Abstract
Purpose of review
The pathophysiological understanding of kidney related disorders has profoundly increased, however tissue- and cell-specific treatments in this field remain scarce. Advances in nanomedicine enable alteration of pharmacokinetics and targeted treatments improving efficiency and reducing toxicity. This review addresses recent developments of nanocarriers used for various purposes in the broad field of kidney disease, which may pave a path to therapeutic and diagnostic solutions for kidney disease.
Recent findings
Controlled delivery of antiproliferative medications enables improved treatment of polycystic kidney disease and fibrosis. Directed anti-inflammatory treatment mitigated glomerulonephritis and tubulointerstitial nephritis. Multiple injury pathways in AKI have been targeted, with therapeutic solutions for oxidative stress, mitochondrial dysfunction, local inflammation and improving self-repair mechanisms. In addition to the treatment development, non-invasive extremely early detection methods (minutes after ischemic insult) have been demonstrated as well. Sustained release of therapies to reduce ischemia reperfusion injury, and new aspects for immunosuppression bring hope to improving kidney transplant outcomes. The latest breakthroughs in gene therapy are made achievable with engineered targeted delivery of DNA and siRNA for new treatments of kidney disease.
Summary
The advances in nanotechnology and pathophysiological understanding of kidney diseases show potential for translatable therapeutic and diagnostic interventions in multiple etiologies of kidney disease.
Keywords: nanoparticles, targeting, reduced-toxicity, oxidative-stress, ischemia-reperfusion-injury
Introduction
Despite a profound increase in the pathophysiological understanding of kidney related disorders, treatment options are notably limited. Regardless of the specific etiology, the standard line of treatment remains limited to risk factor control for chronic kidney diseases (CKD), hemodynamic stabilization to allow for self-repair mechanism of acute kidney injury (AKI), and systemic immunosuppression for some glomerular diseases. Non-optimal pharmacokinetics, off-target toxicity, low efficacy, and molecular instability are a few of the current challenges preventing development of positive therapeutic outcomes. Nanotechnology has recently emerged as a potential solution to these issues that could additionally present new treatment strategies for kidney disorders.
Nanoparticles are customizable therapeutic delivery vehicles possessing physicochemical properties that can be designed to controllably alter drug pharmacokinetics and pharmacodynamics (PK/PD), allowing for less toxicity, higher efficacy, improved stability, and thus new applications (Fig 1) [1]. These vehicles come in a variety of forms but are most commonly observed as dense solid core metallic or polymeric spheres or as self-assembled micelles or vesicles, which are respectively solid or hollow core spherical aggregates of lipid or polymer amphiphiles (Fig 2). The diverse types of nanoparticles and their wide range of compositions and applications are not the topic of this review and have been thoroughly discussed elsewhere [1–4]. While small molecules are an indispensable part of modern treatment, limitations in their development and administration can be overcome using nanoparticles. As an example, the field of cancer therapy has widely explored and benefited from nanoparticle-based formulations, where their enhanced organ, tissue, and cell specific targeting play a vital role in safer and FDA-approved chemotherapies[2]. Importantly, nanoparticles are an essential component of cutting-edge therapeutic strategies involving delivery of biologics and gene therapy, the latter of which was recently evidenced by the success of mRNA vaccine [5].
Figure 1.
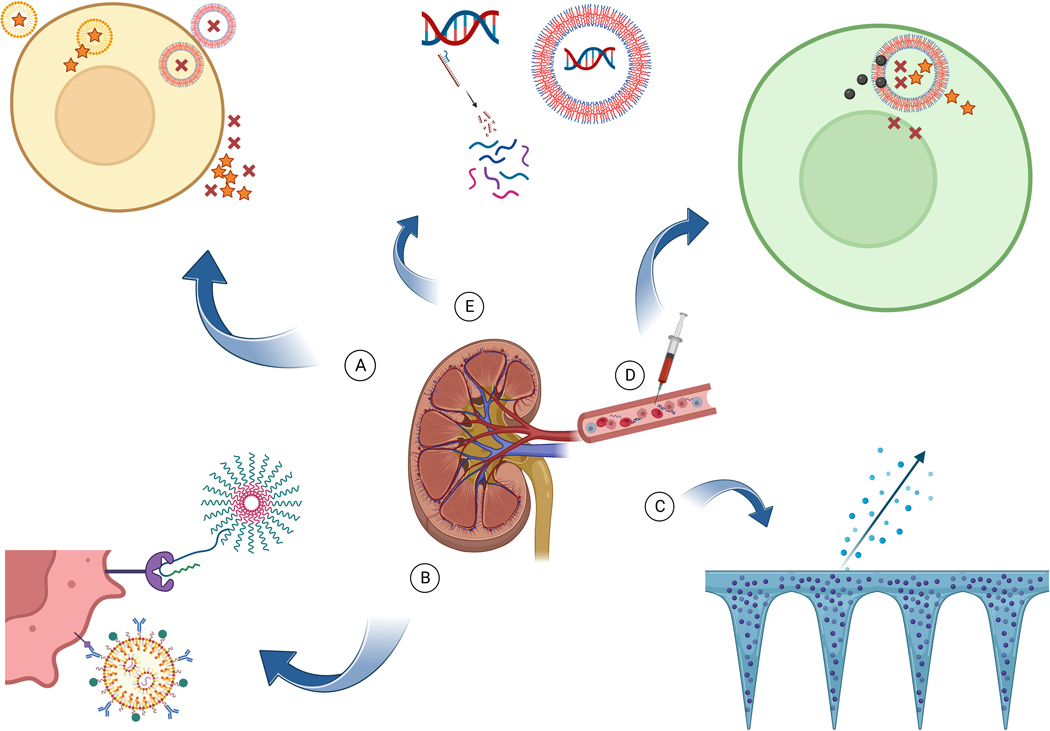
Advantages of nanoparticles
A. Therapeutic molecules are not taken up intracellularly although when loaded in nanoparticles they enter the cell and are released inside.
B. Specific cellular targeting with ligands (antibody, peptide etc.) specific to the cell/condition
C. Different forms of nanostructures can allow local sustained release of therapeutics.
D. Several different molecules are co-delivered to the cell for synergistic effect.
E. Nucleic acids (DNA/RNA) are degraded when exposed in the serum although remain protected when loaded in nanoparticles.
Figure 2.
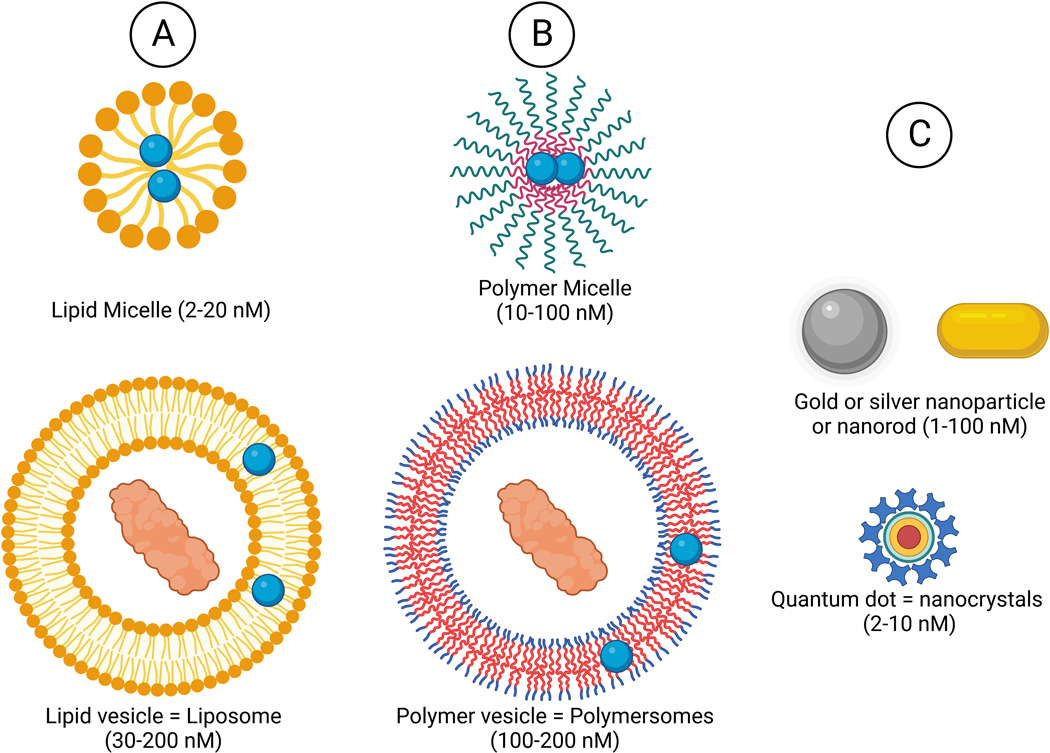
Main types of nanoparticle composition and morphology
A. Lipid
B. Synthetic and biological polymer
C. Inorganic
Vesicle shaped nanoparticle (polymersome, liposome) - hydrophilic core can load hydrophilic molecules and hydrophobic bilayer can load hydrophobic molecules.
Micelles - Hydrophobic core can load hydrophobic molecules.
Inorganic nanoparticles - therapeutic effect of the element itself, and inorganic element core with additional therapeutics
Compared to cancer and infectious disease, the utility of nanoparticles in kidney related disorders has received considerably less attention. Though slowly and steadily new therapeutics are being discovered for kidney diseases, simultaneous exploration of nanotechnology focused on kidney targeted drug delivery systems should elevate the search for better and safer kidney medicine [6–8]. Here we bring an updated review of recent seminal breakthroughs in the field of kidney disease focused nanomedicine (Table 1).
Table 1:
Summary of recent nanoparticle formulations for kidney disease
| Indication | material | Morphology (nM) | cargo | Targeting Ligand (target) | Targeted cell type | result | Ref. |
|---|---|---|---|---|---|---|---|
| ADPKD | DSPE-PEG2k | Micelle (11–16) |
Rapamycin or salsalate | [KKEEE]3K (megalin) |
PTEC | NP combined with free metformin inhibited in-vitro cell proliferation and cyst growth | [1] |
| ADPKD | DSPE-PEG2k | Micelle (10–12) | Various mTOR inhibitors | CKDSPKSSKSIRFIPVST | CCD | Enhanced in-vitro antiproliferative effect | [2] |
| ADPKD | Chitosan-DSPE-PEG2k | Micelle (155) | Metformin | [KKEEE]3K (megalin) | PTEC | Reduced cystic % and kidney weight compared to free drug | [3] |
| DN | Gold (HAuCl4) | (157) | Gold | - | - | reduced in vitro ROS & apoptosis through SIRT3-SOD2 signaling | [4] |
| DN | Zinc oxide | Spherical (<50) | Zinc oxide | - | - | Slowed DN via interplay of autophagy and Nrf2/TXNIP/NLRP3 inflammasome signaling | [5] |
| GN | Lipid, DSPE-PEG | Spherical (114) |
Celastrol | VHPKQHRGGSKGC (VCAM-1) | GEC | Improved renal outcome, anti-inflammatory effect through eNOS increase, reduction of VCAM-1 expression. | [6] |
| TIN | Gold nanoparticles | Spherical (11–25) |
Gold nanoparticles | - | PTEC | Prevented tubule-interstitial injury, anti-inflammatory activity, reduced fibrosis, | [7] |
| Fibrosis | [2-(diisopropylamino) ethyl methacrylate] (PDPA)/PEG | Micelle (59) | Nitric oxide donor: (DNIC; [Fe2(μ-SEt)2(NO)4]) | pH-responsive polymer | Fibrotic kidney tissue | Suppressed myofibroblast activation and collagen I production | [8] |
| Fibrosis | Poly(lactic-co-glycolic acid) | Vesicle (70–80) |
Sorafenib | SLYQTDDRNDYI & RDYHPRDHTATW | Myo-fibroblast | Alleviates renal fibrosis | [9] |
| Fibrosis | Chitosan/GFP | Polyplex (150) |
Metformin | Chitosan (megalin) | RTEC | Improved anti-apoptotic, anti-inflammatory and anti-fibrotic effects | [10] |
| AKI | Gold | spherical (2–3) |
N-acetylcysteine | Passive [size] | Kidney | Reduced kidney ROS, inflammation, AKI and rodent mortality post rhabdomyolysis | [11] |
| AKI | Polyvinylpyrrolidone-curcumin | Micelles (5–8) | Curcumin and Zirconium-89 | Curcumin, size | Kidney | Reduced ROS, mitochondrial damage and AKI, PET-CT imaging | [12] |
| AKI | PEG-b-PAGA + (PDMAEMA-r-PAAPBA)-b-PPBAE | Spherical (141) | Curcumin | pH reactive size de-shelling and ROS reactive release | RTEC | Activated autophagy, reduced mitochondrial injury, ROS, ER (endoplasmic reticulum) stress, apoptosis, and AKI | [13] |
| AKI | D-α-tocopherol PEG1K succinate | Micelle (120 ) | Celastrol | Albumin coating | RTEC | Reduced systemic adverse effects while alleviating ischemia-reperfusion injury | [14] |
| AKI | Chitosan | Spherical (4.5) | Prussian blue | - | - | ROS reduction and reduced rodent mortality from AKI (rhabdomyolysis or cisplatin induced) | [15] |
| AKI | Solutol HS15 (12-hydroxystearic acid-PEG) | Micelle (13) | Myricetin | Size | Kidney | Reduced AKI, reduced ROS, inhibited cisplatin-induced activation of the DNA damage-cGAS−STING pathway. | [16] |
| AKI | Germanene | Nanosheet (0.8) |
Hydrogen | Passive | RTEC | Reduced ROS and AKI compared to NAC | [17] |
| AKI | PEGylated melanin | Spherical (25–40) | PJ34 (PARP-1 inhibitor) and melanin | Antibody (GPR97) | Injured RTEC | Reduced ROS, apoptosis, inflammation, activity of Keap-1/Nrf2/HO-1 & PARP-1/AIF pathways | [18] |
| AKI | Mn3O4 | Flower (110) | Mn3O4 | Passive | Kidney | Reduced ROS, cfDNA, apoptosis and AKI | [19] |
| AKI | Hyaluronic-acid, bilirubin | Micelle (85) | Prodrug of calcium ion chelator | Hyaluronic acid (CD44) | injured PTEC | Reduced ROS, intra cellular calcium, ER stress, apoptosis pathways, TNFα and AKI | [20] |
| AKI | PEG methyl ether-bl-poly(lactide-co-glycolide) | Spherical (442) | Formoterol (long acting β2 agonist) | Passive | PTEC | Kidney expression and reduced myocardial expression of mitochondrial biogenesis | [21] |
| AKI | Gallic acid-gallium polyvinyl pyrrolidone | spherical (25) |
Gallium | - | - | Reduced intracellular free iron, mitochondrial damage, and AKI, inhibited cisplatin induced ferroptosis in vitro | [22] |
| AKI | Phenylene-cyclam, hexa-methylene-bi-sacryl-amide | Spherical polyplex (127) |
p53 siRNA, CXCR4a | Poly[amido amine] CXCR4 antagonist (CXCR) | RTEC | Reduced p53 mRNA and AKI | [23] |
| Indication | Material | Morphology (nM) | Cargo | Targeting Ligand (target) | Targeted cell type | result | Ref. |
| AKI | Chitosan modified with α-cyclam-ptoluic acid | Polyplex (129) |
p53 siRNA, α-cyclam-ptoluic acid (CXCR4a) | α-cyclam-ptoluic acid (CXCR4) | Injured RTEC | Reduced p53 transcription, apoptosis, macrophage and neutrophil infiltration, and AKI | [24] |
| AKI | Polyethyleneimine | Polymer | CXCR4 antagonist | Poly hydroxyl | RTEC | Increased uptake to IRI kidney | [25] |
| AKI | M13 DNA strands | rDON | IL-33 cytokine | Passive [size] | Kidney | Increased kidney Tregs and recovery from IRI, macrophages shifted from M1 to M2 | [26] |
| AKI detection | Aza-boron-dipyrromethene | Brush shaped polyplex (4) | aza-boron-dipyrromethene (aza-BODIPY) |
cRGD | Kidney | Renal-cleared probe for non-invasive diagnosis of renal IRI, with 10x brightness then previous study | [27] |
| AKI detection | Activatable small molecule | Small molecule | NIR-II probe | (Externalized phosphatidylserine and activate caspase) | Apoptotic cells | Detected AKI 24h post IV cisplatin, while Cr and BUN were not yet significantly elevated | [28] |
| AKI | M13mp18 DNA | rDON | Gold Nanorods |
Passive [size] (NIR-II triggered by miR21 upregulation) |
Kidney | Allowed detection of AKI 10 minutes after IRI and increased ROS scavenger function instantaneously | [29] |
| IRI | M13 DNA | rDON (2*60*90) | Anti C5a aptamers | Passive [size] | Kidney | Reduced renal and systemic complement inflammation, ROS and AKI 24h post IRI | [30] |
| ACR | N Polystyrene | Spherical (500) | Isothiocyanate | (MARCO) | inflammatory monocytes | Reduced ACR and mortality compared to no-immunosuppression | [31] |
| RCC | Poly-L-lysine hydrobromide | Spherical (3–4) | yitrium (90Y) | L-Serine residues (KIM1) | Injured RTEC | Reduced kidney tumor growth, reduced nephrotoxicity | [32] |
| RCC | DSPE-PEG2k | Micelle (14) | HIF2α siRNA | peptide derived from CD27 (CD70) | Clear cell RCC | ~70% gene knockdown of HIF2 and its downstream genes | [33] |
| DNA/RNA delivery | Polyethyleneimine, sorbitol | polyplex (69–228) |
Plasmid DNA | Chitobionic acid (Vimentin) | PTEC | Enhanced transfection efficiency | [34] |
ACR – Acute cellular rejection, AIF - apoptosis-inducing factor, CCD – Cortical collecting duct, CD - Cluster of differentiation, cGAS - Cyclic GMP–AMP synthase, DN – Diabetic nephropathy, DSPE - 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine, eNOS - Endothelial NO synthase, GEC – Glomerular endothelial cells, GFP – Green fluorescent protein, HIF - Hypoxia-inducible factor, KEAP1 - Kelch-like ECH-associated protein 1, KIM1 - Kidney Injury Molecule-1, MΦ - inflammatory monocytes, mTOR- Mammalian target of rapamycin, NLRP3 - nod, LRR and pyrin domain-containing protein 3, NRF2 - nuclear factor erythroid 2–related factor 2, PAAPBA- poly2-(acrylamido)phenylboronic acid, PAGA- poly N-acryloyl glucosamine, PARP-1 - Poly [ADP-ribose] polymerase 1, PDPA - poly(2-(diisopropylamino)ethyl methacrylate, PDMAEMA- poly-2-(dimethylamino)ethyl methacrylate, PEG – Polyethylene glycol, PPBAE- poly(beta-amino esters), rDON - Rectangular DNA origami nanocarriers, SOD2 - superoxide dismutase 2, STING - stimulator of interferon genes, TNFα - Tumor necrosis factor α, Treg – regulatory T cell, TXNIP - thioredoxin-interacting protein, VCAM-1 - Vascular cell adhesion protein 1
Chronic kidney Disease (CKD)
Multiple etiologies lead to CKD, resulting in reduced kidney function. The common endpoint phenotype is interstitial fibrosis, with a common groom prognosis and need for dialysis or transplant. The reduced renal clearance aggravates pharmacokinetic and toxicity limitations of current therapeutics. Recent advances in nanoparticle facilitated, controlled, and targeted delivery of small molecules and biologics have opened new paradigms for multidirectional interventions.
Autosomal Dominant Polycystic Kidney Disease (ADPKD), is caused by a second hit mutation in the PKD genes, resulting in increased proliferation of renal tubular epithelial cells (RTEC) and leading to cyst formation and eventually end stage renal disease. Direct M-TOR inhibitors (M-TORi) and Metformin have been shown in experimental models to reduce proliferation of the RTEC in ADPKD, however their clinical applications are limited due to off-target toxicity. M-TORi or Metformin loaded peptide amphiphile micelles, targeted to proximal RTEC (PTEC) or to the cortical collecting duct, significantly improved reduction of epithelial cell proliferation and cyst growth compared to the free drugs [9–11]*. Encapsulating these ~14 nm nanoparticles into larger 130 nm chitosan nanoparticles for protection from low pH environments allowed for oral administration with similar positive effects, overcoming the need for chronic parental administration in a lifelong condition [11].
Diabetic Nephropathy is the most common etiology for end stage renal disease (ESRD). While the focus in diabetes has for decades been on blood glucose control for prevention of complications, new therapies improving clinical outcomes have started to evolve. However, specific treatments designed for diabetic nephropathy damage pathways are rare. Chronic exposure to elevated blood sugars generates advanced glycation end products, with constant oxidative stress resulting in kidney damage. The use of gold salt for the treatment of various medical conditions has for decades been attempted and balanced against the many side effects including nephrotoxicity. Recently, gold nanoparticles [12] and zinc oxide nanoparticles targeted to PTEC [13] were both found to reduce diabetic kidney injury. These results are encouraging, although further development is needed to address chronic disease.
Glomerular Nephropathy, as opposed to many CKD etiologies, is commonly treated. That said, the main treatment of all types of glomerular nephropathy is systemic immunosuppression, which is associated with a broad range of adverse effects and remains an ongoing controversial subject as the use of corticosteroid treatment for IgA nephropathy. Nanoparticles targeted to inflamed glomerular endothelium were loaded with a traditional Chinese root extract possessing anti-inflammatory effects, which reduced kidney disfunction and proteinuria in advanced IgA nephropathy. The anti-inflammatory properties were shown to be driven by eNOS increased expression and VCAM-1 expression reduction [14]*.
Tubulointerstitial nephropathy (TIN) is caused by multiple etiologies, often by commonly used medications such as nonsteroidal anti-inflammatory agents, antibiotics, and proton pump inhibitors. This nephropathy does not necessarily cause glomerular damage, thus serum creatinine remains normal, although tubular dysfunction, including non-nephrotic proteinuria, eventually evolves to fibrosis and CKD. Since there are no available specific treatments for TIN, there has been interest in assessing the role of gold nanoparticles (AuNP) in alleviating this often-misdiagnosed syndrome. Peres et al. [15]* demonstrated AuNP delivery to ameliorate the reduction of megalin-mediated albumin endocytosis in PTECs, preventing tubular albuminuria and switching from a pro-inflammatory to an anti-inflammatory profile. This was measured by reduction in the Th1 (IFN-γ) and Th17 (IL-17) responses and an increase in the Th2 (IL-4) response. They also demonstrated lowered collagen deposition associated with a decrease in urinary γ-GT and LDH activities.
Kidney Interstitial Fibrosis, the common end point of most nephropathies, bares the heaviest negative prognostic value among histologic findings. Myofibroblasts are key players in renal fibrosis. Nitric oxide (NO) is toxic to myofibroblasts, although its low bioavailability and broad spectrum of effects on other tissues prevents systemic NO use for fibrosis. NO donor molecules loaded in pH responsive micelles were designed for payload release at acidic conditions (in areas of ongoing tissue damage as well as intracellularly, in the lysosome). These nanoparticles had increased uptake in fibrotic kidneys and significantly reduced fibrosis [16]*. Several nanoparticle formulations have shown promise in the kidney by repurposing antifibrotics that were already approved for other indications. Targeted peptide-based nanoparticles loaded with the multi-kinase inhibitor Sorafenib, which is FDA approved for treatment of several carcinomas, were found to suppress myofibroblast activation and reduce kidney fibrosis [17]*. Metformin has antifibrotic effects on kidneys and other organs, although requires a dose five times higher than used for its usual diabetes treatment. Furthermore, reduced GFR increases the risk of metformin associated lactic acidosis preventing this treatment for fibrosis. Once loaded within nanoparticles and targeted to RTEC, metformin demonstrated superior anti-apoptotic, anti-inflammatory, and anti-fibrotic effects compared to its free form [18]*.
Acute Kidney injury (AKI)
Few clinical tools exist to reduce AKI aside from hemodynamic stabilization and avoidance from additional nephrotoxic insults. Although the pathophysiology of the tubular damage has been extensively studied to reveal several pathways responsible for maladaptive AKI recovery, translation into clinical interventions has yet to occur. It is this setting that brings multiple attempts to utilize nanomedicine to achieve safe therapeutic interventions.
Increased oxidative stress and accumulation of reactive oxygen species (ROS) in RTEC are a critical aspect of the AKI damage cascade. The vast majority of AKI is prerenal and derives from hypovolemia or has evolved into ischemic acute tubular necrosis (ATN), therefore, most nanomedicine interventions have been tested on an Ischemia reperfusion injury (IRI) model. N-Acetyl Cysteine (NAC) is effective in tubular ROS reduction and minimization of rodent renal injury. However, low bioavailability and minimal renal accumulation hamper any clinical benefit and recommendations for contrast induced nephropathy prevention were removed from the guidelines. Gold nanoclusters capped with NAC demonstrated improved antioxidative and anti-inflammatory effects compared to free NAC, achieving AKI reduction and reduced rodent mortality [19]*. Several other substances that were traditionally known to act as antioxidants have been loaded into nanoparticles targeted to RTEC, successfully reducing oxidative stress in the injured kidney, and mitigating kidney dysfunction [20–26]*. A promising strategy has been to co-load within nanocarriers both ROS scavengers along with drugs used to treat other damage mechanisms to further reduce inflammation [27]* or intracellular calcium, which is believed itself to trigger multiple damage mechanisms [28]**.
Mitochondrial dysfunction is an additional element involved in the AKI process, initiating apoptosis pathways, and several mechanisms of mitochondrial dysfunction have been addressed using nanocarriers. Formoterol, a long-acting β-agonist (LABA) commonly used in obstructive pulmonary disease, has been shown to stimulate mitochondrial biogenesis. Although the use of LABA improved AKI and podocyte damage in-vitro, minimal renal uptake of these drugs and significant cardiac adverse effects have prevented their use. Formoterol nanoparticles improved drug accumulation in PTEC and demonstrated renal mitochondrial biogenesis while myocardial mitochondrial biogenesis was decreased [29]*. Another significant cell death pathway contributing to AKI is ferroptosis. Iron chelators can prevent AKI by reducing the utilization of intracellular free iron. However, systemic toxicity precludes them from being used. Iron chelating nanoparticles ameliorated AKI by reducing ferroptosis while evading toxicity of systemic iron chelation [30]*. Excess ROS causes mitochondrial dysfunction and apoptosis triggering through excessive activation of PARP-1. RTEC targeted nanocarriers loaded with an inhibitor of PARP-1 ameliorated AKI by reducing apoptosis [26]*.
Renal inflammation plays a notorious role in AKI, although systemic treatment with anti-inflammatory medications can present multiple adverse effects that limit their utility. To address this issue, the co-loading of kidney specific anti-inflammatory agents into nanocarriers together with therapeutics for an additional injury mechanism has been attempted to better mitigate AKI [26, 27, 31, 32]. For example, chemokine receptor 4 (CXCR4) is highly expressed in injured RTEC and enhances leucocyte recruitment during AKI, and thus injected CXCR4a nanoparticles successfully reduced inflammation and AKI [31, 32]*. Surface chemistry optimization of CXCR4a NPs, lead to better kidney targeting [33]*. Cell free DNA (cfDNA) is abundant during AKI and is considered to increase local inflammation. Mn3O4 nanoflowers functioned as cfDNA scavengers reducing kidney damage [27]**.
Renal repair processes are strongly regulated by type 2 innate lymphocytic cells (ILC2) and regulatory T (Treg) cell expansion, causing a shift from pro- to anti-inflammatory macrophage phenotypes. Interleukin-33 (IL33) is known to have protective effects in IRI through its expansion of ILC2 and Tregs. However, IL-33 systemic treatment for IRI is limited due to the cytokine’s short half-life and lack of kidney specificity. Rectangular DNA origami nanocarriers (rDON) are highly programmable, biocompatible, and biodegradable nanostructures that preferentially accumulate in the kidney when constructed at certain dimensions. rDON loaded with IL33 accumulated in kidneys for 48 hours while clearing out of all other organs significantly earlier. Compared to free IL33 treatment, IL33 gradually released from rDONs to improve recovery from ischemic AKI, while kidney Tregs were upregulated, and macrophages shifted from M1 to M2 [34]**.
Early non-invasive detection of acute kidney injury is a growing field of interest. Currently, the use of clinically available biomarkers for kidney function, such as serum creatinine, cause the diagnosis of AKI to lag ~48 hours behind the actual initiation of damage and necessitate constant blood sampling. Thus, methods are needed to detect kidney damage earlier and immediately intervene during high-risk situations. Recently, several molecular optical probes have been developed that use the second near infra-red (NIR-II) spectrum for photoacoustic imaging, which enabled non-invasive AKI detection as early as 10 minutes after injury initiation and long before increases in serum biomarkers [35–37]*. The detection of both injury and improvement can be monitored over 82 hours [36]*. Of note, AKI can be detected ten minutes after ischemia initiation, with the detection triggered by HIF upregulation. Combining this early detection with an instantaneous response from the nano-detectors’ function as a ROS scavenger, which is also potentiated by the HIF upregulation, have a great potential to mitigate AKI before damage escalates [37]**.
Kidney transplantation
ESRD patients are doomed to a life of renal replacement therapy (RRT). Kidney transplantation provides most recipients with increased life span, reduced morbidity, and improved quality of life. However, a shortage of grafts remains a significant problem and the majority of ESRD patients will never get transplanted.
Ischemia Reperfusion Injury, which increases with prolonged cold ischemia time in kidney grafts from deceased donors, remains a major obstacle in the efforts to expand the scope of kidneys that can be utilized for transplant. IRI causes reduced kidney function and increases rates of rejection, both decreasing graft survival. The injury evolves from increased oxidative stress in the first hours, which later induces local inflammation causing further damage. An rDON loaded with anti-C5a aptamers demonstrated effective sequential treatment while the DNA component functioned as a ROS scavenger and competitive binding of C5a halted complement deposition and inflammation upregulation in the later stage [38]*. This type of nanocarrier could be delivered directly to a kidney graft during transplant with potential of improving kidney graft function and reducing rejection.
Acute allograft rejection has been significantly reduced after the incorporation of calcineurin inhibitors in the 1970s, although since then there has been minimal progress in this field, and rejection remains the main cause of graft loss and return to RRT. Systemic immunosuppression often induces foreseeable and devastating complications. Although immunosuppression ideally focuses on T-cell activity, the innate immune system has a crucial role in sensitization, eventually causing rejection. Nanoparticles targeting circulating inflammatory monocytes caused them to sequester in the spleen, where they underwent apoptosis [39]*. This treatment was shown to reduce acute rejection and offers proof of principle to broaden the spectrum of induction treatments in the COVID-19 era and growing worries over the use of lymphocyte depleting agents. Rapamycin is an example of a potent immunosuppressive drug with systemic adverse effects that limit its common use. Burke et al, [40]* recently repurposed rapamycin by using rapamycin loaded polymersomes targeted to diverse antigen presenting cell populations. This resulted in Treg upregulation that switched the mechanism of action for rapamycin from non-specific systemic immunosuppression to instead antigen-specific tolerance in a pancreatic islet-cell model.
Renal sparing treatment for renal cell carcinoma (RCC)
Advanced targeting strategies enabled by nanomedicine have allowed safe and selective antineoplastic treatments for RCC, reducing nephrotoxicity during systemic administration. The use of serine residues as targeting moieties increased the uptake of 90yttrium-loaded poly-L-lysine nanocarriers from 21% to 91% within the total RTEC population. In contrast to the free 90yttrium, targeted 90yttrium-loaded nanoparticles caused no nephrotoxicity while vastly improving the antineoplastic effect on the contralateral kidney [41]*. In another key example, micelles targeted to CD70, which is upregulated in RCC, were loaded with HIF2α small interfering RNA (siRNA) to downregulate oncogenes and enhance the selectivity of tumor treatment [42]*.
Gene therapy
Nanoparticle-based mRNA vaccines against COVID-19 were recently introduced to millions of people worldwide and validated both the immense potential and safety of gene delivery as therapeutic strategy. In the area of kidney disease, gene therapy has demonstrated reversal of the ADPKD phenotype upon re-expression of silenced pkd genes [43]. Several kidney specific RNA/DNA carriers have been developed for the delivery of genes in the treatment of kidney disease. Nanocarriers loaded with DNA plasmids were targeted to PTEC using a ligand to Vimentin, with improved RTEC transfection rates and less toxicity [44]*. Tumor suppressor gene p53, which regulates apoptosis and has a significant role in AKI pathophysiology, was recently silenced via siRNA delivery. siRNA loaded in RTEC-specific nanocarriers blocked p53 transcription, reducing apoptosis and mitigating AKI [31, 32]*.
Conclusions
Nanomedicine enables controlled delivery of substances in a targeted manner, allowing to alter pharmacokinetics, reduce toxicity, improve efficacy and stability, and thus overcome obstacles for improved treatment [1].
Recent advances in nanotechnology coupled with improved pathophysiological understanding of kidney related disorders have together made it possible to develop translatable therapeutic and diagnostic interventions for multiple etiologies of kidney disease. A few notable examples of progress in this field were the improved delivery of antiproliferative medications to treat ADPKD and fibrosis, directed anti-inflammatory treatment for GN and TIN, and reduction of multiple injury pathways in AKI including oxidative stress, mitochondrial dysfunction, and local inflammation. Additional nanoparticle-mediated advances include the sustained release of therapies to reduce IRI applicable after transplant, introduction of new aspects of anti-rejection treatment, targeted delivery of DNA and siRNA, as well as non-invasive ultra-early diagnosis of AKI. Some developments in therapeutics for processes like diabetic nephropathy and fibrosis were tested in models with acute disease conditions and necessitate further development to better address treatment under the chronically evolving conditions associated with diabetic nephropathy.
Targeting specific organs has been growing more common, with multiple successful attempts of targeting RTECs. Single cell analysis grants us profound knowledge of kidney disease pathophysiology, to the cellular level. Therefore, there is still a great need to improve the targeting ability to other specific cells involved in diseases, as well as more accurately assess the specific cellular uptake. There is a need for more collaborative approaches between nano/bioengineers and clinical experts to address concurrent needs and avoid common misconceptions. For example, many researchers in the field assume that nanoparticles of size 50–200 nM accumulate in the tubular region by penetrating the glomerular filtration barrier (GFB). A better understanding of kidney microanatomy will clarify that the GFB size barrier, comprised of the basement membrane proteoglycans and the podocyte slit diaphragm (and not the endothelial fenestrations) is ~5 nM. Thus, the tubular uptake of larger particles, even in a state of disease, must occur at the basolateral RTEC membrane via the peritubular capillaries. Such nanoparticles cannot target podocytes through the GFB for similar reasons. These misconceptions have great implications regarding engineering of nanocarriers and targeting ligands. Crosstalk between all the stakeholders and inclusion of multiple expertise in peer review and collaborative projects shall help in realizing the benefit of Nanomedicine for better therapeutic outcomes. Another possibility for misinterpretation of targeted delivery could derive from particle instability. Although vesicular shaped particles tend to be more stable, micelles are by far more commonly used. Micelles at times tend to breakdown and release their payload, or even breakdown to multiple smaller micelles and components. On rare occasions, fluorescent tags and other traceable markers are used to monitor the biodistribution of both the micellar carrier as well as the payload simultaneously to assess stability during delivery. The stability of the nanocarrier formulation can have crucial importance on the cellular targeting and reproducibility of the nanotherapy and should be thoroughly characterized (Fig 3) [45].
Figure 3.
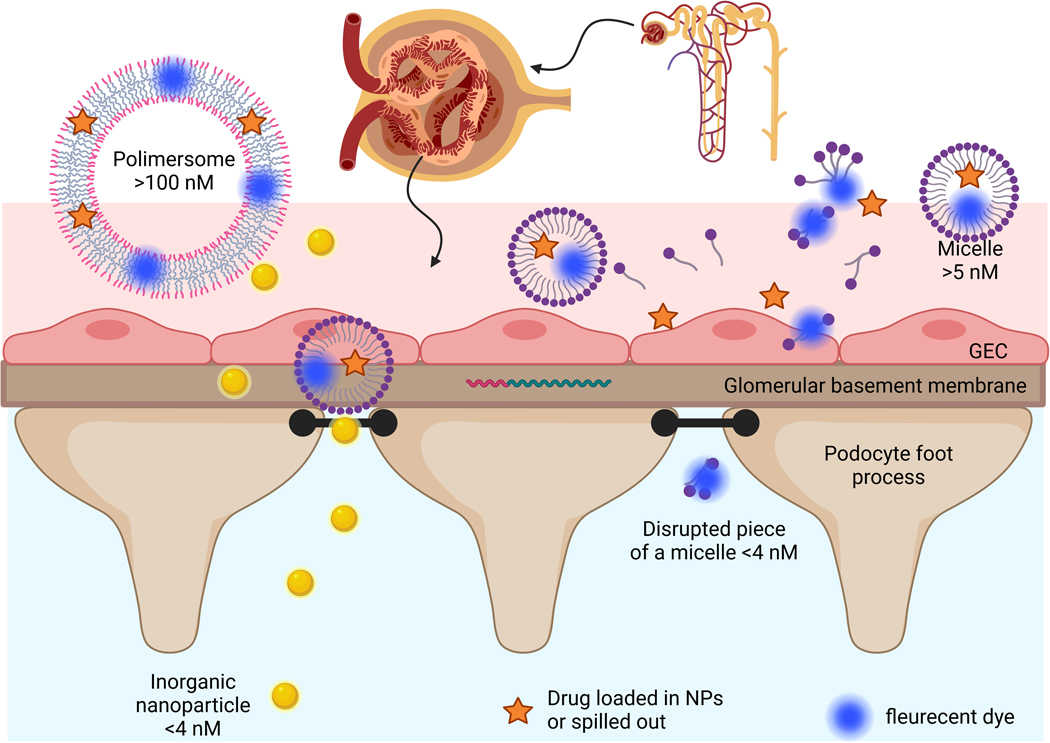
Particles delivery through the glomerular filtration barrier (GBF): Gold nanoparticles of 3 nM diameter flow freely through the GBF, large vesicle shaped particles of 100 nM diameter do not flow through, Micelles of 20 nM are less stable and some are disrupted to different sized clusters/micelles with drug spilled out to the serum although some of the dye remains in the clusters and continues to flow through the GBF.
While hundreds of articles assessing nano-medications in oncology and other fields are published annually, over the same period of time only a few dozen publications present such strategies in kidney science. The time has come to combine the large wealth of renal knowledge with rapidly evolving capabilities in nanotechnology to progress translational science and treatment improvement in kidney disease.
Key points.
The pathophysiological understanding of kidney related disorders has profoundly increased, however tissue- and cell-specific treatments in this field remain scarce.
Advances in nanomedicine enable alteration of pharmacokinetics and targeted treatments improving efficiency and reducing toxicity.
Controlled delivery of antiproliferative medications enables improved treatment of polycystic kidney disease and fibrosis, while directed anti-inflammatory treatment mitigates glomerulonephritis and tubulointerstitial nephritis.
Multiple injury pathways in AKI have been targeted, with therapeutic solutions for oxidative stress, mitochondrial dysfunction, local inflammation and improving self-repair mechanisms.
There is need to improve the targeting ability to specific kidney cells besides RTECs, to thoroughly characterize nanocarrier formulation stability and to improve crosstalk between multiple expertise in collaborative nanomedicine kidney focused projects.
Acknowledgements
• Financial support and sponsorship:
Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number TL1DK132769. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of interest: none.
References
- [1].Amna T, Hassan MS, Gharsan FN et al. Nanotechnology in drug delivery systems: Ways to boost bioavailability of drugs. In: Nanotechnology for Infectious Diseases. Springer; 2022. pp. 223–236. [Google Scholar]
- [2].Vincent MP, Navidzadeh JO, Bobbala S, Scott EA. Leveraging self-assembled nanobiomaterials for improved cancer immunotherapy. Cancer Cell 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Modak M, Frey MA, Yi S et al. Employment of targeted nanoparticles for imaging of cellular processes in cardiovascular disease. Current opinion in biotechnology 2020; 66:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Frey M, Bobbala S, Karabin N, Scott E. Influences of nanocarrier morphology on therapeutic immunomodulation. Nanomedicine 2018; 13:1795–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Huang X, Kong N, Zhang X et al. The landscape of mRNA nanomedicine. Nature Medicine 2022; 28:2273–2287. [DOI] [PubMed] [Google Scholar]
- [6].Chen DQ, Guo Y, Li X et al. Small molecules as modulators of regulated cell death against ischemia/reperfusion injury. Medicinal Research Reviews 2022; 42:2067–2101. [DOI] [PubMed] [Google Scholar]
- [7].Sharma S L. Pablo J, Tolentino KT et al. Further Exploration of the Benzimidazole Scaffold as TRPC5 Inhibitors: Identification of 1-Alkyl-2-(pyrrolidin-1-yl)-1H-benzo [d] imidazoles as Potent and Selective Inhibitors. ChemMedChem 2022; 17:e202200151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Geo HN, Murugan DD, Chik Z et al. Renal Nano-drug delivery for acute kidney Injury: Current status and future perspectives. Journal of Controlled Release 2022. [DOI] [PubMed] [Google Scholar]
- [9]. Jiang K, Huang Y, Chung EJ. Combining Metformin and Drug-Loaded Kidney-Targeting Micelles for Polycystic Kidney Disease. Cellular and Molecular Bioengineering 2022:1–13. *This study demonstrates a synergistic effect of free metformin and nanoparticle loaded rapamycin or salsalate, effectively reducing cyst formation. Each of these medications separately given as a free drug was found to cause off target toxicity at the dose needed for effective ADPKD treatment.
- [10]. Cox A, Tung M, Li H et al. In Vitro Delivery of mTOR inhibitors by Kidney-Targeted Micelles for Autosomal Dominant Polycystic Kidney Disease. SLAS technology 2023. *This study demonstrates the enhanced antiproliferative effect of various mTOR-i loaded in nanoparticles targeted to cortical collecting ducts.
- [11].Huang Y, Wang J, Chin D et al. Oral Delivery of Kidney Targeting Nanotherapeutics for Polycystic Kidney Disease. bioRxiv 2022:2022.2010. 2018.512444. [Google Scholar]
- [12].Yu Y, Gao J, Jiang L, Wang J. Antidiabetic nephropathy effects of synthesized gold nanoparticles through mitigation of oxidative stress. Arabian Journal of Chemistry 2021; 14:103007. [Google Scholar]
- [13].Abd El-Khalik SR, Nasif E, Arakeep HM, Rabah H. The prospective ameliorative role of zinc oxide nanoparticles in STZ-induced diabetic nephropathy in rats: Mechanistic targeting of autophagy and regulating Nrf2/TXNIP/NLRP3 inflammasome signaling. Biological Trace Element Research 2022:1–11. [DOI] [PubMed] [Google Scholar]
- [14]. Wu Q, Wang J, Wang Y et al. Targeted delivery of celastrol to glomerular endothelium and podocytes for chronic kidney disease treatment. Nano Research 2022; 15:3556–3568. *This study demonstrated successful treatment of glomerular disease with nanoparticles targeted to glomerular endothelium. They shed light on the anti-inflammatory properties of a traditional Chinese root extract (Celastrol) driven by eNOS increased expression and VCAM-1 expression reduction.
- [15]. Peres RA, Silva-Aguiar RP, Teixeira DE et al. Gold nanoparticles reduce tubule-interstitial injury and proteinuria in a murine model of subclinical acute kidney injury. Biochimica et Biophysica Acta (BBA)-General Subjects 2023; 1867:130314. *This study demonstrates a therapeutic option for treating tubulointerstitial nephritis and presented an immune response shift secondary to the use of gold nanoparticles.
- [16]. Lee T-Y, Lu H-H, Cheng H-T et al. Delivery of nitric oxide with a pH-responsive nanocarrier for the treatment of renal fibrosis. Journal of Controlled Release 2023; 354:417–428. *This study demonstrates effective reduction of kidney fibrosis by delivering nitric oxide releasing molecules to fibrotic areas, with intracellular release of these molecules.
- [17]. Cheng H-T, Huang H-C, Lee T-Y et al. Delivery of sorafenib by myofibroblast-targeted nanoparticles for the treatment of renal fibrosis. Journal of Controlled Release 2022; 346:169–179. *This study demonstrated specific targeting developed to myofibroblasts. They also demonstrated effective reduction of fibrosis by delivering an FDA approved antineoplastic drug to the myofibroblasts.
- [18]. Sun H, Shi K, Zuo B et al. Kidney-Targeted Drug Delivery System Based on Metformin-Grafted Chitosan for Renal Fibrosis Therapy. Molecular Pharmaceutics 2022; 19:3075–3084. *This study demonstrated effective fibrosis reduction by using metformin loaded nanoparticles. This drug was found effective for fibrosis treatment although toxic in the effective dose needed with free drug delivery.
- [19]. Zhang D-Y, Tu T, Younis MR et al. Clinically translatable gold nanozymes with broad spectrum antioxidant and anti-inflammatory activity for alleviating acute kidney injury. Theranostics 2021; 11:9904. *This study successfully attempted to improve bioavailability of NAC; a known effective antioxidant previously used for AKI prevention although found not effective in the clinical setting. The use of this nanoparticle reduced rhabdomyolysis kidney damage and reduced rodent mortality.
- [20]. Wei H, Jiang D, Yu B et al. Nanostructured polyvinylpyrrolidone-curcumin conjugates allowed for kidney-targeted treatment of cisplatin induced acute kidney injury. Bioactive Materials 2023; 19:282–291. *This study demonstrated a dual-responsive drug delivery system: acidic environment caused de-shelling (smaller size and negative to positive charge) and ROS induced drug release. Loaded curcumin effectively alleviated mitochondria injury and reduced AKI.
- [21]. Lan T, Guo H, Lu X et al. Dual-Responsive Curcumin-Loaded Nanoparticles for the Treatment of Cisplatin-Induced Acute Kidney Injury. Biomacromolecules 2022; 23:5253–5266. * This study effectively utilized curcumins antioxidative effect to reduce mitochondrial damage and cisplatin induced kidney damage. In addition to this effect, the curcumin improved specific targeting to kidneys compared to PVP nanoparticles without loaded curcumin.
- [22]. Qin S, Wu B, Gong T et al. Targeted delivery via albumin corona nanocomplex to renal tubules to alleviate acute kidney injury. Journal of Controlled Release 2022; 349:401–412. *This study emphasized the advantage of albumin coated nanoparticles demonstrating improved kidney targeting. Celastrol , a traditional Chinese remedy was safely and effectively used to alleviated IRI.
- [23]. Zhang D-Y, Liu H, Zhu KS et al. Prussian blue-based theranostics for ameliorating acute kidney injury. Journal of Nanobiotechnology 2021; 19:1–14. *The use of Prussian blue loaded nanoparticles resulted in ROS reduction and reduced rodent mortality from AKI.
- [24]. Qi X, Wang J, Fei F et al. Myricetin-Loaded Nanomicelles Protect against Cisplatin-Induced Acute Kidney Injury by Inhibiting the DNA Damage-cGAS–STING Signaling Pathway. Molecular Pharmaceutics 2022; 20:136–146. *This study demonstrated a double mechanism of AKI reduction while Myricetin loaded nanoparticles reduced ROS as well as inhibited cisplatin-induced activation of the DNA damage-cGAS−STING pathway.
- [25]. Chen Z, Qi F, Qiu W et al. Hydrogenated Germanene Nanosheets as an Antioxidative Defense Agent for Acute Kidney Injury Treatment. Advanced Science 2022; 9:2202933. *This study presents a successful attempt to engineer a nanosheet structure from hydrogenated germanene as an efficient ROS scavenger, significantly reducing AKI compared to NAC.
- [26]. Zhao X, Sun J, Dong J et al. An auto-photoacoustic melanin-based drug delivery nano-platform for self-monitoring of acute kidney injury therapy via a triple-collaborative strategy. Acta Biomaterialia 2022; 147:327–341. *This study demonstrates a combination of specific antibody (GPR97) mediated targeting to injured RTEC, as well as combining two remedies (PJ34; a PARP-1 inhibitor, and melanin; a ROS scavenger) to intervene in 3 separate AKI pathways (activation of the Keap-1/Nrf2/HO-1 pathway, antiapoptotic effect and anti-inflammatory effect).
- [27]. Meng L, Feng J, Gao J et al. Reactive Oxygen Species-and Cell-Free DNA-Scavenging Mn3O4 Nanozymes for Acute Kidney Injury Therapy. ACS Applied Materials & Interfaces 2022; 14:50649–50663. ** This study successfully incorporated two different pathways of kidney damage: reduction of oxidative stress (ROS scavenging) and inflammation reduction. cfDNA is found abundant during AKI and thought to further trigger inflammation, thus cfDNA scavenging reduces local inflammation. The ROS reduction functioned in a cascade manner by first uptaking O2- to H2O2 and further hydrolyzing the latter.
- [28]. Wang Y, Pu M, Yan J et al. 1, 2-Bis (2-aminophenoxy) ethane-N, N, N′, N′-tetraacetic Acid Acetoxymethyl Ester Loaded Reactive Oxygen Species Responsive Hyaluronic Acid–Bilirubin Nanoparticles for Acute Kidney Injury Therapy via Alleviating Calcium Overload Mediated Endoplasmic Reticulum Stress. ACS nano 2022. **This study combined treatment of two mechanisms for kidney injury; direct reduction of oxidative stress with a natural antioxidant (bilirubin) normally not found intracellularly in the target RTEC, alongside intracellular calcium reduction known to trigger multiple different injury mechanisms. In addition, it incorporated a ROS reactive safety mechanism allowing for the calcium chelator to be released only in the intracellular oxidative environment, thus reducing risk of off target toxicity.
- [29]. Vallorz EL, Blohm-Mangone K, Schnellmann RG, Mansour HM. Formoterol PLGA-PEG nanoparticles induce mitochondrial biogenesis in renal proximal tubules. The AAPS journal 2021; 23:88. *This study demonstrated that the nanocarriers allowed repurposing of LABA, commonly used for COPD, by PTEC targeting and thus elimination of cardiac adverse effects in a systemic administration. The LABA nanoparticles safely caused renal mitochondrial biogenesis.
- [30]. Xie X, Zhang Y, Su X et al. Targeting iron metabolism using gallium nanoparticles to suppress ferroptosis and effectively mitigate acute kidney injury. Nano Research 2022; 15:6315–6327. *This study used gallium loaded nanoparticles as an intracellular iron chelator, evading the toxicity of systemic iron chelation. This allowed to ameliorate AKI by reduction of ferroptosis and mitochondrial damage.
- [31]. Tang W, Chen Y, Jang H-S et al. Preferential siRNA delivery to injured kidneys for combination treatment of acute kidney injury. Journal of Controlled Release 2022; 341:300–313. *This study presents a combination delivery of a cytokine antagonist for prevention of local leucocyte recruitment, combined with small interrupting RNA reducing apoptosis for synergistic protective effect in cisplatin induced AKI.
- [32]. Tang W, Panja S, Jogdeo CM et al. Modified chitosan for effective renal delivery of siRNA to treat acute kidney injury. Biomaterials 2022; 285:121562. *This study presents RTEC targeting with loaded siRNA successfully downregulating transcription of tumor suppressor gene p53, found involved in AKI pathophysiology.
- [33]. Tang W, Panja S, Jogdeo CM et al. Study of Renal Accumulation of Targeted Polycations in Acute Kidney Injury. Biomacromolecules 2022; 23:2064–2074. *This study assessed simple modifications with polycataion groups to improve RTEC targeting. Hydroxyl groups were found to be the best in minimizing serum protein adsorption causing maximal uptake to ischemic RTEC.
- [34]. Li W, Wang C, Lv H et al. A DNA nanoraft-based cytokine delivery platform for alleviation of acute kidney injury. ACS nano 2021; 15:18237–18249. **As opposed to the vast majority of nanomedicine interventions for AKI, that focus on reducing the damage which is often already complete at the time of diagnosis, this study enhances the self-repair mechanism to improve recovery.
- [35]. Yao C, Chen Y, Zhao M et al. A Bright, Renal-Clearable NIR-II Brush Macromolecular Probe with Long Blood Circulation Time for Kidney Disease Bioimaging. Angewandte Chemie International Edition 2022; 61:e202114273. *This study designed a nanoprobe for noninvasive early recognition of AKI, with ~10 fold brighter fluorescence than previously reported PEGylated second near infra-red (NIR-II) renal-clearable probes.
- [36]. Weng J, Wang Y, Zhang Y, Ye D. An activatable near-infrared fluorescence probe for in vivo imaging of acute kidney injury by targeting phosphatidylserine and caspase-3. Journal of the American Chemical Society 2021; 143:18294–18304. *This study demonstrates the use of NIR-II fluorescence for noninvasive early AKI detection. It is triggered by ischemic induced caspase activation as early as 24 hours post AKI, at a time that creatinine was still near normal.
- [37]. Xu Y, Zhang Q, Chen R et al. NIR-II Photoacoustic-Active DNA Origami Nanoantenna for Early Diagnosis and Smart Therapy of Acute Kidney Injury. Journal of the American Chemical Society 2022. **This study utilizes an injectable biodegradable nanodevice (rDON) for a dual purpose: early noninvasive detection of ischemic kidney injury and injury triggered treatment. Injury was detected at 10 minutes after the ischemic insult (~48 hours prior to creatinine elevation) triggered by HIF upregulation. After injury detection and photoacoustic readout, there is also increased function as a ROS scavenger by the DNA scaffold, reducing the kidney injury as early as possible.
- [38]. Chen Q, Ding F, Zhang S et al. Sequential therapy of acute kidney injury with a DNA nanodevice. Nano Letters 2021; 21:4394–4402. *This study utilizes a designed DNA origami nanodevice for sequential two step treatment of kidney damage; early ROS scavenging and reduction of inflammation upregulation by complement binding.
- [39]. Lai C, Chadban SJ, Loh YW et al. Targeting inflammatory monocytes by immune-modifying nanoparticles prevents acute kidney allograft rejection. Kidney International 2022; 102:1090–1102. *This study presents proof of principle to broaden the spectrum of induction treatments for transplant, by targeting the innate immune system, and thus reducing sensitization and rejection in the early stage. This has great significance in the COVID-19 era and growing worries regarding use of lymphocyte depleting agents.
- [40]. Burke JA, Zhang X, Bobbala S et al. Subcutaneous nanotherapy repurposes the immunosuppressive mechanism of rapamycin to enhance allogeneic islet graft viability. Nature nanotechnology 2022; 17:319–330. *This study successfully demonstrated repurposing of a well know immunosuppression drug with multiple toxicities, by targeting rapamycin to dendritic cells. This resulted in antigen-specific tolerance, which could have huge significant implications for the future of transplant.
- [41]. Katsumi H, Kitada S, Yasuoka S et al. L-Serine-Modified Poly-L-Lysine as a Biodegradable Kidney-Targeted Drug Carrier for the Efficient Radionuclide Therapy of Renal Cell Carcinoma. Pharmaceutics 2022; 14:1946. *This study demonstrated a simple modification to previously characterized nanoparticles increased RTEC uptake, from 21% to 91%, and improved biodegradability. These nanoparticles loaded with 90yttrium showed reduced nephrotoxicity to the healthy kidney and vast improvement of the antineoplastic effect on the contralateral kidney.
- [42]. Trac N, Oh HS, Jones LI et al. CD70-Targeted Micelles Enhance HIF2α siRNA Delivery and Inhibit Oncogenic Functions in Patient-Derived Clear Cell Renal Carcinoma Cells. Molecules 2022; 27:8457. *This study presents RCC specific targeting, delivering HIF2α siRNA, and causing oncogene downregulation. This is an example of tumor specific treatment with renal sparing of the healthy kidney.
- [43].Dong K, Zhang C, Tian X et al. Renal plasticity revealed through reversal of polycystic kidney disease in mice. Nature genetics 2021; 53:1649–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44]. Mathew AP, Uthaman S, Bae EH et al. Vimentin Targeted Nano-gene Carrier for Treatment of Renal Diseases. Journal of Korean Medical Science 2021; 36. * This study presents a potential kidney specific gene delivery system. Ligands for vimentin were used to target the DNA nanocarriers to PTECs, and co-loading of hyperosmotic sorbitol increased RTEC transfection with reduced toxicity.
- [45].Ghezzi M, Pescina S, Padula C et al. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. Journal of Controlled Release 2021; 332:312–336. [DOI] [PubMed] [Google Scholar]


