Abstract
Background
Anti-CD19 chimeric antigen receptors (CARs) T-cell therapy has been shown to have excellent efficacy in patients with relapsed/refractory (R/R) B-cell acute lymphoblastic leukemia (ALL). But many patients are refractory to anti-CD19-CAR T-cell therapy or relapse again.
Methods
Five patients with R/R B-ALL did not respond to anti-CD19-CAR T-cell therapy or had a disease progression again after CAR-T cell therapy. They received a salvage therapy of Blinatumomab. The clinical response, CD19 expression on ALL cells, the proportion of CD3+ T cells, level of cytokine levels of interleukin-6 (IL-6), hematological toxicity, grade of cytokine release syndrome (CRS), and immune effector cell-associated neurotoxic syndrome (ICANS) were observed in salvage therapy of Blinatumomab.
Results
Four patients obtained CR/CRi, even in patients without high expression of CD19 in B-ALL cells, while the other patient received NR after Blinatumomab therapy. The CD19 expression on ALL cells, the proportion of CD3+ T cells, and CD3+CD8+ T cells were deficient in Pt 5, who obtained PR in Blinatumomab therapy. One patient (Pt 3) was diagnosed with grade 0 hematological toxicity. The other four patients were diagnosed with grades 2–3 of hematological toxicity. The CRS was grade 0/one patient, grade 1/three, and grade 2/one. The ICANS was grade 0/four patients, grade 1/one. Rhizopus microsporus pneumonia and cryptococcal encephalopathy in two patients were controlled during Blinatumomab therapy.
Conclusions
Blinatumomab could be an effective and safe salvage therapy in patients with R/R B-ALL who failed/progressed after anti-CD19-CAR T therapy, even in R/R B-ALL patients without high expression of CD19 in B-ALL cells, patients with CNS leukemia or co-infection.
Key messages
Some R/R B-ALL patients did not respond to anti-CD19 CAR T-cell therapy or had a disease progression again. Effective and safe salvage therapy for such patients remains to be explored.
Blinatumomab could be an effective and safe salvage therapy in patients with R/R B-ALL who failed/progressed after anti-CD19-CAR T therapy, even in patients without high expression of CD19 in B-ALL cells.
Blinatumomab could be an effective and safe salvage therapy in patients with R/R B-ALL who failed/progressed after anti-CD19-CAR T therapy, even in patients with CNS leukemia or co-infection.
Keywords: B-cell acute lymphoblastic leukemia, anti-CD19-CAR T therapy, blinatumomab, salvage therapy
Introduction
CD19 as a B lymphocyte marker is almost universally expressed in newly diagnosed B-cell acute lymphoblastic leukemia (B-ALL) and relapsed/refractory (R/R) B-ALL. Immunotherapy targeting CD19 has a higher response rate in R/R B-ALL compared to salvage chemotherapy [1,2]. A successful approach has been the development of bispecific T cell-engaging (BiTE) antibodies against CD19/CD3 with a satisfactory complete remission (CR) rate in R/R B-ALL. Blinatumomab is the first BiTE antibody against CD19/CD3. It specifically binds to CD19+ B cells and CD3+ T cells, causing activation of T cells and then a cytotoxic T cell response against CD19-expressing tumor cells [3–5]. Cytokine release syndrome (CRS) and immune effector cell-associated neurotoxic syndrome (ICANS) are major and common adverse events (AEs) Blinatumomab therapy [6–8]. The incidence and severity of CRS depend upon the disease burden of R/R B-ALL. In addition, another therapy has been shown to have excellent anti-leukemic activities in R/R B-ALL was anti-CD19 chimeric antigen receptors (CAR) T-cell therapy [9–12]. However, many patients with R/R B-ALL are refractory to anti-CD19-CAR T-cell therapy or relapse after this therapy [11,13]. The limited availability of effective treatments for these patients poses significant challenges. Here we describe Blinatumomab’s efficacy and side effects as salvage therapy in five patients with R/R B-ALL.
Patients and methods
Patient characteristics
Five patients who were diagnosed with R/R B-ALL were enrolled in our study. They had all received anti-CD19-CAR T-cell therapy. But they did not respond to anti-CD19-CAR T-cell treatment or had a disease progression again after they obtained CR/CR with incomplete blood count recovery (CRi) to CAR-T cell therapy. All the patients provided informed consent and received a salvage therapy of Blinatumomab. Informed consent was obtained from the participants. The patients agreed to participate in our study. They decided to use their data for our study. All the data and material have been performed following the Declaration of Helsinki and conformed to relevant aspects of the ARRIVE guidelines.
Salvage therapy of Blinatumomab
When the R/R B-ALL patients who obtained CR in their anti-CD19-CAR T-cell therapy had a disease relapsed again, or they did not respond to CAR-T cell therapy, they received salvage therapy of Blinatumomab. Blinatumomab was administered by continuous intravenous drip and dose-escalating manner with 9 μg/d for the first week, followed by 28 μg/d for the remaining three weeks.
Clinical response criteria
The therapy response of Blinatumomab was assessed at the end of the treatment. The evaluation methods included bone marrow (BM) morphology and BM flow cytometry (FCM). Disease status was defined as CR, CRi, and partial remission (PR).
The proportion of CD3+ T cells and cytokine levels in Blinatumomab therapy
The proportion of CD3+ T cells in peripheral blood were observed before salvage therapy of Blinatumomab by FCM. Cytokine levels of interleukin-6 (IL-6) were observed in the salvage therapy of Blinatumomab using respective enzyme-linked immunosorbent assays.
Adverse events (AEs) of Blinatumomab therapy
AEs were observed for 28 d throughout the treatment with Blinatumomab. The hematological toxicity was graded according to the joint ASCO/IDSA consensus guidelines for cancer-related infection risk [14,15]. The cytokine release syndrome (CRS) grade was determined according to the National Cancer Institute Common Terminology Criteria for AE v4.03 [16]. Neurotoxicity syndrome was defined according to immune effector cell-associated neurotoxic syndrome (ICANS) [17].
Follow-up
From the date of salvage therapy of Blinatumomab, follow-up was carried out up to the cutoff date or the date of death. The cutoff date was October 31, 2022, in our study.
Results
Patient characteristics before Blinatumomab therapy
All five patients received anti-CD19-CAR T-cell therapy one to three times when diagnosed with R/R B-ALL. The detailed characteristics of the five patients before their Blinatumomab therapy are shown in Table 1. None of these patients had received Blinatumomab or CD19 monoclonal antibody before salvage therapy of Blinatumomab. The proportion of leukemia cells in BM before Blinatumomab therapy is shown in Table 1.
Table 1.
Baseline characteristics of five patients with R/R ALL.
| A | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Age | Sex | Ph + ALL | Prior lines | Prior allo-HSCT | Prior CAR-T times | Efficacy of CAR-T | Leukemia cells in BM (%) | CD19 expression (%) | Neutrophil (×106/L) | Haemoglobin (g/L) | Platelets (×106/L) | CD3+ (%) | CD3+CD8+ (%) | CD3+CD4+ (%) |
| Pt 1 | 68 | Male | No | 3 | No | 1 time | CR | 82.5 | 73.09 | 2.2 | 101 | 102 | 81 | 42 | 38 |
| Pt 2 | 31 | Male | No | 5 | No | 1 time | NR | 98.2 | 99.44 | 1.1 | 67 | 58 | 90 | 51 | 37 |
| Pt 3 | 25 | Male | No | 4 | No | 3 times | NR | 47.2 | 45.24 | 2.8 | 123 | 123 | 88 | 45 | 42 |
| Pt 4 | 48 | Female | No | 4 | No | 2 times | NR | 22.9 | 59.03 | 1.5 | 117 | 152 | 92 | 39 | 50 |
| Pt 5 | 20 | Male | No | 5 | Yes | 2 times | NR | 54.0 | 32.02 | 1.2 | 86 | 82 | 73 | 25 | 46 |
| B |
|||||||||||||||
| Characteristics | n = 5 | ||||||||||||||
| Age, year | 20–68 | ||||||||||||||
| Sex, male/female | 4/1 | ||||||||||||||
| Ph+ ALL, yes/no | 0/5 | ||||||||||||||
| Prior lines (Including CAR-T) | 3–5 | ||||||||||||||
| Prior allo-HSCT, yes/no | ¼ | ||||||||||||||
| Prior CAR-T times | 1–3 | ||||||||||||||
| Efficacy of last CAR-T, CR/NR | ¼ | ||||||||||||||
| Time of CAR-T to Blinatumomab, months | 1–18 | ||||||||||||||
| Leukemia cells in BM (%) | 22.9–98.2 | ||||||||||||||
| CD19 expression in Leukemia cells (%) | 32.02–99.44 | ||||||||||||||
| Neutrophil (×106/L) | 1.1–2.8 | ||||||||||||||
| Haemoglobin (g/L) | 67–123 | ||||||||||||||
| Platelets (×106/L) | 58–152 | ||||||||||||||
| CD3+ (%) | 73–92 | ||||||||||||||
| CD3+CD8+ (%) | 25–51 | ||||||||||||||
| CD3+CD4+ (%) | 32–50 | ||||||||||||||
Ph+ ALL: Philadelphia chromosome-positive ALL; allo-HSCT: Allogenic hematopoietic stem cell transplantation; CD3+: CD3+ T cells in peripheral blood; CD3+CD8+: CD3+CD8+ T cells in peripheral blood; CD3+CD4+: CD3+CD4+ T cells in peripheral blood
In this study, Pt 2 was an R/R B-ALL patient with high tumor load and central nervous system (CNS) leukemia. He had 98.2% leukemia cells in his bone marrow with 99.44% of CD19 expression in leukemia cells and had 32.2% leukemia cells in his CNS before salvage therapy of Blinatumomab. Because of the low level of platelet (58 × 106/L) at the beginning of salvage therapy, he received only one time of intrathecal chemotherapy before Blinatumomab therapy.
CD19 expression in B-ALL cells before Blinatumomab therapy
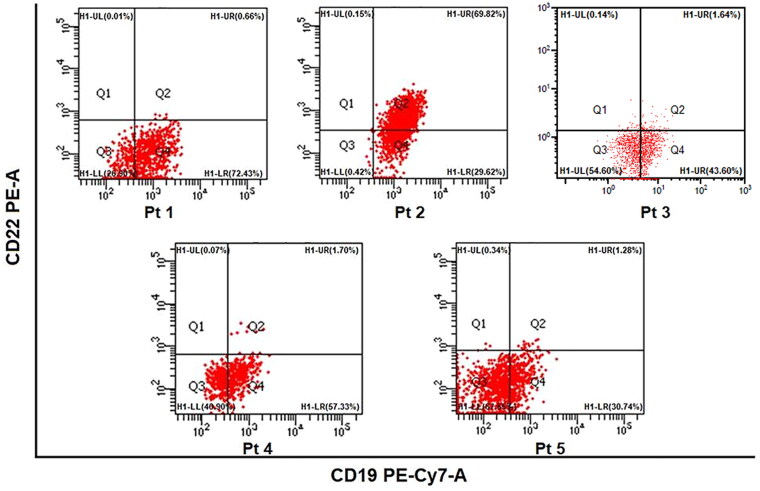
When the five patients with R/R B-ALL were enrolled in their salvage therapy of Blinatumomab, the CD19 terms in B-ALL cells differed. It was 73.09%, 99.44%, 45.24%, 59.03%, and 32.02% in Pt 1 to Pt 5 (Figure 1). Moreover, except for Pt 2, the other four patients had very low CD22 expression in leukemia cells.
Figure 1.
The proportion of CD19 expression in B-ALL cells.
Clinical responses to the Blinatumomab salvage therapy
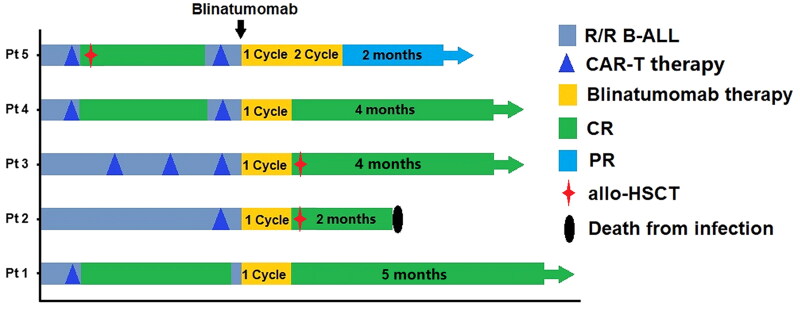
Four patients obtained CR/CRi in the following salvage therapy of Blinatumomab. Three patients (Pt 1, 3, 4) achieved MRD-negative responses, whereas the other patient (Pt 2) was evaluated as CRi with MRD-positive response after one cycle of Blinatumomab therapy. The central nervous system leukemia of Pt 2 also obtained an MRD-negative reply in his cerebrospinal fluid. The other patient (Pt 5) received PR after two cycles of Blinatumomab therapy (Figure 2).
Figure 2.
Responses and survival after Blinatumomab therapy.
The proportion of CD3+ T cells and cytokine levels in Blinatumomab therapy
The proportion of CD3+ T cells, the CD3+CD8+ T cells and the CD3+CD4+ T cells in peripheral blood were observed in Blinatumomab salvage therapy by FCM. The proportion of CD3+ T cells and CD3+CD8+ T cells was more deficient in Pt 5 who obtained PR than that of in the other four patients who obtained CR/CRi in Blinatumomab therapy (Table 2).
Table 2.
Characteristics and outcomes of the five patients in Blinatumomab salvage therapy.
| Number | IL-6 (pg/mL) | CD3+ (%) | CD3+CD8+ (%) | CD3+CD4+ (%) | Hematological toxicity Grade |
CRS Grade | ICANS Grade | Infection | Response to Blinatumomab | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutrophil | Haemoglobin | Platelets | |||||||||
| Pt 1 | 5.81–28.4 | 81–85 | 42–45 | 37–38 | 2 | 1 | 2 | 1 | 0 | RMP | CR MRD− |
| Pt 2 | 13.40–162.5 | 87–91 | 49–52 | 36–39 | 2 | 3 | 3 | 2 | 1 | BP | CR MRD+ |
| Pt 3 | 6.80–4.10 | 88–91 | 45–47 | 41–43 | 1 | 0 | 1 | 1 | 0 | BP, CE | CR MRD− |
| Pt 4 | 4.37–12.72 | 92–96 | 28–39 | 50–63 | 2 | 1 | 0 | 1 | 0 | – | CR MRD− |
| Pt 5 | 6.94–10.5 | 71–73 | 25–28 | 44–47 | 2 | 2 | 3 | 0 | 0 | – | PR |
CD3+: CD3+ T cells in peripheral blood; CD3+CD8+: CD3+CD8+ T cells in peripheral blood; CD3+CD4+: CD3+CD4+ T cells in peripheral blood; MRD: Minimal residual disease; RMP: Rhizopus microsporus pneumonia; BP: Bacterial pneumonia; CE: Cryptococcal encephalopathy.
There was no significant increase in IL-6 levels in peripheral blood. The peak of IL-6 was 28.4 pg/mL, 162.5 pg/mL, 4.10 pg/mL, 12.72 pg/mL, and 10.5 pg/mL in peripheral blood in Blinatumomab therapy (Table 2). The peak of IL-6 was 45.7 pg/mL in the cerebrospinal fluid of Pt 2 with high tumor load and CNS leukemia.
AEs of Blinatumomab therapy
In salvage therapy of Blinatumomab, Pt 2 developed a fever below 38.5 °C, headache, and hepatic dysfunction, Pt 1 developed a fever below 38.0 °C and slight headache. All these AEs recovered quickly before the end of the Blinatumomab therapy.
In this salvage therapy, one patient (Pt 3) was diagnosed with grade 0 of hematological toxicity, and the other four patients (Pt 1, 2, 4, 5) were diagnosed with grades 2–3 of hematological toxicity. Pt 1, 2, 4, 5 received therapy of granulocyte colony-stimulating factor (GCS-F). Except for Pt 5 (PR), hematological toxicity recovered in the other three patients within one week after Blinatumomab therapy (Table 2).
Of these five patients, two (Pt 5) were diagnosed with grade 0 of CRS, and two (Pt 1, 3, 4) were diagnosed with grade 1 of CRS. In contrast, the other patient (Pt 2) was diagnosed with grade 2 of CRS during their salvage therapy of Blinatumomab. Only one patient (Pt 2) was diagnosed with grade 1 of ICANS; the other four were diagnosed with grade 0 of ICANS during this salvage therapy. Pt 2 received two days of dexamethasone therapy (5 mg/d) to relieve his ICANS. No CRS or ICANS-related deaths were observed in our study (Table 2).
Infection and therapy during Blinatumomab therapy
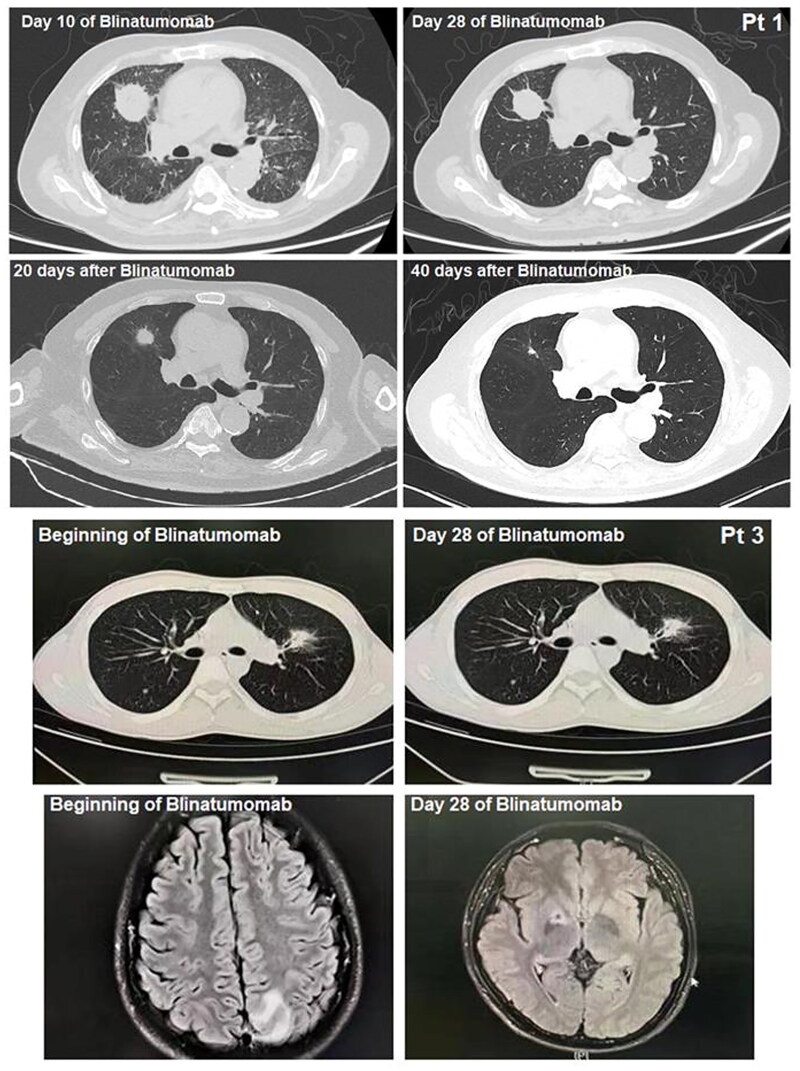
During Blinatumomab therapy, two patients developed invasive fungal disease (IFD). Pt 1 was diagnosed with rhizopus microspores pneumonia by Next-generation sequencing technology (NGS) of bronchoalveolar fluid and pathological examination of infected tissue on day ten of his Blinatumomab therapy. He received isavuconazole orally, amphotericin B intravenously, and amphotericin B atomized. He obtained PR of his rhizopus microspores pneumonia at the end of his Blinatumomab therapy and obtained CR 40 d after his Blinatumomab therapy (Figure 3). Pt 3 was diagnosed with bacterial pneumonia by computed tomography (CT) and cryptococcal encephalopathy of the central nervous system by magnetic resonance imaging (MRI) before his Blinatumomab therapy. He received posaconazole intravenously and obtained CR of his cryptococcal encephalopathy at the end of his Blinatumomab treatment (Figure 3). Pt 2 was diagnosed with bacterial pneumonia by CT and bacterial culture of bronchoalveolar fluid. He received meropenem and obtained CR of his bacterial pneumonia on day 21 of Blinatumomab therapy. Pt 4 and 5 did not develop any infections during their salvage therapy of Blinatumomab.
Figure 3.
Rhizopus microsporus pneumonia of Pt 1, bacterial pneumonia and cryptococcal encephalopathy of central nervous system of Pt 3.
Survival after the salvage therapy of Blinatumomab
After salvage therapy of Blinatumomab, two patients (Pt 2, 3) received allogeneic hematopoietic stem cell transplantation (allo-HSCT). Pt 3 survived in CR on Blinatumomab maintenance therapy until the cutoff date. Pt 2 died of severe IFD two months after allo-HSCT. Two patients (Pt 1, 4) who obtained CR/CRi survived in CR/CRi state, and one patient (Pt 5) with PR survived on maintenance therapy until the cutoff date (Figure 2).
Discussion
There is still no conclusion on the effect of the different order of the application of anti-CD19 CAR T-cell and Blinatumomab therapy on the efficacy of R/R B-ALL. A study proved that prior therapy with Blinatumomab was associated with a significantly higher failure rate to achieve MRD- deep remission or subsequent loss of remission with antigen escape [18]. But another study reported that five patients with R/R B-ALL received anti-CD19 CAR T-cell therapy after antibody-based immunotherapy. In the three patients after prior therapy with Blinatumomab, two patients obtained MRD- remission response to CAR-T cell therapy. The other patient with the active disease died of CRS and ICANS [19]. In the ZUMA-3 phase 1 trial, the efficacy of anti-CD19 CAR T-cell therapy was not compromised by previous therapy with Blinatumomab [20]. However, child patients with R/R B-ALL who had previously received Blinatumomab had a lower survival rate after anti-CD19 CAR T-cell therapy [18]. Data on the influence of Blinatumomab on outcomes of the following anti-CD19 CAR T-cell therapy needed to be more comprehensive in the literature. But the CD19 expression on ALL cells should be assessed before the subsequent anti-CD19 CAR T-cell therapy.
If anti-CD19 CAR T-cell therapy was preferred in patients with R/R B-ALL and Blinatumomab was treated when the disease progressed again, what are the efficacy and side effects? These patients with R/R B-ALL had limited therapy options and poor prognoses [21]. The first study delineated such a challenging therapy but an increasingly more common patient population [22]. In this study, 12 patients with R/R B-ALL who received Blinatumomab and/or Inotuzumab ozogamicin as a salvage therapy progressed after anti-CD19 CAR T-cell therapy. CD19 expression in leukemia cells of the four patients who received Blinatumomab salvage therapy was all positive. Three patients with R/R B-ALL obtained CR response to Blinatumomab salvage therapy; the other patient brought no response (NR)response. Although the sample size was small, encouraging responses were achieved in patients with R/R B-ALL who received Blinatumomab and/or Inotuzumab ozogamicin progressing after anti-CD19 CAR T-cell therapy. There were no severe CRS, ICANS, or cytopenia in the Blinatumomab salvage therapy of these four patients.
For patients with R/R B-ALL, Blinatumomab has been reported a higher CR rate with MRD negativity [5,23,24]. In our study, only five patients with R/R B-ALL received Blinatumomab as salvage therapy after anti-CD19 CAR T-cell therapy. Four patients (4/5, 80%) with R/R B-ALL obtained CR/CRi in the Blinatumomab salvage therapy. The efficacy of salvage therapy with Blinatumomab was satisfactory, even in patients who had a low proportion of CD19 expression in B-ALL cells. In these four patients obtained CR/CRi, CD19 expression in B-ALL cells was 73.09%, 99.44%, 45.24% and 59.03%. The only one patient who obtained PR after two cycles of Blinatumomab therapy had a 32.02% of CD19 expression in B-ALL cells. Moreover, except for Pt 2 (99.44% of CD19 expression), the other four patients had very low CD22 expression in leukemia cells. Therefore, we tried to use Blinatumomab for the salvage therapy in these R/R ALL patients. Little is known about the mechanism of resistance to Blinatumomab therapy. An essential mechanism for treatment failure might be the loss of CD19 antigen before Blinatumomab therapy [25,26], but CD19 antigen loss does not appear to affect the efficacy of Blinatumomab therapy as often as CAR-T therapy [11]. This might be one of the reasons for the satisfactory efficacy of these five patients with Blinatumomab salvage therapy. And the comparison of efficacy and the sequential CD19-targeted therapies between anti-CD19 CAR T-cell therapy and Blinatumomab therapy needs further exploration. As a bispecific antibody targeting CD3 and CD19, Blinatumomab therapy is dependent on CD3+T cells of R/R B-ALL patients. Therefore, we monitored the expression of CD3+T cells in the peripheral blood of patients. The proportion of CD3+T cells in the peripheral blood of Pt 5 who obtained PR was 73%, lower than that of the other four patients who obtained CR/CRi. We should continue to experiment with the efficacy and safety of Blinatumomab salvage therapy, especially in patients with different expression of CD19 in B-ALL cells.
CRS and ICANS are the major complications in Blinatumomab therapy, especially in patients with high tumor load [27,28]. In our study, only one patient (Pt 2) with high tumor load and CNS leukemia was diagnosed with grade 2 of CRS and grade 1 of ICANS during his salvage therapy of Blinatumomab. All this toxicity disappeared quickly, and no CRS or ICANS-related deaths were observed in our study. We monitored changes in IL-6 levels during Blinatumomab therapy to avoid adverse effects associated with higher CRS and ICANS. Although it remains unclear whether Blinatumomab could penetrate the blood-brain barrier [28], it is safe and effective in patients with R/R B-ALL with CNS [29]. In this study, it suggests that efficacy of Blinatumomab in treating CNS leukemia and Blinatumomab might be a potential option for the treatment of CNS leukemia. In our study, Pt 2 with high tumor load and CNS leukemia obtained an MRD-negative response also. Furthermore, the hematological toxicity of Blinatumomab therapy was recovered quickly. In particular, the two patients with rhizopus microspores pneumonia and cryptococcal encephalopathy achieved CR with MRD negativity, and the infection of the patients was also controlled.
The most significant limitation of our study is the small number of patients. Some results were obtained, but not enough to draw definite conclusions. However, our exploration in this respect is still valuable, and we need to continue to validate our results in the clinic.
Conclusion
In conclusion, Blinatumomab could be an effective and safe salvage therapy in patients with R/R B-ALL who failed/progressed after anti-CD19-CAR T therapy, even in R/R B-ALL patients without high expression of CD19 in B-ALL cells, patients with CNS leukemia or co-infection. We need to expand the number of cases to confirm our conclusions.
Acknowledgments
We thank all our patients for their participation in our study.
Funding Statement
This work was partially supported by the Chinese Society of Clinical Oncology Beijing Xisike Clinical Oncology Research Foundation (Y-SY2021QN-0184). Chinese Society of Clinical Oncology Beijing Xisike Clinical Oncology Research Foundation (Y-Young2022-0209). The National Natural Science Foundation of China (81900186). Tianjin Municipal Science and Technology Commission Grant (20JCZDJC00120 and 21JCQNJC00070). The CAMS Innovation Fund for Medical Science (NO.2020-I2M-C&T-A-019).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.
A preprint of this current paper has already published. https://www.researchsquare.com/article/rs-1254282/v1
Author contributions
Designed the study: Qi Deng and Ying Wang; data Analyzed the data and wrote the manuscript: Yao Qi; Data collection: Yao Qi, Hong Liu, Juan Mu, Xin Li, Yin Shi, and Jingyi Li; Data management and case report form management: Hong Liu, Xin Li, Yin Shi, and Jingyi Li. All the authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1–9. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Von Stackelberg A, Locatelli F, Zugmaier G, et al. Phase I/phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol. 2016;34(36):4381–4389. doi: 10.1200/JCO.2016.67.3301. [DOI] [PubMed] [Google Scholar]
- 3.Topp MS, Gökbuget N, Zugmaier G, et al. Phase II trial of the anti-CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. J Clin Oncol. 2014;32(36):4134–4140. doi: 10.1200/JCO.2014.56.3247. [DOI] [PubMed] [Google Scholar]
- 4.Martinelli G, Boissel N, Chevallier P, et al. Complete hematologic and molecular response in adult patients with relapsed/refractory philadelphia chromosome-positive B-precursor acute lymphoblastic leukemia following treatment with blinatumomab: results from a phase II, single-arm, multicenter study. J Clin Oncol. 2017;35(16):1795–1802. doi: 10.1200/JCO.2016.69.3531. [DOI] [PubMed] [Google Scholar]
- 5.Gokbuget N, Dombret H, Bonifacio M, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood. 2018;131(14):1522–1531. doi: 10.1182/blood-2017-08-798322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kantarjian H, Stein A, Gokbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376(9):836–847. doi: 10.1056/NEJMoa1609783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu D, Zhao J.. Cytokine release syndrome: grading, modeling, and new therapy. J Hematol Oncol. 2018;11(1):121. doi: 10.1186/s13045-018-0653-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Topp MS, Gokbuget N, Stein AS, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single arm, phase 2 study. Lancet Oncol. 2015;16(1):57–66. doi: 10.1016/S1470-2045(14)71170-2. [DOI] [PubMed] [Google Scholar]
- 9.Sadelain M, Brentjens R, Rivière I.. The basic principles of chimeric antigen receptor design. Cancer Discover. 2013;3(4):388–398. doi: 10.1158/2159-8290.CD-12-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park JH, Riviere I, Gonen M, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378(5):449–459. doi: 10.1056/NEJMoa1709919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curran KJ, Margossian SP, Kernan NA, et al. Toxicity and response after CD19-specific CAR T-cell therapy in pediatric/young adult relapsed/refractory B-ALL. Blood. 2019;134(26):2361–2368. doi: 10.1182/blood.2019001641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frey NV, Shaw PA, Hexner EO, et al. Optimizing chimeric antigen receptor T-cell therapy for adults with acute lymphoblastic leukemia. J Clin Oncol. 2020;38(5):415–422. doi: 10.1200/JCO.19.01892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taplitz RA, Kennedy EB, Bow EJ, et al. Antimicrobial prophylaxis for adult patients with Cancer-Related immunosuppression: ASCO and IDSA clinical practice guideline update. J Clin Oncol. 2018;36(30):3043–3054. doi: 10.1200/JCO.18.00374. [DOI] [PubMed] [Google Scholar]
- 15.Rejeski K, Perez A, Sesques P, et al. CAR-HEMATOTOX: a model for CAR T-cell-related hematologic toxicity in relapsed/refractory large B-cell lymphoma. Blood. 2021;138(24):2499–2513. doi: 10.1182/blood.2020010543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625–638. doi: 10.1016/j.bbmt.2018.12.758. [DOI] [PubMed] [Google Scholar]
- 18.Pillai V, Muralidharan K, Meng W, et al. CAR T-cell therapy is effective for CD19-dim B-lymphoblastic leukemia but is impacted by prior blinatumomab therapy. Blood Adv. 2019;3(22):3539–3549. doi: 10.1182/bloodadvances.2019000692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Danylesko I, Chowers G, Shouval R, et al. Treatment with anti CD19 chimeric antigen receptor T cells after antibody-based immunotherapy in adults with acute lymphoblastic leukemia. Curr Res Transl Med. 2020;68(1):17–22. doi: 10.1016/j.retram.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Shah BD, Bishop MR, Oluwole OO, et al. End of phase I results of ZUMA-3, a phase 1/2 study of KTE-X19, anti-CD19 chimeric antigen receptor (CAR) T cell therapy, in adult patients (pts) with relapsed/refractory (R/R) acute lymphoblastic leukemia (ALL). J Clin Oncol. 2019;37(15 Suppl):7006–7006. doi: 10.1200/JCO.2019.37.15_suppl.7006. [DOI] [Google Scholar]
- 21.Cao J, Wang G, Cheng H, et al. Potent antileukemia activities of humanized CD19- targeted chimeric antigen receptor T (CAR-T) cells in patients with relapsed/refractory acute lymphoblastic leukemia. Am J Hematol. 2018;93(7):851–858. doi: 10.1002/ajh.25108. [DOI] [PubMed] [Google Scholar]
- 22.Wudhikarn K, Flynn JR, Rivière I, et al. Interventions and outcomes of adult patients with B-ALL progressing after CD19 chimeric antigen receptor T-cell therapy. Blood. 2021;138(7):531–543. doi: 10.1182/blood.2020009515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aboudalle I, Kantarjian HM, Short NJ, et al. Phase II study of blinatumomab in patients with B-cell lineage acute lymphocytic leukemia with positive minimal/measurable residual disease. Blood. 2018;132(Suppl 1):5212–5212. doi: 10.1182/blood-2018-99-119685. [DOI] [Google Scholar]
- 24.Short NJ, Jabbour E, Albitar M, et al. Recommendations for the assessment and management of measurable residual disease in adults with acute lymphoblastic leukemia: a consensus of North American experts. Am J Hematol. 2019;94(2):257–265. doi: 10.1002/ajh.25338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aboudalle I, Kantarjian HM, Short NJ, et al. Long term follow-up on phase 2 study on the efficacy and safety of blinatumomab in adult patients with relapsed refractory B-precursor acute lymphoblastic leukemia. Blood. 2018;132(Suppl 1):4017–4017. doi: 10.1182/blood-2018-99-117507. [DOI] [Google Scholar]
- 26.Jabbour E, Dull J, Yilmaz M, et al. Outcome of patients with relapsed/refractory acute lymphoblastic leukemia after blinatumomab failure: no change in the level of CD19 expression. Am J Hematol. 2018;93(3):371–374. doi: 10.1002/ajh.24987. [DOI] [PubMed] [Google Scholar]
- 27.Topp MS, Gokbuget N, Zugmaier G, et al. Long-term follow-up of hematologic relapse-free survival in a phase 2 study of blinatumomab in patients with MRD in B-lineage ALL. Blood. 2012;120(26):5185–5187. doi: 10.1182/blood-2012-07-441030. [DOI] [PubMed] [Google Scholar]
- 28.Alfayez M, Kantarjian HM, Short NJ, et al. Safety and efficacy of blinatumomab in patients with Central nervous system (CNS) disease: a single institution experience. Blood. 2018;132(Suppl 1):2702–2702. doi: 10.1182/blood-2018-99-117400. [DOI] [Google Scholar]
- 29.Cao H-Y, Chen H, Liu S-B, et al. Case report: blinatumomab therapy for the treatment of B-cell acute lymphoblastic leukemia patients with central nervous system infiltration. Front Immunol. 2023;14:1181620. doi: 10.3389/fimmu.2023.1181620. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.
A preprint of this current paper has already published. https://www.researchsquare.com/article/rs-1254282/v1