Abstract
Introduction
Venous thromboembolism (VTE) is a life-threatening complication occurring in cancer patients. Direct oral anticoagulants (DOACs) or warfarin are widely prescribed for treating cancer-associated VTE. However, data are sparse as to the effectiveness and bleeding complications associated with these medications in elderly patients. The purpose of this study was to compare effectiveness and safety profiles between DOACs and warfarin in elderly cancer patients undergoing chemotherapy.
Methods
Using the Diagnosis Procedure Combination inpatient database, we retrospectively identified cancer patients aged ≥75 years who developed VTE during chemotherapy (n = 4,278, January 2016 to March 2020). Eligible patients were divided into those receiving warfarin (n = 557) and DOACs (n = 3,721). We conducted a 1:4 propensity score matching analysis to adjust for measured confounders. The primary outcome was VTE recurrence requiring hospitalization. Secondary outcomes were major bleeding requiring hospitalization and inhospital death from all causes within 6 months.
Results
The propensity-matched cohort included 557 patients in the warfarin group and 2,278 patients in the DOACs group. The proportion of VTE recurrence requiring hospitalization was lower in the DOACs group (5.3% vs. 7.5%; odds ratio [OR], 0.69; 95% confidence interval [CI], 0.48–0.98). The proportion of recurrent deep vein thrombosis was 6.3% and 4.4%, while that of recurrent pulmonary emboli was 1.3% and 1.3% in the warfarin and DOACs groups, respectively. No statistically significant differences were found in the proportion of major bleeding events requiring hospitalization (1.6% vs. 1.1%; OR, 1.47; 95% CI, 0.62–3.50) or all-cause inhospital mortality (11.1% vs. 9.9%; OR, 1.14; 95% CI, 0.84–1.56) between the DOACs and warfarin groups.
Conclusion
Our findings suggest that DOACs may be more effective than warfarin in terms of VTE recurrence requiring hospitalization and that these medications may be equivalent in terms of safety.
Keywords: Old age, Chemotherapy, Direct oral anticoagulants, Venous thromboembolism, Warfarin
Introduction
Venous thromboembolism (VTE) is a life-threatening complication and the second leading cause of death in cancer patients [1]. Cancer patients have 2–9 fold higher risk of recurrent VTE during anticoagulation therapy and a 2–3 fold higher risk of major bleeding events compared to patients without cancer [2, 3, 4]. Anticoagulants should be carefully selected in treating cancer-associated VTE.
Guidelines have recommended the use of low-molecular-weight heparin as a treatment for cancer-associated VTE. The advent of direct oral anticoagulants (DOACs) has prompted many randomized controlled trials [5, 6, 7, 8, 9], resulting in the inclusion of DOACs within recent guidelines [10, 11, 12]. A network meta-analysis of randomized controlled trials evaluating low-molecular-weight heparin, warfarin, and DOACs for the treatment of cancer-associated VTE showed that the effectiveness and safety of DOACs were equivalent to warfarin and may be equivalent to that of low-molecular-weight heparin [13].
The elderly (those aged 75 years or older) account for a growing proportion of patients with cancer. Age-related declines in kidney and liver function can affect the pharmacokinetics of therapeutic agents [14]. Elderly cancer patients are at higher risk of bleeding events, possibly because of their vulnerability to the adverse effects of chemotherapy as well as potential organ dysfunction and frail health (sarcopenia and undernutrition) associated with advanced age [15].
Although DOACs or warfarin are widely used in practice, their effectiveness and bleeding complications in routine clinical practice in elderly patients with cancer-associated VTE occurring during chemotherapy are still unclear. The aim of this study was to compare the effectiveness and safety profiles between DOACs and warfarin during chemotherapy for elderly cancer patients using a nationwide inpatient database in Japan.
Methods
Data Source
This nationwide retrospective cohort study was performed using the Japanese Diagnosis Procedure Combination database. The database contains discharge abstracts and administrative claims data covering approximately 8,000,000 admissions in more than 1,200 hospitals throughout Japan and includes approximately half of all patients admitted to acute care hospitals in Japan [16]. All 82 academic hospitals are obliged to participate in the database, whereas participation by community hospitals is voluntary.
The database includes the following information: unique hospital identifiers; patient age and sex; smoking history (including both current and former smoking status) at admission; body mass index (BMI) at admission; activities of daily living (ADL) at admission; dates of admission and discharge; length of stay; inhospital mortality; blood transfusions and medications; and interventional/surgical procedures indexed by the original Japanese codes. Diagnoses, comorbidities, and complications are recorded using ICD-10 (International Classification of Diseases, Tenth Revision) codes as well as text data coded in Japanese. The database contains no laboratory data. In a previous validation study, good sensitivity and specificity were shown for the diagnoses and procedures recorded in this database [17].
Patient Selection
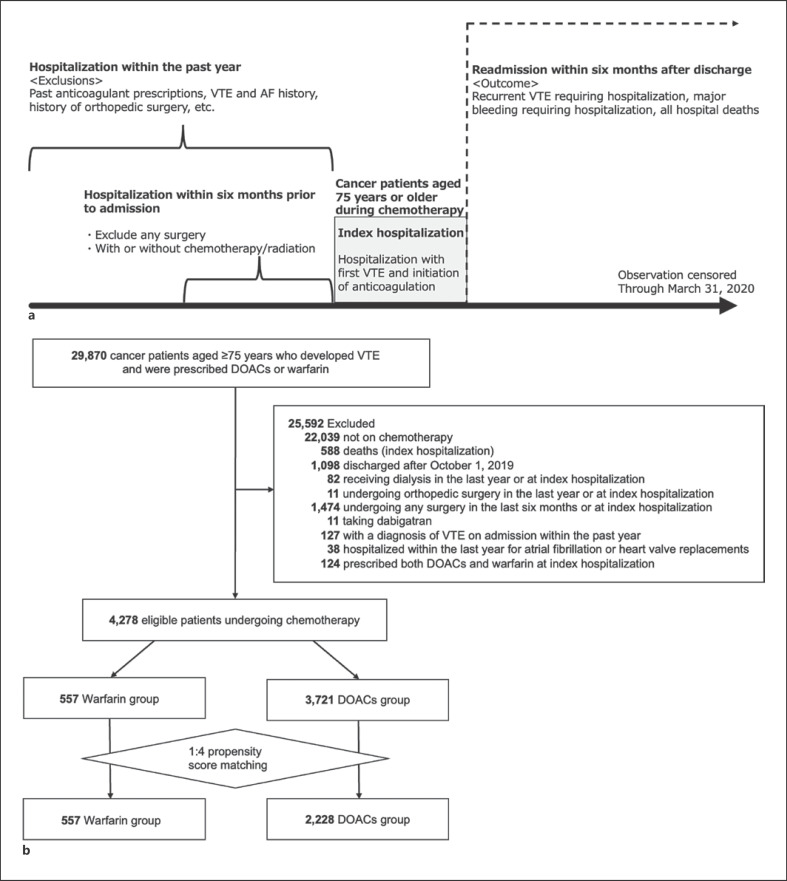
Between January 2016 and March 2020, we identified patients aged 75 years or older with cancer (ICD-10 code, C), who were receiving chemotherapy, and who were diagnosed with VTE (I80.1, I80.2, I80.3, I80.8, I80.9, I82.8, I82.9, I26.0, I26.9) during or shortly after chemotherapy and were prescribed DOACs or warfarin at a participating hospital. The study design is shown in Figure 1a.
Fig. 1.
Schematic diagram depicting study groupings. a Study design. b Flow diagram. AF, atrial fibrillation; DOACs, direct oral anticoagulants; VTE, venous thromboembolism.
The evaluated DOACs included edoxaban, rivaroxaban, and apixaban. In Japan, DOACs have been sequentially approved for the prevention of VTE associated with atrial fibrillation and orthopedic surgery since 2011. However, approval for the treatment or prevention of recurrence of VTE was not obtained until September 2014 for edoxaban, until September 2015 for rivaroxaban, and until December 2015 for apixaban. Therefore, only patients admitted after January 2016 were included in the current study.
Patients undergoing chemotherapy were defined as those receiving chemotherapy at the time of being diagnosed with VTE and prescribed anticoagulants (at the index hospitalization: the index hospitalization was defined as first VTE hospitalization and initiation of anticoagulation) or within 6 months prior to the index hospitalization. The following patients were excluded: (i) those not receiving chemotherapy (currently or recently); (ii) those who died during the index hospitalization; (iii) those who were discharged after October 1, 2019 (as the observation period was terminated on March 31, 2020); (iv) those who underwent dialysis during the last year or at the index hospitalization, (v) those who underwent hip/knee joint replacements or hip fracture surgery during the last year or at the index hospitalization; (vi) those who underwent any surgery during the last 6 months or at the index hospitalization; (vii) those taking dabigatran because dabigatran was not approved for VTE in Japan; (viii) those who received a diagnosis of VTE during hospitalization within the past year; (ix) those who were admitted to hospital within the last year and were diagnosed with atrial fibrillation (I48) or received heart valve replacements, including aortic valve (Z95.2) and mitral valve replacements (Z95.4); and (x) those who were prescribed both DOACs and warfarin at the index hospitalization. We allocated the eligible patients into warfarin and DOAC groups as appropriate.
Outcomes
The primary outcome was recurrent VTE requiring hospitalization, which included recurrent deep vein thrombosis and recurrent pulmonary embolism requiring hospitalization within 180 days of discharge from the index hospitalization. The index date for patient follow-up was the date of discharge from the time of the index hospitalization. The secondary outcomes were (i) major bleeding events requiring hospitalization, (ii) inhospital death from all causes, and (iii) at least one of the following: recurrent VTE, major bleeding event, and inhospital death from all causes (composite outcome). We examined these outcomes that occurred during re-hospitalization within 6 months of discharge from the index hospitalization.
Statistical Analysis
Logistic regression analysis was used to compare outcomes between the two groups. We accounted for clustering within hospitals using generalized estimating equations [18]. All hypothesis tests employed a two-sided statistical significance level of 0.05, and all statistical analyses were performed using Stata/SE 17.0 statistical software (StataCorp, College Station, TX, USA).
Propensity Score Matching
We performed one-to-four propensity score matching between the warfarin and DOACs groups. To estimate propensity scores, we fitted a logistic regression model for warfarin prescription as a function of patient characteristics, treatments, and hospital factors. Patient characteristics comprised year of hospitalization, sex, age, BMI, weight, smoking status (nonsmoker, current/past smoker), hospitalization, ADL at admission, comorbidities, medications prescribed/taken at the hospital, anticancer agents used at the time of the index hospitalization, VTE risk category according to cancer type, metastasis, and the use of radiotherapy and anticancer agents within the past 6 months of the index hospitalization, comorbidities, and baseline medications. BMI was categorized based using World Health Organization classifications: <18.5 kg/m2 (underweight), 18.5–24.9 kg/m2 (normal weight), 25.0–29.9 kg/m2 (overweight), and <30.0 kg/m2 (obese). Normal weight was further divided into low (18.5–21.9) and high (22.0–24.9) normal weight categories. Weight was dichotomized at a demarcation of >60 versus ≤60 kg. This is because, according to medical guidelines, the daily dosage of edoxaban is determined by whether or not weight exceeds 60 kg. ADL at admission was assessed using the Barthel index and was categorized into two groups: <100 and 100. Based on past reports [19], we classified cancer type into the following risk categories (in terms of VTE risk): very high risk (pancreas, stomach), high risk (colon, lung, gynecologic, urinary, blood), and others (head and neck, esophagus, liver, bone, skin, breast, thyroid, etc.). We defined distant metastases as M1 classifications within the TNM stating system, as well as according to classifications of malignant tumors using Union for International Cancer Control guidelines or C77-C80 ICD-10 codes. We calculated c-statistic, set a caliper width at 20% of the standard deviation of the propensity scores, and performed 1:4 nearest neighbor matching without replacement. To estimate the balance of covariates after propensity score matching, we assessed standardized differences to compare patient characteristics between the warfarin and DOAC groups. We considered an absolute standardized difference of over 10% as being out of balance [20, 21, 22]. In the subgroup analysis, we stratified the primary analysis by age (75–79, ≥80 years), metastasis status, and VTE risk category.
Results
We identified 29,870 patients with cancer with a first VTE onset who were aged 75 or older and were prescribed DOACs or warfarin between January 1, 2016, and March 31, 2020. We then excluded 25,592 patients according to the exclusion criteria shown in Figure 1b.
Table 1 shows the baseline characteristics of the unmatched and propensity score-matched patients treated with warfarin and DOACs. Among the unmatched patients, those in the DOAC group were more likely to be treated with antimetabolites, platinum-based medicines, microtubule inhibitors, or molecular targeted drugs and were more likely to have a high risk of VTE according to cancer type, metastasis, the presence of hospital admissions within the last 6 months, and radiotherapy history. The warfarin group was more likely to be older and to have types of cancer that were associated with lower VTE risk. Regarding the year of hospitalization, 2016 and 2017 had a higher proportion of patients in the warfarin group, whereas 2018 and 2019 had a higher proportion of patients in the DOAC group. The proportion of brain metastases (C793) was higher in the DOAC group as compared to the warfarin group both before and after matching: 0.36% versus 2.2% before matching, and 0.36% versus 0.9% after matching.
Table 1.
Baseline characteristics of unmatched and propensity score-matched patients treated with warfarin and DOACs
| Unmatched |
1:4 Propensity score matched |
|||||
|---|---|---|---|---|---|---|
| warfarin n = 557, % | DOACs n = 3,721, % | ASD (%) | warfarin n = 557, % | DOACs n = 2,228, % | ASD (%) | |
| Year of hospitalization | ||||||
| 2016 | 40.6 | 20.4 | 44.9 | 40.6 | 41.5 | 1.9 |
| 2017 | 32.0 | 27.3 | 10.3 | 32.0 | 29.8 | 4.8 |
| 2018 | 18.3 | 31.1 | 29.9 | 18.3 | 18.6 | 0.7 |
| 2019 | 9.2 | 21.3 | 34.2 | 9.2 | 10.1 | 3.3 |
| Age (mean), years | 81.2 | 80.1 | 24.4 | 81.2 | 81.3 | 2.0 |
| Sex (male) | 54.0 | 48.0 | 13.7 | 54.0 | 57.0 | 3.8 |
| BMI, kg/m2 | ||||||
| <18.5 | 11.1 | 12.0 | 2.8 | 11.1 | 11.4 | 0.9 |
| 18.5–21.9 | 31.4 | 32.8 | 3.0 | 31.4 | 31.5 | 0.1 |
| 22.0–24.9 | 32.1 | 30.6 | 3.2 | 32.1 | 32.0 | 0.3 |
| 25.0–29.9 | 18.0 | 18.5 | 1.4 | 18.0 | 17.0 | 2.6 |
| ≥ 30.0 | 4.7 | 3.2 | 7.4 | 4.7 | 5.7 | 4.7 |
| Missing | 2.7 | 2.8 | 0.6 | 2.7 | 2.5 | 1.4 |
| Weight, kg | ||||||
| >60.0 | 67.7 | 70.3 | 5.6 | 67.7 | 66.1 | 3.3 |
| ≤60.0 | 29.8 | 27.8 | 4.3 | 29.8 | 31.2 | 3.1 |
| Missing | 2.5 | 1.9 | 4.3 | 2.5 | 2.6 | 0.8 |
| Smoking | ||||||
| Current/past smoker | 67.0 | 63.9 | 6.4 | 67.0 | 67.9 | 2.0 |
| Nonsmoker | 24.2 | 26.1 | 4.3 | 24.2 | 24.2 | 0.1 |
| Unspecified | 8.8 | 10.0 | 4.1 | 8.8 | 7.9 | 3.2 |
| Hospitalization, day | 31.7 | 26.7 | 7.3 | 31.7 | 31.5 | 0.8 |
| Barthel index | ||||||
| <100 | 40.6 | 37.5 | 6.3 | 40.6 | 39.1 | 3.0 |
| 100 | 48.8 | 50.5 | 3.3 | 48.8 | 50.7 | 3.7 |
| Missing | 10.6 | 12.0 | 4.5 | 10.6 | 10.2 | 1.2 |
| Bleeding | 2.0 | 2.0 | 1.5 | 2.0 | 2.0 | 2.2 |
| Treatments during hospitalization | ||||||
| IVC filter implantation | 3.0 | 4.0 | 5.9 | 3.0 | 4.0 | 4.7 |
| Central venous catheter | 4.0 | 5.0 | 3.5 | 4.0 | 4.0 | 0.7 |
| Thrombolytic agent | 1.0 | 1.0 | 6.0 | 1.0 | 1.0 | 6.0 |
| Erythrocyte transfusion | 28.0 | 30.0 | 3.6 | 28.0 | 27.0 | 3.2 |
| Radiotherapy | 7.0 | 8.0 | 4.0 | 7.0 | 7.0 | 1.6 |
| Anticancer agents | ||||||
| Antimetabolites | 17.0 | 23.0 | 14.8 | 17.0 | 16.0 | 2.7 |
| Platinum-based medicine | 13.0 | 21.0 | 20.9 | 13.0 | 14.0 | 1.7 |
| Microtubule inhibitors | 18.0 | 20.0 | 5.2 | 18.0 | 19.0 | 2.3 |
| Molecular targeted drug | 27.0 | 23.0 | 7.6 | 27.0 | 28.0 | 1.7 |
| Immune checkpoint inhibitor | 1.0 | 1.0 | 5.4 | 1.0 | 1.0 | 1.2 |
| VTE risk category | ||||||
| Very high risk | 8.1 | 10.2 | 7.4 | 8.1 | 8.9 | 2.9 |
| High risk | 47.2 | 52.6 | 10.8 | 47.2 | 46.1 | 2.2 |
| Others | 44.7 | 37.2 | 15.4 | 44.7 | 45.0 | 0.6 |
| Metastasis | 21.0 | 37.0 | 35.1 | 21.0 | 21.0 | 0.3 |
| Treatments before admission | ||||||
| Radiotherapy | 1.0 | 4.0 | 16.8 | 1.0 | 1.0 | 1.7 |
| Anticancer agents | ||||||
| Antimetabolites | 7.0 | 14.0 | 23.4 | 7.0 | 8.0 | 4.5 |
| Platinum-based medicine | 7.0 | 13.0 | 21.6 | 7.0 | 8.0 | 4.0 |
| Microtubule inhibitors | 4.0 | 9.0 | 20.4 | 4.0 | 4.0 | 0.0 |
| Molecular targeted drug | 6.0 | 10.0 | 13.5 | 6.0 | 6.0 | 0.6 |
| Immune checkpoint inhibitor | 1.0 | 1.0 | 6.5 | 1.0 | 0.0 | 4.2 |
DOACs, direct oral anticoagulants; ASD, absolute standardized difference; IVC, inferior vena cava; VTE, venous thromboembolism.
Table 2 shows information on comorbidities and baseline medications. Among unmatched patients, the warfarin group was more likely to have hypertension, congestive heart failure, renal disease, and chronic kidney disease and was more likely to be treated with antiplatelet drugs and antipsychotics. The DOAC group was more likely to be treated with nonsteroidal anti-inflammatory drugs. The C-statistics was 0.6.
Table 2.
Comorbidities and baseline medications in unmatched and propensity score-matched patients treated with warfarin and DOACs
| Unmatched |
1:4 Propensity score matched |
|||||
|---|---|---|---|---|---|---|
| warfarin n = 557, % | DOACs n = 3,721, % | ASD (%) | warfarin n = 557, % | DOACs n = 2,228, % | ASD (%) | |
| Comorbidities | ||||||
| Trauma | 1.0 | 1.0 | 0.7 | 1.0 | 1.0 | 0.8 |
| Hypertension | 39.0 | 33.0 | 12.0 | 39.0 | 39.0 | 1.4 |
| Dyslipidemia | 13.0 | 11.0 | 6.2 | 13.0 | 14.0 | 2.5 |
| Myocardial infarction | 1.0 | 1.0 | 5.7 | 1.0 | 1.0 | 1.7 |
| Congestive heart failure | 12.0 | 8.0 | 14.1 | 12.0 | 12.0 | 1.4 |
| Peripheral vascular disease | 2.0 | 1.0 | 5.6 | 2.0 | 2.0 | 3.8 |
| Cerebrovascular disease | 6.0 | 6.0 | 3.2 | 6.0 | 7.0 | 5.6 |
| Dementia | 5.0 | 4.0 | 8.6 | 5.0 | 5.0 | 0.8 |
| Chronic pulmonary disease | 6.0 | 6.0 | 2.6 | 6.0 | 6.0 | 0.6 |
| Rheumatic disease | 1.0 | 2.0 | 3.2 | 1.0 | 1.0 | 0.0 |
| Peptic ulcer disease | 6.0 | 7.0 | 1.1 | 6.0 | 6.0 | 1.1 |
| Mild liver disease | 6.0 | 4.0 | 8.0 | 6.0 | 6.0 | 0.7 |
| Diabetes without chronic complications | 18.0 | 16.0 | 4.6 | 18.0 | 19.0 | 1.8 |
| Diabetes with chronic complications | 2.0 | 2.0 | 3.5 | 2.0 | 3.0 | 2.3 |
| Renal disease | 8.0 | 2.0 | 24.2 | 8.0 | 8.0 | 0.8 |
| Chronic kidney disease | 7.0 | 2.0 | 21.9 | 7.0 | 7.0 | 0.0 |
| Psychoses | 3.0 | 2.0 | 6.3 | 3.0 | 2.0 | 0.9 |
| Baseline medications | ||||||
| Antiplatelet drugs | 22.0 | 16.0 | 15.1 | 22.0 | 24.0 | 5.4 |
| Nonsteroidal anti-inflammatory drugs | 25.0 | 32.0 | 15.6 | 25.0 | 25.0 | 1.5 |
| Corticosteroids | 36.0 | 39.0 | 5.6 | 36.0 | 37.0 | 2.4 |
| PPI/Potassium-competitive acid blockers | 62.0 | 63.0 | 2.0 | 62.0 | 65.0 | 6.2 |
| Antipsychotics | 15.0 | 19.0 | 12.1 | 15.0 | 16.0 | 2.6 |
| Statins | 21.0 | 19.0 | 3.8 | 21.0 | 22.0 | 3.3 |
| Antihypertensive drugsa | 38.0 | 35.0 | 7.8 | 38.0 | 40.0 | 3.8 |
| Estrogen preparations | 2.0 | 2.0 | 5.4 | 2.0 | 2.0 | 0.9 |
| CYP3A4 strong inhibitors | 2.0 | 2.0 | 4.1 | 3.0 | 3.0 | 1.1 |
| CYP3A4 medium inhibitors | 9.0 | 8.0 | 3.0 | 9.0 | 9.0 | 0.9 |
DOACs, direct oral anticoagulants; ASD, absolute standardized difference; PPI, proton pump inhibitors; CYP, cytochrome P450.
Antihypertensive drugs included angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, β-blockers, and Ca-blockers.
Table 3 shows a comparison of outcomes between the warfarin and DOAC groups in the propensity score-matched population. For the primary outcome, the DOAC group had a statistically significantly lower proportion of recurrent VTEs requiring hospitalization as compared to the warfarin group (5.3% vs. 7.5%; odds ratio [OR], 0.69; 95% confidence interval [CI], 0.48–0.98; p = 0.043). There was no statistically significant difference in major bleeding requiring hospitalization between the two groups (1.6% vs. 1.1%; OR, 1.47; 95% CI, 0.62–3.50; p = 0.39). For the secondary outcome, there was no statistically significant difference in death on rehospitalization within 6 months of discharge from the time of the index hospitalization (11.1% vs. 9.9%; OR, 1.14; 95% CI, 0.84–1.56; p = 0.40) between the two groups.
Table 3.
Thromboembolism, major bleeding, and all-cause inhospital death within 6 months after discharge in propensity score-matched patients treated with warfarin and DOACs
| 1:4 Propensity score matched | |||||||
|---|---|---|---|---|---|---|---|
| warfarin |
DOACs |
OR | 95% CI | p value | |||
| n = 557 | n = 2,228 | ||||||
| Recurrent VTE | 42 | (7.5%) | 118 | (5.3%) | 0.69 | (0.48–0.98) | 0.043 |
| Recurrent deep vein thrombosis | 35 | (6.3%) | 97 | (4.4%) | 0.68 | (0.46–1.01) | |
| Recurrent pulmonary embolism | 7 | (1.3%) | 29 | (1.3%) | 1.04 | (0.45–2.38) | |
| Major bleeding | 6 | (1.1%) | 35 | (1.6%) | 1.47 | (0.62–3.50) | 0.39 |
| Intracranial hemorrhage | 1 | (0.2%) | 12 | (0.5%) | 3.01 | (0.39–23.2) | |
| Gastrointestinal bleeding | 5 | (0.9%) | 17 | (0.8%) | 0.85 | (0.31–2.31) | |
| Other major bleeding | 2 | (0.4%) | 6 | (0.3%) | 0.74 | (0.15–3.72) | |
| All-cause inhospital death | 55 | (9.9%) | 248 | (11.1%) | 1.14 | (0.84–1.56) | 0.40 |
| Composite outcomea | 93 | (16.7%) | 376 | (16.9%) | 1.01 | (0.79–1.30) | 0.92 |
DOACs, direct oral anticoagulants; OR, odds ratio; CI, confidence interval.
Composite outcome, at least one of the following: recurrent VTE, major bleeding event, and all-cause inhospital death.
Online supplementary Tables 1–6 (for all online suppl. material, see www.karger.com/doi/10.1159/000528606) show the results of the subgroup analyses. DOACs were associated with reduced recurrent VTE requiring hospitalization in patients aged 80 or older, in those without metastases, and in those with a high or very high risk of VTE (according to cancer type). DOACs were associated with major bleeding requiring hospitalization in patients aged 80 or older, in those without metastases, and in those with a low risk of VTE due to cancer type. DOACs were associated with all-cause inhospital mortality within 6 months in patients aged 75–79 and in those without metastases.
Discussion
In this study, we compared the effectiveness and safety of DOACs and warfarin in cancer patients with VTE aged 75 or older who were (currently or recently) undergoing chemotherapy using a nationwide Japanese inpatient database. Analysis after propensity score matching showed that the proportion of recurrent VTE requiring hospitalization was lower in the DOAC group, but that the risks of bleeding requiring hospitalization, all-cause inhospital mortality. The composite outcome was not significantly different between the two groups.
Recently, RCTs have evaluated cancer-associated VTEs, comparing only low-molecular-weight heparins with DOACs, and there has been no direct comparison between warfarin and DOACs. Moreover, although a recent observational study compared DOACs and warfarin in active cancers, the mean age was approximately 64 years [23]. To our knowledge, this study is the first large retrospective cohort study comparing DOACs and warfarin in cancer patients aged 75 or older during the course of chemotherapy.
In the present study, patient characteristics prior to matching included the following features. The percentage of patients with impaired renal function and chronic renal failure were each 2.0% in the DOAC group, while the respective corresponding values were 8.0% and 7.0% in the warfarin group. This indicates that warfarin tended to be prescribed to patients with renal impairment. The reason for this may be that warfarin can be used without dosage adjustment regardless of renal function.
The proportion of metastasis was 21.0% in the warfarin group (vs. 37.0% in the DOAC group), which means that patients with advanced cancer were more likely to receive DOACs. In evaluating risk categories for developing VTE by cancer type, high risk was more prevalent in the DOAC group and low risk was more prevalent in the warfarin group. In terms of the types of anticancer agents prescribed, the DAOCs group was prescribed a higher proportion of antimetabolites, platinum, microtubule inhibitors, and molecularly targeted agents during the past 6 months of hospitalization (assessed at the time of the index hospitalization). Antimetabolites such as fluoropyrimidines have been reported to prolong the prothrombin time-international normalized ratio in combination with warfarin [24, 25, 26]. Therefore, DOACs may be chosen over warfarin when starting new agents during these anticancer treatments.
Platinum drugs, microtubule inhibitors, molecular-targeted drugs, and immune checkpoint inhibitors were also included as covariates because these drugs have been reported to be associated with a risk of developing thrombus [27, 28, 29, 30]. Propensity score matching was able to balance all of values to within 10%, even for covariates that had an absolute standardized difference of 10% or greater before matching.
Analysis after propensity score matching showed that the proportion of recurrent VTE requiring hospitalization was lower in the DOAC group than in the warfarin group, whereas the other outcomes did not significantly differ between the two groups. Thus, given the net clinical benefit, the differences in efficacy and safety between DOACs and warfarin were little and clinically irrelevant in elderly patients with cancer receiving chemotherapy. A previous study followed patients with active cancer for 12 months and found that the rates of recurrent VTE were 4% and 7% and that the rates of major bleeding were 3% and 3% in the edoxaban and warfarin groups, respectively. In this previous study, active cancer was defined as the presence of measurable solid tumors (other than nonmelanoma skin cancer or hematologic malignancies) [6]. With regard to studies in the elderly that are not limited to cancer, previous systematic reviews and meta-analyses incorporated data from 3,665 elderly patients aged 75 or older receiving acute VTE treatment and showed that DOACs were superior to warfarin in terms of preventing recurrent VTE and that these medications were equally safe [31].
Our current study is not directly comparable to previous studies because we focused on subjects with cancer who were elderly and undergoing chemotherapy. However, the results of previous studies analyzing cancer patients or elderly patients support the findings of the present study. Current guidelines recommend that DOACs should not be used or should be administered with caution because of the high associated risk of major bleeding, especially as seen in cases of gastrointestinal and urologic malignancies [10]. Based on the results of the present study, we believe that the same caution should be exercised when using warfarin and DOACs, since the degree of bleeding was comparable between the warfarin and DOAC groups. In particular, warfarin has a narrow therapeutic range, and there are large individual differences in its effectiveness and side effects. Moreover, it has been reported that the anticoagulant effects of warfarin were enhanced by interactions with anticancer drugs, such as fluoropyrimidines and tyrosine kinase inhibitors, via the drug-metabolizing enzyme CYP2C9 [32, 33, 34]. Therefore, when warfarin is used to treat VTE during these chemotherapy regimens, the prothrombin time-international normalized ratio should be carefully monitored for over-prolongation to avoid bleeding associated with increased warfarin action.
The results of the subgroup analyses suggest that the use of DOACs may be effective for reducing VTE occurrence requiring hospitalization in older patients without metastasis and in those with higher risk cancer types. The results also suggest that elderly patients are at higher risk for major bleeding requiring hospitalization, but that those with metastases or at high risk of VTE may not be at increased risk. All-cause inhospital mortality within 6 months was lower in older patients with metastasis.
This study had some limitations. First, it was not possible to ascertain patients who started anticoagulants in the outpatient setting during their first VTE hospitalization. Therefore, we considered first VTE onset by excluding patients with an existing diagnosis of VTE or atrial fibrillation, those previously undergoing orthopedic surgery, or those with anticoagulants prescribed in hospitalizations within the past year. Second, specifics on outpatient chemotherapy were not included in the collected database. However, we were able to confirm that the patients in this study received chemotherapy within the past 6 months on admission for first VTE onset. Third, in the present data, it was not possible to consistently identify and classify cancer stage due to many missing values in the TNM classification and cancer stage items; however, the target patients were limited to those undergoing chemotherapy, and metastasis was adjusted as a covariate. Fourth, since laboratory values were not available, it was not known whether patients required dosage adjustment based on renal function while taking DOACs. However, dialysis patients were excluded, and reduced renal function or chronic renal failure was adjusted as covariates. Fifth, we were unable to obtain information on outcomes without hospitalization to an index hospital due to the nature of the database. Because information on outpatient events and deaths at home was lacking, the outcomes might have been underestimated. In addition, the exclusion of deaths during index hospitalization might also have led to an underestimation of mortality. However, since these underestimations would occur similarly in both groups, we consider that the results were robust. Sixth, we were unable to confirm adherence after anticoagulants were prescribed on admission for VTE onset or after discharge from the hospital. However, we believe that this potential bias would not skew the results, as adherence would likely have been similar in both medication groups.
Conclusion
In elderly patients with cancer, DOACs during chemotherapy were associated with a lower proportion of VTE recurrences requiring hospitalization after discharge compared with warfarin. Therefore, caution should be exercised when using either medication, in consideration of patient characteristics. Our findings, which should be confirmed in future research, inform research directions and medication guidelines in elderly cancer patients undergoing chemotherapy.
Statement of Ethics
This study was approved by the Institutional Review Board of The University of Tokyo, which waived the requirement for informed consent because of the anonymity of the patient database, approval number: 3501-(3). This research was conducted in accordance with the principles of the Declaration of Helsinki and its later amendments.
Conflict of Interest Statement
The authors have no actual or potential conflicts of interest to declare.
Funding Sources
This work was supported by grants from the Ministry of Health, Labour and Welfare, Japan (21AA2007 and 22AA2003), and the Ministry of Education, Culture, Sports, Science and Technology, Japan (20H03907).
Author Contributions
Chikako Iwai and Taisuke Jo designed the study, analyzed and interpreted the data, and prepared the manuscript. Takaaki Konishi analyzed and interpreted the data. Ryosuke Kumazawa, Hiroki Matsui, and Kiyohide Fushimi collected and interpreted the data. Hideo Yasunaga designed the study, collected and interpreted the data, and prepared the manuscript. All authors approved the final manuscript.
Data Availability Statement
The data analyzed during the current study are not publicly available due to contracts with the hospitals providing data to the database. Further inquiries on data can be directed to the corresponding author.
Supplementary Material
Supplementary data
Funding Statement
This work was supported by grants from the Ministry of Health, Labour and Welfare, Japan (21AA2007 and 22AA2003), and the Ministry of Education, Culture, Sports, Science and Technology, Japan (20H03907).
References
- 1.Khorana AA. Venous thromboembolism and prognosis in cancer. Thromb Res. 2010 Jun;125((6)):490–493. doi: 10.1016/j.thromres.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hutten BA, Prins MH, Gent M, Ginsberg J, Tijssen JG, Büller HR. Incidence of recurrent thromboembolic and bleeding complications among patients with venous thromboembolism in relation to both malignancy and achieved international normalized ratio a retrospective analysis. J Clin Oncol. 2000 Sep;18((17)):3078–3083. doi: 10.1200/JCO.2000.18.17.3078. [DOI] [PubMed] [Google Scholar]
- 3.Chee CE, Ashrani AA, Marks RS, Petterson TM, Bailey KR, Melton LJ, et al. Predictors of venous thromboembolism recurrence and bleeding among active cancer patients a population-based cohort study. Blood. 2014 Jun;123((25)):3972–8. doi: 10.1182/blood-2014-01-549733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kakkar AK, Levine M, Pinedo HM, Wolff R, Wong J. Venous thrombosis in cancer patients insights from the FRONTLINE survey. Oncologist. 2003;8((4)):381–388. doi: 10.1634/theoncologist.8-4-381. [DOI] [PubMed] [Google Scholar]
- 5.Raskob GE, van Es N, Segers A, Angchaisuksiri P, Oh D, Boda Z, et al. Edoxaban for venous thromboembolism in patients with cancer results from a non-inferiority subgroup analysis of the Hokusai-VTE randomised, double-blind, double-dummy trial. Lancet Haematol. 2016 Aug;3((8)):e379–e387. doi: 10.1016/S2352-3026(16)30057-6. [DOI] [PubMed] [Google Scholar]
- 6.Prins MH, Lensing AWA, Brighton TA, Lyons RM, Rehm J, Trajanovic M, et al. Oral rivaroxaban versus enoxaparin with vitamin K antagonist for the treatment of symptomatic venous thromboembolism in patients with cancer (EINSTEIN-DVT and EINSTEIN-PE) a pooled subgroup analysis of two randomised controlled trials. Lancet Haematol. 2014 Oct;1((1)):e37–e46. doi: 10.1016/S2352-3026(14)70018-3. [DOI] [PubMed] [Google Scholar]
- 7.Agnelli G, Buller HR, Cohen A, Gallus AS, Lee TC, Pak R, et al. Oral apixaban for the treatment of venous thromboembolism in cancer patients results from the AMPLIFY trial. J Thromb Haemost. 2015 Dec;13((12)):2187–2191. doi: 10.1111/jth.13153. [DOI] [PubMed] [Google Scholar]
- 8.Raskob GE, van Es N, Verhamme P, Carrier M, Di Nisio M, Garcia D, et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018 Feb;378((7)):615–624. doi: 10.1056/NEJMoa1711948. [DOI] [PubMed] [Google Scholar]
- 9.Young AM, Marshall A, Thirlwall J, Chapman O, Lokare A, Hill C, et al. Comparison of an oral factor xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism results of a randomized trial (SELECT-D) J Clin Oncol. 2018 Jul;36((20)):2017–2023. doi: 10.1200/JCO.2018.78.8034. [DOI] [PubMed] [Google Scholar]
- 10.Farge D, Frere C, Connors JM, Ay C, Khorana AA, Munoz A, et al. 2019 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2019;20((10)):e566–e581. doi: 10.1016/S1470-2045(19)30336-5. [DOI] [PubMed] [Google Scholar]
- 11.Streiff MB, Holmstrom B, Angelini D, Ashrani A, Elshoury A, Fanikos J, et al. Cancer-associated venous thromboembolic disease version 2.2021 NCCN clinical practice guidelines in oncology. JNCCN. 2021 Oct 15;19((10)):1181–1201. doi: 10.6004/jnccn.2021.0047. [DOI] [PubMed] [Google Scholar]
- 12.Key NS, Khorana AA, Kuderer NM, Bohlke K, Lee AYY, Arcelus JI, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer ASCO clinical practice guideline update. J Clin Oncol. 2020 Feb;38((5)):496–520. doi: 10.1200/JCO.19.01461. [DOI] [PubMed] [Google Scholar]
- 13.Vedovati MC, Germini F, Agnelli G, Becattini C. Direct oral anticoagulants in patients with VTE and cancer a systematic review and meta-analysis. Chest. 2015 Feb;147((2)):475–483. doi: 10.1378/chest.14-0402. [DOI] [PubMed] [Google Scholar]
- 14.Klotz U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev. 2009 May;41((2)):67–76. doi: 10.1080/03602530902722679. [DOI] [PubMed] [Google Scholar]
- 15.Sebuhyan M, Crichi B, Abdallah NA, Bonnet C, Deville L, Marjanovic Z, et al. Drug-drug interaction (DDI) with direct oral anticoagulant (DOAC) in patients with cancer. J Med Vasc. 2020 Nov;45((6S)):6S31–6S38. doi: 10.1016/S2542-4513(20)30517-4. [DOI] [PubMed] [Google Scholar]
- 16.Yasunaga H. Real World data in Japan chapter II the diagnosis procedure combination database. Ann Clin Epidemiol. 2019;1((3)):76–79. [Google Scholar]
- 17.Yamana H, Moriwaki M, Horiguchi H, Kodan M, Fushimi K, Yasunaga H. Validity of diagnoses and laboratory data in Japanese administrative data. J Epidemiol. 2017 Oct;27((10)):476–482. doi: 10.1016/j.je.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hubbard AE, Ahern J, Fleischer NL, Van der Laan M, Lippman SA, Jewell N, et al. To GEE or not to GEE comparing population average and mixed models for estimating the associations between neighborhood risk factors and health. Epidemiology. 2010 Jul;21((4)):467–474. doi: 10.1097/EDE.0b013e3181caeb90. [DOI] [PubMed] [Google Scholar]
- 19.Mulder FI, Candeloro M, Kamphuisen PW, Di Nisio M, Bossuyt PM, Guman N, et al. The Khorana score for prediction of venous thromboembolism in cancer patients a systematic review and meta-analysis. Haematologica. 2019 Jun;104((6)):1277–1287. doi: 10.3324/haematol.2018.209114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Normand ST, Landrum MB, Guadagnoli E, Ayanian JZ, Ryan TJ, Cleary PD, et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly a matched analysis using propensity scores. J Clin Epidemiol. 2001 Apr;54((4)):387–398. doi: 10.1016/s0895-4356(00)00321-8. [DOI] [PubMed] [Google Scholar]
- 21.Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simulat Comput. 2009 May;38((6)):1228–1234. [Google Scholar]
- 22.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011 May;46((3)):399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dawwas GK, Dietrich E, Smith SM, Davis K, Park H. Comparative effectiveness and safety of direct-acting oral anticoagulants and warfarin in patients with venous thromboembolism and active cancer an observational analysis. Clin Ther. 2020 Sep;42((9)):e161–e176. doi: 10.1016/j.clinthera.2020.06.022. [DOI] [PubMed] [Google Scholar]
- 24.Seifter EJ, Brooks BJ, Urba WJ. Possible interactions between warfarin and antineoplastic drugs. Cancer Treat Rep. 1985 Feb;69((2)):244–245. [PubMed] [Google Scholar]
- 25.Wajima T, Mukhopadhyay P. Possible interactions between warfarin and 5-fluorouracil. Am J Hematol. 1992 Jul;40((3)):238. doi: 10.1002/ajh.2830400317. [DOI] [PubMed] [Google Scholar]
- 26.Buyck HCE, Buckley N, Leslie MD, Plowman PN. Capecitabine-induced potentiation of warfarin. Clin Oncol. 2003 Aug;15((5)):297. doi: 10.1016/s0936-6555(03)00111-0. [DOI] [PubMed] [Google Scholar]
- 27.Seng S, Liu Z, Chiu SK, Proverbs-Singh T, Sonpavde G, Choueiri TK, et al. Risk of venous thromboembolism in patients with cancer treated with Cisplatin a systematic review and meta-analysis. J Clin Oncol. 2012 Dec;30((35)):4416–4426. doi: 10.1200/JCO.2012.42.4358. [DOI] [PubMed] [Google Scholar]
- 28.Nalluri, Chu D, Keresztes R, Zhu X, Wu S. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients a meta-analysis. JAMA. 2008 Nov;300((19)):2277–2285. doi: 10.1001/jama.2008.656. [DOI] [PubMed] [Google Scholar]
- 29.Ando Y, Hayashi T, Sugimoto R, Nishibe S, Ito K, Kawada K, et al. Risk factors for cancer-associated thrombosis in patients undergoing treatment with immune checkpoint inhibitors. Invest New Drugs. 2020 Aug;38((4)):1200–1206. doi: 10.1007/s10637-019-00881-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niikura R, Yasunaga H, Yamada A, Matsui H, Fushimi K, Hirata Y, et al. Factors predicting adverse events associated with therapeutic colonoscopy for colorectal neoplasia a retrospective nationwide study in Japan. Gastrointest Endosc. 2016 Dec;84((6)):971.e6–982.e6. doi: 10.1016/j.gie.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 31.Chaudhary R, Pagali S, Garg J, Murad MH, Wysokinski WE, McBane RD. DOACs versus VKAs in older adults treated for acute venous thromboembolism systematic review and meta-analysis. J Am Geriatr Soc. 2020 Sep;68((9)):2021–2026. doi: 10.1111/jgs.16549. [DOI] [PubMed] [Google Scholar]
- 32.Holbrook AM, Pereira JA, Labiris R, McDonald H, Douketis JD, Crowther M, et al. Systematic overview of warfarin and its drug and food interactions. Arch Intern Med. 2005 May;165((10)):1095–1106. doi: 10.1001/archinte.165.10.1095. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi H, Wilkinson GR, Nutescu EA, Morita T, Ritchie MD, Scordo MG, et al. Different contributions of polymorphisms in VKORC1 and CYP2C9 to intra- and inter-population differences in maintenance dose of warfarin in Japanese Caucasians and African-Americans. Pharmacogenet Genomics. 2006 Feb;16((2)):101–110. doi: 10.1097/01.fpc.0000184955.08453.a8. [DOI] [PubMed] [Google Scholar]
- 34.Hiraide M, Minowa Y, Nakano Y, Suzuki K, Shiga T, Nishio M, et al. Drug interactions between tyrosine kinase inhibitors (gefitinib and erlotinib) and warfarin assessment of international normalized ratio elevation characteristics and in vitro CYP2C9 activity. J Oncol Pharm Pract. 2019 Oct;25((7)):1599–607. doi: 10.1177/1078155218801061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Data Availability Statement
The data analyzed during the current study are not publicly available due to contracts with the hospitals providing data to the database. Further inquiries on data can be directed to the corresponding author.