PURPOSE:
Cascade genetic risk evaluation in families with hereditary cancer can reduce the burden of disease but the rate of germline genetic testing in relatives of patients at risk is low.
METHODS:
We identified all 277 women diagnosed with breast cancer in Georgia in 2017 who linked to a clinically actionable germline pathogenic variant through a Surveillance, Epidemiology, and End Results registry-variant linkage initiative. We surveyed them, and then invited eligible respondents to an online platform hosted by a navigator that offered cancer genetic risk education and germline genetic testing to untested relatives. We randomly assigned patient-family clusters at the time of the patient enrollment offer to free versus $50 (USD) test cost. Patients invited relatives to join the study through personalized e-mail. Enrolled relatives received online cancer genetic education and the opportunity to order clinical germline genetic testing through the platform. The primary outcome was the number of relatives who ordered genetic testing.
RESULTS:
One hundred twenty-five of 277 patients completed surveys (45.2%). Most respondents were eligible for the trial offer (113 of 125; 90.4%). In the free testing arm, 20 of 56 eligible patients participated (35.7% of eligible respondents) and they invited 28 relatives: 12 relatives enrolled and 10 ordered testing. In the $50 (USD) arm, 16 of 57 eligible patients participated (28.1%) and they invited 38 relatives: 18 relatives enrolled and 17 ordered testing.
CONCLUSION:
Cascade genetic testing in families with hereditary cancer syndromes accrued through a population-based cancer registry can be achieved through an online platform that offers genetic risk education and low-cost testing to relatives. A modest charge did not appear to influence the percentage of participating patients, numbers of participating relatives, and numbers of relatives who received genetic testing.
INTRODUCTION
Cascade genetic risk evaluation in families with hereditary cancer syndromes (HCS)—the performance of genetic risk education and testing in blood relatives of individuals who have identified genetic mutations—has been endorsed by professional organizations and clinical experts because it can reduce the burden of disease through targeted early detection and prevention strategies.1-4 However, several studies have demonstrated that germline genetic testing in family members of patients at risk is low.5-7 Directly engaging family members of patients with cancer who carry a germline pathogenic variant (PV) for genetic risk evaluation and management may be a cost-effective approach.3,8 Current clinical practice is largely limited to a genetic counselor providing a family letter to the patient with details about the identified PV and options for genetic testing. The patient is then responsible for sharing this information with family members. Enhancing the clinic-based approach to cascade testing may not be practical because of a national shortage of genetic counselors in the face of increasing demand to engage patients after diagnosis of cancer.9,10
We lead the Genetic Information and Family Testing (GIFT) trial, a pragmatic, cancer registry–based, randomized clinical trial intended to increase rates of cascade genetic risk evaluation in families with HCS.11 The GIFT clinical trial aims to develop and deliver a personalized, virtual, family-centered genetic risk education and testing platform to all first- and second-degree relatives of all adult patients diagnosed with cancer in Georgia and California in 2018-2019 who tested positive for a clinically actionable PV in a cancer susceptibility gene. GIFT will examine the effects of two design features: level of personalized family genetic risk education support (online platform with or without a human navigator) and cost of genetic testing offered to relatives (free of charge or $50 USD) on (1) cancer patients' assessment of communication with relatives about genetic cancer risk; (2) relatives' receipt of genetic testing through the GIFT platform; and (3) relatives' completion of formal clinical genetic risk evaluation after trial participation. We report here on the results of a pilot study that informed the GIFT Trial Protocol, trial materials including participant surveys, and features of the online platform for patients and relatives. The pilot study also addressed whether testing cost (free of charge v $50 USD) would have a large effect on enrollment of patients or their relatives into a clinical trial.
METHODS
We previously described the Georgia California Genetic Testing Linkage Initiative, which linked all female patients with breast cancer or ovarian cancer diagnosed from January 1, 2013, to December 31, 2017, in Georgia and California and reported to one of the four Surveillance, Epidemiology, and End Results (SEER) registries that provided statewide coverage (in Georgia, the Georgia Cancer Registry, and in California, the Los Angeles Cancer Surveillance Program, the Greater Bay Area Cancer Registry, and the Cancer Registry of Greater California) to germline genetic testing results reported through 2019 from the four laboratories (Ambry Genetics, Aliso Viejo, CA; GeneDx, Gaithersburg, MD; Invitae, San Francisco, CA; and Myriad Genetics, Salt Lake City, UT) that performed the majority of clinical testing in the regions.12 Using a third-party honest broker that supports the SEER Surveillance Program (Information Management Services, Inc), we identified all women diagnosed with breast cancer in Georgia in 2017 who linked to a clinically actionable germline PV (N = 277) identified through the Initiative data infrastructure (Fig 1). We included patients who tested positive for a PV or likely PV in any of a large set of cancer-associated genes that are commonly evaluated by the partnering laboratories (see Appendix Table A1 [online only] for a list of genes and patient report of PV). We also added a 5% sample of patients without a PV to the cohort to mask the knowledge of PV status to the registry survey research field team.
FIG 1.
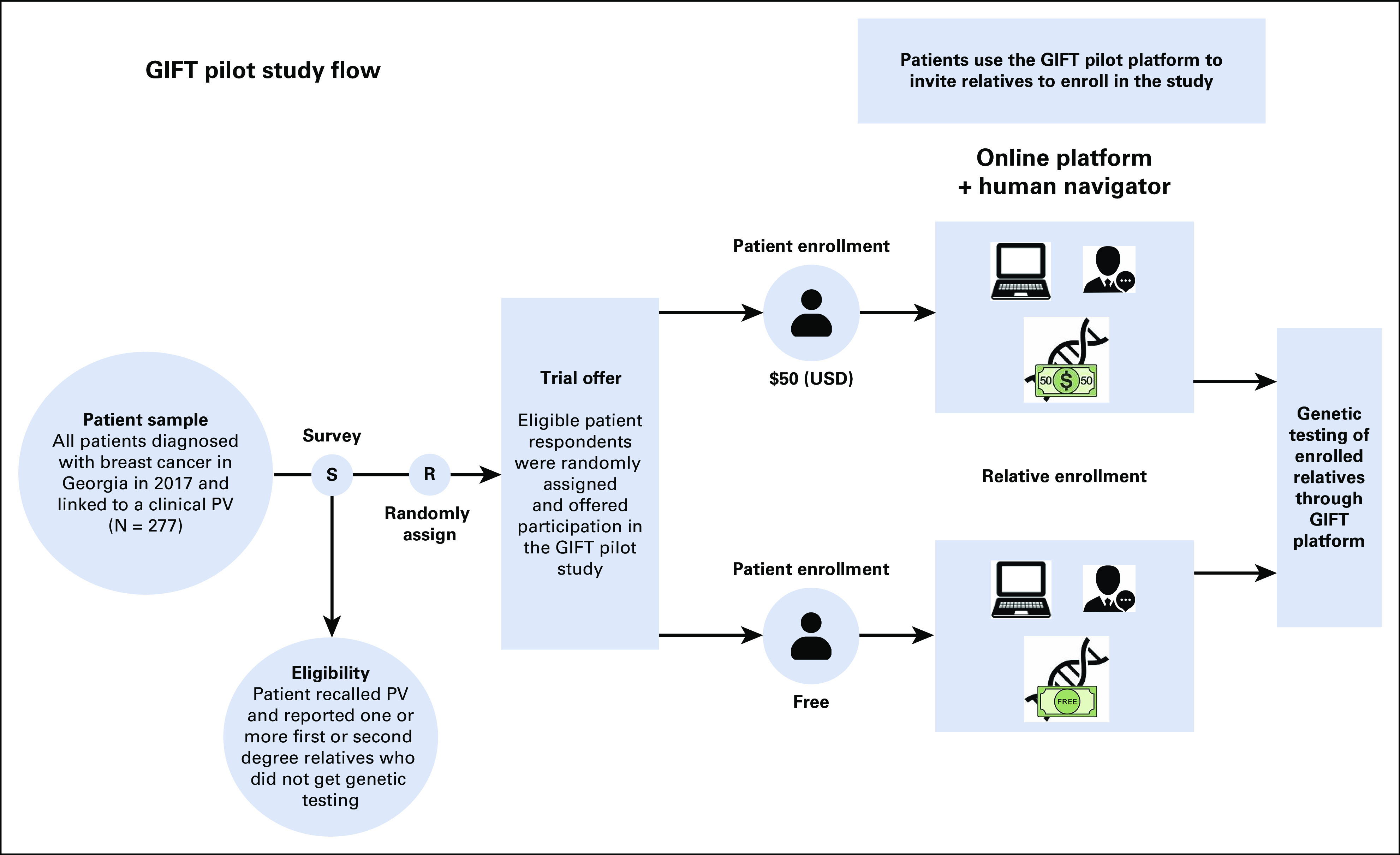
The pilot study design. PV, pathogenic variant.
Registry research staff mailed packets in October 2021 to these patients with a letter that described the study, a $20 (USD) cash incentive, and a request to complete a survey that collected information about their cancer history including receipt and results of germline genetic testing, the family structure of first- and second-degree relatives (eg, the number and types of relatives on both the maternal and paternal sides), whether relatives received genetic testing, and patient perspectives about communication with family members about cancer and genetic risk. Registry staff subsequently invited eligible respondent patients (those who reported receipt of germline genetic testing and a PV result and who had at least one first- or second-degree relative who did not receive genetic testing) to participate in the GIFT pilot intervention phase of the study. The intervention was an online platform with a human navigator that offered cancer genetic risk education and germline genetic testing to all untested first- and second-degree relatives (Fig 1). We randomly assigned patient-family clusters at the time of the patient enrollment offer letter to two different test costs to relatives (free v $50 USD) to explore the impact of test cost on uptake of the intervention and testing in families. Thus, randomization was concealed to patients at the time of the offer letter. Patients were block-randomized to ensure balance between the two study arms at any point throughout the study. Using a block size of four with two arms (F = free and L = low cost), there were six total arm arrangements, (FFLL, FLFL, FLLF, LLFF, LFLF, and LFFL). The six blocks were repeatedly randomly ordered and patients completing the survey were assigned to the next available arm within the current randomized block. We chose the $50 (USD) price point because several companies offered testing at that price to relatives of patients who tested positive for a PV with the company-processed test.13-16 Patients consented and enrolled online and were offered online education addressing key facts about hereditary cancer and role of genetic testing for family members. Patients had the option to create and send a personalized e-mail informing relatives about the GIFT study and offering them the opportunity to join the study to receive (free or $50 USD) genetic testing. The e-mails to family members included a link to the study website along with a unique access code. Interested invited relatives who visited the study site and entered their access code could then learn about the study goals and procedures. Interested relatives were subsequently screened for eligibility for the study (eg, confirming age 18 years or older, their relationship to the patient, and that they had not already had genetic testing for cancer risk) and, if eligible, had the opportunity to provide online informed consent to join the study. Relatives who enrolled received an online education program consisting of key facts about hereditary cancer, the benefits of knowing about personal cancer risk, and the role of genetic testing. They also had the opportunity to request home-delivered genetic testing to assess cancer risk directly through the platform. We offered clinical germline genetic testing and results follow-up to relatives through our partnering laboratory, Color Health Inc, Burlingame, CA, a Clinical Laboratory Improvement Amendments–certified laboratory, using Color's commercially available hereditary cancer multigene panel of 30 genes. Relatives interested in testing could follow a link directly from the GIFT study site into the Color system where they could request genetic testing (at the assigned price).
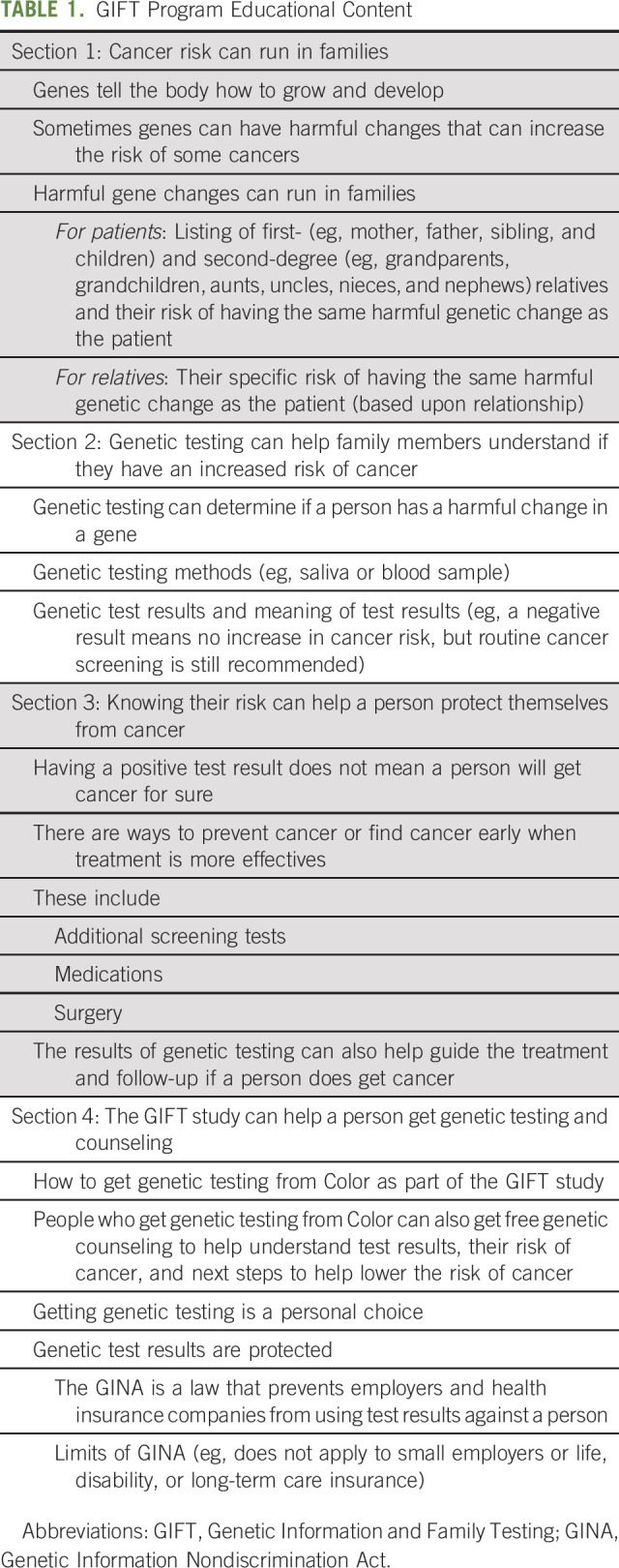
The educational content for the GIFT program for patients and family members was developed by investigators and patient advocates and was informed by the ASCO guidelines for cancer risk counseling.17 Information was presented in the form of a written online family health summary, which consisted of four sections that presented key facts related to inherited cancer risk and genetic testing. The section content and key information highlighted in each section of the GIFT family health summary is shown in Table 1. The information in the GIFT family health summary was written at a sixth-grade reading level. Each section of the GIFT family health summary was presented to program users in a linear fashion. We estimated the time needed to read the GIFT educational content was approximately 15 minutes.
TABLE 1.
GIFT Program Educational Content
All patient and relative participants could request assistance from a human navigator through their study participation. The navigator was a bilingual, bachelor-level research assistant working under the supervision and support of a clinician team consisting of a certified genetic counselor (R.H.) and supervising medical oncologist (A.W.K.). The navigator responded to requests for assistance from patients and relatives regarding their use of the online platform or test ordering. She also proactively reached out to patients to encourage invitation of all eligible relatives. Finally, the navigator proactively reached out to (1) invited relatives to encourage enrollment and (2) enrolled relatives to support them through their experiences using the GIFT program and ordering their genetic test. All proactive outreach was done at days 7, 10, and 14 after enrollment/invitation.
Measures
Clinical variables (stage and biologic subtype) were ascertained through the Georgia SEER Registry. Survey measures included patient characteristics and patient report of family structure and questions about communication with relatives about their genetic test results developed by study investigators. We asked patients their opinions about the need for support with communication with relatives regarding genetic cancer risk and test results. We also quantified aspects of participant use of the online platform through paradata and relatives' ordering of genetic testing through the Color Health provider portal—the primary outcome of the study. Finally, we collected baseline information from invited and enrolled relatives including their family role and sex.
Analytic Plan
After completion of the pilot study, the honest broker removed the 5% sample to create the analytic data set of 277 women for analysis. All analyses were descriptive, given the limited sample size. Chi square and ANOVA tests were used for testing comparisons. We described characteristics of the respondent patients and their report and appraisal of communication with family members about their genetic test results. We then described the flow and outcomes of patients and relatives through the intervention study. This study was reviewed and approved by the institutional review boards at the University of Michigan, Emory University, and the Georgia Department of Public Health.
RESULTS
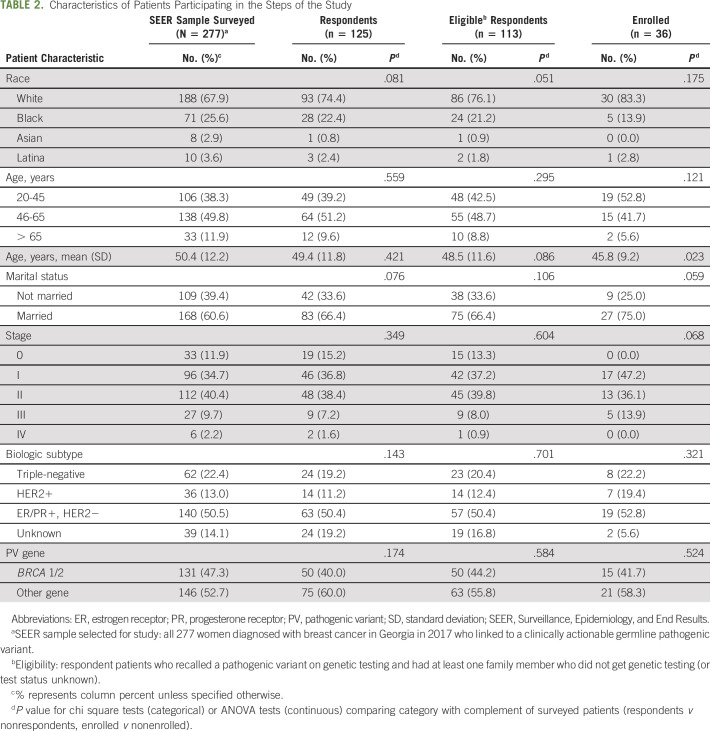
We mailed surveys on average 4.4 years after diagnosis (SD, 0.2 years; range, 4.0-4.7 years). Surveys were completed by 125 of 277 patients (45.2%) on average 23.9 days after initial contact (SD, 28.0). The median age of sampled patients was 50.4 years (IQR, 16.5); 60.6% were married; 67.9% were Caucasian, 25.6% were African American, 2.9% were Asian, and 3.6% were Hispanic (Table 2). The distribution of clinical variables in the patients selected for the pilot study (inception cohort) reflected the cancer registry–based sample of patients with clinically detected PVs: 75.1% were stage 1 or 2, and 50.5% had tumors that were ER/PR-positive, HER2−. About half (47.1%) linked to BRCA1 or BRCA2 versus other PV (52.7%). Compared with the sampled inception cohort (patients selected from the SEER registry database), enrolled patients were more likely to be White (83.3% v 67.9%; P = .175) and of younger age (mean, 45.8 v 50.4; P = .023).
TABLE 2.
Characteristics of Patients Participating in the Steps of the Study
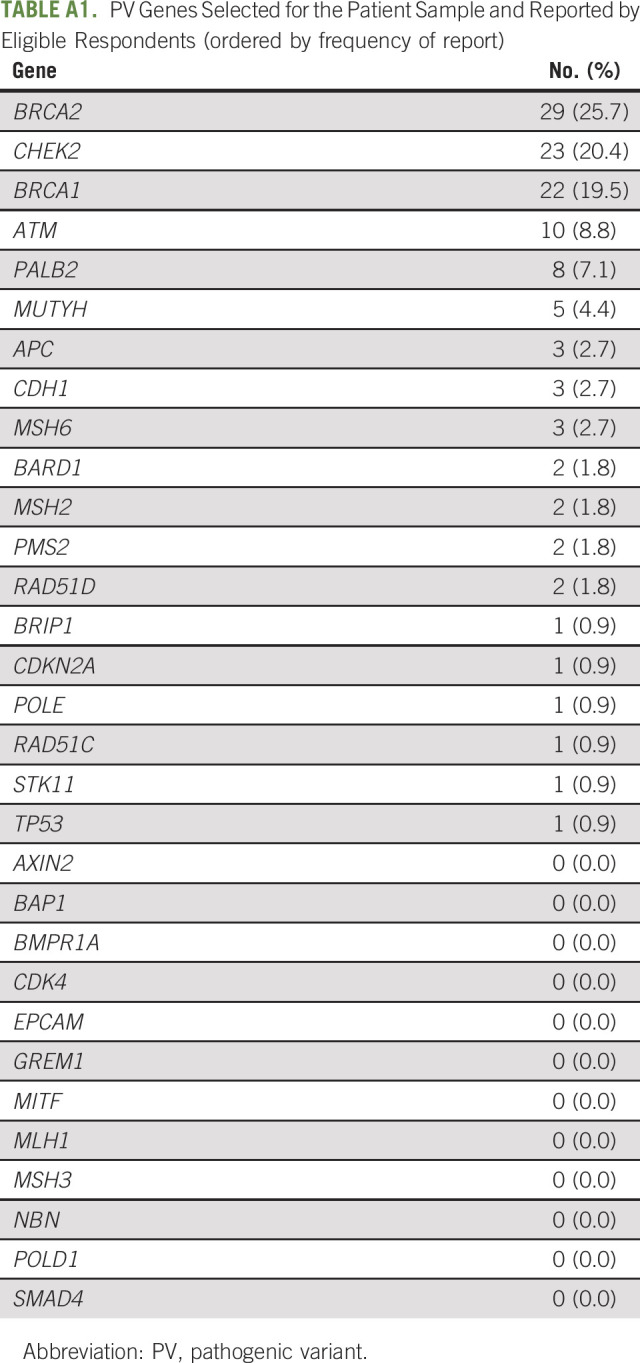
The most common PVs reported by patients were BRCA2 (25.7%), CHEK2 (20.4%), BRCA1 (19.5%), ATM (8.8%), and PALB2 (7.1%; see Appendix Table A1 for full list of PVs). The median number of eligible relatives reported by patients (adult relatives who had not yet undergone testing or test status unknown) was 8.0 (IQR, 10). Among enrolled patients, the median number of eligible relatives was 8.5 (IQR, 7.5).
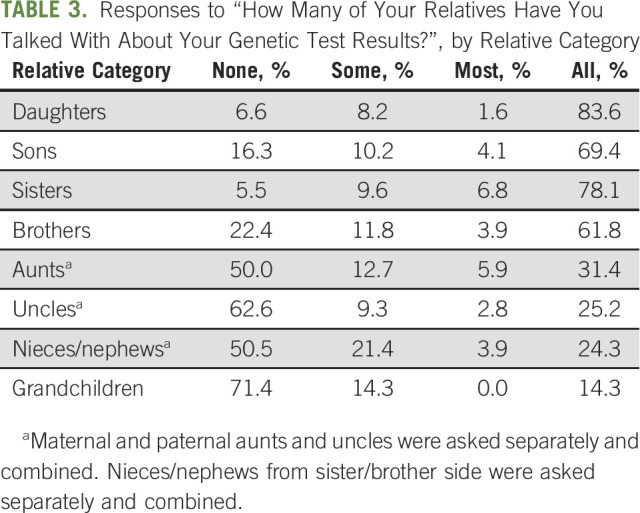
On baseline survey (before engagement of the intervention by the study team), disclosure of genetic test results to first-degree relatives was high, with higher rates for female versus male relatives (Table 3): 73.0% of patients reported that they discussed their test results with their mother quite a bit/very much versus 68.6% with their father (P < .001); 83.6% of patients reported that they discussed results with all their daughters versus 69.4% with all their sons (P < .001); 78.1% discussed their results with all sisters versus 61.8% with all brothers (P < .001). The disclosure of results to second-degree relatives was much lower: only 31.4%, 25.2%, and 24.3% disclosed results to all aunts, all uncles, or all nieces and nephews, respectively.
TABLE 3.
Responses to “How Many of Your Relatives Have You Talked With About Your Genetic Test Results?”, by Relative Category
Most respondents were subsequently eligible for the trial offer on the basis of survey responses (113 of 125; 90.4%; 12 of 13 who were not eligible did not confirm receipt of genetic testing with a PV test result and only one reported a PV but had no eligible relatives). Figure 2 shows the flow of participants through the study, including details of the enrollment of participants, allocation to study arms, and disposition status. Overall, the results were similar between the free test arm and $50 (USD) test arm. In the free testing arm (n = 56), 20 patient participants (35.7%) invited 28 relatives; 12 relatives enrolled and 10 relatives ordered testing. In the $50 (USD) arm (N = 57), 16 (28.1%) patient participants invited 38 relatives, of whom 18 enrolled and 17 ordered testing. There was moderate participant-initiated use of the navigator during the intervention period: a total of 38 help requests were received. The most common help requests included questions from patients about which relatives they could invite, questions from both patients and relatives about confidentiality and details regarding the testing process, and requests from relatives for assistance during the test ordering process.
FIG 2.
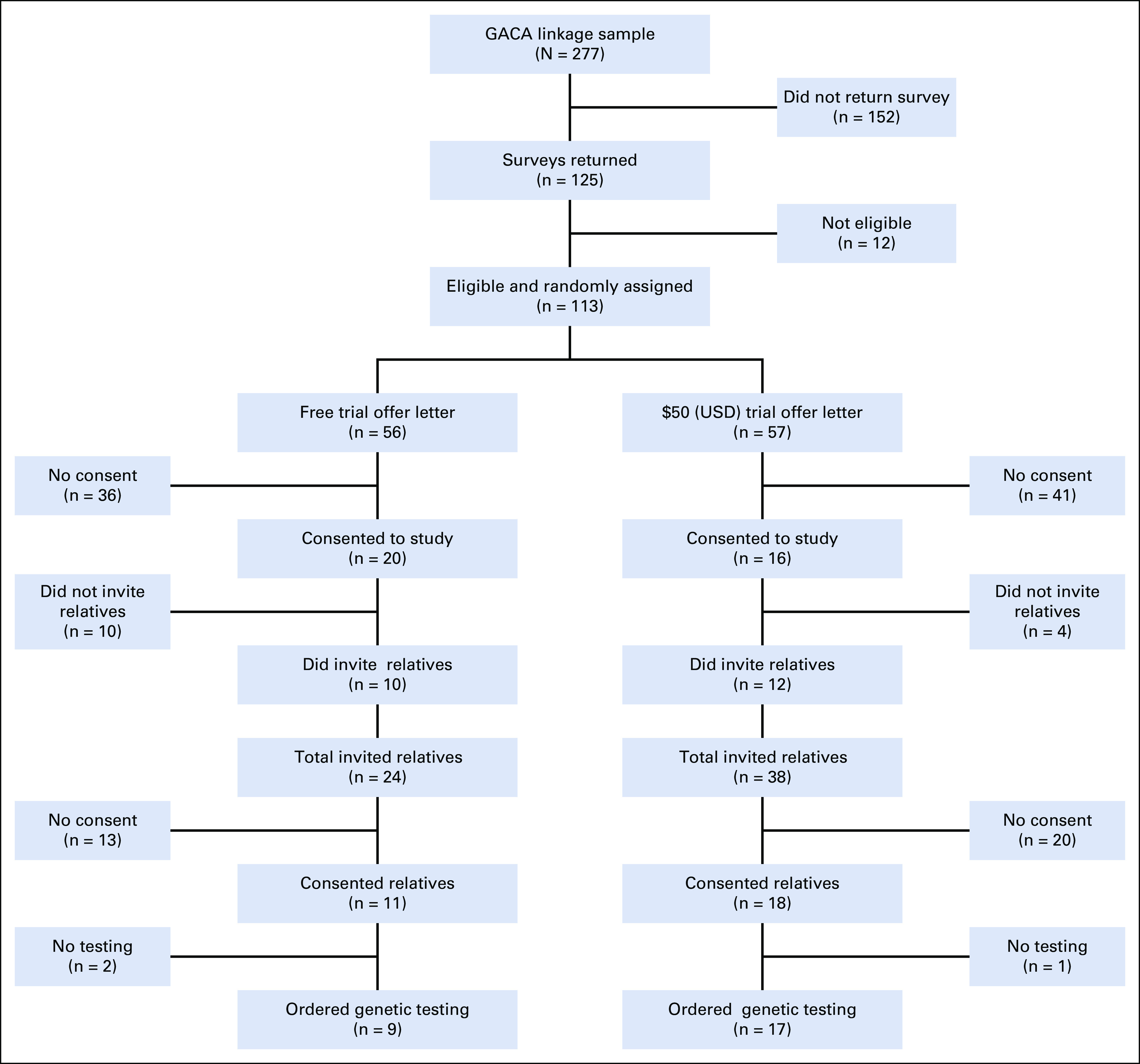
The pilot study flow diagram detailing the enrollment of participants, allocation to study arms, and disposition status.
Of the 66 relatives invited by the patient participants across both arms, 47 (71.2%) were first-degree (eight parents, 22 siblings, and 17 children) and about half of the relatives were men (47.0%). Of the 27 relatives who ordered testing, 60.0% were first-degree and about half were men (46.7%).
DISCUSSION
In this pilot study of cascade genetic testing in a SEER registry–identified cohort of female patients diagnosed with breast cancer who tested positive for a pathogenic variant, we found that most patients surveyed about 4 years after diagnosis had disclosed their genetic test results to first-degree relatives but fewer had disclosed results to second-degree relatives. Despite considerable amounts of communication between patients and family members about test results, patients reported that many relatives had not received genetic testing, or that their testing status was unknown to the patient. A substantial number of patients engaged with our intervention: about half responded to our initial survey, most of the respondents were eligible for the intervention, and about one-third of eligible respondent patients enrolled. Engagement of relatives among enrolled patients was modest. Enrolled patients reported a median number of untested (or testing status unknown) relatives of 8.5, but on average, about two relatives were invited per enrolled patient to join the intervention and about half of invited relatives enrolled (and nearly all ordered genetic tests). There were no substantial differences between test cost arms (free v $50 USD) in patient enrollment or the number of relatives invited, enrolled, or tested. There was an even split between male and female relatives invited, enrolled, and tested.
Our findings are consistent with other studies that suggest that cascade genetic testing in families with HCS appears low despite considerable communication between patient and relatives. Reasons for this large gap include the lack of cancer genetic education programs for relatives in families with HCS,18 the paucity of genetic counseling resources in clinical practice, and patient privacy concerns and need for their consent to engage relatives.8,19 Furthermore, there are few financial incentives to support practice-based clinicians to engage family members, many of whom reside in distant locations and receive health care in many different health systems. A few studies in limited practice settings have been published that attempt to close the gap in cascade testing through clinic-based strategies with moderate success.20-22 But the findings from these studies reinforce the challenges clinicians face to facilitate genetic risk evaluation in family members of a patient diagnosed with cancer in their practice who tests positive for a pathogenic variant.
Our GIFT trial will address this challenge by leveraging a unique SEER-based data infrastructure that linked uniform clinical information to germline genetic test results for all patients diagnosed in Georgia and California from 2013 to 2019 with test reporting through 2021. GIFT will directly engage all adult cancer survivors selected from the data infrastructure who were diagnosed in 2018-2019 and who tested positive for PV to offer an online platform of personalized genetic risk education and low-cost testing to all untested first- and second-degree adult relatives. Building on our prior work,5 which demonstrated a 48% cascade testing rate by relatives offered an online, low-cost cascade genetic testing process, we are partnering with a Clinical Laboratory Improvement Amendments–certified laboratory, Color Health, Inc, to support relative-initiated testing and result disclosure by laboratory-affiliated genetic counselors. Our pilot study results underscore the opportunity to close the gap in cascade genetic testing by directly engaging cancer survivors, even years after diagnosis. Most patient respondents agreed that it was important for family members to understand their own genetic risk and would have liked more support to make it easier for family members to get tested. However, our results highlight challenges including patients' ability and confidence to engage relatives, patients' willingness to facilitate direct engagement of their family members, and relatives' preparedness to consider and complete genetic testing. This reinforces the need to develop a robust pretest education program for relatives and facilitate convenient, low-cost genetic testing and follow-up.
Aspects of the pilot study merit comment. Strengths include sampling patients from a Georgia cancer registry–based infrastructure that identified patients who tested positive for a PV, the use of an online platform hosted by a human navigator, and the partnership with Color Health Inc—an experienced internet provider of clinical-grade germline genetic testing including structured results reporting to clients. Limitations include the sample of female patients with breast cancer only and a long period between diagnosis and patient contact. Although the test status of patients was concealed to staff, random assignment of patients to the two study arms was not. Also, patient and relative enrollment was lower than estimated in our IRB-approved study protocol because of the need to efficiently generate results from the pilot study within the timeline of the GIFT clinical trial development phase. Specifically, the modest patient survey response rate and observed enrollment bias (younger age and lower minority participation) was partly the result of a short period of follow-up of patients to complete surveys and enroll in the study. Additionally, relatives' uptake of the intervention was constrained by a one-month limit between enrollment of relatives and completion of test ordering; this likely contributed to the modest observed uptake.
In conclusion, cascade genetic testing in families with HCS accrued through a population-based cancer registry can be achieved through an online platform that offers genetic risk education and low-cost testing to relatives. A $50 (USD) testing cost (v free of charge) did not substantively reduce patient or relative enrollment into the pilot. Our GIFT randomized clinical trial will identify strategies of engagement and features of an online platform experience that can maximize cascade genetic risk evaluation and are scalable at the population level.
ACKNOWLEDGMENT
The authors acknowledge the Facing Hereditary Cancer Empowered (FORCE) patient and family advocacy organization, under the leadership of Sue Friedman and Diane Rose, for their assistance with user testing of an early prototype of the GIFT pilot intervention. The authors also acknowledge the invaluable contributions of the U-M Center for Health Communications Research staff—including Stefanie Goodell, Shelly Chang, Diane Egleston, Elizabeth Hershey, Colleen Leh, Ian Moore, Jeffrey Rosczyk, and Jill Solomon—for their work building and supporting a robust family communication and genetic testing platform. Finally, the authors acknowledge the staff of the Georgia Center for Cancer Statistics at Emory University, including Mackenzie Crawford and Richard Claxton, for their work recruiting the patient sample for this ambitious pilot study.
APPENDIX
TABLE A1.
PV Genes Selected for the Patient Sample and Reported by Eligible Respondents (ordered by frequency of report)
Allison Kurian
Other Relationship: Ambry Genetics, Color Genomics, GeneDx/BioReference, InVitae, Genentech, Myriad Genetics, Adela
No other potential conflicts of interest were reported.
SUPPORT
The Rogel Cancer Center Fund for Discovery, University of Michigan; the collection of cancer incidence data in Georgia was supported by contract HHSN261201800003I, Task Order HHSN26100001 from the NCI, and cooperative agreement 6NU58DP006352-05-01 from the CDC.
K.W. and L.A. contributed equally to this work.
AUTHOR CONTRIBUTIONS
Conception and design: Steven J. Katz, Paul Abrahamse, Rachel Hodan, Allison W. Kurian, Aaron Rankin, Rachel S. Tocco, Kevin C. Ward, Lawrence C. An
Financial support: Steven J. Katz
Administrative support: Aaron Rankin
Provision of study materials or patients: Aaron Rankin, Kevin C. Ward
Collection and assembly of data: Steven J. Katz, Rachel Hodan, Allison W. Kurian, Aaron Rankin, Rachel S. Tocco, Sonia Rios-Ventura, Kevin C. Ward, Lawrence C. An
Data analysis and interpretation: Steven J. Katz, Paul Abrahamse MA, Rachel Hodan, Allison W. Kurian, Rachel S. Tocco, Kevin C. Ward, Lawrence C. An
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Cascade Genetic Risk Education and Testing in Families With Hereditary Cancer Syndromes: A Pilot Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Allison Kurian
Other Relationship: Ambry Genetics, Color Genomics, GeneDx/BioReference, InVitae, Genentech, Myriad Genetics, Adela
No other potential conflicts of interest were reported.
REFERENCES
- 1.Committee on Gynecologic Practice : ACOG Committee Opinion No. 727: Cascade testing: Testing women for known hereditary genetic mutations associated with cancer. Obstet Gynecol 131:e31-e34, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Network NCC : Genetic/familial high-risk assessment: Breast, ovarian and Pancreatic (Version 2.2022). 2021. https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf [Google Scholar]
- 3.Offit K, Tkachuk KA, Stadler ZK, et al. : Cascading after peridiagnostic cancer genetic testing: An alternative to population-based screening. J Clin Oncol 38:1398-1408, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samimi G, Bernardini MQ, Brody LC, et al. : Traceback: A proposed framework to increase identification and genetic counseling of BRCA1 and BRCA2 mutation Carriers through family-based outreach. J Clin Oncol 35:2329-2337, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caswell-Jin JL, Zimmer AD, Stedden W, et al. : Cascade genetic testing of relatives for hereditary cancer risk: Results of an online initiative. J Natl Cancer Inst 111:95-98, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griffin NE, Buchanan TR, Smith SH, et al. : Low rates of cascade genetic testing among families with hereditary gynecologic cancer: An opportunity to improve cancer prevention. Gynecol Oncol 156:140-146, 2020 [DOI] [PubMed] [Google Scholar]
- 7.Whitaker KD, Obeid E, Daly MB, et al. : Cascade genetic testing for hereditary cancer risk: An underutilized tool for cancer prevention. JCO Precis Oncol 5:1387-1396, 2021 [DOI] [PubMed] [Google Scholar]
- 8.Kurian AW, Katz SJ: Emerging opportunity of cascade genetic testing for population-wide cancer prevention and control. J Clin Oncol 38:1371-1374, 2020 [DOI] [PubMed] [Google Scholar]
- 9.Dragojlovic N, Borle K, Kopac N, et al. : The composition and capacity of the clinical genetics workforce in high-income countries: A scoping review. Genet Med 22:1437-1449, 2020 [DOI] [PubMed] [Google Scholar]
- 10.Raspa M, Moultrie R, Toth D, et al. : Barriers and facilitators to genetic service delivery models: Scoping review. Interact J Med Res 10:e23523, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.gov Clinicaltrials : The Genetic Information and Family Testing (GIFT) Study—Study Record Detail. NIH. 2021. https://clinicaltrials.gov/ct2/show/NCT05552664?titles=GIFT&draw=2&rank=1 [Google Scholar]
- 12.Kurian AW, Ward KC, Abrahamse P, et al. : Time trends in receipt of germline genetic testing and results for women diagnosed with breast cancer or ovarian cancer, 2012-2019. J Clin Oncol 39:1631-1640, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Color's Family Testing Program : Color. 2023. https://www.color.com/family-testing-program [Google Scholar]
- 14.Flexible Follow-Up Testing : Invitae. 2023. https://www.invitae.com/en/providers/follow-up-testing [Google Scholar]
- 15.Our Tests - CancerNext—Expanded : Ambry Genetics. 2023. https://www.ambrygen.com/providers/genetic-testing/28/oncology/cancernext-expanded [Google Scholar]
- 16.Targeted Varient Testing . Gene Dx. 2023. https://www.genedx.com/tests/targeted-variant-testing [Google Scholar]
- 17.Oncology ASoC : Special Issues in Cancer Risk Counseling. ASCO, 2022. https://www.asco.org/news-initiatives/current-initiatives/genetics-toolkit/special-issues-cancer-risk-counseling [Google Scholar]
- 18.Bednar EM, Sun CC, McCurdy S, et al. : Assessing relatives' readiness for hereditary cancer cascade genetic testing. Genet Med 22:719-726, 2020 [DOI] [PubMed] [Google Scholar]
- 19.Schwartz MD: Identification of BRCA1 and BRCA2 mutation Carriers through a traceback framework: Consent, privacy, and autonomy. J Clin Oncol 35:2226-2228, 2017 [DOI] [PubMed] [Google Scholar]
- 20.Frey MK, Kahn RM, Chapman-Davis E, et al. : Prospective feasibility trial of a novel Strategy of facilitated cascade genetic testing using telephone counseling. J Clin Oncol 38:1389-1397, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nitecki R, Moss HA, Watson CH, et al. : Facilitated cascade testing (FaCT): A randomized controlled trial. Int J Gynecol Cancer 31:779-783, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Makhnoon S, Tran G, Levin B, et al. : Uptake of cancer risk management strategies among women who undergo cascade genetic testing for breast cancer susceptibility genes. Cancer 127:3605-3613, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]