Abstract
Background and Aims
Nitrogen enrichment affects biodiversity, plant functional traits and ecosystem functions. However, the direct and indirect effects of nitrogen addition and biodiversity on the links between plant traits and ecosystem functions have been largely overlooked, even though multidimensional characteristics of plant functional traits are probably critical predictors of ecosystem functions.
Methods
To investigate the mechanism underlying the links between plant trait identity, diversity, network topology and above- and below-ground biomass along a plant species richness gradient under different nitrogen addition levels, a common garden experiment was conducted in which those driving factors were manipulated.
Key Results
The study found that nitrogen addition increased above-ground biomass but not below-ground biomass, while species richness was positively associated with above- and below-ground biomass. Nitrogen addition had minor effects on plant trait identity and diversity, and on the connectivity and complexity of the trait networks. However, species richness increased above-ground biomass mainly by increasing leaf trait diversity and network modularity, and enhanced below-ground biomass through an increase in root nitrogen concentration and network modularity.
Conclusions
The results demonstrate the mechanistic links between community biomass and plant trait identity, diversity and network topology, and show that the trait network architecture could be an indicator of the effects of global changes on ecosystem functions as importantly as trait identity and diversity.
Keywords: Biodiversity, nitrogen deposition, community biomass, trait identity, functional diversity, trait network
INTRODUCTION
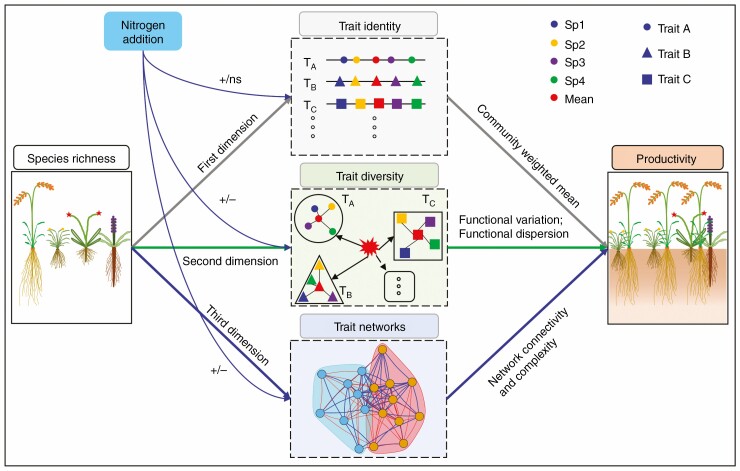
Plant functional traits, usually defined as the core attributes that are closely related to plant colonization, survival, growth and death, significantly affect ecosystem functions, and are closely associated with the response of ecosystem functions to environmental changes (Diaz et al., 1998; Diaz and Cabido, 2001; Reich et al., 2003). It has been shown that the identity of plant functional traits are the key drivers of ecosystem functioning (Weiher et al., 1999; Cornelissen et al., 2003; Bardgett et al., 2014). For example, communities with high acquisition traits, such as high specific leaf area and leaf nitrogen (N) concentration, are able to grow fast and uptake resources quickly, resulting in high community productivity (Lambers et al., 2008; Bardgett et al., 2014; Cortois et al., 2016; Li et al., 2020). Meanwhile, the dissimilarity of plant functional traits in communities can reduce niche overlap due to the divergence in resource acquisition and use strategies, enhance resource use efficiency, and then increase community productivity (Petchey and Gaston, 2006; Roscher et al., 2013). However, the positive relationships between functional diversity and community productivity are not ubiquitous (Finegan et al., 2015; Hao et al., 2020), presumably due to the functional redundancy of plant life histories (Li et al., 2022). Recent studies have referred that plant trait networks are assumed to constitute the backbone for the flow of energy and nutrients throughout the entirety of plant communities (Mason and Donovan, 2015; Li et al., 2021, 2022; Liu et al., 2022), can provide higher resolution of the correlative architecture of suites of plant traits, and are probably critical predictors of community productivity in response to environmental changes (Li et al., 2022; Wang et al., 2022). To the best of our knowledge, a framework quantifying the relationship between community productivity and plant trait identity, trait diversity and trait network is lacking (Fig. 1).
Fig. 1.
Conceptual diagram of the influences of nitrogen addition and plant diversity on community productivity through changes in multidimensional functional trait characteristics. Arrows indicate associations between different attributes, point colours indicate different plant species and point shapes indicate different functional traits. +, a positive effect; ‒, a negative effect; ns, a non-significant effect. TA, trait A; TB, trait B; TC, trait C. Sp1, Sp2, Sp3 and Sp4 represent species 1, species 2, species 3 and species 4, respectively.
Biodiversity usually enhances community productivity through selection effects and niche complementarity (Loreau, 1998; Loreau and Hector, 2001; Cardinale et al., 2007; Prieto et al., 2015). Plants with high nutrient acquisition traits, such as high leaf area, specific leaf area and specific root length, generally show higher productivity (Lambers et al., 2008; Bardgett et al., 2014; Cortois et al., 2016; Li et al., 2020). Hence, the mixed communities that contain these plant species would result in high community productivity (Fig. 1). Plant traits in the mixed communities showed significant differences among species due to niche differentiation, resulting in higher functional diversity (Petchey and Gaston, 2006; Roscher et al., 2013; Bongers et al., 2021). Thus, a high functional diversity in the mixed communities generally promotes above-ground productivity because of the complementary use of light (Wang et al., 2020a) and enhances below-ground productivity because of the complementary use of soil nutrients (Oram et al., 2018) (Fig. 1). Moreover, a recent study showed that the connectivity and complexity of a plant trait network were positively correlated with species richness due to niche differentiation (Li et al., 2022). Thus, mixed communities with high network connectivity and complexity may increase community productivity through a more efficient energy and nutrient flow (Fig. 1). Therefore, we predict that both plant trait identity, diversity and network topology could regulate the effects of biodiversity on community productivity.
Nitrogen deposition is considerably enhanced due to the increased combustion of fossil fuels and direct agricultural inputs (Galloway et al., 2008; Liu et al., 2013; Penuelas et al., 2013). There is growing evidence that this eutrophication increases soil-available N and phosphorous but decreases biodiversity in grasslands (Harpole et al., 2016; Wang et al., 2021). Increased soil nutrients enhance leaf area, specific leaf area, and leaf and root N concentration (Zhou et al., 2018; Zhang et al., 2019), resulting in an increase in community productivity (Fig. 1). Alternatively, the effect of N addition on community productivity has been correlated with either increased or decreased functional diversity depending on whether N addition increased or decreased plant species richness in the mixed communities (de Bello et al., 2021). For example, N addition may reduce functional diversity by decreasing plant species richness (Harpole et al., 2016). However, N addition can also increase functional diversity due to the divergent response of species with different resource use strategies when N addition does not change species richness (Palmroth et al., 2014; Zhang et al., 2019) (Fig. 1). Moreover, N addition may reduce the connectivity and complexity of plant trait network due to an N-induced decrease in species richness (Li et al., 2022). Alternatively, N-related plant traits may be closely linked to each other but show weaker associations with other traits due to release of nutrient stress (Li et al., 2022), resulting in a decrease in network connectivity but an enhancement in network modularity after N addition (Fig. 1). Therefore, in response to N addition, we predict that N addition could influence community productivity by regulating plant trait identity, diversity and network topology.
Plants synchronize their resource capture strategies above and below ground (i.e. light and nutrients) through the resource acquisition and retention function of leaves and roots (Mommer and Weemstra, 2012). For example, leaf traits that allow rapid photosynthesis, such as high maximum photosynthetic rate and high leaf N, increase water and nutrient demand, resulting in high above-ground productivity (Zhang et al., 2019). Meanwhile, these traits may be coupled with root traits that facilitate leaf photosynthetic requirements regarding nutrient acquisition, such as high specific root length and low root diameter ensuring a large root absorptive surface (Mommer and Weemstra, 2012; Freschet et al., 2020), leading to high root production. According to this hypothesis, leaf and root traits are associated not only with above-ground productivity but also with below-ground productivity.
Here we report on a 3-year common garden experiment to investigate the effects of N addition and plant species richness on above- and below-ground biomass based on plant trait identity, diversity and network in assembled grassland communities. The common garden experiment manipulated plant species richness with five levels (one, two, four, six and eight plant species) and N addition treatment with two levels (0 and 6 g N m‒2 year‒1). This design allowed us to answer the following two questions: (1) By which plant trait characteristics (i.e. trait identity, diversity and network) does species richness affect above- and below-ground biomass? (2) Does N addition influence the relationship between plant species richness, functional trait characteristics (i.e. identity, diversity and network) and community biomass?
MATERIALS AND METHODS
Study site and experimental design
Our study site (40°10ʹ45″N, 116°26ʹ13″E, and 50 m above sea level) is located at the Station of the Institute of Grassland, Flowers and Ecology on Xiaotang Mountain in Beijing, China. Mean annual precipitation is 526 mm, mean annual temperature is 11.8 °C (2000–2018). The common garden experiment was established in 2019, using a split-plot experiment design with N addition and plant species richness as the main treatment factors. The experiment comprised the following: 2 N levels (0 and 6 g N m‒2 year‒1) × 4 replicate blocks × 17 combinations of species per N treatment/replicate (four combinations per level of species richness with one, two, four and six species and one with eight species) = 136 pipes (Supplementary Data Fig. S1). Each pipe (30 cm in diameter and 50 cm in height; PVC sealed pipes) was filled with uniformly mixed soil (natural soil with stones and roots sieved out) with sand (in a 3 : 1 soil/sand ratio), and then buried in the ground without any shelters. Eight species were selected in our study to build assembled grassland communities, including Poa annua (Poaceae), Carex breviculmis (Cyperaceae), Medicago sativa (Fabaceae), Astragalus adsurgens (Fabaceae), Dianthus barbatus (Caryophyllaceae), Penstemon campanulatus (Plantaginaceae), Chrysanthemum maximum (Asteraceae) and Allium schoenoprasum (Liliaceae) (Wang et al., 2023). Sowing rate was determined by the germination rate of each species to manipulate the species with the same original proportion in the mixed communities (Wang et al., 2023). Seeds of different species were well mixed and distributed randomly in the pipes, maintained at around 60 individuals in each pipe at the beginning of experiment, and were not re-sown later (Weisser et al., 2017). Nitrogen was added as urea (12.86 g m−2 year−1), dissolved in N-free water, and then applied by spraying on 1 May of each year; the control treatment received an equal amount of N-free water. Additional information regarding the experimental design is provided in Wang et al. (2023).
Biomass and functional trait measurements
To measure the community and individual species biomass above and below-ground, the above-ground part in each pipe was clipped at the peak of the growing season in early September from 2019 to 2021; roots in each pipe were extracted by sieving and washing through a 0.5-mm mesh in 2021. The above-ground part and roots in each pipe were oven-dried at 65 °C to a constant weight and then pooled together to calculate above- and below-ground biomass. Moreover, 3-year above-ground biomass was averaged in our analysis, and we then calculated total biomass by combining mean above-ground biomass with below-ground biomass.
Three healthy individuals of each plant species were selected to measure plant height and species-level leaf traits in each pipe. Two leaves from each individual were chosen to measure leaf traits at the peak growing season. These traits were measured using standard methods according to Pérez-Harguindeguy et al. (2013). Spread leaves were scanned and analysed using a Li-Cor 310 (Li-Cor Inc., USA) to determine leaf length, mean width, maximum width and area. Leaves were weighed fresh, dried for 48 h at 65 °C and re-weighed, leaf dry matter content (LDMC, g dry mass g−1 fresh mass) was calculated. Specific leaf area (SLA, cm2 dry mass g−1) was calculated as the ratio of leaf area to its dry mass. Oven-dried leaf and root samples were ground, and the concentrations of leaf carbon (C), leaf N, root C and root N were determined using an elemental analyser (Vario MACRO Cube, Elementar, Germany). The concentrations of leaf and root phosphorus (P) were determined by inductively coupled plasma atomic emission spectroscopy (AutoAnalyzer 3, Germany). Finally, leaf and root C/N, C/P and N/P ratios were calculated.
Quantifying functional trait identity, diversity and network
Twelve leaf and six root traits were selected to calculate the community-weighted mean (CWM, trait identity) for each pipe using the mean biomass-weighted values for each species. Principal component analysis (PCA) was used to distinguish the leaf and root traits based on community-weighted mean traits using the ‘vegan’ package in R software (Supplementary Data Fig. S2). The first PCA axis for leaf traits captured 58 % of the variation in 12 leaf traits (Fig. S2a) and was positively correlated with leaf acquisitive traits (i.e. SLA and leaf N concentration). The first PCA axis for root traits captured 57 % of the variation in six root traits (Fig. S2b) and was positively correlated with root conservative traits (i.e. root C concentration and C/P ratio). Second, we calculated functional diversity as biomass-weighted trait dispersion to represent the range and distribution of traits in niche space using the ‘FD’ package (Laliberte and Legendre, 2010). Finally, network analysis for plant traits was conducted in R with the ‘igraph’ and ‘psych’ packages (R Core Team, 2021). We calculated Spearman correlations by using the plant traits in four plant combinations across four replicates (n = 16) in each N addition and species richness level, and then built the trait network based on random matrix theory (Li et al., 2022; Wang et al., 2022). To simplify and better visualize networks, significant interactions at P ≤ 0.05 are presented (Fig. S3). To describe the overall topology of plant trait networks, edge density, average path length, average clustering coefficient and modularity were used to quantify network ‘connectivity’ and ‘complexity’ (Li et al., 2022).
Statistical analyses
We applied linear mixed-effects models using ‘lme’ function (package ‘nlme’) (Pinheiro et al., 2007) to test the effect of N addition and plant species richness on above-ground biomass across years; N addition and species richness were set as fixed effects, and year, block and plant combinations were set as random effects. We also used linear mixed-effects models to examine the effects of N addition and plant species richness on below-ground biomass, the community-weighted mean and diversity of leaf and root traits, and network parameters; N addition and species richness were set as fixed effects, and block and plant combinations were set as random effects. In addition, individual relationships between species richness and above- and below-ground biomass, trait identity and diversity, and network parameters were analysed and plotted with linear mixed-effects models, and the block and plant combinations were set as random effects. We then calculated the correlation coefficients between trait identity, trait diversity and network parameters. Moreover, we evaluated the relationships among above- and below-ground biomass, trait identity and diversity, and network parameters using linear mixed-effects models.
Moreover, structural equation models (SEMs) were used to compare the hypothesized associations of N addition and species richness with total biomass in R with the ‘piecewise-SEM’ package (Lefcheck, 2016). First, we established an a priori framework based on the hypothesized causal relationships among these variables (Fig. 1). Second, the relationships between all variables were examined by bivariate correlations to ensure that a linear model was appropriate. Finally, models with lower Fisher’s C and Akaike information criterion (AIC), and higher P values (>0.05) were selected in our analysis. Statistical analyses were conducted and graphs were prepared in R 3.4.1 (R Core Team, 2021). Effects were considered significant at P ≤ 0.05 or marginally significant at 0.05 < P ≤ 0.10.
RESULTS
Biomass and functional traits respond to N addition and plant diversity
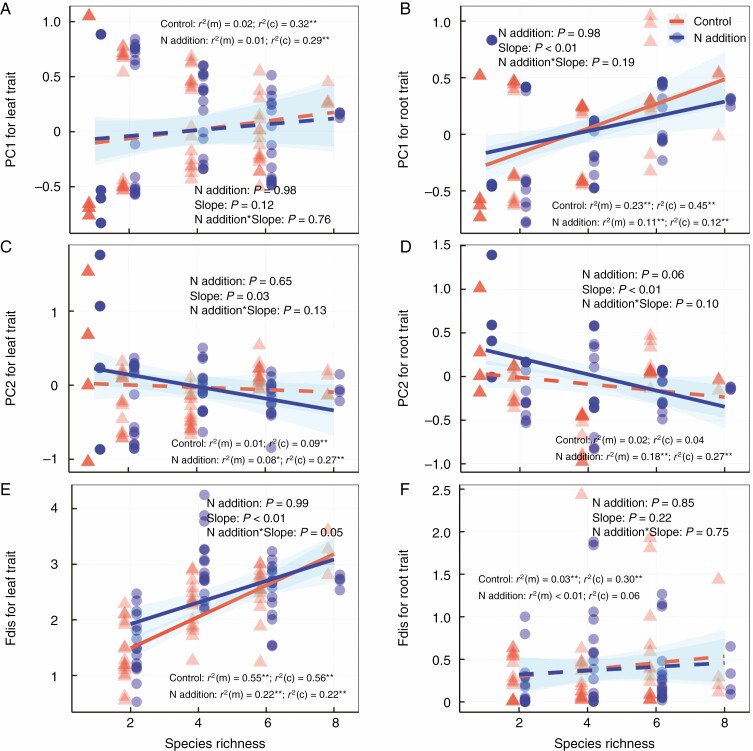
In our common garden experiment, N addition increased above-ground biomass (Fig. 2A), but had minor effects on below-ground biomass (Fig. 2B). Species richness was positively correlated with above- and below-ground biomass (Fig. 2). Nitrogen addition had minor effects on most leaf and root traits, except for leaf area and SLA (Supplementary Data Table S1). Species richness showed significant relationships with most leaf traits and root N concentration, but showed no significant correlations with other root traits (Table S1). The effects of N addition and species richness on biomass and functional traits were largely independent of each other (Fig. 2 and Table S1). Moreover, N addition did not significantly change the first and second component of leaf and root traits, or the diversity of leaf and root traits (Fig. 3). Species richness was positively correlated with the first component of root trait identity (Fig. 3B) and the diversity of leaf traits (Fig. 3E), but negatively correlated with the second component of leaf and root trait identity under N addition treatment (Fig. 3C, D).
Fig. 2.
Relationship between species richness and (A) above-ground and (B) below-ground biomass. Control, orange points and lines; nitrogen addition, blue points and lines. Solid lines represent significant (P ≤ 0.05) relationships. The shaded area indicates 95 % confidence intervals of the regression lines. r2(m) and r2(c) represent model variations explained by fixed effects and the combination of fixed and random effects, respectively. Asterisks indicate linear regressions are significant *P ≤ 0.05, **P ≤ 0.01.
Fig. 3.
Relationships between species richness and (A) PC1 for leaf trait identity, (B) PC1 for root trait identity, (C) PC2 for leaf trait identity, (D) PC2 for root trait identity, (E) diversity of leaf traits and (F) diversity of root traits. Control, orange points and lines; nitrogen addition, blue points and lines. Solid and dashed lines represent significant (P ≤ 0.05) and non-significant (P > 0.05) relationships, respectively. The shaded area indicates 95 % confidence intervals of the regression lines. r2(m) and r2(c) represent model variations explained by fixed effects and the combination of fixed and random effects, respectively. Asterisks indicate linear regressions are significant: *P ≤ 0.05, **P ≤ 0.01. PC1, the first component of principal component analysis; PC2, the second component of principal component analysis; Fdis, functional diversity.
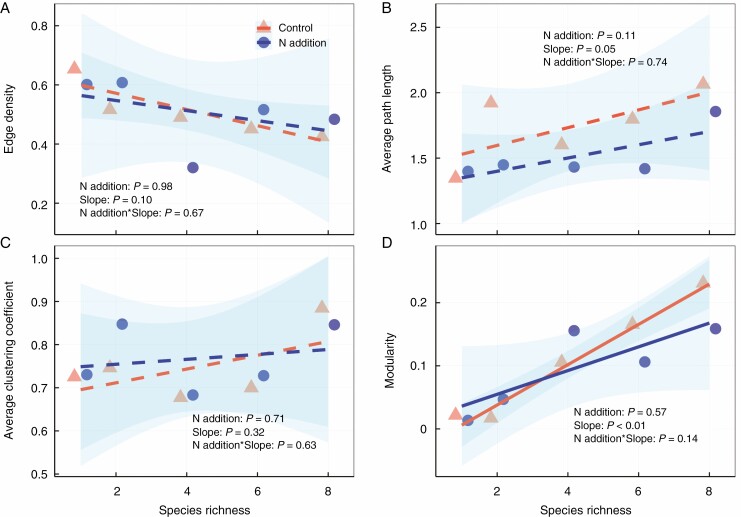
In addition to quantifying the effects of N addition and species richness on the number of associations among functional traits, network analysis revealed shifts in trait network topology (Fig. 4 and Supplementary Data Fig. S3). Nitrogen addition had minor effects on network connectivity and complexity, including edge density, average path length, average clustering coefficient and modularity (Fig. 4 and Table S2). However, species richness showed a positive correlation with network modularity (Fig. 4D), but showed no significant correlations with edge density, average path length or average clustering coefficient (Fig. 4A–C).
Fig. 4.
Relationships between the network parameters and species richness. Control, orange points and lines; nitrogen addition, blue points and lines. Solid and dashed lines represent significant (P ≤ 0.05) and non-significant (P > 0.05) relationships, respectively. The shaded area indicates 95 % confidence intervals of the regression lines.
Relationships between plant traits and community biomass
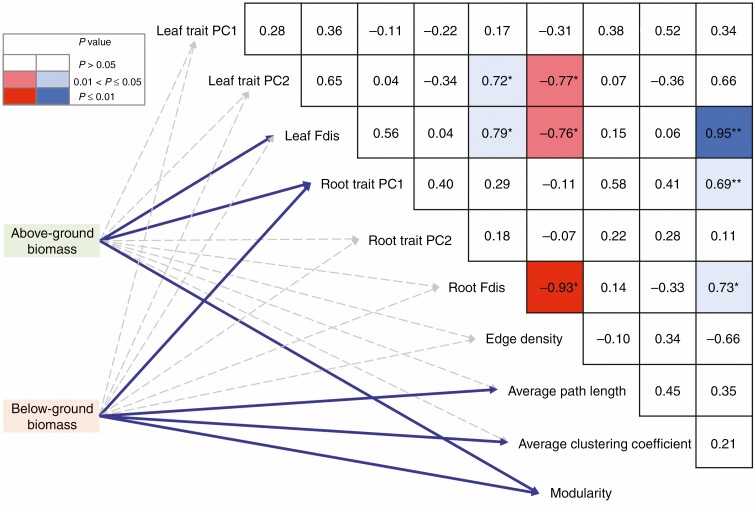
Our results showed that above- and below-ground biomass were closely correlated with leaf and root trait characteristics (Fig. 5). Specifically, above-ground biomass was positively associated with the diversity of leaf traits, the first component of root trait identity and network modularity; below-ground biomass was positively correlated with the first component of root trait identity, average path length, average clustering coefficient and modularity (Fig. 5). In addition, we also found that leaf trait diversity was positively associated with the diversity of root traits. Leaf and root trait diversity were negatively correlated with edge density, but they were positively correlated with network modularity (Fig. 5).
Fig. 5.
Correlations between biomass and trait attributes. Edge width corresponds to the absolute value of the correlation coefficient determined by the linear mixed-effects models. Solid and dashed lines denote significant (P ≤ 0.05) and non-significant (P > 0.05) correlations, respectively. Pairwise comparisons of trait attributes are shown in the triangle. Colours indicate correlation types. Asterisks indicate coefficients are significant: *P ≤ 0.05; **P ≤ 0.01. PC1, the first component of principal component analysis; PC2, the second component of principal component analysis; Fdis, functional diversity.
The SEM revealed that species richness increased above- and below-ground biomass by regulating trait identity, diversity and network modularity (Fig. 6 and Supplementary Data Fig. S4). Species richness increased above-ground biomass by enhancing leaf trait diversity and network modularity (Fig. 6). Consistent with bivariate results, below-ground biomass was positively related to leaf trait identity (i.e. SLA), root trait identity (i.e. root N concentration) and network modularity (Figs S4 and S5). Species richness indirectly increased below-ground biomass mainly through the enhancement in the first component of root trait identity and network modularity (Fig. 6).
Fig. 6.
Structural equation model exploring the effects of plant species richness and nitrogen addition on the links between plant functional traits and community biomass. Boxes represent measured variables and arrows represent relationships among variables. Blue arrows represent positive pathways. Solid arrows represent significant (P ≤ 0.05) pathways. The thickness of the arrows is proportional to the magnitude of standardized path coefficients. Standardized path coefficients are given next to each path. Asterisks indicate coefficients are significant: *0.01 < P ≤ 0.05; **P ≤ 0.01. PC1, the first component of principal component analysis; Fdis, functional diversity.
DISCUSSION
In our common garden experiment, intrinsic links between plant trait identity, diversity, and network topology and community biomass were identified, and the mechanisms underlying functional traits that regulate the effects of N addition and plant species richness on community biomass were clarified. Leaf trait diversity and the first component of root trait identity increased along a species richness gradient. Similarly, the complexity of the trait network was positively related to species richness. Notably, species richness indirectly increased above-ground biomass by increasing leaf trait diversity and modular complexity, and species richness indirectly enhanced below-ground biomass through the enhancement in the first component of root trait identity and network modularity. However, N addition had minor effects on leaf and root trait identity and diversity, and on the connectivity and complexity of the trait network. Therefore, our results provide a novel perspective to understand the relationship between biodiversity and ecosystem functioning within the context of global changes.
Biodiversity increases community biomass by regulating plant traits
Above-ground biomass was positively related to leaf trait diversity but showed no significant relationship with leaf trait identity. The results are consistent with those of previous studies and indicate that increases in plant species richness lead to increases in leaf trait diversity because of competitive relaxation via species partitioning of light (Wang et al., 2020b; Bongers et al., 2021). Moreover, our study revealed that trait network modularity was the other key factors driving community biomass, and it was positively related to above- and below-ground biomass. Modularity represents the difference between the fractions of connections among traits within a given module, with a greater tendency for leaf traits to cluster into modules due to niche differentiation along the plant species richness gradients (Li et al., 2022), Hence, high network modularity may lead to a more efficient flow of energy and nutrients between above- and below-ground parts, resulting in a higher above- and below-ground biomass. In contrast, our study found that below-ground biomass was positively related to root trait identity (i.e. root N concentration) rather than to root trait diversity. This result is inconsistent with a previous study (Oram et al., 2018) and indicates that plant roots in the mixed communities showed no significant complementarity for acquisition of organic or mineral nutrients, possibly due to the space limitation of each pipe. As a result, increasing plant species richness did not affect root trait diversity. However, root N concentration increased with plant species richness (Supplementary Data Table S1), which may enhance the metabolic capacity of roots (Fig. S5; Bardgett et al., 2014; Zhang et al., 2019), resulting in an increase in below-ground biomass.
As expected, plants synchronize their functional traits and biomass above and below ground. High SLA, high leaf N concentration and low leaf dry matter content allowed rapid photosynthesis, and increased nutrient demand, resulting in high above-ground biomass (Supplementary Data Fig. S5). Meanwhile, these traits were coupled with root traits that facilitate leaf photosynthetic requirements relating to nutrient acquisition, such as a high root N concentration and N/P ratio ensuring a large root absorptive capacity (Fig. S5), leading to high below-ground biomass. Hence, above- and below-ground biomass were not only related to leaf traits but also related to root traits.
N addition has minor effects on plant traits
Nitrogen addition had minor effects on most leaf and root traits but enhanced above-ground biomass. Our results showed that above-ground biomass was positively correlated with SLA. High specific leaf area allowed rapid photosynthesis (Reich et al., 2003; Zhang et al., 2019), and thus enhanced SLA increased above-ground biomass after N addition. Moreover, N addition showed that minor effects on plant traits might result from two pathways working independently and/or in combination. First, previous studies showed that N addition significantly changed grassland ecosystem functions when achieved reached 10 g m−2 year−1 (Tian et al., 2016; Wang et al., 2020a). We added 6 g N m−2 year−1 in our 3-year common garden experiment. Nitrogen addition only significantly changed those plant traits that are sensitive to environmental changes, but showed no significant effects on most plant traits. Second, as legumes were the dominant species in the mixed communities in our study (Wang et al., 2023), and their traits were insensitive to N addition as growth was not limited by N (Bordeleau and Prévost, 1994), this led to no significant changes in community trait values after N addition.
Moreover, functional diversity represents niche complementarity, which was induced by species richness in the mixed communities (Petchey and Gaston, 2006; Roscher et al., 2013), while N addition showed no significant effect on species richness in our experiment (Wang et al., 2023), which led to no significant change in functional diversity under N addition. Similarly, N addition had minor effects on most plant traits, which may lead to no significant differences in the energy flow and nutrient cycle of plants, resulting in no significant changes in the connectivity and complexity of the trait network. In addition, it has been shown that the connectivity and complexity of a trait network are related to species richness due to niche differentiation (Li et al., 2022). Thus, N addition showed minor effects on the connectivity and complexity of the trait network due to no significant N-induced species richness loss.
Limitations of our study
Although our analysis provides novel perspectives to understand potential links among functional traits and ecosystem functioning under global changes, the study has two weaknesses. First, few functional traits were involved in this study, especially root traits. The leaf traits selected were not directly related to plant photosynthesis, such as leaf area, SLA and leaf N concentration. As a result, the trait network may not sufficiently represent the energy flow and nutrient cycle of plants. Hence, more leaf physiological traits that are directly associated with productivity (i.e. leaf photosynthesis and respiration rate, chlorophyll content, and trace element content) should be measured in the future. In addition, the root traits selected here were all related to element concentration, and thus additional traits related to root architecture and morphology should be measured (Bardgett et al., 2014). Second, the effects of biodiversity and/or global changes on plant functional traits fluctuated with inter-annual dynamics of environmental conditions (Lü et al., 2016; Salo et al., 2019; Zhang et al., 2019). Our analysis is based mostly on data collected in a single year, which may blur inter-annual variations in the effects of biodiversity and global changes on community biomass and functional traits. Overall, the regulation of functional trait identity, diversity and network on the biodiversity–ecosystem functioning relationships needs to be examined through multiple-year field experiments in natural grasslands.
CONCLUSIONS
Our common garden experiment represents an important step forward in enhancing our understanding of the biodiversity–ecosystem functioning relationship by demonstrating mechanistic links between functional traits (i.e. trait identity, diversity and network) and above- and below-ground biomass under different N addition levels. Leaf trait diversity, root trait identity and trait network modularity increased with increasing plant species richness, whereas N addition had minor effects on trait identity, diversity and network topology. In particular, species richness enhanced above-ground biomass by increasing leaf trait diversity and network modularity, and increased below-ground biomass mainly by increasing root trait identity (i.e. root N concentration) and network modularity. Overall, the regulation effects of plant trait networks on above- and below-ground biomass provide new insights into understanding the effects of global changes on ecosystem functioning.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Table S1: Results showing the effect of nitrogen addition and plant species richness on functional traits. Table S2: Results showing the effect of nitrogen addition and plant species richness on the architecture of functional trait networks. Figure S1: Design and photo of the experiments. Figure S2: Results of principal component analysis for functional leaf and root traits. Figure S3: The connectivity and complexity of functional trait networks under control and nitrogen addition treatment across levels of plant species richness. Figure S4: Structural equation model exploring the effects of plant species richness and nitrogen addition on total biomass. Figure S5: Pairwise comparisons of plant traits and biomass.
ACKNOWLEDGEMENTS
We thank the staff at the Xiaotangshan station of the Institute of Grassland, Flowers and Ecology for providing logistical support in the field.
Contributor Information
Chao Wang, Institute of Grassland, Flowers and Ecology, Beijing Academy of Agriculture and Forestry Sciences, Beijing 100097, China.
Yanhui Hou, Institute of Grassland, Flowers and Ecology, Beijing Academy of Agriculture and Forestry Sciences, Beijing 100097, China.
Yanxia Hu, Institute of Grassland, Flowers and Ecology, Beijing Academy of Agriculture and Forestry Sciences, Beijing 100097, China.
Ruilun Zheng, Institute of Grassland, Flowers and Ecology, Beijing Academy of Agriculture and Forestry Sciences, Beijing 100097, China.
Xiaona Li, Institute of Grassland, Flowers and Ecology, Beijing Academy of Agriculture and Forestry Sciences, Beijing 100097, China.
Conflict of interest
The authors declare that they have no competing interests.
FUNDING
This study was supported by Natural Science Foundation of Beijing Municipality (Grant No. 5232006), Beijing Academy of Agriculture and Forestry Sciences Special Project for Innovation (Grant No. KJCX20230111) and National Natural Science Foundation of China (Grant No. 31901173).
LITERATURE CITED
- Bardgett RD, Mommer L, De Vries FT.. 2014. Going underground: root traits as drivers of ecosystem processes. Trends in Ecology and Evolution 29: 692–699. doi: 10.1016/j.tree.2014.10.006. [DOI] [PubMed] [Google Scholar]
- de Bello FD, Lavorel S, Hallett LM, et al. 2021. Functional trait effects on ecosystem stability: assembling the jigsaw puzzle. Trends in Ecology and Evolution 36: 822–836. doi: 10.1016/j.tree.2021.05.001. [DOI] [PubMed] [Google Scholar]
- Bongers FJ, Schmid B, Bruelheide H, et al. 2021. Functional diversity effects on productivity increase with age in a forest biodiversity experiment. Nature Ecology and Evolution 5: 1594–1603. doi: 10.1038/s41559-021-01564-3. [DOI] [PubMed] [Google Scholar]
- Bordeleau L, Prévost D.. 1994. Nodulation and nitrogen fixation in extreme environments. Plant and Soil 161: 115–125. doi: 10.1007/BF02183092. [DOI] [Google Scholar]
- Cardinale BJ, Wright JP, Cadotte MW, Carroll IT, Hector A, Srivastava DS, Loreau M, Weis J.. 2007. Impacts of plant diversity on biomass production increase through time because of species complementarity. Proceedings of the National Academy of Sciences USA 104: 18123–18128. doi: 10.1073/pnas.0709069104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen JHC, Lavorel S, Garnier E, et al. 2003. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Australian Journal of Botany 51: 335–380. doi: 10.1071/bt02124. [DOI] [Google Scholar]
- Cortois R, Schroeder-Georgi T, Weigelt A, van der Putten WH, De Deyn GB.. 2016. Plant–soil feedbacks: role of plant functional group and plant traits. Journal of Ecology 104: 1608–1617. doi: 10.1111/1365-2745.12643. [DOI] [Google Scholar]
- Diaz S, Cabido M.. 2001. Vive la difference: plant functional diversity matters to ecosystem processes. Trends in Ecology and Evolution 16: 646–655. doi: 10.1016/s0169-5347(01)02283-2. [DOI] [Google Scholar]
- Diaz S, Cabido M, Casanoves F.. 1998. Plant functional traits and environmental filters at a regional scale. Journal of Vegetation Science 9: 113–122. doi: 10.2307/3237229. [DOI] [Google Scholar]
- Finegan B, Pena-Claros M, Oliveira AD, et al. 2015. Does functional trait diversity predict above-ground biomass and productivity of tropical forests? Testing three alternative hypotheses. Journal of Ecology 103: 191–201. doi: 10.1111/1365-2745.12346. [DOI] [Google Scholar]
- Freschet GT, Roumet C, Comas LH, et al. 2020. Root traits as drivers of plant and ecosystem functioning: Current understanding, pitfalls and future research needs. New Phytologist 232: 1123–1158. doi: 10.1111/nph.17072. [DOI] [PubMed] [Google Scholar]
- Galloway J, Townsend AR, Erisman JW, et al. 2008. Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320: 889–892. doi: 10.1126/science.1136674. [DOI] [PubMed] [Google Scholar]
- Hao M, Messier C, Geng Y, Zhang C, Gadow KV.. 2020. Functional traits influence biomass and productivity through multiple mechanisms in a temperate secondary forest. European Journal of Forest Research 139: 959–968. doi: 10.1007/s10342-020-01298-0. [DOI] [Google Scholar]
- Harpole WS, Sullivan LL, Lind EM, et al. 2016. Addition of multiple limiting resources reduces grassland diversity. Nature 537: 93–96. doi: 10.1038/nature19324. [DOI] [PubMed] [Google Scholar]
- Laliberte E, Legendre P.. 2010. A distance-based framework for measuring functional diversity from multiple traits. Ecology 91: 299–305. doi: 10.1890/08-2244.1. [DOI] [PubMed] [Google Scholar]
- Lambers H, Raven JA, Shaver GR, Smith SE.. 2008. Plant nutrient-acquisition strategies change with soil age. Trends in Ecology and Evolution 23: 95–103. doi: 10.1016/j.tree.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Lefcheck JS. 2016. piecewiseSEM: Piecewise structural equation modelling in R for ecology, evolution, and systematics. Methods in Ecology and Evolution 7: 573–579. doi: 10.1111/2041-210X.12512. [DOI] [Google Scholar]
- Li Y, Reich PB, Schmid B, et al. 2020. Leaf size of woody dicots predicts ecosystem primary productivity. Ecology Letters 23: 1003–1013. doi: 10.1111/ele.13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liu C, Xu L, Li M, Zhang J, He N.. 2021. Leaf trait networks based on global data: representing variation and adaptation in plants. Frontiers in Plant Science 12: 710530. doi: 10.3389/fpls.2021.710530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liu C, Sack L, et al. 2022. Leaf trait network architecture shifts with species-richness and climate across forests at continental scale. Ecology Letters 25: 1442–1457. doi: 10.1111/ele.14009. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhang Y, Han W, et al. 2013. Enhanced nitrogen deposition over China. Nature 494: 459–462. doi: 10.1038/nature11917. [DOI] [PubMed] [Google Scholar]
- Liu C, Li Y, He N.. 2022. Differential adaptation of lianas and trees in wet and dry forests revealed by trait correlation networks. Ecological Indicators 135: 108564. doi: 10.1016/j.ecolind.2022.108564. [DOI] [Google Scholar]
- Loreau M. 1998. Separating sampling and other effects in biodiversity experiments. Oikos 82: 600–604. doi: 10.2307/3546381. [DOI] [Google Scholar]
- Loreau M, Hector A.. 2001. Partitioning selection and complementarity in biodiversity experiments. Nature 412: 72–76. doi: 10.1038/35083573. [DOI] [PubMed] [Google Scholar]
- Lü X, Zhou G, Wang Y, Song X.. 2016. Effects of changing precipitation and warming on functional traits of zonal Stipa plants from Inner Mongolian grassland. Journal of Meteorological Research 30: 412–425. doi: 10.1007/s13351-016-5091-5. [DOI] [Google Scholar]
- Mason CM, Donovan LA.. 2015. Evolution of the leaf economics spectrum in herbs: evidence from environmental divergences in leaf physiology across Helianthus (Asteraceae). Evolution 69: 2705–2720. doi: 10.1111/evo.12768. [DOI] [PubMed] [Google Scholar]
- Mommer L, Weemstra M.. 2012. The role of roots in the resource economics spectrum. New Phytologist 195: 725–727. doi: 10.1111/j.1469-8137.2012.04247.x. [DOI] [PubMed] [Google Scholar]
- Oram NJ, Ravenek JM, et al. 2018. Below-ground complementarity effects in a grassland biodiversity experiment are related to deep-rooting species. Journal of Ecology 106: 265–277. doi: 10.1111/1365-2745.12877. [DOI] [Google Scholar]
- Palmroth S, Bach LH, Nordin A, Palmqvist K.. 2014. Nitrogen-addition effects on leaf traits and photosynthetic carbon gain of boreal forest understory shrubs. Oecologia 175: 457–470. doi: 10.1007/s00442-014-2923-9. [DOI] [PubMed] [Google Scholar]
- Penuelas J, et al. 2013. Human-induced nitrogen–phosphorus imbalances alter natural and managed ecosystems across the globe. Nature Communications 4: 1–10. doi: 10.1038/ncomms3934. [DOI] [PubMed] [Google Scholar]
- Pérez-Harguindeguy N, et al. 2013. New handbook for standardised measurement of plant functional traits worldwide. Australian Journal of Botany 64: 715. doi: 10.1071/BT12225. [DOI] [Google Scholar]
- Petchey OL, Gaston KJ.. 2006. Functional diversity: back to basics and looking forward. Ecology Letters 9: 741–758. doi: 10.1111/j.1461-0248.2006.00924.x. [DOI] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D.. 2007. Nlme: Linear and nonlinear mixed effects models. R Package Version 3: 1–89. https://CRAN.R-project.org/package=nlme [Google Scholar]
- Prieto I, Violle C, Barre P, Durand JL, Ghesquiere M, Litrico I.. 2015. Complementary effects of species and genetic diversity on productivity and stability of sown grasslands. Nature Plants 1: 15033. doi: 10.1038/nplants.2015.33. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. 2021. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Reich PB, Wright IJ, Cavender-Bares J, et al. 2003. The evolution of plant functional variation: traits, spectra, and strategies. International Journal of Plant Sciences 164: 143–S164. doi: 10.1086/374368. [DOI] [Google Scholar]
- Roscher C, Schumacher J, Lipowsky A, et al. 2013. A functional trait-based approach to understand community assembly and diversity–productivity relationships over 7 years in experimental grasslands. Perspectives in Plant Ecology, Evolution and Systematics 15: 139–149. doi: 10.1016/j.ppees.2013.02.004. [DOI] [Google Scholar]
- Salo T, Mattila J, Eklöf J.. 2019. Long-term warming affects ecosystem functioning through species turnover and intraspecific trait variation. Oikos 129: 283–295. doi: 10.1111/oik.06698. [DOI] [Google Scholar]
- Tian D, Wang H, Sun J, Niu S.. 2016. Global evidence on nitrogen saturation of terrestrial ecosystem net primary productivity. Environmental Research Letters 11: 024012. doi: 10.1088/1748-9326/11/2/024012. [DOI] [Google Scholar]
- Wang C, Ren F, Zhou X, et al. 2020a. Variations in the nitrogen saturation threshold of soil respiration in grassland ecosystems. Biogeochemistry 148: 3111–3324. doi: 10.1007/s10533-020-00661-y. [DOI] [Google Scholar]
- Wang C, Tang Y, Li X, Zhang W, Zhao C, Li C.. 2020b. Negative impacts of plant diversity loss on carbon sequestration exacerbate over time in grasslands. Environmental Research Letters 15: 104055. doi: 10.1088/1748-9326/abaf88. [DOI] [Google Scholar]
- Wang R, Yang J, Liu H, et al. 2021. Nitrogen enrichment buffers phosphorus limitation by mobilizing mineral-bound soil phosphorus in grasslands. Ecology 103: e3616. doi: 10.1002/ecy.3616. [DOI] [PubMed] [Google Scholar]
- Wang L, Rao Q, Su H, et al. 2022. Linking the network topology of plant traits with community structure, functioning, and adaptive strategies of submerged macrophytes. Science of the Total Environment 850: 158092. doi: 10.1016/j.scitotenv.2022.158092. [DOI] [PubMed] [Google Scholar]
- Wang C, Li X, Hu Y, Zheng R, Hou Y.. 2023. Nitrogen addition weakens the biodiversity – multifunctionality relationships across soil profiles in a grassland assemblage. Agriculture, Ecosystems and Environment 342: 108241. doi: 10.1016/j.agee.2022.108241. [DOI] [Google Scholar]
- Weiher E, van der Werf A, Thompson K, et al. 1999. Challenging Theophrastus: a common core list of plant traits for functional ecology. Journal of Vegetation Science 10: 609–620. doi: 10.2307/3237076. [DOI] [Google Scholar]
- Weisser WW, Roscher C, Meyer ST, et al. 2017. Biodiversity effects on ecosystem functioning in a 15-year grassland experiment: Patterns, mechanisms, and open questions. Basic Applied Ecology 23: 1–73. doi: 10.1016/j.baae.2017.06.002. [DOI] [Google Scholar]
- Zhang D, Peng Y, Li F, et al. 2019. Trait identity and functional diversity co-drive response of ecosystem productivity to nitrogen enrichment. Journal of Ecology 107: 2402–2414. doi: 10.1111/1365-2745.13184. [DOI] [Google Scholar]
- Zhou X, Guo Z, Zhang P, Du G.. 2018. Shift in community functional composition following nitrogen fertilization in an alpine meadow through intraspecific trait variation and community composition change. Plant and Soil 431: 289–302. doi: 10.1007/s11104-018-3771-x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.