Abstract
Background
The optimal duration of thromboprophylaxis after total hip or knee replacement, or hip fracture repair remains controversial. It is common practice to administer prophylaxis using low‐molecular‐weight heparin (LMWH) or unfractionated heparin (UFH) until discharge from hospital, usually seven to 14 days after surgery. International guidelines recommend extending thromboprophylaxis for up to 35 days following major orthopaedic surgery but the recommendation is weak due to moderate quality evidence. In addition, recent oral anticoagulants that exert effect by direct inhibition of thrombin or activated factor X lack the need for monitoring and have few known drug interactions. Interest in this topic remains high.
Objectives
To assess the effects of extended‐duration anticoagulant thromboprophylaxis for the prevention of venous thromboembolism (VTE) in people undergoing elective hip or knee replacement surgery, or hip fracture repair.
Search methods
The Cochrane Vascular Information Specialist searched the Specialised Register (last searched May 2015) and CENTRAL (2015, Issue 4). Clinical trials databases were searched for ongoing or unpublished studies.
Selection criteria
Randomised controlled trials assessing extended‐duration thromboprophylaxis (five to seven weeks) using accepted prophylactic doses of LMWH, UFH, vitamin K antagonists (VKA) or direct oral anticoagulants (DOAC) compared with short‐duration thromboprophylaxis (seven to 14 days) followed by placebo, no treatment or similar extended‐duration thromboprophylaxis with LMWH, UFH, VKA or DOACs in participants undergoing hip or knee replacement or hip fracture repair.
Data collection and analysis
We independently selected trials and extracted data. Disagreements were resolved by discussion. We performed fixed‐effect model meta‐analyses with odds ratios (ORs) and 95% confidence intervals (CIs). We used a random‐effects model when there was heterogeneity.
Main results
We included 16 studies (24,930 participants); six compared heparin with placebo, one compared VKA with placebo, two compared DOAC with placebo, one compared VKA with heparin, five compared DOAC with heparin and one compared anticoagulants chosen at investigators' discretion with placebo. Three trials included participants undergoing knee replacement. No studies assessed hip fracture repair.
Trials were generally of good methodological quality. The main reason for unclear risk of bias was insufficient reporting. The quality of evidence according to GRADE was generally moderate, as some comparisons included a single study, low number of events or heterogeneity between studies leading to wide CIs.
We showed no difference between extended‐duration heparin and placebo in symptomatic VTE (OR 0.59, 95% CI 0.35 to 1.01; 2329 participants; 5 studies; high quality evidence), symptomatic deep vein thrombosis (DVT) (OR 0.73, 95% CI 0.39 to 1.38; 2019 participants; 4 studies; moderate quality evidence), symptomatic pulmonary embolism (PE) (OR 0.61, 95% CI 0.16 to 2.33; 1595 participants; 3 studies; low quality evidence) and major bleeding (OR 0.59, 95% CI 0.14 to 2.46; 2500 participants; 5 studies; moderate quality evidence). Minor bleeding was increased in the heparin group (OR 2.01, 95% CI 1.43 to 2.81; 2500 participants; 5 studies; high quality evidence). Clinically relevant non‐major bleeding was not reported.
We showed no difference between extended‐duration VKA and placebo (one study, 360 participants) for symptomatic VTE (OR 0.10, 95% CI 0.01 to 1.94; moderate quality evidence), symptomatic DVT (OR 0.13, 95% CI 0.01 to 2.62; moderate quality evidence), symptomatic PE (OR 0.32, 95% CI 0.01 to 7.84; moderate quality evidence) and major bleeding (OR 2.89, 95% CI 0.12 to 71.31; low quality evidence). Clinically relevant non‐major bleeding and minor bleeding were not reported.
Extended‐duration DOAC showed reduced symptomatic VTE (OR 0.20, 95% CI 0.06 to 0.68; 2419 participants; 1 study; moderate quality evidence) and symptomatic DVT (OR 0.18, 95% CI 0.04 to 0.81; 2459 participants; 2 studies; high quality evidence) compared to placebo. No differences were found for symptomatic PE (OR 0.25, 95% CI 0.03 to 2.25; 1733 participants; 1 study; low quality evidence), major bleeding (OR 1.00, 95% CI 0.06 to 16.02; 2457 participants; 1 study; low quality evidence), clinically relevant non‐major bleeding (OR 1.22, 95% CI 0.76 to 1.95; 2457 participants; 1 study; moderate quality evidence) and minor bleeding (OR 1.18, 95% CI 0.74 to 1.88; 2457 participants; 1 study; moderate quality evidence).
We showed no difference between extended‐duration anticoagulants chosen at investigators' discretion and placebo (one study, 557 participants, low quality evidence) for symptomatic VTE (OR 0.50, 95% CI 0.09 to 2.74), symptomatic DVT (OR 0.33, 95% CI 0.03 to 3.21), symptomatic PE (OR 1.00, 95% CI 0.06 to 16.13), and major bleeding (OR 5.05, 95% CI 0.24 to 105.76). Clinically relevant non‐major bleeding and minor bleeding were not reported.
We showed no difference between extended‐duration VKA and heparin (one study, low quality evidence) for symptomatic VTE (OR 1.64, 95% CI 0.85 to 3.16; 1279 participants), symptomatic DVT (OR 1.36, 95% CI 0.69 to 2.68; 1279 participants), symptomatic PE (OR 9.16, 95% CI 0.49 to 170.42; 1279 participants), major bleeding (OR 3.87, 95% CI 1.91 to 7.85; 1272 participants) and minor bleeding (OR 1.33, 95% CI 0.64 to 2.76; 1279 participants). Clinically relevant non‐major bleeding was not reported.
We showed no difference between extended‐duration DOAC and heparin for symptomatic VTE (OR 0.70, 95% CI 0.28 to 1.70; 15,977 participants; 5 studies; low quality evidence), symptomatic DVT (OR 0.60, 95% CI 0.11 to 3.27; 15,977 participants; 5 studies; low quality evidence), symptomatic PE (OR 0.91, 95% CI 0.43 to 1.94; 14,731 participants; 5 studies; moderate quality evidence), major bleeding (OR 1.11, 95% CI 0.79 to 1.54; 16,199 participants; 5 studies; high quality evidence), clinically relevant non‐major bleeding (OR 1.08, 95% CI 0.90 to 1.28; 15,241 participants; 4 studies; high quality evidence) and minor bleeding (OR 0.95, 95% CI 0.82 to 1.10; 11,766 participants; 4 studies; high quality evidence).
Authors' conclusions
Moderate quality evidence suggests extended‐duration anticoagulants to prevent VTE should be considered for people undergoing hip replacement surgery, although the benefit should be weighed against the increased risk of minor bleeding. Further studies are needed to better understand the association between VTE and extended‐duration oral anticoagulants in relation to knee replacement and hip fracture repair, as well as outcomes such as distal and proximal DVT, reoperation, wound infection and healing.
Keywords: Humans; Anticoagulants; Anticoagulants/therapeutic use; Arthroplasty, Replacement, Hip; Arthroplasty, Replacement, Hip/adverse effects; Arthroplasty, Replacement, Knee; Arthroplasty, Replacement, Knee/adverse effects; Hemorrhage; Hemorrhage/chemically induced; Heparin; Heparin/therapeutic use; Hip Fractures; Hip Fractures/surgery; Randomized Controlled Trials as Topic; Venous Thromboembolism; Venous Thromboembolism/prevention & control; Vitamin K; Vitamin K/antagonists & inhibitors
Plain language summary
Anticoagulants taken for longer periods to prevent deep vein thrombosis or pulmonary embolism after hip or knee replacement
Background
Patients undergoing surgery have an increased risk of developing blood clots in their veins. These clots may be in the deep veins of the leg (deep vein thrombosis (DVT)) or travel to the lungs (pulmonary embolism (PE)). Venous thromboembolism (VTE) is the combined term for DVT and PE. Prevention of these blood clots (prophylaxis) after surgery may reduce the risk of postoperative vein clots. These potential benefits, however, have to be balanced against the associated risks of bleeding. The optimal duration of prophylaxis after total hip or knee replacement, or hip fracture repair remains controversial. It is common practice to administer prophylaxis using drugs such as low‐molecular‐weight heparin and unfractionated heparin (anticoagulants) until discharge from hospital and for a minimum of seven to 14 days after surgery. Current international guidelines recommend extending prophylaxis for up to 35 days following major orthopaedic surgery but recognise that the recommendation is weak due to moderate quality evidence. In addition, new oral anticoagulants (direct oral anticoagulants (DOAC)) show potential benefits such as taking tablets by mouth instead of injection, lack of frequent monitoring and few known drug interactions. Interest in this topic therefore remains high.
Study characteristics and key results
A total of 16 studies were included, with 24,930 randomised participants (current until May 2015). The main outcomes of interest were symptomatic (showing symptoms) VTE, including DVT and PE, and bleeding (major, clinically relevant non‐major and minor bleeding). Six studies compared heparin with placebo, one compared the vitamin K antagonist (VKA) warfarin with placebo, two compared DOAC with placebo, one compared VKA with heparin, five compared DOAC with heparin and one study compared using a variety of anticoagulant treatments with placebo. Only three trials included participants undergoing knee replacement and no studies included participants undergoing hip fracture repair.
For the comparison heparin versus placebo (six studies) no differences were found between the study arms for symptomatic VTE, symptomatic DVT, symptomatic PE and major bleeding. Minor bleeding was increased in the heparin group. Clinically relevant non‐major bleeding was not reported.
The comparison VKA versus placebo (one study) and the comparison placebo with anticoagulants chosen at the discretion of the investigators (one study) showed no differences between the study arms for symptomatic VTE, symptomatic DVT, symptomatic PE and major bleeding. Clinically relevant non‐major bleeding and minor bleeding were not reported.
For the comparison DOACs versus placebo (two studies), reduced symptomatic VTE and symptomatic DVT were found in favour of the DOAC but no differences were found for symptomatic PE, major bleeding, clinically relevant non‐major bleeding and minor bleeding.
Comparing extended‐duration VKA with extended‐duration LMWH (one study) there was no difference between the study arms for symptomatic VTE, symptomatic DVT, symptomatic PE, major bleeding and minor bleeding. Clinically relevant non‐major bleeding was not reported.
Comparing extended‐duration DOAC with extended‐duration LMWH (five studies) there was no difference between the study arms for symptomatic VTE, symptomatic DVT, symptomatic PE, major bleeding, clinically relevant non‐major bleeding and minor bleeding.
Quality of the evidence
Overall, the included studies were of good methodological quality, with the majority of studies having little risk of bias due to study design and reporting. The majority of concerns came from lack of reporting of specific details. The quality of the evidence was generally moderate, either because only one study was included in a comparison, because of few events or because there were a lot of differences between the findings of the studies meaning that the data were difficult to interpret. Further studies are needed to better understand the relationship between VTE and extended‐duration oral anticoagulants for knee replacement and hip fracture repair as well as outcomes such as DVT below the knee and above the knee, reoperation, wound infection and healing.
Summary of findings
Summary of findings for the main comparison. Heparin compared to placebo for prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair.
| Heparin compared to placebo for prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair | ||||||
| Patient or population: people requiring prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair Setting: hospital and outpatient setting Intervention: heparin Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with heparin | |||||
| Symptomatic VTE (DVT and PE) Treatment duration 28 ‐ 42 days | Study population | OR 0.59 (0.35 to 1.01) | 2329 (5 RCTs) | ⊕⊕⊕⊕ HIGH | — | |
| 33 per 1000 | 20 per 1000 (12 to 33) | |||||
| Symptomatic DVT (proximal or distal) Treatment duration 28 ‐ 42 days | Study population | OR 0.73 (0.39 to 1.38) | 2019 (4 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | — | |
| 24 per 1000 | 18 per 1000 (9 to 33) | |||||
| Symptomatic PE Treatment duration 28 ‐ 42 days | Study population | OR 0.61 (0.16 to 2.33) | 1595 (3 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | — | |
| 6 per 1000 | 4 per 1000 (1 to 15) | |||||
| Bleeding ‐ major Treatment duration 28 ‐ 42 days | Study population | OR 0.59 (0.14 to 2.46) | 2500 (5 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | — | |
| 4 per 1000 | 2 per 1000 (0 to 9) | |||||
| Clinically relevant non‐major bleeding Treatment duration 28 ‐ 42 days | see comment | — | — | — | not reported | |
| Bleeding ‐ minor Treatment duration 28 ‐ 42 days | Study population | OR 2.01 (1.43 to 2.81) | 2500 (5 RCTs) | ⊕⊕⊕⊕ HIGH | — | |
| 46 per 1000 | 88 per 1000 (65 to 119) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DVT: deep vein thrombosis; OR: odds ratio; PE: pulmonary embolism; RCT: randomised controlled trial; VTE: venous thromboembolism | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded by one level, low number of events leading to imprecision of results 2 Downgraded by one level, some heterogeneity present (I2 = 49%) leading to wide CIs
Summary of findings 2. Vitamin K antagonists compared to placebo for prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair.
| Vitamin K antagonists compared to placebo for prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair | ||||||
| Patient or population: people requiring prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair Setting: hospital and outpatient setting Intervention: vitamin K antagonists Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with vitamin K antagonists | |||||
| Symptomatic VTE (DVT and PE) Treatment duration 28 ‐ 42 days | Study population | OR 0.10 (0.01 to 1.94) | 360 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | — | |
| 23 per 1000 | 2 per 1000 (0 to 43) | |||||
| Symptomatic DVT (proximal or distal) Treatment duration 28 ‐ 42 days | Study population | OR 0.13 (0.01 to 2.62) | 360 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | — | |
| 17 per 1000 | 2 per 1000 (0 to 43) | |||||
| Symptomatic PE Treatment duration 28 ‐ 42 days | Study population | OR 0.32 (0.01 to 7.84) | 360 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | — | |
| 6 per 1000 | 2 per 1000 (0 to 43) | |||||
| Bleeding ‐ major Treatment duration 28 ‐ 42 days | see comment | OR 2.89 (0.12 to 71.31) | 360 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | no events recorded in placebo group | |
| Clinically relevant non‐major bleeding Treatment duration 28 ‐ 42 days | see comment | — | — | — | not reported | |
| Minor bleeding Treatment duration 28 ‐ 42 days | see comment | — | — | — | not reported | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DVT: deep vein thrombosis; OR: odds ratio; PE: pulmonary embolism; RCT: randomised controlled trial; VTE: venous thromboembolism | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded by one level, results from a single study only so heterogeneity could not be assessed 2 Downgraded by one level, number of events small leading to wide CI and imprecision of results
Summary of findings 3. DOAC compared to placebo for prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair.
| DOAC compared to placebo for prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair | ||||||
| Patient or population: people requiring prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair Setting: hospital and outpatient setting Intervention: DOAC Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with DOAC | |||||
| Symptomatic VTE (DVT and PE) Treatment duration 28 ‐ 42 days | Study population | OR 0.20 (0.06 to 0.68) | 2419 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | — | |
| 12 per 1000 | 3 per 1000 (1 to 8) | |||||
| Symptomatic DVT (proximal or distal) Treatment duration 28 ‐ 42 days | Study population | OR 0.18 (0.04 to 0.81) | 2459 (2 RCTs) | ⊕⊕⊕⊕ HIGH | — | |
| 9 per 1000 | 2 per 1000 (0 to 7) | |||||
| Symptomatic PE Treatment duration 28 ‐ 42 days | Study population | OR 0.25 (0.03 to 2.25) | 1733 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | — | |
| 5 per 1000 | 1 per 1000 (0 to 10) | |||||
| Bleeding ‐ major Treatment duration 28 ‐ 42 days | Study population | OR 1.00 (0.06 to 16.02) | 2457 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | — | |
| 1 per 1000 | 1 per 1000 (0 to 13) | |||||
| Bleeding‐ clinically relevant non‐major Treatment duration 28 ‐ 42 days | Study population | OR 1.22 (0.76 to 1.95) | 2457 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | — | |
| 27 per 1000 | 33 per 1000 (21 to 51) | |||||
| Bleeding ‐ minor Treatment duration 28 ‐ 42 days | Study population | OR 1.18 (0.74 to 1.88) | 2457 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | — | |
| 28 per 1000 | 32 per 1000 (21 to 51) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DOAC: direct oral anticoagulant; DVT: deep vein thrombosis; OR: odds ratio; PE: pulmonary embolism; RCT: randomised controlled trial; VTE: venous thromboembolism | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded by one level, results from a single study so heterogeneity cannot be assessed 2 Downgraded by one level, low number of events leading to wide CI and imprecision of results
Summary of findings 4. Anticoagulants (chosen at investigators' discretion) compared to placebo for prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair.
| Anticoagulants (chosen at investigators' discretion) compared to placebo for prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair | ||||||
| Patient or population: people requiring prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair Setting: hospital and outpatient setting Intervention: anticoagulant (chosen at investigators' discretion) Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with anticoagulant (chosen at investigators' discretion) | |||||
| Symptomatic VTE (DVT and PE) Treatment duration 28 ‐ 42 days | Study population | OR 0.50 (0.09 to 2.74) | 557 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | — | |
| 14 per 1000 | 7 per 1000 (1 to 38) | |||||
| Symptomatic DVT (proximal or distal) Treatment duration 28 ‐ 42 days | Study population | OR 0.33 (0.03 to 3.21) | 557 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | — | |
| 11 per 1000 | 4 per 1000 (0 to 34) | |||||
| Symptomatic PE Treatment duration 28 ‐ 42 days | Study population | OR 1.00 (0.06 to 16.13) | 557 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | — | |
| 4 per 1000 | 4 per 1000 (0 to 55) | |||||
| Bleeding ‐ major Treatment duration 28 ‐ 42 days | see comment | OR 5.05 (0.24 to 105.76) | 557 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | no major bleeding recorded in the placebo groups | |
| Clinically relevant non‐major bleeding Treatment duration 28 ‐ 42 days | see comment | — | — | — | not reported | |
| Minor bleeding Treatment duration 28 ‐ 42 days | see comment | — | — | — | not reported | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DVT: deep vein thrombosis; OR: odds ratio; PE: pulmonary embolism; RCT: randomised controlled trial; VTE: venous thromboembolism | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded by one level, results from a single study so heterogeneity could not be assessed 2 Downgraded by one level, low number of events leading to wide CIs and imprecision of results
Summary of findings 5. Vitamin K antagonists compared to heparin for prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair.
| Vitamin K antagonists compared to heparin for prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair | ||||||
| Patient or population: people requiring prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair Setting: hospital and outpatient setting Intervention: vitamin K antagonists Comparison: heparin | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with heparin | Risk with vitamin K antagonists | |||||
| Symptomatic VTE (DVT and PE) Treatment duration 28 ‐ 42 days | Study population | OR 1.64 (0.85 to 3.16) | 1279 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | — | |
| 23 per 1000 | 38 per 1000 (20 to 70) | |||||
| Symptomatic DVT (proximal or distal) Treatment duration 28 ‐ 42 days | Study population | OR 1.36 (0.69 to 2.68) | 1279 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | — | |
| 23 per 1000 | 31 per 1000 (16 to 60) | |||||
| Symptomatic PE Treatment duration 28 ‐ 42 days | see comment | OR 9.16 (0.49 to 170.42) | 1279 (1 RCT) | ⊕⊕⊝⊝ LOW 1 3 | no cases of symptomatic PE reported in the heparin study arm | |
| Bleeding ‐ major Treatment duration 28 ‐ 42 days | Study population | OR 3.87 (1.91 to 7.85) | 1272 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | — | |
| 16 per 1000 | 58 per 1000 (30 to 111) | |||||
| Bleeding ‐ clinically indicated non‐major Treatment duration 28 ‐ 42 days |
see comment | — | — | — | clinically indicated non‐major bleeding events not reported in single included study in this comparison | |
| Bleeding ‐ minor Treatment duration 28 ‐ 42 days | Study population | OR 1.33 (0.64 to 2.76) | 1279 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | — | |
| 20 per 1000 | 27 per 1000 (13 to 54) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DVT: deep vein thrombosis; OR: odds ratio; PE: pulmonary embolism; RCT: randomised controlled trial; VKA: vitamin K antagonist; VTE: venous thromboembolism | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded by one level, single study so heterogeneity could not be assessed 2 Downgraded by one level, wide CI 3 Downgraded by one level, low number of events leading to imprecision of results
Summary of findings 6. DOAC compared to heparin for people requiring prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair.
| DOAC compared to heparin for people requiring prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair | ||||||
| Patient or population: people requiring prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair Setting: hospital and outpatient setting Intervention: DOAC Comparison: heparin | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with heparin | Risk with DOAC | |||||
| Symptomatic VTE (DVT and PE) Treatment duration 28 ‐ 42 days | Study population | OR 0.70 (0.28 to 1.70) | 15977 (5 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | — | |
| 4 per 1000 | 3 per 1000 (1 to 8) | |||||
| Symptomatic DVT (proximal or distal) Treatment duration 28 ‐ 42 days |
Study population | OR 0.60 (0.11 to 3.27) | 15977 (5 RCTs) | ⊕⊕⊝⊝ LOW 2 3 | — | |
| 3 per 1000 | 2 per 1000 (0 to 9) | |||||
| Symptomatic PE Treatment duration 28 ‐ 42 days | Study population | OR 0.91 (0.43 to 1.94) | 14731 (5 RCTs) | ⊕⊕⊕⊝ MODERATE 2 | — | |
| 2 per 1000 | 2 per 1000 (1 to 4) | |||||
| Bleeding ‐ major Treatment duration 28 ‐ 42 days |
Study population | OR 1.11 (0.79 to 1.54) | 16199 (5 RCTs) | ⊕⊕⊕⊕ HIGH | — | |
| 8 per 1000 | 9 per 1000 (7 to 13) | |||||
| Bleeding ‐ clinically relevant, non‐major Treatment duration 28 ‐ 42 days | Study population | OR 1.08 (0.90 to 1.28) | 15241 (4 RCTs) | ⊕⊕⊕⊕ HIGH | — | |
| 33 per 1000 | 36 per 1000 (30 to 42) | |||||
| Bleeding ‐ minor Treatment duration 28 ‐ 42 days |
Study population | OR 0.95 (0.82 to 1.10) | 11766 (4 RCTs) | ⊕⊕⊕⊕ HIGH | — | |
| 66 per 1000 | 63 per 1000 (55 to 72) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DOAC: direct oral anticoagulant; DVT: deep vein thrombosis; OR: odds ratio; PE: pulmonary embolism; RCT: randomised controlled trial; VTE: venous thromboembolism | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded by one level for inconsistency (heterogeneity, I2 = 55%) 2 Downgraded by one level for imprecision due to low number of events leading to wide CI 3 Downgraded by one level for inconsistency (heterogeneity, I2 = 65%)
Background
Description of the condition
Venous thromboembolism describes the formation of a clot in the deep veins (deep vein thrombosis or DVT ‐ usually of the lower extremities) and the subsequent embolisation of the clot to the pulmonary circulation (pulmonary embolisation or PE) or both. DVT of the lower limbs is associated with localised pain, swelling and erythema as well as the development of pulmonary emboli (PE), and the more localised and chronic post thrombotic syndrome. PE presents with shortness of breath, pain on inspiration, tachycardia and right heart overload, and if untreated, can lead to circulatory collapse and death.
The incidence of VTE, in mostly white populations, is between 100 and 200 per 100,000 person years (Heit 2015; White 2003). Of these, it is estimated that 45 to 117 per 100,000 person years are due to DVT (without PE) and 29 to 78 per 100,000 person years are due to PE (with or without DVT) (Heit 2015). Recurrent VTE occurs in approximately 7.4% of patients by one year, rising to 30.4% of patients by 10 years (Cushman 2007; Heit 2015; White 2003).
Although DVT and PE can occur spontaneously, there are many risk factors for VTE, including periods of inactivity, dehydration, hospitalisation, trauma, clotting disorders and previous thrombosis, varicose veins with phlebitis, pregnancy, oral combined hormonal contraceptives, malignancy, obesity, smoking and age (NICE 2010).
Prophylactic strategies in those deemed to be at risk (for example those undergoing surgical procedures or prolonged hospital inpatient stays) are recommended by international guidelines published by the National Institute for Health and Care Excellence (NICE) (NICE 2010; NICE 2012), The American College of Chest Physicians (ACCP) (Guyatt 2012) and the Scottish Intercollegiate Guidelines Network (SIGN) (SIGN 2010) and include the use of anticoagulation such as LMWH, UFH, oral direct factor Xa inhibitor (rivaroxaban), oral direct thrombin inhibitor (dabigatran), pentasaccharides (fondaparinux), and mechanical compression such as compression stockings and intermittent pneumatic compression devices.
Description of the intervention
It is common practice to administer prophylaxis until discharge from hospital, and for a minimum of seven to 14 days after surgery. However, in patients receiving in‐hospital prophylaxis, the prevalence of venographic DVT (a blood clot in the leg detected by venography) is still 15% to 30% at the time of hospital discharge (Eriksson 1997; Mohr 1993; Nurmohamed 1992) while an additional 10% to 25% of patients develop new asymptomatic DVT during the next three to four weeks (Dahl 1997; Fragmin Trial; French Study; Hirsh 1998). It is estimated, based on the available literature, that fewer than 10% of patients with venographically documented DVT will develop symptomatic VTE.
Extended‐duration prophylaxis is prophylaxis which starts at admission and continues well beyond discharge, typically for an additional 21 to 28 days after discharge, leading to a period of prophylaxis of approximately 35 days (NICE 2010). Randomised trials have demonstrated that extending prophylaxis beyond the time of hospital discharge substantially reduces the risk of developing new asymptomatic thrombi at 30 to 45 days (Dahl 1997; Fragmin Trial; French Study) and this has led these investigators to recommend that prophylaxis of longer duration should be used in all patients undergoing total hip replacement (THR).
However, two prospective studies, conducted in patients without known proximal DVT at the time of discharge from hospital, demonstrated that the incidence of new out‐of‐hospital symptomatic DVT or pulmonary embolism (PE) without extended‐duration prophylaxis was only approximately 2% after three months of follow‐up (Leclerc 1998; Robinson 1997). This data suggest that the majority of asymptomatic thrombi remain clinically silent irrespective of whether extended‐duration prophylaxis is given. Meanwhile, the impact of extended‐duration prophylaxis on symptomatic VTE remains to be clarified. Heit 2000 showed that a significant reduction in symptomatic VTE could not be demonstrated and therefore concluded that prophylaxis confined to the in‐hospital phase is adequate in most patients.
Why it is important to do this review
Current ACCP and NICE guidelines (Guyatt 2012; NICE 2010) for the prevention of VTE recommend extending thromboprophylaxis for up to 35 days following major orthopaedic surgery but recognise that the recommendation is weak due to moderate quality evidence (Guyatt 2012). In addition, recent oral anticoagulants that exert effect by direct inhibition of thrombin or activated factor X lack the need for monitoring and have few known drug interactions. Differences between anticoagulants for the extended duration also warrant further investigation. Interest in this topic therefore remains high.
Objectives
To assess the effects of extended‐duration anticoagulant thromboprophylaxis for the prevention of venous thromboembolism (VTE) in people undergoing elective hip or knee replacement surgery, or hip fracture repair.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled studies which assessed extended duration of anticoagulant thromboprophylaxis for the prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair.
We defined extended‐duration thromboprophylaxis as thromboprophylaxis of duration of five to seven weeks.
We included studies which used objective methods (e.g. ultrasound, venogram, V‐Q scan) to confirm the diagnosis of symptomatic and asymptomatic VTE (DVT or PE). We included data on asymptomatic DVT from these studies only if screening was performed using ascending lower‐limb contrast venography. We included open‐label, as well as double‐blind studies.
Types of participants
Patients undergoing elective total hip or knee replacement surgery or hip fracture repair. Patients undergoing revisions of a previous hip or knee replacement were included.
Types of interventions
We included studies which assessed extended‐duration thromboprophylaxis (five to seven weeks) using accepted prophylactic doses of anticoagulants LMWH, UFH, vitamin K antagonists or direct oral anticoagulants (DOACs) compared with short duration thromboprophylaxis therapy (seven to 14 days) followed by placebo or no treatment or similar extended duration of thromboprophylaxis therapy with anticoagulants LMWH, UFH, vitamin K antagonists or DOACs. Other antithrombotic therapy used in the prevention of VTE in major orthopaedic surgery was excluded.
Types of outcome measures
Primary outcomes
Symptomatic VTE (including DVT and PE)
Secondary outcomes
Total VTE (symptomatic or asymptomatic)
Asymptomatic DVT
Asymptomatic proximal and distal DVT
All‐cause mortality
Adverse events
Bleeding events (major, clinically relevant non‐major bleeding and minor bleeding)
Reoperation
Wound infection
Wound healing
Search methods for identification of studies
Electronic searches
The Cochrane Vascular Information Specialist (CIS) searched the Specialised Register (last searched May 2015) and the Cochrane Central Register of Controlled Trials (CENTRAL) 2015, Issue 4, part of The Cochrane Library, www.cochranelibrary.com. See Appendix 1 for details of the search strategy used to search CENTRAL. The Specialised Register is maintained by the CIS and is constructed from weekly electronic searches of MEDLINE, EMBASE, CINAHL, AMED, and through handsearching relevant journals. The full list of the databases, journals and conference proceedings which have been searched, as well as the search strategies used are described in the Specialised Register section of the Cochrane Vascular module in The Cochrane Library (www.cochranelibrary.com).
The CIS searched the following trial databases for details of ongoing and unpublished studies;
World Health Organization International Clinical Trials Registry http://apps.who.int/trialsearch/
ClinicalTrials.gov http://clinicaltrials.gov/
ISRCTN Register http://www.isrctn.com/
Searching other resources
We searched references sections of relevant reports for further studies.
Data collection and analysis
Selection of studies
Studies identified in the search were individually evaluated by two review authors (RF and MS) based on title, abstract or full report for possible inclusion and any disagreements resolved by discussion.
Data extraction and management
Two review authors (RF and MS) independently extracted data on study characteristics, efficacy and safety outcomes. Extracted information included study design, country, setting, intention‐to‐treat methods, number of participants randomised, number of participants excluded post‐randomisation, losses to follow up, age, sex, inclusion criteria, exclusion criteria, treatment and control details and duration of treatment, primary and secondary outcomes, funding, and how VTE outcomes were confirmed using objective methods. Data extracted for study outcomes included symptomatic VTE (DVT and PE), total VTE (symptomatic and asymptomatic), asymptomatic DVT documented by ascending lower limb contrast venography (stratified by proximal (popliteal vein and above) and distal (calf vein) DVT, where possible), all‐cause mortality, adverse events and bleeding events (major, clinically relevant non‐major and minor bleeding). We also extracted data for reoperation, wound infection and wound healing. We accepted the primary study authors' definitions for DVT, PE and bleeding events. Any disagreements were resolved through discussion.
Assessment of risk of bias in included studies
RF and MS independently assessed the methodological quality of included studies using the 'Risk of bias' tool according to Higgins 2011. The following domains were assessed: selection bias (random sequence generation, allocation concealment), performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment), attrition bias (incomplete outcome data), reporting bias (selective reporting) and other bias. We classified the domains as low risk, high risk, or unclear risk according to Higgins 2011. Disagreements were resolved by discussion.
Measures of treatment effect
We divided studies into six groups based on the treatment profile.
Heparin (LMWH or UFH) versus placebo
Vitamin K antagonists versus placebo
DOACs versus placebo
Anticoagulant chosen at investigators' discretion versus placebo
Vitamin K antagonists versus heparin (LMWH or UFH)
DOACs versus heparin (LMWH or UFH)
We pooled data for each of the comparisons for the outcomes of symptomatic VTE, total VTE, asymptomatic DVT documented by ascending lower limb contrast venography, all‐cause mortality, adverse events, bleeding events, reoperation, wound infection and wound healing. The pooled data for each outcome were used to create a meta‐analysis by calculating odds ratios (ORs) with 95% confidence intervals (CIs), as all outcomes were dichotomous. We used fixed‐effect models, unless there was evidence of a large amount of heterogeneity (see Assessment of heterogeneity), in which case a random‐effects model was implemented.
Unit of analysis issues
The unit of analysis was the individual patient.
Dealing with missing data
Where appropriate we used all randomised participants for the analysis. However, many of the included studies had many participants excluded after randomisation, creating a large disparity between the number of participants randomised and the number available for assessment of VTE at the end of the study. After discussion between the review authors, we decided it was generally inappropriate to include all randomised participants in our analysis, and would use the 'intention‐to‐treat' populations as reported by the studies. This population generally consisted of all participants that received treatment and had evaluable testing of VTE at the end of the study. If these values were not available, we utilised the reported per‐protocol data. Where necessary, we contacted study authors to provide missing data.
Assessment of heterogeneity
A test for heterogeneity examines the null hypothesis that all studies are evaluating the same effect. For each included meta‐analysis we obtained a value comparing the test statistic with a Chi2 distribution. To help readers assess the consistency of results of studies in a meta‐analysis, Review Manager software (RevMan) (RevMan 2014) includes a method (I2 statistic) that describes the percentage of total variation across studies due to heterogeneity rather than by chance. A value of 0% indicates no observed heterogeneity, and larger values show increasing heterogeneity (Higgins 2003). For the purposes of this review, if a meta‐analysis was found to have an I2 value of > 50%, we calculated the ORs using a random‐effects model instead of a fixed‐effect model.
Assessment of reporting biases
To detect reporting bias we planned to construct funnel plots for meta‐analyses that included at least 10 studies, as funnel plots with fewer than 10 studies lack the power to distinguish chance from real asymmetry (Egger 1997).
Data synthesis
We planned to use fixed‐effect models for each meta‐analysis to pool data, unless there was evidence of heterogeneity (see Assessment of heterogeneity), in which case we planned to use a random‐effects model to derive the ORs and 95% CIs.
Subgroup analysis and investigation of heterogeneity
We pre‐specified the following subgroup analyses for the primary outcome symptomatic VTE:
hip replacement versus knee replacement versus hip fracture repair;
according to duration of in‐hospital prophylaxis (up to 10 days, 10 to 14 days, 15 days or more) in trials comparing extended anticoagulant thromboprophylaxis versus placebo or no treatment;
performing mandatory discharge venography versus not performing mandatory discharge venography in trials comparing extended anticoagulant thromboprophylaxis versus placebo or no treatment;
patients undergoing revisions of a previous hip or knee replacement.
Sensitivity analysis
We conducted sensitivity analyses to further explore the robustness of our results. To identify any study that may have exerted a disproportionate influence on the summary treatment effect, we removed studies from the analysis that accounted for over 50% of the weighted summary statistic, where three or more studies were included, to see if these heavily‐weighted studies altered the findings. We also planned to examine the effect of excluding studies that were at high risk of bias from the analysis, based on the 'Risk of bias' tool. We planned to carry out sensitivity analysis only when two or more studies remained in the analysis after the removal of the studies in question.
Summary of findings
We constructed a 'Summary of findings' table for each comparison using the GRADEpro GDT software (GRADEpro GDT 2015) to present the main findings of the review. We included the outcomes symptomatic VTE, symptomatic DVT, symptomatic PE, major bleeding, clinically relevant non‐major bleeding and minor bleeding in the 'Summary of findings' table. We calculated assumed control intervention risks from the mean number of events in the control groups of the selected studies for each outcome. The system developed by the Grading of Recommendation, Assessment, Development and Evaluation Working Group (GRADE working group) was used for grading the quality of evidence as high, moderate, low and very low, based on within‐study risk of bias, directness of evidence, heterogeneity, precision of effects estimates, and risk of publication bias (Atkins 2004). For completeness, additional 'Summary of findings' tables were created for the remaining outcomes of this review and presented in the appendices.
Results
Description of studies
Results of the search
See Figure 1
1.
Study flow diagram.
Included studies
We included a total of 16 studies with 24,930 randomised participants (ADVANCE 3; Barrellier 2010; Dahl 1997; DaPP Study; EXTEND Study; Fragmin Trial; French Study; Heit 2000; Kolb 2003; Prandoni 2002; RECORD 1 Trial; RECORD 2 Trial; RE‐NOVATE II Trial; RE‐NOVATE Trial; SACRE Study; Zhang 2014). Nearly all the included randomised studies were multicentre, with just three recruiting from only a single centre (French Study; Prandoni 2002; Zhang 2014). Three studies were conducted in France (Barrellier 2010; French Study; SACRE Study), one study in Norway (Dahl 1997), one in Denmark (DaPP Study), one in the US (Heit 2000) and another in the US and Canada (Fragmin Trial), one in Germany and Czech Republic (Kolb 2003), one in Italy (Prandoni 2002), one in China (Zhang 2014) and six that involved centres in multiple countries, ranging from 16 up to 27 countries (ADVANCE 3; EXTEND Study; RECORD 1 Trial; RECORD 2 Trial; RE‐NOVATE II Trial; RE‐NOVATE Trial). The duration of treatment ranged between the studies, with the majority having treatment periods for an average of 35 days (ADVANCE 3; Barrellier 2010; Dahl 1997; DaPP Study; EXTEND Study; Fragmin Trial; French Study; RECORD 1 Trial; RECORD 2 Trial; Zhang 2014). Both RE‐NOVATE II Trial and RE‐NOVATE Trial had treatment periods of 28 to 35 days. One study had a four‐week post‐discharge period on top of a median nine‐day hospital phase (Prandoni 2002). Two studies had six‐week treatment periods (Heit 2000; SACRE Study), with the final study having a treatment period "up to 42 days" (Kolb 2003).
Heparin versus placebo
A total of six trials with 3221 participants compared heparin with placebo (Dahl 1997; DaPP Study; Fragmin Trial; French Study; Heit 2000; Kolb 2003). Four of the studies evaluated participants undergoing total hip replacement (Dahl 1997; DaPP Study; Fragmin Trial; French Study), two evaluated a combined group of hip and knee replacements (Heit 2000; Kolb 2003). Heit 2000 presented results for the hip and knee replacement groups separately, while Kolb 2003 presented results for the combined group only.
Five included studies evaluated LMWH versus placebo: three evaluated dalteparin (Dahl 1997; DaPP Study; Fragmin Trial), one evaluated enoxaparin (French Study) and one evaluated the certoparin (Kolb 2003). Heit 2000 compared the anticoagulant ardeparin sodium versus placebo. It should be noted that ardeparin has been removed from the market in the US since 2000, but not for reasons of efficacy or safety (Dotzel 2002).
Duration of the in‐hospital/initial phase ranged from four days to 14 days. Heit 2000 reported four to 10 days, while Dahl 1997 and DaPP Study reported seven days for the initial phase. The French Study and Kolb 2003 had 14 days for their initial phase. The Fragmin Trial did not report this timeframe. At the time of discharge from the hospital or at the end of the initial phase Dahl 1997, Fragmin Trial and the French Study had mandatory venography to test for DVT. The DaPP Study did state that they identified those with DVT at the end of the initial phase, but did not state their methods. While Heit 2000 and Kolb 2003 did not use objective methods to test for DVT at the end of the initial phase of the trial.
Vitamin K antagonists versus placebo
One trial with 360 participants compared the VKA antagonist warfarin with placebo (Prandoni 2002). Prandoni 2002 evaluated participants undergoing total hip replacement. Duration of the in‐hospital/initial phase had a median of nine days (Prandoni 2002). At the time of discharge from the hospital Prandoni 2002 performed ultrasonography on all participants to determine incidence of DVT before the second phase of the trial.
DOACs versus placebo
Two trials with 2549 participants compared DOACs with placebo (RECORD 2 Trial; Zhang 2014). Both studies evaluated participants undergoing total hip replacement (RECORD 2 Trial; Zhang 2014).
The RECORD 2 Trial compared the oral anticoagulant rivaroxaban with enoxaparin in the initial phase, then the enoxaparin group began taking placebo. Zhang 2014 evaluated rivaroxaban compared with no treatment. Duration of the in‐hospital/initial phase was reported by Zhang 2014 as seven days for the initial phase while RECORD 2 Trial reported 10 to 14 days. At the end of the initial phase Zhang 2014 performed ultrasonography on all participants to determine incidence of DVT before the second phase of the trial. The RECORD 2 Trial did not use objective methods to test for DVT at the end of the initial phase of the trial.
Anticoagulant treatments chosen at the investigators' discretion versus placebo
One trial with 857 participants compared thromboprophylaxis using anticoagulant treatments chosen at the investigators' discretion with placebo in participants undergoing total knee replacement (Barrellier 2010). The anticoagulation treatment was either heparin, enoxaparin, dalteparin, tinzaparin, nadroparin or fondaparinux (Barrellier 2010). Duration of the in‐hospital/initial phase was reported as 10 days. At the end of the initial phase Barrellier 2010 performed ultrasonography on all participants to determine incidence of DVT before the second phase of the trial.
Vitamin K antagonists versus heparin
One study with a total of 1289 randomised participants evaluated acenocoumarol versus reviparin sodium (SACRE Study). All participants began on the LMWH reviparin sodium until 3 ± 1 days after surgery when participants were randomised to either continue on reviparin sodium or cross over to acenocoumarol for six weeks. Participants showing clinical signs or symptoms of DVT, PE or major bleeding were not randomised. It is not clear whether those experiencing clinical signs or symptoms of DVT and PE at the randomisation stage underwent objective testing using venography or duplex scanning. The SACRE Study randomised participants undergoing a total hip replacement.
DOACs versus heparin
Five studies with a total of 16,654 randomised participants evaluated DOAC versus heparin therapy (ADVANCE 3; EXTEND Study; RECORD 1 Trial; RE‐NOVATE II Trial; RE‐NOVATE Trial). All five studies evaluated participants undergoing a total hip replacement. One study compared apixaban versus enoxaparin (ADVANCE 3). One study evaluated ximelagatran versus enoxaparin (EXTEND Study), although this study was terminated early due to safety concerns with ximelagatran. Two studies compared dabigatran etexilate with enoxaparin, one with two doses of dabigatran of 220 mg and 150 mg (RE‐NOVATE Trial) and the other only evaluated the 220 mg dosage (RE‐NOVATE II Trial). Rivaroxaban was compared to enoxaparin in one study (RECORD 1 Trial). As these five studies compared two different anticoagulant treatments and randomised participants prior to surgery, there was no initial phase and objective assessment of VTE after initial phase as seen in the studies described above comparing anticoagulants versus placebo.
Excluded studies
A total of seven studies were assessed as excluded (Comp 2001; EPCAT II; Kristensen 1990; Manganelli 1998; NPHDO Study Group; PENTHIFRA PLUS Study; Swedish Study). Six studies had extended duration, but did not meet the five week minimum requirement (Comp 2001; EPCAT II; Manganelli 1998; NPHDO Study Group; PENTHIFRA PLUS Study; Swedish Study). Kristensen 1990 did not evaluate a treatment comparison within the scope of this review: heparin plus indomethacin versus heparin plus placebo.
A total of 175 records were deemed not relevant mainly due to the duration of the thromboprophylaxis being 10 to 14 days and not extended duration.
Risk of bias in included studies
See Figure 2 and Figure 3 for further information on risk of bias.
2.
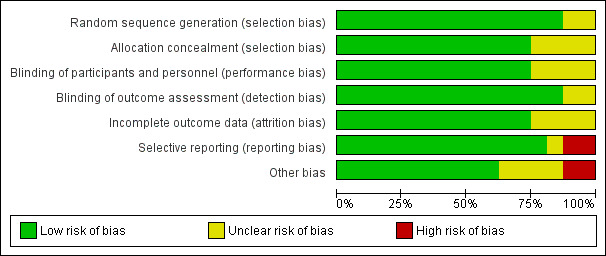
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
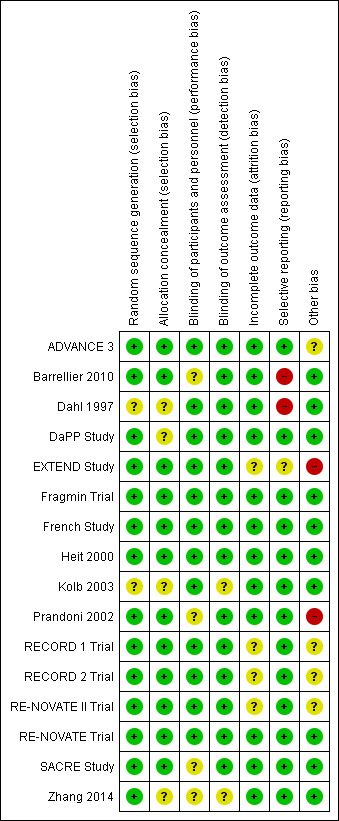
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Overall risk of bias was moderate to low with the largest concerns being some studies either not reporting on listed outcomes or reporting outcomes they did not pre‐define, leading to selective reporting bias, and other bias, mainly stemming from early termination or potential conflicts of interest due to the involvement of pharmaceutical companies.
Allocation
Adequate random sequence generation was described in the majority of studies, but two studies did not provide enough detail to make an assessment, and were rated as unclear (Dahl 1997; Kolb 2003).
Likewise, nearly all studies provided enough information to determine if allocation concealment was adequate, yet four studies did not give enough detail (Dahl 1997; DaPP Study; Kolb 2003; Zhang 2014).
Blinding
Performance bias was generally low as most studies were double‐blind and utilised a placebo. However, four studies were given an unclear rating as one study was open‐label (Barrellier 2010) and three studies did not state either way if they were open‐label or double‐blind, but as no placebo was acknowledged, they were most likely open‐label (Prandoni 2002; SACRE Study; Zhang 2014). It is unclear if knowing the treatment would have an effect on the outcomes.
All studies, except for two (Kolb 2003; Zhang 2014) used a blinded assessor or committee to evaluate at least some, if not all, outcomes. Kolb 2003 and Zhang 2014 may have used blinded assessors, but no details were given.
Incomplete outcome data
Twelve of the 16 included studies had low risk of attrition bias as all participants were accounted for in the analysis, and thorough explanations were given for exclusions. Four studies, while all participants were accounted for, did not give adequate explanations for why some participants stopped taking their medication or never started (EXTEND Study; RECORD 1 Trial; RECORD 2 Trial; RE‐NOVATE II Trial).
Selective reporting
The majority of studies had no evidence of reporting bias, but two were rated as high risk of bias because one study had bleeding events listed as an outcome but did not fully report the number of events (Barrellier 2010) and the other reported on several outcomes that were not listed as a pre‐planned outcome (Dahl 1997). The EXTEND Study was given an unclear rating as defined outcomes were not reported on after early termination of the trial.
Other potential sources of bias
Two studies were given a high risk of other bias as one study was terminated early due to safety issues and also the funding pharmaceutical company was highly involved in the study design, data collection and analysis (EXTEND Study), and the other study was also terminated early because they had a very high statistical significance with the first 360 enrolled participants (Prandoni 2002). A further four studies also had pharmaceutical companies as funding bodies that were very involved in the design, data collection and analysis of the studies, which could be a conflict of interest (ADVANCE 3; RECORD 1 Trial; RECORD 2 Trial; RE‐NOVATE II Trial).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
Heparin versus placebo
See Table 1 and Appendix 2
Symptomatic VTE (DVT and PE)
Five studies assessed symptomatic VTE (Dahl 1997; Fragmin Trial; French Study; Heit 2000; Kolb 2003) and the fixed‐effect model found no difference between the study arms: OR 0.59, 95% CI 0.35 to 1.01; participants = 2329; studies = 5; I2 = 0%; high quality evidence; Analysis 1.1.
1.1. Analysis.
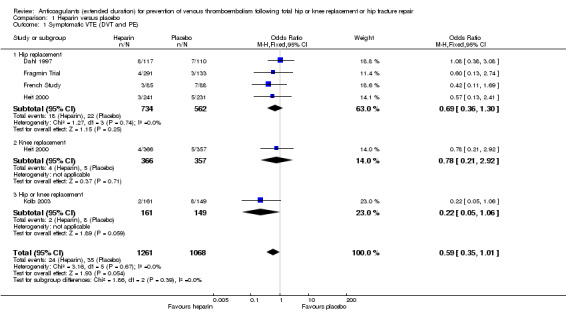
Comparison 1 Heparin versus placebo, Outcome 1 Symptomatic VTE (DVT and PE).
Subgroup analysis by hip or knee replacement showed no differences between the subgroups and no differences between heparin and placebo were observed for the individual surgery groups (Analysis 1.1).
The findings did not change in studies with an in‐hospital or initial phase of 10 to 14 days, or with less than 10 days of in‐hospital/initial phase or when the studies that did not have mandatory, objective detection of DVT at hospital discharge were excluded (data analyses not shown).
Symptomatic DVT
Four studies assessed symptomatic DVT (distal or proximal) (Dahl 1997; Fragmin Trial; French Study; Heit 2000) and the fixed‐effect model found no difference between the study arms: OR 0.73, 95% CI 0.39 to 1.38; participants = 2019; studies = 4; I2 = 0%; moderate quality evidence; Analysis 1.2.
1.2. Analysis.
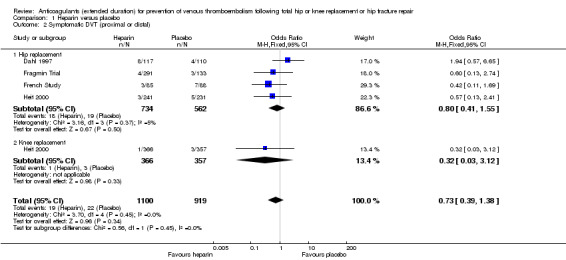
Comparison 1 Heparin versus placebo, Outcome 2 Symptomatic DVT (proximal or distal).
Subgroup analysis by hip or knee replacement showed no differences between the subgroups and no differences between heparin and placebo were observed for the individual surgery groups (Analysis 1.2).
The findings did not change in studies with an in‐hospital or initial phase of 10 to 14 days, or with less than 10 days of in‐hospital/initial phase or when the studies that did not have mandatory, objective detection of DVT at hospital discharge were excluded (data analyses not shown).
Symptomatic PE
The fixed‐effect model evaluating three studies for symptomatic PE (Dahl 1997; French Study; Heit 2000), showed no difference between heparin or placebo (OR 0.61, 95% CI 0.16 to 2.33; participants = 1595; studies = 3; I2 = 49%; low quality evidence) Analysis 1.3.
1.3. Analysis.
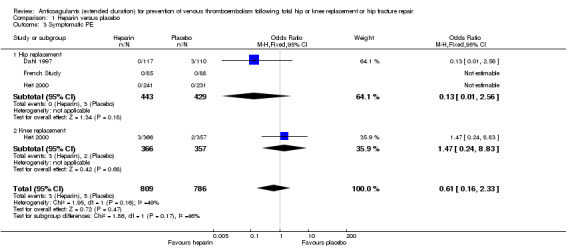
Comparison 1 Heparin versus placebo, Outcome 3 Symptomatic PE.
Subgroup analysis by hip or knee replacement showed no differences between the subgroups and no differences between heparin and placebo were observed for the individual surgery groups (Analysis 1.3).
The findings did not change in studies with an in‐hospital or initial phase of 10 to 14 days, or with less than 10 days of in‐hospital/initial phase or when the studies that did not have mandatory, objective detection of DVT at hospital discharge were excluded (data analyses not shown).
Regarding sensitivity analysis, Dahl 1997 was found to account for 64.1% of the participants within this outcome, but after its removal no difference was seen in the findings.
Total VTE (symptomatic or asymptomatic)
For the six reporting studies (Dahl 1997; DaPP Study; Fragmin Trial; French Study; Heit 2000; Kolb 2003), the fixed‐effect model found decreased odds of any VTE event in favour of heparin (OR 0.39, 95% CI 0.28 to 0.56; participants = 2544; studies = 6; I2 = 0%; high quality evidence) Analysis 1.4.
1.4. Analysis.
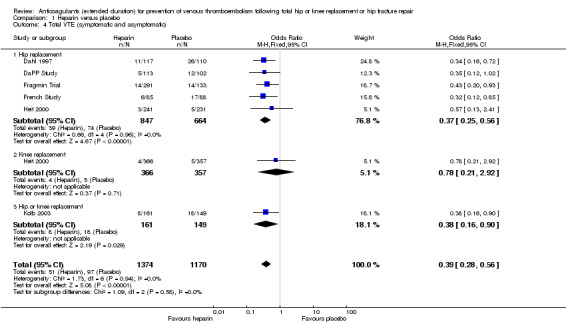
Comparison 1 Heparin versus placebo, Outcome 4 Total VTE (symptomatic and asymptomatic).
For the five studies reporting on hip replacement, the fixed‐effect model found decreased odds in favour of heparin (OR 0.37, 95% CI 0.25 to 0.56; participants = 1511; studies = 5; I2 = 0%) Analysis 1.4. The single study reporting on knee replacement found no difference between the study arms (OR 0.78, 95% CI 0.21 to 2.92; participants = 723) Analysis 1.4. The single study reporting on a combined group of hip or knee replacement participants found decreased odds in favour of heparin (OR 0.38, 95% CI 0.16 to 0.90; participants = 310) Analysis 1.4.
When comparing the subgroup of studies evaluating 10 to 14 day in‐hospital/initial phase treatment duration and those that included objective DVT screening at discharge, the findings were still in favour of heparin treatment (data analyses not shown).
Asymptomatic DVT
In the five studies that reported on asymptomatic DVT (Dahl 1997; DaPP Study; Fragmin Trial; French Study; Kolb 2003), the fixed‐effect model showed a decreased odds favouring the heparin treatment group (OR 0.38, 95% CI 0.24 to 0.60; participants = 1304; studies = 5; I2 = 0%; high quality evidence) Analysis 1.5.
1.5. Analysis.
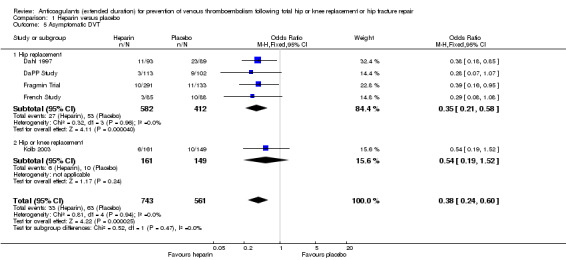
Comparison 1 Heparin versus placebo, Outcome 5 Asymptomatic DVT.
When evaluating hip replacement only, the four included studies retained a decreased odds of asymptomatic DVT (OR 0.35, 95% CI 0.21 to 0.58; participants = 994; studies = 4; I2 = 0%) Analysis 1.5. The single study reporting on a combined group of hip or knee replacement participants found no difference between the study arms (OR 0.54, 95% CI 0.19 to 1.52; participants = 310) Analysis 1.5.
When comparing the subgroup of studies evaluating 10 to 14 day in‐hospital/initial phase treatment duration and those that included objective DVT screening at discharge, the findings were still in favour of heparin treatment (data analyses not shown).
Asymptomatic proximal DVT
None of the included studies reported on asymptomatic proximal DVT. Many did report asymptomatic or proximal DVT separately, but given the provided data, it was not possible to determine which events fell into both categories.
Asymptomatic distal DVT
No studies reported asymptomatic distal DVT. See above description. Many did report asymptomatic or distal DVT separately, but given the provided data, it was not possible to determine which events fell into both categories.
All‐cause mortality
For the five included studies that evaluated all‐cause mortality for heparin versus placebo (Dahl 1997; Fragmin Trial; French Study; Heit 2000; Kolb 2003), there was no difference between the study arms (OR 1.01, 95% CI 0.31 to 3.26; participants = 2518; studies = 5; I2 = 0%; moderate quality evidence) Analysis 1.6.
1.6. Analysis.
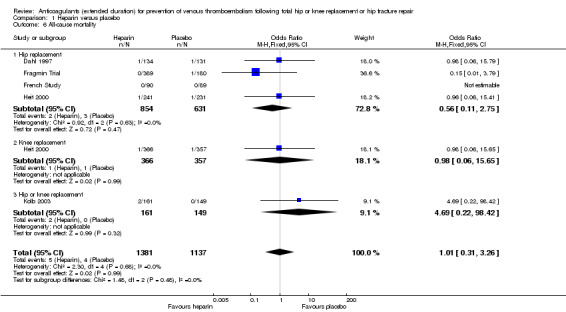
Comparison 1 Heparin versus placebo, Outcome 6 All‐cause mortality.
There was also no difference in the four studies evaluating participants undergoing hip replacement (OR 0.56, 95% CI 0.11 to 2.75; participants = 1485; studies = 4; I2 = 0%) Analysis 1.6, or in the one study undergoing knee replacement (OR 0.98, 95% CI 0.06 to 15.65; participants = 723) Analysis 1.6. The single study reporting on a combined group of hip or knee replacement participants also found no difference between the study arms (OR 4.69, 95% CI 0.22 to 98.42; participants = 310) Analysis 1.6.
When only evaluating studies that had an in‐hospital/initial phase of 10 to 14 days or studies that included objective DVT verification at discharge, there was still no difference between study arms (data analysis not shown).
Adverse events
Only two studies evaluating participants undergoing hip replacement reported on adverse events (DaPP Study; French Study) and found no difference between study arms (OR 1.06, 95% CI 0.68 to 1.64; participants = 460; I2 = 4%; moderate quality evidence Analysis 1.7), although it should be noted that there was minimal or no description of the criteria for adverse event reporting, and it varied greatly between the two reporting studies.
1.7. Analysis.
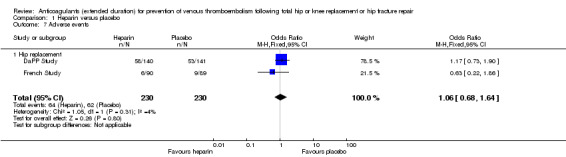
Comparison 1 Heparin versus placebo, Outcome 7 Adverse events.
Too few studies remained for subgroup analysis by initial phase duration or objective DVT verification at discharge (data analyses not shown).
Bleeding events (major, clinically relevant non‐major, minor)
Major bleeding was reported in five studies (DaPP Study; Fragmin Trial; French Study; Heit 2000; Kolb 2003) and minor bleeding was reported in five studies (DaPP Study; Fragmin Trial; French Study; Heit 2000; Kolb 2003). Clinically relevant non‐major bleeding was not reported by the included studies comparing heparin and placebo.
There was no difference in major bleeding between the study arms (OR 0.59, 95% CI 0.14 to 2.46; participants = 2500; studies = 5; I2 = 0%; moderate quality evidence Analysis 1.8.
1.8. Analysis.
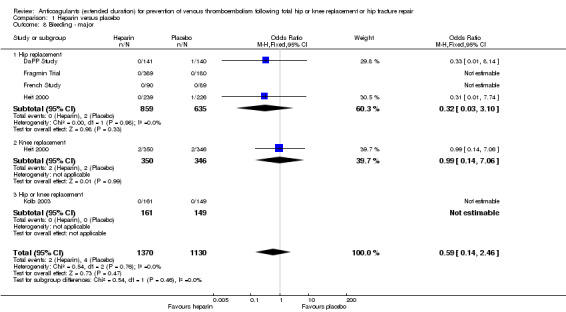
Comparison 1 Heparin versus placebo, Outcome 8 Bleeding ‐ major.
For participants undergoing hip replacement, there was no difference in major bleeding (OR 0.32, 95% CI 0.03 to 3.10; participants = 1494; studies = 4; I2 = 0% Analysis 1.8. There was no difference in major bleeding in participants undergoing knee replacement (OR 0.99, 95% CI 0.14 to 7.06; participants = 696; studies = 1; I2 = 0% Analysis 1.8). The single study reporting on a combined group of hip or knee replacement participants reported no major bleeding in either study arm (0/161 heparin versus 0/149 placebo).
There was an increased odds of minor bleeding in the heparin treatment group compared with placebo (OR 2.01, 95% CI 1.43 to 2.81; participants = 2500; studies = 5; I2 = 0%; high quality evidence) Analysis 1.9.
1.9. Analysis.
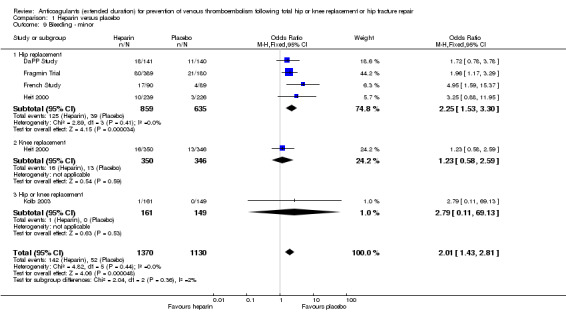
Comparison 1 Heparin versus placebo, Outcome 9 Bleeding ‐ minor.
For participants undergoing hip replacement, there was an increased odds of minor bleeding in the heparin treatment group compared with placebo (OR 2.25, 95% CI 1.53 to 3.30; participants = 1494; studies = 4; I2 = 0%) Analysis 1.9. There was no difference in minor bleeding in participants undergoing knee replacement (OR 1.23, 95% CI 0.58 to 2.59; participants = 696; studies = 1 Analysis 1.9). The single study reporting on a combined group of hip and knee replacement participants also reported no difference in minor bleeding in both study arms (OR 2.79, 95% CI 0.11 to 69.13; participants = 310; studies = 1; I2 = 0%) Analysis 1.9.
There were no changes to the findings when only comparing studies with a in‐hospital/initial phase of 10 to 14 days or studies that had objective DVT testing at discharge (data analyses not shown).
Reoperation
The DaPP Study reported that two participants required reoperation but did not report from which study arm. Dahl 1997 reported reoperation due to bleeding to be a safety outcome but did not present data. The Fragmin Trial, Heit 2000 and Kolb 2003 did not report on reoperation. The French Study reported that no reoperations were required following bleeding Analysis 1.10.
1.10. Analysis.
Comparison 1 Heparin versus placebo, Outcome 10 Reoperation.
Wound infection
DaPP Study, French Study, Heit 2000 and Kolb 2003 did not report on wound infection. Dahl 1997 reported that the majority of adverse events were luxation of prosthesis and infection but did not report specific numbers for the study arms. The Fragmin Trial reported wound infection as part of complications associated with wound haematoma but no specific details for infection were provided.
Wound healing
None of the six studies comparing heparin with placebo reported wound healing (Dahl 1997; DaPP Study; Fragmin Trial; French Study; Heit 2000; Kolb 2003).
Vitamin K antagonists versus placebo
One study evaluated vitamin K antagonist versus placebo (Prandoni 2002), in participants undergoing hip replacement, and therefore no subgroup analysis between hip and knee replacement was undertaken. We found no difference between the treatment groups for any of the evaluated outcomes except for total VTE. See Table 2 and Appendix 3.
Symptomatic VTE (DVT and PE)
No difference between VKA and placebo was observed for symptomatic VTE (OR 0.10, 95% CI 0.01 to 1.94; participants = 360; moderate quality evidence) Analysis 2.1.
2.1. Analysis.
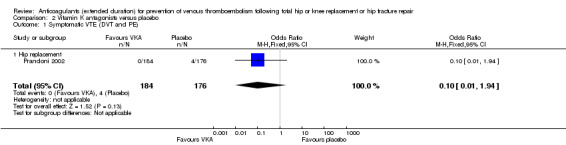
Comparison 2 Vitamin K antagonists versus placebo, Outcome 1 Symptomatic VTE (DVT and PE).
Symptomatic DVT
No difference between VKA and placebo was observed for symptomatic DVT (OR 0.13, 95% CI 0.01 to 2.62; participants = 360; moderate quality evidence) Analysis 2.2.
2.2. Analysis.
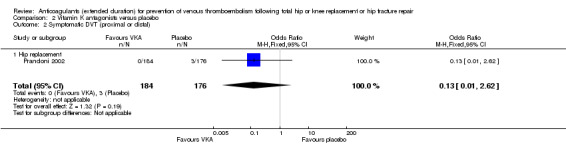
Comparison 2 Vitamin K antagonists versus placebo, Outcome 2 Symptomatic DVT (proximal or distal).
Symptomatic PE
No difference between VKA and placebo was observed for symptomatic PE (OR 0.32, 95% CI 0.01 to 7.84; participants = 360; moderate quality evidence) Analysis 2.3.
2.3. Analysis.
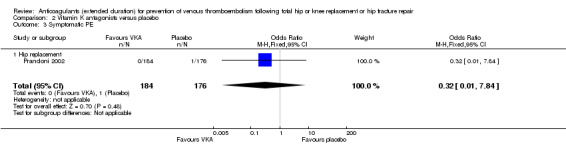
Comparison 2 Vitamin K antagonists versus placebo, Outcome 3 Symptomatic PE.
Total VTE (symptomatic or asymptomatic)
Prandoni 2002 showed decreased odds of any VTE event in favour of VKA versus placebo (OR 0.10, 95% CI 0.01 to 0.81; participants = 360; moderate quality evidence) Analysis 2.4.
2.4. Analysis.
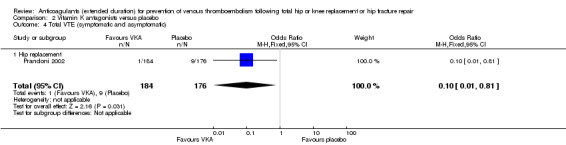
Comparison 2 Vitamin K antagonists versus placebo, Outcome 4 Total VTE (symptomatic and asymptomatic).
Asymptomatic DVT
Prandoni 2002 did not report on asymptomatic DVT.
Asymptomatic proximal DVT
Prandoni 2002 did not report on asymptomatic proximal DVT.
Asymptomatic distal DVT
Prandoni 2002 did not report on asymptomatic distal DVT.
All‐cause mortality
Prandoni 2002 reported no deaths in either study arm (0/184 VKA versus 0/176 placebo) Analysis 2.5.
2.5. Analysis.

Comparison 2 Vitamin K antagonists versus placebo, Outcome 5 All‐cause mortality.
Adverse events
Prandoni 2002 reported no adverse events in either study arm (0/184 VKA versus 0/176 placebo) Analysis 2.6.
2.6. Analysis.
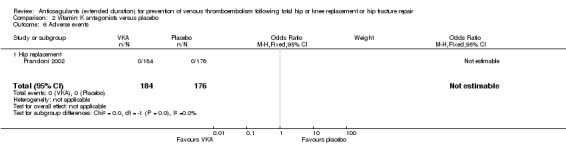
Comparison 2 Vitamin K antagonists versus placebo, Outcome 6 Adverse events.
Bleeding events (major, clinically relevant non‐major, minor)
There were no differences between the study arms in major bleeding (OR 2.89, 95% CI 0.12 to 71.31; participants = 360; low quality evidence) Analysis 2.7. Prandoni 2002 did not report clinically relevant non‐major bleeding or minor bleeding.
2.7. Analysis.
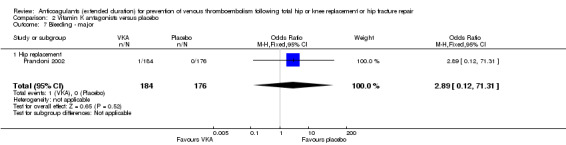
Comparison 2 Vitamin K antagonists versus placebo, Outcome 7 Bleeding ‐ major.
Reoperation
Prandoni 2002 did not report on reoperation.
Wound infection
Prandoni 2002 did not report on wound infection
Wound healing
Prandoni 2002 did not report on wound healing.
DOACs versus placebo
Two studies evaluated DOACs versus placebo (RECORD 2 Trial; Zhang 2014) in participants undergoing hip replacement, and therefore no subgroup analysis between hip and knee replacement was undertaken. Zhang 2014 reported assessment of symptomatic DVT only and is therefore only included in the analysis for symptomatic DVT. See Table 3 and Appendix 4.
Symptomatic VTE (DVT and PE)
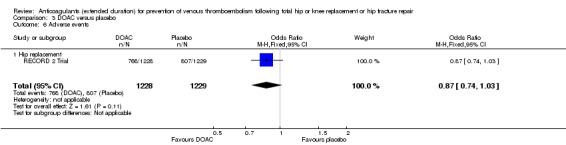
The RECORD 2 Trial reported on symptomatic VTE showing a reduced odds of VTE in favour of the DOAC treatment (OR 0.20, 95% CI 0.06 to 0.68; participants = 2419; moderate quality evidence) Analysis 3.1.
3.1. Analysis.
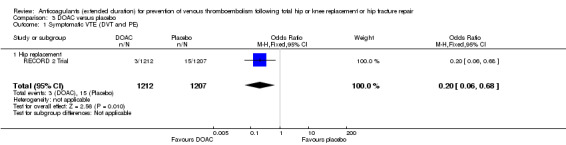
Comparison 3 DOAC versus placebo, Outcome 1 Symptomatic VTE (DVT and PE).
Symptomatic DVT
A reduced odds of symptomatic DVT in favour of DOAC treatment was observed (OR 0.18, 95% CI 0.04 to 0.81; participants = 2459; studies = 2; I2 = not applicable; high quality evidence) Analysis 3.2
3.2. Analysis.
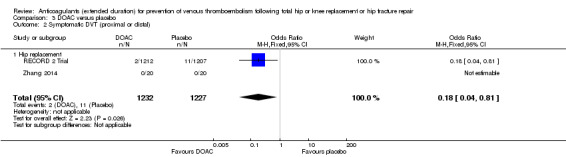
Comparison 3 DOAC versus placebo, Outcome 2 Symptomatic DVT (proximal or distal).
Symptomatic PE
No difference between DOACs and placebo was observed for symptomatic PE (OR 0.25, 95% CI 0.03 to 2.25; participants = 1733; studies = 1; low quality evidence) Analysis 3.3.
3.3. Analysis.
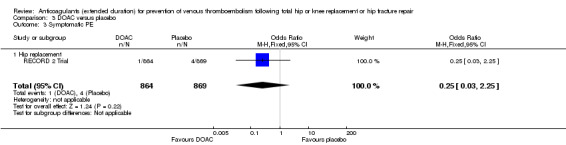
Comparison 3 DOAC versus placebo, Outcome 3 Symptomatic PE.
Total VTE (symptomatic or asymptomatic)
The RECORD 2 Trial showed decreased odds of any VTE event in favour of DOAC treatment versus placebo (OR 0.19, 95% CI 0.11 to 0.33; moderate quality evidence) Analysis 3.4.
3.4. Analysis.
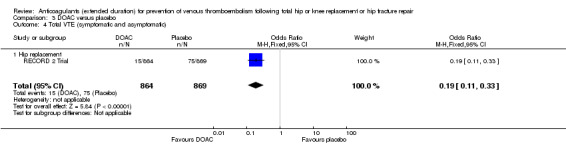
Comparison 3 DOAC versus placebo, Outcome 4 Total VTE (symptomatic and asymptomatic).
Asymptomatic DVT
The RECORD 2 Trial and Zhang 2014 do not report on asymptomatic DVT.
Asymptomatic proximal DVT
The RECORD 2 Trial and Zhang 2014 do not report on asymptomatic proximal DVT.
Asymptomatic distal DVT
The RECORD 2 Trial and Zhang 2014 do not report on asymptomatic distal DVT.
All‐cause mortality
There were no differences between the study arms in all‐cause mortality (OR 0.33, 95% CI 0.07 to 1.66; participants = 1733; studies = 1; low quality evidence Analysis 3.5.
3.5. Analysis.
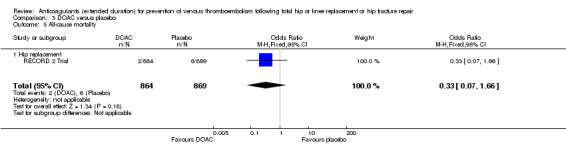
Comparison 3 DOAC versus placebo, Outcome 5 All‐cause mortality.
Adverse events
The RECORD 2 Trial reported no differences in adverse events (OR 0.87, 95% CI 0.74 to 1.03; participants = 2457; moderate quality evidence) Analysis 3.6.
3.6. Analysis.
Comparison 3 DOAC versus placebo, Outcome 6 Adverse events.
Bleeding events (major, clinically relevant non‐major, minor)
There were no differences in any of the bleeding event categories reported by RECORD 2 Trial; major bleeding (OR 1.00, 95% CI 0.06 to 16.02; participants = 2457; low quality evidence Analysis 3.7); clinically relevant bleeding (OR 1.22, 95% CI 0.76 to 1.95; participants = 2457; moderate quality evidence Analysis 3.8); minor bleeding (OR 1.18, 95% CI 0.74 to 1.88; participants = 2457; moderate quality evidence Analysis 3.9.
3.7. Analysis.
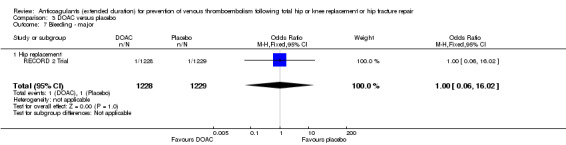
Comparison 3 DOAC versus placebo, Outcome 7 Bleeding ‐ major.
3.8. Analysis.

Comparison 3 DOAC versus placebo, Outcome 8 Bleeding‐ clinically relevant non‐major.
3.9. Analysis.
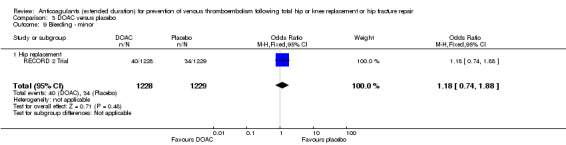
Comparison 3 DOAC versus placebo, Outcome 9 Bleeding ‐ minor.
Reoperation
The RECORD 2 Trial reported reoperation following bleeding; no cases of reoperation (0/1228 DOAC versus 0/1229 placebo) were reported Analysis 3.10.
3.10. Analysis.
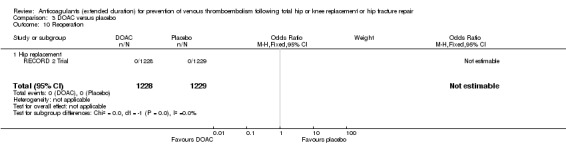
Comparison 3 DOAC versus placebo, Outcome 10 Reoperation.
Wound infection
The RECORD 2 Trial reported no differences in post‐operative wound infections (OR 1.34, 95% CI 0.46 to 3.86; participants = 2457; low quality evidence) Analysis 3.11.
3.11. Analysis.

Comparison 3 DOAC versus placebo, Outcome 11 Wound infection.
Wound healing
The RECORD 2 Trial and Zhang 2014 did not report wound healing.
Anticoagulant treatments chosen at the investigators' discretion versus placebo
One study evaluated anticoagulant treatment chosen at the investigators' discretion versus placebo (Barrellier 2010) in participants undergoing knee replacement, and therefore no subgroup analysis between hip and knee replacement was undertaken. The anticoagulation treatment was either heparin, enoxaparin, dalteparin, tinzaparin, nadroparin or fondaparinux, at the discretion of the investigator at the study location. See Table 4 and Appendix 5.
Symptomatic VTE (DVT and PE)
No difference between anticoagulant treatment and placebo was observed for symptomatic VTE (OR 0.50, 95% CI 0.09 to 2.74; participants = 557; low quality evidence) Analysis 4.1.
4.1. Analysis.
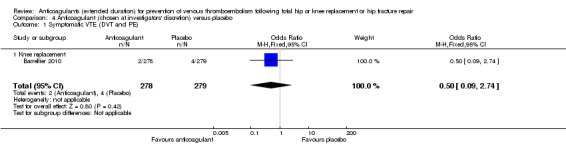
Comparison 4 Anticoagulant (chosen at investigators' discretion) versus placebo, Outcome 1 Symptomatic VTE (DVT and PE).
Symptomatic DVT
No difference between anticoagulant treatment and placebo was observed for symptomatic DVT (OR 0.33, 95% CI 0.03 to 3.21; participants = 557; low quality evidence) Analysis 4.2.
4.2. Analysis.
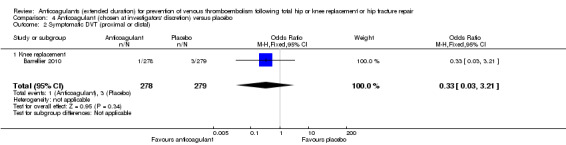
Comparison 4 Anticoagulant (chosen at investigators' discretion) versus placebo, Outcome 2 Symptomatic DVT (proximal or distal).
Symptomatic PE
No difference between anticoagulant treatment and placebo was observed for symptomatic PE (OR 1.00, 95% CI 0.06 to 16.13; participants = 557; low quality evidence) Analysis 4.3.
4.3. Analysis.
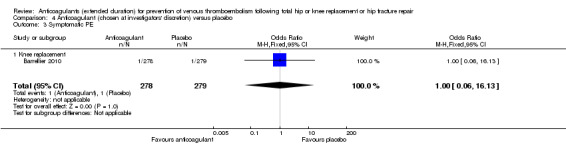
Comparison 4 Anticoagulant (chosen at investigators' discretion) versus placebo, Outcome 3 Symptomatic PE.
Total VTE (symptomatic or asymptomatic)
The Barrellier 2010 study showed decreased odds of any VTE event in favour of anticoagulant treatment versus placebo (OR 0.26, 95% CI 0.14 to 0.50; participants = 557; moderate quality evidence) Analysis 4.4.
4.4. Analysis.
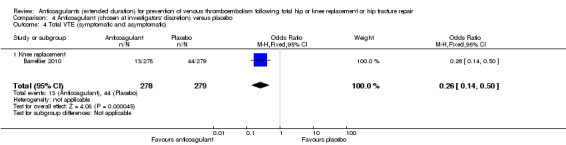
Comparison 4 Anticoagulant (chosen at investigators' discretion) versus placebo, Outcome 4 Total VTE (symptomatic and asymptomatic).
Asymptomatic DVT
Barrellier 2010 showed a significant difference in new cases of asymptomatic DVT favouring anticoagulant treatment compared to placebo (OR 0.26, 95% CI 0.13 to 0.54; participants = 557; moderate quality evidence) Analysis 4.5. Barrellier 2010 reported on asymptomatic distal DVT cases only.
4.5. Analysis.
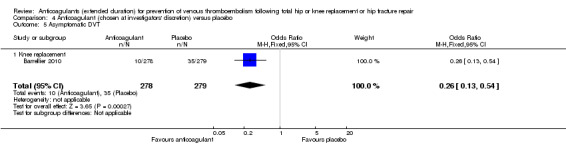
Comparison 4 Anticoagulant (chosen at investigators' discretion) versus placebo, Outcome 5 Asymptomatic DVT.
Asymptomatic proximal DVT
Barrellier 2010 did not report asymptomatic proximal DVT.
Asymptomatic distal DVT
Barrellier 2010 reported asymptomatic distal DVT as described above (Analysis 4.6).
4.6. Analysis.
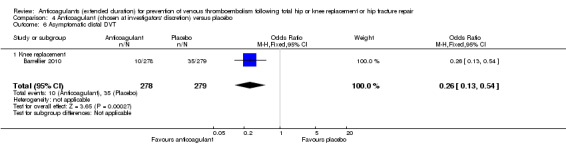
Comparison 4 Anticoagulant (chosen at investigators' discretion) versus placebo, Outcome 6 Asymptomatic distal DVT.
All‐cause mortality
Barrellier 2010 reported no deaths in either study arm (0/422 anticoagulant treatment versus 0/420 placebo) Analysis 4.7.
4.7. Analysis.

Comparison 4 Anticoagulant (chosen at investigators' discretion) versus placebo, Outcome 7 All‐cause mortality.
Adverse events
Barrellier 2010 did not report adverse events.
Bleeding events (major, clinically relevant non‐major, minor)
There were no differences between the treatment groups in major bleeding (OR 5.05, 95% CI 0.24 to 105.76; participants = 557; low quality evidence). Analysis 4.8. Barrellier 2010 did not report clinically relevant non‐major bleeding or minor bleeding.
4.8. Analysis.
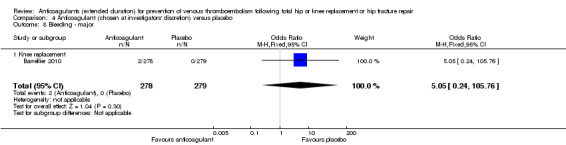
Comparison 4 Anticoagulant (chosen at investigators' discretion) versus placebo, Outcome 8 Bleeding ‐ major.
Reoperation
Barrellier 2010 reported on reoperation as part of their definition of major bleeding outcome (bleeding leading to reoperation) but did not report this data separately.
Wound infection
Barrellier 2010 did not report on wound infection.
Wound healing
Barrellier 2010 did not report on wound healing.
Vitamin K antagonists versus heparin
One study evaluated vitamin K antagonists versus heparin (SACRE Study) in participants undergoing hip replacement, and therefore no subgroup analysis between hip and knee replacement was undertaken. We found no difference between the treatment groups for any of the evaluated outcomes. See Table 5 and Appendix 6.
Symptomatic VTE (DVT and PE)
No difference was observed in symptomatic VTE between the treatment groups (OR 1.64, 95% CI 0.85 to 3.16; participants = 1279; low quality evidence) Analysis 5.1.
5.1. Analysis.
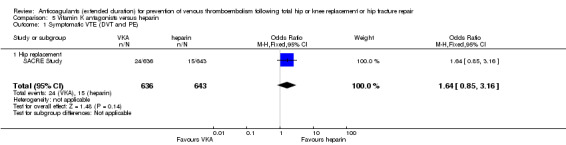
Comparison 5 Vitamin K antagonists versus heparin, Outcome 1 Symptomatic VTE (DVT and PE).
Symptomatic DVT
There was also no difference between the treatment groups for symptomatic DVT (OR 1.36, 95% CI 0.69 to 2.68; participants = 1279; low quality evidence) Analysis 5.2.
5.2. Analysis.

Comparison 5 Vitamin K antagonists versus heparin, Outcome 2 Symptomatic DVT (proximal or distal).
Symptomatic PE
No cases of PE were reported in the heparin group (0/643) compared with 4 cases in the VKA group (4/636) . No difference was found between the treatment groups for the outcome of symptomatic PE (OR 9.16, 95% CI 0.49 to 170.42; participants = 1279; low quality evidence) Analysis 5.3.
5.3. Analysis.
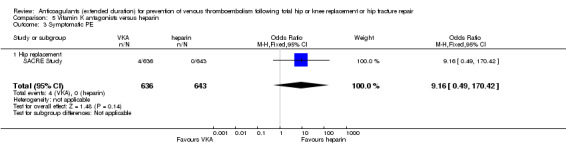
Comparison 5 Vitamin K antagonists versus heparin, Outcome 3 Symptomatic PE.
Total VTE (symptomatic or asymptomatic)
There was no difference in total VTE between the treatment groups (OR 1.64, 95% CI 0.85 to 3.16; participants = 1279; low quality evidence) Analysis 5.4 .
5.4. Analysis.
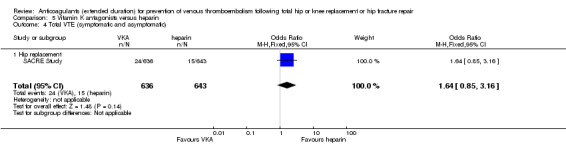
Comparison 5 Vitamin K antagonists versus heparin, Outcome 4 Total VTE (symptomatic and asymptomatic).
Asymptomatic DVT
The SACRE Study did not report asymptomatic DVT.
Asymptomatic proximal DVT
The SACRE Study did not report asymptomatic proximal DVT.
Asymptomatic distal DVT
The SACRE Study did not report asymptomatic distal DVT.
All‐cause mortality
No cases of death were reported in the heparin group (0/643) compared with 2 cases in the VKA group (2/636). There was no difference in all‐cause mortality between the treatment groups (OR 5.07, 95% CI 0.24 to 105.83; participants = 1279; low quality evidence) Analysis 5.5.
5.5. Analysis.
Comparison 5 Vitamin K antagonists versus heparin, Outcome 5 All‐cause mortality.
Adverse events
The SACRE Study did not report adverse events.
Bleeding events (major, clinically relevant non‐major, minor)
There were no differences between the treatment groups in major or minor bleeding (OR 3.87, 95% CI 1.91 to 7.85; participants = 1272; low quality evidence, Analysis 5.6, and OR 1.33, 95% CI 0.64 to 2.76; participants = 1279; low quality evidence Analysis 5.7, respectively). The SACRE Study did not report clinically relevant non‐major bleeding.
5.6. Analysis.
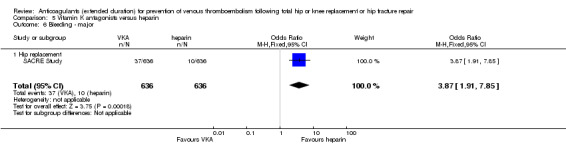
Comparison 5 Vitamin K antagonists versus heparin, Outcome 6 Bleeding ‐ major.
5.7. Analysis.
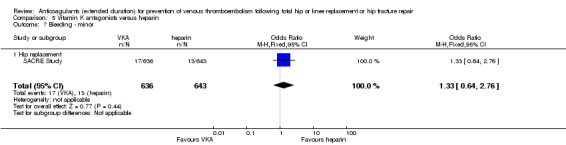
Comparison 5 Vitamin K antagonists versus heparin, Outcome 7 Bleeding ‐ minor.
Reoperation
The SACRE Study reported on reoperation as part of their definition of major bleeding outcome (bleeding leading to reoperation; reoperation was required in 11 participants (two in the reviparin group and nine in the acecoumarol group (OR 4.60, 95% CI 0.99 to 21.38; participants = 1279; low quality evidence).
Wound infection
The SACRE Study did not report wound infection.
Wound healing
The SACRE Study did not report wound healing.
DOACs versus heparin
Five studies evaluated DOACs versus heparin therapy (ADVANCE 3; EXTEND Study; RECORD 1 Trial; RE‐NOVATE II Trial; RE‐NOVATE Trial). The studies evaluating DOACs versus heparin were only performed in participants undergoing hip replacement, and therefore no subgroup analysis between hip and knee replacement was undertaken. As these studies compared two different anticoagulant treatments and randomised participants prior to surgery, there was no initial phase and objective assessment of VTE after the initial phase. Therefore these subgroup analyses were not possible. We found no difference between the treatment groups for most of the evaluated outcomes. Due to heterogeneity in the data, some of the meta‐analyses in this treatment profile used random‐effects models. See Table 6 and Appendix 7.
Symptomatic VTE (DVT and PE)
The random‐effects model evaluating five studies (ADVANCE 3; EXTEND Study; RECORD 1 Trial; RE‐NOVATE II Trial; RE‐NOVATE Trial) found no difference in symptomatic VTE between the treatment groups (OR 0.70, 95% CI 0.28 to 1.70; participants = 15,977; I2 = 55%; low quality evidence) Analysis 6.1.
6.1. Analysis.
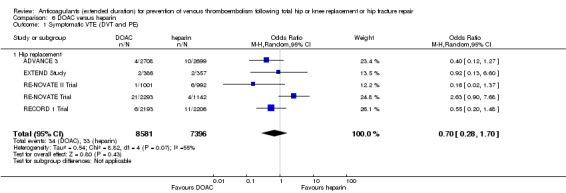
Comparison 6 DOAC versus heparin, Outcome 1 Symptomatic VTE (DVT and PE).
Symptomatic DVT
There was no difference between the treatment groups for symptomatic DVT, as evaluated by the random‐effects model in five studies (ADVANCE 3; EXTEND Study; RECORD 1 Trial; RE‐NOVATE II Trial; RE‐NOVATE Trial) (OR 0.60, 95% CI 0.11 to 3.27; participants = 15,977; I2 = 65%; low quality evidence) Analysis 6.2.
6.2. Analysis.
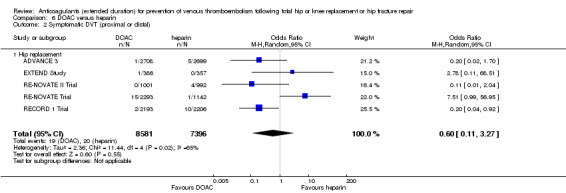
Comparison 6 DOAC versus heparin, Outcome 2 Symptomatic DVT (proximal or distal).
Symptomatic PE
No difference was found between the DOAC and LMWH treatment groups for the outcome of symptomatic PE (OR 0.91, 95% CI 0.43 to 1.94; participants = 14,731; studies = 5; I2 = 0%; moderate quality evidence) Analysis 6.3.
6.3. Analysis.
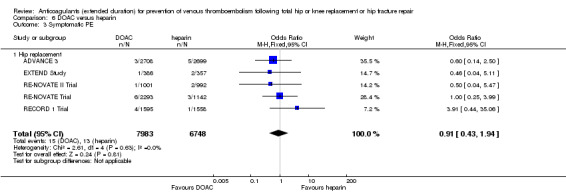
Comparison 6 DOAC versus heparin, Outcome 3 Symptomatic PE.
Total VTE (symptomatic or asymptomatic)
There was significant difference in total VTE in the random‐effects model evaluating four studies favouring DOACs (ADVANCE 3; RECORD 1 Trial; RE‐NOVATE II Trial; RE‐NOVATE Trial) (OR 0.53, 95% CI 0.29 to 0.97; participants = 12,447; I2 = 87%; moderate quality evidence) Analysis 6.4.
6.4. Analysis.
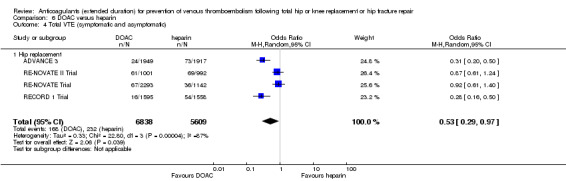
Comparison 6 DOAC versus heparin, Outcome 4 Total VTE (symptomatic and asymptomatic).
Asymptomatic DVT
In the random‐effects model, there was no difference in asymptomatic DVT between treatment groups in the two reporting studies (ADVANCE 3; RE‐NOVATE Trial) (OR 0.56, 95% CI 0.19 to 1.59; participants = 6559; I2 = 92%; low quality evidence) Analysis 6.5.
6.5. Analysis.
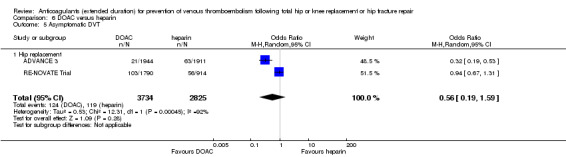
Comparison 6 DOAC versus heparin, Outcome 5 Asymptomatic DVT.
Asymptomatic proximal DVT
Only a single study (RE‐NOVATE Trial) reported on asymptomatic proximal DVT (OR 0.73, 95% CI 0.46 to 1.15; participants = 2704; moderate quality evidence) Analysis 6.6.
6.6. Analysis.
Comparison 6 DOAC versus heparin, Outcome 6 Asymptomatic proximal DVT.
Asymptomatic distal DVT
As for asymptomatic proximal DVT, a single study (RE‐NOVATE Trial) reported on asymptomatic distal DVT (OR 1.22, 95% CI 0.75 to 1.99; participants = 2639; moderate quality evidence) Analysis 6.7.
6.7. Analysis.
Comparison 6 DOAC versus heparin, Outcome 7 Asymptomatic distal DVT.
All‐cause mortality
For the five reporting studies (ADVANCE 3; EXTEND Study; RECORD 1 Trial; RE‐NOVATE II Trial; RE‐NOVATE Trial), there was no difference in all‐cause mortality between the treatment groups based on the fixed‐effect model ((OR 1.63, 95% CI 0.64 to 4.16; participants = 14,966; studies = 5; I2 = 0%; moderate quality evidence) Analysis 6.8. Regarding sensitivity analysis, the RECORD 1 Trial was found to account for 56% of the participants within this outcome, but after its removal no difference was seen in the findings.
6.8. Analysis.
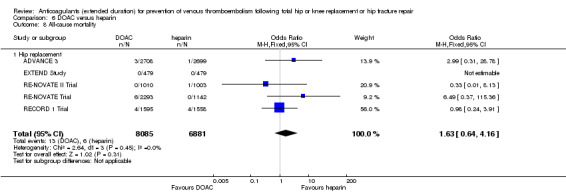
Comparison 6 DOAC versus heparin, Outcome 8 All‐cause mortality.
Adverse events
There was also no difference in adverse events between treatment groups in the three studies reporting adverse events (RECORD 1 Trial; RE‐NOVATE II Trial; RE‐NOVATE Trial) (OR 0.96, 95% CI 0.88 to 1.05; participants = 9908; studies = 3; I2 = 0%; high quality evidence) Analysis 6.9. Regarding sensitivity analysis, the RECORD 1 Trial was found to account for 50.8% of the participants within this outcome, but after its removal no difference was seen in the findings.
6.9. Analysis.
Comparison 6 DOAC versus heparin, Outcome 9 Adverse events.
Bleeding events (major, clinically relevant non‐major, minor)
Major bleeding was reported in five studies (ADVANCE 3; EXTEND Study; RECORD 1 Trial; RE‐NOVATE II Trial; RE‐NOVATE Trial), clinically relevant non‐major bleeding was reported in four studies (ADVANCE 3; RECORD 1 Trial; RE‐NOVATE II Trial; RE‐NOVATE Trial), and minor bleeding was reported in four studies (ADVANCE 3; EXTEND Study; RE‐NOVATE II Trial; RE‐NOVATE Trial).
There were no differences in any of the bleeding event categories within the studies reporting on bleeding events; major bleeding, OR 1.11, 95% CI 0.79 to 1.54; participants = 16,199; studies = 5; I2 = 21%, high quality evidence Analysis 6.10; clinically relevant bleeding OR 1.08, 95% CI 0.90 to 1.28; participants = 15,241; studies = 4; I2 = 7%, high quality evidence) Analysis 6.11; minor bleeding OR 0.95, 95% CI 0.82 to 1.10; participants = 11,766; studies = 4; I2 = 0%, high quality evidence Analysis 6.12.
6.10. Analysis.
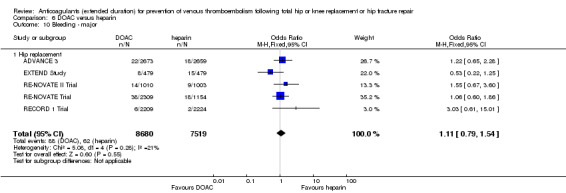
Comparison 6 DOAC versus heparin, Outcome 10 Bleeding ‐ major.
6.11. Analysis.
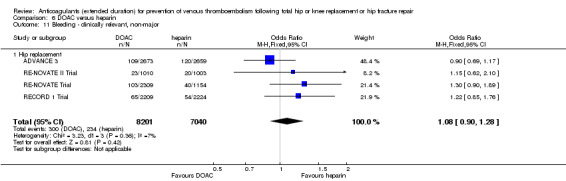
Comparison 6 DOAC versus heparin, Outcome 11 Bleeding ‐ clinically relevant, non‐major.
6.12. Analysis.
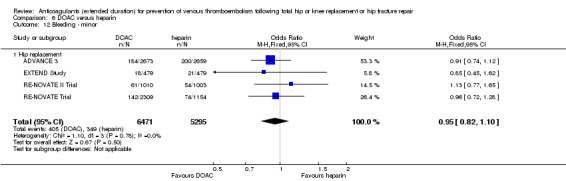
Comparison 6 DOAC versus heparin, Outcome 12 Bleeding ‐ minor.
For minor bleeding, ADVANCE 3 accounted for 53.3% of the participants, and when removed in a sensitivity analysis there were no differences in the association.
Reoperation
Four studies reported on reoperation relating to bleeding (ADVANCE 3; RECORD 1 Trial; RE‐NOVATE II Trial; RE‐NOVATE Trial) showing no differences between the study arms (OR 1.06, 95% CI 0.34 to 3.24; participants = 15241; studies = 4; I2 = 0%; moderate quality evidence) Analysis 6.13.
6.13. Analysis.
Comparison 6 DOAC versus heparin, Outcome 13 Reoperation.
The RE‐NOVATE Trial accounted for > 50 % of the weight and when removed in a sensitivity analysis there were no differences in the association.
Wound infection
Three studies reported on wound infection (RECORD 1 Trial; RE‐NOVATE II Trial; RE‐NOVATE Trial). The RE‐NOVATE Trial reported one participant who died of septicaemia and GI bleeding following ischaemic bowel resection but reported no other cases of wound infection. Pooling RECORD 1 Trial and RE‐NOVATE II Trial showed no differences in wound infections between the treatment arms (OR 0.89, 95% CI 0.46 to 1.72; participants = 6446; studies = 2; I2 = 0%; moderate quality evidence) Analysis 6.14.
6.14. Analysis.
Comparison 6 DOAC versus heparin, Outcome 14 Wound infection.
Wound healing
None of the five studies included in this comparison reported on wound healing (ADVANCE 3; EXTEND Study; RECORD 1 Trial; RE‐NOVATE II Trial; RE‐NOVATE Trial).
Reporting bias, subgroup and sensitivity analysis
We did not construct funnel plots for meta analyses because the meta analyses did not include at least 10 studies.
We planned to apply subgroup analysis to examine participants undergoing revisions of a previous hip or knee replacement or hip fracture repair, but we unfortunately found this to be impossible because these data were not reported separately. As the duration of the in‐hospital/initial phase ranged from four days to 14 days, subgroup analysis for 15 days or more was not required. Results of the other planned subgroup analyses are reported above. We did not consider any studies to be of low quality based on the 'Risk of bias' tool and therefore did not perform the planned sensitivity analysis for this reason.
Discussion
Summary of main results
We have shown that extended‐duration prophylaxis with DOACs in patients undergoing major hip or knee replacement surgery significantly reduces the risk of symptomatic VTE. This benefit is achieved with no excess adverse events or major bleeding but with increased minor bleeding. A significant reduction in PE or mortality could not be shown but the direction of the treatment effect for these outcomes was consistent with that seen for the primary outcome, suggesting that a similar reduction for these outcomes might be expected.
Our findings of a significant treatment benefit of extended‐duration prophylaxis for prevention of symptomatic VTE are based on a pooled analysis of both hip and knee replacement trials. However, our meta‐analysis cannot draw a fully informed conclusion for participants undergoing knee replacement as far fewer studies evaluated these types of participants separately, as compared with hip replacement.
When comparing extended‐duration anticoagulants (DOACs and VKA) versus extended‐duration LMWHs there were no differences between the treatments in symptomatic and asymptomatic VTE and showed decreased odds in total VTE for DOACs versus LMWH. There was also no difference in mortality, adverse events or bleeding events. These findings support the use of less invasive oral anticoagulants versus the more invasive LMWHs which often have to be administered by injection into subcutaneous tissue. However, it needs to be kept in mind that several of the meta‐analyses were underpowered and showed heterogeneity. We hope with the inclusion of new studies in future updates of this review we will be better able to draw stronger conclusions regarding these treatment comparisons.
One widely recognised impediment to the more widespread use of effective prophylaxis in patients undergoing hip or knee replacement is concern about the risk of bleeding. In this regard, the data from our review are reassuring in that, even with sustained use for up to six weeks, prophylactic‐dose anticoagulants is not associated with an excess in major bleeding in all comparisons except for the comparison of VKA versus heparin.
Overall completeness and applicability of evidence
This review included a total of 16 studies with nearly 25,000 randomised participants. Despite this large number of included studies some comparisons included a single study only, or few events were recorded, which led to wide confidence intervals, and difficulties interpreting the data.
There are other antithrombotic therapies besides the ones evaluated in this review that might be used for prevention of VTE in major orthopaedic surgery or out‐of‐hospital prophylaxis, for example aspirin, which were not addressed in this review. We plan to include these in future updates of this review.
Although compliance with extended‐duration prophylaxis seemed to be 90% or higher in most trials included in our meta‐analyses, the definition of compliance varied among the studies and not all studies reported the level of compliance with randomised treatment allocation that was achieved. However, the probable impact of lack of compliance is to reduce the power of a randomised trial to detect a significant treatment benefit. Therefore an even greater benefit of extended‐duration prophylaxis might be realised in populations in which higher levels of compliance are achieved.
Wang 2014 reported that surgical site infections and reoperations in the three months following joint replacements may be associated with anticoagulant use and this may be a barrier to extended thromboprophylaxis but our meta‐analyses are underpowered to draw conclusions on this issue due to a lack of reporting of wound infections, wound healing and reoperations in the studies included in this review.
Quality of the evidence
Despite examining the totality of the evidence by pooling results from all the available properly randomised trials, the total number of participants randomly assigned and the number of outcome events was modest. Therefore, our meta‐analyses potentially lacked statistical power to provide precise estimates of frequency and treatment effect for clinically important outcomes such as PE. However, DVT and PE represent clinical manifestations of the same underlying disease process. Therefore, strategies that are effective for the prevention of DVT, especially those proximally located, are likely also to be effective for the prevention of non‐fatal and fatal PE.
There was a lot of variation in the design of studies included in our meta‐analyses. However, differences among trials are inevitable since individual trials look at different populations with different treatment protocols, and there is always some heterogeneity, even within individual trials (Lau 1998; Thompson 1994). Differences in trial design do not necessarily preclude pooling of their results since, in a meta‐analysis, individual participants are directly compared only with other participants within the same trial, and not across the trials. The validity of our approach is further supported by the external consistency of our findings with the results of individual trials.
The quality of the evidence varied by comparison and was on average moderate. Heterogeneity was found between studies comparing DOAC and heparin, and for some outcomes, such as PE and mortality, few events were recorded during the study periods leading to imprecision. For three comparisons, only one study was identified. See Table 1; Table 2; Table 3; Table 4; Table 5; Table 6 for further details.
Potential biases in the review process
Every effort was made to limit potential biases in the review through duplication of study selection, data extraction and assessment of risk of bias by two review authors. Also, we utilised pre‐designed data extraction forms and discussed any disagreements and undertook a meticulous and exhaustive search for both published and unpublished studies.
Despite these techniques to reduce bias, there are still areas of concern. One issue was with the total numbers of participants used in the meta‐analyses. Most of the included studies had large numbers of participants that were excluded after randomisation. This was due mainly to detecting DVT at discharge from the hospital, non‐compliance, adverse events and non‐evaluable objective testing of DVT. Due to these large numbers of exclusions it was inappropriate to use all randomised participants, and we therefore attempted to use the most reliable intention‐to‐treat population as reported by the study authors. This method required us to adapt the numbers used by the study authors, which did vary between studies.
We did not stratify according to the use of mechanical prophylaxis since, in most studies, the use of mechanical prophylaxis was left to the discretion of the local investigator and is usually not reported separately in the final study report. However, this should not lead to any bias because the use of mechanical methods is likely to be balanced between the two groups as a result of proper use of randomisation.
Agreements and disagreements with other studies or reviews
A recently published meta‐analysis evaluated extended duration using new oral anticoagulants versus extended duration using LMWHs (Liew 2014). The four included studies are also included in our review. For symptomatic VTE they found a risk ratio in favour of oral anticoagulants of 0.42 (95% CI 0.21 to 0.86). Although Liew 2014 included RE‐NOVATE Trial in their review, they did not include this study in the evaluation of symptomatic VTE, which led to the major differences in our findings, as the two dabigatran treatment groups had a large number of VTEs, compared with the LMWH group. Liew 2014 also did not include the SACRE Study. Liew 2014 found no difference in mortality or bleeding events, which is similar to our own review.
A meta‐analysis published in 2001 compared extended‐duration prophylaxis to short duration followed by placebo or no treatment (Eikelboom 2001). Eikelboom 2001 included nine studies, five of which were also included in our review and the other four we excluded because we deemed they did not meet the five‐week treatment‐duration requirement for this review. Eikelboom 2001's findings were similar to ours, with a decreased odds of symptomatic DVT in the extended duration treatment group (OR 0.38, 95% CI 0.24 to 0.61), no difference in major bleeding but an increase in minor bleeding.
Authors' conclusions
Implications for practice.
Extended‐duration thromboprophylaxis should be considered for people undergoing hip or knee replacement, but the risk of increased minor bleeding should be carefully considered.
Implications for research.
Continued research in this area is needed, specifically as newer, and possibly safer medications become available. Oral anticoagulants could be a promising alternative to LMWHs that require daily injections, but the evidence is currently insufficient. Specifically, further research is required to better understand the association between VTE and extended‐duration oral anticoagulants in relation to knee replacement and hip fracture repair as well as outcomes such as distal and proximal DVT, reoperation, wound infection and healing, regarding the optimal duration of extended therapy, possibility of combining therapies and effects of extended therapies on length of hospital stay. An assessment of cost‐effectiveness as part of the further research is also warranted.
Feedback
Anticoagulant feedback, 14 February 2011
Summary
Feedback received on this protocol, and other reviews and protocols on anticoagulants, is available on the Cochrane Editorial Unit website at http://www.editorial‐unit.cochrane.org/anticoagulants‐feedback.
History
Protocol first published: Issue 2, 2003 Review first published: Issue 3, 2016
| Date | Event | Description |
|---|---|---|
| 14 February 2011 | Amended | Link to anticoagulant feedback added |
| 1 October 2008 | Amended | Converted to new review format. |
| 11 January 2002 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The review authors thank Dr DJ Quinlan, Dr JW Eikelboom, and Dr JD Douketis for their work on the protocol of this review and Prof G Stansby and Dr R Hughes for their clinical input.
Appendices
Appendix 1. CENTRAL search strategy
| #1 | MeSH descriptor: [Thrombosis] this term only | 1309 |
| #2 | MeSH descriptor: [Thromboembolism] this term only | 1067 |
| #3 | MeSH descriptor: [Venous Thromboembolism] this term only | 461 |
| #4 | MeSH descriptor: [Venous Thrombosis] this term only | 1060 |
| #5 | (thromboprophyla* or thrombus* or thrombotic* or thrombolic* or thromboemboli* or thrombos* or embol*):ti,ab,kw | 17234 |
| #6 | MeSH descriptor: [Pulmonary Embolism] explode all trees | 944 |
| #7 | PE or DVT or VTE:ti,ab,kw | 4161 |
| #8 | (vein* or ven*) near thromb*:ti,ab,kw | 6917 |
| #9 | #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 | 20147 |
| #10 | MeSH descriptor: [Anticoagulants] explode all trees | 4058 |
| #11 | anticoagul* or anti‐coagu* or antithrombotic* | 9328 |
| #12 | warfarin or (vitamin near/3 antagonist*) or VKA or Nicoumalone or phenindione or acenocoumarol* or Sinthrome or dicoumarol* or nicoumalone or phenprocoumon or Marcoumar or Marcumar or Falithrom or AVK or bishydroxycoumarin* or coumarin* or coumadin* or phenprocoumon* | 4024 |
| #13 | Ximelagatran or Exanta or Exarta or H 376/95 or dabigatran or rivaroxaban or Xarelto | 874 |
| #14 | fondaparinux or Arixtra or BAY59‐7939 or TTP889 or odiparcil or LY517717 or YM150 or DU‐176b | 395 |
| #15 | apixaban or betrixaban or edoxaban or idraparinux | 402 |
| #16 | LMWH or UFH or heparin or nadroparin* or fraxiparin* or enoxaparin | 9899 |
| #17 | Clexane or klexane or lovenox or dalteparin or Fragmin or ardeparin | 762 |
| #18 | normiflo or tinzaparin or logiparin or Innohep or certoparin or sandoparin or reviparin or clivarin* | 432 |
| #19 | danaproid or danaparoid or antixarin or ardeparin* or bemiparin* | 136 |
| #20 | Zibor or cy 222 or embolex or monoembolex or parnaparin* | 128 |
| #21 | rd 11885 or tedelparin or Kabi‐2165 or Kabi 2165 | 78 |
| #22 | emt‐966 or emt‐967 or "pk‐10 169" or pk‐10169 or pk10169 or cy‐216 or cy216 | 83 |
| #23 | seleparin* or tedegliparin or seleparin* or tedegliparin* | 19 |
| #24 | wy90493 or "wy 90493" or "kb 101" or kb101 | 21 |
| #25 | lomoparan or orgaran or parnaparin or fluxum or lohepa or lowhepa or "op 2123" or parvoparin or AVE5026 | 113 |
| #26 | #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 | 17458 |
| #27 | MeSH descriptor: [Hip] explode all trees | 309 |
| #28 | MeSH descriptor: [Knee] explode all trees | 584 |
| #29 | hip:ti,ab,kw (Word variations have been searched) | 11495 |
| #30 | knee:ti,ab,kw (Word variations have been searched) | 13133 |
| #31 | orthop?edic | 6896 |
| #32 | MeSH descriptor: [Orthopedic Procedures] explode all trees | 10000 |
| #33 | #27 or #28 or #29 or #30 or #31 or #32 | 31372 |
| #34 | #9 and #26 and #33 | 1182 |
Appendix 2. Additional Summary of findings table: Heparin compared to placebo for prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair
| Heparin compared to placebo for prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair | ||||||
| Patient or population: people requiring prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair Setting: hospital and outpatient setting Intervention: heparin Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with heparin | |||||
| Total VTE (symptomatic and asymptomatic) Treatment duration 28 ‐ 42 days |
Study population | OR 0.39 (0.28 to 0.56) | 2544 (6 RCTs) | ⊕⊕⊕⊕ HIGH | — | |
| 83 per 1000 | 34 per 1000 (25 to 48) | |||||
| Asymptomatic DVT Treatment duration 28 ‐ 42 days |
Study population | OR 0.38 (0.24 to 0.60) | 1304 (5 RCTs) | ⊕⊕⊕⊕ HIGH | — | |
| 112 per 1000 | 46 per 1000 (29 to 71) | |||||
| Asymptomatic proximal DVT Treatment duration 28 ‐ 42 days |
see comment | not estimable | — | — | asymptomatic and proximal DVT reported separately but due to available data it was not possible to determine which events fell into which category | |
| Asymptomatic distal DVT Treatment duration 28 ‐ 42 days |
see comment | not estimable | — | — | asymptomatic and distal DVT reported separately but due to available data it was not possible to determine which events fell into which category | |
| All‐cause mortality Treatment duration 28 ‐ 42 days |
Study population | OR 1.01 (0.31 to 3.26) | 2518 (5 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | — | |
| 4 per 1000 | 4 per 1000 (1 to 11) | |||||
| Adverse events Treatment duration 28 ‐ 42 days |
Study population | OR 1.06 (0.68 to 1.64) | 460 (2 RCTs) | ⊕⊕⊕⊝ MODERATE 2 | — | |
| 270 per 1000 | 281 per 1000 (201 to 377) | |||||
| Reoperation Treatment duration 28 ‐ 42 days | see comment | — | 179 (1 RCT) | — | one study reported no operations in either study arm. Three studies did not report on reoperation and two studies did not report sufficient data to analyse | |
| Wound infection Treatment duration 28 ‐ 42 days |
see comment | — | — | — | four studies did not report on wound infection. Two studies did not provide specific details for wound infection | |
| Wound healing Treatment duration 28 ‐ 42 days |
see comment | — | — | — | not reported | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DVT: deep vein thrombosis; OR: odds ratio; PE: pulmonary embolism; RCT: randomised controlled trial; VTE: venous thromboembolism | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded by one level, low number of events leading to imprecision of results 2 Downgraded by one level due to imprecision
Appendix 3. Additional Summary of findings table: Vitamin K antagonists compared to placebo for prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair
| Vitamin K antagonists compared to placebo for prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair | ||||||
| Patient or population: people requiring prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair Setting: hospital and outpatient setting Intervention: vitamin K antagonists Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with vitamin K antagonists | |||||
| Total VTE (symptomatic and asymptomatic) Treatment duration 28 ‐ 42 days | Study population | OR 0.10 (0.01 to 0.81) | 360 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | — | |
| 51 per 1000 | 5 per 1000 (1 to 42) | |||||
| Asymptomatic DVT Treatment duration 28 ‐ 42 days |
see comment | — | — | — | not reported in single included study in this comparison | |
| Asymptomatic proximal DVT Treatment duration 28 ‐ 42 days |
see comment | — | — | — | not reported in single included study in this comparison | |
| Asymptomatic distal DVT Treatment duration 28 ‐ 42 days |
see comment | — | — | — | not reported in single included study in this comparison | |
| All‐cause mortality Treatment duration 28 ‐ 42 days |
see comment | — | 360 (1 RCT) | — | single included study reported no deaths in either study arm so not possible to assess risk | |
| Adverse events Treatment duration 28 ‐ 42 days |
see comment | — | 360 (1 RCT) | — | single included study reported no adverse events in either study arm so not possible to assess risk | |
| Reoperation Treatment duration 28 ‐ 42 days | see comment | — | — | — | not reported in single included study in this comparison | |
| Wound infection Treatment duration 28 ‐ 42 days |
see comment | — | — | — | not reported in single included study in this comparison | |
| Wound healing Treatment duration 28 ‐ 42 days |
see comment | — | — | — | not reported in single included study in this comparison | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DVT: deep vein thrombosis; OR: odds ratio; PE: pulmonary embolism; RCT: randomised controlled trial; VTE: venous thromboembolism | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded by one level, results from a single study only so heterogeneity could not be assessed
Appendix 4. Additional Summary of findings table: DOAC compared to placebo for prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair
| DOAC compared to placebo for prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair | ||||||
| Patient or population: people requiring prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair Setting: hospital and outpatient setting Intervention: DOAC Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with DOAC | |||||
| Total VTE (symptomatic and asymptomatic) Treatment duration 28 ‐ 42 days |
Study population | OR 0.19 (0.11 to 0.33) | 1733 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | — | |
| 86 per 1000 | 18 per 1000 (10 to 30) | |||||
| Asymptomatic DVT Treatment duration 28 ‐ 42 days |
see comment | — | — | — | not reported in single included study in this comparison | |
| Asymptomatic proximal DVT Treatment duration 28 ‐ 42 days |
see comment | — | — | — | not reported in single included study in this comparison | |
| Asymptomatic distal DVT Treatment duration 28 ‐ 42 days |
see comment | — | — | — | not reported in single included study in this comparison | |
| All‐cause mortality Treatment duration 28 ‐ 42 days |
Study population | OR 0.33 (0.07 to 1.66) | 1733 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | — | |
| 7 per 1000 | 2 per 1000 (0 to 11) | |||||
| Adverse events Treatment duration 28 ‐ 42 days |
Study population | OR 0.87 (0.74 to 1.03) | 2457 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | — | |
| 657 per 1000 | 625 per 1000 (586 to 663) | |||||
| Reoperation Treatment duration 28 ‐ 42 days | see comment | — | 2457 (1 RCT) | — | single study reported no cases of reoperation in the study arms | |
| Wound infection Treatment duration 28 ‐ 42 days |
Study population | OR 1.34 (0.46 to 3.86) | 2457 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | — | |
| 5 per 1000 | 7 per 1000 (2 to 19) | |||||
| Wound healing Treatment duration 28 ‐ 42 days |
see comment | — | — | — | not reported in single included study in this comparison | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DOAC: direct oral anticoagulant; DVT: deep vein thrombosis; OR: odds ratio; PE: pulmonary embolism; RCT: randomised controlled trial; VTE: venous thromboembolism | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded by one level, results from a single study so heterogeneity cannot be assessed 2 Downgraded by one level, low number of events leading to wide CI and imprecision of results
Appendix 5. Additional Summary of findings table: Anticoagulants (chosen at investigators' discretion) compared to placebo for prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair
| Anticoagulants (chosen at investigators' discretion) compared to placebo for prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair | ||||||
| Patient or population: people requiring prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair Setting: hospital and outpatient setting Intervention: anticoagulant (chosen at investigators' discretion) Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with anticoagulant (chosen at investigators' discretion) | |||||
| Total VTE (symptomatic and asymptomatic) Treatment duration 28 ‐ 42 days |
Study population | OR 0.26 (0.14 to 0.50) | 557 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | — | |
| 158 per 1000 | 46 per 1000 (26 to 86) | |||||
| Asymptomatic DVT Treatment duration 28 ‐ 42 days |
Study population | OR 0.26 (0.13 to 0.54) | 557 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | — | |
| 125 per 1000 | 36 per 1000 (18 to 72) | |||||
| Asymptomatic proximal DVT Treatment duration 28 ‐ 42 days |
see comment | — | — | — | not reported in single included study in this comparison | |
| Asymptomatic distal DVT Treatment duration 28 ‐ 42 days |
Study population | OR 0.26 (0.13 to 0.54) | 557 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | — | |
| 125 per 1000 | 36 per 1000 (18 to 72) | |||||
| All‐cause mortality Treatment duration 28 ‐ 42 days |
see comment | — | 842 (1 RCT) | — | the single included study reported no deaths in either study arm | |
| Adverse events Treatment duration 28 ‐ 42 days |
see comment | — | — | — | not reported in single included study in this comparison | |
| Reoperation Treatment duration 28 ‐ 42 days | see comment | — | — | — | outcome reported as part of the definition of the outcome major bleeding but data not reported separately | |
| Wound infection Treatment duration 28 ‐ 42 days |
see comment | — | — | — | not reported in single included study in this comparison | |
| Wound healing Treatment duration 28 ‐ 42 days |
see comment | — | — | — | not reported in single included study in this comparison | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Ccnfidence interval; DVT: deep vein thrombosis; OR: odds ratio; PE: pulmonary embolism; RCT: randomised controlled trial; VTE: venous thromboembolism | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded by one level, results from a single study so heterogeneity could not be assessed
Appendix 6. Additional Summary of findings table: Vitamin K antagonists compared to heparin for prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair
| Vitamin K antagonists compared to heparin for prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair | ||||||
| Patient or population: people requiring prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair Setting: hospital and outpatient setting Intervention: vitamin K antagonists Comparison: heparin | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with heparin | Risk with vitamin K antagonists | |||||
| Total VTE (symptomatic and asymptomatic) Treatment duration 28 ‐ 42 days |
Study population | OR 1.64 (0.85 to 3.16) | 1279 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | — | |
| 23 per 1000 | 38 per 1000 (20 to 70) | |||||
| Asymptomatic DVT Treatment duration 28 ‐ 42 days |
see comment | — | — | — | not reported in single included study in this comparison | |
| Asymptomatic proximal DVT Treatment duration 28 ‐ 42 days |
see comment | — | — | — | not reported in single included study in this comparison | |
| Asymptomatic distal DVT Treatment duration 28 ‐ 42 days |
see comment | — | — | — | not reported in single included study in this comparison | |
| All‐cause mortality Treatment duration 28 ‐ 42 days |
see comment | OR 5.07 (0.24 to 105.83) | 1279 (1 RCT) | ⊕⊕⊝⊝ LOW 1 3 | no cases of death reported in the heparin study arm | |
| Adverse events Treatment duration 28 ‐ 42 days |
see comment | — | — | — | not reported in single included study in this comparison | |
| Reoperation Treatment duration 28 ‐ 42 days | Study population | OR 4.60 (0.99 to 21.38) | 1279 (1 RCT) | ⊕⊕⊝⊝ LOW 1 3 | — | |
| 3 per 1000 | 14 per 1000 (3 to 63) | |||||
| Wound infection Treatment duration 28 ‐ 42 days |
see comment | — | — | — | not reported in single included study in this comparison | |
| Wound healing Treatment duration 28 ‐ 42 days |
see comment | — | — | — | not reported in single included study in this comparison | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DVT: deep vein thrombosis; OR: odds ratio; PE: pulmonary embolism; RCT: randomised controlled trial; VKA: vitamin K antagonist; VTE: venous thromboembolism | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded by one level, single study so heterogeneity could not be assessed 2 Downgraded by one level, wide CI 3 Downgraded by one level, low number of events leading to imprecision of results
Appendix 7. Additional Summary of findings table: DOAC compared to heparin for people requiring prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair
| DOAC compared to heparin for people requiring prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair | ||||||
| Patient or population: people requiring prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair Setting: hospital and outpatient setting Intervention: DOAC Comparison: heparin | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with heparin | Risk with DOAC | |||||
| Total VTE (symptomatic and asymptomatic) Treatment duration 28 ‐ 42 days |
Study population | OR 0.53 (0.29 to 0.97) | 12447 (4 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | — | |
| 41 per 1000 | 22 per 1000 (12 to 40) | |||||
| Asymptomatic DVT Treatment duration 28 ‐ 42 days |
Study population | OR 0.56 (0.19 to 1.59) | 6559 (2 RCTs) | ⊕⊕⊝⊝ LOW 2 | — | |
| 42 per 1000 | 24 per 1000 (8 to 65) | |||||
| Asymptomatic proximal DVT Treatment duration 28 ‐ 42 days |
Study population | OR 0.73 (0.46 to 1.15) | 2704 (1 RCT) | ⊕⊕⊕⊝ MODERATE 3 | — | |
| 35 per 1000 | 26 per 1000 (16 to 40) | |||||
| Asymptomatic distal DVT Treatment duration 28 ‐ 42 days |
Study population | OR 1.22 (0.75 to 1.99) | 2639 (1 RCT) | ⊕⊕⊕⊝ MODERATE 3 | — | |
| 27 per 1000 | 33 per 1000 (20 to 52) | |||||
| All‐cause mortality Treatment duration 28 ‐ 42 days |
Study population | OR 1.63 (0.64 to 4.16) | 14966 (5 RCTs) | ⊕⊕⊕⊝ MODERATE 4 | — | |
| 1 per 1000 | 1 per 1000 (1 to 4) | |||||
| Adverse events Treatment duration 28 ‐ 42 days |
Study population | OR 0.96 (0.88 to 1.05) | 9908 (3 RCTs) | ⊕⊕⊕⊕ HIGH | — | |
| 691 per 1000 | 682 per 1000 (663 to 701) | |||||
| Reoperation Treatment duration 28 ‐ 42 days |
Study population | OR 1.06 (0.34 to 3.24) | 15241 (4 RCTs) | ⊕⊕⊕⊝ MODERATE 4 | — | |
| 1 per 1000 | 1 per 1000 (0 to 2) | |||||
| Wound infection Treatment duration 28 ‐ 42 days |
Study population | OR 0.89 (0.46 to 1.72) | 6446 (2 RCTs) | ⊕⊕⊕⊝ MODERATE 4 | — | |
| 6 per 1000 | 5 per 1000 (3 to 10) | |||||
| Wound healing Treatment duration 28 ‐ 42 days |
see comment | — | — | — | not reported in five included studies in this comparison | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DOAC: direct oral anticoagulant; DVT: deep vein thrombosis; OR: odds ratio; PE: pulmonary embolism; RCT: randomised controlled trial; VTE: venous thromboembolism | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded by one level for inconsistency (heterogeneity, I2 = 87%) 2 Downgraded by two levels for serious inconsistency (heterogeneity, I2 = 92%) 3 Downgraded by one level, single included study so unable to assess heterogeneity 4 Downgraded by one level, few events leading to wide CI and imprecision
Data and analyses
Comparison 1. Heparin versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptomatic VTE (DVT and PE) | 5 | 2329 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.35, 1.01] |
| 1.1 Hip replacement | 4 | 1296 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.36, 1.30] |
| 1.2 Knee replacement | 1 | 723 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.21, 2.92] |
| 1.3 Hip or knee replacement | 1 | 310 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.05, 1.06] |
| 2 Symptomatic DVT (proximal or distal) | 4 | 2019 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.39, 1.38] |
| 2.1 Hip replacement | 4 | 1296 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.41, 1.55] |
| 2.2 Knee replacement | 1 | 723 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.03, 3.12] |
| 3 Symptomatic PE | 3 | 1595 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.16, 2.33] |
| 3.1 Hip replacement | 3 | 872 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 2.56] |
| 3.2 Knee replacement | 1 | 723 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.24, 8.83] |
| 4 Total VTE (symptomatic and asymptomatic) | 6 | 2544 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.28, 0.56] |
| 4.1 Hip replacement | 5 | 1511 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.25, 0.56] |
| 4.2 Knee replacement | 1 | 723 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.21, 2.92] |
| 4.3 Hip or knee replacement | 1 | 310 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.16, 0.90] |
| 5 Asymptomatic DVT | 5 | 1304 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.24, 0.60] |
| 5.1 Hip replacement | 4 | 994 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.21, 0.58] |
| 5.2 Hip or knee replacement | 1 | 310 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.19, 1.52] |
| 6 All‐cause mortality | 5 | 2518 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.31, 3.26] |
| 6.1 Hip replacement | 4 | 1485 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.11, 2.75] |
| 6.2 Knee replacement | 1 | 723 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.06, 15.65] |
| 6.3 Hip or knee replacement | 1 | 310 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.69 [0.22, 98.42] |
| 7 Adverse events | 2 | 460 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.68, 1.64] |
| 7.1 Hip replacement | 2 | 460 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.68, 1.64] |
| 8 Bleeding ‐ major | 5 | 2500 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.14, 2.46] |
| 8.1 Hip replacement | 4 | 1494 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.03, 3.10] |
| 8.2 Knee replacement | 1 | 696 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.14, 7.06] |
| 8.3 Hip or knee replacement | 1 | 310 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Bleeding ‐ minor | 5 | 2500 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.01 [1.43, 2.81] |
| 9.1 Hip replacement | 4 | 1494 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.25 [1.53, 3.30] |
| 9.2 Knee replacement | 1 | 696 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.58, 2.59] |
| 9.3 Hip or knee replacement | 1 | 310 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.79 [0.11, 69.13] |
| 10 Reoperation | 1 | 179 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Hip replacement | 1 | 179 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Vitamin K antagonists versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptomatic VTE (DVT and PE) | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 1.94] |
| 1.1 Hip replacement | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 1.94] |
| 2 Symptomatic DVT (proximal or distal) | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 2.62] |
| 2.1 Hip replacement | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 2.62] |
| 3 Symptomatic PE | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.84] |
| 3.1 Hip replacement | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.84] |
| 4 Total VTE (symptomatic and asymptomatic) | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 0.81] |
| 4.1 Hip replacement | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 0.81] |
| 5 All‐cause mortality | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Hip replacement | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Adverse events | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 Hip replacement | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Bleeding ‐ major | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.89 [0.12, 71.31] |
| 7.1 Hip replacement | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.89 [0.12, 71.31] |
Comparison 3. DOAC versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptomatic VTE (DVT and PE) | 1 | 2419 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.06, 0.68] |
| 1.1 Hip replacement | 1 | 2419 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.06, 0.68] |
| 2 Symptomatic DVT (proximal or distal) | 2 | 2459 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.04, 0.81] |
| 2.1 Hip replacement | 2 | 2459 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.04, 0.81] |
| 3 Symptomatic PE | 1 | 1733 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.25] |
| 3.1 Hip replacement | 1 | 1733 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.25] |
| 4 Total VTE (symptomatic and asymptomatic) | 1 | 1733 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.11, 0.33] |
| 4.1 Hip replacement | 1 | 1733 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.11, 0.33] |
| 5 All‐cause mortality | 1 | 1733 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.07, 1.66] |
| 5.1 Hip replacement | 1 | 1733 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.07, 1.66] |
| 6 Adverse events | 1 | 2457 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.74, 1.03] |
| 6.1 Hip replacement | 1 | 2457 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.74, 1.03] |
| 7 Bleeding ‐ major | 1 | 2457 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.06, 16.02] |
| 7.1 Hip replacement | 1 | 2457 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.06, 16.02] |
| 8 Bleeding‐ clinically relevant non‐major | 1 | 2457 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.76, 1.95] |
| 8.1 Hip replacement | 1 | 2457 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.76, 1.95] |
| 9 Bleeding ‐ minor | 1 | 2457 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.74, 1.88] |
| 9.1 Hip replacement | 1 | 2457 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.74, 1.88] |
| 10 Reoperation | 1 | 2457 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Hip replacement | 1 | 2457 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Wound infection | 1 | 2457 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.46, 3.86] |
| 11.1 Hip replacement | 1 | 2457 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.46, 3.86] |
Comparison 4. Anticoagulant (chosen at investigators' discretion) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptomatic VTE (DVT and PE) | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.09, 2.74] |
| 1.1 Knee replacement | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.09, 2.74] |
| 2 Symptomatic DVT (proximal or distal) | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.03, 3.21] |
| 2.1 Knee replacement | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.03, 3.21] |
| 3 Symptomatic PE | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.06, 16.13] |
| 3.1 Knee replacement | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.06, 16.13] |
| 4 Total VTE (symptomatic and asymptomatic) | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.14, 0.50] |
| 4.1 Knee replacement | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.14, 0.50] |
| 5 Asymptomatic DVT | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.13, 0.54] |
| 5.1 Knee replacement | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.13, 0.54] |
| 6 Asymptomatic distal DVT | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.13, 0.54] |
| 6.1 Knee replacement | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.13, 0.54] |
| 7 All‐cause mortality | 1 | 842 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 Knee replacement | 1 | 842 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Bleeding ‐ major | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.05 [0.24, 105.76] |
| 8.1 Knee replacement | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.05 [0.24, 105.76] |
Comparison 5. Vitamin K antagonists versus heparin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptomatic VTE (DVT and PE) | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.85, 3.16] |
| 1.1 Hip replacement | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.85, 3.16] |
| 2 Symptomatic DVT (proximal or distal) | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.69, 2.68] |
| 2.1 Hip replacement | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.69, 2.68] |
| 3 Symptomatic PE | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 9.16 [0.49, 170.42] |
| 3.1 Hip replacement | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 9.16 [0.49, 170.42] |
| 4 Total VTE (symptomatic and asymptomatic) | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.85, 3.16] |
| 4.1 Hip replacement | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.85, 3.16] |
| 5 All‐cause mortality | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.07 [0.24, 105.83] |
| 5.1 Hip replacement | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.07 [0.24, 105.83] |
| 6 Bleeding ‐ major | 1 | 1272 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.87 [1.91, 7.85] |
| 6.1 Hip replacement | 1 | 1272 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.87 [1.91, 7.85] |
| 7 Bleeding ‐ minor | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.64, 2.76] |
| 7.1 Hip replacement | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.64, 2.76] |
| 8 Reoperation | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.60 [0.99, 21.38] |
| 8.1 Hip replacement | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.60 [0.99, 21.38] |
5.8. Analysis.
Comparison 5 Vitamin K antagonists versus heparin, Outcome 8 Reoperation.
Comparison 6. DOAC versus heparin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptomatic VTE (DVT and PE) | 5 | 15977 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.28, 1.70] |
| 1.1 Hip replacement | 5 | 15977 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.28, 1.70] |
| 2 Symptomatic DVT (proximal or distal) | 5 | 15977 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.11, 3.27] |
| 2.1 Hip replacement | 5 | 15977 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.11, 3.27] |
| 3 Symptomatic PE | 5 | 14731 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.43, 1.94] |
| 3.1 Hip replacement | 5 | 14731 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.43, 1.94] |
| 4 Total VTE (symptomatic and asymptomatic) | 4 | 12447 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.29, 0.97] |
| 4.1 Hip replacement | 4 | 12447 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.29, 0.97] |
| 5 Asymptomatic DVT | 2 | 6559 | Odds Ratio (M‐H, Random, 95% CI) | 0.56 [0.19, 1.59] |
| 5.1 Hip replacement | 2 | 6559 | Odds Ratio (M‐H, Random, 95% CI) | 0.56 [0.19, 1.59] |
| 6 Asymptomatic proximal DVT | 1 | 2704 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.46, 1.15] |
| 6.1 Hip replacement | 1 | 2704 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.46, 1.15] |
| 7 Asymptomatic distal DVT | 1 | 2639 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.75, 1.99] |
| 7.1 Hip replacement | 1 | 2639 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.75, 1.99] |
| 8 All‐cause mortality | 5 | 14966 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.63 [0.64, 4.16] |
| 8.1 Hip replacement | 5 | 14966 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.63 [0.64, 4.16] |
| 9 Adverse events | 3 | 9908 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.88, 1.05] |
| 9.1 Hip replacement | 3 | 9908 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.88, 1.05] |
| 10 Bleeding ‐ major | 5 | 16199 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.79, 1.54] |
| 10.1 Hip replacement | 5 | 16199 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.79, 1.54] |
| 11 Bleeding ‐ clinically relevant, non‐major | 4 | 15241 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.90, 1.28] |
| 11.1 Hip replacement | 4 | 15241 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.90, 1.28] |
| 12 Bleeding ‐ minor | 4 | 11766 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.82, 1.10] |
| 12.1 Hip replacement | 4 | 11766 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.82, 1.10] |
| 13 Reoperation | 4 | 15241 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.34, 3.24] |
| 13.1 Hip replacement | 4 | 15241 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.34, 3.24] |
| 14 Wound infection | 2 | 6446 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.46, 1.72] |
| 14.1 Hip replacement | 2 | 6446 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.46, 1.72] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
ADVANCE 3.
| Methods |
Study design: Randomised, double‐blind, double‐dummy, clinical trial Country: Multi‐Country (21) Setting: Multicentre (160 sites); home and hospital; March 2007 to May 2009 Intention‐to‐treat: Yes, primary analysis on all except those that had no or venograms or uninterpretable venography, also per‐protocol efficacy analysis excluding protocol violations ‐ protocol violations not described ‐ safety data ITT minus those that did not receive at least one dose For the purposes of our meta‐analyses we used a mixture of all randomised and ITT population, as reported appropriate by the study authors |
|
| Participants |
Number randomised: Total n = 5407 (apixaban n = 2708; enoxaparin n = 2699) Exclusions post randomisation: Total n = 1616 (Apixaban: 363 had no venography, 396 had uninterpretable venograms (efficacy), 35 did not have at least one dose (safety). Enoxaparin: 40 did not receive at least one dose (safety); 364 no venography, 418 uninterpretable venogram (efficacy)) Losses to follow up: No reports of loss to follow up Age mean (range): Apixaban 60.9 (19 ‐ 92); enoxaparin 60.6 (19 ‐ 93) Sex (%F): Apixaban 52.8%; enoxaparin 53.8% Inclusion criteria: Patients scheduled to undergo elective total hip replacement or revision of previously inserted hip replacement Exclusion criteria: Main reasons for exclusion: active bleeding; contraindication to anticoagulant prophylaxis; need for ongoing anticoagulant or antiplatelet treatment |
|
| Interventions |
Treatment: 2.5 mg apixaban, orally, twice daily, initiated 12 ‐ 24 hours after surgery Control: 40 mg enoxaparin, subcutaneously, once daily, initiated 12 hours before surgery Duration: Prophylaxis continued for 35 days (32 to 38 day range) after surgery; follow‐up evaluations also occurred at 65 and 95 days after surgery |
|
| Outcomes |
Primary: Composite of asymptomatic or symptomatic DVT, nonfatal PE, or death from any cause; bleeding during treatment period (classed as major, clinically relevant non‐major and minor bleeding, and composite of major and clinically relevant) Secondary: Major VTE: composite of symptomatic or asymptomatic proximal DVT, nonfatal PE or death related to VTE Bleeding definitions: Major bleeding ‐ acute, clinically overt bleeding accompanied by one or more of the following: a decrease in haemoglobin level of 2 g/dL or more over 24 hours; transfusion of 2 or more units of packed red cells; bleeding into the operated joint necessitating reoperation or intervention; intramuscular bleeding; fatal bleeding; Minor bleeding ‐ clinically overt but did not meet the criteria for major or clinically relevant non‐major bleeding; Clinically relevant non‐major bleeding ‐ acute, clinically overt episodes such as wound haematoma, bruising or ecchymosis, gastrointestinal bleeding, haemoptysis, haematuria or epistaxis that did not meet the criteria for major bleeding |
|
| Notes |
Funding: Bristol‐Myers Squibb and Pfizer Method of VTE evaluation/confirmation: bilateral venography was performed after 35 days |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using interactive telephone system, randomisation schedule generated at the randomisation centre of Bristol‐Myers Squib with the use of SAS software and was stratified according to study site, with a block size of four |
| Allocation concealment (selection bias) | Low risk | Interactive telephone system |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as double‐blind, double‐dummy, participants received either placebo tablets or injections based on treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All VTE, bleeding and adverse events were reviewed by an independent, blinded, adjudication committee |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All losses‐to‐follow‐up were recorded per treatment group with adequate reasoning |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on |
| Other bias | Unclear risk | Reasons for protocol violations not described; Funded by Bristol‐Myers Squibb and Pfizer; data were collected and monitored by the sponsors, and data and safety monitoring board were given a fee by the sponsors |
Barrellier 2010.
| Methods |
Study design: Randomised, open‐label, prospective, non‐inferiority, parallel group trial Country: France Setting: Multicentre (17 centres); hospital and home; June 2004 to June 2007 Intention‐to‐treat: No For the purposes of our meta‐analyses we used the per‐protocol population, as reported by the study authors |
|
| Participants |
Number randomised: Total n = 857 (extended n = 430; short n = 427)* Exclusions post randomisation: Extended: exclusion criteria: 7, not treated: 13; short: exclusion criteria 2, consent withdrawal 4 not treated: 33 Losses to follow up: Extended n = 1; short n = 1 Age mean years (SD): Extended 70.9 (8.1); short 70.1 (8.6) Sex % M: Extended 37.9%; short 35.4% Inclusion criteria: 45 years or older; scheduled for a first total unilateral knee arthroplasty Exclusion criteria: History of confirmed symptomatic venous thromboembolism at any time, stroke or myocardial infarction within the previous month, current active bleed, gastrointestinal bleeding or hemorrhagic stroke within the previous six months, brain, spinal, opthalmological or other major surgery within the previous month, active cancer, renal impairment, hepatic impairment, a contraindication to anticoagulant therapy, hypersensitivity to heparin and patients who required therapeutic anticoagulation |
|
| Interventions |
Treatment: Anticoagulant treatment was chosen by the investigator and could be: heparin (5000 U, two to three times per day), enoxaparin (4000 IU), dalteparin (5000 IU), tinzaparin (4500 IU), body‐weight adjusted nadroparin, fondaparinux (2.5 mg) Control: No specific control, only short duration prophylaxis Duration: 10 ± 2 days 'short thromboprophylaxis'; 35 ± 5 days 'extended thromboprophylaxis' |
|
| Outcomes |
Primary: Composite of proximal DVT, symptomatic DVT, non‐fatal symptomatic PE, major bleeding, heparin‐induced thrombocytopenia, all‐cause mortality Secondary: Ultrasonographic distal DVT Bleeding definitions: Major bleeding fatal bleeding, bleeding that was intracranial, intraocular, retroperitoneal, gastrointestinal or intra‐articular, bleeding leading to reoperation, or bleeding requiring cessation of treatment |
|
| Notes |
Funding: Caen University Hospital with unrestricted grant from the French Health Ministry Method of VTE evaluation/confirmation: All participants were examined for DVT by bilateral whole‐leg ultrasonography on Day 35 ± 5, or earlier if thrombosis was clinically suspected *For the analysis, we only included participants that had negative ultrasonographic scans at discharge, as most comparative studies excluded these participants with positive ultrasonographic scans at discharge |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Concealed randomisation was performed using a centralised telephone system, according to a permuted block design with block size of four, with stratification by centre and by the presence or absence of distal deep‐vein thrombosis on whole leg ultrasonography |
| Allocation concealment (selection bias) | Low risk | "Concealed randomization was performed using a centralized telephone system" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Sonographer was not blinded; Primary outcomes were reviewed by a central, independent, blinded adjudication committee |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for |
| Selective reporting (reporting bias) | High risk | Safety outcomes reported as "the bleeding risk was very low (0.7%)", no further details provided |
| Other bias | Low risk | Sponsored by the Caen University Hospital with an unrestricted grant from the French Health Ministry ‐ which had no other role in the study |
Dahl 1997.
| Methods |
Study design: Randomised, prospective, double‐blind, placebo‐controlled trial Country: Norway Setting: Multicentre, hospital and home, January 1993 ‐ June 1994 Intention‐to‐treat: Yes, after withdrawals removed. For the purposes of our meta‐analyses we used the reported ITT population |
|
| Participants |
Number randomised: Total n = 265 (extended LMWH n = 134; placebo n = 131) Exclusions post randomisation: Total n = 38 (extended LMWH n = 17; placebo n = 21) withdrawn for reasons other than DVT or PE (21/38 due to adverse events: 10 extended LMWH and 11 placebo) Losses to follow up: No losses to follow‐up all participants received treatment until the day of final visit Age mean years: Extended LMWH 70.98, placebo 71.4 Sex %F: Extended LMWH 68.5%; placebo 73.6% Inclusion criteria: 18 years or older; admitted to hospital for elective primary or secondary hip replacement; obtained written consent form Exclusion criteria: Known renal or liver insufficiency; cerebral bleeding less than three months before surgery or known haemorrhagic diathesis; eye or ear surgery within one month before surgery; severe hypertension; septic endocarditis; threatened arterial circulation in the leg; body weight less than 40 kg; anticoagulant therapy less than one week before surgery; known hypersensitivity to heparin, low‐molecular‐weight heparin, dextran or contrast media; pregnancy or breastfeeding; inability to comply with study protocol; previous surgery within study |
|
| Interventions |
Treatment: Dalteparin 5000 IU, injections once daily for four weeks Control: Initial treatment with dalteparin 5000 IU followed by placebo (sodium chloride) injections, once daily Duration: 35 days (7 days initial treatment + 28 days continued treatment) |
|
| Outcomes |
Primary: Verified VTE on days 7 and 35 Secondary: Haematological assessment; safety: reoperation due to bleeding, wound haematoma and local haematoma at injection site |
|
| Notes |
Funding: Not reported Method of VTE evaluation/confirmation: DVT verified by bilateral venography and PE verified by perfusion ventilation or chest X‐ray |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information given to determine adequate randomisation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information given to determine adequate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as double‐blinded and gave placebo subcutaneous injections |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Venograms, X‐rays and V‐Q scans evaluated after the study by a blinded specialist |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for, ITT and PP analysis |
| Selective reporting (reporting bias) | High risk | VAS patient acceptability reported but not reported as pre‐planned, as was pain at injection site and serious adverse events, bleeding not reported as outcomes |
| Other bias | Low risk | No evidence of other bias |
DaPP Study.
| Methods |
Study design: Randomised, double‐blind, placebo‐controlled, parallel group, prospective trial Country: Denmark Setting: Multicentre; hospital and home; January to November 1994 Intention‐to‐treat: Yes For the purposes of our meta‐analyses we used the reported ITT population |
|
| Participants |
Number randomised: Total n = 281 (extended LMWH n = 140; placebo n = 141) Exclusions post randomisation: Total n = 66 (extended LMWH n = 27; placebo n = 39); 39 due to inadequate or missing phlebographies, 14 withdrew consent, 8 adverse events, 2 placebo patients with symptoms of PE had inconclusive lung scans, 2 withdrawn due to reoperation and 1 used other anticoagulant drug Losses to follow up: Not reported Age median years (range): Extended LMWH 68 (30 ‐ 94); placebo 70 (28 ‐ 91) Sex M/F: Extended LMWH 66/74; placebo 62/79 Inclusion criteria: All participants admitted for total hip replacement; age 18 years or older Exclusion criteria: Previous surgery in the study; simultaneous participation in another pharmacological study; informed consent not obtained; high probability for drop‐out; renal insufficiency; hepatic insufficiency; prothrombin < 0.7; platelet count < 100 x 109/L; treatment with oral anticoagulants or heparin within seven days before inclusion; hypersensitivity to heparin, LMWH or contrast media; documented bleeding within three months prior to surgery; intracranial bleeding within three months prior to surgery; eye, ear or CNS surgery within one month prior to surgery; hypertension with diastolic pressure > 120 mmHg; septic endocarditis; body weight < 40 kg; known pregnancy or lactation |
|
| Interventions |
Treatment: Dalteparin subcutaneous injections (5000 anti‐Xa), once daily Control: Initial treatment with dalteparin (5000 anti‐Xa) followed by placebo (sodium chloride) injections, once daily placebo subcutaneous injections, once daily, started after discharge Duration: 35 days after surgery |
|
| Outcomes |
Primary: DVT and PE Secondary: Bleeding complications and adverse events; haemological analysis Bleeding definitions: No definition for major bleeding was provided |
|
| Notes |
Funding: Not reported Method of VTE evaluation/confirmation: Bilateral ascending phlebography at the end of treatment to detect DVT; PE detected by perfusion/ventilation lung scan or pulmonary angiography |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed with a separate randomisation list for each centre |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information given to determine adequate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo injections, saline, given to control participants |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All venograms were evaluated by a panel of three radiologists who were unaware of the result of the randomisation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for, similar numbers in groups, no missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on |
| Other bias | Low risk | No indication of other bias |
EXTEND Study.
| Methods |
Study design: Randomised, double‐blind, parallel‐group study Country: Multi‐country (16 countries) Setting: Multicentre; hospital and home; September 2005 to February 2006 Intention‐to‐treat: No analysis performed as study stopped prematurely For the purposes of our meta‐analyses we used the per‐protocol population as reported by the authors |
|
| Participants |
Number randomised: Total n = 1158; 641 completed at time of termination (ximelagatran n = 580; enoxaparin n = 578) Exclusions post randomisation: Total n = 150 (ximelagatran n = 70; enoxaparin n = 80) Not treated: 23 melagatran, 16 enoxaparin; Premature stop: 47 melagatran, 64 enoxaparin Losses to follow up: Study terminated early Age median years (range): Hip replacement: ximelagatran 64.7 (24 ‐ 89); enoxaparin 63.9 (21 ‐ 89); Fracture surgery: ximelagatran 73.1 (44 ‐ 91); enoxaparin 70.7 (26 ‐ 94) Sex M/F: Hip replacement: ximelagatran 229/250; enoxaparin 211/268; Fracture surgery: Ximelagatran 17/60; enoxaparin 23/50 Inclusion criteria: 18 years or older; undergoing primary elective unilateral total hip replacement or surgery for hip fracture Exclusion criteria: "same as in the previously reported phase III studies in orthopaedic surgery on ximelagatran" |
|
| Interventions |
Treatment 1: 3 mg ximelagatran subcutaneously 4 ‐ 8 hours after surgery and twice daily for up to 2 days post‐op, followed by 24 mg oral ximelagatran twice daily Treatment 2: 40 mg enoxaparin subcutaneous once daily starting the night before surgery or post‐operatively Duration: 32 ‐ 38 days after surgery; Randomised within 5 days before surgery |
|
| Outcomes |
Primary: Efficacy outcomes: composite of proximal DVT, any clinically suspected and objectively confirmed DVT and/or PE, VTE‐related death or death where VTE could not be ruled out. Safety outcomes: major bleeding events, transfusions of whole blood and packed red blood cells, injection site haematomas > 2 cm, clinically verified and adjudicated myocardial infarction, evidence of hepatic injury Secondary: No distinction between primary and secondary Bleeding definitions: Major bleeding ‐ transfusions of whole blood and packed red blood cells, injection site haematomas > 2 cm, clinically verified and adjudicated myocardial infarction, evidence of hepatic injury |
|
| Notes |
Funding: AstraZeneca, Sweden; employees of AstraZeneca contributed to study design, interpretation of results and decision to submit paper Method of VTE evaluation/confirmation: bilateral compression ultrasound (CUS) of the legs at the end of treatment period |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Interactive web‐based randomisation system; stratified by type of surgery |
| Allocation concealment (selection bias) | Low risk | Used web‐based randomisation system |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes adjudicated by a blinded, independent committee |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT numbers do not add up; insufficient information given |
| Selective reporting (reporting bias) | Unclear risk | No details of the VTE events were provided |
| Other bias | High risk | Terminated early due to safety issues, AstraZeneca supported trial ‐ had influence on study design, interpretation of results and decision to submit paper |
Fragmin Trial.
| Methods |
Study design: Randomised, double‐blind, clinical trial Country: USA and Canada Setting: Multicentre (18 centres); hospital and home Intention‐to‐treat: Yes, ITT: VTE analysis performed on participants who had successful venography, safety analysis with those who had successful venogram at day six For the purposes of our meta‐analyses we used the reported ITT population |
|
| Participants |
Number randomised: Total n = 991 but only n = 569 received treatment (pre‐op dalteparin n = 199; post‐op dalteparin n = 190; warfarin/placebo n = 180) Exclusions post randomisation: Total n = 293: 94 refused participation, 40 adverse event in‐hospital, 12 unable to self inject, 42 other reasons, plus 105 no or inadequate venography Losses to follow up: No reports of losses to follow up but could be part of 42 other reasons for no venography Age mean years (SD): Pre‐op dalteparin 62 (12); post‐op dalteparin 63 (12); warfarin/placebo 63 (12) Sex M/F: Pre‐op dalteparin 106/93; post‐op dalteparin 87/103; warfarin/placebo 94/86 Inclusion criteria: Aged 18 years or older; scheduled for elective unilateral total hip replacement; gave informed consent Exclusion criteria: Documented bleeding within three months before surgery; known hypersensitivity to heparin, LMWH, warfarin or contrast media; defective haemostasis; ongoing anticoagulant therapy; pregnancy or breastfeeding; clinically significant hepatic dysfunction; renal insufficiency; severe hypertension; septic endocarditis; weight less than 40 kg; eye, ear or central nervous system surgery within one month before hip surgery; diseases with unfavourable prognosis or concurrent disease making study participation medically complicated; simultaneous participant in another study or receiving any investigation drug 30 days or less before surgery; previous randomisation into this study; use of pneumatic compression stockings during study period |
|
| Interventions |
Treatment 1: Dalteparin sodium, subcutaneous, 5000 IU once daily, commenced two hours before surgery plus placebo capsules while in hospital Treatment 2: Dalteparin sodium, subcutaneous, 5000 IU once daily, commenced four hours after surgery plus placebo capsules while in hospital Control: Placebo Duration: 35 ± 2 days |
|
| Outcomes |
Primary: Venogram‐confirmed DVT and proximal DVT Secondary: PE, bleeding complications, death Bleeding definitions: Major bleeding ‐ clinically overt, associated with a decrease in haemoglobin of 20 g/L or more; if it required a blood transfusion of 2 or more units; intracranial, intraocular, intraspinal or intraperitoneal; occurred into a prosthetic joint Minor bleeding ‐ clinically overt but not meeting criteria for major |
|
| Notes |
Funding: A grant‐in‐aid by Pharmacia & Upjohn to the University of Calgary Method of DVT and PE evaluation/confirmation: Participants with VTE symptoms received objective testing for confirmation; bilateral ascending venography was performed at discharge and at the end of the study period for all participants |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation performed using computer‐derived treatment schedule, divided into consecutive blocks, stratified by treatment centre |
| Allocation concealment (selection bias) | Low risk | Randomisation by computer‐derived methods |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Used placebo dalteparin injections and placebo warfarin capsules to maintain blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Venograms were interpreted by a blinded central reader as well as a local radiologist; disagreements resolved by a second blinded interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were accounted for |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on |
| Other bias | Low risk | No indication of other bias |
French Study.
| Methods |
Study design: Randomised, double‐blind, placebo controlled trial Country: France Setting: Single centre; hospital and home; August 1991 to June 1994 Intention‐to‐treat: Yes, ITT and per‐protocol, although based on second bilateral venography For the purposes of our meta‐analyses we used the reported ITT population |
|
| Participants |
Number randomised: Total n = 179 (extended LMWH n = 90; placebo n = 89) Exclusions post randomisation: Total n = 6 (extended LMWH n = 5; placebo n = 1), had no second venogram and excluded from efficacy analysis Losses to follow up: No losses to follow up; all participants included in safety analysis Age mean years (SD): Extended LMWH 70 (9.1); placebo 68 (8.2) Sex M/F: Extended LMWH 47/43; placebo 55/34 Inclusion criteria: Underwent primary total hip replacement or conversion/reversion of total hip replacement; received prophylactic treatment with enoxaparin for post‐op VTE; > 45 years of age; body weight 45 ‐ 95 kg; could walk unassisted using crutches; were free of DVT as assessed by bilateral ascending contrast venography performed within five days before discharge; had to be available for a follow‐up visit at day 21 ± 2 days post discharge Exclusion criteria: History of documented DVT or PE within last six months; active cancer; underlying bleeding disorders or haemostasis abnormalities; prothrombin time < 60% or activated partial thromboplastin time > 8 seconds or longer than control subjects; active gastroduodenal ulcer; history of hypersensitivity to heparin or to contrast media; renal or hepatic insufficiency; uncontrolled hypertension; recent stroke; inability to give informed consent |
|
| Interventions |
Treatment: 40 mg enoxaparin, subcutaneous, daily Control: 40 mg enoxaparin, subcutaneous, daily during hospitalisation (14 ± 1 day) followed by placebo (isotonic saline) injections Duration: 35 days after surgery |
|
| Outcomes |
Primary: DVT and/or PE Secondary: Onset of proximal or distal DVT; safety: death, major and minor haemorrhages, adverse events Bleeding definitions: Major bleeding ‐ overt and was associated with a decrease in haemoglobin concentration of 2 g/dL or more compared with the last postoperative value, or a need for transfusion of two or more units of packed red blood cells, or if it was retroperitoneal or intracranial. Minor bleeding ‐ overt but did not meet the other criteria for major bleeding |
|
| Notes |
Funding: Not reported Method of VTE evaluation/confirmation: Clinical evaluation of symptoms and clinical signs of DVT and bilateral venographic evaluation at day 35 post‐surgery |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was balanced in blocks of four by means of a computer‐generated randomization schedule." |
| Allocation concealment (selection bias) | Low risk | Used computer‐generated randomisation schedule |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label during hospitalisation period when all patients receiving enoxaparin, then double‐blinded after randomisation to extended duration or placebo using saline injections for the placebo group; "The double‐blind conditions (for patients, nurses, attending physicians, and investigators) were maintained until the database was locked." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Venograms were independently evaluated by two blinded radiologists |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on |
| Other bias | Low risk | No indication of other bias |
Heit 2000.
| Methods |
Study design: Randomised, double‐blind, placebo‐controlled trial Country: US Setting: Multicentre (33 centres); hospital and home; November 1994 to November 1997 Intention‐to‐treat: Yes For the purposes of our analyses we used all participants randomised, as reported by the study authors |
|
| Participants |
Number randomised: Total n = 1195 (Extended LMWH n = 607; Placebo n = 588) Exclusions post randomisation: Unclear Losses to follow up: Unclear Age mean years (SD): Extended LMWH 65 (11); placebo 66 (11) Sex M/F: Extended LMWH 265/342; placebo 275/313 Inclusion criteria: Aged 18 years or older; received elective primary or revision unilateral total hip replacement, primary unilateral or bilateral total knee replacement Exclusion criteria: 'Pregnant, lactating or women of childbearing age; patients with clinical bleeding disorder; uncontrolled hypertension' severely impaired hepatic or renal function; active alcohol or drug abuse; patients who could not comply with home injections or complete a 10‐week post‐op follow‐up; patients receiving warfarin or thrombolytic therapy; had a major surgery within previous seven days; had major orthopaedic surgery involving lower extremities in previous six weeks; history of substantial internal bleeding, active peptic ulcer, myocardial infarction or stroke; intracranial or intraocular surgery in previous eight weeks; patients planning to undergo staged bilateral total knee replacement with anticipated interval of less than 10 weeks; hypersensitivity to heparin, pork products, metabisulphite, methylparaben or propylparaben; weight great than 120 kg; prolonged activated partial thromboplastin time or prothrombin time at baseline; baseline platelet count less than 100 x 109/L; history of VTE; current use of dextran sulphate, desmopressin acetate, other LMWH, oral anticoagulants, thrombolytic agents or external pneumatic compression |
|
| Interventions |
Treatment: Ardeparin sodium 100 anti‐Xa IU/kg weight, subcutaneous injections, daily, starting within 24 hours after surgery Control: Four to 10 days ardeparin sodium 100 anti‐Xa IU/kg weight (starting within 24 hours after surgery), daily, subcutaneous injection, followed by placebo injections from time of discharge Duration: Six weeks after surgery |
|
| Outcomes |
Primary: Incidence of symptomatic, objectively documented DVT or PE or death Secondary: The incidence of major and minor bleeding and thrombocytopenia Bleeding definitions: Major bleeding ‐ overt bleeding associated with haemoglobin decrement of at least 20 g/L or transfusion of at least 2 units of blood products, any intracranial, retroperitoneal, intraocular or mediastinal bleeding that occurred after at least one dose of post‐discharge study drug Minor bleeding ‐ overt bleeding not meeting the criteria for major bleeding |
|
| Notes |
Funding: Wyeth‐Ayerst Research, Philadelphia, US ‐ performed statistical analysis, data interpretation and manuscript preparation done by writing committee, sponsor did not have prior right of approval for final manuscript publication Method of VTE evaluation/confirmation: Compression duplex ultrasonography or venography, ventilation perfusion lung scanning or pulmonary angiography |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified by clinical centre, type of surgery and history of VTE; block randomisation derived from a randomisation table |
| Allocation concealment (selection bias) | Low risk | Allocation done in consecutively numbered, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind phase of study achieved by giving placebo injections in identical Tubex cartridges containing 0.5 mL ardeparin sodium or placebo (sodium chloride solution) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes assessed by blinded, central adjudication committee |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for, although > 15% withdrew in both groups |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on |
| Other bias | Low risk | No indication of other bias |
Kolb 2003.
| Methods |
Study design: Randomised, double blind, placebo controlled trial Country: Germany and Czech Republic Setting: Multicentre (13 centres), hospital and home Intention‐to‐treat: Yes and per‐protocol For the purposes of our analyses we used all participants randomised, as reported by the study authors |
|
| Participants |
Number randomised: Total n = 310 (extended LMWH n = 161; placebo n = 149) Exclusions post randomisation: Total n = 37: protocol violation: 8, adverse events:10, withdrawal of consent: 19 Losses to follow up: None Age mean years (SD): Extended LMWH 78.1 (8.4); placebo 75.8 (8.4) Sex M/F: Extended LMWH 25/136; placebo 29/120 Inclusion criteria: Participants undergoing endoprosthetic joint replacement or osteosynthesis of the lower limb Exclusion criteria: Age under 18 years; hypersensitivity against heparin; clinical conditions with increased risk of bleeding; haemorrhagic diathesis; platelet count < 100.000/ul; concomitant treatment with anticoagulants or platelet inhibitors; renal or hepatic insufficiency; hypertension with systolic values > 200 mmHg and diastolic values > 105 mmHg despite treatment; malignancy; endocarditis lenta; drug abuse; pregnancy; participation in a clinical trial during the last four weeks; thromboembolic complications between start of treatment and randomisation; discontinuation of study medication due to adverse events; withdrawal of consent |
|
| Interventions |
Treatment: Certoparin 3000 u anti‐Xa Control: Certoparin 3000 u anti‐Xa for 14 days then placebo Duration: 42 days |
|
| Outcomes |
Primary: Composite of symptomatic or asymptomatic DVT (proximal and/or distal), symptomatic PE and deaths related to VTE Secondary: Coagulation parameters Bleeding definition ‐ not provided |
|
| Notes |
Funding: Supported by Novartis Pharma, Germany, test kits for fibrin monomers and Ddimer sponsored by Roche Diagnostics Germany, protein C resistance kits sponsored by Dada Behring Germany Method of VTE evaluation/confirmation: DVT was screened for by compression and duplex ultrasonography every week and confirmed by ascending leg and pelvic venography (whenever possible). PE was verified by pulmonary angiography, spiral CT or perfusion lung scanning |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information provided on sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, participants given placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided on assessor blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for, although treatment group was not indicated for loss‐to‐follow‐ups |
| Selective reporting (reporting bias) | Low risk | All defined outcomes were reported on |
| Other bias | Low risk | No indication of other bias |
Prandoni 2002.
| Methods |
Study design: Prospective randomised controlled trial Country: Italy Setting: Single centre; hospital and home; September 1998 to December 2000 Intention‐to‐treat: Yes For the purposes of our meta‐analyses we used the reported ITT population |
|
| Participants |
Number randomised: Total n = 360 (extended duration n = 184; control n = 176) Exclusions post randomisation: Total n = 6: three protocol violations per group but all were available for clinical follow‐up Losses to follow up: None Age median years (range): Extended duration 68 (48 ‐ 82); control 69 (44 ‐ 87) Sex M/F: Extended duration 83/101; control 79/97 Inclusion criteria: Underwent elective total hip replacement; received warfarin prophylaxis during hospitalisation Exclusion criteria: Previous hip surgery on the same side; history of thromboembolic disorder; needing long‐term anticoagulation; were unavailable for long‐term follow‐up; refused to give written informed consent; developed VTE complications, asymptomatic proximal DVT or major bleeding during hospitalisation period |
|
| Interventions |
Treatment: 5 mg/d of warfarin sodium prophylaxis starting two days pre‐op; dosage adjusted to increase international normalised ration between 2.0 and 3.0 Control: 5 mg/d of warfarin sodium prophylaxis starting two days pre‐op; dosage adjusted to increase international normalised ration between 2.0 and 3.0, discontinued at discharge Duration: 4 weeks after discharge (Median nine day hospitalisation plus four weeks extended treatment) |
|
| Outcomes |
Primary: Composite of symptomatic VTE and asymptomatic proximal DVT during first four weeks of follow up, efficacy during complete three months follow up, clinical parameters, also reports on major bleeding, death and other adverse events Secondary: Not specifically defined Bleeding definitions: Major bleeding ‐ clinically overt and associated with either a decrease in haemoglobin of at least 2.0 g/dL or a need of transfusion of two or more units of blood, was intracranial or retroperitoneal, resulted in permanent discontinuation of treatment |
|
| Notes |
Funding: Not reported Method of VTE evaluation/confirmation: compression ultrasonography for DVT; PE confirmed by ventilation perfusion, spiral CT, abnormal finding on angiography or autopsy |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Computer‐generated list |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No discussion of blinding and no placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Ultrasound was performed by a blinded operator; cause of death adjudicated by a blinded physician and all outcomes evaluated by a blinded committee |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for and no loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on |
| Other bias | High risk | Terminated early "after inclusion of the first 360 patients because a statistically significant and clinically relevant superiority of extended over short‐term thromboprophylaxis was observed" |
RE‐NOVATE II Trial.
| Methods |
Study design: Randomised, double‐blind, double‐dummy, non‐inferiority, active‐controlled trial Country: 19 countries in Europe, North America, India, Australia, New Zealand and South Africa Setting: Multicentre (108 locations); hospital and home; March 2008 to May 2009 Intention‐to‐treat: Modified intention‐to‐treat: all participants who had surgery, received treatment and had evaluable venograms For the purposes of our meta‐analyses we used the reported ITT population of participants that underwent surgery |
|
| Participants |
Number randomised: Total n = 2055 (dabigatran n = 1036; enoxaparin n = 1019) Exclusions post randomisation: Total n = 477 (dabigatran n = 243; enoxaparin n = 234: not treated: dabigatran 25 and enoxaparin 16. Dabigatran 9 and enoxaparin 11 did not have surgery, dabigatran 209 and enoxaparin 207 no or not evaluable venogram Losses to follow up: All participants accounted for Age mean years (SD): Dabigatran 62 (12); enoxaparin 62 (11) Sex F%: Dabigatran 53.6%; enoxaparin 50.0% Inclusion criteria: 18 years or older; scheduled to undergo a primary, unilateral, elective total hip replacement; > 40 kg body weight; gave written informed consent Exclusion criteria: History of bleeding diathesis; excessive risk of bleeding as judged by investigators; major surgery or trauma within three months of enrolment; recent unstable cardiovascular disease; any history of haemorrhagic stroke or any of the following intracranial pathologies: bleeding, neoplasm, atriovenous malformation or aneurysm; ongoing treatment for VTE; clinical relevant bleeding within six months of enrolment; gastric or duodenal ulcer within one year of enrolment; liver disease expected to have any potential impact on survival; active liver disease or liver disease decreasing survival; known severe renal insufficiency; elevated creatinine that contraindicates venography; Treatment with anticoagulants, clopidogrel, ticlopidine, abciximab, aspiring or NSAID within seven days prior to hip replacement or anticipated need of such medication; anticipated required use of intermittent pneumatic compression and electric stimulation of lower leg; active malignant disease or current cytostatic treatment; pre‐menopausal women who are pregnant or nursing, or are of child‐bearing potential and are not practising or do not plan to continue practising acceptable methods of birth control; allergy to radio opaque contrast media, heparins or dabigatran; contraindications to enoxaparin; participation in a clinical trial during the last 30 days; leg amputee; known alcohol or drug abuse which would interfere with study completion; previous participation in this study; history of thrombocytopaenia |
|
| Interventions |
Treatment 1: 220 mg dabigatran etexilate (2 x 110 mg tablets), orally, once daily, initial dose of 110 mg on the day of surgery plus placebo injection identical to enoxaparin treatment Treatment 2: 40 mg enoxaparin, subcutaneous, once daily, initial dose evening before surgery plus placebo tablets identical to dabigatran treatment Duration: 28 ‐ 35 days |
|
| Outcomes |
Primary: Composite of total VTE and all‐cause mortality (VTE includes both proximal and distal DVT, symptomatic DVT, PE) Secondary: Major VTE and VTE‐related death, proximal DVT, total DVT, symptomatic DVT, PE, all‐cause mortality, bleeding events, lab parameters and adverse events Bleeding definitions: Major bleeding ‐ fatal, clinically overt associated with loss of haemoglobin greater than or equal to 20 g/L or leading to transfusion of greater than or equal to 2 units of packed cells or whole blood; symptomatic retroperitoneal, intracranial, intraocular or intraspinal; requiring treatment cessation; leading to reoperation. Clinically relevant bleeding ‐ spontaneous skin haematoma greater than or equal to 25 cm2; wound haematoma greater than or equal to 100 cm2; spontaneous nose bleed lasting longer than 5 min; macroscopic haematuria spontaneous or lasting longer than 24 hours if associated with an intervention; spontaneous rectal bleeding (more than a spot on toilet paper); gingival bleeding lasting longer than 5 min; any other bleeding event considered clinically relevant by the investigator Minor bleeding ‐ any other bleeding events that were not classified as major or clinically relevant |
|
| Notes |
Funding: Boehringer Ingelheim, Sweden Method of VTE evaluation/confirmation: DVT confirmed/detected by bilateral venography or compression ultrasound or autopsy; PE confirmed by pulmonary V‐Q scintigraphy, chest X‐ray, angiography, spiral CT or autopsy |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by central computer‐generated system, stratified by centre, prepared in blocks of six |
| Allocation concealment (selection bias) | Low risk | Utilised central computer‐generated system |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both treatment groups received one study drug and one placebo identical in appearance to the other active treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Efficacy outcomes confirmed by blinded, central adjudication committee |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All participants were accounted for, with similar numbers in each treatment group, but no description was given for excluded participants that did not take study medication |
| Selective reporting (reporting bias) | Low risk | No protocol but all expected outcomes reported |
| Other bias | Unclear risk | Study sponsors were involved in the design and conduct of the trial. The data were collected and analysed by the sponsors of the study |
RE‐NOVATE Trial.
| Methods |
Study design: Randomised, double‐blind, active‐controlled trial Country: Multi‐country (16 countries) in Europe, Australia and South Africa Setting: Multicentre (115 centres); hospital and home; November 2006 to July 2006 Intention‐to‐treat: No, per‐protocol: excluding not treated, no surgery, and inadequate or no venogram For the purposes of our meta‐analyses we used the reported ITT population of participants that underwent surgery |
|
| Participants |
Number randomised: Total n = 3493 (220 mg dabigatran n = 1157; 150 mg dabigatran n = 1174; enoxaparin n = 1162) Exclusions post randomisation: Total n = 322 (220 mg dabigatran n = 108; 150 mg dabigatran n = 102; enoxaparin n = 112), reason for all: did not complete study Losses to follow up: Not specified Age mean years (SD): 220 mg dabigatran 65 (10); 150 mg dabigatran 63 (11); enoxaparin 64 (11) Sex %F: 220 mg dabigatran 56%; 150 mg dabigatran 57%; enoxaparin 56% Inclusion criteria: 18 years or older; scheduled to undergo a primary, unilateral, elective total hip replacement; > 40 kg body weight; gave written informed consent Exclusion criteria: Patients with an excessive risk of bleeding; active malignant disease or current cytostatic treatment; known severe renal insufficiency; liver disease expected to have any potential impact on survival, or elevated AST or ALT > 2 x upper limit of normal; recent unstable cardiovascular disease or history of myocardial infarction within the last three months; pre‐menopausal women who are pregnant or nursing, or are of child‐bearing potential and are not practising or do not plan to continue practising acceptable methods of birth control; allergy to radio opaque contrast media or iodine, heparins (including heparin‐induced thrombocytopenia) or dabigatran; contraindications to enoxaparin; participation in a clinical trial during the last 30 days |
|
| Interventions |
Treatment 1*: 150 mg dabigatran etexilate, orally, once daily, starting with half dose on day of surgery plus one placebo pill identical to other treatment and subcutaneous placebo injection Treatment 2*: 220 mg dabigatran etexilate, orally, once daily, starting with half dose on day of surgery plus one placebo pill identical to other treatment subcutaneous placebo injection Control: 40 mg enoxaparin, subcutaneous, once daily plus two placebo pills identical in appearance to active treatments Duration: 28 ‐ 35 days (average 33 days) *For the analyses within this review, the 150 mg and 220 mg treatment groups were combined as both dosages are considered normal therapeutic dosages. |
|
| Outcomes |
Primary: Composite of total VTE and all‐cause mortality; VTE includes both proximal and distal DVT, symptomatic DVT, PE Secondary: Major VTE and VTE‐related death, proximal DVT, total DVT, symptomatic DVT, PE, all‐cause mortality, bleeding events Bleeding definitions: Major bleeding ‐ fatal, clinically overt associated with loss of haemoglobin greater than or equal to 20g/L or leading to transfusion of greater than or equal to 2 units of packed cells or whole blood; symptomatic retroperitoneal, intracranial, intraocular or intraspinal; requiring treatment cessation; leading to reoperation Clinically relevant bleeding ‐ spontaneous skin haematoma greater than or equal to 25 cm2; wound haematoma greater than or equal to 100 cm2; spontaneous nose bleed lasting longer than 5 min; macroscopic haematuria spontaneous or lasting longer than 24 hours if associated with an intervention; spontaneous rectal bleeding (more than a spot on toilet paper); gingival bleeding lasting longer than 5 min; any other bleeding event considered clinically relevant by the investigator. Minor bleeding ‐ any other bleeding events that were not classified as major or clinically relevant |
|
| Notes |
Funding: Steering committee and sponsor responsible for study design, data collection and analysis done by sponsor, independent data and safety committee monitored study, steering committee had overall responsibility for all aspects and final responsibility for decision to submit paper Method of VTE evaluation/confirmation: DVT detected and confirmed by bilateral venography; PE confirmed by pulmonary V‐Q scintigraphy, chest X‐ray, angiography, spiral CT or autopsy |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by central computer‐generated system, stratified by centre, prepared in blocks of six |
| Allocation concealment (selection bias) | Low risk | Utilised central computer‐generated system |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All treatment groups received one study drug and one placebo identical in appearance to the other active treatment as well as a subcutaneous injection |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Efficacy outcomes initially assessed locally, then confirmed by blinded, central adjudication committee |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All exclusions were reported with reasons and by study group |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on |
| Other bias | Low risk | No indication of other bias |
RECORD 1 Trial.
| Methods |
Study design: Randomised, double‐blind trial Country: Multi‐country (27 countries) Setting: Multi‐centre; hospital and home; February 2006 to March 2007 Intention‐to‐treat: Yes, modified ITT and per‐protocol analysis For the purposes of our meta‐analyses we used the reported modified ITT population of participants that underwent surgery |
|
| Participants |
Number randomised: Total n = 4541 (rivaroxaban n = 2266; enoxaparin n = 2275) Exclusions post randomisation: Total n = 1177 (rivaroxaban n = 580; enoxaparin n = 597); 57 rivaroxaban and 51 enoxaparin randomised but not included in safety analysis ‐ did not receive study drug but no further description; excluded from ITT if not treated and did not undergo surgery and had no suitable venograms (additional 523 rivaroxaban, 546 enoxaparin) Losses to follow up: No reports of loss‐to‐follow‐up Age mean years (range): Rivaroxaban 63.1 (18 ‐ 91); enoxaparin 63.3 (18 ‐ 93) Sex F %: Rivaroxaban 55.2%; enoxaparin 55.8% Inclusion criteria: Men and women 18 years or older; scheduled to undergo elective total hip replacement Exclusion criteria: Scheduled to undergo staged, bilateral hip arthroplasty; pregnant or breastfeeding; active bleeding or a high risk of bleeding; contraindication for prophylaxis with enoxaparin; conditions preventing bilateral venography; substantial liver disease; severe renal impairment; concomitant use of protease inhibitors for the treatment of HIV; planned intermittent pneumatic compression; requirement for anticoagulant therapy that could not be stopped |
|
| Interventions |
Treatment 1: 10 mg rivaroxaban, orally, once daily beginning after surgery Control: 40 mg enoxaparin, subcutaneously, once daily beginning the evening before surgery Duration: 35 days (range 31 ‐ 39); mean duration 33.4 days in rivaroxaban and 33.7 in enoxaparin group ‐ venography took place |
|
| Outcomes |
Primary: Composite DVT (symptomatic or detected by venography), non‐fatal PE or death from any cause at day 36 Secondary: Major VTE (proximal DVT, non‐fatal PE or death from VTE), major bleeding, DVT, symptomatic VTE, death, lab values and cardiovascular events Bleeding definitions: Major bleeding ‐ fatal, occurred in a critical organ (e.g., retroperitoneal, intracranial, intraocular, and intraspinal bleeding), or required reoperation or extrasurgical‐site bleeding that was clinically overt and was associated with a fall in the haemoglobin level of at least 2 g per decilitre or that required transfusion of 2 or more units of whole blood or packed cells |
|
| Notes |
Funding: Bayer Healthcare and Johnson & Johnson: data collected and analysed by sponsors, steering committee designed and supervised, all authors contributing had access to all data and analysis and vouch for accuracy and completeness of data reported Method of VTE evaluation/confirmation: Mandatory bilateral venography the day after the last dose of the study drug |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation generated in permuted blocks and stratification according to centre by a central telephone system with a computer‐generated randomised list |
| Allocation concealment (selection bias) | Low risk | Use of telephone system and computer‐generated randomisation list |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients received study medication plus placebo tablets or injection |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes assessed by a central, blinded adjudication committee |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 57 rivaroxaban and 51 enoxaparin randomised but not included in safety analysis ‐ did not receive study drug but no reasons given why |
| Selective reporting (reporting bias) | Low risk | No protocol provided but all outcomes reported on |
| Other bias | Unclear risk | Data was collected and analysed by the sponsors: Bayer HealthCare and Johnson & Johnson |
RECORD 2 Trial.
| Methods |
Study design: Randomised, double‐blind, controlled trial Country: Multinational (21 countries) Setting: Multicentre (123 centres); hospital and home; February 2006 to April 2007 Intention‐to‐treat: Modified ITT: not‐treated, did not receive surgery, no readable venogram For the purposes of our meta‐analyses we used the reported modified ITT population of participants that underwent surgery |
|
| Participants |
Number randomised: Total n = 2509 (rivaroxaban n = 1252; enoxaparin n = 1257) Exclusions post randomisation: Total n = 90 (rivaroxaban n = 40; enoxaparin n = 50). Rivaroxaban: 24, enoxaparin: 28 ‐ not taken study medication (no reason why); rivaroxaban 16 and enoxaparin 22 did not receive surgery Losses to follow up: Not reported Age mean years (SD): Rivaroxaban 61.4 (13.2); enoxaparin 61.6 (13.7) Sex %F: Rivaroxaban 54.3% enoxaparin 53.0% Inclusion criteria: Aged 18 or older; scheduled to undergo elective total hip replacement Exclusion criteria: Scheduled to undergo staged, bilateral hip arthroplasty; pregnant or breastfeeding; active bleeding or a high risk of bleeding; contraindication for prophylaxis with enoxaparin; conditions preventing bilateral venography; substantial liver disease; severe renal impairment; concomitant use of protease inhibitors for the treatment of HIV; use of fibrinolytic therapy; planned intermittent pneumatic compression; requirement for anticoagulant therapy that could not be stopped |
|
| Interventions |
Treatment: 10 mg rivaroxaban, orally, once daily beginning after surgery plus placebo injections for 10 ‐ 14 days Control: 40 mg enoxaparin, subcutaneously, once daily beginning the evening before surgery and continued for 10 ‐ 14 days and received placebo tablets for the entire study period Duration: 31 ‐ 39 days |
|
| Outcomes |
Primary: Composite of any DVT, nonfatal PE and all‐cause mortality; incidence of major bleeding events Secondary: Major VTE (composite of proximal DVT, non‐fatal PE and VTE‐related death); DVT (proximal and distal), symptomatic VTE, on‐treatment bleeding, death Bleeding definitions: Major bleeding ‐ fatal, occurred in a critical organ (e.g., retroperitoneal, intracranial, intraocular, and intraspinal bleeding), or required reoperation or extrasurgical‐site bleeding that was clinically overt and was associated with a fall in the haemoglobin level of at least 2 g per decilitre or that required transfusion of 2 or more units of whole blood or packed cells |
|
| Notes |
Funding: Bayer HealthCare AG, Johnson & Johnson Pharmaceutical Research and Development LLC; sponsors involved in design and conduct of trial, data collection and analysis; all authors had full access to data and analyses and vouch for accuracy and completeness of data and were involved in decision to submit the manuscript Method of VTE evaluation/confirmation: DVT confirmed by bilateral venography; PE confirmed by perfusion/ventilation lung scintigraphy, angiography, chest X‐ray or spiral CT or autopsy |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation generated in permuted blocks and stratification according to centre by a central telephone system with a computer‐generated randomised list |
| Allocation concealment (selection bias) | Low risk | Use of telephone system and computer‐generated randomisation list |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients received study medication plus placebo tablets or injection |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes assessed by a central, blinded adjudication committee |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All participants were accounted for, with similar numbers in each treatment group, but no description was given for excluded participants that did not take study medication |
| Selective reporting (reporting bias) | Low risk | No protocol provided but all outcomes reported on |
| Other bias | Unclear risk | Funded by Bayer HealthCare and Johnson & Johnson ‐ study sponsors were involved in the design and conduct of the trial. The data were collected and analysed by the sponsors of the study |
SACRE Study.
| Methods |
Study design: Randomised trial Country: France Setting: Multicentre (65 centres); hospital and home; September 1997 to October 1999 Intention‐to‐treat: Yes, and per‐protocol For the purposes of our meta‐analyses we used the reported ITT population |
|
| Participants |
Number randomised: Total n = 1289 (LMWH n = 644; anticoagulant n = 645) Exclusions post randomisation: Total n = 10 (LMWH n = 1; anticoagulant n = 9); no treatment 7 (1 reviparin, 6 acenocoumarol); event at randomisation: 3 acenocoumarol Losses to follow up: Not reported Age mean years (SD): LMWH 66 (11); anticoagulant 65 (12) Sex %F: LMWH 51%; anticoagulant 50% Inclusion criteria: 18 years or older; scheduled to undergo elective unilateral primary total hip replacement Exclusion criteria: Femoral neck fracture; current active bleeding or disorders contraindicating anticoagulant therapy; a history of DVT or PE; heparin‐induced thrombocytopaenia, peptic ulcer, allergy to radiopaque contrast medium; use of aspirin or ticlopidine hydrochloride; renal insufficiency; liver failure; acute endocarditis; recent stroke; uncontrolled hypertension; pregnancy; alcoholism; inability to follow instructions |
|
| Interventions |
Treatment: Fixed‐dose subcutaneous LMWH reviparin sodium, 4200 anti‐Xa IU, beginning 12 hours preoperatively Treatment 2: After initial LMWH treatment as described above, crossed over to adjusted‐dose oral anticoagulant acenocoumarol, international normalised ratio, 2 ‐ 3 Duration: 6 weeks after surgery |
|
| Outcomes |
Primary: Combined clinical events of symptomatic thromboembolic event, major haemorrhage or death Secondary: Minor bleeding Bleeding definitions: Major bleeding ‐ clinically overt and was associated with a decrease in haemoglobin level of more than 20 g/L or required a transfusion of 2 U or more of packed red blood cells after randomisation or was digestive, intracranial, retroperitoneal or intraocular or was located at the surgical site and required reoperation or, according to the investigator's opinion, led to discontinuation of the treatment. Minor bleeding ‐ clinically overt but not major |
|
| Notes |
Funding: Supported by Knoll France, Investigators received USD 400 per patient included in the study and PI received final grant of USD 4000 Method of VTE evaluation/confirmation: Suspected DVT confirmed by venography or duplex scanning; suspected PE confirmed by ventilation‐perfusion or angiography |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...randomized computer‐derived treatment schedule" performed at a central location, stratified by each centre, balanced in blocks of four |
| Allocation concealment (selection bias) | Low risk | Utilised a centralised computer‐derived randomisation schedule |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No mention of blinding of participants or personnel; most likely unblinded as one treatment given subcutaneously and the other orally |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes interpreted by a blinded adjudication committee |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Table 1. clearly demonstrates missing participants and reasons, although it should be noted the anticoagulant treatment group had a higher rate of protocol violations (statistical tests not performed) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on |
| Other bias | Low risk | No indication of other bias |
Zhang 2014.
| Methods |
Study design: Randomised trial Country: China Setting: Single centre; hospital and outpatient clinic; June 2012 and May 2013 Intention‐to‐treat: Yes For the purposes of our analyses we used all participants randomised, as reported by the study authors |
|
| Participants |
Number randomised: Total n = 40 (extended n = 20; short n = 20) Exclusions post randomisation: Not stated Losses to follow up: None Age mean years (range): Extended 60.1 (37 ‐ 76); short 61.3 (42 ‐ 78) Sex (F/M): Extended 11/9; Short 12/8 Inclusion criteria: All patients admitted to the Center for Bone and Joint Health between June 2012 and May 2013, who were diagnosed with osteonecrosis of the femoral head, scheduled for elective total hip replacement and provided informed consent Exclusion criteria: Had Doppler ultrasound performed 48 hrs prior to the surgery showing DVT; had undergone surgery recently, a history of active major bleeding or a tendency to bleed; had recent use of anticoagulants, antiplatelets or antifibrinogenics; were pregnant or nursing baby or did not take measures to prevent pregnancy; had severe kidney or liver dysfunction or other disease that could interfere with drug metabolism or blood coagulation |
|
| Interventions |
Treatment: Oral rivaroxaban 10 mg once daily started within 6 ‐ 10 hrs after the surgery for 35 days Control: Oral rivaroxaban 10 mg once daily started within 6 ‐ 10 hrs after the surgery for 7 days followed by no treatment Duration: 35 days post surgery |
|
| Outcomes |
Primary: Efficacy: haemostatic parameters including thrombin‐antithrombin complexes, prothrombin fragment 1 and 2, D‐dimer and fibrinogen Secondary: DVT Outcomes were recorded pre‐operatively (within 48 hours before surgery) and at 1, 7, and 35 days after surgery |
|
| Notes |
Funding: Not reported Method of VTE evaluation/confirmation: lower limb ultrasonography |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using random numbers generated by computer prior to the surgery |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No mention of blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for, similar numbers in groups, no missing outcome data |
| Selective reporting (reporting bias) | Low risk | All listed outcomes reported on |
| Other bias | Low risk | No indication of other bias |
DVT: deep vein thrombosis F: female IU: international unit LMWH: low‐molecular‐weight heparin M: male NSAID: non‐steroidal anti‐inflammatory drug PE: pulmonary embolism post‐op: post‐operatively, after operation pre‐op: prior to operation VAS: visual analogue scale V‐Q: ventilation–perfusion VTE: venous thromboembolism
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Comp 2001 | Treatment duration only four weeks |
| EPCAT II | Treatment duration only 30 days |
| Kristensen 1990 | Treatment not within scope of review: heparin plus indomethacin versus heparin plus placebo |
| Manganelli 1998 | Treatment duration only 30 days |
| NPHDO Study Group | Treatment duration was extended but not to five weeks |
| PENTHIFRA PLUS Study | Treatment duration only four weeks |
| Swedish Study | Treatment duration for only one month |
Differences between protocol and review
New authors have taken over this review.
The outcome 'Total VTE' was added as several studies did not report VTE (specifically DVT) as symptomatic or asymptomatic, making it difficult to place within the previously listed outcome definitions. This way more data could be combined for comparison, but needed to be done with caution as the outcomes would be more heterogenous.
'Clinically relevant non‐major bleeding' was added to the bleeding outcomes as this was commonly reported in the newer, larger trials, and does not fit well within major or minor bleeding categories.
The outcomes 'Wound infection', 'Wound healing' and 'Reoperation following surgery' were added as they are deemed important outcomes for orthopaedic surgeons (Wang 2014).
Calculation of number needed to treat for an additional beneficial outcome (NNTB) and needed to treat for an additional harmful outcome (NNTH) were removed as the calculations in a meta‐analysis setting can be misleading and should be treated with caution.
The method of evaluating study quality has changed since the protocol was published; we used the Cochrane 'Risk of bias' tool (Higgins 2011). We have also added 'Summary of findings' tables.
In the 'Sensitivity analysis' section, we have removed the indication to compare results using a fixed and random‐effects model, as we are using the I2 statistic to decide the appropriateness of the use of the different model types.
Contributions of authors
RF and MS selected the studies, extracted the data, performed the statistical analyses and wrote the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Incentive Award funding to Cochrane Vascular. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
RF: none known. MS: none known. MS is a member of Cochrane Vascular's editorial staff. To prevent any conflict of interest issues editorial decisions and activities related to this review were carried out by other editorial staff where appropriate.
New
References
References to studies included in this review
ADVANCE 3 {published data only}
- Lassen MR, Gallus A, Raskob GE, Pineo G, Chen D, Ramirez LM, et al. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. New England Journal of Medicine 2010;363(26):2487‐98. [DOI] [PubMed] [Google Scholar]
- NCT00423319. Study of an investigational drug for the prevention of thrombosis‐related events following hip replacement surgery (ADVANCE‐3). https://clinicaltrials.gov/ct2/show/NCT00423319?term=NCT00423319&rank=1 (accessed 30 August 2015).
- Pineo GF, Gallus AS, Raskob GE, Chen D, Ramirez L‐M, Ramacciotti E, et al. Apixaban after hip or knee arthroplasty versus enoxaparin: Efficacy and safety in key clinical subgroups. Journal of Thrombosis and Haemostasis 2013;11(3):444‐51. [DOI] [PubMed] [Google Scholar]
- Raskob GE, Gallus AS, Pineo GF, Chen D, Ramirez LM, Lassen MR. Apixaban versus enoxaparin for thromboprophylaxis after joint replacement surgery: pooled analysis of major venous thromboembolism and bleeding in 8,464 patients from the advance 2 and 3 trials [Abstract No. 192]. Blood. 2010; Vol. 116. [DOI] [PubMed]
- Raskob GE, Gallus AS, Pineo GF, Chen D, Ramirez LM, Wright RT, et al. Apixaban versus enoxaparin for thromboprophylaxis after hip or knee replacement: pooled analysis of major venous thromboembolism and bleeding in 8464 patients from the ADVANCE‐2 and ADVANCE‐3 trials. Journal of Bone and Joint Surgery. British Volume 2012;94(2):257‐64. [DOI] [PubMed] [Google Scholar]
Barrellier 2010 {published data only}
- Barrellier MT, Lebel B, Parienti JJ, Mismetti P, Dutheil JJ, Vielpeau C, et al. Short versus extended thromboprophylaxis after total knee arthroplasty: a randomized comparison. Thrombosis Research 2010;126(4):e298‐304. [DOI] [PubMed] [Google Scholar]
Dahl 1997 {published data only}
- Anon. Dalteparin new treatment duration: new dosage. Is prolonged prophylaxis needed?. Prescrire International 2000;9(45):197‐98. [PubMed] [Google Scholar]
- Arnesen H, Dahl OE, Aspelin T, Seljeflot I, Kierulf P, Lyberg T. Sustained prothrombotic profile after hip replacement surgery: the influence of prolonged prophylaxis with dalteparin. Journal of Thrombosis and Haemostasis 2003;1(5):971‐5. [DOI] [PubMed] [Google Scholar]
- Dahl OE, Andreassen G, Aspelin T, Muller C, Mathiesen P, Nyhus S, et al. Prolonged thromboprophylaxis following hip replacement surgery‐‐results of a double‐blind, prospective, randomised, placebo‐controlled study with dalteparin (Fragmin). Thrombosis and Haemostasis 1997;77(1):26‐31. [PubMed] [Google Scholar]
- Dahl OE, Andreassen G, Muller C, Mathisen P, Nyhus S, Aspelin T, et al. The effect of prolonged thromboprophylaxis with dalteparin on the frequency of deep vein thrombosis (DVT) and pulmonary embolism (PE) 35 days after hip replacement surgery (HRS). Thrombosis and Haemostasis 1995;73(6):1094‐Abstract No 743. [Google Scholar]
DaPP Study {published data only}
- Lassen MR, Borris LC. Prolonged thromboprophylaxis with low molecular weight heparin (Fragmin) after elective total hip arthroplasty ‐ a placebo controlled study. Thrombosis and Haemostasis. 1995; Vol. 73, issue 6:1104.
- Lassen MR, Borris LC, Anderson BS, Jensen HP, Skejo Bro HP, Andersen G, et al. Efficacy and safety of prolonged thromboprophylaxis with a low molecular weight heparin (dalteparin) after total hip arthroplasty‐‐the Danish Prolonged Prophylaxis (DaPP) Study. Thrombosis Research 1998;89(6):281‐7. [DOI] [PubMed] [Google Scholar]
EXTEND Study {published data only}
- Agnelli G, Eriksson BI, Cohen AT, Bergqvist D, Dahl OE, Lassen MR, et al. Safety assessment of new antithrombotic agents: Lessons from the EXTEND study on ximelagatran. Thrombosis Research 2009;123(3):488‐94. [DOI] [PubMed] [Google Scholar]
Fragmin Trial {published data only}
- Hull RD. New insights into extended prophylaxis after orthopaedic surgery ‐ the North American Fragmin Trial experience. Haemostasis. 2000; Vol. 30, issue Suppl 2:95‐100. [DOI] [PubMed]
- Hull RD, Pineo GF, Francis C, Bergqvist, D, Fellenius C, Soderberg K, et al. Low‐molecular‐weight heparin prophylaxis using dalteparin extended out‐of‐hospital vs in‐hospital warfarin/out‐of‐hospital placebo in hip arthroplasty patients: a double‐blind, randomized comparison. North American Fragmin Trial Investigators. Archives of Internal Medicine 2000;160(14):2208‐15. [DOI] [PubMed] [Google Scholar]
French Study {published data only}
- Planes A, Vochelle N. The post‐hospital discharge venous thrombosis risk of the orthopedic patient. Orthopedics 1997;20(2 Suppl):18‐21. [PubMed] [Google Scholar]
- Planes A, Vochelle N, Compan D, Weisslinger N, Huet Y. Efficacy and safety of prolonged administration of enoxaparin in the prevention of deep venous thrombosis after elective total hip replacement. Orthopaedic Transactions. 1996; Vol. 20, issue 1:274. [DOI] [PubMed]
- Planes A, Vochelle N, Darmon J‐Y, Fagola M, Bellaud M, Compan D, et al. Efficacy and safety of postdischarge administration of enoxaparin in the prevention of deep venous thrombosis after total hip replacement. A prospective randomised double‐blind placebo‐controlled trial. Drugs 1996;52(Suppl 7):47‐54. [DOI] [PubMed] [Google Scholar]
- Planes A, Vochelle N, Darmon J‐Y, Fagola M, Bellaud M, Huet Y. Risk of deep‐venous thrombosis after hospital discharge in patients having undergone total hip replacement: double‐blind randomised comparison of enoxaparin versus placebo. Lancet 1996;348(9022):224‐8. [DOI] [PubMed] [Google Scholar]
- Planes A, Vochelle N, Darmon JY. Out‐of‐hospital prophylaxis with low‐molecular‐weight heparin in hip surgery: the French study‐‐venographic outcome at 35 days. Chest 1998;114(2 Suppl Evidence):125S‐9S. [DOI] [PubMed] [Google Scholar]
Heit 2000 {published data only}
- Heit JA, Elliott CG, Trowbridge AA, Hirsh J, Gent M. Extended out‐of‐hospital LMWH venous thromboembolism prophylaxis after total hip or knee replacement surgery. Blood 1998;92:500a. [DOI] [PubMed] [Google Scholar]
- Heit JA, Elliott CG, Trowbridge AA, Morrey BF, Gent M, Hirsh J. Ardeparin sodium for extended out‐of‐hospital prophylaxis against venous thromboembolism after total hip or knee replacement. A randomized, double‐blind, placebo‐controlled trial. Annals of Internal Medicine 2000;132(11):853‐61. [DOI] [PubMed] [Google Scholar]
Kolb 2003 {published data only}
- Kolb G, Bodamer I, Galster H, Grambach K, Koudela K, Eisele RR, et al. Prolonged prophylaxis with the low molecular weight heparin certoparin reduces the rate of venous thromboembolism after orthopedic surgery. Journal of Thrombosis and Haemostasis. 2003; Vol. Suppl:Abstract: P1868. [DOI] [PubMed]
- Kolb G, Bodamer I, Galster H, Seidlmayer C, Grambach K, Koudela K, et al. Reduction of venous thromboembolism following prolonged prophylaxis with the low molecular weight heparin Certoparin after endoprothetic joint replacement or osteosynthesis of the lower limb in elderly patients. Thrombosis and Haemostasis 2003;90(6):1100‐5. [DOI] [PubMed] [Google Scholar]
Prandoni 2002 {published data only}
- Prandoni P, Bruchi O, Sabbion P, Tanduo C, Scudeller A, Sardella C, et al. Prolonged thromboprophylaxis with oral anticoagulants after total hip arthroplasty: a prospective controlled randomized study. Archives of Internal Medicine 2002;162(17):1966‐71. [DOI] [PubMed] [Google Scholar]
RECORD 1 Trial {published data only}
- Anon. Prevention of thromboembolism after knee replacement surgery: rivaroxaban one tablet/once daily superior to twice daily injectable enoxaparin in preventing venous blood clots after total knee replacement surgery in pivotal phase III trial. http://www.bayer.ca/ 2008.
- Cohn DM, Hermanides J, Devries JH, Kamphuisen PW, Kuhls S, Homering M, et al. Stress‐induced hyperglycaemia and venous thromboembolism following total hip or total knee arthroplasty. Analysis from the RECORD trials. Thrombosis and Haemostasis 2012;107(2):225‐231. [DOI] [PubMed] [Google Scholar]
- Eriksson BI, Borris LC, Friedman RJ, Haas S, Huisman MV, Kakkar AK, et al. Oral rivaroxaban compared with subcutaneous enoxaparin for extended thromboprophylaxis after total hip arthroplasty: The RECORD1 trial. Blood (ASH Annual Meeting Abstracts) 2007;110(11):6. [Google Scholar]
- Eriksson BI, Borris LC, Friedman RJ, Haas S, Huisman MV, Kakkar AK, et al. Oral rivaroxaban versus subcutaneous enoxaparin for extended thromboprophylaxis after total hip replacement: RECORD1 (abstract 222). British Journal of Haematology 2008;141(Suppl 1):82. [Google Scholar]
- Eriksson BI, Borris LC, Friedman RJ, Haas S, Huisman MV, Kakkar AK, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. New England Journal of Medicine 2008;358(26):2765‐75. [DOI] [PubMed] [Google Scholar]
- Eriksson BI, Kakkar AK, Turpie AG, Gent M, Bandel TJ, Homering M, et al. Oral rivaroxaban for the prevention of symptomatic venous thromboembolism after elective hip and knee replacement. Journal of Bone and Joint Surgery. British Volume 2009;91(5):636‐44. [DOI] [PubMed] [Google Scholar]
- Eriksson BI, Rosencher N, Friedman RJ, Homering M, Dahl OE. Concomitant use of medication with antiplatelet effects in patients receiving either rivaroxaban or enoxaparin after total hip or knee arthroplasty. Thrombosis Research 2012;130:147‐51. [DOI] [PubMed] [Google Scholar]
- Haas S, Borris LC, Friedman RJ, Huisman MV, Kakkar AK, Geerts W, et al. Rivaroxaban, an oral, direct factor Xa inhibitor in extended prophylaxis of thromboembolism after total hip replacement: RECORD1 (abstract O58). Pathophysiology of Haemostasis and Thrombosis 2008;36(Suppl 1):A15. [Google Scholar]
- Lassen MR, Gent M, Kakkar AK, Eriksson BI, Homering M, Berkowitz SD, et al. The effects of rivaroxaban on the complications of surgery after total hip or knee replacement: Results from the RECORD programme. Journal of Bone and Joint Surgery. British Volume 2012;94(11):1573‐8. [DOI] [PubMed] [Google Scholar]
- NCT00329628. Rivaroxaban (10mg) given once daily in patients undergoing total hip replacement compared to enoxaparin. https://clinicaltrials.gov/ct2/show/NCT00329628?term=NCT00329628&rank=1 (accessed 30 August 2015).
- Rosencher N, Llau JV, Mueck W, Loewe A, Berkowitz SD, Homering M. Incidence of neuraxial haematoma after total hip or knee surgery: RECORD programme (rivaroxaban vs. enoxaparin). Acta Anaesthesiologica Scandinavica 2013;57(5):565‐72. [DOI] [PubMed] [Google Scholar]
RECORD 2 Trial {published data only}
- Kakkar AK, Brenner B, Dahl OE, Eriksson BI, Mouret P, Bandel TJ, et al. A phase III study of extended thromboprophylaxis with oral rivaroxaban versus short‐term subcutaneous enoxaparin after total hip replacement: RECORD2 (abstract O59). Pathophysiology of Haemostasis and Thrombosis 2008;36(Suppl 1):A15. [Google Scholar]
- Kakkar AK, Brenner B, Dahl OE, Eriksson BI, Mouret P, Muntz J, et al. Extended duration rivaroxaban versus short‐term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double‐blind, randomised controlled trial. Lancet 2008;372(9632):31‐9. [DOI] [PubMed] [Google Scholar]
- Kakkar AK, Brenner B, Dahl OE, Eriksson BI, Mouret P, Muntz J, et al. Extended thromboprophylaxis with rivaroxaban compared with short‐term thromboprophylaxis with enoxaparin after total hip arthroplasty: The RECORD2 trial. Blood (ASH Annual Meeting Abstracts) 2007;110(11):307. [Google Scholar]
- Kakkar AK, Brenner B, Dahl OE, Eriksson BI, Mouret P, Muntz J, et al. RECORD2: extended thromboprophylaxis with rivaroxaban versus short‐term thromboprophylaxis with enoxaparin after total hip replacement (abstract 176). British Journal of Haematology 2008;141(Suppl 1):65. [Google Scholar]
- Mouret P, Kakkar AK, Brenner B, Dahl OE, Eriksson BI, Muntz J, et al. Extended thrombosis prophylaxis with rivaroxaban in comparison to short‐term thrombosis prophylaxis with enoxaparin after total hip replacement: The RECORD2 study. Medizinische Klinik 2008;103(3):15. [Google Scholar]
- NCT00332020. Regulation of coagulation in orthopedic surgery to prevent DVT and PE, a controlled, double‐blind, randomized study of BAY 59‐7939 in the extended prevention of VTE in patients undergoing elective total hip replacement (RECORD 2). https://clinicaltrials.gov/ct2/show/NCT00332020?term=NCT00332020&rank=1 (accessed 30 August 2015).
RE‐NOVATE II Trial {published data only}
- Eriksson BI, Dahl OE, Huo MH, Kurth AA, Hantel S, Hermansson K, et al. Oral dabigatran versus enoxaparin for thromboprophylaxis after primary total hip arthroplasty (RE‐NOVATE II): A randomised, double‐blind, non‐inferiority trial. Thrombosis and Haemostasis 2011;105(4):721‐9. [DOI] [PubMed] [Google Scholar]
- Huo M, Eriksson B, Dahl O, Kurth A, Hantel S, Hermasson K, et al. Oral dabigatran versus enoxaparin for thromboprophylaxis after primary total hip arthroplasty: the RE‐NOVATE II randomised trial [Abstract No. 0564]. Haematologica 2010; Vol. 92:233.
- NCT00657150. Dabigatran etexilate compared with enoxaparin in prevention of venous thromboembolism (VTE) following total hip arthroplasty. https://clinicaltrials.gov/ct2/show/NCT00657150?term=RE‐NOVATE&rank=1 (accessed 30 August 2015).
RE‐NOVATE Trial {published data only}
- Eriksson BI, Dahl OE, Rosencher N, Kurth AA, Dijk CN, Frostick SP, et al. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double‐blind, non‐inferiority trial. Lancet 2007;370(9591):949‐56. [DOI] [PubMed] [Google Scholar]
- Eriksson BI, Dahl OE, Rosencher N, Kurth AA, Dijk N, Frostick SP, et al. Dabigatran etexilate is effective and safe for the extended prevention of venous thromboembolism following total hip replacement (abstract OW‐049). Journal of Thrombosis and Haemostasis 2007;5(Suppl 2):OW‐049. [DOI] [PubMed] [Google Scholar]
- NCT00168818. Dabigatran etexilate in extended VTE prevention after hip replacement surgery. https://clinicaltrials.gov/ct2/show/NCT00168818?term=RE‐NOVATE&rank=2 (accessed 30 August 2015).
SACRE Study {published data only}
- Samama CM, Vray M, Barre J, Fiessinger JN, Rosencher N, Lecompte T, et al. Extended venous thromboembolism prophylaxis after total hip replacement: a comparison of low‐molecular‐weight heparin with oral anticoagulant. Archives of Internal Medicine 2002;162(19):2191‐6. [DOI] [PubMed] [Google Scholar]
Zhang 2014 {published data only}
- Zhang H, Lin J, Li H, Guan Z, Zhou D, Kon B, et al. Effects of thromboprophylaxis duration on coagulation indicators after total hip replacement. National Medical Journal of China 2014;94(7):525‐8. [PubMed] [Google Scholar]
References to studies excluded from this review
Comp 2001 {published data only}
- Comp PC, Spiro TE, Friedman RJ, Whitsett TL, Johnson GJ, Gardiner GA, et al. Prolonged enoxaparin therapy to prevent venous thromboembolism after primary hip or knee replacement. Enoxaparin Clinical Trial Group. Journal of Bone & Joint Surgery ‐ American Volume 2001;83‐A(3):336‐45. [DOI] [PubMed] [Google Scholar]
- Spiro TE. A double‐blind multicenter clinical trial comparing long term enoxaparin and placebo treatments in the prevention of venous thromboembolic disease after hip and knee replacement surgery. Blood. 1997; Vol. 90, issue Suppl 1:295a.
EPCAT II {published data only}
- NCT01720108. Extended venous thromboembolism prophylaxis comparing rivaroxaban to aspirin following total hip and knee arthroplasty (EPCAT II). https://clinicaltrials.gov/ct2/show/NCT01720108 (accessed 30 August 2015).
Kristensen 1990 {published data only}
- Kristensen SS, Pedersen P, Pedersen NW, Schmidt SA, Kjaersgaard‐Andersen P. Combined treatment with indomethacin and low‐dose heparin after total hip replacement. Journal of Bone and Joint Surgery. American Volume 1990;72:447‐9. [DOI] [PubMed] [Google Scholar]
Manganelli 1998 {published data only}
- Manganelli D, Pazzagli M, Mazzantini D, Punzi G, Manca M, Vignali C, et al. Prolonged prophylaxis with unfractioned heparin is effective to reduce delayed deep vein thrombosis in total hip replacement. Respiration 1998;65(5):369‐74. [DOI] [PubMed] [Google Scholar]
- Palla A, Manganelli D, Rossi G. Prolonged prophylaxis with unfractioned heparin (UH) is effective to reduce delayed deep vein thrombosis (DVT) in total hip replacement (THR). European Respiratory Journal. 1997; Vol. 10, issue Suppl 25:5S. [DOI] [PubMed]
NPHDO Study Group {published data only}
- Haentjens P, Delince P, The Belgian Nadroparin Post‐Hospital Discharge In Orthopedics (NPHDO) Study Group. Prevention of venous thromboembolism after hospital discharge. Continued pharmacologic prophylaxis versus no prophylaxis in patients undergoing total hip replacement. Hip International 2001;11(1):25‐36. [Google Scholar]
- Haentjens P, The Belgian Nadroparin Post‐Hospital Discharge in Orthopedics (NPHDO) Study Group. Post‐hospital discharge prevention of deep vein thrombosis with nadroparin calcium after elective total hip replacement. British Journal of Anaesthesia 1999;82(Suppl 1):78. [Google Scholar]
- The Belgian Nadroparin Post Hospital Discharge in Orthopedics (NPHDO) Study Group. Post hospital discharge prevention of deep vein thrombosis with nadroparin calcium after total hip arthroplasty. Haemostasis. 1998; Vol. 28, issue Suppl 2:292.
PENTHIFRA PLUS Study {published data only}
- Anon. A multicenter, multinational, randomized, double‐blind study of fondaparinux sodium (Org31540/SR90107A) versus placebo for the prolonged prevention of VTE in hip fracture surgery. (PENTIHFRA PLUS). Study No: EFC4582. GlaxoSmithKline Clinical Trial Register 2005.
- Bauer KA, Eriksson BI, Lassen MR, Turpie AGG. No episode of thrombocytopenia after four‐week administration of fondaparinux, a new synthetic and selective inhibitor of factor Xa, in the PENTHIFRA‐PLUS study. Journal of Thrombosis and Haemostasis 2003;1(Suppl 1):P2050. [Google Scholar]
- Dobesh PP. Novel concepts: emerging data and the role of extended prophylaxis following hip fracture surgery. American Journal of Health‐System Pharmacy 2003;60(22 Suppl 7):S15‐9. [DOI] [PubMed] [Google Scholar]
- Eriksson BI. A multicenter, randomized, placebo‐controlled, double‐blind study of fondaparinux for the prolonged prevention of venous thromboembolism in hip fracture surgery. Abstracts of the Sicot/Sirot XXII World Congress 2002:318.
- Eriksson BI, Lassen MR. Consistency of efficacy of extended thromboprophylaxis with fondaparinux (arixtra) in prevention of venous thromboembolism (VTE) after hip fracture surgery according to different composite efficacy endpoints: the PENTHIFRA‐PLUS study. Journal of Thrombosis and Haemostasis 2003;1(Suppl 1):Abstract P2064. [Google Scholar]
- Eriksson BI, Lassen MR. Duration of prophylaxis against venous thromboembolism with fondaparinux after hip fracture surgery: A multicenter, randomized, placebo‐controlled, double‐blind study. Archives of Internal Medicine 2003 Jun 9;163(11):1337‐42. [DOI] [PubMed] [Google Scholar]
- Eriksson BI, Lassen MR, Colwell CW, Jr. Efficacy of fondaparinux for thromboprophylaxis in hip fracture patients. Journal of Arthroplasty 2004;19(7 Suppl 2):78‐81. [DOI] [PubMed] [Google Scholar]
- Lassen M, Bauer KA, Eriksson BI, Turpie AGG. Absence of transaminase increase after 4‐week administration of fondaparinux (Arixtra®) a new synthetic and selective inhibitor of factor Xa in the PENTHIFRA‐PLUS study. Journal of Thrombosis and Haemostasis 2003;1(Suppl 1):Abstract number: P2052. [Google Scholar]
Swedish Study {published data only}
- Bergqvist D, Benoni G, Bjorgell O, Fredin H, Hedlundh U, Nicolas S, et al. Low‐molecular‐weight heparin (enoxaparin) as prophylaxis against venous thromboembolism after total hip replacement. New England Journal of Medicine 1996;335(10):696‐700. [DOI] [PubMed] [Google Scholar]
- Bergqvist D, Jönsson B. Cost‐effectiveness of prolonged out‐of‐hospital prophylaxis with low‐molecular‐weight heparin following total hip replacement. Haemostasis 2000;30 Suppl 2:130‐5. [DOI] [PubMed] [Google Scholar]
- Nilsson PE, Bergqvist D, Benoni G, Bjorgell O, Fredin H, Hedlund U, et al. The post‐discharge prophylactic management of the orthopedic patient with low‐molecular‐weight heparin: enoxaparin. Orthopedics 1997;20 Suppl:22‐5. [PubMed] [Google Scholar]
Additional references
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cushman 2007
- Cushman M. Epidemiology and risk factors for venous thrombosis. Seminars in Hematology 2007;44(2):62‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dotzel 2002
- Dotzel, MM. Determination that ardeparin sodium injection was not withdrawn from sale for reasons of safety or effectiveness. US Food and Drug Administration 2002:http://www.fda.gov/ohrms/dockets/98fr/052302b.htm (date accessed September 2015).
Egger 1997
- Egger M, Davey SG, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eikelboom 2001
- Eikelboom JW, Quinlan DJ, Douketis JD. Extended‐duration prophylaxis against venous thromboembolism after total hip or knee replacement: a meta‐analysis of the randomised trials. Lancet 2001;358(9275):9‐15. [DOI] [PubMed] [Google Scholar]
Eriksson 1997
- Eriksson BI, Wille‐Jorgensen P, Kalebo P, Mouret P, Rosencher N, Bosch P, et al. A comparison of recombinant hirudin with a low‐molecular‐weight heparin to prevent thromboembolic complications after total hip replacement [see comments]. New England Journal of Medicine 1997;337(19):1329‐35. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime, Inc). Available from www.gradepro.org. GRADEpro Guideline Development Tool (GRADEpro GDT). McMaster University (developed by Evidence Prime, Inc). Available from www.gradepro.org, 2015.
Guyatt 2012
- Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ, American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest 2012;141(2 Suppl):7S‐47S. [DOI: 10.1378/chest.1412S3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heit 2015
- Heit JA. Epidemiology of venous thromboembolism. Nature Reviews Cardiology 2015;12:464–74. [DOI: 10.1038/nrcardio.2015.83] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hirsh 1998
- Hirsh J. Evidence for the needs of out‐of‐hospital thrombosis prophylaxis: introduction. Chest 1998;114(2 Suppl Evidence):113S‐4S. [DOI] [PubMed] [Google Scholar]
Lau 1998
- Lau J, Ioannidis JP, Schmid CH. Summing up evidence: one answer is not always enough. Lancet 1998;351(9096):123‐7. [DOI] [PubMed] [Google Scholar]
Leclerc 1998
- Leclerc JR, Gent M, Hirsh J, Geerts WH, Ginsberg JS. The incidence of symptomatic venous thromboembolism after enoxaparin prophylaxis in lower extremity arthroplasty: a cohort study of 1,984 patients. Chest 1998;114(2 Suppl II):115S‐8S. [DOI] [PubMed] [Google Scholar]
Liew 2014
- Liew A. Extended‐duration new oral anticoagulants for venous thromboprophylaxis in patients undergoing total hip arthroplasty: A meta‐analysis of the randomized controlled trials. Journal of Thrombosis and Haemostasis 2014;12(1):107‐9. [DOI] [PubMed] [Google Scholar]
Mohr 1993
- Mohr DN, Silverstein MD, Murtaugh PA, Harrison JM. Prophylactic agents for venous thrombosis in elective hip surgery: Meta‐analysis of studies using venographic assessment. Archives of Internal Medicine 1993;153(19):2221‐8. [PubMed] [Google Scholar]
NICE 2010
- National Clinical Guideline Centre. Venous thromboembolism: reducing the risk of venous thromboembolism (deep vein thrombosis and pulmonary embolism) in patients admitted to hospital. Methods evidence and guidance. http://www.nice.org.uk/guidance/cg92/evidence/cg92‐venous‐thromboembolism‐reducing‐the‐risk‐full‐guideline3 (accessed September 2015).
NICE 2012
- National Clinical Guideline Centre. Venous thromboembolic diseases: the management of venous thromboembolic diseases and the role of thrombophilia testing. Clinical Guideline. Methods, evidence and recommendations. http://www.nice.org.uk/guidance/cg144/evidence/cg144‐venous‐thromboembolic‐diseases‐full‐guideline3 (accessed September 2015).
Nurmohamed 1992
- Nurmohamed MT, Rosendaal FR, Buller HR, Dekker E, Hommes DW, Vandenbroucke JP, et al. Low‐molecular‐weight heparin versus standard heparin in general and orthopaedic surgery: a meta‐analysis. Lancet 1992;340(8812):152‐6. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robinson 1997
- Robinson KS, Anderson DR, Gross M, Petrie D, Leighton R, Stanish W, et al. Ultrasonographic screening before hospital discharge for deep venous thrombosis after arthroplasty: the post‐arthroplasty screening study. A randomized, controlled trial. Annals of Internal Medicine 1997;127(6):439‐45. [DOI] [PubMed] [Google Scholar]
SIGN 2010
- Scottish Intercollegiate Guidelines Network. Thrombosis Guidelines Prevention and management of venous thromboembolism. A national clinical guideline. http://www.sign.ac.uk/pdf/sign122.pdf (accessed September 2015).
Thompson 1994
- Thompson SG. Why sources of heterogeneity in meta‐analysis should be investigated. BMJ (Clinical research ed.) 1994;309(6965):1351‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2014
- Wang Z, Anderson FA Jr, Ward M, Bhattacharyya T. Surgical site infections and other postoperative complications following prophylactic anticoagulation in total joint arthroplasty. PLoS ONE 2014;9(4):e91755. [10.1371/journal.pone.0091755. eCollection 2014] [DOI] [PMC free article] [PubMed] [Google Scholar]
White 2003
- White RH. The epidemiology of venous thromboembolism. Circulation 2003;107:I‐4–I‐8. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Quinlan 2002
- Quinlan DJ, Eikelboom JW, Douketis JD. Anticoagulants (extended duration) for prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair. Cochrane Database of Systematic Reviews 2002, Issue 2. [DOI: 10.1002/14651858.CD004179] [DOI] [PMC free article] [PubMed] [Google Scholar]


