Summary
Technological advances have driven many recent advances in developmental biology. Light sheet imaging can reveal single-cell dynamics in living three-dimensional tissues, whereas single-cell genomic methods open the door to a complete catalogue of cell types and gene expression states. An equally powerful but complementary set of approaches are also becoming available to define development processes from the bottom up. These synthetic approaches aim to reconstruct the minimal developmental patterns, signaling processes, and gene networks that produce the basic set of developmental operations: spatial polarization, morphogen interpretation, tissue movement, and cellular memory. In this review we discuss recent approaches at the intersection of synthetic biology and development, including synthetic circuits to deliver and record signaling stimuli and synthetic reconstitution of pattern formation on multicellular scales.
1. Introduction: The emerging interface between synthetic biology and development
For many biologists, embryogenesis is the greatest show on earth. The rapid development from a single cell to a complex, patterned multicellular organism encapsulates many of the features that capture the imagination about life: the formation of exquisite molecular patterns and physical structures and the elaboration and maintenance of the diverse cellular types required for a functional organism. Many recent technological advances have thus focused on watching the show with ever-greater resolution. For example, light-sheet microscopy enables the developmental biologist to observe every cellular actor from all angles in its full three-dimensional context. At the same time, single-cell sequencing methods provide more detailed information about these individuals than ever before, offering new insights into the richness of cell types that the embryo generates. Collectively, these methods have generated new datasets with unprecedented resolution, and these data have in turn enabled increasingly rigorous quantitative models which link properties of cell signaling networks to macroscopic developmental outcomes.
Nevertheless, a developmental biologist may not be content to merely watch the show, but from time to time might also wish to try their hand at writing or directing an episode. Here, a different class of approaches are needed. We might require a rudimentary cast and set – a developmental model system where environmental variables can be controlled or altered, and different cellular actors can be assembled. We may also wish to revise the script and characters: altering the rules for cell signaling or physical interactions to observe how the final state changes. Achieving these goals would deepen our understanding of developmental processes by delineating minimal sets of components that can perform a desired function and pointing the way to correct errors when they occur.
Fortunately, a different and complementary set of methods from synthetic biology are increasingly available to help the developmental biologist achieve their show-running goals. Synthetic biology is a discipline focused on engineering complex biological functions from well-defined and predictable parts. From its roots in constructing bacterial gene networks that act as oscillators1 or toggle switches2, synthetic biology has grown to encompass approaches for building eukaryotic signaling circuits3,4, ligand-receptor signaling5–7, and cell-cell adhesion8, processes that are all deeply relevant to our understanding of development. A second thrust has been the establishment of synthetic interfaces: engineered proteins and genes that, for example, enable the experimentalist to deliver stimuli to a cell using light9–11, or that record particular signaling states into persistent changes in a cell’s DNA sequence12–15 or protein contents16,17. These interfaces present exciting new opportunities to perturb and monitor developmental processes.
In this review, we describe current advances at the intersection of synthetic and developmental biology. First, we highlight new advances in engineering developmental “input/output interfaces”: optogenetic tools to apply precise inputs to developmental systems and synthetic recording systems for measuring new outputs such as cell lineage and signaling histories. We then review advances in understanding three developmental processes that have proven to be especially amenable to synthetic manipulation: morphogen signaling, cell fate specification, and tissue patterning and self-organization. We close by discussing emerging future applications at the interface of synthetic and developmental biology.
2. Synthetic interfaces to developmental systems
Where in developmental biology might engineering-based approaches have the biggest impact? One context where questions meet tools is in the context of designing synthetic “interfaces” – engineered proteins or gene networks that enable the experimentalist to either deliver a stimulus to a cell of interest (input interfaces) or to record a specific cellular response (output interfaces). Synthetic interfaces are also among the simplest synthetic systems, serving primarily as relays that convert light, chemical, or biological signals to gene expression.
In development, cells often encounter transient stimuli that may trigger a fate choice hours or days later. The ability to deliver such a stimulus to a particular set of cells, or to track cells that receive an endogenous stimulus over time, would be particularly powerful. Synthetic interfaces also provide exceptional capacity for reuse. For example, an optogenetic interface can be used to deliver signals that vary in timing, intensity, or spatial range, enabling the experimentalist to scan a wide range of perturbative effects. In this section we focus on how cell engineering can be used to build circuits for precise input control and output measurement in developmental systems.
2.1. Engineering the inputs: optogenetics for controlling developmental systems
We focus first on one class of synthetic input interface: optogenetic tools that enable light-based control over developmental processes (Figure 1A). Optogenetics is ideally suited for probing developmental systems18–22. Light delivery is feasible for any developmental model system that is amenable to imaging studies (e.g., Drosophila and zebrafish embryos; the mouse pre-implantation embryo; stem cell-based models like gastruloids and organoids). Light can be applied and removed at will, can be patterned with high spatial resolution, and is largely non-interacting with most developmental cell types. We note that excellent resources, including reviews23,24 and the OptoBase web portal25, are already available that describe in detail the large and growing toolbox of light-sensitive protein domains that make up the optogenetic toolbox – we point the interested reader to those resources and focus here on their applications in developmental biology.
Figure 1. Engineering the inputs: synthetic strategies for delivering designed stimuli to developmental systems.
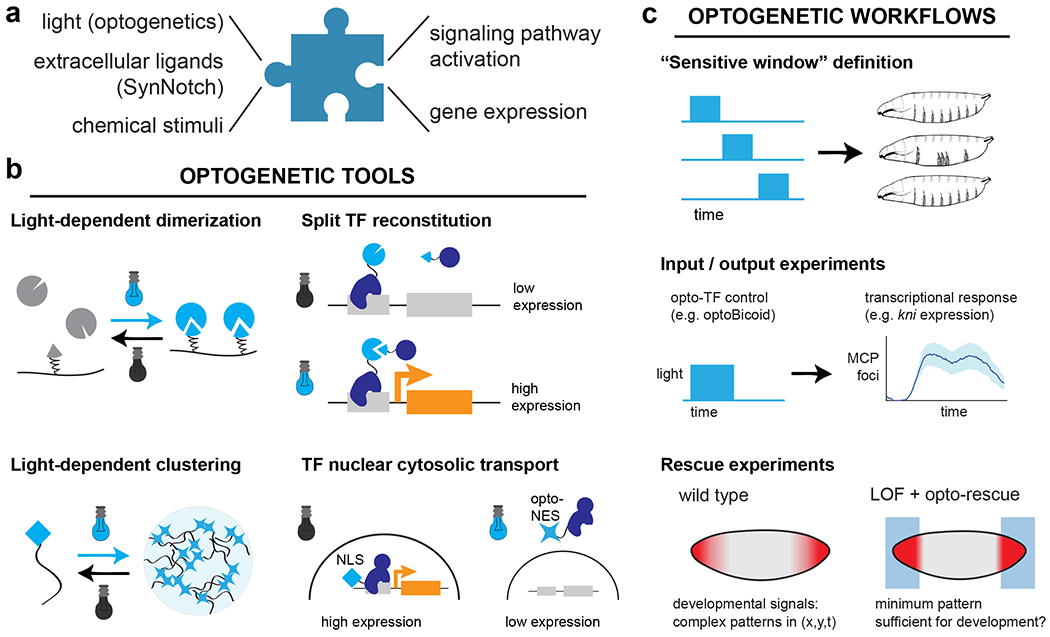
(a) Synthetic input interfaces aim to link a desired stimulus of interest to defined cellular outcomes. Stimuli may be either controllable and engineered (e.g., such as light or chemical stimuli) or may represent external factors presented by other cells (e.g., surface proteins presented by neighboring cells factors that they secrete). (b) Optogenetic tools constitute a major class of synthetic interfaces. The basic toolset includes many different forms of light-driven protein protein interaction, such as light-based dimerization and clustering. These tools can be applied to control diverse developmental processes, including light-induced expression of target genes of interest. (c) The availability of synthetic input interfaces necessitates new experimental workflows that take advantage of the ability to deliver precise spatiotemporal stimuli. For example, varying the period of illumination can define sensitive windows to a signaling cue, and coupling rapid input variation with live biosensors can reveal the magnitude and timescale of downstream processes. Recent studies have also begun to define which stimuli that are sufficient to rescue the loss of an endogenous developmental pattern.
2.1.1. Optogenetic control of developmental processes
Over the past few years, the use of optogenetics in developmental cell signaling has exploded. Many classical developmental signaling pathways – including BMP26, FGF27–29, Ras30–32, Wnt33,34, Nodal18, and Notch/Delta35,36 signaling – have now been placed under some form of optogenetic control. But the devil is in the details: because each pathway may be activated by different molecular events, the mechanistic basis for optogenetic control can vary (Figure 1B). For many receptor-driven signaling events (e.g., BMP, Nodal and FGFR), light-controlled dimerization between receptor subunits has proven to be a powerful strategy18,26,29. Dimerization can be readily achieved upon blue light illumination using fusion to the light-oxygen-voltage sensing (LOV) domains from V. frigida Aureochrome 1 (VfAU1) or the N. crassa Vivid protein (VVD), ~150 amino acid domains that homodimerize upon blue light stimulation29,37–39.
Protein clustering is also known to play a crucial role in the activation of many signaling pathways and can be robustly achieved using variants of the of A. sativa Cry2 photolyase homology region (PHR) domain that oligomerize upon blue light stimulation40,41. Light-triggered clustering proved to potently modulate FGFR27,28, Wnt34, and Notch/Delta signaling35, although in the case of Notch/Delta clustering proved to be inhibitory, rather than activating. Finally, Notch/Delta signaling has also been successfully manipulated by directly controlling the nuclear localization of the Notch intracellular domain using the combined action of a bifunctional optogenetic tool: the Zdark/LANS system, in which the LANS-tagged protein both dissociates from the mitochondrial outer membrane upon illumination and undergoes light-induced nuclear import36. Taken together, these diverse successes demonstrate that a vast array of developmental processes are accessible to optogenetic control.
Cell-type specific control over gene expression is one of the major tools in the developmental biologist’s arsenal, and it was recognized early on that extending this capability to light-based gene expression would be quite powerful. One major class of tools rely on light-controlled variants of the yeast Gal4 transcription factor, which has long been a mainstay in developmental biology since its original development in Drosophila42. Gal4 binds DNA as a dimer, and early efforts sought to replace this constitutive dimerization with light using the VVD blue light-activated homodimerization domain38. The resulting LightOn system (also termed GAVPO) was successfully applied for light-induced Cre recombination or insulin secretion in a transgenic mouse38, as well as to interrogate the decision of cultured mouse embryonic stem cells to different into mesendoderm or neural ectoderm43. Most recently, the VVD-derived pMag/nMag heterodimerization system brought high-quality optogenetic Gal4 control back to the fly in the “ShineGal4” system39,44,45. GAVPO-based tools proved functional but toxic in zebrafish, and an alternative approach was developed using a bacterial transcription factor, EL222, which dimerizes and binds DNA in a light-sensitive manner46. Fusions of EL222’s DNA binding domain with eukaryotic transcriptional activation domains led to potent light-dependent gene expression in zebrafish embryos47–49.
What about endogenous transcription factors? It is attractive not just to control gene expression from an engineered Gal4-responsive promoter, but also to drive endogenous programs of gene expression from a naturally produced transcription factor. A variety of optogenetic nuclear import-export systems can provide this function, including the LEXY system for light-inducible nuclear protein export50 and the LANS51 and LINUS52 systems for import. These approaches are beginning to see application in a variety of cell and developmental contexts as well, including nuclear shuttling of the YAP transcription factor53 and optogenetic control of the Bicoid morphogen54 and Twist transcription factor55 in Drosophila embryos. Finally, higher-order assembly of transcription factors is emerging as an organizing principle for gene regulation, and light-inducible clustering tools have recently begun to be used to control developmental transcription factors, including the transcription factor Bicoid56 and the pioneer factor Zelda57. In summary, developmental optogenetics has entered a period of rapid growth, with tools available or within reach for almost any process in developmental signaling or gene expression.
2.1.2. Experimental workflows for optogenetic developmental biology
The optogenetic strategies described above have also been coupled to innovative experimental workflows to gain insights into developmental systems. One of the most straightforward uses of light-sensitive signaling proteins has been to precisely define the temporal windows of an effect (e.g., the window in which cells can differentiate in response to a signaling cue) (Figure 1C, top). This experimental approach takes advantage of the rapid timescale with which light can be applied and removed and is particularly well suited to light-sensitive proteins that rapidly revert to their inactive states after a shift to darkness. A similar approach of varying the illumination time was used to map the essential temporal requirements for the transcription factors Bicoid and Zelda in early Drosophila embryogenesis56,57, assess when the embryo is most sensitive to ectopic Erk signaling in Drosophila30 and zebrafish58, and define the relationship between signal timing and gene expression for Nodal and BMP signaling in zebrafish18,26.
A second group of studies have begun to focus in more precisely at relationship between signaling and the dynamics of developmental gene expression, using a combination of optogenetic stimulation with live-cell transcription biosensors such as the MS2/MCP system59 (Figure 1C, middle). Light can be used to acutely trigger nuclear import/export of a developmental transcription factors within seconds-minutes, raising the possibility that the dynamics of these distinct regulatory processes can be separated in time. Many exciting early examples of this framework have appeared in Drosophila studies, which has proven to be an excellent test bed for coupling optogenetic stimulation and live transcriptional biosensors. For example, Viswanathan and colleagues linked the light-triggered nuclear import of the Notch intracellular domain, a transcriptional activator, to live biosensors of the sim target gene36. These experiments revealed sim transcriptional adaptation on a ~30 min timescale despite sustained nuclear accumulation of the transcription factor, with subsequent experiments pointing to a role for the transcription factor Twist in regulating adaptation. McFann and colleagues combined optogenetic Erk activation, live transcriptional imaging, and genetic perturbations to define a negative regulatory circuit on mesoderm specification that acts through hkb, a target of Erk signaling60. Finally, Singh, Wu, and colleagues performed similar acute optogenetic perturbations of the Bicoid transcription factor while monitoring all four canonical Bicoid-responsive gap genes54. They observed diverse timescales of transcriptional response ranging from 3 min–1 h, including a paradoxical response from the kni gene, which was rapidly transcribed upon a decrease in nuclear concentration of its activator Bcd. These experiments suggest that there is much to learn from acutely perturbing transcription factors while monitoring signal flow through developmental gene networks.
Finally, a third class of studies capitalizes on the power of optogenetics for applying local spatial stimuli, which could in principle take the form of different geometric “blocks” of light, continuous gradients, or even noisy or discontinuous patterns (Figure 1C, bottom). Spatial stimuli are exceptionally well suited to studying developmental signaling and tissue morphogenesis, processes that define the body’s coordinate system, drive organized patterns of cell movement, and trigger tissue-specific gene expression. De Renzis and colleagues pioneered the use of spatial optogenetic stimulation using the Drosophila ventral furrow as a model system. Light-inducible recruitment of a lipid phosphatase drove dramatic changes in illuminated cell shape due to neighboring cells’ morphogenetic movements19, whereas recruitment of a Rho GTP exchange factor (RhoGEF) triggered local apical constriction and invagination of cells in the illuminated region61. In both cases, a close correspondence was observed between the geometry of the illuminated region and the aspect ratio of individual cells. These approaches have been further extended to investigate differences in light-induced morphogenesis between different embryo regions62 or at a subcellular scale by comparing light-induced tissue movements along apical, basal, and lateral cell surfaces in the Drosophila wing disc63.
Perhaps the highest bar that can be satisfied by a synthetic developmental pattern is to demonstrate that it can fully compensate for the loss of the corresponding endogenous pattern. This complete replacement can be exceedingly difficult to achieve, because it may require a synthetic system whose activity covers the entire dynamic range of the natural pattern, the ability to precisely replicate the natural pattern in space and time, and a loss-of-function background that is otherwise fully competent for normal development.
Despite these strict requirements, light-based rescue has been demonstrated for one developmental process: the terminal pattern of Ras/Erk signaling in the Drosophila embryo32. This context proved ideal for a few reasons. First, the endogenous pattern can be eliminated without additional consequences. The terminal pattern, a pair of inward-facing gradients from the anterior and posterior poles of the embryo, is established by a ligand and receptor tyrosine kinase that are dispensable for the remainder of the fly life cycle64. Second, the timing and spatial range of the endogenous signal has been quantified rigorously65, and is comparatively easy to replicate with a pattern of light. Finally, the OptoSOS system was previously shown to have exceptional dynamic range (from 0-200% of the natural pattern), and can be tuned to intermediate levels by varying the intensity or duration of light pulses30. The authors found that even an all-or-none light stimulus delivered to the embryonic termini led to successful completion of embryogenesis in many of the illuminated embryos despite loss of the signaling gradient present in the endogenous pattern. Such developmental reconstitution is only a first step towards defining the mechanistic basis by which different doses of Erk signaling dictates developmental phenotypes.
2.2. Engineering the outputs: recording signaling cues, gene expression and cell lineage
Embryonic development is dynamic: cells move in relation to one another and experience complex, time-varying combinations of external signals before adopting their final positions and fates. It thus remains challenging to link the signals that a cell experiences along its journey to its ultimate transcriptional and morphogenic fate. While fluorescent reporter cell lines can illuminate some of these transient signals, they typically only reflect recent activity of a pathway of interest, making it difficult to bridge the timescales of signaling (typically minutes to hours) and cell fates/phenotypes (many hours to days). Here we outline how synthetic biology may define a new generation of “smart” reporters that can track stimulated cells with higher resolution and over longer periods of time.
Ideally, such a smart reporter would contain two components. The first is some kind of “recorder” system – a gene/protein network that can be triggered under well-defined conditions to result in a long-term cellular change (Figure 2A). The second component is a “reader” – a system that activates the recorder only in response to a desired stimulus, such as a particular combination of upstream signaling pathways or gene expression states. Together, a reader-recorder pair could connect measurements of signaling dynamics to endpoint phenotypes (e.g., the number or identity of cells in an eventual differentiated tissue), or be combined with methods such as single-cell RNA sequencing66, MERFISH67, or slide-seq68 to relate early signaling events to subsequent cellular identity. Here we review the current state of reader and recorder systems that may ultimately enable the experimentalist to connect transient stimuli to eventual cell phenotypes at unprecedented resolution.
Figure 2. Engineering the outputs: detecting and recording complex cell states.

(a) Synthetic output interfaces aim to selectively sense a particular cellular state – e.g., whether a specific combination of genes is expressed, or if a pathway is transiently activated. They then transduce the signal to a long-lived reporter, such as a permanent genetic modification or stable protein filament. (b) Scratch pad recorders target Cas9 cutting and error-prone repairs to defined, transcriptionally active sequences in the genome; these can later be sequenced to report on cell lineage. (c) Ticker-tape recorders perform sequential operations that can be read out over time, providing a linear temporal history of cellular states. DNA-based ticker tapes use prime editors to sequentially modify DNA sequences, whereas protein-based ticker tapes form long-lived protein polymers with differentially labeled subunits. (d) Readers can provide detailed information about the combination or dynamics of active pathways in a cell. Recombinase-based circuits can detect complex combinations of upstream inputs, provided they are linked to recombinase expression. Conversely, a pulse-detecting gene circuit offers the first opportunity to stably record transient signaling dynamics.
2.2.1. DNA-based molecular recorders
The predominant strategy for molecular recording uses CRISPR-targeted DNA mutations to encode lineage information within a cell’s genome. This approach can be understood as an extension of lineage tracing by mapping somatic mutations. A cell stochastically occurs mutations in its genome over time, which are inherited by all its progeny and therefore encode information about their lineage. Synthetic DNA-recording circuits work by concentrating somatic mutation at a targeted recording locus, for example using CRISPR-based genome editing. A cell is engineered to harbor a ‘scratchpad’ DNA locus at which edits will be performed; it also must express both the Cas9 protein and a guide RNA (gRNA) that targets Cas9 to the scratchpad. Repair of Cas9-generated double stranded DNA breaks is error-prone, so insertion-deletion (indel) mutations accumulate within the scratchpad (Figure 2B). This overall strategy has been implemented successfully to annotate lineage phylogeny in zebrafish12,69, mouse13, and fly embryos14.
CRISPR-based molecular recorders all share the same basic principle, but their detailed implementations vary depending on the desired mutation rates and total number of edits. For example, the zebrafish-based GESTALT system features a 257 bp recording scratchpad with 10 optimized target sites, each corresponding to a separate gRNA69. Because the recording locus is embedded within the 3’ UTR of an EGFP gene, single cell scratch pads can be retrieved within a droplet-based scRNA sequencing pipeline. GESTALT was optimized for rapid recording to match the fast pace of early zebrafish development, and most scratchpad edits appear to occur prior to dome stage (4.3 hours post-fertilization), after which their scratchpads are exhausted.
In the mouse, editing rates must be scaled to achieve lineage tracing over a proportionally longer developmental time window. Chan and colleagues addressed this challenge by identifying three target-guide RNA pairs which show a broad dynamic range of editing rates depending on the location and frequency of sequence mismatches13. They further improved the recording channel capacity by integrating up to 20 copies of the scratchpad recording cassette, each with a unique ‘integration barcode’ that allows copies to be disambiguated during sequencing. Leeper and colleagues adopted an alternative approach in mice termed “homing CRISPR”70. In typical Cas9-based genome editing, a guide RNA loses the ability to recognize and re-edit its target site after one to several indels accumulate. Homing CRISPR combines the gRNA and scratchpad into a single unit by adding the protospacer-adjacent motif (PAM) to the gRNA gene. By targeting its own sequence, the gRNA maintains fidelity as it self-edits and evolves.
In each of the preceding cases, DNA edits are transcribed and read out using single-cell RNA sequencing of dissociated cells. This approach is powerful because it enables cell lineage to be linked to the full transcriptome of single cells; however, it also destroys information about cells’ spatial position within the tissue. A related technique, MEMOIR, seeks to overcome this limitation by imaging cell lineages in situ using multiplexed smFISH15. MEMOIR features 28 scratchpads, each with a shared protospacer target sequence and a unique barcode sequence which can be imaged using corresponding single molecule fluorescence in situ hybridization (smFISH) probes. Cas9 mutation of the target causes ‘collapse’ of the scratchpad and a loss of signal when it no longer binds its corresponding smFISH probe. A successor method, intMEMOIR, dispenses with Cas9 entirely and instead uses the serine integrase Bxb1 to irreversibly recombine a recorder sequence from an initial state to one of two distinct edited states14. This element therefore comprises a trinary memory element, or ‘trit’. intMEMOIR features an array of 10 trits, whose states can be imaged via independent smFISH probe barcodes. intMEMOIR was deployed in Drosophila to trace the lineage of neuronal cell types to their progenitor neuroblast cells. In both flavors of MEMOIR, spatial information comes at the price of transcriptomic depth, as smFISH cannot yet achieve full transcriptomic depth at scale.
2.2.2. “Tickertape” recorders link signaling states to long-lived responses
An ideal molecular recorder would not just integrate a pathway’s activity over time but would leave a time-resolved history of signaling states. Proof of concept of such a ‘tickertape’ recorder was recently demonstrated using CRISPR-Cas9 prime editing technology to insert targeted barcode sequences into a recording locus71. A prime editor is a fusion protein comprising a reverse transcriptase and a Cas9 nickase (a Cas9 with one catalytic site deactivated). Prime editing guide RNAS (pegRNAs) are also specialized to include an additional template sequence adjacent to the targeting protospacer sequence. When the Cas9 nicks the target site, the reverse transcripatase appends the template pegRNA sequence next to the targeting sequence within the host genome.
In a recent preprint, Choi, Chen, and colleagues adapted prime editing to realize a tickertape recorder by designing a recording locus that sequential orders prime edits72 (Figure 2C, upper). Specifically, it features a repeated array of 5’ truncated protospacer target sites, where only the first site has the complete protospacer sequence. The pegRNAs are designed such that upon prime editing, the reverse transcriptase inserts a template barcode, disrupts the 3’ end of the current target site, and completes the 5’ end of the subsequent site. The net effect is therefore to record the active pegRNA via its barcode at the current site shift and shift the tickertape ‘write-head’ to the following site. The sequence of barcodes therefore encodes the order in which they were inserted. Choi, Chen, et al validated this technique using serial transfections in cell culture, although the resulting tickertape recording was only revealed in averaged measurements from many cells, presumably due to the current inefficiency of prime editing. Future improvements are likely necessary to provide tickertapes with single-cell resolution for developmental applications.
Inferring signaling dynamics from such a tickertape further requires that the availability of barcoded pegRNAs somehow depends on the cell’s signaling state. In a simultaneous effort, the same group reported ENGRAM: a technology for parallel recording of signaling pathway activity using prime editing73. ENGRAM uses the endoribonuclease Csy4 to excise barcoded pegRNAs from the 3’ UTR of synthetic mRNAs74. By placing the host mRNAs under the control of pathway-specific sentinel promoters, barcode insertions at the recording locus can be linked to their corresponding pathway. To validate ENGRAM, the authors simultaneously recorded barcodes corresponding to Wnt, NF-κB, and Tet-On responsive promoters.
In principle, ENGRAM is compatible with a sequential tickertape recorder, opening the possibility of encoding a complete history of a cell’s signaling dynamics directly in DNA. However, the low editing efficiency of existing prime editors is an important technical barrier. ENGRAM labeled less than 5% of cells in validation studies after 48 hours at saturating doses of Wnt or NF-κB; tracing developmentally relevant signals will require higher fidelity and temporal resolution. Sequence-reconstructions of tickertape events also required ensemble averaging over populations of cells. This may not be possible in a developmental context where it can be difficult to know which subpopulations should be averaged. In sum, while the remarkable information bandwidth of DNA-based tickertapes makes them attractive, a next-generation of improved prime editors is likely needed to unlock this strategy’s full potential.
Might it be possible to record a cell’s history in other polymers than nucleic acids? Two groups recently reported development of protein-based tickertapes to encode the history of neuronal activity within a growing protein fiber (Figure 2C, lower). Lin, Li, and collaborators used a fusion protein of the kinase domain of Pak4 and its inhibitor Inka1 (together, iPAK4). iPAK4 polymerizes within cells into stable linear crystals with internal pores that permit inclusion of fluorescent tags16. Alternating wash-ins of two different halo-tag dyes labels the growing fiber with stripes, creating a temporal basis. A GFP-iPAK4 fusion is placed under the cFos immediate earlier gene promoter, so that neuronal activity is recorded by inclusion of GFP within the growing fiber. Imaging the iPAK4 ticker tape in cultured cells after a 12-hour recording period was able to resolve signaling dynamics with sub-hour temporal resolution. Linghu and collaborators simultaneously developed a protein ticker using the same basic principle (cFos-dependent incorporation of labeled monomers into a growing protein chain), but a different chemistry17. Their ticker tape comprised of 1POK monomers fused to ‘insulating’ maltose binding protein (MBP) domains (which prevent lateral aggregation), and short epitope tags which are resolved by immunohistochemistry. Their 1POK ‘expression recording islands’ (XRIs) demonstrated longer recordings than iPAK4, at the cost of lower temporal resolution. In principle, both protein fiber tickertapes could be adapted to record developmental signals by expressing labeled monomers under signal-responsive promoters. Recorder toxicity might post a challenge: while protein tickertapes did not disrupt neural signaling, they may be more problematic when embedded in mitotically active, migratory cells of the embryo.
2.2.3. “Readers” for detecting dynamic and combinatorial cellular signals
What synthetic circuits could endow a cell to read a specific signaling state of interest? The simplest case is a classical pathway-specific promoter, which can highlight whether a single pathway is on in a particular cell at a particular time. Yet more sophisticated circuits could be quite useful. Combinatorial circuits could selectively label cells in which multiple pathways are active simultaneously75, and dynamic circuits could define subpopulations that experience transient pulses of signals76. An ideal signaling reader would be specific to a desired stimulus condition, and would detect stimuli only during a user-defined developmental time period.
Implementing this kind of signal detection is precisely where synthetic biology excels. One may use a gene network that acts as a logic gate to define which cells experience a particular combination of developmental cues (e.g., an AND gate that triggers gene expression only in the presence of signals A and B). Synthetic logic gates have been produced using a variety of biological components, including combinations of engineered transcription factors77, RNA-based logic78, or programmable DNA binding domains termed transcription activator-like effectors (TALEs)79. Even more complex logic functions have been constructed using sets of orthogonal recombinases in the so-called BLADE system80 (Figure 2D, upper). These circuits work by excising or inverting sections of DNA at specific sites, so that only the presence of specific combinations of recombinases would enable expression of specific gene programs. Eight orthogonal pairs of recombinases and DNA elements were identified and used to construct more than 100 logic functions that selectively respond to states of up to three distinct inputs. An important caveat is that these circuits are typically single use, since any recombinase would typically rearrange genetic elements in the circuit, altering its initial state. (The permanent DNA modification produced by recombinase-based logic also blurs the line between reader and recorder circuits.) Nevertheless, the logic gates described above make it possible for an experimentalist to define virtually any logical cellular state (e.g., cell in which pathway A and B are on but C is off) and, with high fidelity, label only the cells in that state.
Synthetic gene circuits can also be implemented to specifically detect the dynamics of a signal. Dynamic-sensitive gene circuits build on decades of work cataloguing ‘network motifs’ – the signal processing functions conferred by modules of a small number of interacting biochemical systems81. Feedforward loops, a subset of network motifs that contain combinations of fast and slow (or direct and indirect) regulatory links, were predicted to be especially sensitive to the dynamics of a signal82. Based on this logic, Ravindran et al. screened a small library of feedforward networks for the ability to selectively detect signaling pulses, identifying one incoherent feedforward circuit with robust pulse-detecting capabilities76 (Figure 2D, lower). The authors went on to implement this circuit experimentally for the Erk signaling pathway using a single synthetic target gene cassette: an Erk pathway-responsive promoter83 driving the expression of a chimeric transcription factor fused to an Erk-triggered nuclear export tag (the ‘kinase translocation reporter’, or KTR)84. Only upon a rise in Erk activity is the chimeric transcription factor expressed; a subsequent fall of Erk activity is necessary to trigger its nuclear import and subsequent expression of a fluorescent reporter. The authors demonstrated that Erk pulses, but not high or low activity states, were capable of triggering gene expression, opening the door to selectively defining the subpopulation of cells exhibiting Erk pulses without requiring live-cell imaging or tracking, a simple workflow for developmental studies.
Despite these exciting proofs of principle, few studies have yet connected any of the reader and recorder systems in a functional developmental context. We eagerly await the work of a next generation of developmental synthetic biologists interested in bridging the timescales of signaling and cell fate response using molecular readers and recorders.
4. Reconstituting the emergent properties of developmental systems
In its purest form, synthetic biology proposes to build complex biological functions from the bottom up. Like classical in vitro biochemical reconstitution, which has been instrumental in defining how minimal biochemical circuits can give rise to emergent properties ranging from stable oscillations to traveling waves85,86, synthetic approaches offer the chance to define how tissues might generate gradients, self-organize, polarize, and adopt long-lived differentiated states using minimal sets of well-defined components. Synthetic reconstitution can also enable the experimentalist to monitor how emergent properties are altered when system parameters (e.g., the concentrations or affinities of components) are systematically altered, which can be essential understanding both the functions and limitations of a biochemical or genetic network.
Pioneering efforts in synthetic biology were focused at the cellular scale, first in bacteria2,87 and subsequently in mammalian cells88. In recent years, an explosion of interest in synthetic multicellularity and pattern formation has brought engineered networks to simple multicellular contexts and even classical developmental model systems. Here, we highlight examples in which these tools have been used to engineer developmental processes and functions in eukaryotic cells.
4.1. Synthetic morphogen gradients
The question of how cells obtain information about their physical location in an embryo remains one of the central questions of developmental biology89. A major organizing concept has been the idea of a morphogen90 – a substance (e.g., small molecule or protein ligand) that diffuses across a field of cells to alter the behavior of cells that sense it. In their simplest form, morphogen gradients can be generated by a sender cell releasing a soluble factor that diffuses over the length of multiple cells, with receiver cells sensing this factor and triggering concentration-dependent responses. However, the situation is often complicated by regulatory feedbacks. Morphogens may not freely diffuse but instead interact with other molecules such as inhibitors, shuttling proteins, or coreceptors91. Morphogen gradients are also further modified by positive or negative feedback loops triggered in the cells that receive them, resulting in increased or decreased expression of receptors, ligands, or other system components. Due to their central role in embryo patterning, morphogen gradients have been a major recent testbed for synthetic developmental approaches.
Initial success in reconstituting morphogen signaling was obtained using cell culture models. Li and colleagues reconstituted Sonic Hedgehog (Shh) gradients in an engineered cell culture model92. The authors generated a “sender” cell line in which ligand production could be induced and a “receiver” cell line which produces a fluorescent reporter upon ligand detection and pathway activation. Even though the diffusible ligand was not itself labeled, receiver-cell measurements and cell patterning experiments revealed that Shh gradient formation required cell-cell contact and is likely to occur via lateral movement in the cell layer as opposed to free bulk diffusion. Furthermore, varying the ligand production rate and the receiver cell network architecture revealed mechanisms that ensure the pathway’s robustness to ligand levels and rapid progression to a steady-state gradient. These insights complement studies of how signaling feedbacks in vivo can confer robustness93,94 and scale-invariance95 to morphogen patterns.
Whereas Li et al reconstituted a morphogen gradient out of natural components, another approach is to build fully synthetic morphogen/receptor systems. The SynNotch system7 presents an exemplary design strategy. In Notch signaling, activation of the receptor by a surface-presented ligand on a neighboring cell triggers cleavage and release of an intracellular domain, which then acts as a transcriptional activator. SynNotch adapts this architecture to instead release a synthetic transcriptional activator (e.g. Gal4, rtTA) in response to an engineered ligand-receptor interaction (e.g. GFP/anti-GFP nanobody). Toda et al adapted SynNotch for the detection of diffusible ligands by capturing the ligand with a membrane-bound anchor on neighboring cells. While this two-step binding mode might be seen as an inconvenience of the synthetic system, it likely improved performance, as weak surface tethering of ligands has been found to be essential for proper gradient formation both in synthetic and natural systems8,96. Indeed, tuning the number of anchors present in the cell population was sufficient to modify the range of the established gradients. Using two orthogonal SynNotch with cognate ligands and anchors – and implementing positive feedback via SynNotch induced expression of more ligand and negative feedback via secretion of inhibitors – the authors could increase the complexity of the synthetic morphogen network.
While a pure in vitro reconstitution can probe quantitative aspects of morphogen patterns, it cannot directly address the biological implications of pattern features. Can a synthetic morphogen system functionally compensate for the loss of an endogenous pattern to coordinate tissue morphogenesis? This question was resoundingly addressed by a recent study to engineer synthetic replacement of the Dpp morphogen gradient in the Drosophila wing disc96 (the fly homolog of vertebrate BMP ligands). Stapornwongkul and colleagues designed a synthetic morphogen system comprising a diffusible GFP dimer ‘morphogen’, and a synthetic receptor pair which senses GFP via extracellular anti-GFP nanobody. Because the synthetic receptor pair further carries intracellular copies of Dpp receptor domains (one with tkv, another with put), they initiate Dpp signaling upon heterodimerization in the presence of a GFP morphogen. The authors regulated protein clearance by adding a membrane-tethered anti-GFP nanobody, thereby creating a detectable GFP dimer gradient. Replacing the endogenous Dpp pathway with this synthetic GFP morphogen system was sufficient to rescue the formation of recognizable wing structures. Tuning the affinity of the nanobody co-receptors, their expression levels, and their ability to laterally diffuse on the membrane (e.g., using a transmembrane domain versus GPI anchor protein) allowed the authors to refine the system such that the resulting GFP gradient was sufficient to induce wildtype-like target gene expression and wing morphology.
4.2. Synthetic circuits for cell sorting and tissue self-organization
While diffusible ligands can establish the coordinate axes of a developmental model system, much of the actual structural shaping of the embryo requires self-organized changes in cellular properties: cell shape, cell-cell adhesion, mechanical forces, and cell movement97. Synthetic developmental biologists have begun to mold their own self-organizing tissues by combining synthetic contact-dependent signaling with response programs that themselves regulate cell-cell interactions. Signaling thus drives physical changes that further alter signaling, forming feedback circuits that result in emergent tissue-scale properties.
The SynNotch synthetic contact-dependent signaling system proved to be instrumental as a platform for programming tissue-scale patterning7. These receptors have two primary advantages. First, signaling only proceeds at direct cell-cell contacts, avoiding undesired interactions between secreting and sensing cells over long distances. Second, SynNotch receptors directly link a cell-cell contact event to a transcriptional response, so cells may be engineered to express multiple SynNotch systems without crosstalk between any shared components (assuming distinct ligand recognition and transcription factors). A network of SynNotch systems was thus used to produce concentric rings of gene expression responses in 2D cultured cells, seeded by a central group of GFP-expressing cells to first initiate SynNotch activation (Figure 3B).
Figure 3. Synthetic reconstitution of developmental processes.

(a) Synthetic morphogen gradients can be constructed by placing ‘sender’ (morphogen secreting) and ‘receiver’ (receptor-expressing) cells nearby one another. Recent evidence suggests that simple diffusion-based transport of soluble ligands is unlikely to be sufficient in many cases, and surface transport plays a key role in gradient formation. Engineered, surface associated GFP gradients can rescue pattern formation in the wing. (b) Synthetic cell-cell communication and differential adhesion can drive pattern formation in simple ‘tissues’ composed of engineered cells. SynNotch receptors that link specific cell-cell interactions to expression of homotypic adhesion molecules can drive patterns of gene expression in 2D and cell sorting and patterning in 3D. (c) Synthetic multi-stable gene circuits exhibit long-term memory and the potential to implement complex differentiation landscapes. Simple gene circuits containing 2 or 3 transcription factors produced up to 3 or 7 stable gene expression states, respectively, that persisted over many cell generations.
A follow-up study went still further, extending synthetic tissue morphogenesis to a 3-dimensional context and avoiding the requirement for pattern seeding8. The authors placed the expression of different sets of cadherins under the control of SynNotch-based signaling circuits, hypothesizing that the resulting changes in cell-cell adhesion would enable cells to sort into distinct self-organized patterns (Figure 3B). This differential adhesion hypothesis is a classical model of multicellular self-organization, and prior reconstitution studies have shown cell sorting in response to differential cadherin expression98,99; the study was the first to use synthetic signaling-based control to achieve programmable self-organization. Indeed, connecting two engineered “cell types” (defined by different SynNotch receptors linked to distinct cell-cell adhesion molecules) enabled the generation of tissue spheroids with two- or three-layered spatial organization. Moreover, a circuit resembling the lateral inhibition of the original endogenous Notch/Delta system (i.e. a double-negative feedback loop) was able to produce multilayered and asymmetric spheroid structures starting from a single cell type100. The resulting synthetic tissues exhibited true self-organization capabilities ranging from spontaneous symmetry breaking and pattern formation to self-healing upon mechanical splitting.
4.3. Synthetic cell fate determination
One major consequence of developmental signaling is to trigger appropriate cell fate choices. But how do cells achieve irreversible commitment to a particular fate? Here, again, synthetic biology is poised to produce insights. The ability to toggle between stable gene expression states is a classic challenge in synthetic biology2, belying a long history of interest in how to achieve stable acquisition of a gene expression program. More recently, studies have established synthetic control over epigenetic modification to produce heritable expression states in mammalian cells101–105. For example, Park et al engineered mammalian cells to use an orthogonal form of epigenetic modification, N6-methyladenine (m6A). Cells expressing synthetic factors that read and wrote m6A modification could produce stable epigenetic memory and gene expression control.
Yet despite these impressive capabilities, a gap has remained between the simple picture afforded by two-state synthetic gene circuits (i.e., a two-state paradigm where a gene may be either on or off) and the potentially richer landscape of cell states that is traversed during development. For example, a single population of pluripotent cells often gives rise to more than two distinct fates, suggesting the existence of multi-stable circuits with more than two possible states.
One recent study suggests a first bridge between the simple synthetic picture and the more complex landscape of developmental cell fate transitions105 (Figure 3C). Taking inspiration from classical developmental transcription factors (e.g., Sox2, Oct4, and Sox17), the authors observed that developmental transcription factors are often connected in autoregulatory feedback loops, but that homo- and hetero-dimers formed between these transcription factors often exert different – or even opposing – effects on their target genes. To explore the possible consequences of these forms of regulation, the authors engineered synthetic zinc finger transcription factors that could homo- and heterodimerize as well as regulate their own expression. The authors found that seven distinct stable cell states could be produced using only 3 of the synthetic transcription factors, and that varying the initial concentration or stability of each transcription factor could predictably alter the number of stable states. The wealth of tools and predictive control afforded by modern synthetic transcriptional memory systems suggests that reconstitution or re-engineering of developmental cell fates in vivo may soon be within reach.
5. Summary and outlook
We have laid out a few directions where advances in synthetic biology and cell engineering are already beginning to make an impact in developmental model systems. These areas include (1) synthetic interfaces for delivering cues to cells of interest and measuring their responses and (2) synthetic circuits for producing morphogen gradients, stable cell fate decisions, and tissue self-organization. These are by no means an exhaustive list! There are many other developmental questions that could be addressed using engineering principles (e.g., spontaneous symmetry breaking and polarization along a single body axis)106,107. We chose to highlight examples that are particularly mature – where the synthetic tools are already well described and the applications to development are clear.
Nevertheless, our reader will likely realize that we have highlighted very few studies in which synthetic tools were already applied to a developmental model system. Many of the case studies described here fall one step short: they rely on toy models such as immortalized cell lines that are argued to function in analogy to developmental systems. We predict that this line will continue to blur; as synthetic approaches are increasingly applied in vivo or in stem-cell derived micropatterns108, organoids109, or gastruloids106, we will learn more about the sufficiency of synthetic tools to recapitulate true developmental processes.
We have described many synthetic approaches that can perform developmentally relevant functions in isolation: secreting a localized morphogen, recording a transient signaling state, or forming a tissue-scale pattern. We predict that future studies will begin to combine these modules to achieve more complex developmental outcomes. One may envision linking patterning and cell fate circuits to define the spatial organization of functional cell types or combining optogenetic stimulation with a morphogen module to polarize development along a user-defined body axis. Experimental feedback control is also a powerful technique for shaping and controlling the response of biological systems110–112; when coupled to optogenetic control in a developmental model system, feedback control over light inputs could enable a new generation of precision tools for manipulating morphogenesis and tissue patterning. The future is bright for synthetic developmental biology.
Funding Sources:
This work was supported by NSF grant 1750663 and NIH Grant U01DK127429 (to J.E.T.), the Lewis Sigler Scholars program (to H.M.), and the NSF Center for the Physics of Biological Function PHY-1734030 (to B.R.).
References
- 1.Elowitz MB & Leibler S A synthetic oscillatory network of transcriptional regulators. Nature 403, 335–338 (2000). [DOI] [PubMed] [Google Scholar]
- 2.Gardner TS, Cantor CR & Collins JJ Construction of a genetic toggle switch in Escherichia coli. Nature 403, 339–342 (2000). [DOI] [PubMed] [Google Scholar]
- 3.Ng AH et al. Modular and tunable biological feedback control using a de novo protein switch. Nature 572, 265–269 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Gao XJ, Chong LS, Kim MS & Elowitz MB Programmable protein circuits in living cells. Science 361, 1252–1258 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manhas J, Edelstein HI, Leonard JN & Morsut L The evolution of synthetic receptor systems. Nat. Chem. Biol 1–12 (2022) doi: 10.1038/s41589-021-00926-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daringer NM, Dudek RM, Schwarz KA & Leonard JN Modular Extracellular Sensor Architecture for Engineering Mammalian Cell-based Devices. ACS Synth. Biol 3, 892–902 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morsut L et al. Engineering Customized Cell Sensing and Response Behaviors Using Synthetic Notch Receptors. Cell 164, 780–791 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toda S, Blauch LR, Tang SKY, Morsut L & Lim WA Programming self-organizing multicellular structures with synthetic cell-cell signaling. Science 361, 156–162 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu YI et al. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature 461, 104–8 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levskaya A, Weiner OD, Lim WA & Voigt CA Spatiotemporal control of cell signalling using a light-switchable protein interaction. Nature 461, 997–1001 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kennedy MJ et al. Rapid blue-light-mediated induction of protein interactions in living cells. Nat Methods 7, 973–5 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raj B et al. Simultaneous single-cell profiling of lineages and cell types in the vertebrate brain. Nat. Biotechnol 36, 442–450 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan MM et al. Molecular recording of mammalian embryogenesis. Nature 570, 77–82 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chow KK et al. Imaging cell lineage with a synthetic digital recording system. Science 372, (2021). [DOI] [PubMed] [Google Scholar]
- 15.Frieda KL et al. Synthetic recording and in situ readout of lineage information in single cells. Nature 541, 107–111 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin D et al. Time-tagged ticker tapes for intracellular recordings. 2021.10.13.463862 (2021) doi: 10.1101/2021.10.13.463862. [DOI] [PMC free article] [PubMed]
- 17.Linghu C et al. Recording of cellular physiological histories along optically readable self-assembling protein chains. 2021.10.13.464006 (2021) doi: 10.1101/2021.10.13.464006. [DOI] [PMC free article] [PubMed]
- 18.Sako K et al. Optogenetic Control of Nodal Signaling Reveals a Temporal Pattern of Nodal Signaling Regulating Cell Fate Specification during Gastrulation. Cell Rep 16, 866–77 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Guglielmi G, Barry JD, Huber W & De Renzis S An Optogenetic Method to Modulate Cell Contractility during Tissue Morphogenesis. Dev Cell 35, 646–60 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guglielmi G, Falk HJ & De Renzis S Optogenetic Control of Protein Function: From Intracellular Processes to Tissue Morphogenesis. Trends Cell Biol 26, 864–874 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson HE & Toettcher JE Illuminating developmental biology with cellular optogenetics. Curr Opin Biotechnol 52, 42–48 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buckley CE et al. Reversible Optogenetic Control of Subcellular Protein Localization in a Live Vertebrate Embryo. Dev Cell 36, 117–126 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farahani PE, Reed EH, Underhill EJ, Aoki K & Toettcher JE Signaling, Deconstructed: Using Optogenetics to Dissect and Direct Information Flow in Biological Systems. Annu Rev Biomed Eng (2021) doi: 10.1146/annurev-bioeng-083120-111648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Repina NA, Rosenbloom A, Mukherjee A, Schaffer DV & Kane RS At Light Speed: Advances in Optogenetic Systems for Regulating Cell Signaling and Behavior. Annu Rev Chem Biomol Eng 8, 13–39 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolar K, Knobloch C, Stork H, Znidaric M & Weber W OptoBase: A Web Platform for Molecular Optogenetics. ACS Synth Biol 7, 1825–1828 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Rogers KW, ElGamacy M, Jordan BM & Muller P Optogenetic investigation of BMP target gene expression diversity. Elife 9, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim N et al. Spatiotemporal control of fibroblast growth factor receptor signals by blue light. Chem Biol 21, 903–12 (2014). [DOI] [PubMed] [Google Scholar]
- 28.Dine E, Gil AA, Uribe G, Brangwynne CP & Toettcher JE Protein Phase Separation Provides Long-Term Memory of Transient Spatial Stimuli. Cell Syst 6, 655–663 e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grusch M et al. Spatio-temporally precise activation of engineered receptor tyrosine kinases by light. EMBO J 33, 1713–26 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson HE et al. The Spatiotemporal Limits of Developmental Erk Signaling. Dev Cell 40, 185–192 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson HE & Toettcher JE Signaling Dynamics Control Cell Fate in the Early Drosophila Embryo. Dev Cell 48, 361–370 e3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson HE, Djabrayan NJV, Shvartsman SY & Toettcher JE Optogenetic Rescue of a Patterning Mutant. Curr Biol 30, 3414–3424 e3 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenbloom AB et al. beta-Catenin signaling dynamics regulate cell fate in differentiating neural stem cells. Proc Natl Acad Sci U A 117, 28828–28837 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Repina NA et al. Optogenetic control of Wnt signaling for modeling early embryogenic patterning with human pluripotent stem cells. bioRxiv 665695 (2019) doi: 10.1101/665695. [DOI] [Google Scholar]
- 35.Viswanathan R et al. Optogenetic inhibition of Delta reveals digital Notch signalling output during tissue differentiation. EMBO Rep 20, e47999 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Viswanathan R, Hartmann J, Pallares Cartes C & De Renzis S Desensitisation of Notch signalling through dynamic adaptation in the nucleus. EMBO J 40, e107245 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitra D, Yang X & Moffat K Crystal Structures of Aureochrome1 LOV Suggest New Design Strategies for Optogenetics. Structure 20, 698–706 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X, Chen X & Yang Y Spatiotemporal control of gene expression by a light-switchable transgene system. Nat Methods 9, 266–9 (2012). [DOI] [PubMed] [Google Scholar]
- 39.Benedetti L et al. Optimized Vivid-derived Magnets photodimerizers for subcellular optogenetics in mammalian cells. Elife 9, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taslimi A et al. An optimized optogenetic clustering tool for probing protein interaction and function. Nat Commun 5, 4925 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bugaj LJ, Choksi AT, Mesuda CK, Kane RS & Schaffer DV Optogenetic protein clustering and signaling activation in mammalian cells. Nat Methods 10, 249–52 (2013). [DOI] [PubMed] [Google Scholar]
- 42.Brand AH & Perrimon N Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401–15 (1993). [DOI] [PubMed] [Google Scholar]
- 43.Sokolik C et al. Transcription factor competition allows embryonic stem cells to distinguish authentic signals from noise. Cell Syst 1, 117–129 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.di Pietro F et al. Rapid and robust optogenetic control of gene expression in Drosophila. Dev Cell 56, 3393–3404 e7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kawano F, Suzuki H, Furuya A & Sato M Engineered pairs of distinct photoswitches for optogenetic control of cellular proteins. Nat. Commun 6, 6256 (2015). [DOI] [PubMed] [Google Scholar]
- 46.Zoltowski BD, Motta-Mena LB & Gardner KH Blue light-induced dimerization of a bacterial LOV-HTH DNA-binding protein. Biochemistry 52, 6653–61 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reade A et al. TAEL: a zebrafish-optimized optogenetic gene expression system with fine spatial and temporal control. Development 144, 345–355 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.LaBelle J et al. TAEL 2.0: An Improved Optogenetic Expression System for Zebrafish. Zebrafish 18, 20–28 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Motta-Mena LB et al. An optogenetic gene expression system with rapid activation and deactivation kinetics. Nat Chem Biol 10, 196–202 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Niopek D, Wehler P, Roensch J, Eils R & Di Ventura B Optogenetic control of nuclear protein export. Nat Commun 7, 10624 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yumerefendi H et al. Control of Protein Activity and Cell Fate Specification via Light-Mediated Nuclear Translocation. PLoS One 10, e0128443 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Niopek D et al. Engineering light-inducible nuclear localization signals for precise spatiotemporal control of protein dynamics in living cells. Nat Commun 5, 4404 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dowbaj AM et al. An optogenetic method for interrogating YAP1 and TAZ nuclear-cytoplasmic shuttling. J Cell Sci 134, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singh AP et al. Optogenetic control of the Bicoid morphogen reveals fast and slow modes of gap gene regulation. bioRxiv 2021.10.13.464280 (2021) doi: 10.1101/2021.10.13.464280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kogler AC et al. Extremely rapid and reversible optogenetic perturbation of nuclear proteins in living embryos. Dev Cell 56, 2348–2363 e8 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang A, Amourda C, Zhang S, Tolwinski NS & Saunders TE Decoding temporal interpretation of the morphogen Bicoid in the early Drosophila embryo. Elife 6, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McDaniel SL et al. Continued Activity of the Pioneer Factor Zelda Is Required to Drive Zygotic Genome Activation. Mol Cell 74, 185–195 e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patel AL et al. Optimizing photoswitchable MEK. Proc Natl Acad Sci U A 116, 25756–25763 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garcia HG, Tikhonov M, Lin A & Gregor T Quantitative imaging of transcription in living Drosophila embryos links polymerase activity to patterning. Curr Biol 23, 2140–5 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McFann S, Dutta S, Toettcher JE & Shvartsman SY Temporal integration of inductive cues on the way to gastrulation. Proc Natl Acad Sci U A 118, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Izquierdo E, Quinkler T & De Renzis S Guided morphogenesis through optogenetic activation of Rho signalling during early Drosophila embryogenesis. Nat. Commun 9, 2366 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rich A, Fehon RG & Glotzer M Rho1 activation recapitulates early gastrulation events in the ventral, but not dorsal, epithelium of Drosophila embryos. eLife 9, e56893 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sui L et al. Differential lateral and basal tension drive folding of Drosophila wing discs through two distinct mechanisms. Nat. Commun 9, 4620 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schüpbach T & Wieschaus E Maternal-effect mutations altering the anterior-posterior pattern of the Drosophila embryo. Rouxs Arch. Dev. Biol 195, 302–317 (1986). [DOI] [PubMed] [Google Scholar]
- 65.Coppey M, Boettiger AN, Berezhkovskii AM & Shvartsman SY Nuclear Trapping Shapes the Terminal Gradient in the Drosophila Embryo. Curr. Biol 18, 915–919 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tang F et al. mRNA-Seq whole-transcriptome analysis of a single cell. Nat. Methods 6, 377–382 (2009). [DOI] [PubMed] [Google Scholar]
- 67.Chen KH, Boettiger AN, Moffitt JR, Wang S & Zhuang X Spatially resolved, highly multiplexed RNA profiling in single cells. Science 348, aaa6090 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Slide-seq: A scalable technology for measuring genome-wide expression at high spatial resolution. 10.1126/science.aaw1219. [DOI] [PMC free article] [PubMed]
- 69.McKenna A et al. Whole-organism lineage tracing by combinatorial and cumulative genome editing. Science 353, aaf7907 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leeper K et al. Lineage barcoding in mice with homing CRISPR. Nat. Protoc 16, 2088–2108 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Anzalone AV et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 576, 149–157 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Choi J et al. A temporally resolved, multiplex molecular recorder based on sequential genome editing. 2021.11.05.467388 (2021) doi: 10.1101/2021.11.05.467388. [DOI]
- 73.Chen W et al. Multiplex genomic recording of enhancer and signal transduction activity in mammalian cells. 2021.11.05.467434 (2021) doi: 10.1101/2021.11.05.467434. [DOI]
- 74.Liu Y et al. Enhancing prime editing by Csy4-mediated processing of pegRNA. Cell Res. 31, 1134–1136 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sonnen KF et al. Modulation of Phase Shift between Wnt and Notch Signaling Oscillations Controls Mesoderm Segmentation. Cell 172, 1079–1090.e12 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ravindran PT, McFann S, Thornton RH & Toettcher JE A synthetic gene circuit for imaging-free detection of signaling pulses. Cell Syst. 13, 131–142.e13 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bashor CJ et al. Complex signal processing in synthetic gene circuits using cooperative regulatory assemblies. Science 364, 593–597 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Win MN & Smolke CD Higher-Order Cellular Information Processing with Synthetic RNA Devices. Science 322, 456–460 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gaber R et al. Designable DNA-binding domains enable construction of logic circuits in mammalian cells. Nat. Chem. Biol 10, 203–208 (2014). [DOI] [PubMed] [Google Scholar]
- 80.Weinberg BH et al. Large-scale design of robust genetic circuits with multiple inputs and outputs for mammalian cells. Nat. Biotechnol 35, 453–462 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alon U Network motifs: theory and experimental approaches. Nat Rev Genet 8, 450–61 (2007). [DOI] [PubMed] [Google Scholar]
- 82.Mangan S & Alon U Structure and function of the feed-forward loop network motif. Proc Natl Acad Sci U A 100, 11980–5 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ravindran PT, Wilson MZ, Jena SG & Toettcher JE Engineering combinatorial and dynamic decoders using synthetic immediate-early genes. Commun Biol 3, 436 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Regot S, Hughey JJ, Bajar BT, Carrasco S & Covert MW High-sensitivity measurements of multiple kinase activities in live single cells. Cell 157, 1724–34 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Reconstitution of Circadian Oscillation of Cyanobacterial KaiC Phosphorylation in Vitro. 10.1126/science.1108451. [DOI] [PubMed]
- 86.Ramm B, Heermann T & Schwille P The E. coli MinCDE system in the regulation of protein patterns and gradients. Cell. Mol. Life Sci 76, 4245–4273 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Elowitz MB & Leibler S A synthetic oscillatory network of transcriptional regulators. Nature 403, 335–338 (2000). [DOI] [PubMed] [Google Scholar]
- 88.Tigges M, Marquez-Lago TT, Stelling J & Fussenegger M A tunable synthetic mammalian oscillator. Nature 457, 309–312 (2009). [DOI] [PubMed] [Google Scholar]
- 89.Lawrence PA Morphogens: how big is the big picture? Nat. Cell Biol 3, E151–E154 (2001). [DOI] [PubMed] [Google Scholar]
- 90.Turing AM The chemical basis of morphogenesis. Philos. Trans. R. Soc. Lond. B. Biol. Sci 237, 37–72 (1952). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rogers KW & Schier AF Morphogen Gradients: From Generation to Interpretation. Annu. Rev. Cell Dev. Biol 27, 377–407 (2011). [DOI] [PubMed] [Google Scholar]
- 92.Li P et al. Morphogen gradient reconstitution reveals Hedgehog pathway design principles. Science 360, 543–548 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rogers KW et al. Nodal patterning without Lefty inhibitory feedback is functional but fragile. eLife 6, e28785 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lord ND, Carte AN, Abitua PB & Schier AF The pattern of nodal morphogen signaling is shaped by co-receptor expression. eLife 10, e54894 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Almuedo-Castillo M et al. Scale-invariant patterning by size-dependent inhibition of Nodal signalling. Nat. Cell Biol 20, 1032–1042 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stapornwongkul KS, de Gennes M, Cocconi L, Salbreux G & Vincent J-P Patterning and growth control in vivo by an engineered GFP gradient. Science 370, 321–327 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gilmour D, Rembold M & Leptin M From morphogen to morphogenesis and back. Nature 541, 311–320 (2017). [DOI] [PubMed] [Google Scholar]
- 98.Duguay D, Foty RA & Steinberg MS Cadherin-mediated cell adhesion and tissue segregation: qualitative and quantitative determinants. Dev. Biol 253, 309–323 (2003). [DOI] [PubMed] [Google Scholar]
- 99.Steinberg MS & Takeichi M Experimental specification of cell sorting, tissue spreading, and specific spatial patterning by quantitative differences in cadherin expression. Proc. Natl. Acad. Sci 91, 206–209 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Matsuda M, Koga M, Woltjen K, Nishida E & Ebisuya M Synthetic lateral inhibition governs cell-type bifurcation with robust ratios. Nat. Commun 6, 6195 (2015). [DOI] [PubMed] [Google Scholar]
- 101.Bintu L et al. Dynamics of epigenetic regulation at the single-cell level. Science 351, 720–724 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Van MV, Fujimori T & Bintu L Nanobody-mediated control of gene expression and epigenetic memory. Nat. Commun 12, 537 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nuñez JK et al. Genome-wide programmable transcriptional memory by CRISPR-based epigenome editing. Cell 184, 2503–2519.e17 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Park M, Patel N, Keung AJ & Khalil AS Engineering Epigenetic Regulation Using Synthetic Read-Write Modules. Cell 176, 227–238.e20 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhu R, del Rio-Salgado JM, Garcia-Ojalvo J & Elowitz MB Synthetic multistability in mammalian cells. Science 375, eabg9765. [DOI] [PubMed] [Google Scholar]
- 106.Beccari L et al. Multi-axial self-organization properties of mouse embryonic stem cells into gastruloids. Nature 562, 272–276 (2018). [DOI] [PubMed] [Google Scholar]
- 107.Chau AH, Walter JM, Gerardin J, Tang C & Lim WA Designing synthetic regulatory networks capable of self-organizing cell polarization. Cell 151, 320–32 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Warmflash A, Sorre B, Etoc F, Siggia ED & Brivanlou AH A method to recapitulate early embryonic spatial patterning in human embryonic stem cells. Nat. Methods 11, 847–854 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lancaster MA et al. Cerebral organoids model human brain development and microcephaly. Nature 501, 373–379 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Toettcher JE, Gong D, Lim WA & Weiner OD Light-based feedback for controlling intracellular signaling dynamics. Nat Methods 8, 837–9 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Milias-Argeitis A et al. In silico feedback for in vivo regulation of a gene expression circuit. Nat Biotechnol 29, 1114–6 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Harrigan P, Madhani HD & El-Samad H Real-Time Genetic Compensation Defines the Dynamic Demands of Feedback Control. Cell 175, 877–886.e10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]


