Abstract
Aims
Musculoskeletal infection is a devastating complication in both trauma and elective orthopaedic surgeries that can result in significant morbidity. Aim of this study was to assess the effectiveness and complications of local antibiotic impregnated dissolvable synthetic calcium sulphate beads (Stimulan Rapid Cure) in the hands of different surgeons from multiple centres in surgically managed bone and joint infections.
Methods
Between January 2019 and December 2022, 106 patients with bone and joint infections were treated by five surgeons in five hospitals. Surgical debridement and calcium sulphate bead insertion was performed for local elution of antibiotics in high concentration. In all, 100 patients were available for follow-up at regular intervals. Choice of antibiotic was tailor made for each patient in consultation with microbiologist based on the organism grown on culture and the sensitivity. In majority of our cases, we used a combination of vancomycin and culture sensitive heat stable antibiotic after a thorough debridement of the site. Primary wound closure was achieved in 99 patients and a split skin graft closure was done in one patient. Mean follow-up was 20 months (12 to 30).
Results
Overall, six out of 106 patients (5.6%) presented with sepsis and poorly controlled comorbid conditions, and died in the hospital within few days of index surgery. Out of the remaining 100 patients, control of infection was achieved in 95 patients (95%). Persistence of infection was noted in five (5%) patients. Out of these 95 patients that had good control of infection, four patients (4.2%) with gap nonunion needed Masquelet procedure to achieve union.
Conclusion
Our multicentre experience confirmed that surgical debridement along with calcium sulphate bead insertion was effective in treating bone and joint infections without any side effects and complications.
Cite this article: Bone Jt Open 2023;4(7):516–522.
Keywords: Stimulan, calcium sulphate beads, bone and joint infection, antibiotic delivery system, treatment of osteomyelitis, local antibiotics, joint infections, antibiotics, infections, surgical debridement, calcium sulphate, organisms, vancomycin, nonunion and bone, sepsis
Introduction
Musculoskeletal infection is a difficult problem and challenging condition to treat and achieve control. The mainstay of treatment is surgical debridement followed by procedures like Ilizarov and Masquelet techniques. All these techniques generally combine parenteral antibiotics in high doses and for prolonged duration, to achieve effective concentrations at the site of infection. This may result in systemic antibiotic toxicity. To reduce such problems, local antibiotic delivery systems were introduced.
The local delivery of antimicrobial agents at the infection site in orthopaedics is based on the need for high concentrations of these drugs to kill planktonic and bio-film based bacteria.1 Most of the debridements are intralesional and leave the resected surfaces exposed to the contaminating bacteria. The debris that remains in the wound includes small biofilm fragments and a few organisms that are usually unresponsive to antimicrobial agents in the given concentrations.1,2 Pharmacokinetic factors, including alterations in local tissue perfusion, make the local antibiotic delivery system as the best method of achieving an extremely high antimicrobial level.1,2
Various preparations for local antibiotic delivery are available to treat fracture related infections and in joint arthroplasties. The materials can be broadly grouped into polymethyl methacrylate (PMMA)-based and non-PMMA-based.1,2 The non-PMMA materials are further classified based on their composition into protein- and peptide-based, bone graft and graft substitutes, and synthetic polymers.1 Antibiotic eluting scaffolds have recently been classified into natural, synthetic, and composite materials for the treatment of osteomyelitis.2
We present our experience of using antibiotic impregnated dissolvable synthetic pure calcium sulphate beads (Stimulan Rapid Cure; Biocomposites, UK) for delivering high concentration of antibiotics at the site of infection.
Methods
This prospective multicentre study was conducted at five teaching hospitals (Yashoda Hospital, Apollo Hospital, KIMS Hospital, Care Hospitals, Banjara Hills, and Gachibowli, Hyderabad, India) after obtaining institutional ethical approvals and consent was obtained from all the patients. The study period was between January 2019 and December 2022.
Inclusion criteria
All patients with bone and joint infections that needed surgical intervention, and who may benefit from a local antibiotic delivery system, namely postoperative infections following closed and open fractures, spontaneous bone infections and native joint infections) were included in the study.
Exclusion criteria
All patients who had both calcium sulphate beads and PMMA, periprosthetic joint infections (PJIs), and diabetic foot infections were excluded.
Antibiotics were discontinued five days prior to definitive surgical treatment, if it was safe to do so. Preoperative pus swabs/joint aspirate samples were sent for culture and sensitivity. Intraoperative fluid and tissue samples were taken again in all the cases for culture and sensitivity. Antibiotic therapy for the perioperative period included second-generation cephalosporin (cefuroxime) and aminoglycoside (amikacin) that were given only after fluid and tissue samples were collected. They were continued till the culture results were available.
All the procedures were performed by experienced surgeons (PM, SRN, VPG, SK, SKG) from the five teaching hospitals. Thorough surgical debridement along with decompression or sequestrectomy and saucerization was performed as needed. Copious lavage with normal saline was performed in all cases. Pulse lavage was used in majority of the patients. All the implants (plates, screws, and nails) were removed in postoperative patients, except in four patients with acute postoperative infection (within six weeks). For the local delivery, broad spectrum antibiotics like vancomycin and gentamicin were used when the organism/sensitivity was not known. Appropriate antibiotics along with vancomycin were mixed for the preparation of calcium sulphate beads when the organism and sensitivities were known. Wounds were closed primarily after achieving haemostasis in 99 patients, and one patient needed a split skin graft. Closed suction drain was not used. Postoperatively, for patients who grew organisms that were sensitive to local antibiotics, intravenous antibiotics were discontinued once cultures were available. In patients in whom the organism was not sensitive to the antibiotic inserted in calcium sulphate beads, IV antibiotics were continued for six weeks. All the patients had clinical and radiological follow-up at regular intervals.
Results
In all, 106 patients had calcium sulphate bead insertion for controlling/treating infection in our study. A total of six patients (5.6%) who died of sepsis in the immediate postoperative period were excluded, as there was no possibility of knowing the effectiveness of local antibiotics without long term follow-up, while 100 patients were followed-up at regular intervals. Mean follow-up was 20 months (12 to 30). There were 67 male patients (67%) and 33 female patients (33%), with a mean age of 50 years (11 to 79). Control of infection was achieved in 95 out of 100 patients (95%). Persistence of infection was noted in five patients (5%).
The infection control rate for the spontaneous infection group was 96.4% and for postoperative infections was 94.4%, respectively. Although we were able to control infection in 94.4% of patients, in four patients (4%) with gap nonunions, we had to perform additional Masquelet procedures to achieve bone healing. These patients had no growth from intraoperative cultures sent during stage 1 Masquelet procedure. Three patients were with open fractures (two involved in a road traffic accident, and one who sustained a farm-based injury), and two patients suffered from diabetes. One patient tested positive for hepatitis B surface antigen. Prolonged ooze beyond two weeks was noted in six patients (6%) who settled spontaneously within six weeks. There were no fractures noted following treatment of osteomyelitis in our series.
Control of infection in 95 patients (95%) was confirmed based on clinical improvement (no local signs of infection), laboratory results (CRP, ESR, and white blood cell (WBC) count), and radiological findings. These parameters could not be applied to the patients who died of sepsis within few days of index surgery and hence were excluded. The six patients who died were suffering from uncontrolled diabetes and other comorbidities: five had gram-negative made up of sepsis with Pseudomonas aeruginosa (two patients), Klebsiella pneumoniae (two patients), Escherichia coli (one patient), and methicillin-resistant Staphylococcus aureus (MRSA; one patient).
These patients had undergone the following procedures: proximal femoral nailing for intertrochanteric fractures (two patients), plating for distal femoral fracture (one patient), intramedullary nailing for open tibial fracture (one patient), plating for proximal tibial fracture (one patient), and proximal humerus plating (one patient).
Out of 72 postoperative infection patients (72%), 60 (83%) underwent index surgery elsewhere. Overall, eight out of the 60 patients (13%) had initial surgical treatment in other countries, and 12 (17%) had index surgery at one of the study group hospitals. A total of 28 patients (28%) had spontaneous bone/joint infections.
Clinical presentation
In the spontaneous infection group of 28 patients, 22 (78.6%) presented with chronic osteomyelitis (Figure 1), and six (21.4%) presented with acute osteomyelitis. In the postoperative infection group of 78 patients, the most common presentation was of chronic osteomyelitis in 46 patients (58.9%), followed by delayed hematogenous infection (Figure 2) in 20 (25.6%), and fulminant sepsis in six (5.6%). (Table I). Osteomyelitis was noted mostly in the femur (46; 42.9%), followed by the tibia (26; 24.2%). Proximal femur was involved in 22 patients and shaft of tibia in 13 patients. Septic arthritis was noted in the hip (two), knee (five), and shoulder (two). (Table II). The commonest anatomical presentation of osteomyelitis, as per Cierny-Mader classification,3 was permeative/stable (type 3; 82 patients (82%)) followed by medullary (type 1; eight patients (8%)). The most common host type, as per the Cierny-Mader classification,3 was of type B in 44 patients (44%), followed by type A in 40 patients (40%), while the remaining 16 patients (16%) were type C host. (Table III). Most common comorbid conditions were diabetes mellitus (36 patients) and hypertension (18 patients). Overall, 22 of these 36 patients had two comorbid conditions, and four patients had three comorbid conditions. Four patients were diagnosed to have coronary artery disease and four patients had chronic kidney disease (Table IV). The most common organism isolated was methicillin-sensitive S. aureus (MSSA) in 28 (27.7%) samples, followed by Klebsiella pneumoniae in 13 (12.8%) samples. There were eight samples (7.9%) that grew two organisms. Skin flora were noted in two samples (1.9%). There was no growth in 27 samples (26.7%) (Table V). The commonest antibiotic that was used in Stimulan was vancomycin in 90 patients (89.1%), followed by gentamicin in ten patients (9.9%) (Table VI). The most frequently used quantity of Stimulan was 10 cc in 54 patients (53.4%), followed by 5 cc in 38 patients (37.6%) (Table VII).
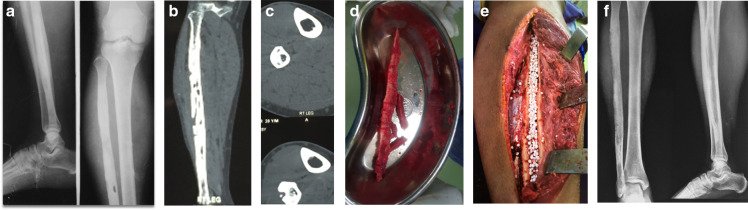
Fig. 1.
a) 28-year-old male with fibular osteomyelitis. Anteroposterior (AP) and lateral views demonstrating diaphyseal radiolucent lesion in the shaft and sclerosis of entire fibula. b) Coronal CT section demonstrating multiple radiolucent lesions and sclerosis of entire fibula suggestive of extensive fibular osteomyelitis. c) Axial CT sections demonstrating fibular osteomyelitis with sequestrum and cortical defect. d) Intraoperative picture of sequestrum removed from fibula. e) Intraoperative picture following sequestrectomy and saucerization of fibular osteomyelitis and Stimulan insertion. f) Follow-up radiograph, with AP and lateral views demonstrating good healing.
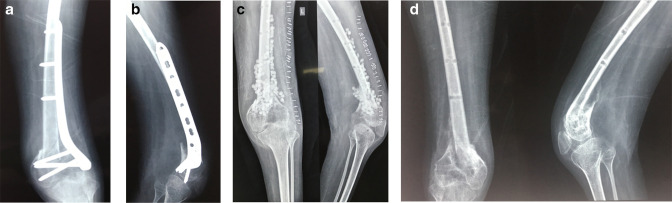
Fig. 2.
a) 29-year-old male with post-polio residual paralysis and type 1 open fracture. Following debridement and ORIF with reversed proximal tibial plate (to match the hypoplastic bone). Presented a year later with pseudomonas infection following closed injury and haematoma formation. b) Lateral view of distal femur with knee, demonstrating the distal femoral fracture and plate fixation. c) Anteroposterior (AP) and lateral views of distal femur with knee, following wound debridement, implant removal, and Stimulan insertion. d) AP and lateral views of distal femur with knee demonstrating good healing of distal femoral fracture at six months' follow-up.
Table I.
Clinical presentation of infection.
| Spontaneous | N (total = 28 patients) | Postoperative | N (= total 78 patients) |
|---|---|---|---|
| Acute osteomyelitis | 6 | Acute infection | 4 |
| Chronic osteomyelitis | 22 | Septic shock | 6 |
| Delayed haematogenous infection | 20 | ||
| Chronic osteomyelitis | 46 | ||
| Septic arthritis | 2 |
Table II.
Site of infection.
| Site | Data |
|---|---|
| Femur, n (%) | 46 (42.9) |
| Proximal femur, n | 22 |
| Shaft femur, n | 9 |
| Distal femur, n | 15 |
| Tibia, n (%) | 26 (24.2) |
| Proximal tibia, n | 6 |
| Shaft tibia, n | 13 |
| Distal tibia, n | 7 |
| Fibula, n | 2 |
| Proximal humerus, n | 4 |
| Ulna, n | 10 |
| Radius, n | 4 |
| Calcaneum | 6 |
| Hip Joint | 2 |
| Knee joint | 5 |
| Shoulder joint | 2 |
Table III.
Classification of osteomyelitis, as per Cierny-Mader classification.2
| Anatomical type | Patients, n (%) | Host type | Patients, n (%) |
|---|---|---|---|
| 1 (medullary) | 8 (8) | A | 40 (40) |
| 2 (superficial) | 4 (4) | B | 44 (44) |
| BL | 24 | ||
| BS | 20 | ||
| 3 (permeative/stable) | 82 (82) | C | 16 (16) |
| 4 (permeative/unstable) | 6 (6) |
Table IV.
Comorbidities.
| Variable | Patients, n (%) |
|---|---|
| Diabetes mellitus | 36 (36) |
| Hypertension | 18 (18) |
| Coronary artery disease | 4 (4) |
| Chronic kidney disease | 4 (4) |
Table V.
Organisms grown on cultures.
| Organisms | Patients, n (%) |
|---|---|
| Meticillin-sensitive Staphylococcus aureus | 28 (27.7) |
| Klebsiella pneumoniae | 13 (12.8) |
| Pseudomonas aeruginosa | 5 (4.9) |
| Escherichia coli | 5 (4.9) |
| Enterococcus faecalis | 5 (4.9) |
| Staphylococccus epidermidis | 1 (0.99) |
| Streptococcus | 1 (0.99) |
| Methicillin-resistant S. aureus | 2 (1.9) |
| Methicillin-resistant Staphylococcus arneri | 1 (0.9) |
| Proteus irabilis | 1 (0.9) |
| Mycobacteria tuberculosis | 1 (0.9) |
| Atypical mycobacteria | 1 (0.9) |
| Skin flora | 2 (1.9) |
| Two organisms | 8 (7.9) |
| No growth | 27 (26.7) |
Table VI.
Antibiotics used in Stimulan.
| Antibiotic | Patients, n (%) |
|---|---|
| Vancomycin | 90 (89.1) |
| Gentamicin | 10 (9.9) |
| Meropenem | 4 (3.9) |
| Augmentin | 2 (1.9) |
| Amikacin | 2 (1.9) |
| Colistin | 2 (1.9) |
| Tigecycline | 1 (0.99) |
Table VII.
Quantity of Stimulan.
| Quantity, cc | Patients, n (%) |
|---|---|
| 5 | 38 (37.6) |
| 10 | 54 (53.4) |
| 15 | 6 (5.9) |
| 20 | 2 (1.9) |
| 30 | 1 (0.99) |
Complications
Delayed wound healing was noted in two patients who went on to heal with regular dressings. In six patients, wound ooze was noted for over two weeks that settled without any further intervention within six weeks. In two patients with infected nonunion following implant removal, debridement, and Stimulan insertion, fracture healing was noted at the follow-up that went on to complete union later. In four patients (4%) with infected nonunion and bone loss, infection control was achieved with surgical debridement and Stimulan, but needed additional two stage Masquelet procedure for large bone defects to achieve union. Persistence of infection was noted in five patients (5%) (Table VIII). These five patients were managing their condition with dressings and antibiotics as needed and they were not keen for any further interventions at the last follow-up.
Table VIII.
Complications.
| Complication | Patients, % |
|---|---|
| Delayed wound healing | 2 |
| Prolonged postoperative ooze | 2 |
| Nonunion (needed 2 stage Masquelet procedure to achieve union) | 4 |
| Persistence of infection | 5 |
The majority of the patients with diabetes mellitus (28 out of 36) had grown gram-negative organisms in cultures. Six patients (6%) who presented with septicemia died of sepsis-related complications during initial phases of the treatment; five of those who died were suffering from uncontrolled diabetes and other comorbidities such as chronic kidney disease or coronary artery disease. They were critically ill prior to surgery, and none of these six or any other patients demonstrated any features suggestive of antibiotic toxicity, hypersensitivity, or allergic reaction in our study.
Discussion
Osteomyelitis is defined as inflammatory disease of the bone that is caused by infection. It can occur following trauma (after open fracture), can be postoperative (after fracture fixation or joint arthroplasty), can be secondary to hematogenous spread, or can be spontaneous.4 Osteomyelitis has always been a challenging condition to treat and to achieve a cure. Although there are various newer treatment methods available in the last two decades, we still have many hurdles to get on top of this difficult problem.1,2
The most important factors that influence the treatment of osteomyelitis are the condition of the host, the functional impairment caused by the disease, the site of involvement, and the extent of bony necrosis.2,3
The mainstay of osteomyelitis treatment is thorough surgical debridement. However, the other quintessential factors are identification of the causal organism and appropriate antibiotic therapy for adequate duration and relevant dead space management.4
There are various options available for dead space management in the form of bone grafts, PMMA, calcium sulphate, and calcium phosphate substitutes. Both PMMA and calcium substitutes have the advantage of delivering high concentration of antibiotics for a prolonged period, in a controlled environment, without additional risk of systemic side-effects of prolonged antibiotic use.2,4 However, PMMA beads need removal especially in infected nonunions and chronic osteomyelitis with large bone defects.2,4
In the current study, Stimulan was used to deliver high concentration of antibiotics locally. Stimulan is an absorbable calcium sulphate that can be directly applied at the site of infection, for control of infection and dead space management in infected nonunions, osteomyelitis, and PJIs.5 Stimulan has the advantage of complete absorption within six to eight weeks, and second surgery for removal is not needed.
Our results can be compared to other published studies like the Oxford studies by Ferguson et al,6 published in 2014 and 2016 with the use of Osteoset and Cerament G, respectively. In their study with Osteoset T (calcium sulphate with tobramycin), infection resolved in 97.9% of cases. The authors also reported prolonged ooze in 15.4%. Fractures were noted in 4.6% of the 195 cases. With Cerament G (calcium sulphate/hydroxyapatite biocomposite with gentamicin), they were able to eradicate infection in 96% with first surgery and the recurrences were cured of infection with repeat surgeries.7
Tarar et al,8 in their systematic review on hypercalcemia with use of calcium sulphate in PJIs, reviewed 1,049 patients and 44 (4.2%) reported hypercalcemia, with 41 (3.91%) transient in nature and three (0.28%) required management, including one with intensive care unit admission. They concluded that calcium sulphate beads are safe and effective against PJIs.
Maale et al,9 in their study on elution profile and safety of Stimulan, demonstrated that it could be used as a platform for local delivery of vancomycin and tobramycin at therapeutic concentrations. There were no adverse reactions and no prolonged wound discharge in any of the patients.
Metsemakers et al,10 in their review, concluded that for the successful treatment of bone and joint infection, in addition to bony stability and soft-tissue cover, successful debridement, and irrigation, along with systemic and local antibiotics, are useful. They also recommended prospective multicentre studies to ensure the safety profile and evaluate the possible toxicity with high local antibiotic concentrations.
Ene et al,11 in their review of ceramics, concluded that calcium sulphate has demonstrated optimal degradation, mechanical and antibiotic elution characteristics and has a definite place in the future management strategies of musculoskeletal infection management.
Jiang et al,12 in their retrospective review of 34 patients, followed the technique of cortical bone windowing, eggshell-like debridement and implantation of antibiotic-loaded calcium sulphate for localized (Cierny-Mader type III) calcaneal osteomyelitis. They were able to eradicate infection in 29 patients (85%) with the above procedure and recurrent infections were cured with further procedures.
Tao et al,13 in their retrospective study, reviewed treatment of paediatric osteomyelitis with the use of antibiotic impregnated calcium sulfate in 21 patients, and infection recurrence was 0% at 31 months follow-up.
Shi et al,14 in their systematic review of 16 studies and 917 patients, assessed the effectiveness of radical debridement combined with antibiotic loaded calcium sulfate for chronic osteomyelitis and confirmed overall eradication rate of 92%. The overall reoperation rate was 9%, refracture rate was 2%, delayed wound healing rate was 20%, and rate of aseptic wound leakage was 12%.
Elhessy et al,15 in their retrospective study, suggested that intramedullary injection of vancomycin and tobramycin loaded calcium sulfate can be used as a single stage procedure for the treatment of long bone intramedullary chronic osteomyelitis.
He et al,16 in their review article on current concepts of treatment of fracture related infection recommended the “Oxford group” protocol for local implantation of calcium sulfate with antibiotics for intramedullary (type I), localized (type III), and diffuse (type IV) infections, while soft-tissue coverage is recommended for superficial infection (type II).
To the best of our knowledge, this is the first clinical study in a large series on calcium sulphate usage and its effectiveness in controlling bone and joint infection in chronic osteomyelitis and postoperative infections.
Limitations
Although this study is prospective in nature, it does have few limitations. A comparative study between two different antibiotic delivery systems would have been ideal. However, during the study period, Stimulan was the only product available for us. It may also be argued that in certain situations, thorough debridement and systemic antibiotics alone would have controlled the infection. However, the success depends on patient compliance for oral/intravenous use of systemic antibiotics for prolonged periods, and it is important to monitor the side effects of high dose of systemic antibiotics.
In conclusion, our multicentre experience suggests that along with a thorough debridement and appropriate antibiotic loaded in calcium sulphate beads for local delivery has been very effective in treating bone and joint infections without any major side-effects and complications.
With increase in the availability and cost effectiveness of various antibiotic delivery systems, it would be appropriate to conduct a prospective randomized controlled trial between different systems for establishing the most efficacious system with least complications.
Take home message
- Calcium sulphate beads are effective in controlling bone and joint infection, without any significant side-effects or complications.
Author contributions
P. Mereddy: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Validation, Visualization, Writing - original draft, Writing - review & editing.
S. R. Nallamilli: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Validation, Writing - review & editing.
V. P. Gowda: Conceptualization, Data curation, Formal analysis, Investigation, Writing - review & editing.
S. Kasha: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing - review & editing.
Shashi Kanth Godey: Data Curation, Formal Analysis, Investigation, Methodology, Writing - Review and Editing
R. R. Nallamilli: Data curation, Formal analysis, Investigation, Methodology, Writing - review & editing.
R. GPRK: Data curation, Formal analysis, Investigation, Methodology.
V. G. R. Meda: Data curation, Formal analysis, Investigation, Methodology, Writing - review & editing.
Funding statement
The author(s) received no financial or material support for the research, authorship, and/or publication of this article.
Data sharing
The data that support the findings for this study are available to other researchers from the corresponding author upon reasonable request.
Acknowledgements
We would like to thank Dr Swathi, consultant microbiologist at Yashoda Hospital, Hyderabad, for her constant inputs during the treatment, and Dr Vamshi Krishna Terala and Dr Saad, orthopaedic surgeons, for their initial contribution to this study.
Ethical review statement
This study was approved by the Institutional Review Board (ID: RP/PO/12-2018)
Open access funding
The authors report that the open access funding for this manuscript was self-funded.
Follow P. Mereddy @mereddy_praveen
© 2023 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/
Contributor Information
Praveen Mereddy, Email: praveenmereddy3@gmail.com.
Somasekhar R. Nallamilli, Email: nreddy12@yahoo.com.
Veda P. Gowda, Email: docveda@gmail.com.
Srinivas Kasha, Email: drsrinivaskasha@gmail.com.
Shashi K. Godey, Email: shashikanthg@gmail.com.
Rajyalakshmi R. Nallamilli, Email: nrajyalakshmireddy@gmail.com.
Rohit GPRK, Email: gprkrohit@yahoo.com.
Venu G. R. Meda, Email: meda.venu@gmail.com.
References
- 1.McLaren AC, Gutierrez FN, Martin M, McLemore R. Musculoskeletal Infection. Vol. 2. OKU, AAOS: Musculoskeletal Infection Society; pp. 95–116. Vol. [Google Scholar]
- 2. Kyriacou H, Kamaraj A, Khan WS. Developments in antibiotic-eluting scaffolds for the treatment of osteomyelitis. Applied Sciences. 10(7):2244. doi: 10.3390/app10072244. [DOI] [Google Scholar]
- 3. Cierny G, Mader JT, Penninck JJ. A clinical staging system for adult osteomyelitis. Clin Orthop Relat Res. 2003;(414):7–24. doi: 10.1097/01.blo.0000088564.81746.62. [DOI] [PubMed] [Google Scholar]
- 4. De Grado GF, Keller L, Idoux-Gillet WQ, et al. Bone substitutes: a review of their characteristics, clinical use, and perspectives for large bone defects management. Journal of Tissue Engg. 2018;9:1–18. doi: 10.1177/2041731418776819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.No authors listed [3 July 2023]. http://www.biocomposites.com date last. accessed.
- 6. Ferguson JY, Dudareva M, Riley ND, Stubbs D, Atkins BL, McNally MA. The use of a biodegradable antibiotic-loaded calcium sulphate carrier containing tobramycin for the treatment of chronic osteomyelitis: a series of 195 cases. Bone Joint J. 2014;96-B(6):829–836. doi: 10.1302/0301-620X.96B6.32756. [DOI] [PubMed] [Google Scholar]
- 7. McNally MA, Ferguson JY, Lau ACK, et al. Single-stage treatment of chronic osteomyelitis with a new absorbable, gentamicin-loaded, calcium sulphate/hydroxyapatite biocomposite: a prospective series of 100 cases. Bone Joint J. 2016;98-B(9):1289–1296. doi: 10.1302/0301-620X.98B9.38057. [DOI] [PubMed] [Google Scholar]
- 8. Tarar MY, Toe KKZ, Javed K, Shah N, Khalid A. The risk of iatrogenic hypercalcemia in patients undergoing calcium sulphate beads implantation in prosthetic joint surgery: a systematic review. Cureus. 2021;13(10):e18777. doi: 10.7759/cureus.18777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maale GE, Eager JJ, Mohammadi DK, Calderon FA. Elution profiles of synthetic CaSO4 hemihydrate beads loaded with vancomycin and tobramycin. Eur J Drug Metab Pharmacokinet. 2020;45(4):547–555. doi: 10.1007/s13318-020-00622-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Metsemakers W-J, Fragomen AT, Moriarty TF, et al. Evidence-based recommendations for local antimicrobial strategies and dead space management in fracture-related infection. J Orthop Trauma. 2020;34(1):18–29. doi: 10.1097/BOT.0000000000001615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ene R, Nica M, Ene D, Cursaru A, Cirstoiu C. Review of calcium-sulphate-based ceramics and synthetic bone substitutes used for antibiotic delivery in PJI and osteomyelitis treatment. EFORT Open Rev. 2021;6(5):297–304. doi: 10.1302/2058-5241.6.200083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jiang N, Zhao X-Q, Wang L, Lin Q-R, Hu Y-J, Yu B. Single-stage debridement with implantation of antibiotic-loaded calcium sulphate in 34 cases of localized calcaneal osteomyelitis. Acta Orthop. 2020;91(3):353–359. doi: 10.1080/17453674.2020.1745423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tao R, Wu J-Q, Luo J-W, et al. Antibiotic-impregnated calcium sulfate for the treatment of pediatric hematogenous osteomyelitis. BMC Pediatr. 2022;22(1):732. doi: 10.1186/s12887-022-03791-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shi X, Wu Y, Ni H, et al. Antibiotic-loaded calcium sulfate in clinical treatment of chronic osteomyelitis: a systematic review and meta-analysis. J Orthop Surg Res. 2022;17(1):104. doi: 10.1186/s13018-022-02980-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Elhessy AH, Rivera JC, Shu HT, Andrews T-J, Herzenberg JE, Conway JD. Intramedullary canal injection of vancomycin- and tobramycin-loaded calcium sulfate: a novel technique for the treatment of chronic intramedullary osteomyelitis. Strategies Trauma Limb Reconstr. 2022;17(2):123–130. doi: 10.5005/jp-journals-10080-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. He S-Y, Yu B, Jiang N. Current concepts of fracture-related infection. Int J Clin Pract. 2023;2023:4839701. doi: 10.1155/2023/4839701. [DOI] [PMC free article] [PubMed] [Google Scholar]