Abstract
Nitrophorins are heme proteins used by blood feeding insects to deliver nitric oxide (NO) to a victim, leading to vasodilation and antiplatelet activity. Cimex lectularius (bedbug) nitrophorin (cNP) accomplishes this with a cysteine ligated ferric (Fe(III)) heme. In the acidic environment of the insect’s salivary glands, NO binds tightly to cNP. During a blood meal, cNP-NO is delivered to the feeding site where dilution and increased pH lead to NO release. In a previous study, cNP was shown to not only bind heme, but to also nitrosate the proximal cysteine, leading to Cys-NO (SNO) formation. SNO formation requires oxidation of the proximal cysteine, which was proposed to be metal-assisted through accompanying reduction of ferric heme and formation of Fe(II)-NO. Here, we report the 1.6 angstrom crystal structure of cNP first chemically reduced and then exposed to NO, and show that Fe(II)-NO is formed but SNO is not, supporting a metal-assisted SNO formation mechanism. Crystallographic and spectroscopic studies of mutated cNP show that steric crowding of the proximal site inhibits SNO formation while a sterically relaxed proximal site enhances SNO formation, providing insight into specificity for this poorly understood modification. Experiments examining the pH dependence for NO implicate direct protonation of the proximal cysteine as the underlying mechanism. At lower pH, thiol heme ligation predominates, leading to a smaller trans effect and 60-fold enhanced NO affinity (Kd = 70 nM). Unexpectedly, we find that thiol formation interferes with SNO formation, suggesting cNP-SNO is unlikely to form in the insect salivary glands.
Keywords: heme protein, nitrophorin, S-nitrosocysteine, nitrosylation, nitric oxide
Graphical Abstract Synopsis
Bedbug nitrophorin is a heme protein with proximal cysteine that binds nitric oxide for delivery to victims and undergoes metal assisted S-nitrosation. Crystallographic and spectroscopic analyses reveal proximal cysteine protonation at the low pH of bedbug salivary glands competes with S-nitrosation. Proximal site crowding inhibits S-nitrosation formation.
1. Introduction
Nitric oxide regulates broad swaths of physiology in mammals, including blood pressure, wound healing, immune response, memory formation, and sexual function. NO, with an unpaired electron, is a free radical molecule, making it an odd choice for a critical regulatory role in biology. Certain blood-feeding insects deliver nitric oxide from their salivary glands to the tissues of unsuspecting victims, giving rise to local vasodilation, reduced blood coagulation, and improved feeding for the insect. Delivery is via nitrophorins, a family of small heme proteins that bind NO with pH dependence, binding tighter at the low pH of the insect saliva and weaker at the neutral pH of blood-bearing mammals, allowing for storage, delivery, and release. Nitrophorin heme is stabilized in the ferric state where NO affinity is sufficiently tight for storage and transport but still sufficiently weak for release to the victim.
The nitrophorins from Rhodnius prolixus, the kissing bug, were the first to be discovered and are the best characterized [1-3]. The Rhodnius nitrophorins incorporate heme into an eight-stranded antiparallel beta barrel (lipocalin family) and make use of a histidine in the proximal site for heme coordination. There are seven different nitrophorins expressed in the Rhodnius salivary gland (rNP1 – rNP7), each with differing affinities and release rates for nitric oxide, allowing for NO release to spread over a larger time span than would be possible for any individual protein. The mechanism by which the Rhodnius nitrophorins achieve high NO affinity at the low pH of the insect salivary gland and low affinity at the higher pH of a victim’s tissues has been extensively characterized through ultra-high-resolution X-ray crystallography [4-8], stopped-flow spectroscopy and flash photolysis [9-11], NMR spectroscopy [12, 13], electrochemistry [14-16], and computational modeling [17]. These studies reveal that the Rhodnius nitrophorins make use of buried charged residues to stabilize heme in the ferric state, and have two mobile loops that collapse over the heme pocket at low pH, forming a series of hydrogen bonds between protonated glutamate and aspartate side chains, trapping NO in the heme pocket. At higher pH, the carboxylic acid groups become deprotonated, leading to charge repulsion, loop displacement, an open distal heme pocket, and NO release. Interestingly, the Rhodnius nitrophorins are multifunctional: at higher pH the more open distal pocket allows for histamine binding, stabilized by the same carboxylate groups on the mobile loops that seal the heme pocket at low pH. Histamine is released by the victim in response to the insect bite, but the inflammatory response to its release and accompanying itching are dampened through binding to the nitrophorins, allowing the insect to go undetected while feeding [7, 18-20]. Additionally, rNP2 directly interferes with blood coagulation [21].
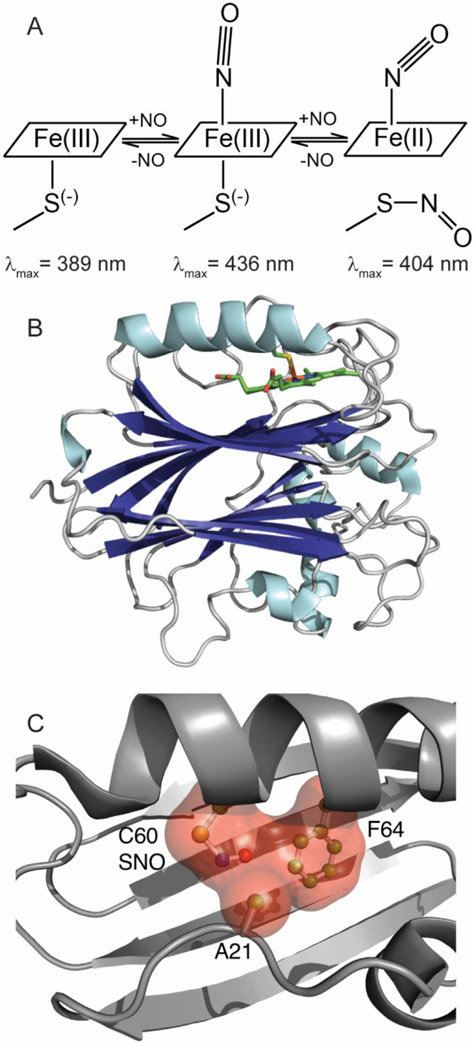
The nitrophorin from Cimex lectularius (cNP) evolved independently, have a different fold, and have unusual heme chemistry. Like the Rhodnius nitrophorins, cNP is a ferric heme protein but with a proximal cysteine rather than histidine. NO binding is again pH sensitive with tighter binding at lower pH. However, NO can bind not only to iron on the distal side of the cNP heme but can also react with the proximal cysteine to form an S-nitrosothiol. S-nitrosation (also called S-nitrosylation) is prevalent in vivo and may regulate numerous pathways [22]. Formation requires 1-electron oxidation of cysteine, leading to the neutral S-nitroso group, often referred to as a SNO modification. For cNP, SNO formation appears to be metal assisted through simultaneous reduction of heme iron. Stopped flow spectroscopy revealed sequential binding events that were completely reversible, with NO first binding to the distal side of ferric heme, followed by proximal cysteine release and reaction with NO (Fig. 1, [23]). Critically, heme is reduced in the reaction, yielding the Fe(II) (ferrous) nitrosyl complex, presumably through oxidation of the proximal cysteine, yielding a thiyl radical that can react with the NO radical to yield a SNO modification. Reversal of the reaction releases two NO molecules and restores cNP to the ferric state.
Fig. 1.
Cartoons illustrating structure and NO binding mechanism for Cimex nitrophorin. (A) NO binding is initially to the ferric protein, yielding a six-coordinate nitrosyl complex. A second NO molecule binds at higher NO concentrations, yielding a five-coordinate ferrous nitrosyl complex and S-nitrosocysteine. All steps are reversible. Soret band absorption maxima are shown for each complex. (B) Overall fold for cNP (PDB entry 1YTF). (C) Contacts among Cys-NO, Ala 21 and Phe 64 in the proximal pocket.
Here, we pursue the underlying mechanism for pH dependence in NO affinity for cNP and examine reduced cNP to test the proposed mechanism for SNO formation. We also examine how proximal pocket environment influences SNO formation and stability since little is known about the chemistry of this protein modification. We find that pH dependence is likely due to direct protonation of the proximal cysteine, that steric crowding greatly influences SNO stability and that coordinated heme reduction / cysteine oxidation takes place but is likely disfavored in the insect salivary gland.
2. Materials and Methods
2.1. Protein preparation
Expression and purification of recombinant wild type cNP and the A21V and F64V mutants were carried out as described previously [4, 23, 24]. Briefly, the apoproteins were expressed in E. coli as inclusion bodies, solubilized in 6 M guanidine hydrochloride, refolded under reducing conditions and dialyzed against 100 mM sodium phosphate buffer at pH 7. Heme was incorporated by adding a 10 mM hemin chloride (Sigma) solution until the ratio of the absorbance at 280 nm and 398 nm reached ~1:1.2. The protein was purified on Q-Sepharose ion exchange and Sephacryl S-100 columns, concentrated by ultrafiltration and equilibrated with 20 mM Tris.HCl buffer at pH 7.5. Aliquots of this solution were flash frozen in liquid N2 and stored at −80 °C. Frozen aliquots were thawed on ice and used for all experiments. Typical protein yield was ~70 mg/L of cell culture.
The A21V and F64V mutants were prepared using the QuikChange II site-directed mutagenesis kit (Stratagene) and plasmid pETcNP as a template, following the manufacturer’s recommendations. Primers were designed using the OligoPerfect™ primer design (Invitrogen) tool and obtained from the Midland Certified Reagent Company. For A21V, the forward and reverse primers used were:
5’-AGTGGACATGAACGTGTCCCAACGAATCTCGAAGAG-3’ and 5’-TCACCTGTACTTGCACAGGGTTGCTTAGAGCTTCTC-3’.
For F64V, the forward and reverse primers used were:
5’-GGCCCAGCTTGCGTGAAAAACGTCCAAAGCCTCCTTACA-3’ and 5’-CCGGGTCGAACGCACTTTTTGCAGGTTTCGGAGGAATGT-3’.
Altered codons are underlined and italicized. Mutated gene constructs were sequenced by the Arizona Genetics Core (University of Arizona).
2.2. Crystal preparations for structure determinations and crystal spectroscopy
Plate-like monoclinic crystals of wild type, A21V and F64V proteins were grown from 23% PEG 4000, 200 mM Li2SO4, 100 mM Tris.HCl, pH 8.5, and 15% ethanol, using the hanging drop method at room temperature. Typical crystals used for data collection were 0.4 × 0.4 × 0.2 mm and were in space group P21 with unit cell dimensions approximately a = 49.1 Å, b = 41.5 Å, c = 65.4 Å, β = 95.4°, with one copy of cNP in the crystal asymmetric unit. Unliganded ferric cNP crystals were equilibrated in 40% PEG 4000, 100 mM Tris.HCl, pH 8.5 for 10 minutes and flash frozen in liquid N2. NO/SNO complexes of the mutated proteins were obtained by first washing a crystal anaerobically for 1 h in an argon-saturated solution of 40% PEG 4000, 100 mM sodium citrate, pH 5.6, moving the crystal to a similar soaking solution saturated with NO for 20 min, and flash-freezing the crystal in liquid N2. NO was generated in the soaking solution in situ by using Diethylamine NONOate (DEA/NO) (kindly provided by Dr. Katrina Miranda, Department of Chemistry and Biochemistry, University of Arizona). A concentrated stock solution of DEA/NO in 10 mM NaOH was added into the soaking solution to generate ~2 mM NO as the final concentration.
Crystals of ferrous cNP were prepared by transferring the ferric cNP crystals to a septum closed test tube containing deoxygenated solution of 35% PEG 4000 buffered with 100 mM Tris.HCl pH 7 or 100 mM sodium citrate pH 5.6, or 100 mM Glycine/NaOH pH 9, and supplemented with sodium dithionite to a final concentration of 10 mM. The CO and NO complexes were obtained anaerobically by 10-min equilibration of the ferrous cNP crystals in 35% PEG 4000, 100 mM Tris.HCl pH 7.0 saturated with gaseous CO or NO at room temperature in a septum closed test tube. NO gas was purchased from Nathenson Co. and was purified by bubbling through a 1 molar solution of NaOH. All crystals for X-ray data collection and crystal spectroscopy were flash frozen in liquid nitrogen and measured at 100 K. Crystals of unliganded ferrous cNP, which were particularly sensitive to re-oxidation to the ferric state, were flash-frozen anaerobically in a glove box.
2.3. Crystal structure determinations
Diffraction measurements were obtained at beam line 9-2, Stanford SSRL, or beam line 14 BM-C, Argonne APS. All data sets were processed with d*TREK [25]. The structures were determined by molecular replacement using the ferric aqua structure (PDB entry 1NTF [23]), referred to herein as cNP(III)-H2O and isomorphous with the present crystals. Models were adjusted using the programs O [26] or COOT [27, 28], refined with Refmac5 [29] as incorporated into CCP4 [30], using standard constraints from the CCP4 library [30] except for the heme axial ligands, which were left unrestrained. Bond lengths, bond angles and torsional angles for the SNO moiety on proximal Cys 60 were restrained during refinement to values found in small-molecule crystal structures S-nitroso-N-acetyl-penicillamine, S-nitrosocaptopril, S-nitroso-L-cysteine ethyl ester hydrochloride [31-33], as previously described for S-nitroso thioredoxin (PDB entry 2IFQ) [34]. The C-S-N-O torsion angle was restrained to be 0° or 180° (planar). Wild type cNP(II)-NO/SNO (PDB entry 1Y21)[23] was re-refined with the above restraints for the sake of consistency. The ferrous cNP(II)-CO and unliganded structures were refined with anisotropic temperature factors. Heme distortions were measured with the Normal-Coordinate Structural Decomposition method [35]. All structure figures were prepared with Pymol (DeLano Scientific LLC) or ChimeraX [36].
2.4. UV-visible solution and crystal spectroscopy
Absorption spectra of frozen single crystals of cNP complexes were measured using a microspectrophotometer equipped with a CCD array detector (Spectral Instruments, Inc., Tucson, AZ) and focusing optics (4DXray Systems, Uppsala, Sweden) mounted on an optical bench. The optical elements were focused to produce a 15 μm incident beam generated by a xenon lamp light source. Crystals were mounted in cryoloops (Hampton Research), frozen in liquid nitrogen and positioned on the spectrophotometer in a 100 K nitrogen cryostream (Oxford CryoSystems, Oxford, UK). Spectra were measured in the wavelength range 450-700 nm with the incident beam approximately normal to the face of the plate-like crystals, and using crystals less than 50 μm thick, as thicker crystals exceeded the upper limit of detection by the spectrophotometer.
Solution spectra were measured at room temperature using a Cary 50 Bio UV-visible spectrophotometer. Ferrous cNP solution samples were prepared by injecting degassed 10 mg/ml protein solution into septum protected cuvettes containing argon saturated 100 mM buffer solutions of sodium phosphate pH 7, Tris.HCl pH 8, or Glycine/NaOH pH 9, each supplemented with 10 mM sodium dithionite. Ferrous complexes of cNP with NO or CO in solution were obtained anaerobically by passing CO or NO above the surface of the solutions containing ferrous cNP at pH 7 in a septum closed cuvette at room temperature.
2.5. cNP(III)-NO dissociation constants
Samples for UV-visible spectroscopy were prepared by injecting degassed protein solution into a septum-protected quartz cuvette containing argon saturated 100 mM Tris.HCl, pH 7.4, or 100 mM sodium citrate, pH 5.5, with minimal head space at room temperature (22 °C). NO titrations were performed using alkaline solutions of DEA/NO, which are stable. DEA/NO dissociates to the free amine and NO in a pH-dependent manner following first order kinetics. DEA/NO stock solutions were prepared fresh in 10 mM NaOH and quantified by absorbance at 250 nm using the extinction coefficient ε250 = 8000 M−1 cm−1, a value adjusted to account for incomplete release of NO [37]. For titration, an aliquot of stock DEA/NO in 10 mM NaOH was added to the cuvette using a Hamilton syringe. At pH 7.4, complete degradation of DEA/NO was assumed to occur after 20 min and to release two mole of NO per mole of DEA/NO, while at pH 5.5, decomposition was assumed to be complete after 5 min. Approximately 2 μM protein was used in all titration experiments. All additions were performed with Hamilton gas tight syringes and cuvettes that were septum capped and secured with parafilm. Absolute and difference spectral scans (250 nm – 700 nm) were recorded after addition of each aliquot of NO using a Cary-50 UV-Visible Spectrophotometer (Varian).
For measurements at pH 7.4, the normalized absorbance change was plotted against free NO concentration and the data were fitted to a hyperbolic function describing a simple one-ligand-one-binding site equilibrium:
| (Eq. 1) |
where is the Fe (III)-NO concentration, is the total protein concentration, is the free NO concentration, and is the NO dissociation constant. All fitting was with SigmaPlot (SPSS, Inc., Chicago, IL). Titrations were repeated five times and reported as the mean ± SD of the independent measurements.
For measurements at pH 5.5, equation 1 could not be used due to the tighter NO binding and substantial NO depletion at lower NO concentrations. We therefore turned to a mutually depleting model [16]:
| (Eq. 2) |
where is the change in absorbance at a given wavelength for a specific total ligand concentration , is the maximal absorbance change at the same wavelength, is the total protein concentration and is the NO dissociation constant. Titrations were repeated three times and reported as the mean ± SD of the independent measurements.
The concentration dependence for Cys 60-SNO formation was examined using the same approach as for Fe(III)-NO formation except that higher concentrations of NO were used and a protein concentration of ~5 μM.
3. Results
3.1. Ferrous cNP binds only one molecule of NO
S-nitrosation requires a 1-electron oxidation of sulfur, which we previously proposed occurs for Cys 60 of cNP via reduction of heme iron (Fig. 1, [23]). If this model is correct, addition of NO to ferrous cNP (cNP (II)) should yield nitrosyl heme (cNP(II)-NO) without modification of Cys 60, since heme reduction has already occurred. To test this possibility, we reduced cNP crystals with dithionite, prepared their NO and CO complexes, and determined their structures (Table 1). The resulting structures were to high resolution (1.30 – 1.65 Å) and yielded models with excellent stereochemistry.
Table 1.
Crystallographic data.
| cNP(II)- NO |
cNP(II)- CO |
cNP(II) | A21V(II)- NO/SNO |
F64V(II)- NO/SNO |
A21V(III) | F64V(III) | |
|---|---|---|---|---|---|---|---|
| Data Measurement | |||||||
| pH | 7.0 | 7.0 | 7.0 | 5.6 | 5.6 | 8.5 | 8.5 |
| X-ray source | SSRL 9-2 | APS 14BM-C | APS 14BM-C | SSRL 9-2 | SSRL 9-2 | SSRL 9-2 | SSRL 9-2 |
| Wavelength (Å) | 0.9798 | 0.9000 | 0.9000 | 0.9798 | 0.9798 | 0.9798 | 0.9798 |
| Resolution (Å) | 1.65 | 1.45 | 1.30 | 1.68 | 1.65 | 1.55 | 1.65 |
| Observed reflections | 84263 | 166682 | 503943 | 158351 | 176907 | 212447 | 166462 |
| Unique reflections | 30416 | 44739 | 64243 | 30208 | 32033 | 38982 | 32298 |
| Completeness (%)a | 95.5 (83.5) | 96.7 (99.9) | 99.3 (99.8) | 99.5 (100) | 99.9 (100) | 99.9 (100) | 99.5 (99.4) |
| I/ σ(I)a | 16.5 (4.2) | 10.4 (1.6) | 17.6 (2.4) | 13.6 (4.2) | 17.3 (3.9) | 15.4 (4.6) | 11.8 (3.8) |
| a | 0.036 (0.19) | 0.073 (0.27) | 0.067 (0.33) | 0.055 (0.27) | 0.038 (0.34) | 0.046 (0.25) | 0.063 (0.30) |
| Structure Refinement | |||||||
| /b | 0.20/0.25 | 0.20/0.26 | 0.18/0.21 | 0.17/0.22 | 0.18/0.22 | 0.22/0.26 | 0.20/0.25 |
| rmsd bonds (Å) | 0.02 | 0.02 | 0.019 | 0.020 | 0.021 | 0.021 | 0.020 |
| rmsd angles (°) | 1.9 | 1.8 | 1.8 | 1.9 | 2.1 | 2.1 | 1.9 |
| Ramachandran outliers (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| PDB entry | 1YJH | 1SI6 | 2IMQ | 4L20 | 4L21 | 4L1Y | 4L1Z |
Overall (outer shell).
Calculated from 5% of the data excluded from refinement.
The ferrous structures were very similar to their ferric counterparts, displaying RMSD values for Cα positions of 0.2 – 0.3 Å after superpositioning. cNP(II)-NO displayed clear electron density for nitrosyl heme (Fig. 2A). The Fe-NO group was unrestrained during refinement and yielded a geometry consistent with a ferrous NO complex with an Fe ─ NO bond length of 1.87 Å and an Fe ─ N ─ O bond angle of 122°[38]. This bent geometry is stabilized through a hydrogen bond between the NO nitrogen and a well-ordered water molecule (2.98 Å, Fig. 2A), which is additionally stabilized through hydrogen bonding to the Val 42 carbonyl oxygen and the Thr 87 hydroxyl group. Importantly, the sulfur atom of Cys 60 was released from heme (3.07 Å away from the Fe atom) and showed no evidence of S-nitrosation. Overall, the structure is very similar to that for cNP(II)-NO/SNO except for a minor collapse of the proximal heme pocket due to the absence of SNO and its steric conflict with Ala 21. In the presence of SNO, residues 19-23 shifted outward by ~1 Å [23]. Partially disordered Gln 56, with its side chain located in a similar position as in the cNP(III)-H2O structure [23], forms a weak hydrogen bond with the sulfur atom of Cys 60.
Fig. 2.
Structures of ferrous cNP. Shown are the heme, Cys 60 and distal pocket solvent and ligand molecules, with electron density (2Fo-Fc coefficients, 1.5 σ). The propionate group attached to pyrrole ring A has been omitted for clarity. The nitrosyl complex has released the proximal thiol but is unmodified (no SNO). (A) cNP(II)-NO. (B) cNP(II)-CO. (C) cNP(II).
The porphyrin system is moderately deformed in the structure, displaying small non-planer distortions for ruffling (0.25 Å), saddling (0.51 Å) and doming (0.20 Å). The heme Fe atom is shifted 0.16 Å out of the mean plane of the porphyrin system toward the distal NO ligand, and about 0.7 Å from its position in the cNP(III)-H2O structure. Additionally, as a consequence of losing coordination to Cys 60, the overall heme molecule is shifted toward the distal pocket by ~0.3 Å with respect to its position in the cNP(III)-H2O structure.
3.2. Ferrous cNP-CO and unliganded structures
The structure for cNP(II)-CO was determined since CO only binds to ferrous heme, providing a convenient check on heme oxidation state. The electron density for the CO molecule was clear but more spherical than expected at 1.45 Å resolution, indicating greater mobility for CO than for NO, which was anchored through hydrogen bonding. The CO ligand was modeled in a linear geometry (Fig. 2b) with an Fe ─ CO bond distance of 1.84 Å. Electron density maps calculated with CO omitted from the model confirm that a diatomic ligand was attached to the heme iron; however, the Fe ─ C ─ O bond angle could not be accurately determined. Two out of three water molecules observed in the distal pocket of cNP(III)-H2O were expelled in cNP(II)-CO, while the third water molecule remained hydrogen bonded to Val 42 and Thr 87 in much the same way as in the NO complex, but now 4.7 Å away from CO (Fig. 2B). CO binding did not induce significant changes in the distal pocket except for the phenyl ring of Phe 49, which was rotated ~20° with respect to its position in the cNP(III)-H2O structure, due to contact with CO.
The electron density for the Fe ─ S bond was weaker than in the cNP(III)-H2O structure and the bond stretched from 2.42 to 2.53 Å in the final model. The partially ordered hydrogen bond between Gln 56 and the sulfur atom of Cys 60 remains evident. The heme is more planar than in cNP(II)-NO, displaying values for ruffling, saddling and doming of only 0.15, 0.33 and 0.14 Å, respectively. Likewise, the heme iron atom is only 0.02 Å out of the mean plane of the porphyrin system.
The structure of ferrous cNP in the absence of added ligand (pH 7) was determined to 1.3 Å resolution and displayed excellent electron density for the heme pocket. The distal binding site is empty in the structure (Fig. 2C), as expected. The electron density between heme iron and the proximal Cys 60 sulfur was weak and the Fe-S distance refined to a moderately long 2.77 Å. Nonetheless, the heme iron was displaced out of the heme plane toward the proximal sulfur by 0.16 Å, consistent with weak Fe-S coordination. The porphyrin ring was slightly distorted, displaying ruffling, saddling and doming values of 0.26 Å, 0.20 Å and 0.06 Å, respectively. Gln 56 is again partially disordered but appears to form a weak hydrogen bond with Cys 60.
We sought the structure of cNP(III)-NO by including only a single NO equivalent. Although the cNP(III)-NO was clearly formed as indicated by crystal spectroscopy (described below), the complex was photoreduced to cNP(II)-NO in the X-ray beam under all conditions examined, including those where the X-ray intensity, exposure time, and wavelength were varied, and after testing multiple cryosolvents. We also examined cNP(II) at pH 9.0 to search for structural changes associated with pH-dependent changes in NO affinity; however, the heme and Cys 60 became highly disordered at this pH and the structures were not further pursued.
3.3. UV-visible spectra for cNP(II) complexes in crystal and solution
Reduction of ferric cNP with dithionite, and binding of NO or CO, were confirmed spectroscopically in both crystal and solution (Fig. 3). For cNP crystals, only the visible portion of the spectra (450 to 700 nm) could be measured due to the high optical density of the crystals. In general, absorption maxima in the crystal, recorded at 100 K, were sharper than those recorded in solution at room temperature, and were shifted to shorter wavelengths by 2 – 4 nm. These effects were most likely due to the difference in temperature; crystal spectra of nitrophorin 4 from Rhodnius prolixus recorded at room temperature, for example, were identical to those from nitrophorin 4 in solution [4].
Fig. 3.
Electronic absorption spectra of cNP complexes in solution and crystal. Shown are spectra recorded in solution (25 °C, ~15 μM cNP) between 350 and 700 nm (thin lines), and in the crystal (100 K) between 450 and 700 nm (inset, thick lines). Normalized solution spectra are also included in the insets for reference (thin lines, ~40 μM cNP). Peaks labeled in the inset are for the crystal spectra. All spectra are recorded at pH 7.0 unless otherwise noted. Where cNP was chemically reduced, the UV regions of the spectra are obscured by the reducing agent, sodium dithionite. (A) cNP(III)-H2O. (B) Dithionite reduced cNP(II) at pH 7.0 (solid line), pH 8.0 (dotted line), pH 9.0 (dashed line), and at pH 7.0 in the crystal (inset, thick line). (C) cNP(II)-NO (dithionite reduced). (D) cNP(II)-NO/SNO (formed from cNP(III) by addition of two NO equivalents). (E) cNP(II)-CO (dithionite reduced). (F) cNP(III)-NO (formed with one NO equivalent).
cNP(II) and its NO and CO complexes displayed unexpected pH-dependent spectral features. For cNP(III) at pH 7.0, spectra were as expected both in solution and in the crystal, with a Soret maximum at 389 nm in solution and good agreement between crystal and solution in the Q-band region (Fig. 3A). The prominent charge transfer band at 643 nm (crystal) is characteristic for the ferric high-spin state. Upon reduction, the Soret band shifted to 420 nm and displayed a strong shoulder at ~408 nm (Fig. 3B). Raising the pH led to an increase in the 408 nm peak, loss of the 420 nm peak, and the appearance of a shoulder at 450 nm, suggesting that a pH-dependent change in heme coordination was taking place, possibly involving protonation of the proximal cysteine. An isosbestic point at 438 nm is consistent with transition between two states. No such spectral change was observed for cNP(III) between pH values 6 and 9 (not shown); however, the pH dependence for NO binding affinity for cNP(III) [23] is also consistent with a key titratable group in the heme pocket.
The Soret band for cNP(II)-CO was sharply blue-shifted from the expected position. Proteins in the P450 family, which also have thiol-ligated hemes, all display Soret bands for the CO complex at ~450 nm. For cNP(II)-CO, the Soret band is quite sharp, as is typical for CO complexes, but dramatically shifted to 419 nm (Fig. 3E).
For cNP(III)-NO, obtained through addition of one equivalent of NO, the Soret band lies at 437 nm (Fig. 3F), a value typical for 6-coordinant low-spin species. Unfortunately, we were unable to obtain a structure of cNP(III)-NO for comparison, due to photoreduction in the X-ray beam (described above). As with cNP(III), the cNP(III)-NO complex spectra were insensitive to pH. However, addition of NO to chemically reduced cNP yields a cNP(II)-NO complex with a Soret maxima at 405 nm (Fig. 3C), a value more typical of a five-coordinate complex with proximal ligand missing, as was found crystallographically (Figure 2A). cNP(II)-NO/SNO, formed from addition of two NO equivalents to cNP(III), displays absorption spectra very similar, but not identical, to cNP(II)-NO (Figs. 3C and 3D). In cNP(II)-NO/SNO, the Soret is slightly blue-shifted to 402 nm, and the α/β bands switch in prominence, with α (~566 nm) more prominent in cNP(II)-NO/SNO and β (−536 nm) more prominent in cNP(II)-NO.
3.4. Proximal heme pocket crowding and low pH hinder Cys 60-SNO formation
To begin understanding how cNP functions as an NO transporter, we measured binding affinity at pH 5.5, the approximate pH of bedbug saliva, and pH 7.4, the pH of mammalian blood.[18, 23, 24] As expected, NO binding at lower pH was tighter, facilitating binding and storage in the salivary gland (, Fig. 4). Binding was ~60-fold weaker at higher pH (), facilitating release of NO to the victim. Care was taken in these measurements to avoid NO concentrations leading to appreciable SNO formation.
Fig. 4.
Titration of cNP with NO at pH 5.5 and 7.4. (A) Change in absorption at 437 nm (pH 5.5), plotted against total NO concentration and fitted to a mutually depleting model (see methods), yielding (n = 3). Inset: difference spectra. (B) Change in absorption at 437 nm (pH 7.4), plotted against the free NO concentration and fitted to a simple hyperbola, yielding (n = 5). Inset: difference spectra.
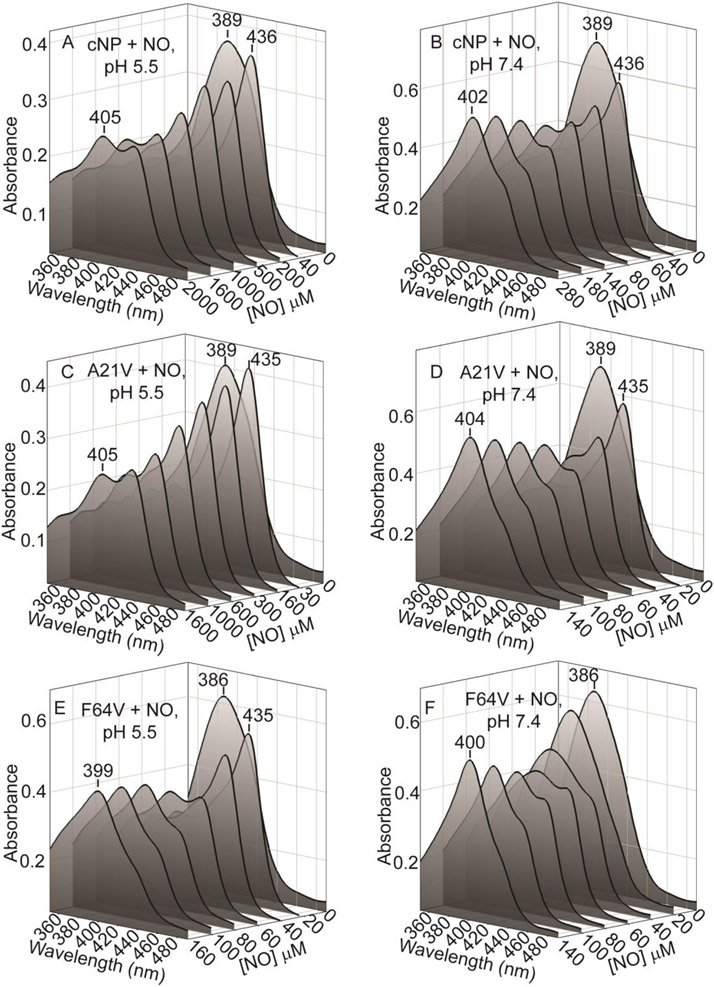
We then asked how pH and proximal pocket crowding effected SNO formation, creating mutants A21V and F64V. Ala 21 and Phe 64 lie near proximal heme ligand Cys 60, contacting the Cys 60 SNO in the cNP(II) NO/SNO complex (Fig. 1). Mutant A21V was designed to better fill the proximal pocket while mutant F64V was designed to relieve strain in the pocket. We examined SNO formation at pH values of 5.5 and 7.4 to explore the role of SNO in NO transport (Fig. 5). Upon titrating the wild type cNP at pH 5.5 (8 μM), the cNP(III)-NO complex was completely formed upon addition of 40 μM NO, indicated by the peak at 436 nm (Fig. 5A). However, SNO formation was disfavored at higher NO concentrations and only 50% SNO formation could be achieved even at saturating NO concentration (2 mM, Fig. 5A), as indicated by the equal peaks at 436 nm (cNP (III)-NO) and at 405 nm (cNP (II)-NO/SNO). Thus, unexpectedly, Cys 60 SNO appears unlikely to form to significant levels in the insect salivary glands with their low pH, possibly due to protonation of Cys 60. In contrast, titrating the wild type protein at pH 7.4 led to complete cNP(II)-NO/SNO formation at ~280 μM NO (Figure 5B), with an apparent of ~100 μM (the approximate value for 50% conversion).
Fig. 5.
NO titration with full spectra for wild type and mutant proteins. (A) cNP wild type, pH 5.5. The cNP(II)-NO/SNO species (405 nm) is ~50% at saturating NO concentrations. (B) cNP wild type, pH 7.4. The cNP(II)-NO/SNO Soret band (405 nm) is fully present at an NO concentration of 300 μM. (C) A21V, pH 5.5. The A21V(II)-NO/SNO species (405 nm) is ~50% at saturating NO concentrations. (D) A21V, pH 7.4. The A21V(II)-NO/SNO Soret band (405 nm) is fully present at an NO concentration of 140 μM. (E) F64V, pH 5.5. The F64V(II)-NO/SNO Soret band (399 nm) is fully present at an NO concentration of 160 μM (F) F64V, pH 7.4. The F64V(II)-NO/SNO Soret band (400 nm) is fully present at an NO concentration of 140 μM. Protein concentrations varied from 4-8 μM.
The A21V mutant behaved much like the wild type protein at pH 5.5, displaying ~50% Cys 60-SNO formation with near saturating NO concentration (Fig. 5C). The protein is slightly more prone to SNO formation than wild type at pH 7.4, showing complete conversion at 140 μM NO (apparent ~50 μM, Fig. 5D). In contrast, NO titration of the F64V mutant results in complete SNO formation at both pH 5.5 and pH 7.4 with ~150 μM NO (apparent ~50 μM, Figs. 5E and 5F). Unlike with wild type and A21V cNP, an independent Fe(III)-NO peak was not detected at pH 7.4, suggesting the NO concentrations needed for heme binding and SNO formation are similar in F64V and all three species (unliganded, cNP(III)-NO and cNP(II)-NO/SNO) are present at intermediate NO concentrations.
3.5. Crystal structures of cNP(II)-NO/SNO complexes for the A21V and F64V proteins
Crystal structures of A21V and F64V as unliganded and Fe(II)-NO/SNO complexes were prepared similarly to the wild type protein, yielding structures ranging from 1.55 to 1.68 Å resolution (Table 1). cNP consists of a large beta-sandwich motif with heme inserted between one face of the sandwich and an alpha helix near the N-terminus, referred to as the proximal helix (Fig. 1B). Both Cys 60 and Phe 64, are part of the proximal helix while Ala 21 is provided by the loop connecting two parallel beta strands, referred to as the proximal loop. Overall, the wild type and mutant structures are very similar, displaying RMS deviations for Cα positions of 0.3 – 0.4 Å. The heme was well ordered in all structures and slightly domed toward the distal pocket in the NO/SNO complexes (Fig. 6).
Figure 6.
Structures of A21V and F64V mutant cNP proteins, highlighting nitrosyl heme and proximal SNO geometry. (A) A21V, (2Fo-Fc coefficients, 1.0 σ). (B) F64V, (2Fo-Fc coefficients, 1.2 σ). In both structures, the Fe-N-O group has geometry consistent with a ferrous nitrosyl complex (Fe-N-O bond angle ~124° and Fe-N bond length ~1.8 Å). (C) Cross-eyed stereo view of the proximal heme pocket of A21V (magenta) superposed on cNP wild type (green, PDB entry 1Y21). Val 21 is pointing away from the heme face and the Cys 60 SNO moiety is in the trans conformation. (D) Cross-eyed stereo view of the proximal heme pocket of F64V (purple) superposed on cNP wild type (green). Val 64 is in a similar position to that of Phe 64 and the Cys 60 SNO moiety is in the cis conformation, similarly to the wild type complex.
Minor changes occurred in each of the unliganded protein heme pockets. In the structure of A21V, Val 21 is now in contact with the sulfur of Cys 60 (3.4 Å), causing it to shift slightly off axis with respect to the heme iron, which does not move. The coordinate bond to heme iron remains the same length (2.43 Å). In wild type, Phe 64 contacts heme, inducing a small, local distortion. Removal of this contact in the F64V mutant led to heme incorporating into the heme pocket in a flipped orientation (180° rotation along the axis passing through the α, γ meso positions) such that the vinyl group positions are exchanged. The vinyl group in this new position fills the void created by the F64V mutation and would be in steric conflict with the Phe side chain if it were present. A slight heme translation and loss of distortion accompanies this flipped heme position in F64V, and the Fe-S bond is slightly longer (2.47 Å) in the model. Heme in all three proteins (wild type and two mutants) appears to fully occupy only one of the two possible orientations. The distal ligand position is unoccupied in both mutant protein structures. That heme orientation can be changed through modification of the heme pocket has been previously shown for the nitrophorin from Rhodnius prolixus by Walker and colleagues (see, for example, [13]).
Addition of NO led to crystals displaying 5-coordinate heme for both mutant proteins, with NO in the distal pocket and Cys 60-SNO fully formed in the proximal pocket and detached from heme (Fig. 6), as previously described for the wild type protein. The Fe-N-O bond angles refined to 122° for A21V (Fe-N bond length 1.79 Å), and 125° for F64F (Fe-N bond length 1.80 Å), indicating that both were Fe(II)-NO bonds (Fig. 1A). The heme iron shifts toward the distal pocket and adopts a heme conformation that is slightly domed toward the distal pocket.
The Cys 60-SNO group was well defined in both proteins and each displayed geometry consistent with a planar SNO modification (Figs. 6 and 7, [23, 31, 32, 34]). SNO formation appeared complete in both proteins despite the lower pH used for forming the complex, which results in only 50% SNO formation for A21V in solution (Fig. 5). The reason for greater conversion in the crystal is unclear but may have to do with affinity differences for SNO in the crystal or from the high PEG concentration in solution while soaking the crystal, which could lead to higher NO concentrations. Cys 90, the only other cysteine in the protein, was completely reduced and unmodified in both proteins, highlighting the specificity for metal-assisted S-nitrosation.
Fig. 7.
Structures for A21V and F64V SNO moieties along with schematic representation of S-nitroso cysteine. The S-N-O linkage has a partial double bond-character, leading to a planar SNO geometry and CB-S-N-O bond dihedral () of either 180° (trans) or 0° (cis). Optimal values for and are ~165° and ~−70°, respectively. (A) trans geometry. (B) cis geometry. (C) 2Fo-Fc electron density for A21V (1.2σ). (D) 2Fo-Fc electron density for F64V (1.2σ).
The SNO groups were planar, as expected, due to partial conjugation through the S-NO bond, much as occurs in a peptide bond. Both cis and trans conformations are possible (Fig. 7). The SNO modification in F64V-NO/SNO adopts the cis conformation and is quite similar to that for the wild type protein (Fig. 7D). However, the proximal helix shifts ~0.5 Å away from the heme iron, in the direction of the cavity formed by the Phe to Val mutation. The SNO moiety rotates slightly toward Val 64 and remains in contact with the valine side chain. The proximal loop, which is free to move, shifts ~1.5 Å into the proximal pocket, allowing Ala to be in contact with the SNO moiety. The loop is also better ordered than in the other structures. Thus, the SNO modification occupies a larger pocket without strain in F64V while retaining van der Waals contact with hydrophobic groups and excluding water. This stable arrangement may explain why SNO formation is favored in F64V as compared with the wild type protein.
In contrast, A21V-NO/SNO adapts to its more constrained proximal pocket in two ways. First, the SNO moiety switches to the trans conformation, with oxygen pointing toward Val 21 (Figs. 6C and 7C). Second, Val 21 is shifted below the SNO by ~1.5 Å, creating a sandwich for the SNO moiety between the Val 21 side chain and the heme, and causing a local distortion in both heme and Val 21 (Fig. 6C).
4. Discussion
The nitrophorin from Cimex lectularius (cNP) has a pH dependence for NO binding to a ferric heme that assists the insect in storing NO at the low pH of its salivary glands and releasing NO to a victim while feeding, hindering blood coagulation and inducing local vasodilation. Unique to cNP is a metal-assisted S-nitrosation of the proximal cysteine, Cys 60 [23]. Here, we explore the mechanisms behind pH dependent NO delivery, heme assisted S-nitrosation, and the effect of steric crowding on S-nitrosothiol formation and stability. We find that Cys 60 is likely protonated at low pH, enhancing NO binding to heme and interfering with S-nitrosothiol formation. Steric crowding also interferes with S-nitrosation while an uncrowded, hydrophobic, proximal pocket enhances S-nitrosation. A model for obligate linking of heme reduction with S-nitrosation is supported.
4.1. Proximal cysteine nitrosation in cNP requires sulfur oxidation by ferric heme
cNP is a ferric heme thiolate protein. When exposed to NO, two NO molecules can bind, one to heme in the distal site and one to the proximal cysteine, forming an S-nitrosyl adduct (SNO). Previous stopped flow experiments suggested these were sequential events: NO first bound to the ferric heme, which then released and oxidized the proximal cysteine, generating a thiyl radical that can then react with NO, itself a radical, to yield SNO (Fig. 1). Here, we tested this proposed mechanism by first chemically reducing the heme, yielding ferrous heme, and adding NO. The resulting crystal structure (Fig. 2) and crystal spectra (Fig. 3) clearly show a ferrous five-coordinate nitrosyl heme with unmodified proximal cysteine now detached from the heme.
Two important themes emerge from these results. First, these results highlight that SNO formation requires oxidation of the cysteine sulfur for SNO formation to occur. SNO formation, often called S-nitrosylation, is widely studied as a signaling mechanism in cells producing NO (reviewed in [22]) but how a thiyl radical is produced in such cells, allowing for SNO formation, is poorly described. Here, a metal-assisted mechanism is in play: ferric heme, in contact with proximal Cys 60, directly oxidizes the Cys 60 sulfur, yielding a thiyl radical and SNO formation, becoming reduced to ferrous heme in the process. Importantly, Cys 90, which lies in a hydrophobic pocket near the protein surface, remains unmodified by NO, highlighting the need for an oxidant for SNO formation.
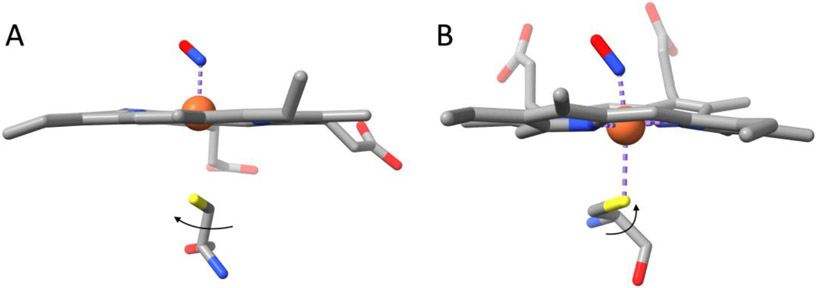
Second, heme coordination geometry is apparently important for thiyl radical formation and S-nitrosation. SNO formation has not been described for other heme-thiolate proteins, even those that produce NO such as nitric oxide synthase (NOS, see, for example, [39]). The reason for this may be linked to the cysteine coordination geometry. In cNP, the cysteine is placed such that the coordinating sulfur atom cannot readily rotate about the Cα-Cβ bond to follow heme iron if it pulls away, which is exactly what happens when NO binds to the distal coordination site (Fig. 8). The iron in the nitrosyl complex moves toward the distal pocket, leaving a detached cysteine ligand behind on the proximal side. This arrangement apparently facilitates oxidation of the thiolate to thiyl radical, which is then trapped through reaction with a second molecule of NO, forming SNO. The resulting five coordinate Fe(II)-NO complex is extremely stable, having a dissociation constant in the picomolar range [40], further facilitating SNO formation. In contrast, NOS, cytochrome P450, and related proteins, have a proximal cysteine that is rotated by about 90° with respect to the proximal cysteine in cNP (Fig. 8). In this orientation, the cysteine sulfur is free to rotate about the Cα-Cβ bond toward the heme iron and remain in contact, even if the heme iron moves toward the distal side of the heme for ligation with NO. In the example shown in Fig. 8, the ferric nitrosyl complex with neuronal NOS (nNOS) was prepared, photoreduced in the X-ray beam (confirmed by crystal spectroscopy), and the structure of the ferrous nitrosyl complex determined [39]. The complex is therefore directly comparable to the ferrous nitrosyl complex of cNP with the major difference being that the proximal cysteine is free to follow heme iron movement and remain coordinated to the iron. SNO formation does not occur. Similar results have been reported for endothelial NOS [41], bacterial NOS [42], and nitric oxide reductase [43]. SNO formation in a synthetic heme thiolate small molecule was proposed based on extensive kinetic and spectroscopic analyses, following a mechanism equivalent to that shown in Fig. 1 [44]. The authors also noted that SNO formation does not occur in cytochrome P450cam.
Fig. 8.
Comparison of proximal thiol orientation in cNP and NOS nitrosyl complexes. (A) cNP(II)-NO. The proximal cysteine sulfur is oriented such that it cannot rotate about the Cα-Cβ bond to follow heme iron as it moves to the distal pocket. (B) NOS (PDB entry 3HSP, [39]). The proximal cysteine sulfur can rotate toward the heme to retain coordination with heme iron.
4.2. The pH dependence for NO binding may be through protonation of the proximal cysteine
A key feature of cNP is its increased affinity for NO at the lower pH of the bedbug salivary gland and decreased affinity at the higher pH of a victim’s blood, leading to NO release, local vasodilation, and improved feeding for the insect. How this is achieved remains unclear. For the nitrophorin from Rhodnius prolixus (rNP), the kissing bug, tighter binding at low pH is achieved through a conformational change that closes the heme pocket and traps NO inside [5, 6, 9, 17]. For cNP, which has a completely different fold than rNP, no such change in conformation is seen at different pH values [23]. So how is NO affinity modulated?
Several lines of evidence suggest that proximal cysteine Cys 60 exists in a thiol/thiolate equilibrium in cNP and that protonation of Cys 60 is responsible for enhancing NO affinity at lower pH. First, the cNP(II)-CO complex (pH 7.0) has an unusually long bond between heme iron and proximal cysteine sulfur (2.53 Å) and an unusually blue-shifted Soret maxima (420 nm). Typically, the heme iron – cysteine sulfur bond length is ~2.4 Å for a ferrous-CO complex (see, for example, cytochrome P450CAM-CO [45]). Protonation of proximal cysteine, generating the thiol adduct, would be expected to lengthen the Fe-S bond and has been suggested to lead to a blue-shift in the Soret band, as occurs in cytochrome P420CAM [46] and, likely, in cNP. Second, the Soret band shift of cNP(II) observed as pH is increased (Fig. 3B) is consistent with protonation and thiol formation at lower pH. Third, decreased S-nitrosation at lower pH is also consistent with thiol formation. Fourth, stopped flow measurements display two on rates for NO at lower pH but only one at higher pH, consistent with a Cys 60 thiol/thiolate mixture at low pH but only thiolate at neutral pH [47]. Finally, proximal ligand strength is a primary factor in controlling distal ligand affinity (and vice versa, [48]). Thiolate (RS−) ligand can exert a much larger trans effect on the distal ligand, weakening the distal bond strength, than either thiol or thioether ligands [46, 49].
Taken together, these factors suggest the increased NO affinity seen at lower pH in cNP(III)-NO is due to protonation of proximal Cys 60, yielding the smaller trans effect of thiol ligation, while the weaker NO affinity seen at higher pH is due to deprotonation of Cys 60, yielding the larger trans effect of thiolate ligation. One additional factor likely increases NO affinity even further: NO binding to heme iron should shift heme iron from doming towards the proximal side of the heme (0.36 Å, [23]) to doming on the distal side of the heme, further lengthening and weakening the Cys 60 S-Fe bond, since the Cys 60 orientation prevents the sulfur atom from following the heme iron (Fig. 8).
4.3. Steric crowding interferes with SNO formation
Although S-nitrosation is prevalent in biology and regulates numerous processes, the factors governing SNO formation and stability remains unclear (see, for example, [50, 51]). Here, we examine how the local environment influences metal-assisted SNO formation in cNP. The hydrophobic proximal heme pocket where SNO resides in cNP(II) NO/SNO expands on SNO formation, making room for the SNO modification. We asked if additional space would facilitate SNO formation (mutant F64V) and if additional crowding would hamper SNO formation (A21V). Creating additional room for SNO in the proximal pocket with the F64V mutant clearly increased SNO formation, particularly at low pH (Fig. 5). For wild type cNP, only 50% of the protein could be S-nitrosated at pH 5.5, whereas for F64V, complete S-nitrosation occurred with apparent ~ 50 μM. SNO formation was similar for the two proteins at pH 7.4.
Additional crowding in the proximal heme pocket through the A21V mutation led to a protein that behaved similarly to wild type with respect to SNO formation. However, structurally, the SNO modification went from cis planar to trans planar (Fig. 6). The CSNO moiety is planar due to electron delocalization through the C-S-N-O π bonds. The stabilization energy for this is substantial: the barrier for rotating about the C-S-N-O dihedral is estimated to be 12 kcal/mol [52], meaning the geometry on formation (cis or trans) likely persists, and that formation in the A21V mutant occurs from the opposite side of the Cys 60 sulfur in A21V as compared to the wild type protein.
4.4. Conclusion
We conclude that cNP has evolved to disfavor SNO formation in the insect salivary gland, perhaps to limit unwanted and deleterious side reactions. Saturation of the distal site at lower pH requires only nanomolar NO concentration (), well separated from the NO levels required for SNO formation.
Highlights.
The bedbug delivers nitric oxide to victims to increase blood flow
Nitric oxide is delivered via nitrophorin using a ferric heme
Binding affinity is increased at low pH by protonating the heme proximal cysteine
Proximal cysteine can also be nitrosated after oxidation by heme iron
Crowding in the proximal heme pocket hinders S-nitrosothiol formation
Acknowledgements
We thank Jacqueline Brailey for protein purification, Abreeza Zegeer for crystal preparations, Dr. Katrina Miranda for the kind gift of DEA/NO, Robert E. Berry for discussions on titration data fitting and Dr. Gordon Tollin and Dr. James T. Hazzard for discussions on SNO formation and stability. This work was supported by grants from the National Institutes of Health (HL062969 (W.R.M), GM117357 (W.R.M) and U54 CA143924 (W.R.M.). Diffraction measurements at BioCars Sector 14, Advanced Photon Source, Argonne National Laboratory, were supported by DOE Contract W-31-109-Eng-38 and NCRR Grant RR07707. Portions of this research were carried out at the Stanford Synchrotron Radiation Laboratory, a national user facility operated by Stanford University on behalf of the U.S. Department of Energy, Office of Basic Energy Sciences. The SSRL Structural Molecular Biology Program is supported by the Department of Energy, Office of Biological and Environmental Research, and by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program, and the National Institute of General Medical Sciences.
Abbreviations
- cNP
nitrophorin from Cimex lectularius
- cNP(II)
ferrous cNP
- cNP(III)
ferric cNP
- cNP(II)-NO
ferrous cNP with heme-ligated nitric oxide and unmodified proximal cysteine
- cNP(II)-NO/SNO
ferrous cNP with heme-ligated nitric oxide and S-nitrosated proximal cysteine
- rNP
nitrophorin from Rhodnius prolixus
- SNO
S-nitrosocysteine
Footnotes
Dedication
This manuscript is written in memory of our friend and colleague, Professor Frances Ann Walker. Together, we unraveled the chemistry and biochemistry of nitric oxide transport by the nitrophorins, ferriheme proteins that deliver nitric oxide from the salivary glands of certain blood-feeding insects to the tissues of unsuspecting victims, giving rise to vasodilation, reduced blood coagulation, and improved feeding for the insect. Ann brought her immense understanding of heme chemistry to bear on the problem of nitric oxide transport by the nitrophorins, teaching us much about heme function along the way. Her enthusiasm for impactful science as well as for student and colleague success was a joy to us all. She is missed.
CrediT authorship contribution statement
Hemant Badgandi: Conceptualization, Methodology, Investigation, Formal Analysis, Writing – original draft. Andrzej Weichsel: Conceptualization, Methodology, Investigation, Formal Analysis, Writing – original draft. William R. Montfort: Conceptualization, Methodology, Formal Analysis, Writing – original draft, review & editing, Funding aquisition.
Declaration of Competing Interest
The authors declare no competing financial conflicts.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data availability
Data will be made available on request. Coordinates and structure factors have been deposited in the Protein Data Bank as entries 4L1Y, 4L1Z, 4L20, 4L21, 1YJH, 1SI6 and 2IMQ.
References
- [1].Walker FA. Nitric oxide interaction with insect nitrophorins and thoughts on the electron configuration of the {FeNO}6 complex. J. Inorg. Biochem 2005, 99:216–236. [DOI] [PubMed] [Google Scholar]
- [2].Montfort WR, Weichsel A, Andersen JF. Nitrophorins and related antihemostatic lipocalins from Rhodnius prolixus and other blood-sucking arthropods. Biochim. Biophys. Acta 2000, 1482:110–118. [DOI] [PubMed] [Google Scholar]
- [3].Andersen JF. Structure and mechanism in salivary proteins from blood-feeding arthropods. Toxicon. 2010, 56:1120–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Maes EM, Roberts SA, Weichsel A, Montfort WR. Ultrahigh Resolution Structures of Nitrophorin 4: Heme Distortion in Ferrous CO and NO Complexes. Biochemistry. 2005, 44:12690–12699. [DOI] [PubMed] [Google Scholar]
- [5].Kondrashov DA, Roberts SA, Weichsel A, Montfort WR. Protein functional cycle viewed at atomic resolution: conformational change and mobility in nitrophorin 4 as a function of pH and NO binding. Biochemistry. 2004, 43:13637–13647. [DOI] [PubMed] [Google Scholar]
- [6].Weichsel A, Andersen JF, Roberts SA, Montfort WR. Reversible nitric oxide binding to nitrophorin 4 from Rhodnius prolixus involves complete distal pocket burial. Nat. Struct. Biol 2000, 7:551–554. [DOI] [PubMed] [Google Scholar]
- [7].Roberts SA, Weichsel A, Qiu Y, Shelnutt JA, Walker FA, Montfort WR. Ligand-Induced Heme Ruffling and Bent NO Geometry in Ultra-High Resolution Structures of Nitrophorin 4. Biochemistry. 2001, 40:11327–11337. [DOI] [PubMed] [Google Scholar]
- [8].Andersen JF, Montfort WR. Crystal structures of nitrophorin 2: A trifunctional antihemostatic protein from the saliva of Rhodnius prolixus. J. Biol. Chem 2000, 275:30496–30503. [DOI] [PubMed] [Google Scholar]
- [9].Maes EM, Weichsel A, Andersen JF, Shepley D, Montfort WR. Role of binding site loops in controlling nitric oxide release: structure and kinetics of mutant forms of nitrophorin 4. Biochemistry. 2004, 43:6679–6690. [DOI] [PubMed] [Google Scholar]
- [10].Andersen JF, Ding XD, Balfour C, Shokhireva TK, Champagne DE, Walker FA, et al. Kinetics and equilibria in ligand binding by nitrophorins 1-4: Evidence for stabilization of a NO-ferriheme complex through a ligand-induced conformational trap. Biochemistry. 2000, 39:10118–10131. [DOI] [PubMed] [Google Scholar]
- [11].Kubo M, Gruia F, Benabbas A, Barabanschikov A, Montfort WR, Maes EM, et al. Low-frequency mode activity of heme: femtosecond coherence spectroscopy of iron porphine halides and nitrophorin. J. Am. Chem. Soc 2008, 130:9800–9811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Shokhireva T, Weichsel A, Smith KM, Berry RE, Shokhirev NV, Balfour CA, et al. Assignment of the ferriheme resonances of the low-spin complexes of nitrophorins 1 and 4 by (1)H and (13)C NMR spectroscopy: comparison to structural data obtained from X-ray crystallography. Inorg. Chem 2007, 46:2041–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Shokhireva TK, Berry RE, Zhang H, Shokhirev NV, Walker FA. NMR studies of nitrophorin distal pocket side chain effects on the heme orientation and seating of NP2 as compared to NP1. J. Inorg. Biochem 2011, 105:1238–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shokhireva T, Berry RE, Uno E, Balfour CA, Zhang H, Walker FA. Electrochemical and NMR spectroscopic studies of distal pocket mutants of nitrophorin 2: stability, structure, and dynamics of axial ligand complexes. Proc. Natl. Acad. Sci. USA 2003, 100:3778–3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Berry RE, Shokhirev MN, Ho AY, Yang F, Shokhireva TK, Zhang H, et al. Effect of mutation of carboxyl side-chain amino acids near the heme on the midpoint potentials and ligand binding constants of nitrophorin 2 and its NO, histamine, and imidazole complexes. J. Am. Chem. Soc 2009, 131:2313–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Berry RE, Ding XD, Shokhireva T, Weichsel A, Montfort WR, Walker FA. Axial ligand complexes of the Rhodnius nitrophorins: reduction potentials, binding constants, EPR spectra, and structures of the 4-iodopyrazole and imidazole complexes of NP4. J. Biol. Inorg. Chem 2004, 9:135–144. [DOI] [PubMed] [Google Scholar]
- [17].Kondrashov DA, Montfort WR. Nonequilibrium dynamics simulations of nitric oxide release: comparative study of nitrophorin and myoglobin. J. Phys. Chem. B 2007, 111:9244–9252. [DOI] [PubMed] [Google Scholar]
- [18].Valenzuela JG, Walker FA, Ribeiro JMC. A salivary nitrophorin (nitric-oxide-carrying hemoprotein) in the bedbug Cimex lectularius. J. Exp. Biol 1995, 198:1519–1526. [DOI] [PubMed] [Google Scholar]
- [19].Ribeiro JMC, Walker FA. High affinity histamine-binding and antihistaminic activity of the salivary nitric oxide-carrying heme protein (nitrophorin) of Rhodnius prolixus. J. Exp. Med 1994, 180:2251–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Weichsel A, Andersen JF, Champagne DE, Walker FA, Montfort WR. Crystal structures of a nitric oxide transport protein from a blood-sucking insect. Nat. Struct. Biol 1998, 5:304–309. [DOI] [PubMed] [Google Scholar]
- [21].Mizurini DM, Francischetti IM, Andersen JF, Monteiro RQ. Nitrophorin 2, a factor IX(a)-directed anticoagulant, inhibits arterial thrombosis without impairing haemostasis. Thromb Haemost. 2010, 104:1116–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: purview and parameters. Nat. Rev. Mol. Cell Biol 2005, 6:150–166. [DOI] [PubMed] [Google Scholar]
- [23].Weichsel A, Maes EM, Andersen JF, Valenzuela JG, Shokhireva T, Walker FA, et al. Heme-assisted S-nitrosation of a proximal thiolate in a nitric oxide transport protein. Proc. Natl. Acad. Sci. USA 2005, 102:594–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Valenzuela JG, Ribeiro JMC. Purification and cloning of the salivary nitrophorin from the hemipteran Cimex lectularius. J. Exp. Biol 1998, 201:2659–2664. [DOI] [PubMed] [Google Scholar]
- [25].Pflugrath JW. The finer things in X-ray diffraction data collection. Acta Crystallogr. 1999, D55:1718–1725. [DOI] [PubMed] [Google Scholar]
- [26].Jones TA, Zou JY, Cowan SW, Kjelgard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. 1991, A47:110–119. [DOI] [PubMed] [Google Scholar]
- [27].Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr 2004, 60:2126–2132. [DOI] [PubMed] [Google Scholar]
- [28].Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr 2010, 66:486–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Murshudov GN, Vagin AA, Dodson EJ. Refinement of Macromolecular Structures by the Maximum-Likelihood Method. Acta Crystallogr. 1997, D53:240–255. [DOI] [PubMed] [Google Scholar]
- [30].Collaborative Computational Project Number 4. The CCP4 Suite: Programs for Protein Crystallography. Acta Crystallogr. D Biol. Crystallogr 1994, D50:760–763. [DOI] [PubMed] [Google Scholar]
- [31].Bartberger MD, Houk KN, Powell SC, Mannion JD, Lo KY, Stamler JS, et al. Theory, spectroscopy, and crystallographic analysis of S-nitrosothiols: Conformational distribution dictates spectroscopic behavior. J. Am. Chem. Soc 2000, 122:5889–5890. [Google Scholar]
- [32].Arulsamy N, Bohle DS, Butt JA, Irvine GJ, Jordan PA, Sagan E. Interrelationships between conformational dynamics and the redox chemistry of S-nitrosothiols. J. Am. Chem. Soc 1999, 121:7115–7123. [Google Scholar]
- [33].Yi J, Coppens P, Powell DR, Richter-Addo GB. Linkage Isomerization in Nitrosothiols (RSNOs): The X-ray Crystal Structure of an S-nitrosocysteine and DFT Analysis of its Metastable MS1 and MS2 Isomers. Comments Inorg Chem. 2016, 36:81–91. [Google Scholar]
- [34].Weichsel A, Brailey JL, Montfort WR. Buried S-Nitrosocysteine Revealed in Crystal Structures of Human Thioredoxin. Biochemistry. 2007, 46:1219–1227. [DOI] [PubMed] [Google Scholar]
- [35].Jentzen W, Song X-Z, Shelnutt JA. Structural Characterization of Synthetic and Protein-Bound Porphyrins in Terms of the Lowest-Frequency Normal Coordinates of the Macrocycle. J. Phys. Chem. B 1997, 101:1684–1699. [Google Scholar]
- [36].Pettersen EF, Goddard TD, Huang CC, Meng EC, Couch GS, Croll TI, et al. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci. 2021, 30:70–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Maragos CM, Morley D, Wink DA, Dunams TM, Saavedra JE, Hoffman A, et al. Complexes of .NO with nucleophiles as agents for the controlled biological release of nitric oxide. Vasorelaxant effects. J. Med. Chem 1991, 34:3242–3247. [DOI] [PubMed] [Google Scholar]
- [38].Wyllie GR, Schulz CE, Scheidt WR. Five- to six-coordination in (nitrosyl)iron(II) porphyrinates: effects of binding the sixth ligand. Inorg Chem. 2003, 42:5722–5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Doukov T, Li H, Soltis M, Poulos TL. Single crystal structural and absorption spectral characterizations of nitric oxide synthase complexed with N(omega)-hydroxy-L-arginine and diatomic ligands. Biochemistry. 2009, 48:10246–10254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Tsai AL, Berka V, Martin E, Olson JS. A "sliding scale rule" for selectivity among NO, CO, and O(2) by heme protein sensors. Biochemistry. 2012, 51:172–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Li H, Raman CS, Martasek P, Masters BS, Poulos TL. Crystallographic studies on endothelial nitric oxide synthase complexed with nitric oxide and mechanism-based inhibitors. Biochemistry. 2001, 40:5399–5406. [DOI] [PubMed] [Google Scholar]
- [42].Pant K, Crane BR. Nitrosyl-heme structures of Bacillus subtilis nitric oxide synthase have implications for understanding substrate oxidation. Biochemistry. 2006, 45:2537–2544. [DOI] [PubMed] [Google Scholar]
- [43].Shimizu H, Obayashi E, Gomi Y, Arakawa H, Park SY, Nakamura H, et al. Proton delivery in NO reduction by fungal nitric-oxide reductase. Cryogenic crystallography, spectroscopy, and kinetics of ferric-NO complexes of wild-type and mutant enzymes. J. Biol. Chem 2000, 275:4816–4826. [DOI] [PubMed] [Google Scholar]
- [44].Franke A, Stochel G, Suzuki N, Higuchi T, Okuzono K, van Eldik R. Mechanistic studies on the binding of nitric oxide to a synthetic heme-thiolate complex relevant to cytochrome p450. J Am Chem Soc. 2005, 127:5360–5375. [DOI] [PubMed] [Google Scholar]
- [45].Raag R, Poulos TL. Crystal structure of the carbon monoxide-substrate-cytochrome P-450CAM ternary complex. Biochemistry. 1989, 28:7586–7592. [DOI] [PubMed] [Google Scholar]
- [46].Perera R, Sono M, Sigman JA, Pfister TD, Lu Y, Dawson JH. Neutral thiol as a proximal ligand to ferrous heme iron: implications for heme proteins that lose cysteine thiolate ligation on reduction. Proc. Natl. Acad. Sci. USA 2003, 100:3641–4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Badgandi HB. Biology Facilitated by Heme Proteins as seen in Cimex Nitrophorin and Ecdysone Inducible Protein 75: The University of Arizona; 2009. [Google Scholar]
- [48].Spiro TG, Zgierski MZ, Kozlowski PM. Stereoelectronic factors in CO, NO and O2 binding to heme from vibrational spectroscopy and DFT analysis. Coord. Chem. Rev 2001, 219-221:923–936. [Google Scholar]
- [49].Hu S, Kincaid JR. Resonance Raman Characterization of Nitric Oxide Adducts of Cytochrome P450cam: The Effect of Substrate Structure on the Iron-Ligand Vibrations. J. Am. Chem. Soc 1991, 113:2843–2850. [Google Scholar]
- [50].Pratyush P, Pokharel S, Saigo H, Kc DB. pLMSNOSite: an ensemble-based approach for predicting protein S-nitrosylation sites by integrating supervised word embedding and embedding from pre-trained protein language model. BMC Bioinformatics. 2023, 24:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Stomberski CT, Hess DT, Stamler JS. Protein S-Nitrosylation: Determinants of Specificity and Enzymatic Regulation of S-Nitrosothiol-Based Signaling. Antioxid Redox Signal. 2019, 30:1331–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zhao YL, Houk KN. Thionitroxides, RSNHO(*): The Structure of the SNO Moiety in "S-Nitrosohemoglobin", A Possible NO Reservoir and Transporter. J. Am. Chem. Soc 2006, 128:1422–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request. Coordinates and structure factors have been deposited in the Protein Data Bank as entries 4L1Y, 4L1Z, 4L20, 4L21, 1YJH, 1SI6 and 2IMQ.