PURPOSE
Optimal comprehensive survivorship care is insufficiently delivered. To increase patient empowerment and maximize the uptake of multidisciplinary supportive care strategies to serve all survivorship needs, we implemented a proactive survivorship care pathway for patients with early breast cancer at the end of primary treatment phase.
METHODS
Pathway components included (1) a personalized survivorship care plan (SCP), (2) face-to-face survivorship education seminars and personalized consultation for supportive care referrals (Transition Day), (3) a mobile app delivering personalized education and self-management advice, and (4) decision aids for physicians focused on supportive care needs. A mixed-methods process evaluation was performed according to the Reach, Effectiveness, Adoption, Implementation and Maintenance framework including administrative data review, pathway experience survey (patient, physician, and organization), and focus group. The primary objective was patient-perceived satisfaction with the pathway (predefined progression criteria for pathway continuation ≥70%).
RESULTS
Over 6 months, 321 patients were eligible for the pathway and received a SCP and 98 (30%) attended the Transition Day. Among 126 patients surveyed, 77 (66.1%) responded. 70.1% received the SCP, 51.9% attended the Transition Day, and 59.7% accessed the mobile app. 96.1% of patients were very or completely satisfied with the overall pathway, whereas perceived usefulness was 64.8% for the SCP, 90% for the Transition Day, and 65.2% for the mobile app. Pathway implementation seemed to be positively experienced by physicians and the organization.
CONCLUSION
Patients were satisfied with a proactive survivorship care pathway, and the majority reported that its components were useful in supporting their needs. This study can inform the implementation of survivorship care pathways in other centers.
INTRODUCTION
Survival rates after early-stage breast cancer (BC) now exceed 80% at 10 years, thanks to early detection and more suitable multimodal diagnostic and therapeutic strategies.1,2 Nevertheless, survivors of BC often face physical, psychologic, and social burdens that are direct byproducts of cancer and its treatments.3-6 This represents up to 50% of patients living with at least one distressing long-term physical symptom,3 30% facing emotional distress,5 and 20% struggling to rejoin the workplace.4
CONTEXT
Key Objective
How can we improve the delivery of comprehensive survivorship care after breast cancer (BC) treatment?
Knowledge Generated
We have implemented a proactive survivorship care pathway for patients with early BC at the end of their primary treatment phase. Pathway components included: (1) a personalized survivorship care plan, (2) face-to-face survivorship education seminars and personalized consultation for supportive care referrals (Transition Day), (3) a mobile app delivering personalized education and self-management advice, and (4) decision aids for physicians focused on supportive care needs.
Relevance
In our pilot implementation study, patients were satisfied with the survivorship care pathway and the majority reported that they were useful in supporting their needs. This study may inform the implementation of survivorship care pathways in other institutions.
Significant efforts have been made to develop a survivorship framework to guide clinical care delivery.7-9 It is now consensual that optimal comprehensive survivorship care delivery needs to go beyond screening of recurrences and new malignancies and include health promotion, identification and management of physical and psychosocial needs, and proper attention to concomitant chronic health conditions.8,10-12
However, a comprehensive approach that serves all survivorship domains is insufficiently delivered in clinical practice.13,14 This may be related to implementation challenges such as lack of health care professional awareness and training and prioritization of survivorship, organizational barriers including the need for dedicated time slots in clinics to allow for multidisciplinary assessment and management involving a range of subspecialty professionals and supportive care experts, articulation with appropriate referral networks, and reimbursement issues.15-19
To address these unmet needs, we cocreated with multiple stakeholders, including patients, a proactive care pathway in the institution to empower and support survivors of BC. During the pilot implementation phase,20 we conducted a mixed-methods study to describe patient experience regarding perceived satisfaction and usefulness of the pathway and to study the implementation process guided by the Reach, Effectiveness, Adoption, Implementation, and Maintenance (RE-AIM) framework.21
METHODS
Intervention—Survivorship Care Pathway
Intervention Development and Core Components.
A multidisciplinary team comprising breast medical oncologists, radiation oncologists, patient representatives, gynecologists, implementation science researchers, sociologists, nurses, psychologists, psychiatrists, sexologists, nutritionists, physical therapists, hospital managers, and additional supportive care symptom management experts was created to develop the pathway components and plan its implementation according to pre-existing institutional workflows. The operational team met periodically every 21 days for 6 months before implementation and every 28 days thereafter to monitor the implementation. Three representatives of the team (M.A.F., I.V.-L., and E.M.) officially met with patient representatives at four key moments to foster cocreation: (1) at conceptualization to identify survivors' needs, (2) during development of pathway components giving input and ideas of components to prioritize and how to model them, (3) just before implementation, and (4) after implementation. In addition, a patient representative was a member of the operational team (J.A.).
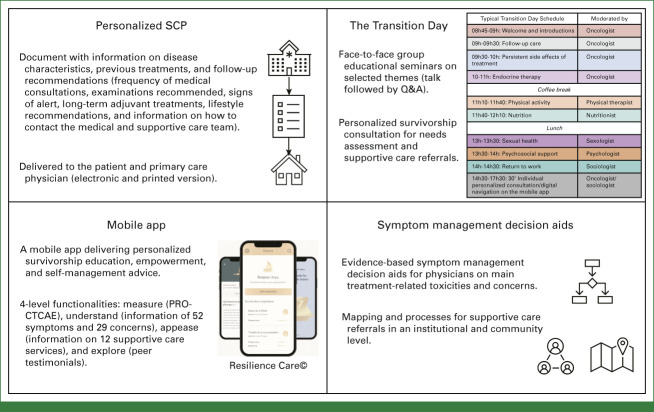
The final pathway included four components: (1) personalized survivorship care plan (SCP), (2) face-to-face survivorship education seminars and personalized survivorship consultation for needs assessment and supportive care referrals (Transition Day), (3) mobile app delivering personalized education and self-management advice, and (4) decision aids for physicians focused on supportive care needs (Fig 1). A detailed description of each component is available in the Supplementary Material (Data Supplement, online only, Survivorship Care Pathway Components).
FIG 1.
Pathway components (personalized SCP, the Transition Day, mobile app, and decision aids for physicians focused on supportive care needs). Digital navigation on the mobile application is offered during The Transition Day. For detailed information in each one of the components refer to the Supplementary Material (Data Supplement, Pages 3-7). PRO-CTCAE, patient-reported outcome Common Terminology Criteria for Adverse Events; Q&A, question and answer; SCP, survivorship care plan.
Setting, Participants, and Inclusion Workflow.
The pathway and all its components were systematically proposed in a real-world setting for all patients with early BC (stages I-III) who had completed their primary treatment (surgery with or without systemic therapy with or without radiotherapy). A pathway manager was responsible for screening and flagging all eligible patients to the assisting physician with a reminder of pathway eligibility 1 day before the end of the treatment visit (ie, visit preceding postprimary treatment follow-up). Patients were informed about the pathway and offered participation/access to its components by (1) their treating oncologist at the end of the treatment visit, (2) e-mail invite sent by the pathway manager, and (3) written information inside the SCP document.
Implementation Evaluation.
Guided by the RE-AIM framework (Supplementary Table 1, Data Supplement), we used a combination of quantitative and qualitative methods for process evaluation assessing the reach, potential for adoption, while identifying barriers to implementation and strategies to permanently integrate the pathway into the current model of care at our institution. The effectiveness of this pathway in BC outcomes was not formally evaluated in this study. Nevertheless, patients, physicians, and organizational experiences were assessed, as well as the capacity of the pathway to detect supportive care needs.
This study was approved by the Institutional Scientific Committee and registered in the French Health Data Hub (No. F20211025144254). No written informed consent was required; nevertheless, all participants received a participation informative form explaining the study scope and detailed aspects regarding voluntary participation, data rights, and data protection.
Data Sources
Administrative data review included the number of patients who completed their primary treatment and were therefore eligible for the pathway, number of SCPs delivered, and Transition Day data (number of patients attending and reasons for declining participation, time interval between the end of treatment and attendance, number of seminars delivered and experts who delivered, and survivorship care unmet needs detected).
Pathway Experience Survey
An online Pathway Experience Survey was conducted to understand pathway delivery, perceived utility, and acceptability at three levels: patients, physicians, and organization.
The Patient Pathway Experience Survey was sent to all patients who had finished their primary BC treatment, regardless of their participation in the pathway activities, to allow evaluation of reach. Surveys were sent 4 weeks after the end of the treatment visit or after participation in the Transition Day.
Patients were asked to rate their satisfaction with the overall pathway and perceived usefulness of each component on a 5-point Likert scale. Questions regarding pathway delivery (access, delivery method, content, and format) were also included. An open field was used to collect additional suggestions and remarks. Clinical, sociodemographic, and health literacy aspects22 and previous engagement with technology were also collected.
The Physician Pathway Experience Survey was sent to BC physicians who referred patients to the pathway to assess their engagement with the pathway and perceived utility and acceptability of each component, and suggestions for improvement were collected.
The Organization Pathway Experience Survey was sent to the Implementation Multidisciplinary Team to better understand their experience and satisfaction with their work activities, as well as team behavior spirit, adjustments to the general routine, and work performed in the organization after pathway implementation.
Focus Groups
Focus groups were conducted with a sample of survivors of BC who were asked to test the mobile app by teleconference and guided by a predefined script (Focus Group Guide—Supplementary Material, Data Supplement). Focus groups aimed to explore barriers and facilitators for engaging with a mobile app; insights into perceived utility, acceptability, and health impacts; preferred format, length, and language of the content; factors influencing engagement; and suggestions for improvement. The focus groups were moderated by a trained sociologist unknown to the research participants and were conducted until thematic saturation was reached.
Statistical and Qualitative Analyses
The primary study outcome was patient satisfaction and perceived usefulness of the proactive pathway, as assessed in the Patient Pathway Experience Survey. Following recommendations for sample size estimations for pilot studies23-25 and mirroring similar pilot implementation studies,26-32 a minimum of 50 answers to the overall survey were prespecified as needed to provide an initial assessment of patient experience/satisfaction. Pathway progression criteria were defined as at least 70% of the patients receiving the entire pathway reporting to be very satisfied or completely satisfied.
Continuous data were summarized as means and standard deviations, and categorical data as frequencies and percentages.
Qualitative data were recorded after the patients provided verbal consent and transcribed for thematic analysis33 using NVivo version 12.0.
RESULTS
Administrative Data Review
From October 2021 to April 2022, 321 patients were eligible for the pathway and received an SCP (75% delivered in real time) and 98 (30%) patients attended the Transition Day. Tracked reasons for refusals to attend the Transition Day included lack of interest (56%), distance constraints (21%), time constraints (10%), language barrier (7%), and mobility constraints (6%).
A total of 153 seminars were delivered during 17 Transition Days. Intervention fidelity (ie, delivery as planned) was maintained during 96% of the seminars, and owing to a temporary unavailability of physical therapists, six practical seminars of physical activity were replaced by a practice of mindfulness delivered by a trained psychologist. Nevertheless, educational information on the benefits of physical activity to survivors of BC was still delivered in all Transition Days. Most patients (57%) attended the Transition Day <2 months after the end of their primary treatment, 10% between 2 and 2.9 months, and 23% more than 3 months.
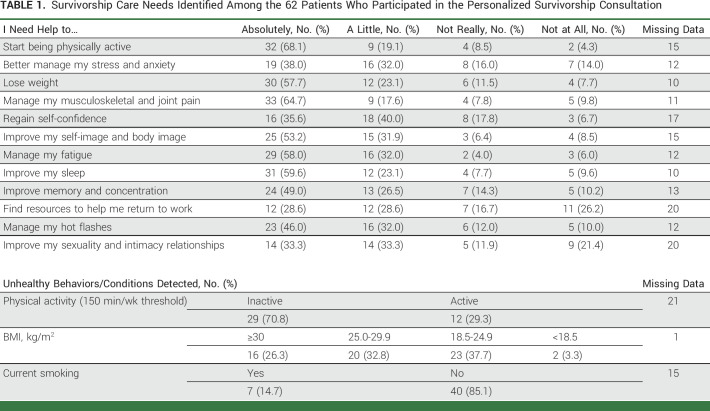
During the first 6 months of the pathway, 288 needs (including physical and psychosocial domains) and 72 unhealthy conditions were detected among 62 patients who participated in the personalized consultation (Table 1).
TABLE 1.
Survivorship Care Needs Identified Among the 62 Patients Who Participated in the Personalized Survivorship Consultation
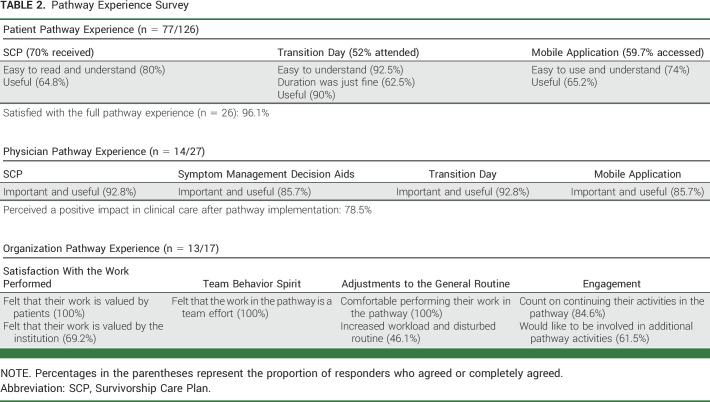
A summary of the Pathway Experience Survey at the patient, physician, and organizational levels is presented in Table 2.
TABLE 2.
Pathway Experience Survey
Patient Pathway Experience Survey
Among the 126 patients surveyed, 77 (61.1%) responded; 54 (70.1%) received the SCP, 40 (51.9%) attended the Transition Day, and 46 (59.7%) accessed the mobile app. The clinical and sociodemographic data of the survey participants are presented in Supplementary Table 2 (Data Supplement).
Twenty-six (33.8%) patients who answered the survey participated in the full pathway experience (SCP, Transition Day, and mobile app), with 25 patients (96.1%) reporting to be completely satisfied or very satisfied.
Regarding specific aspects of each pathway component, from the 54 patients who received the SCP, 44 (81.5%) agreed or completely agreed that the document was easy to read and understand and 35 (64.8%) agreed with its usefulness. From the 40 patients who attended the Transition Day, 37 (92.5%) agreed or completely agreed that the seminars were easy to understand, 36 (90.0%) agreed with its usefulness, and 25 (62.5%) agreed that the duration was just fine. Among the 46 mobile app users, 44 (73.9%) agreed or completely agreed that it was easy to use and understand and 30 (65.2%) agreed with its usefulness.
Of note, a very low proportion of patients considered the pathway components as not useful (three patients for the SCP, two patients for the mobile app, and one patient for the Transition Day). Importantly, seven patients (9.1%) reported that they were not invited to attend the Transition Day and 18 (23.4%) did not receive information on how to access the mobile app. Of the 77 patients who answered the survey, 37 (48.0%) agreed or completely agreed that they would have liked the possibility of participating in the Transition Day virtually. Of patients who attended the in-person Transition Day, 16 (40.0%) agreed or completely agreed with the virtual format.
The main themes emerging from the free-text responses were (1) feeling of having their needs addressed, (2) feeling of not being abandoned, and (3) satisfaction with the care team. For negative experiences, the emerging themes detected were (1) need for improvement in intervention delivery and (2) need for a continuous care (Supplementary Table 3, Data Supplement).
Physician Pathway Experience Survey
Among the 27 oncologists involved in BC care, 14 (51.8%) replied to the survey. Thirteen physicians (92.8%) agreed or completely agreed on the importance and utility of the SCP, 12 (85.7%) for decision aids, 13 (92.8%) for the themes explored in the Transition Day, and 12 (85.7%) for the content of the mobile app. In addition, even if there was no real-time transmission of the symptoms reported in the mobile app to the health care providers, seven physicians (50.0%) agreed or completely agreed that the presence of the mobile app facilitated symptom management discussions with the patient. Eleven physicians (78.6%) agreed that the whole pathway had a positive impact in clinics.
Organizational Experience
Among the 17 members of the Multidisciplinary Implementation Team, 13 (76.5%) responded to the survey. Their responses indicated a high level of satisfaction with the work performed in the pathway, full agreement that the patients valued their work, that the pathway was a result of a team effort, and that they felt comfortable performing their work in the pathway. Moreover, 11 (84.6%) responders expected to continue their activities in the pathway, and eight (61.5%) would like to be involved in more pathway-related activities. Increased workload and disturbance of the general routine were reported by six (46.1%) responders.
Focus Groups
Seventeen patients from diverse professional and socioeconomic background (Supplementary Table 4, Data Supplement) participated in three focus groups after testing the mobile app. There was a consensus regarding the acceptability and perception of the usefulness of a mobile app delivering survivorship education, especially during adjuvant endocrine therapy. The emergent themes and quotes are provided in Supplementary Table 5 (Data Supplement).
DISCUSSION
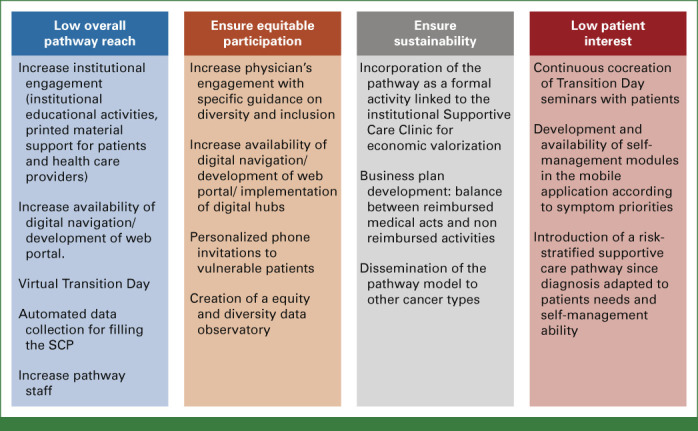
This study describes our institutional experience of cocreating and delivering a proactive survivorship care pathway for survivors of BC. Our pathway enabled (1) delivery of a clear and structured personalized SCP to the patient and primary care physician, (2) in-person and at-home patient education and self-management, (3) promotion of healthy behaviors, (4) physical and psychosocial toxicity management and activation of multidisciplinary internal and external referrals, and (5) articulation with community services and primary health care physicians. With 96.1% of patients who received the whole pathway reporting to be very satisfied or completely satisfied, this study demonstrated preliminary patient satisfaction and perceived usefulness of a proactive survivorship care pathway. A total of 288 needs and 72 unhealthy conditions were identified and addressed in 62 patients. According to the RE-AIM evaluation,21 during this pilot process evaluation, our pathway presented a moderate reach, with moderate to high acceptance rates. Key actionable factors influencing reach and acceptance were identified, including physician engagement, patient interests and preferences, and logistics and equity considerations (Appendix Fig A1, online only).
Several lessons can be noted from this pilot implementation phase.
Our workflow highlights the advantages of leveraging team medicine34 to cocreate a purposeful, evidence-based, and deliverable pathway. Survivorship care is multidisciplinary8 and, therefore, should be built on a network with various stakeholders, including supportive care specialists, psychosocial support professionals, administrative staff, community and primary care services, and patient representatives. Strategies such as (1) building institutional symptom management decision aids, (2) defining clear and structured referral processes, and (3) hosting educational events to increase awareness of survivorship issues may be a starting block to building this multidisciplinary dynamic. This exercise also helped us identify gaps in institutional supportive care resources and where specific recruitment or externalization of care was needed. Institutional leadership played an important role in the implementation of this pilot study by prioritizing survivorship care and facilitating funding and resources.
Nevertheless, multidisciplinary dynamics, institutional support, and implementing the pathway may not necessarily lead to optimal reach and acceptance. As observed in our results, only 30% of eligible patients attended the Transition Day and 60% used the mobile app. In addition, only 51% of the physicians responded to our survey, perhaps reflecting their low engagement. Although a positive organizational experience was observed, 41% of the multidisciplinary implementation team highlighted disturbances in work routines after pathway implementation. This is not surprising since previous literature has demonstrated that even robust survivorship programs face challenges and implementation struggles.35 Actions that can be implemented to help overcome these challenges include educational activities for increasing physician awareness, inclusion of digital solutions for minimizing time and distance-related burden (eg, virtual Transition Day), formal economic valorization with protected time for pathway delivery, and continuous cocreation of pathway components with patients to ensure the that they respond to their needs. Moreover, an upfront identification of patients with very low clinical complexity and high self-management skills and who do not need extensive pathway resources is desirable.
We acknowledge that our survey responders mainly included highly educated patients with high health literacy levels.36,37 Vulnerable patients are often excluded from health care innovations and might have limited access to digital devices.38,39 There is a major need to ensure that our pathway is accessible, inclusive, and positively experienced by all patients. Specific guidance for health care professionals for diversity and inclusion in pathway activities, availability of nurses and digital navigators, technology hubs, and personalized support for vulnerable patients since diagnosis are actions that can be implemented to ensure equitable pathway access.16,40-42 Furthermore, a diversity data observatory with a minimum set of sociodemographic characteristics of the participants in each pathway component is being created.
The overall positive experience reported by our patients when participating in the pathway activities probably reflects care coordination and survivorship care need. Importantly, a previous systematic review of 24 articles comprising 11 nonrandomized and 13 randomized studies concluded that the delivery of a SCP alone is unlikely to achieve the desired benefits, and a more comprehensive approach to survivorship care delivery is needed.43,44 On the contrary, randomized clinical studies have also demonstrated that survivorship education can have a positive impact on emotional distress45 and a proactive screening and management of treatment-related side effects can improve needs assessment46 and symptom control.47 Considering these data, our pathway was designed to be a multimodal strategy including not only a personalized SCP but also care coordination and supportive care referrals added to education and self-management support delivered in person and digitally by a mobile app.
The creation of new care pathway delivery methods and provision of novel technologies and infrastructures to support self-management have been flagged as research priorities in survivorship care.9,48 This study revealed the promising acceptability and perceived usefulness of the pilot version of our mobile app and highlighted suggestions for increasing its adoption. Randomized clinical studies have demonstrated that digital health may play an important role in facilitating patient empowerment and improving symptom management in survivors of BC.49-54 However, these were mainly fragmented, symptom-specific interventions, and the role of a comprehensive digital companion allowing continuous education, self-management, and monitoring is not yet established in cancer survivors.55,56 Moreover, social determinants of health may affect the adoption of digital health, requiring the personalization of digital devices and navigation.57-59 On the basis of the initial feedback of this study, the mobile app was refined with encouraging feedback, evidence-based digital self-management programs, and biosensor connections. It has also been enriched with additional educational content and remote monitoring features to accompany the patient from the time of diagnosis and primary treatment phase to survivorship care. A detailed evaluation of the usage of the mobile app according to clinical and sociodemographic characteristics is planned in a future study.
Finally, although we could deliver our pathway in a very early post-treatment survivorship phase (<3 months after the end of treatment), we believe that to be transformative, survivorship care should start at the time of cancer diagnosis.8,15,18,60 This would allow prevention or early recognition of treatment-related side effects and concerns and addressing of patient's needs across the whole cancer care continuum in a timely manner, potentially facilitating reach and acceptability. Previous data have demonstrated considerable interindividuality in the onset, persistence, and longitudinal evolution of treatment-related symptoms,5,61,62 and therefore, some patients may need more care and resources than others. Stratifying patients to different pathways of care according to the individual risk of toxicity development could be a suitable model of care.60 Considering individual's ability to self-manage and social determinants of health in the stratification model may be crucial, especially when using digital health to enable self-management and facilitate care delivery.
The present study describes the implementation workflow of a BC survivorship care pathway and highlights implementation challenges and suggestions for program improvements, which may be useful for cancer centers building survivorship care programs. Our findings suggest that the pathway is acceptable and perceived as useful for patients starting the post-treatment survivorship phase, but ensuring reach is challenging. The implementation seemed to be well-perceived by physicians and the organization. Limitations should be acknowledged, including the small sample size, single-institution experience, and proportion of highly educated participants.
The evolution of this model toward the implementation of a continuous personalized, proactive care pathway from the moment of diagnosis is ongoing and will be tested in future clinical trials.
ACKNOWLEDGMENT
We thank all the patients who participated in this study and all participants who contributed to the implementation of this pathway, particularly Sandra Doucène, Alexis Imbert, Christine Pailler, Sofia Rivera, Sophie Bockel, Guillaume Louvel, Karima Mezaib, Khalida Berkane, Pascal Rouby, Jessica Klein, Muriel Helou, Ariane Cavaviuti, Capucine Sallard Lallane Berdoutico, Estelle Favre, Alice Maurel, Severine Guezennec, Valérie Lapierre, Daniele Presti, Pietro Lapidari, and Nathalie Al Rassy. This work was funded by a Conquer Cancer – Breast Cancer Research Foundation Career Development Award for Diversity and Inclusion, supported by Breast Cancer Research Foundation. Any opinions, findings, and conclusions expressed in this material are those of the author(s) and do not necessarily reflect those of the American Society of Clinical Oncology or Conquer Cancer, or Breast Cancer Research Foundation.
APPENDIX
FIG A1.
Implementation barriers and strategies to overcome.
Maria Alice Franzoi
Research Funding: Resilience Care (Inst)
Marion Aupomerol
Honoraria: Lilly
Expert Testimony: Novartis
Travel, Accommodations, Expenses: Menarini
Davide Soldato
Honoraria: AstraZeneca
Travel, Accommodations, Expenses: Novartis
Antonio Di Meglio
Expert Testimony: Kephren, techspert.io
Léonor Fasse
Employment: GlaxoSmithKline
Consulting or Advisory Role: Pierre Fabre
Research Funding: GlaxoSmithKline, Amgen Foundation (Inst)
Céline Lazorthes
Employment: Resilience Care
Leadership: Resilience Care
Stock and Other Ownership Interests: Resilience Care
Mario di Palma
Honoraria: AstraZeneca, Novartis
Consulting or Advisory Role: Sandoz
Speakers' Bureau: Amgen, Kyowa Kirin International, MSD Oncology, Mundipharma, Sandoz, Roche
Research Funding: Bayer, Sandoz (Inst), Pierre Fabre (Inst), Fresenius Kabi, Astellas Pharma (Inst), Janssen Oncology (Inst), Roche (Inst), Sanofi (Inst)
Travel, Accommodations, Expenses: Pfizer, Novartis
Suzette Delaloge
Consulting or Advisory Role: AstraZeneca (Inst), Sanofi (Inst), Besins Healthcare (Inst), Rappta Therapeutics (Inst), Gilead Sciences (Inst)
Research Funding: AstraZeneca (Inst), Pfizer (Inst), Roche/Genentech (Inst), Puma Biotechnology (Inst), Lilly (Inst), Novartis (Inst), Sanofi (Inst), Exact Sciences (Inst), Bristol Myers Squibb (Inst), Taiho Pharmaceutical (Inst)
Travel, Accommodations, Expenses: Pfizer, AstraZeneca, Novartis (Inst)
Barbara Pistilli
Consulting or Advisory Role: Puma Biotechnology, Pierre Fabre, Novartis, Myriad Genetics, AstraZeneca, Daiichi Sankyo/UCB Japan
Research Funding: Pfizer (Inst), Puma Biotechnology (Inst), Merus (Inst), Daiichi-Sankyo (Inst), Gilead Sciences (Inst), AstraZeneca (Inst)
Travel, Accommodations, Expenses: Pfizer, AstraZeneca, MSD Oncology, Novartis, Pierre Fabre, Daiichi Sankyo Europe GmbH
Florian Scotté
Honoraria: Leo Phar, Viatris, Pharmanovia, Amgen, Gilead Sciences, BMS GmbH & Co. KG, GlaxoSmithKline
Ines Vaz-Luis
Honoraria: AstraZeneca (Inst), Amgen (Inst), Pfizer (Inst), Novartis (Inst), Sandoz
Research Funding: Resilience Care (Inst)
No other potential conflicts of interest were reported.
DISCLAIMER
Any opinions, findings, and conclusions expressed in this material are those of the author(s) and do not necessarily reflect those of the American Society of Clinical Oncology or Conquer Cancer, or Breast Cancer Research Foundation.
PRIOR PRESENTATION
Presented in part as a poster at the 2022 ASCO Quality Care Symposium.
SUPPORT
M.A.F. is supported by a Conquer Cancer—Breast Cancer Research Foundation Career Development Award for Diversity and Inclusion in Breast Cancer Research. This study is supported by the Gustave Roussy Foundation (INTERVAL to I.V.-L.).
AUTHOR CONTRIBUTIONS
Conception and design: Maria Alice Franzoi, Lena Degousée, Marion Aupomerol, Antonio Di Meglio, Camila Chiodi, Hajer Chaouachi, Joana Ribeiro, Jean Bernard Le-Provost, Johanna Arvis, Anne de Jesus, Bruno Raynard, Suzette Delaloge, Ines Vaz-Luis
Financial support: Ines Vaz-Luis
Administrative support: Maria Alice Franzoi, Aude Barbier, Florian Scotté, Ines Vaz-Luis
Provision of study materials or patients: Diane Boinon, Léonor Fasse, Jean Bernard Le-Provost, Anne de Jesus, Suzette Delaloge, Barbara Pistilli
Collection and assembly of data: Maria Alice Franzoi, Patricia Miguel Semedo, Aude Barbier, Joana Ribeiro, Suzette Delaloge, Barbara Pistilli, Ines Vaz-Luis
Data analysis and interpretation: Maria Alice Franzoi, Elise Martin, Patricia Miguel Semedo, Davide Soldato, Antonio Di Meglio, Nathalie Renvoisé, Diane Boinon, Léonor Fasse, Céline Lazorthes, Mario di Palma, Arnaud Pagès, Suzette Delaloge, Florian Scotté, Ines Vaz-Luis
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Implementing a PROACTive Care Pathway to Empower and Support Survivors of Breast Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Maria Alice Franzoi
Research Funding: Resilience Care (Inst)
Marion Aupomerol
Honoraria: Lilly
Expert Testimony: Novartis
Travel, Accommodations, Expenses: Menarini
Davide Soldato
Honoraria: AstraZeneca
Travel, Accommodations, Expenses: Novartis
Antonio Di Meglio
Expert Testimony: Kephren, techspert.io
Léonor Fasse
Employment: GlaxoSmithKline
Consulting or Advisory Role: Pierre Fabre
Research Funding: GlaxoSmithKline, Amgen Foundation (Inst)
Céline Lazorthes
Employment: Resilience Care
Leadership: Resilience Care
Stock and Other Ownership Interests: Resilience Care
Mario di Palma
Honoraria: AstraZeneca, Novartis
Consulting or Advisory Role: Sandoz
Speakers' Bureau: Amgen, Kyowa Kirin International, MSD Oncology, Mundipharma, Sandoz, Roche
Research Funding: Bayer, Sandoz (Inst), Pierre Fabre (Inst), Fresenius Kabi, Astellas Pharma (Inst), Janssen Oncology (Inst), Roche (Inst), Sanofi (Inst)
Travel, Accommodations, Expenses: Pfizer, Novartis
Suzette Delaloge
Consulting or Advisory Role: AstraZeneca (Inst), Sanofi (Inst), Besins Healthcare (Inst), Rappta Therapeutics (Inst), Gilead Sciences (Inst)
Research Funding: AstraZeneca (Inst), Pfizer (Inst), Roche/Genentech (Inst), Puma Biotechnology (Inst), Lilly (Inst), Novartis (Inst), Sanofi (Inst), Exact Sciences (Inst), Bristol Myers Squibb (Inst), Taiho Pharmaceutical (Inst)
Travel, Accommodations, Expenses: Pfizer, AstraZeneca, Novartis (Inst)
Barbara Pistilli
Consulting or Advisory Role: Puma Biotechnology, Pierre Fabre, Novartis, Myriad Genetics, AstraZeneca, Daiichi Sankyo/UCB Japan
Research Funding: Pfizer (Inst), Puma Biotechnology (Inst), Merus (Inst), Daiichi-Sankyo (Inst), Gilead Sciences (Inst), AstraZeneca (Inst)
Travel, Accommodations, Expenses: Pfizer, AstraZeneca, MSD Oncology, Novartis, Pierre Fabre, Daiichi Sankyo Europe GmbH
Florian Scotté
Honoraria: Leo Phar, Viatris, Pharmanovia, Amgen, Gilead Sciences, BMS GmbH & Co. KG, GlaxoSmithKline
Ines Vaz-Luis
Honoraria: AstraZeneca (Inst), Amgen (Inst), Pfizer (Inst), Novartis (Inst), Sandoz
Research Funding: Resilience Care (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Shapiro CL: Cancer survivorship. N Engl J Med 379:2438-2450, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fuchs HE, et al. : Cancer statistics, 2022. CA Cancer J Clin 72:7-33, 2022 [DOI] [PubMed] [Google Scholar]
- 3.Ferreira AR, Di Meglio A, Pistilli B, et al. : Differential impact of endocrine therapy and chemotherapy on quality of life of breast cancer survivors: A prospective patient-reported outcomes analysis. Ann Oncol 30:1784-1795, 2019 [DOI] [PubMed] [Google Scholar]
- 4.Dumas A, Vaz Luis I, Bovagnet T, et al. : Impact of breast cancer treatment on employment: Results of a multicenter prospective cohort study (CANTO). J Clin Oncol 38:734-743, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charles C, Bardet A, Larive A, et al. : Characterization of depressive symptoms trajectories after breast cancer diagnosis in women in France. JAMA Netw Open 5:e225118, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pistilli B, Paci A, Ferreira AR, et al. : Serum detection of nonadherence to adjuvant tamoxifen and breast cancer recurrence risk. J Clin Oncol 38:2762-2772, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Institute of Medicine and National Research Council : From Cancer Patient to Cancer Survivor: Lost in Transition. 2005. https://nap.nationalacademies.org/catalog/11468/from-cancer-patient-to-cancer-survivor-lost-in-transition [Google Scholar]
- 8.Nekhlyudov L, Mollica MA, Jacobsen PB, et al. : Developing a quality of cancer survivorship care framework: Implications for clinical care, research, and policy. J Natl Cancer Inst 111:1120-1130, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaz-Luis I, Masiero M, Cavaletti G, et al. : ESMO expert consensus statements on cancer survivorship: Promoting high-quality survivorship care and research in Europe. Ann Oncol 33:1119-1133, 2022 [DOI] [PubMed] [Google Scholar]
- 10.Runowicz CD, Leach CR, Henry NL, et al. : American Cancer Society/American Society of Clinical Oncology breast cancer survivorship care guideline. CA Cancer J Clin 66:43-73, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Cardoso F, Kyriakides S, Ohno S, et al. : Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 30:1194-1220, 2019 [DOI] [PubMed] [Google Scholar]
- 12.Khatcheressian JL, Hurley P, Bantug E, et al. : Breast cancer follow-up and management after primary treatment: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 31:961-965, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Brauer ER, Long EF, Petersen L, et al. : Current practice patterns and gaps in guideline-concordant breast cancer survivorship care. J Cancer Surviv 10.1007/s11764-021-01152-1 [epub ahead of print on December 30, 2021] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brauer ER, Ganz PA: Moving the translational needle in breast cancer survivorship: Connecting intervention research to clinical practice. J Clin Oncol 40:2069-2073, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayer DK, Alfano CM: Personalized risk-stratified cancer follow-up care: Its potential for healthier survivors, happier clinicians, and lower costs. J Natl Cancer Inst 111:442-448, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alfano CM, Leach CR, Smith TG, et al. : Equitably improving outcomes for cancer survivors and supporting caregivers: A blueprint for care delivery, research, education, and policy. CA Cancer J Clin 69:35-49, 2019 [DOI] [PubMed] [Google Scholar]
- 17.Rohan EA, Miller N, Bonner F, et al. : Comprehensive cancer control: Promoting survivor health and wellness. Cancer Causes Control 29:1277-1285, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jefford M, Howell D, Li Q, et al. : Improved models of care for cancer survivors. Lancet 399:1551-1560, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emery J, Butow P, Lai-Kwon J, et al. : Management of common clinical problems experienced by survivors of cancer. Lancet 399:1537-1550, 2022 [DOI] [PubMed] [Google Scholar]
- 20.Jc M, Ks D, Na S, et al. : Systematic review of the Exploration, Preparation, Implementation, Sustainment (EPIS) framework. Implement Sci 14:1, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaglio B, Shoup JA, Glasgow RE: The RE-AIM framework: A systematic review of use over time. Am J Public Health 103:e38-e46, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osborne RH, Batterham RW, Elsworth GR, et al. : The grounded psychometric development and initial validation of the Health Literacy Questionnaire (HLQ). BMC Public Health 13:658, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hertzog MA: Considerations in determining sample size for pilot studies. Res Nurs Health 31:180-191, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Julious SA: Sample size of 12 per group rule of thumb for a pilot study. Pharm Stat 4:287-291, 2005 [Google Scholar]
- 25.Lewis M, Bromley K, Sutton CJ, et al. : Determining sample size for progression criteria for pragmatic pilot RCTs: The hypothesis test strikes back! Pilot Feasibility Stud 7:40, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stan DL, Inselman JW, Ridgeway JL, et al. : Pilot implementation to assess the feasibility and care team impact of an app-based interactive care plan to remotely monitor breast cancer survivors. J Cancer Surviv 16:13-23, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smale EM, van Vlijmen B, Colen HBB, et al. : Feasibility of an individualized dispensing program for patients prescribed oral anticancer drugs to prevent waste. JCO Oncol Pract 19:e618-e629, 2023 [DOI] [PubMed] [Google Scholar]
- 28.Shankaran V, Leahy T, Steelquist J, et al. : Pilot feasibility study of an oncology financial navigation program. J Oncol Pract 14:e122-e129, 2018 [DOI] [PubMed] [Google Scholar]
- 29.Petrocchi S, Filipponi C, Montagna G, et al. : A breast cancer smartphone app to navigate the breast cancer journey: Mixed methods study. JMIR Form Res 5:e28668, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leske M, Koczwara B, Blunt J, et al. : Co-designing healthy living after cancer online: An online nutrition, physical activity, and psychosocial intervention for post-treatment cancer survivors. J Cancer Surviv 1-11, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lai-Kwon J, Kelly B, Lane S, et al. : Feasibility, acceptability, and utility of a nurse-led survivorship program for people with metastatic melanoma (MELCARE). Support Care Cancer 30:9587-9596, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teare MD, Dimairo M, Shephard N, et al. : Sample size requirements to estimate key design parameters from external pilot randomised controlled trials: A simulation study. Trials 15:264, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith J, Firth J: Qualitative data analysis: The framework approach. Nurse Res 18:52-62, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Alfano CM, Oeffinger K, Sanft T, et al. : Engaging TEAM medicine in patient care: Redefining cancer survivorship from diagnosis. Am Soc Clin Oncol Educ Book 42:1-11, 2022 [DOI] [PubMed] [Google Scholar]
- 35.Hwang S, Bozkurt B, Huson T, et al. : Identifying strategies for robust survivorship program implementation: A qualitative analysis of cancer programs. JCO Oncol Pract 18:e304-e312, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bo A, Friis K, Osborne RH, et al. : National indicators of health literacy: Ability to understand health information and to engage actively with healthcare providers—A population-based survey among Danish adults. BMC Public Health 14:1095, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beauchamp A, Buchbinder R, Dodson S, et al. : Distribution of health literacy strengths and weaknesses across socio-demographic groups: A cross-sectional survey using the Health Literacy Questionnaire (HLQ). BMC Public Health 15:678, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel MI, Lopez AM, Blackstock W, et al. : Cancer disparities and health equity: A policy statement from the American Society of Clinical Oncology. J Clin Oncol 38:3439-3448, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Penman-Aguilar A, Talih M, Huang D, et al. : Measurement of health disparities, health inequities, and social determinants of health to support the advancement of health equity. J Public Health Manag Pract 22:S33-S42, 2016. (suppl 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Birhiray MN, Birhiray RE: Practical strategies for creating diversity, equity, inclusion, and access in cancer clinical research: DRIVE. Blood Adv 7:1507-1512, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oyer RA, Hurley P, Boehmer L, et al. : Increasing racial and ethnic diversity in cancer clinical trials: An American Society of Clinical Oncology and Association of Community Cancer Centers joint research statement. J Clin Oncol 40:2163-2171, 2022 [DOI] [PubMed] [Google Scholar]
- 42.Alcaraz KI, Wiedt TL, Daniels EC, et al. : Understanding and addressing social determinants to advance cancer health equity in the United States: A blueprint for practice, research, and policy. CA Cancer J Clin 70:31-46, 2020 [DOI] [PubMed] [Google Scholar]
- 43.Stuver R, Faig J, Cadorette M, et al. : Survivorship care plans: Reaching a benchmark and striving to achieve intent. JCO Oncol Pract 16:e1249-e1254, 2020 [DOI] [PubMed] [Google Scholar]
- 44.Jacobsen PB, DeRosa AP, Henderson TO, et al. : Systematic review of the impact of cancer survivorship care plans on health outcomes and health care delivery. J Clin Oncol 36:2088-2100, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bower JE, Partridge AH, Wolff AC, et al. : Targeting depressive symptoms in younger breast cancer survivors: The pathways to wellness randomized controlled trial of mindfulness meditation and survivorship education. J Clin Oncol 39:3473-3484, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Penedo FJ, Medina HN, Moreno PI, et al. : Implementation and feasibility of an electronic health record–integrated patient-reported outcomes symptom and needs monitoring pilot in ambulatory oncology. JCO Oncol Pract 18:e1100-e1113, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ganz PA, Greendale GA, Petersen L, et al. : Managing menopausal symptoms in breast cancer survivors: Results of a randomized controlled trial. J Natl Cancer Inst 92:1054-1064, 2000 [DOI] [PubMed] [Google Scholar]
- 48.Leach CR, Alfano CM, Potts J, et al. : Personalized cancer follow-up care pathways: A Delphi consensus of research priorities. J Natl Cancer Inst 112:1183-1189, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hummel SB, van Lankveld JJDM, Oldenburg HSA, et al. : Internet-based cognitive behavioral therapy for sexual dysfunctions in women treated for breast cancer: Design of a multicenter, randomized controlled trial. BMC Cancer 15:321, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beatty L, Koczwara B, Wade T: Evaluating the efficacy of a self-guided Web-based CBT intervention for reducing cancer-distress: A randomised controlled trial. Support Care Cancer 24:1043-1051, 2016 [DOI] [PubMed] [Google Scholar]
- 51.Abrahams HJG, Gielissen MFM, Donders RRT, et al. : The efficacy of internet-based cognitive behavioral therapy for severely fatigued survivors of breast cancer compared with care as usual: A randomized controlled trial. Cancer 123:3825-3834, 2017 [DOI] [PubMed] [Google Scholar]
- 52.Atema V, van Leeuwen M, Kieffer JM, et al. : Efficacy of internet-based cognitive behavioral therapy for treatment-induced menopausal symptoms in breast cancer survivors: Results of a randomized controlled trial. J Clin Oncol 37:809-822, 2019 [DOI] [PubMed] [Google Scholar]
- 53.Zachariae R, Amidi A, Damholdt MF, et al. : Internet-delivered cognitive-behavioral therapy for insomnia in breast cancer survivors: A randomized controlled trial. J Natl Cancer Inst 110:880-887, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van den Berg SW, Gielissen MFM, Custers JAE, et al. : BREATH: Web-based self-management for psychological adjustment after primary breast cancer—Results of a multicenter randomized controlled trial. J Clin Oncol 33:2763-2771, 2015 [DOI] [PubMed] [Google Scholar]
- 55.van der Hout A, van Uden-Kraan CF, Holtmaat K, et al. : Role of eHealth application Oncokompas in supporting self-management of symptoms and health-related quality of life in cancer survivors: A randomised, controlled trial. Lancet Oncol 21:80-94, 2020 [DOI] [PubMed] [Google Scholar]
- 56.van der Hout A, van Uden-Kraan CF, Holtmaat K, et al. : Reasons for not reaching or using web-based self-management applications, and the use and evaluation of Oncokompas among cancer survivors, in the context of a randomised controlled trial. Internet Interv 25:100429, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Norman CD, Skinner HA: eHEALS: The eHealth Literacy Scale. J Med Internet Res 8:e27, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kayser L, Karnoe A, Furstrand D, et al. : A multidimensional tool based on the eHealth literacy framework: Development and initial validity testing of the eHealth Literacy Questionnaire (eHLQ). J Med Internet Res 20:e36, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beauchamp A, Batterham RW, Dodson S, et al. : Systematic development and implementation of interventions to OPtimise Health Literacy and Access (Ophelia). BMC Public Health 17:230, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Di Meglio A, Havas J, Soldato D, et al. : Development and validation of a predictive model of severe fatigue after breast cancer diagnosis: Toward a personalized framework in survivorship care. J Clin Oncol 40:1111-1123, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Di Meglio A, Havas J, Gbenou AS, et al. : Dynamics of long-term patient-reported quality of life and health behaviors after adjuvant breast cancer chemotherapy. J Clin Oncol 40:3190-3204, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vaz-Luis I, Di Meglio A, Havas J, et al. : Long-term longitudinal patterns of patient-reported fatigue after breast cancer: A group-based trajectory analysis. J Clin Oncol 40:2148-2162, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]