014;71:1556–65. doi: 10.2146/ajhp130031. [DOI] [PubMed] [Google Scholar]
- Mujukian A, Truong A, Tran H, Shane R, Fleshner P, Zaghiyan K. A standardized multimodal analgesia protocol reduces perioperative opioid use in minimally invasive colorectal surgery. J Gastrointest Surg. 2020;24:2286–94. doi: 10.1007/s11605-019-04385-9. [DOI] [PubMed] [Google Scholar]
- National Quality Measures Clearinghouse. Agency for Healthcare Research and Quality; Patient Safety Network Home | PSNet (ahrq.gov) (accessed 2nd January 2022).
- Neuman MD, Bateman BT, Wunsch H. Inappropriate opioid prescription after surgery. Lancet. 2019;393:1547–57. doi: 10.1016/S0140-6736(19)30428-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oderda GM, Gan TJ, Johnson BH, Robinson SB. Effect of opioid-related adverse events on outcomes in selected surgical patients. J Pain Palliat Care Pharmacother. 2013;27:62–70. doi: 10.3109/15360288.2012.751956. [DOI] [PubMed] [Google Scholar]
- Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan; a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation | The EQUATOR Network (equator-network.org) (accessed 22nd March 2022).
- Pruitt LCC, Swords DS, Vijayakumar S, et al. Implementation of a quality improvement initiative to decrease opioid prescribing in general surgery. J Surg Res. 2020;247:514–23. doi: 10.1016/j.jss.2019.09.051. [DOI] [PubMed] [Google Scholar]
- Pullman A, Syrowatka A, Businger A, et al. Development and alpha testing of specifications for a prolonged opioid prescribing electronic clinical quality measure (eCQM) AMIA Annu Symp Proc. 2021;2020:1022–30. [PMC free article] [PubMed] [Google Scholar]
- Rademakers J, Delnoij D, de Boer D. Structure, process of outcome: which contributes most to patients’ overall assessment of healthcare quality? BMJ Qual Saf. 2011;20:326–31. doi: 10.1136/bmjqs.2010.042358. [DOI] [PubMed] [Google Scholar]
- Roughead EE, Lim R, Ramsay E, Moffat AK, Pratt NL. Persistence with opioids post discharge from hospitalisation for surgery in Australian adults: a retrospective cohort study. BMJ Open. 2019;4:e023990. doi: 10.1136/bmjopen-2018-023990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber LZ, Guy GP, Jr, Seth P, Young R, Mattson CL, Mikosz CA, et al. Trends and patterns of geographic variation in opioid prescribing practices by State, United States, 2006–2017. JAMA Network Open. 2019;2:e190665. doi: 10.1001/jamanetworkopen.2019.0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small C, Laycock H. Acute postoperative pain management. British Journal of Surgery. 2020;107:e70–e80. doi: 10.1002/bjs.11477. [DOI] [PubMed] [Google Scholar]
- Standards and Indicators | NICE (accessed 22nd March 2022)
- Stafford C, Francone T, Roberts PL, Ricciardi R. What factors are associated with increased risk for prolonged postoperative opioid usage after colorectal surgery? Surg Endosc. 2018;32:3557–61. doi: 10.1007/s00464-018-6078-3. [DOI] [PubMed] [Google Scholar]
- Stelfox H, Straus S. Measuring quality of care: considering measurement frameworks and needs assessment to guide quality indicator development. J Clin Epidemiol. 2013;66:1320–1327. doi: 10.1016/j.jclinepi.2013.05.018. [DOI] [PubMed] [Google Scholar]
- Srivastava D, Hill S, Carty S et al. BJA 2021 126, 1208-16. Surgery and opioids: evidence based consensus guidelines on the perioperative use of opioids in the UK. British J Anesth. 2021;126:1208-16. [DOI] [PubMed]
- Syrowatka A, Pullman A, Kim W, et al. Re-tooling an Existing Clinical Quality Measure for Chronic Opioid Use to an Electronic Clinical Quality Measure (eCQM) for Post-operative opioid prescribing: development and testing of draft specifications. AMIA Annu Symp Proc. 2021;2020:1200–09. [PMC free article] [PubMed] [Google Scholar]
- Thiele R, Rea K, Turrentine F. Standardization of care: impact of an enhanced recovery protocol on length of stay, complications, and direct costs after colorectal surgery. J Am Coll Surg. 2015;220:430–43. doi: 10.1016/j.jamcollsurg.2014.12.042. [DOI] [PubMed] [Google Scholar]
- Thiels CA, Anderson SS, Ubl DS, et al. Wide variation and overprescription of opioids after elective surgery. Ann Surg. 2017;266:564–73. doi: 10.1097/SLA.0000000000002365. [DOI] [PubMed] [Google Scholar]
- Truong A, Mujukian A, Fleshner P, Zaghiyan K. No pain, more gain: reduced postoperative opioid consumption with a standardized opioid-sparing multimodal analgesia protocol in opioid-tolerant patients undergoing colorectal surgery. Am Surg. 2019;85:1155–58. doi: 10.1177/000313481908501017. [DOI] [PubMed] [Google Scholar]
- Tsui SL, Tong WN, Irwin M, et al. The efficacy, applicability and side-effects of postoperative intravenous patient-controlled morphine analgesia: an audit of 1233 Chinese patients. Anaesth Intensive Care. 1996;24:658–64. doi: 10.1177/0310057X9602400604. [DOI] [PubMed] [Google Scholar]
- Verhamme KMC, Bohnen AM. Are we facing an opioid crisis in Europe? The Lancet Public Health. 2019;4:e483–e484. doi: 10.1016/S2468-2667(19)30156-2. [DOI] [PubMed] [Google Scholar]
- Wang TV, Okose O, Abt NB, Kamani D, Emerick KS, Randolph GW. One institution's experience with self-audit of opioid prescribing practices for common cervical procedures. Head Neck. 2021;43:2385–94. doi: 10.1002/hed.26697. [DOI] [PubMed] [Google Scholar]
- Wick EC, Grant MC, Wu CL. Postoperative multimodal analgesia pain management with nonopioid analgesics and techniques: a review. J Am Med Assoc Surg. 2017;152:691–97. doi: 10.1001/jamasurg.2017.0898. [DOI] [PubMed] [Google Scholar]
- Yap R, Nassif G, Hwang G, et al. Achieving opioid-free major colorectal surgery: is it possible? Dig Surg. 2020;37:376–82. doi: 10.1159/000505516. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary materials 1. Extracted quality indicators
Additional file 2: Supplementary materials 2. Characteristics of papers
Additional file 3: Supplementary materials 3. Quality indicators and themes
Data Availability Statement
All data generated or analysed during this study are included in this published article [and its supplementary information files].