Abstract
This article comments on:
Bishal G. Tamang, Yanqun Zhang, Michelle A. Zambrano and Elizabeth A. Ainsworth Anatomical determinants of gas exchange and hydraulics vary with leaf shape in soybean, Annals of Botany, Volume 131, Issue 6, 9 May 2023, Pages 909–920, https://doi.org/10.1093/aob/mcac118
Keywords: Leaf anatomy, allometry, shape, size, leaf geometry, leaf economics
Over the last two decades, trait-based plant ecology has been dominated by studies designed around the Leaf Economics Spectrum (LES), a framework that collapses variance in leaf traits onto a single axis based on the observed global trade-off between acquisitive and conservative investment strategies (Wright et al., 2004). The introduction of this paradigm reflected a phenomenon characteristic of biological research, wherein high-impact studies seeking universal trade-offs or biological ‘rules’ governing structure, behaviour or function (e.g. Wright et al., 2004; Díaz et al., 2016; and reviewed by Glazier, 2010) inspire detailed responses characterizing ‘exceptions’ to the established theoretical framework (see Agutter and Wheatley, 2004; Isaac and Carbone, 2010). In many cases, the systems that break the rules are based upon replications or empirical tests of global patterns at smaller scales, for example within a genus or species. Many such clade-specific studies show the dissipation (e.g. Niinemets, 2015a), or even reversal (e.g. Anderegg et al., 2018), of LES trends. Despite repeated recognition of the pattern bias introduced by theoretical simplification (e.g. Niinemets et al., 2015b; Chen et al., 2020; Westerband et al., 2021) and exceptional advancements in our capacity to collect and manipulate large, complex datasets over the past decade (Piao et al., 2019; York, 2019; Yang et al., 2020), we have limited our epistemic advancement in thoughtful reductionism by continuing to define organisms by their coarse functional traits. As a result, we risk critically oversimplifying the processes driving complex biological patterns and ontologies by neglecting parameters that contribute mechanistically to those processes.
In this issue of Annals of Botany, Tamang and co-authors explore the drivers of canopy-scale photosynthesis using leaf anatomical and compositional traits quantified across distinct genetic lines of soybeans. They report higher area-based rates of photosynthetic assimilation in thicker, narrower leaves with relatively high leaf mass per area (LMA). As in the works referenced above, this finding contradicts the now classic and universally cited framework for leaf economics described by Wright et al. (2004). While this reversed pattern is common in crop species, for which anthropogenic breeding programmes have selected trait combinations orthologous to the LES (e.g. high investment in both rapid assimilation and long lifespan) and an over-investment in light-harvesting tissues (Hikosaka and Hirose, 1997; Slattery and Ort, 2021), the phenomenon is not constrained to crop species. Several studies have reported intraspecific (Mason and Donovan, 2015; Fletcher et al., 2018) and even intracanopy (Niinements et al., 2015b) deviations from the LES framework.
The failure of the LES to hold within species and individual crowns raises concerns about extrapolations based on allometric simplifications of physiological models, as well as reliance on species means, in trait-based ecology. Here, Tamang et al. (2023) address this point and respect mechanistic processes by allowing the functional proximity of traits to guide and constrain their choice of allometric simplifications used to theoretically define those processes. For example, their approach favours distinct analyses of leaf length, width and thickness, which have more direct links to gas diffusion, light interception and hydraulic resistance relative to constitutive traits such as LMA.
Disentangling area and thickness makes strong physiological sense. For example, a shift in leaf area in the axial plane has dramatic impacts on the bulk surface for light capture, while a shift in leaf thickness alters the internal surface area available for gas exchange (Théroux-Rancourt et al., 2017). Based on the data of Tamang et al., thicker leaves feature a greater proportion of spongy mesophyll, suggesting an even more substantial investment in internal surface area for gas exchange (Turrell, 1933). Increased diffusive surface area may explain this reported phenomenon of increased conductance rates (both internal, liquid-phase leaf hydraulic conductance, Kleaf, and external, gas-phase stomatal conductance, gs; Nobel, 1977; Tomás et al., 2013; Buckley et al., 2015, 2017; Earles et al., 2018) in thicker leaves with smaller projected area. Taken together, these functional considerations provide an explanation at the leaf level for why soybean canopies with narrower leaves (larger length-to-width ratios) show increased productivity.
One perspective that is perhaps under-explored in the Tamang et al. paper is the non-independence of leaf shape and size embedded in the definition of leaf narrowness. By collapsing ‘leaf shape’ into a single geometric proportion of length and width, leaf ratio (LR) serves as an informative natural variable, yet is not representative of shape, but rather geometry (i.e. ‘slenderness’ or ‘fineness’; Niklas, 1994). As soy leaves express compound leaves comprised of lancet leaflets with entire margins, projected leaf area (LA) then has a necessary dependence on length-to-width ratio, as is apparent in the strong, negative correlation between LR to LA in their Supplementary Data. This would not, however, always be the case in leaves with diverse, complex morphologies. Consistent with the analyses of Tamang et al., we explored allometric scaling relationships across a theoretical shape continuum of cotton leaves (Gossypium hirsutum L).
Cotton, not only an important agricultural crop, represents one of the first species for which leaf morphology was comprehensively studied toward understanding leaf development (Hammond, 1941). Leaves of cotton range from semi-stellate with entire margins to highly dissected, deeply lobed (‘okra’) leaves, purposefully developed through the alteration of a single genetic locus (Andres et al., 2016). Given its prominent role in furthering leaf morphometrics (Andres et al., 2017), we used cotton to test the independent effects of shape and size using an index of leaf shape based on a simplified calculation of ‘compactness’ that mathematically corrects for the intrinsic effect of leaf size such that
where LS is a unitless index of shape, P is the leaf perimeter (cm) and A is the leaf area (cm2), both of the latter two measured excluding the leaf petiole. This index estimates deviation from the null model of a perfect circle, such that larger scores denote more complex shapes. We made calculations for LR, LS and LA for leaf projections generated by progressively manipulating the shape of a typical cotton leaf from entire to deeply lobed.
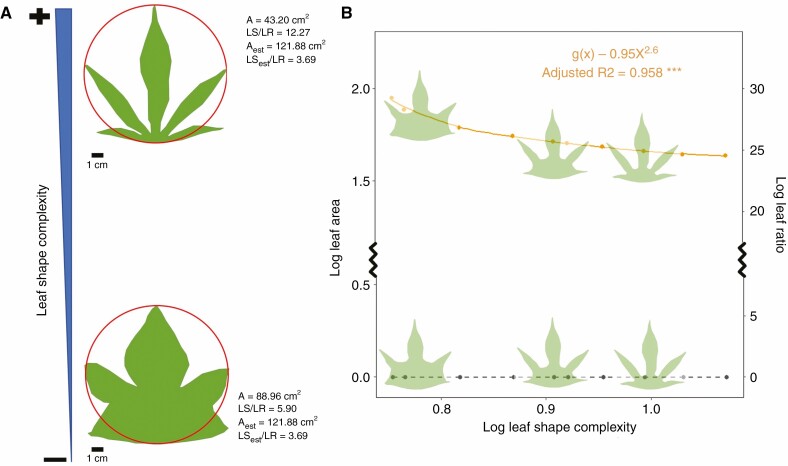
While the index used by Tamang et al. produced a geometric autocorrelation between LR and LA in soy, when parameterized with our simulated morphologically diverse leaves, this autocorrelation was absent such that LR was constant and A decreased with complexity. This change in significance is driven entirely by the introduction of leaf lobing (Fig. 1A). In the case of lobed leaves, LR biases datasets that include diverse leaf shapes, as the degree to which this effect dominates the LR–A autocorrelation is strongly dependent upon leaf complexity. The size-independent shape index, LS, for our highly lobed leaf is over 12-fold greater than LR, while a simple stellate leaf only expresses less than 6-fold difference in LS and LR (Fig. 1A). If we, instead, consider the projected shape based only on leaf length and width (red lines in Fig. 1A) we achieve convergence in LS/LR between the two leaves despite a much lower projected area in the deeply lobed leaf and visible differences in shape. Indeed, for our cotton model, LR could vary independently of LS, such that leaves with more complex margins could maintain constant ‘complexity’ based on the LR reported by Tamang et al. (black points in Fig. 1B), highlighting the possible conflation of shape and size and the inability of dimensions to capture realized leaf shape.
Fig. 1.
Analysis of leaf shape across a theoretical continuum of cotton leaf morphologies. Two leaves with identical ‘shapes’ under the approach of Tamang et al., wherein ‘shape’ is quantified as a geometric proportion of length and width (leaf ratio; LR), can feature disparate ‘shapes’ under our proposed leaf shape complexity (LS) index (A). The effect of lobing or expansion of margin lacunae on shape calculations can be examined based upon the relative outcomes of calculations of LS and LR informed with true leaf area (A) or leaf area estimated from length and width (i.e. ignoring lobing; Aest). While LR and LS remain equal across leaf shapes when leaves are assumed to be ovate (red lines in A) as in the LR model, a size-dependent effect appears for complex leaves such that LR is less predictive of true shape at high shape complexities. As a result, under increasing LS and constant LR, A intrinsically, geometrically decreases (B; yellow), but LR is independent of LS and remains constant across observable shifts in leaf shape (B, black). Fitted linear slopes, multiple R2 and P-values are reported. Asterisks denote *P < 0.05, **P < 0.01 and ***P < 0.001 (α = 0.05).
Notably, the intrinsic link between shape and size extends beyond straightforward, geometric autocorrelation. Even when geometrically correcting for differences in size, we found negative correlations between shape and size across cotton leaves when leaf length and width were held constant (yellow points in Fig. 1B). However, given that our dataset was generated through the physical manipulation of shape, rather than observations in situ, this trend is unlikely to reflect a physiological driver of categorical shifts in LS not captured by LR. Both within and across real canopies, for which dimensions are not strictly constrained, such physiological associations with shape and size, correlative trait networks, and trait multi-functionality (see Sack and Buckley, 2020) undoubtedly complicate these trends. Allometries not captured by this theoretical approach, for example those arising from developmental constraints between leaf dimensions and lobing patterns, may also disrupt expectations based on such trends. Furthermore, it is not clear how metabolic rates, gene expression, phenology or microclimatic shifts mechanistically link to leaf shape at broader scales (e.g. communities and landscapes). Finally, an important consideration when attempting to mechanistically link these allometries to plant physiological performance that is not addressed with this approach is the importance of the spatially explicit nature of plant processes across three dimensions (Earles et al., 2019).
The physical distribution of leaf tissue is undoubtedly important to leaf function with implications for the canopy-scale processes discussed in Tamang et al., and in the broader global ecological and evolutionary context (Nicotra et al., 2011; Poorter et al., 2015). However, the mechanistic linkages between shape and metabolic processes are not comprehensively understood and are in themselves complex. From Tamang et al., ‘universal’ scaling laws do not necessarily explain within-species variation, and, from our theoretical analysis of cotton, relationships between leaf shape and function should be expected to vary across different leaf morphologies. As such, further exploration of these concepts is critical if we are to develop a mechanistic understanding of the impacts of leaf shape on gas exchange capable of informing breeding programmes, as described by Tamang and co-authors, and expanding our understanding of global patterns of leaf economics, evolution and function.
Contributor Information
Grace P John, Department of Biology, University of Florida, 220 Bartram Hall, PO Box 118525, Gainesville, FL 32611-8525, USA.
Claudia J Garnica-Díaz, Department of Biology, University of Florida, 220 Bartram Hall, PO Box 118525, Gainesville, FL 32611-8525, USA.
REFERENCES
- Agutter PS, Wheatley DN.. 2004. Metabolic scaling: consensus or controversy? Theoretical Biology and Medical Modelling 1: 20041101. doi: 10.1186/1742-4682-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderegg LD, Berner LT, Badgley G, Sethi ML, Law BE, HilleRisLambers J.. 2018. Within-species patterns challenge our understanding of the leaf economics spectrum. Ecology Letters 21: 734–744. doi: 10.1111/ele.12945. [DOI] [PubMed] [Google Scholar]
- Andres RJ, Bowman DT, Jones DC, Kuraparthy V.. 2016. Major leaf shapes of cotton: genetics and agronomic effects in crop production. The Journal of Cotton Science 20: 330–340. [Google Scholar]
- Andres RJ, Coneva V, Frank MH, et al. 2017. Modifications to a LATE MERISTEM IDENTITY1 gene are responsible for the major leaf shapes of upland cotton (Gossypium hirsutum L.). Proceedings of the National Academy of Sciences 114: E57–E66. doi: 10.1073/pnas.1613593114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley TN, John GP, Scoffoni C, Sack L.. 2015. How does leaf anatomy influence water transport outside the xylem? Plant Physiology 168: 1616–1635. doi: 10.1104/pp.15.00731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley TN, John GP, Scoffoni C, Sack L.. 2017. The sites of evaporation within leaves. Plant Physiology 173: 1763–1782. doi: 10.1104/pp.16.01605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Sun J, Wang M, et al. 2020. The leaf economics spectrum constrains phenotypic plasticity across a light gradient. Frontiers in Plant Science 11: 735. doi: 10.3389/fpls.2020.00735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz S, Kattge J, Cornelissen J, et al. 2016. The global spectrum of plant form and function. Nature 529: 167–171. doi: 10.1038/nature16489. [DOI] [PubMed] [Google Scholar]
- Earles JM, Buckley TN, Brodersen CR, et al. 2019. Embracing 3D complexity in leaf carbon–water exchange. Trends in Plant Science 24: 15–24. https 10.1016/j.tplants.2018.09.005 [DOI] [PubMed] [Google Scholar]
- Earles JM, Theroux-Rancourt G, Roddy AB, Gilbert ME, McElrone AJ, Brodersen CR.. 2018. Beyond porosity: 3D leaf intercellular airspace traits that impact mesophyll conductance. Plant Physiology 178: 148–162. doi: 10.1104/pp.18.00550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher LR, Cui H, Callahan H, et al. 2018. Evolution of leaf structure and drought tolerance in species of Californian Ceanothus. American Journal of Botany 105: 1672–1687. doi: 10.1002/ajb2.1164. [DOI] [PubMed] [Google Scholar]
- Glazier DS. 2010. A unifying explanation for diverse metabolic scaling in animals and plants. Biological Reviews 85: 111–138. doi: 10.1111/j.1469-185x.2009.00095.x. [DOI] [PubMed] [Google Scholar]
- Hammond D. 1941. The expression of genes for leaf shape in Gossypium hirsutum L. and Gossypium arboreum L. II. American Journal of Botany 28: 124–137. doi: 10.1002/j.1537-2197.1941.tb07952.x. [DOI] [Google Scholar]
- Hikosaka H, Hirose T.. 1997. Leaf angle as a strategy for light competition: optimal and evolutionarily stable light-extinction coefficient within a leaf canopy. Ecoscience 4: 501– 507. doi: 10.1080/11956860.1997.11682429. [DOI] [Google Scholar]
- Isaac NJB, Carbone C.. 2010. Why are metabolic scaling exponents so controversial? Quantifying variance and testing hypotheses. Ecology Letters 13: 728–735. doi: 10.1111/j.1461-0248.2010.01461.x. [DOI] [PubMed] [Google Scholar]
- Mason CM, Donovan LA.. 2015. Evolution of the leaf economics spectrum in herbs: evidence from environmental divergences in leaf physiology across Helianthus (Asteraceae). Evolution 69: 2705–2720. doi: 10.1111/evo.12768. [DOI] [PubMed] [Google Scholar]
- Nicotra AB, Leigh A, Boyce CK, et al. 2011. The evolution and functional significance of leaf shape in the angiosperms. Functional Plant Biology 38: 535353–535552. doi: 10.1071/fp11057. [DOI] [PubMed] [Google Scholar]
- Niinmets U. 2015a. Is there a species spectrum within the world-wide leaf economics spectrum? Major variations in leaf functional traits in the Mediterranean sclerophyll Quercus ilex. New Phytologist 205: 79–96. doi: 10.1111/nph.13001. [DOI] [PubMed] [Google Scholar]
- Niinemets U, Keenan TF, Hallik L.. 2015b. A worldwide analysis of within-canopy variations in leaf structural, chemical and physiological traits across plant functional types. New Phytologist 205: 973–993. doi: 10.1111/nph.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niklas KJ. 1994. Plant allometry: the scaling of form and process. Chicago: University of Chicago Press. [Google Scholar]
- Nobel PS. 1977. Internal leaf area and cellular CO2 resistance: photosynthetic implications of variations with growth conditions and plant species. Physiologia Plantarum 40: 137–144. doi: 10.1111/j.1399-3054.1977.tb01510.x. [DOI] [Google Scholar]
- Piao S, Liu Q, Chen A, et al. 2019. Plant phenology and global climate change: current progresses and challenges. Global Change Biology 25: 1922–1940. doi: 10.1111/gcb.14619. [DOI] [PubMed] [Google Scholar]
- Poorter H, Jagodzinki AM, Ruiz-Peinado R, et al. 2015. How does biomass distribution change with size and differ among species? An analysis for 1200 plant species from five continents. New Phytologist 208: 736–749. doi: 10.1111/nph.13571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack L, Buckley TN.. 2020. Trait multi-functionality in plant stress response. Integrative and Comparative Biology 60: 98–112. doi: 10.1093/icb/icz152. [DOI] [PubMed] [Google Scholar]
- Slattery RA, Ort DR.. 2021. Perspectives on improving light distribution and light use efficiency in crop canopies. Plant Physiology 185: 34–48. doi: 10.1093/plphys/kiaa006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamang, BG, Zhang Y, Zambrano MA, Ainsworth EA.. 2023. Anatomical determinants of gas exchange and hydraulics vary with leaf shape in soybean. Annals of Botany, this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théroux-Rancourt G, Earles JM, Gilbert ME, et al. 2017. The bias of a two-dimensional view: comparing two-dimensional and three-dimensional mesophyll surface area estimates using noninvasive imaging. New Phytologist 215: 1609–1622. doi: 10.1111/nph.14687. [DOI] [PubMed] [Google Scholar]
- Tomás M, Flexas J, Copolovici L, et al. 2013. Importance of leaf anatomy in determining mesophyll diffusion conductance to CO2 across species: quantitative limitations and scaling up by models. Journal of Experimental Botany 64: 2269–2281. doi: 10.1093/jxb/ert086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrell FM. 1933. The internal exposed surface of foliage leaves. Science 78: 536–537. doi: 10.1126/science.78.2032.536-a. [DOI] [PubMed] [Google Scholar]
- Westerband AC, Funk JL, Barton KE.. 2021. Intraspecific trait variation in plants: a renewed focus on its role in ecological processes. Annals of Botany 127: 397–410. doi: 10.1093/aob/mcab011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright I, Reich P, Westoby M, et al. 2004. The worldwide leaf economics spectrum. Nature 428: 821–827. doi: 10.1038/nature02403. [DOI] [PubMed] [Google Scholar]
- Yang W, Feng H, Zhang X, et al. 2020. Crop phenomics and high-throughput phenotyping: past decades, current challenges, and future perspectives. Molecular Plant 13: 187–214. doi: 10.1016/j.molp.2020.01.008. [DOI] [PubMed] [Google Scholar]
- York LM. 2019. Functional phenomics: an emerging field integrating high-throughput phenotyping, physiology, and bioinformatics. Journal of Experimental Botany 70: 379–386. doi: 10.1093/jxb/ery379. [DOI] [PubMed] [Google Scholar]