Abstract
Background
Obesity can pose perioperative challenges related to obesity-associated co-morbidities and technical factors. However, the true impact of obesity on postoperative outcomes is not well established and reports are conflicting. The aim was to perform a systematic review and meta-analysis to explore the effect of obesity on perioperative outcomes for general surgery procedures in distinct obesity subtypes.
Methods
A systematic review was performed for studies reporting postoperative outcomes in relation to BMI in upper gastrointestinal, hepatobiliary and colorectal based on an electronic search using the Cochrane Library, Science Direct, PubMed and Embase up to January 2022. The primary outcome was the incidence of 30-day postoperative mortality among patients with obesity undergoing general surgical procedures in comparison to patients with normal range BMI.
Results
Sixty-two studies, including 1 886 326 patients, were eligible for inclusion. Overall, patients with obesity (including class I/II/II) had lower 30-day mortality rates in comparison to patients with a normal BMI (odds ratio (OR) 0.75, 95 per cent c.i. 0.66 to 0.86, P < 0.0001, I2 = 71 per cent); this was also observed specifically in emergency general surgery (OR 0.83, 95 per cent c.i. 0.79 to 0.87, P < 0.0000001, I2 = 7 per cent). Compared with normal BMI, obesity was positively associated with an increased risk of 30-day postoperative morbidity (OR 1.11, 95 per cent c.i. 1.04 to 1.19, P = 0.002, I2 = 85 per cent). However, there was no significant difference in postoperative morbidity rates between the cohorts of patients with a normal BMI and class I/II obesity (OR 0.98, 95 per cent c.i. 0.92 to 1.04, P = 0.542, I2 = 92 per cent). Overall, the cohort with obesity had a higher rate of postoperative wound infections compared with the non-obese group (OR 1.40, 95 per cent c.i. 1.24 to 1.59, P < 0.0001, I2 = 82 per cent).
Conclusion
These data suggest a possible ‘obesity paradox’ and challenge the assumption that patients with obesity have higher postoperative mortality compared with patients with normal range BMI. Increased BMI alone is not associated with increased perioperative mortality in general surgery, highlighting the importance of more accurate body composition assessment, such as computed tomography anthropometrics, to support perioperative risk stratification and decision-making.
Registration number
CRD42022337442 (PROSPERO https://www.crd.york.ac.uk/prospero/).
Results from this meta-analysis of 62 studies suggest a possible ‘obesity paradox’ and challenges the assumption that patients with obesity have higher postoperative mortality compared with patients with normal range BMI. Increased BMI alone is not associated with increased perioperative mortality in gastrointestinal surgery, highlighting the importance of more accurate body composition assessment, such as computed tomography anthropometrics, to support perioperative risk stratification and decision-making.
Introduction
Obesity is a consequence of complex interactions between genetic, socioeconomic and cultural influences. In the last three decades, the worldwide prevalence of obesity has increased three-fold. In 2016, over 39 per cent of adults aged 18 years and older were overweight and 13 per cent were obese1. In Europe, obesity (BMI greater than or equal to 30 kg/m2) has reached epidemic proportions with the prevalence in men ranging from 4.0 to 28.3 per cent and in women from 6.2 to 36.5 per cent, with considerable geographical variation2. The most widely adopted classification of obesity in the Western world is the WHO criteria. A BMI of less than 18.5 kg/m2 is considered underweight and a BMI of 25–29.9 kg/m2 is considered overweight. The extent of obesity can then be further classified as: class I is specified for a BMI of 30–34.9 kg/m2, class II for a BMI of 35–39.9 kg/m2 and class III applies to those with a BMI greater than or equal to 40 kg/m23. However, this grading system is better suited to Europeans as geographical differences in physiology and subsequently BMI are well documented. Asia-Pacific populations have different body fat distribution and therefore morbidity and mortality can occur in this population group with lower BMIs. To address this variability, in 2000 a BMI scale for Asian adults was proposed with a normal BMI range of 18.5–22.9 kg/m2 and obesity class I defined as a BMI of 25–29.9 kg/m24.
Obesity is associated with several co-morbidities, including diabetes, hypertension, coronary artery disease and increased risk of certain cancers3. Every five-unit increase in BMI above 25 kg/m2 is believed to increase mortality rate by 30 per cent5. The obesity epidemic also impacts surgery and surgical outcomes, not merely because of an increase in the prevalence of obesity but also because of an increase in obesity-related surgical diseases6. Obesity can pose several perioperative challenges, including management of co-morbidities, as well as technical and equipment-related issues7. However, the true impact of obesity on postoperative outcomes is not well established and reports across a wide range of surgical studies are conflicting.
The obesity paradox, which suggests that patients with obesity have more favourable postoperative outcomes compared with those who have a normal BMI, was first conceptualized in relation to cardiac surgery in the early 2000s8,9. Since then, several conflicting studies have reported on the subject of the obesity paradox in general surgery. Mullen et al. prospectively examined patients undergoing non-bariatric general surgery and demonstrated that patients with obesity had a lower risk of mortality compared with patients who have a normal BMI10. These results have been replicated in larger cohort studies comparing general surgical mortality rates between patients with elevated BMI to a non-obese reference group11–13. More recently, obesity was reported to be particularly protective in older adults undergoing emergency surgery14. In contrast, Kassahun et al. found that patients with obesity were more likely to have co-morbidities and were at increased risk of postoperative complications and mortality following emergency laparotomy for high-risk abdominal emergencies15. Similarly, postoperative complication and wound infection rates have also been reported to be higher in general surgical patients with obesity in multi-institutional cohort studies16,17. The aim of this study was to review the existing literature with respect to the impact of obesity on perioperative outcomes among patients undergoing general surgery operations.
Methods
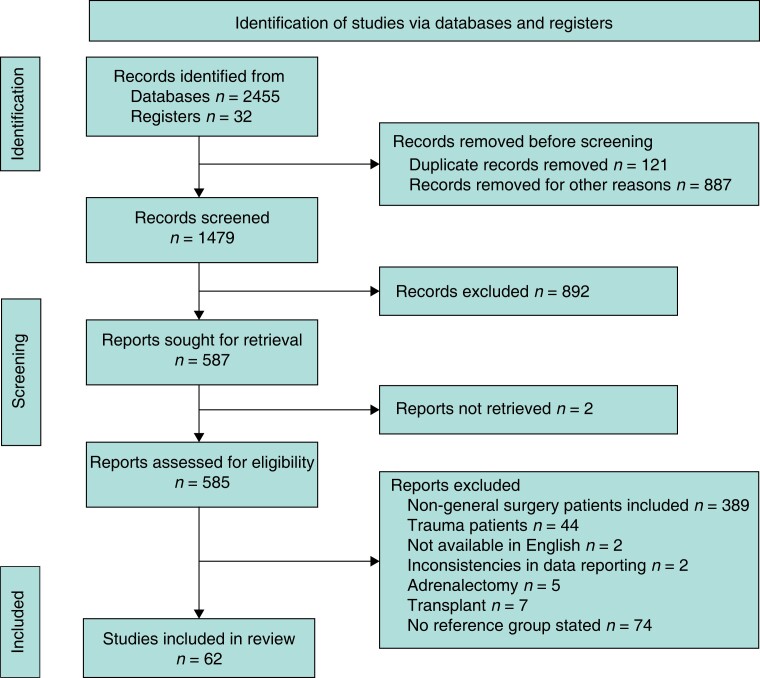
This study was performed following guidance from the PRISMA and Meta-Analysis of Observational Studies in Epidemiology (Fig. 1)18. Prospective registration was performed on PROSPERO (CRD42022337442).
Fig. 1.
PRISMA flowchart
Search strategy
An electronic search was conducted using the Cochrane Library, Science Direct, PubMed and Embase. All studies published to January 2022 were included. The following search terms/MeSH terms were used: (Obesity (MeSH) OR obese OR Body Mass Index OR in high BMI OR elevated BMI OR BMI) AND (general surgery (MeSH) OR surgery OR non bariatric surgery OR colorectal surgery OR gastrointestinal surgery OR GI surgery OR abdominal surgery OR hepatobiliary surgery OR Hepatobiliary (HPB) surgery emergency surgery OR oesophageal surgery OR liver surgery OR gastric surgery) AND (postoperative outcomes (MeSH) OR complications OR outcomes). All titles were initially screened, and appropriate abstracts were reviewed by two independent reviewers (C.C., A.F.). Each publication bibliography and Google Scholar were manually searched for relevant articles. The last date of search was 31 January 2022.
Outcomes
The primary outcome was the incidence of 30-day postoperative mortality among patients with obesity undergoing general surgical procedures in comparison to patients with normal range BMI. Obesity class subgroup categorization into class I, II and III obesity as per the WHO criteria was performed1. Asia-Pacific populations have different body fat distribution and have lower BMI cut-off values. Population-specific values were considered when subgrouping studies into the different BMI classes. The secondary outcomes were the incidence of in-hospital mortality, 30-day postoperative complications (classified as Clavien–Dindo >/= II) and surgical site infection (SSI) (classified as superficial or deep SSI). Clavien–Dindo >/= II morbidities were included to capture all complications requiring pharmacological or surgical intervention as these interventions impact the duration of hospital stay and postoperative patient experience. A subgroup analysis was also performed on studies including emergency general surgery patients. The quality of the studies included was assessed using the Newcastle–Ottawa Scale (NOS)19. The risk of bias was assessed using the Risk of Bias in Non-Randomized Studies of Intervention (ROBINS I Tool)20. Publication bias was assessed using visual inspection of funnel plots.
Inclusion criteria
All studies (observational, non-randomized) meeting the following criteria were suitable for inclusion: studies that included elective and emergency general surgery including upper gastrointestinal (GI), hepatobiliary and colorectal; studies that included a small cohort of non-general surgery patients (that is urology, vascular) with general surgery patients; studies that included non-obese as a reference group and reported the number of patients in individual BMI categories with reported outcomes of interest.
Exclusion criteria
The following exclusion criteria were applied: studies published in languages other than English; studies that focused on non-GI surgery patient groups, including organ transplant and adrenalectomy21; studies on trauma due to complexity of their multiple organ injury and physiology; studies did not present standardized incidence ratio, odds ratios, risk ratios or hazard ratio estimates (with 95 per cent c.i.), standard errors or number of events necessary to calculate these for the outcomes of interest.
Data extraction
Two reviewers (C.C., A.F.) independently screened all titles/abstracts/texts for eligibility according to the above predefined strategy and criteria. Each reviewer extracted the following variables: title and study details (year, design, country), study population characteristics (sample size, subspecialty, elective versus emergency, obesity subtypes, outcomes of interest and number of events). In cases of disagreement, a consensus was reached by discussion and agreed with a third reviewer (C.F.).
Statistical analysis
Statistical analysis was performed using Review Manager 5 (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark). Binary outcome data were reported as odds ratios (OR) with 95 per cent c.i. using the Mantel–Haenszel method. Adjusted odds ratios (OR) reported in the study publication were used when available; otherwise, they were extrapolated from the available data. Weighted mean differences (MDs) were calculated for the effect size on continuous variables. Heterogeneity was assessed using I-squared statistics, with >10 per cent being considered significant heterogeneity. A fixed-effects model was preferred to a random-effects model when there was no heterogeneity, otherwise a random-effects model was used. Pooled estimates of differences were calculated using random-effects models, accounting for potential interstudy heterogeneity. P values of <0.05 were considered significant.
Results
Characteristics of included studies
As outlined in Fig. 1, 62 studies, including 1 886 326 patients, were eligible for inclusion10–17,22–75. All studies were published between 2001 and 2022. Eleven studies were prospectively designed11,12,16,23,43,47,48,52,61,72,73 and the remaining 51 studies were retrospective10,13–15,17,22,24–30,32–42,44–46,49–51,53–60,62–67,74,75. Two of the prospectively designed studies were matched case-controlled43,47 and eight retrospective studies included matched controls28,33,42,44–46,49,54.
Countries of origin included: USA (n = 27), the Netherlands (n = 4), France (n = 4), Japan (n = 6), Germany (n = 5), Switzerland (n = 2), Denmark (n = 1), Chile (n = 1), South Korea (n = 1), Ireland (n = 2), Canada (n = 1), Australia (n = 1), Czech Republic (n = 1), UK (n = 2), Turkey (n = 1), Spain (n = 1) and China (n = 1). The breakdown of the different subspecialties of general surgery was also diverse: emergency general surgery (n = 5), colorectal (n = 34), elective general surgery (n = 9), upper GI (n = 12) and hepatobiliary (n = 2). Of these studies, three involved robotic surgery (colorectal (n = 2) and upper GI (n = 1)). Sixty-seven per cent (n = 42) of the included studies had a NOS of 8 or 9. The remaining 21 studies had a score of 6 or 7 (Table 1).
Table 1.
Characteristics of included studies (n = 62)
| n (%) | |
|---|---|
| Year of publication | |
| 2000–2006 | 9 (15) |
| 2007–2012 | 22 (36) |
| 2013–2017 | 19 (31) |
| 2018–2022 | 12 (18) |
| Median (range) age (years) | 66.6 (17–91) |
| Country of origin | |
| North America | 28 (46) |
| South America | 1 (2) |
| Asia | 8 (12) |
| Europe | 23 (38) |
| Australia | 1 (2) |
| Study design | |
| Prospective | 11 (18) |
| Retrospective | 51 (82) |
| Subspecialty | |
| Emergency general surgery (laparotomy, duodenal/bowel perforation, bowel obstruction) | 5 (8) |
| Elective general surgery (abdominal oncological resections, hernia repair, retroperitoneal dissection, liver surgery, cholecystectomy) | 9 (15) |
| Colorectal (colorectal resections, pouch surgery, rectal prolapse surgery) | 34 (56) |
| Upper gastrointestinal (gastrectomy, oesophagectomy) | 12 (18) |
| Hepatobiliary (liver resection, pancreato-duodenectomy) | 2 (3) |
| Newcastle-Ottawa score | |
| 8–9 | 42 (67) |
| 6–7 | 21 (33) |
Values are n (%) unless otherwise stated.
30-day morbidity
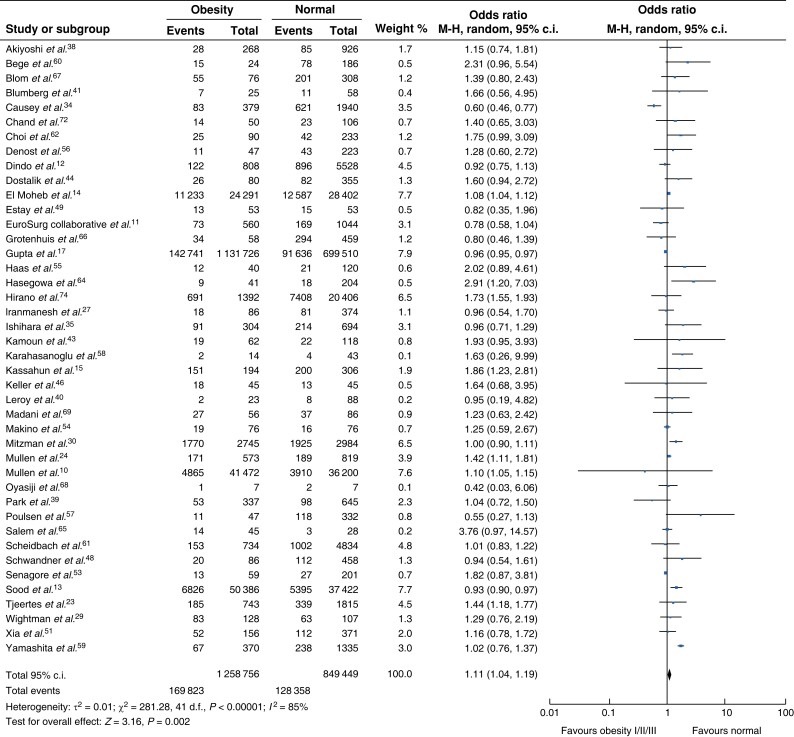
Forty-two studies (Fig. 2) were eligible for inclusion11–17,23,26,35,37–39,42,43,45–47,50–52,54–56,58–60,62–66,70–74. Compared with a normal BMI, obesity was positively associated with an increased risk of 30-day postoperative morbidity, although significant heterogeneity between studies was observed (OR 1.11, 95 per cent c.i. 1.04 to 1.19, P = 0.008, I2 = 79 per cent). Comparing normal BMI to class I obesity10,12,14,17,24,27,29,30,61 or class I/II obesity combined10,13,14,17,24,30, no significant difference in 30-day morbidity was observed (Fig. S1). More favourable outcomes for patients with a normal BMI compared with class II/III obesity combined were observed10,12,14,17,24,27,29,30,61 (OR 1.14, 95 per cent c.i. 1.02 to 1.27, P = 0.022) and results demonstrated significant heterogeneity (I2 = 93 per cent).
Fig. 2.
Thirty-day morbidity rate between non-obese patients and patients with obesity M-H, Mantel-Haenszel method.
Surgical site infection
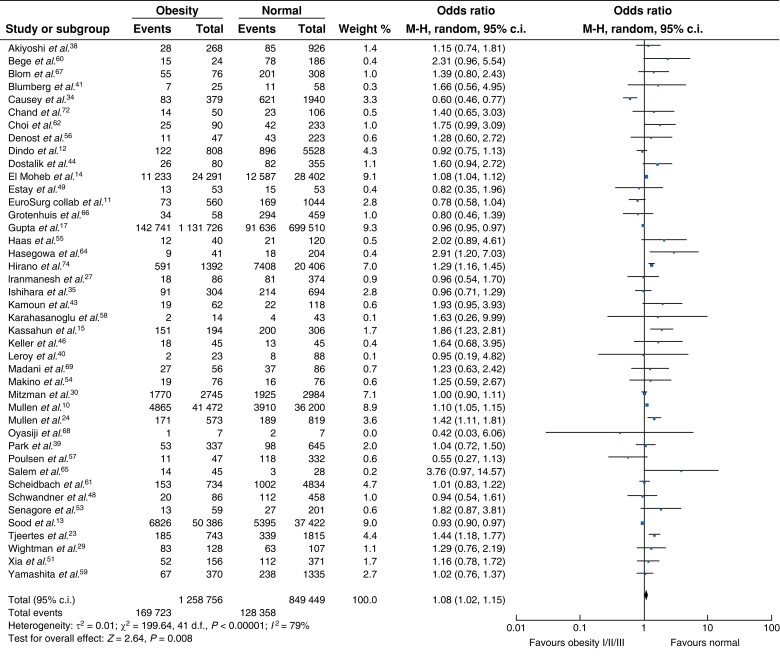
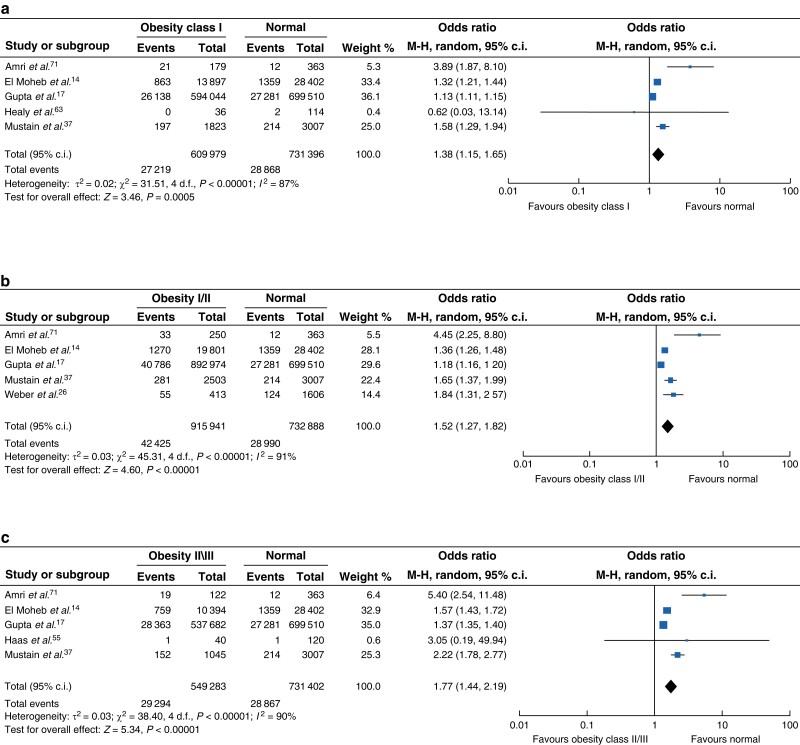
Thirty-six studies reported the incidence of SSI between patient cohorts with and without obesity12–17,23,26,35,37–39,42,43,45–47,50–52,54–56,58–60,62–66,70–73,75. Overall, the non-obese cohort had a statistically lower rate of SSI compared with patients with obesity (Fig. 3), although data were heterogenous (OR 1.40, 95 per cent c.i. 1.24 to 1.59, P < 0.001, I2 = 82 per cent). Five studies compared SSI rates between patients with normal range BMI and patients with class I obesity14,17,37,63,71 and demonstrated a significantly higher rate of SSI (Fig. 4a, OR 1.38, 95 per cent c.i. 1.15 to 1.65, P < 0.005). Compared with patients with a normal range BMI, patients with class I/II obesity had a significantly higher rate of SSI (Fig. 4b, OR 1.52, 95 per cent c.i. 1.27 to 1.82, P < 0.001)14,17,26,37,71. SSI rates among patients with class II/III obesity were also significantly higher compared with those with a normal BMI, however, there was significant heterogeneity between studies14,17,37,55,71 (Fig. 4c, OR 1.77, 95 per cent c.i. 1.44 to 2.19, P < 0.001, I2 = 90 per cent).
Fig. 3.
Surgical site infection among patients with normal BMI compared with obesity M-H, Mantel-Haenszel method.
Fig. 4.
Surgical site infection (SSI) among patients with normal BMI compared with obesity sub-class a SSI among patients with BMI as compared with class I obesity; b SSI among patients with normal BMI as compared with class I/II obesity; c SSI among patients with normal BMI as compared with class II/III obesity. M-H, Mantel-Haenszel method.
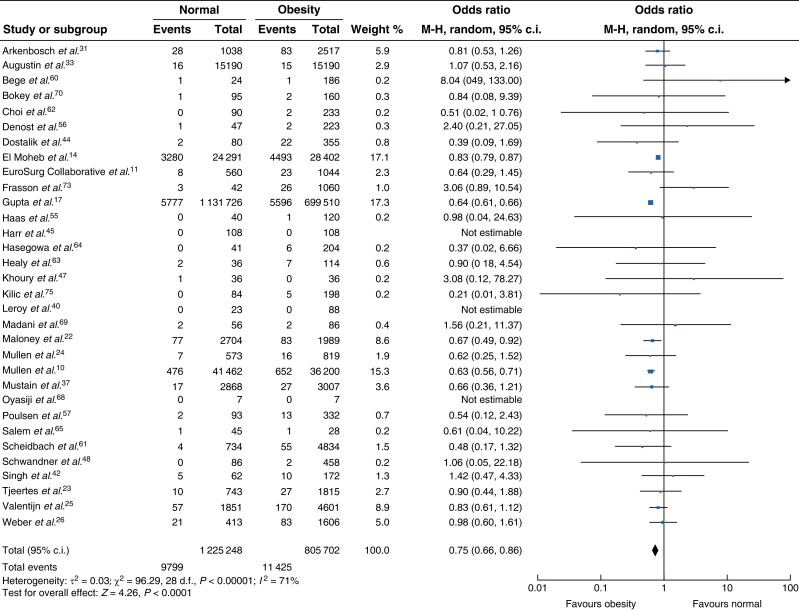
Thirty-day mortality and in-hospital mortality
Thirty-two studies reported 30-day mortality rates between non-obese and obese patient cohorts10,11,14,17,22–26,31,33,37,40,42,44,45,47,48,55–57,60–65,68–70,73,75. Overall, patients with obesity appeared to have significantly lower 30-day mortality rates in comparison to patients with a normal BMI (OR 0.75, 95 per cent c.i. 0.66 to 0.86, P < 0.0001). However, there was significant heterogeneity between the studies I2 = 71 per cent (Fig. 5). Nine studies compared mortality in normal BMI and class I obesity10,14,17,22,24,25,37,61,63. Patients with class I obesity had lower mortality in comparison to normal weight patients (OR 0.69 P < 0.001) with significant heterogeneity between the studies I2 = 83 per cent (Fig. S2a). In the eight studies comparing normal BMI and class I/II obesity combined10,14,17,22–25,37, patients with a normal BMI demonstrated a higher 30-day mortality rate in comparison to patients with class I and/or II obesity (Fig. S2b, OR 0.78, 95 per cent c.i. 0.64 to 0.93, P = 0.007) with significant heterogeneity between the studies (I2 = 94 per cent). This was also demonstrated in the comparison of normal BMI and class II/III obesity combined10,14,17,22,24–26,33,37,61, again favouring higher BMI (Fig. S2c, OR 0.78, 95 per cent c.i. 0.65 to 0.93, P = 0.006, I2 = 87 per cent). Fifteen studies compared the in-hospital mortality between patients with a normal BMI and all classes of obesity combined12,13,15,22,28–30,32,35,36,43,64,66,67,71,74. Comparisons according to the class of obesity were not possible due to limited reporting of obesity classes. Overall, there was no difference in in-hospital mortality between patients with obesity and patients without obesity (Fig. S3, OR 1.15, 95 per cent c.i. 0.79 to 1.67, P = 0.462, I2 = 90 per cent).
Fig. 5.
Thirty-day mortality among patients with normal BMI compared with obesity M-H, Mantel-Haenszel method.
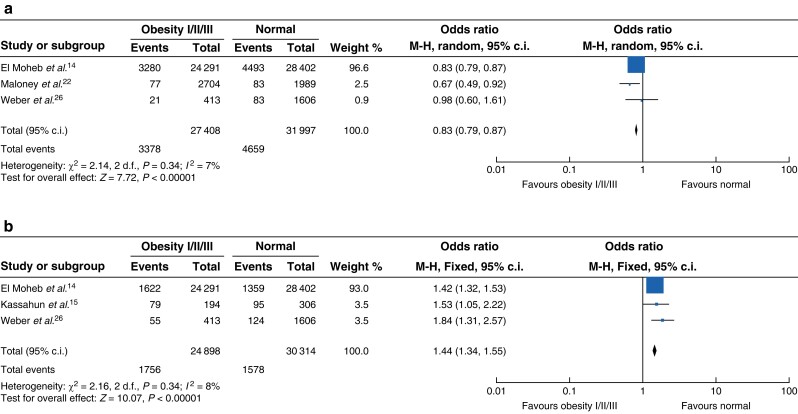
Emergency general surgery
Five studies reported on emergency general surgery patients only14,15,22,26,32. Three studies reported 30-day mortality in emergency general surgery patients with a normal BMI compared with patients with obesity14,22,26. Patients with an elevated BMI had a significantly lower 30-day mortality rate (Fig. 6a, OR 0.83, 95 per cent c.i. 0.79 to 0.87, P < 0.001, I2 = 7 per cent). Subgroup analysis of three suitable studies reporting SSI rates14,15,26 showed that patients with obesity had higher rates of wound infection (OR 1.44, 95 per cent c.i. 1.34 to 1.55, P < 0.001) with little heterogeneity between the studies (I2 = 8 per cent) (Fig. 6b).
Fig. 6.
Outcomes in emergency general surgery comparing normal BMI and obesity (all classes) a Thirty-day mortality following emergency general surgery among patients with a normal BMI as compared with obesity. b Surgical site infections following emergency general surgery among patients with a normal BMI as compared with obesity. M-H, Mantel-Haenszel method.
Discussion
This systematic review and meta-analysis investigated the effect of obesity on postoperative morbidity and mortality following GI surgery. Interestingly, no difference in 30-day morbidity between patients with a normal BMI and obesity was demonstrated, when obesity classes were considered individually. However, when all obesity classes were combined, there was an increased risk of 30-day postoperative morbidity among patients with obesity compared with patients with a normal BMI. Conversely, the 30-day postoperative mortality rate was significantly lower in patients with obesity compared with patients with a normal BMI.
The existence of an ‘obesity paradox’ relative to short-term 30-day survival outcomes among patients undergoing non-bariatric surgery was previously described by Mullen et al.10. Their large, prospective, multi-institutional study of 118 707 patients undergoing non-bariatric surgery demonstrated a reverse J-shaped relationship between BMI and 30-day mortality with the highest event rate observed among those who were underweight and those with severe obesity, and the lowest rates among those who were classed as overweight and moderately obese10. These results concurred with a single institution study of 6336 patients from Switzerland examining the impact of obesity on elective general surgery outcomes. Obesity was reported to be neither protective of or a risk factor for death or complications in patients undergoing elective surgery12. The long-term validation of the ‘obesity paradox’ was further reported by two general surgery studies with median follow-up times of over 5 years23,25. The concept that obesity incurs a reduced risk perioperatively is counterintuitive given that obesity is associated with various co-morbidities, including type 2 diabetes mellitus, hypertension, cerebrovascular events, coronary artery disease and an increased risk of death76,77.
Several biological mechanisms have been proposed to explain this obesity paradox. One such theory relates to the synergistic relationship between metabolomics and the immune response78. Immunological response to trauma or surgical insult initiates an acute phase inflammatory response79. Patients with obesity exhibit chronic, low-grade inflammation at baseline80. This chronically activated ‘meta-inflammatory’ state is the same signalling response required by the hosts’ immune response to respond appropriately to surgical trauma and initiate tissue healing81, suggesting that patients with obesity are primed immunologically to deal with the surgical insult. Another proposed theory on the protective effect of obesity on perioperative outcomes centres on the nutritional reserve available to mount the appropriate stress response to injury. This only applies to the obese and moderately obese cohorts as patients with severe obesity are believed to be ineffective in their energy use resulting in hyperbolic inflammatory responses, oxidative stress and immunosuppression10. The concept that patients with obesity have an increased metabolic reserve was analysed in a cohort of patients with obesity admitted to ICU following trauma where the authors demonstrated that in obesity, abundant fat stores are not effectively used as a fuel source and there is greater reliance on other fuel sources, such as endogenous protein82.
Findings from this meta-analysis suggest that when all classes of obesity are grouped together and compared with patients with a normal BMI, there is a slight increased risk in 30-day postoperative mortality in the non-obese group. A recent study demonstrated that in-patient mortality in patients undergoing emergency laparotomy varied according to weight classification, with patients with a BMI >40 having the worst outcome15. However, following adjustment for specific co-morbidities, BMI itself was not found to be an independent factor predictive of in-hospital mortality15. Similarly, Yanquez et al. suggested that increasing age combined with higher BMI was positively associated with morbidity and mortality, however, BMI itself was not an independent factor predicting 30-day complications83. A previous meta-analysis of 10 observational studies examined the perioperative outcomes of rectal cancer surgery in obese and non-obese cohorts and concluded that obesity increases the conversion rate (from laparoscopy to open) and postoperative morbidity of rectal cancer surgery but does not influence pathological results. However, six of the studies included underweight and overweight patients in the non-obese reference group, which may limit the generalizability of the results84. Similarly, a recent multicentre Japanese study observed that patients with a BMI >30 have an increased risk of postoperative complications following laparoscopic colorectal surgery (OR 2.6)59. Furthermore, a multicentre collaborative study conducted in the UK and Ireland suggested that overweight and obese patients undergoing surgery for GI malignancy, but not benign disease, were at increased risk of major postoperative complications compared with those of normal weight. These findings were explained by the fact that those undergoing surgery for benign conditions are likely to be subject to a selection bias of fitter patients for generally lower-risk procedures16. It was not possible to examine patients with benign and malignant conditions in this meta-analysis due to the heterogeneity of included studies, however, a follow-up European collaborative study (EuroSurg Collaborative) concluded that obesity was not found to be associated with major complications following GI surgery11.
Subgroup analysis of 36 eligible studies in the present study indicated that SSI rates were significantly lower in patients with a normal BMI (OR 0.71). The positive association between SSI and increased BMI is an observation that is echoed throughout the literature23,26,59. Wound infection rates in patients with obesity could be attributed to excess adipose tissue, which has low regional oxygen tension and is therefore susceptible to impaired wound healing and infection12. Furthermore, immune dysregulation and chronic cytokine secretion associated with obesity results in an immunosuppressive state, which likely contributes to higher rates of wound infection80. The tension on suture lines might be stressed in patients with obesity due to increased subcutaneous fat tissue, which would explain why laparoscopic surgery is advantageous in reducing SSI compared with open surgery in elective general surgery12,85.
This meta-analysis of 1 846 920 patients challenges the assumption that patients with obesity have a higher incidence and severity of major complications perioperatively compared with non-obese patients. Obesity defined by BMI does not appear to be a major risk factor in GI surgery. Given that body fat increases and muscle mass decreases with age, BMI may not accurately reflect changes in body fat or muscle mass and does not provide data on body fat distribution86. Abdominal obesity characterized by visceral fat accumulation measured using computed tomography (CT) may be a more accurate predictor of metabolic dysregulation in obesity86. Furthermore, BMI does not correctly capture the subset of patients with ‘metabolically healthy’ obesity, who do not experience the expected metabolic complications of obesity, most likely due to less visceral fat and preserved insulin sensitivity87,88. Conversely, patients with a normal BMI could be harbouring a disproportionately high mortality risk due to central obesity characterized by excessive visceral fat89.
There has been a shift in interest towards using more precise measures of body composition such as CT-derived abdominal measurement to accurately predict postsurgical outcomes. Kuritzkes et al. used CT-derived anthropometric results to predict postoperative morbidity in 264 patients undergoing colon resection for cancer and revealed that visceral fat accumulation, not BMI, predicts morbidity following elective surgery for colon cancer90. The clinical validity of visceral fat accumulation in predicting postoperative complications was corroborated by several other studies91,92. More recent studies indicate that the visceral to subcutaneous fat ratio and visceral to total fat ratio may be a more accurate predictive marker of postoperative outcomes because these values capture adipose tissue distribution and the technical challenges associated with central adiposity93,94. Fleming et al. reported that subcutaneous fat, which is considered relatively benign, was associated with higher levels of cytokines with anti-inflammatory properties, including interleukin 2 (IL-2) and IL-10, whereas patients with a high visceral to total fat ratio who developed recurrence had higher levels of the proinflammatory IL-6 and TNFα93. These findings suggest that different body composition profiles display their own unique inflammatory landscape, which may impact on postoperative outcomes.
The strength of this study lies in the large number of studies available for analysis spanning over two decades. Obesity subclassification according to BMI class also provides a more precise estimate of perioperative risk. However, there are several limitations to this study. The majority of the studies included were retrospective in nature and therefore subject to recall and information bias, particularly when relying on self-reported measurements and retrospective data from chart reviews. Similarly, there may be an element of selection bias present in the studies as patients with obesity may have been carefully selected for elective surgery because they displayed ‘healthy’ parameters. Subgroup analysis of emergency general surgery procedures was performed in an attempt to filter out patients who were carefully selected for elective surgery due to a healthier phenotype. As sarcopenia and an underweight BMI are consistently associated with poorer surgical outcomes in the literature, it is important to note that 21 of the studies included underweight patients in the non-obese comparator group26,32,42–50,53,54,57,59,60,62,67,69,70,72.
Supplementary Material
Contributor Information
Carolyn Cullinane, Department of Colorectal Surgery, University Hospital Waterford, Waterford, Ireland.
Anna Fullard, Department of General and Colorectal Surgery, University of Limerick Hospital Group, Limerick, Ireland.
Stefanie M Croghan, Department of Urology, Royal College of Surgeons Ireland, St Stephen’s Green, Dublin, Ireland.
Jessie A Elliott, Department of Surgery, Trinity St. James’s Cancer Institute, Trinity College Dublin, and St. James’s Hospital, Dublin, Ireland.
Christina A Fleming, Department of General and Colorectal Surgery, University of Limerick Hospital Group, Limerick, Ireland; Progress Women in Surgery Fellowship, Royal College of Surgeons in Ireland, Dublin, Ireland.
Funding
The authors have no funding to declare.
Disclosure
The authors declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS Open online.
Data availability
All data generated or analysed during this study are included in this manuscript (and its supplementary evidence). The data sets generated during the current study are available upon request from the corresponding author.
Author contributions
CC, SC, AF, JE and CF all conceptualised the project. CC, AF and CF performed data collection and data analysis. CC drafted the manuscript. AF, SC, JE and CF provided manuscript editing. JE and CF supervised the project.
References
- 1. World Health Organization . Obesity and Overweight Key Facts. 2021 [cited 23 May 2022]; Available from:https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
- 2. Berghöfer A, Pischon T, Reinhold T, Apovian CM, Sharma AM, Willich SNet al. Obesity prevalence from a European perspective: a systematic review. BMC Public Health 2008;8:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Haslam DW, James WP. Obesity. Lancet 2005;366:1197–1209 [DOI] [PubMed] [Google Scholar]
- 4. Suastika K. Update in the management of obesity. Acta Med Indones 2006;38:231–237 [PubMed] [Google Scholar]
- 5. Prospective Studies Collaboration, Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson Jet al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 2009;373:1083–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hawn MT, Bian J, Leeth RR, Ritchie G, Allen N, Bland KIet al. Impact of obesity on resource utilization for general surgical procedures. Ann Surg 2005;241:821–826; discussion 826–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lascano CA, Kaidar-Person O, Szomstein S, Rosenthal R, Wexner SD. Challenges of laparoscopic colectomy in the obese patient: a review. Am J Surg 2006;192:357–365 [DOI] [PubMed] [Google Scholar]
- 8. Gruberg L, Weissman NJ, Waksman R, Fuchs S, Deible R, Pinnow EEet al. The impact of obesity on the short-term and long-term outcomes after percutaneous coronary intervention: the obesity paradox? J Am Coll Cardiol 2002;39:578–584 [DOI] [PubMed] [Google Scholar]
- 9. Gurm HS, Brennan DM, Booth J, Tcheng JE, Lincoff AM, Topol EJ. Impact of body mass index on outcome after percutaneous coronary intervention (the obesity paradox). Am J Cardiol 2002;90:42–45 [DOI] [PubMed] [Google Scholar]
- 10. Mullen JT, Moorman DW, Davenport DL. The obesity paradox: body mass index and outcomes in patients undergoing nonbariatric general surgery. Ann Surg 2009;250:166–172 [DOI] [PubMed] [Google Scholar]
- 11. EuroSurg . Body mass index and complications following major gastrointestinal surgery: a prospective, international cohort study and meta-analysis. Colorectal Dis 2018;20:O215–O225 [DOI] [PubMed] [Google Scholar]
- 12. Dindo D, Muller MK, Weber M, Clavien P-A. Obesity in general elective surgery. Lancet 2003;361:2032–2035 [DOI] [PubMed] [Google Scholar]
- 13. Sood A, Abdollah F, Sammon JD, Majumder K, Schmid M, Peabody JOet al. The effect of body mass index on perioperative outcomes after major surgery: results from the national surgical quality improvement program (ACS-NSQIP) 2005–2011. World J Surg 2015;39:2376–2385 [DOI] [PubMed] [Google Scholar]
- 14. El Moheb M, Jia Z, Qin H, El Hechi MW, Nordestgaard AT, Lee JMet al. The obesity paradox in elderly patients undergoing emergency surgery: a nationwide analysis. J Surg Res 2021;265:195–203 [DOI] [PubMed] [Google Scholar]
- 15. Kassahun WT, Mehdorn M, Babel J. The impact of obesity on surgical outcomes in patients undergoing emergency laparotomy for high-risk abdominal emergencies. BMC Surg 2022;22:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. STARSurg Collaborative. Multicentre prospective cohort study of body mass index and postoperative complications following gastrointestinal surgery. Br J Surg 2016;103:1157–1172. doi: 10.1002/bjs.10203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gupta M, Dugan A, Chacon E, Davenport DL, Shah MB, Marti Fet al. Detailed perioperative risk among patients with extreme obesity undergoing nonbariatric general surgery. Surgery 2020;168:462–470 [DOI] [PubMed] [Google Scholar]
- 18. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CDet al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–605 [DOI] [PubMed] [Google Scholar]
- 20. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan Met al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Danwang C, Agbor VN, Bigna JJ. Obesity and postoperative outcomes of the patients with laparoscopic adrenalectomy: a systematic review and meta-analysis. BMC Surg 2020;20:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maloney SR, Reinke CE, Nimeri AA, Ayuso SA, Christmas AB, Hetherington Tet al. The obesity paradox in emergency general surgery patients. Am Surg 2021;88:3134820968524. [DOI] [PubMed] [Google Scholar]
- 23. Tjeertes EK, Hoeks SSE, Beks SSBJC, Valentijn TTM, Hoofwijk AAGM, Stolker RJRJ. Obesity–a risk factor for postoperative complications in general surgery? BMC Anesthesiol 2015;15:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mullen JT, Davenport DL, Hutter MM, Hosokawa PW, Henderson WG, Khuri SFet al. Impact of body mass index on perioperative outcomes in patients undergoing major intra-abdominal cancer surgery. Ann Surg Oncol 2008;15:2164–2172 [DOI] [PubMed] [Google Scholar]
- 25. Valentijn TM, Galal W, Hoeks SE, van Gestel YR, Verhagen HJ, Stolker RJ. Impact of obesity on postoperative and long-term outcomes in a general surgery population: a retrospective cohort study. World J Surg 2013;37:2561–2568 [DOI] [PubMed] [Google Scholar]
- 26. Weber KT, Chung PJ, La Gamma N, Procaccino JA, Alfonso AE, Coppa Get al. Effect of body mass index on outcomes after surgery for perforated diverticulitis. J Surg Res 2020;247:220–226 [DOI] [PubMed] [Google Scholar]
- 27. Iranmanesh P, Delaune V, Meyer J, Liot E, Konrad B, Ris Fet al. Comparison of outcomes between obese and non-obese patients in a colorectal enhanced recovery after surgery (ERAS) program: a single-center cohort study. Dig Surg 2020;37:420–427 [DOI] [PubMed] [Google Scholar]
- 28. Klasen J, Junger A, Hartmann B, Jost A, Benson M, Virabjan Tet al. Increased body mass index and peri-operative risk in patients undergoing non-cardiac surgery. Obes Surg 2004;14:275–281 [DOI] [PubMed] [Google Scholar]
- 29. Wightman SC, Posner MC, Patti MG, Ganai S, Watson S, Prachand Vet al. Extremes of body mass index and postoperative complications after esophagectomy. Dis Esophagus 2017;30:1–6 [DOI] [PubMed] [Google Scholar]
- 30. Mitzman B, Schipper PH, Edwards MA, Kim S, Ferguson MKet al. Complications after esophagectomy are associated with extremes of body mass index. Ann Thorac Surg 2018;106:973–980 [DOI] [PubMed] [Google Scholar]
- 31. Arkenbosch JHC, van Erning FN, Rutten HJ, Zimmerman D, de Wilt JHW, Beijer S. The association between body mass index and postoperative complications, 30-day mortality and long-term survival in Dutch patients with colorectal cancer. Eur J Surg Oncol 2019;45:160–166 [DOI] [PubMed] [Google Scholar]
- 32. Ferrada P, Anand RJ, Malhotra A, Aboutanos M. Obesity does not increase mortality after emergency surgery. J Obes 2014;2014:492127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Augustin T, Moslim MA, Brethauer S, Aminian A, Kroh M, Schneider Eet al. Obesity and its implications for morbidity and mortality after cholecystectomy: a matched NSQIP analysis. Am J Surg 2017;213:539–543 [DOI] [PubMed] [Google Scholar]
- 34. Causey MW, Johnson EK, Miller S, Martin M, Maykel J, Steele SR. The impact of obesity on outcomes following major surgery for Crohn's disease: an American College of Surgeons national surgical quality improvement program assessment. Dis Colon Rectum 2011;54:1488–1495 [DOI] [PubMed] [Google Scholar]
- 35. Ishihara A, Tanaka S, Shinkawa H, Yoshida H, Takemura S, Amano Ret al. Superiority of laparoscopic liver resection to open liver resection in obese individuals with hepatocellular carcinoma: a retrospective study. Ann Gastroenterol Surg 2022;6:135–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pausch T, Hartwig W, Hinz U, Swolana T, Bundy BD, Hackert Tet al. Cachexia but not obesity worsens the postoperative outcome after pancreatoduodenectomy in pancreatic cancer. Surgery 2012;152:S81–S88 [DOI] [PubMed] [Google Scholar]
- 37. Mustain WC, Davenport DL, Hourigan JS, Vargas HD. Obesity and laparoscopic colectomy: outcomes from the ACS-NSQIP database. Dis Colon Rectum 2012;55:429–435 [DOI] [PubMed] [Google Scholar]
- 38. Akiyoshi T, Ueno M, Fukunaga Y, Nagayama S, Fujimoto Y, Konishi Tet al. Effect of body mass index on short-term outcomes of patients undergoing laparoscopic resection for colorectal cancer: a single institution experience in Japan. Surg Laparosc Endosc Percutan Tech 2011;21:409–414 [DOI] [PubMed] [Google Scholar]
- 39. Park JW, Lim S-W, Choi HS, Jeong S-Y, Oh JH, Lim S-Bet al. The impact of obesity on outcomes of laparoscopic surgery for colorectal cancer in Asians. Surg Endosc 2010;24:1679–1685 [DOI] [PubMed] [Google Scholar]
- 40. Leroy J, Ananian P, Rubino F, Claudon B, Mutter D, Marescaux J. The impact of obesity on technical feasibility and postoperative outcomes of laparoscopic left colectomy. Ann Surg 2005;241:69–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Blumberg D. Laparoscopic colectomy performed using a completely intracorporeal technique is associated with similar outcome in obese and thin patients. Surg Laparosc Endosc Percutan Tech 2009;19:57–61 [DOI] [PubMed] [Google Scholar]
- 42. Singh A, Muthukumarasamy G, Pawa N, Riaz AA, Hendricks JB, Motson RW. Laparoscopic colorectal cancer surgery in obese patients. Colorectal Dis 2011;13:878–883 [DOI] [PubMed] [Google Scholar]
- 43. Kamoun S, Alves A, Bretagnol F, Lefevre JH, Valleur P, Panis Y. Outcomes of laparoscopic colorectal surgery in obese and nonobese patients: a case-matched study of 180 patients. Am J Surg 2009;198:450–455 [DOI] [PubMed] [Google Scholar]
- 44. Dostalik J, Martínek L, Vávra P, Andel P, Gunka I, Gunková P. Laparoscopic colorectal surgery in obese patients. Obes Surg 2005;15:1328–1331 [DOI] [PubMed] [Google Scholar]
- 45. Harr JN, Luka S, Kankaria A, Juo Y-Y, Agarwal S, Obias V. Robotic-assisted colorectal surgery in obese patients: a case-matched series. Surg Endosc 2017;31:2813–2819 [DOI] [PubMed] [Google Scholar]
- 46. Keller DS, Madhoun N, Flores-Gonzalez JR, Ibarra S, Tahilramani R, Haas EM. Effect of BMI on short-term outcomes with robotic-assisted laparoscopic surgery: a case-matched study. J Gastrointest Surg 2016;20:488–493 [DOI] [PubMed] [Google Scholar]
- 47. Khoury W, Kiran RP, Jessie T, Geisler D, Remzi FH. Is the laparoscopic approach to colectomy safe for the morbidly obese? Surg Endosc 2010;24:1336–1340 [DOI] [PubMed] [Google Scholar]
- 48. Schwandner O, Farke S, Schiedeck THK, Bruch H-P. Laparoscopic colorectal surgery in obese and nonobese patients: do differences in body mass indices lead to different outcomes? Surg Endosc 2004;18:1452–1456 [DOI] [PubMed] [Google Scholar]
- 49. Estay C, Zarate AJ, Castro M, Kronberg U, López-Köstner F, Wainstein C. Does obesity increase early postoperative complications after laparoscopic colorectal surgery? Results from a single center. Surg Endosc 2014;28:2090–2096 [DOI] [PubMed] [Google Scholar]
- 50. Pikarsky AJ, Saida Y, Yamaguchi T, Martinez S, Chen W, Weiss EGet al. Is obesity a high-risk factor for laparoscopic colorectal surgery? Surg Endosc 2002;16:855–858 [DOI] [PubMed] [Google Scholar]
- 51. Xia X, Huang C, Jiang T, Cen G, Cao J, Huang Ket al. Is laparoscopic colorectal cancer surgery associated with an increased risk in obese patients? A retrospective study from China. World J Surg Oncol 2014;12:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tuech JJ, Regenet N, Hennekinne S, Pessaux P, Bergamaschi R, Arnaud J-P. Laparoscopic colectomy for sigmoid diverticulitis in obese and nonobese patients: a prospective comparative study. Surg Endosc 2001;15:1427–1430 [DOI] [PubMed] [Google Scholar]
- 53. Senagore AJ, Delaney CP, Madboulay K, Brady KM, Fazio CV. Laparoscopic colectomy in obese and nonobese patients. J Gastrointest Surg 2003;7:558–561 [DOI] [PubMed] [Google Scholar]
- 54. Makino T, Trencheva K, Shukla PJ, Rubino F, Zhuo C, Pavoor RSet al. The influence of obesity on short- and long-term outcomes after laparoscopic surgery for colon cancer: a case-matched study of 152 patients. Surgery 2014;156:661–668 [DOI] [PubMed] [Google Scholar]
- 55. Haas E, Aminian A, Nieto J, Pedraza R, Martinez C, Patel CBet al. Minimally invasive colorectal surgery in the morbidly obese: does high body mass index lead to poorer outcomes? Surg Curr Res 2013;03:3486–3494 [Google Scholar]
- 56. Denost Q, Quintane L, Buscail E, Martenot M, Laurent C, Rullier E. Short- and long-term impact of body mass index on laparoscopic rectal cancer surgery. Colorectal Dis 2013;15:463–469 [DOI] [PubMed] [Google Scholar]
- 57. Poulsen M, Ovesen H. Is laparoscopic colorectal cancer surgery in obese patients associated with an increased risk? Short-term results from a single center study of 425 patients. J Gastrointest Surg 2012;16:1554–1558 [DOI] [PubMed] [Google Scholar]
- 58. Karahasanoglu T, Hamzaoglu I, Baca B, Aytac E, Kirbiyik E. Impact of increased body mass index on laparoscopic surgery for rectal cancer. Eur Surg Res 2011;46:87–93 [DOI] [PubMed] [Google Scholar]
- 59. Yamashita M, Tominaga T, Nonaka T, Fukda A, Moriyama M, Oyama Set al. Impact of obesity on short-term outcomes of laparoscopic colorectal surgery for Japanese patients with colorectal cancer: a multicenter study. Asian J Endosc Surg 2021;14:432–442 [DOI] [PubMed] [Google Scholar]
- 60. Bege T, Lelong B, Francon D, Turrini O, Guiramand J, Delpero J-R. Impact of obesity on short-term results of laparoscopic rectal cancer resection. Surg Endosc 2009;23:1460–1464 [DOI] [PubMed] [Google Scholar]
- 61. Scheidbach H, Benedix F, Hügel O, Kose D, Köckerling F, Lippert H. Laparoscopic approach to colorectal procedures in the obese patient: risk factor or benefit? Obes Surg 2008;18:66–70 [DOI] [PubMed] [Google Scholar]
- 62. Choi BJ, Jeong WJ, Kim S-J, Lee SC. Impact of obesity on the short-term outcomes of single-port laparoscopic colectomy for colorectal cancer in the Asian population: a retrospective cohort study. Medicine (Baltimore) 2017;96:e6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Healy LA, Ryan AM, Gopinath B, Rowley S, Byrne PJ, Reynolds JVet al. Impact of obesity on outcomes in the management of localized adenocarcinoma of the esophagus and esophagogastric junction. J Thorac Cardiovasc Surg 2007;134:1284–1291 [DOI] [PubMed] [Google Scholar]
- 64. Hasegawa T, Kubo N, Ohira M, Sakurai K, Toyokawa T, Yamashita Yet al. Impact of body mass index on surgical outcomes after esophagectomy for patients with esophageal squamous cell carcinoma. J Gastrointest Surg 2015;19:226–233 [DOI] [PubMed] [Google Scholar]
- 65. Salem AI, Thau MR, Strom TJ, Abbott AM, Saeed N, Almhanna Ket al. Effect of body mass index on operative outcome after robotic-assisted Ivor-Lewis esophagectomy: retrospective analysis of 129 cases at a single high-volume tertiary care center. Dis Esophagus 2017;30:1–7 [DOI] [PubMed] [Google Scholar]
- 66. Grotenhuis BA, Wijnhoven BPL, Hötte GJ, van der Stok EP, Tilanus HW, van Lanschot JJBet al. Prognostic value of body mass index on short-term and long-term outcome after resection of esophageal cancer. World J Surg 2010;34:2621–2627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Blom RL, Lagarde SM, Klinkenbijl JHG, Busch ORC, van Berge Henegouwen MI. A high body mass index in esophageal cancer patients does not influence postoperative outcome or long-term survival. Ann Surg Oncol 2012;19:766–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Oyasiji T, Baldwin K, Katz SC, Espat NJ, Somasundar P. Feasibility of purely laparoscopic resection of locally advanced rectal cancer in obese patients. World J Surg Oncol 2012;10:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Madani K, Zhao R, Lim HJ, Casson SM, Casson AG. Obesity is not associated with adverse outcome following surgical resection of oesophageal adenocarcinoma. Eur J Cardiothorac Surg 2010;38:604–608 [DOI] [PubMed] [Google Scholar]
- 70. Bokey L, Chapuis PH, Dent OF. Impact of obesity on complications after resection for rectal cancer. Colorectal Dis 2014;16:896–906 [DOI] [PubMed] [Google Scholar]
- 71. Amri R, Bordeianou LG, Sylla P, Berger DL. Obesity, outcomes and quality of care: body mass index increases the risk of wound-related complications in colon cancer surgery. Am J Surg 2014;207:17–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chand M, De’Ath HD, Siddiqui M, Mehta C, Rasheed S, Bromilow Jet al. Obese patients have similar short-term outcomes to non-obese in laparoscopic colorectal surgery. World J Gastrointest Surg 2015;7:261–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Frasson M, Granero-Castro P, Ramos Rodríguez JL, Flor-Lorente B, Braithwaite M, Martí Martínez Eet al. Risk factors for anastomotic leak and postoperative morbidity and mortality after elective right colectomy for cancer: results from a prospective, multicentric study of 1102 patients. Int J Colorectal Dis 2016;31:105–114 [DOI] [PubMed] [Google Scholar]
- 74. Hirano Y, Kaneko H, Konishi T, Itoh H, Matsuda S, Kawakubo Het al. Impact of body mass index on major complications, multiple complications, in-hospital mortality, and failure to rescue following esophagectomy for esophageal cancer: a nationwide inpatient database study in Japan. Ann Surg 2021;277:e785–792 [DOI] [PubMed] [Google Scholar]
- 75. Kilic A, Schuchert MJ, Pennathur A, Yaeger K, Prasanna V, Luketich JDet al. Impact of obesity on perioperative outcomes of minimally invasive esophagectomy. Ann Thorac Surg 2009;87:412–415 [DOI] [PubMed] [Google Scholar]
- 76. Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash Ret al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med 2006;355:763–778 [DOI] [PubMed] [Google Scholar]
- 77. Mourelo R, Kaidar-Person O, Fajnwaks P, Roa PE, Pinto D, Szomstein Set al. Hemorrhagic and thromboembolic complications after bariatric surgery in patients receiving chronic anticoagulation therapy. Obes Surg 2008;18:167–170 [DOI] [PubMed] [Google Scholar]
- 78. Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006;444:860–867 [DOI] [PubMed] [Google Scholar]
- 79. Moldawer LL, Copeland EM 3rd.. Proinflammatory cytokines, nutritional support, and the cachexia syndrome: interactions and therapeutic options. Cancer 1997;79:1828–1839 [PubMed] [Google Scholar]
- 80. Gil A, María Aguilera C, Gil-Campos M, Cañete R. Altered signalling and gene expression associated with the immune system and the inflammatory response in obesity. Br J Nutr 2007;98:S121–S126 [DOI] [PubMed] [Google Scholar]
- 81. Doyle SL, Lysaght J, Reynolds JV. Obesity and post-operative complications in patients undergoing non-bariatric surgery. Obes Rev 2010;11:875–886 [DOI] [PubMed] [Google Scholar]
- 82. Jeevanandam M, Young DH, Schiller WR. Obesity and the metabolic response to severe multiple trauma in man. J Clin Invest 1991;87:262–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Yanquez FJ, Clements JM, Grauf D, Merchant AM. Synergistic effect of age and body mass index on mortality and morbidity in general surgery. J Surg Res 2013;184:89–100 [DOI] [PubMed] [Google Scholar]
- 84. Qiu Y, Liu Q, Chen G, Wang W, Peng K, Xiao Wet al. Outcome of rectal cancer surgery in obese and nonobese patients: a meta-analysis. World J Surg Oncol 2016;14:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. DeMaria EJ, Sugerman HJ, Kellum JM, Meador JG, Wolfe LG. Results of 281 consecutive total laparoscopic roux-en-Y gastric bypasses to treat morbid obesity. Ann Surg 2002;235:640–645; discussion 645–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Rothman KJ. BMI-related errors in the measurement of obesity. Int J Obes (Lond) 2008;32:S56–S59 [DOI] [PubMed] [Google Scholar]
- 87. Stefan N, Häring H-U, Hu FB, Schulze MB. Metabolically healthy obesity: epidemiology, mechanisms, and clinical implications. Lancet Diabetes Endocrinol 2013;1:152–162 [DOI] [PubMed] [Google Scholar]
- 88. Chen DL, Liess C, Poljak A, Xu A, Zhang J, Thoma Cet al. Phenotypic characterization of insulin-resistant and insulin-sensitive obesity. J Clin Endocrinol Metab 2015;100:4082–4091 [DOI] [PubMed] [Google Scholar]
- 89. Coutinho T, Corrêa de Sá D, Kragelund C, Kanaya AM, Zeller M, Park J-Set al. Central obesity and survival in subjects with coronary artery disease: a systematic review of the literature and collaborative analysis with individual subject data. J Am Coll Cardiol 2011;57:1877–1886 [DOI] [PubMed] [Google Scholar]
- 90. Kuritzkes BA, Pappou EP, Kiran RP, Baser O, Fan L, Guo Xet al. Visceral fat area, not body mass index, predicts postoperative 30-day morbidity in patients undergoing colon resection for cancer. Int J Colorectal Dis 2018;33:1019–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Yamamoto M, Takakura Y, Ikeda S, Itamoto T, Urushihara T, Egi H. Visceral obesity is a significant risk factor for incisional hernia after laparoscopic colorectal surgery: a single-center review. Asian J Endosc Surg 2018;11:373–377 [DOI] [PubMed] [Google Scholar]
- 92. Chen WZ, Chen X-D, Ma L-L, Zhang F-M, Lin J, Zhuang C-Let al. Impact of visceral obesity and sarcopenia on short-term outcomes after colorectal cancer surgery. Dig Dis Sci 2018;63:1620–1630 [DOI] [PubMed] [Google Scholar]
- 93. Fleming CA, O’Connell EP, Kavanagh RG, O’Leary DP, Twomey M, Corrigan MAet al. Body composition, inflammation, and 5-year outcomes in colon cancer. JAMA Network Open 2021;4:e2115274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. He AQ, Li C-Q, Zhang Q, Liu T, Liu J, Liu G. Visceral-to-subcutaneous fat ratio is a potential predictor of postoperative complications in colorectal cancer. Med Sci Monit 2021;27:e930329 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this manuscript (and its supplementary evidence). The data sets generated during the current study are available upon request from the corresponding author.